Abstract
The development of a functional organ requires coordinated programs of cell fate specification, terminal differentiation and morphogenesis. Whereas signaling mechanisms that specify individual cell fates are well documented, little is known about the pathways and molecules that maintain these fates stably as normal development proceeds or how their dysregulation may contribute to altered cell states in diseases such as cancer. In Drosophila, the tyrosine kinase Abelson (Abl) interfaces with multiple signaling pathways to direct epithelial and neuronal morphogenesis during embryonic and retinal development. Here we show that Abl is required for photoreceptor cell fate maintenance, as Abl mutant photoreceptors lose neuronal markers during late pupal stages but do not re-enter a proliferative state or undergo apoptosis. Failure to maintain the differentiated state correlates with impaired trafficking of the Notch receptor and ectopic Notch signaling, and can be suppressed by reducing the genetic dose of Notch or of its downstream transcriptional effector Suppressor of Hairless. Together, these data reveal a novel mechanism for maintaining the terminally differentiated state of Drosophila photoreceptors and suggest that neuronal fates in the fly retina retain plasticity late into development. Given the general evolutionary conservation of developmental signaling mechanisms, Abl-mediated regulation of Notch could be broadly relevant to cell fate maintenance and reprogramming during normal development, regeneration and oncogenic transformation.
Keywords: Retinal development, Dedifferentiation, Pupal eye, Transdifferentiation, Pigment cell, Drosophila
INTRODUCTION
Terminal differentiation involves a complex and coordinated sequence of events through which newly specified cells acquire the unique features needed to carry out their specific functions. Throughout this process, cells must retain molecular memory of their specified fate, or cell state. At present, although the molecular networks that initially specify cell types have been studied at length, the mechanisms that stabilize and maintain cell fates as development proceeds remain poorly understood. Furthermore, the extent to which different cell states retain plasticity during terminal differentiation is an open question, the answer to which has great relevance not only to normal development, but also to tissue regeneration, stem cell renewal and oncogenic transformation.
The Drosophila eye provides a valuable experimental model to study cell fate and plasticity because it develops from an unpatterned epithelium into a stereotyped three-dimensional structure with well-characterized cell types. The adult eye field comprises an array of ∼750 ommatidia, each of which contains eight photoreceptor neurons (R1-R8) that are bordered and supported by non-neuronal cone and pigment cells. Pattern formation begins in the third instar larval eye disk, where a dorsoventral indentation called the morphogenetic furrow (MF) sweeps gradually across the tissue from posterior to anterior; cells anterior to the MF remain undifferentiated and proliferative, whereas cells posterior differentiate and organize into the stereotypically configured ommatidia (reviewed by Roignant and Treisman, 2009). After the initial pattern is defined, the photoreceptors undergo extensive morphogenesis. Axons extend basally and travel through the optic stalk to innervate appropriate regions of the brain, whereas at the other end of the cell, the apical-junctional region undergoes dramatic remodeling and specialization (Longley and Ready, 1995; Ready et al., 1976). Whether and how neuronal fates are actively stabilized as this morphogenetic program unfolds is unknown.
Abelson (Abl) family nonreceptor tyrosine kinases, conserved from invertebrates to humans, serve as key regulators of cell morphogenesis, epithelial integrity, cell motility, proliferation and oncogenesis (reviewed by Pendergast, 2002). In Drosophila, Abl has been best-studied during embryogenesis, during which it interacts with a variety of signaling receptors and actin regulatory proteins to direct neuronal and epithelial morphogenesis (Bashaw et al., 2000; Crowner et al., 2003; Forsthoefel et al., 2005; Grevengoed et al., 2003; Grevengoed et al., 2001; Kuzina et al., 2011; Liebl et al., 2000; Tamada et al., 2012). More recently, roles for Abl as a dynamic regulator of photoreceptor morphogenesis and retinal patterning have been reported. For example, Abl is required for proper targeting of the photoreceptor axons to the larval brain (Xiong et al., 2009), for epithelial planar polarity (Singh et al., 2010) and for photoreceptor apical morphogenesis (Xiong and Rebay, 2011). Thus Abl is a key player in many of the processes that mark the photoreceptor terminal differentiation program.
Here we explore further the role of Drosophila Abl in photoreceptor terminal differentiation and report a novel requirement in maintaining neuronal cell fate. Thus at late pupal stages, Abl mutant photoreceptor cells lose expression of neuronal markers, although they do not undergo apoptosis or re-enter the cell cycle. Abl loss concomitantly disrupts endocytic trafficking of the Notch receptor, leading to a peak of ectopic Notch pathway activation that correlates with the loss of neuronal fate. Reducing the genetic dose of Notch or Suppressor of Hairless dominantly suppresses the loss of neuronal marker expression, suggesting that increased Notch activation provides a molecular mechanism driving neuronal dedifferentiation in Abl mutant photoreceptors. More broadly, our results reveal an unexpected degree of cellular plasticity in the Drosophila retina and raise the possibility that Abl-mediated regulation of Notch signaling could be important for cell fate maintenance and reprogramming in other developmental or pathological contexts.
MATERIALS AND METHODS
Fly genetics
All strains were obtained from the Bloomington Stock Center. The amorphic Abl2 and hypomorphic Abl1 alleles (Henkemeyer et al., 1987) were recombined onto the FRT80B chromosome. To generate Abl mutant clones, Abl2FRT80B/TM6Tubby, Notch264-39/FM7Twist-GFP; Abl2FRT80B/TM6Tubby, and Su(H)2/CyoActin-GFP; Abl2FRT80B/TM6Tubby males were crossed to ey-FLP; GFPnlsFRT80B virgins. Non-tubby third instar larvae or pre-pupae were collected. Genotyped immobile white pre-pupae were aged in a humid chamber at 25°C and harvested at selected time points for dissection. For Notch intracellular domain (NICD) overexpression, GMR-Gal4/CyoActin-GFP; tubulin-Gal80ts males were crossed to UAS-NICD virgins and the progeny were raised at 18°C. White pre-pupae were collected, aged another 48 hours at 18°C and then shifted to 25°C. Control crosses were maintained at 18°C throughout. Eyes were dissected from newly eclosed non-GFP adults.
Immunostaining and antibodies
Late third instar eye and wing imaginal disks and 24/48 hours after puparium formation (APF) pupal eye disks were dissected in S2 cell medium (Gibco, sf-900 II SFM) and fixed for 10 minutes in 4% paraformaldehyde in PBT (PBS with 0.1% Triton X-100). For 72/96 hours pupal and adult eye dissections, heads were cut in half, fixed for 20 minutes, dissected, post-fixed for 10 minutes, washed three times in PBT, blocked in PNT (PBT + 3% normal goat serum) for 1 hour, incubated overnight at 4°C in primary antibodies diluted in PNT, washed three times in PBT, incubated in secondary antibodies diluted in PNT for 2 hours, washed three times in PBT, mounted (Invitrogen, ProLong Antifade Kit), and imaged on a Zeiss 510 confocal microscope. Antibodies from the Developmental Studies Hybridoma Bank (DSHB): mouse anti-Elav (1:50); anti-Notch intracellular domain (1:10); anti-E(Spl) (1:2); anti-Delta (1:1000). Other antibodies: rabbit anti-GFP (1:1000, Invitrogen); rabbit anti-phosphohistone H3 (1:1000, Upstate); rabbit anti-cleaved capase3 (1:1000, Cell Signaling); rabbit anti-Egfr (1:20,000, provided by E. Bach via R. Fehon); mouse anti-GFP (1:100, Invitrogen); DAPI (1:1000, Invitrogen); Cy3/FITC-conjugated secondaries (1:2000, Jackson ImmunoResearch).
Data analysis and quantification
Confocal stacks were rendered into 3D volumes and analyzed using Imaris software (Version 5.7.2, Bitplane). The Imaris spot function was used to manually count the number of cells expressing a given marker. Ommatidial number within a clone was estimated based on the lattice organization of cells, bristle cell number and clone size. Clone size was measured in pixels using ImageJ. The relative Elav+ cell density was calculated as the ratio of Elav+ cell density in an Abl clone (Elav+ cell number/clone size in pixels) to that in its neighboring wild-type clone. Unpaired, two-tailed Student’s t-tests were performed for all statistical comparisons between genotypes, and in all figures the data are plotted as the mean ± s.d.
Live Notch labeling
Dissected 24 hours APF eye disks were washed in cold Schneider’s medium and labeled with mouse anti-Notch C458.2H (1:2 mix of hybridoma culture supernatant in Schneider’s) for 15 minutes on ice, washed three times in cold Schneider’s on ice, transferred to room temperature for 0, 30 or 60 minutes before fixation in 4% paraformaldehyde (in PBS) for 10 minutes., blocked in PNT and stained with Cy3-conjugated secondary (1:2000, Jackson ImmunoResearch).
RESULTS
Abl mutant photoreceptors lose neuronal identity at late pupal stages
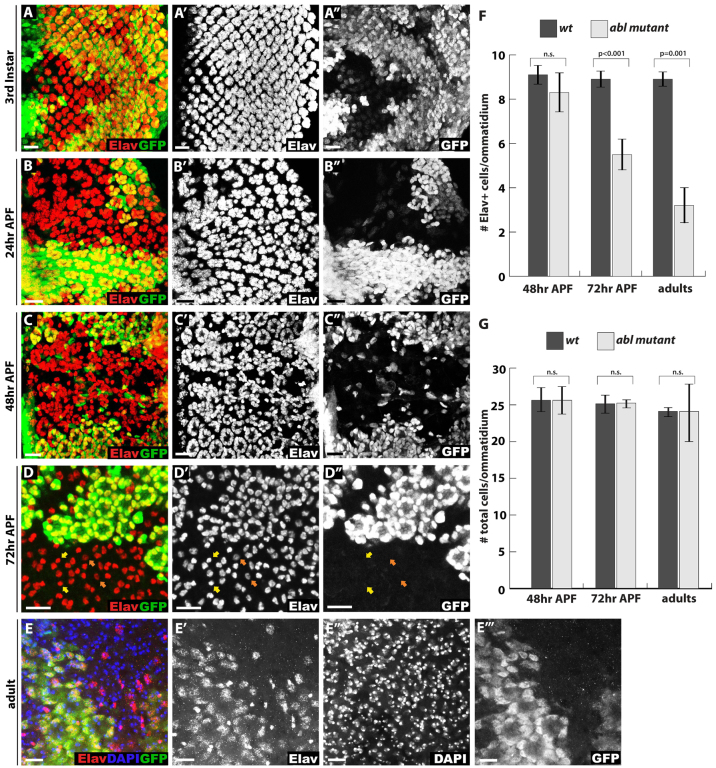
To investigate the consequences of loss of Abl on photoreceptor terminal differentiation, we followed expression of the marker Elav as an indicator of neuronal fate during larval and pupal development. As previously reported (Bennett and Hoffmann, 1992; Singh et al., 2010), photoreceptor cell fate specification occurs relatively normally in the absence of Abl, although modest recruitment delays, occasional missing photoreceptors and irregularities in the ommatidial pattern can be observed in third instar disks (Fig. 1A; data not shown). Similarly at 24 hours APF, although Abl mutant photoreceptor clusters appear more disorganized and irregularly spaced than their wild-type counterparts, the overall density of Elav-positive nuclei remains roughly comparable to wild type (Fig. 1B). By 48 hours APF, Abl mutant Elav-positive photoreceptor nuclei form a disordered solid pattern that lacks the ring-like arrangement and defined ommatidial cluster boundaries typical of wild type (Fig. 1C). As at earlier stages, the numbers of Elav positive nuclei in Abl mutant clones appear neither dramatically decreased nor increased relative to wild type (Fig. 1C,F).
Fig. 1.
Abl mutant photoreceptors lose neuronal cell fate at late pupal stages. (A-D″) Confocal maximal projections spanning the entire apical to basal extent of Drosophila eye disks showing expression of the neuronal marker Elav (red). Lack of GFP (green) marks Abl mutant clones in this and all other figures. (A-A″) At third instar, some Abl mutant clusters appear smaller than wild type with more interommatidial space, suggesting mild cell fate specification defects. (B-B″) At 24 hours APF, the ommatidial organization of Elav+ photoreceptor cells is disrupted in Abl clones, although photoreceptor cell density does not appear significantly decreased compared with wild type. (C-C″) By 48 hours APF, the organization of Elav+ photoreceptors is further disrupted in Abl clones such that mutant cells are dispersed without clear ommatidial boundaries. (D-D″) By 72 hours APF, few Elav+ photoreceptors remain in Abl mutant ommatidia. Bristle cells identified by their small round nuclei and bright Elav staining are unaffected (yellow arrows) and most of the remaining Abl mutant photoreceptor nuclei have weaker Elav staining compared with the wild-type photoreceptors (orange arrows). (E-E″′) A projection image of a whole-mount adult eye stained with anti-Elav (red), the nuclear marker DAPI (blue) and anti-GFP (green) shows few, if any, Elav+ cells in Abl clones, although the nuclei of these ‘former’ photoreceptors are still present. (F,G) Quantification of four clones per stage and genotype, with values plotted as mean ± s.d. In this, and all other figures, pairwise comparisons were performed with an unpaired, two-tailed Student’s t-test. (F) Quantification of Elav+ cell density in Abl and wild-type clones shows a gradual Elav+ cell loss from 48 hours APF to adult. (G) Quantification of overall nuclei density in Abl and wild-type clones shows no decrease or increase of cell numbers at any stage. Scale bars: 10 μm.
A striking change occurs by 72 hours APF, when a significant proportion of Abl mutant photoreceptors have lost expression of the neuronal marker Elav, a phenotype that becomes even more pronounced in the adult (Fig. 1D-F). The bristle cells, distinguished morphologically from the photoreceptors by their smaller, rounder nuclear shape and brighter Elav intensity, do not normally express Abl (Xiong and Rebay, 2011). They are thus unaffected by Abl loss, and so account for a significant fraction of the remaining Elav-positive nuclei (Fig. 1D, examples indicated by yellow arrows) (supplementary material Fig. S1). Among the remaining Abl mutant photoreceptors at 72 hours APF, Elav intensity appears reduced relative to the wild-type photoreceptors, suggesting that the cells might be transitioning toward a non-neuronal state (Fig. 1D, examples indicated by orange arrows). Lack of induction of the cleaved Caspase 3 or phosphohistone H3 markers at 72 hours APF or earlier stages indicates that Abl mutant photoreceptors do not undergo apoptosis or re-enter the cell cycle (supplementary material Fig. S2) and quantification of DAPI-stained nuclei shows similar nuclear densities in wild-type and Abl mutant tissue at all stages examined (Fig. 1E,G). Thus loss of ommatidial organization does not result from increased cell death or proliferation, and although neuronal marker expression is lost late in development, the Abl mutant cells are still present. Taken together, these results suggest Abl function is required in the photoreceptors for maintenance of neuronal fate and that the terminally differentiated state of a Drosophila photoreceptor neuron remains reversible at least until 72 hours APF, about a day before eclosion of the fully developed adult.
Altered endocytic trafficking increases Notch expression in Abl clones
What molecular mechanisms might underlie the loss of neuronal marker expression in Abl mutant photoreceptors? Our previous analysis of Abl loss-of-function phenotypes revealed defects in photoreceptor axon targeting and apical morphogenesis (Xiong et al., 2009; Xiong and Rebay, 2011). Although such morphogenetic defects might theoretically be sufficient to compromise stability of the differentiated state of a photoreceptor neuron, to our knowledge such an outcome has not been reported for mutations that disrupt photoreceptor axon guidance or apical polarity and morphogenesis (Izaddoost et al., 2002; Maurel-Zaffran et al., 2001; Newsome et al., 2000; Pellikka et al., 2002). This suggests that altered morphogenesis is unlikely to be the sole force driving loss of neuronal marker expression in Abl mutant photoreceptors and led us to explore the hypothesis that inappropriate upregulation of a signaling pathway that normally suppresses neuronal differentiation might contribute to the phenotype.
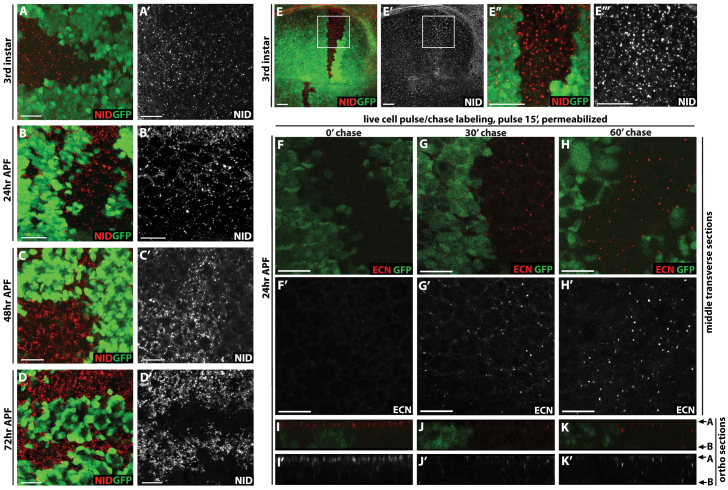
One appealing candidate is Notch, which functions in many contexts to inhibit neuronal differentiation and is required for the determination of all cell types in the Drosophila eye (Cagan and Ready, 1989; Hansen et al., 2010; Shimizu et al., 2008). Because Notch is no longer highly expressed in the pupal photoreceptors (Fehon et al., 1991), we asked whether Notch levels might become abnormally elevated upon Abl loss. In third instar and 24 hours APF disks we observe modest upregulation of Notch expression in Abl clones, and in particular an increase in size and prevalence of Notch-positive cytoplasmic punctae (Fig. 2A,B). The phenotype becomes more prominent at 48 hours and 72 hours APF (Fig. 2C,D), the developmental window in which the photoreceptors lose Elav expression (Fig. 1). The effect on Notch is not eye-specific, as Abl clones in the wing imaginal disk also show increased levels of Notch-positive cytoplasmic punctae (Fig. 2E); nor does it result from genetic background, as clones of the Abl1 allele show similar loss of Elav and increase in Notch expression (supplementary material Fig. S3).
Fig. 2.
Notch expression and endocytic trafficking are altered in Abl clones. (A-D′) Eye and (E) wing disks with Abl clones stained with anti-Notch intracellular domain (NID, red) and anti-GFP (green). All images are maximal projections. (A,A′) A slight increase in punctate Notch staining is detected in Abl clones in a third instar disk. (B,B′) In 24 hours APF disks, Notch staining is slightly more pronounced with some larger puncta (yellow arrows) in the Abl clones than in wild-type tissue. (C,C′) By 48 hours APF, the increased Notch staining in Abl clones relative to wild type is striking. (D,D′) At 72 hours APF, Notch staining appears absent in the wild-type tissue but is present ectopically in Abl mutant clones. (E-E″′) In third instar wing disks, more and bigger Notch puncta are evident in Abl clones than in wild type. E″ and E″′ are magnified views of the regions boxed in E and E′. (F-K′) Live 24 hour APF eye disks carrying Abl clones were pulse labeled with antibody recognizing the extracellular domain of Notch (anti-ECN, Red), washed, and either fixed immediately (F,I) or chased for 30 (G,J) or 60 (H,K) minutes to follow Notch trafficking. Detergent was added to permeabilize the tissue for visualization of internalized Notch protein. (F-H) Single optical sections across the middle plane of the epithelium. (I-K) Orthogonal sections of the same disk shown in F-H, respectively, with apical and basal surfaces marked by arrows on the right. (F) Internal Notch staining is absent with no chase, indicating all labeled receptor is at the cell surface. (G) After 30 minutes chase, some Notch puncta are seen in Abl clones. (H) After 60 minutes chase, more and brighter Notch puncta are evident in Abl clones than in wild-type. (I) With no chase, most Notch localizes to the apical-junctional surface, with little or no detectable cytoplasmic staining. (J) After 30 minutes chase, Notch is barely detectable at the apical-junctional surface, while cytoplasmic puncta become evident. (K) After 60 minutes chase, brightly stained Notch puncta are apparent in Abl clones. Scale bars: 10 μm. A, apical; B, basal.
Endocytic trafficking provides a key mechanism for regulating Notch protein levels and signaling activity (reviewed by Fortini and Bilder, 2009; Yamamoto et al., 2010). The noticeable association of ectopic Notch with cytoplasmic punctate structures raised the possibility that loss of Abl might block Notch degradation by altering its endocytic trafficking. To test this, we compared the trafficking of surface-labeled Notch in Abl mutant versus wild-type tissue. Live eye disks carrying Abl clones were pulse-labeled with an antibody recognizing the extracellular domain of Notch and then chased for various amounts of time. With no chase, equivalent levels of surface-labeled Notch were seen in adjacent Abl mutant and wild-type clones (Fig. 2F,I; supplementary material Fig. S4). After a 30 minute chase, Notch was essentially fully depleted from the apical surface and cytoplasmic punctae were observed more basally in both wild-type and mutant cells, although the number and size of the Notch-positive endocytic structures were greater in Abl mutant clones (Fig. 2G,J). By 60 minutes, little labeled Notch was detectable in wild-type tissue, whereas the cytoplasmic punctae remained prominent in Abl clones, suggesting a block in endocytic trafficking and a failure to downregulate Notch (Fig. 2H,K).
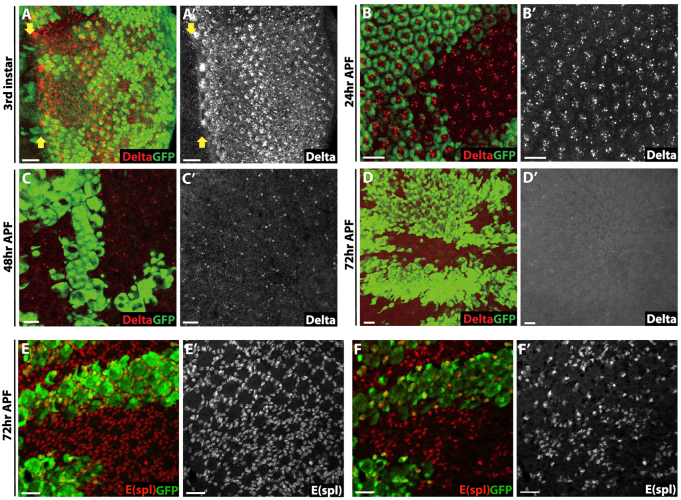
To ascertain whether loss of Abl broadly perturbs trafficking and levels of cell-surface proteins, we examined the expression of the Notch ligand Delta and the Epidermal growth factor receptor (Egfr). At early stages Delta levels were not altered (Fig. 3A-C). At 72 hours APF, the stage when the ectopic Notch and loss of Elav phenotypes become most striking (Fig. 1D, Fig. 2D), Delta was undetectable in either wild-type or Abl mutant tissue (Fig. 3D). Similarly, Egfr expression appeared neither increased nor altered in Abl mutant clones (supplementary material Fig. S5). Together these results support the argument for a more specific effect of Abl in Notch downregulation, and suggest that any activation of ectopic Notch signaling at 72 hours APF occurs independently of binding Delta ligand. This interpretation is consistent with prior work implicating endocytic trafficking in both ligand-dependent and ligand-independent Notch signaling (Fortini and Bilder, 2009; Lu and Bilder, 2005; Vaccari et al., 2008).
Fig. 3.
Delta-independent activation of Notch signaling in Abl clones. (A-D′) Delta expression in third instar (A), 24 hours APF (B), 48 hours APF (C) and 72 hours APF (D) eye disks. All pictures are confocal maximal projections. The MF is marked by yellow arrows in A. Delta protein is normally expressed in Abl clones, with its level decreasing gradually during development. At 72 hours APF, when Notch is highly expressed in Abl clones, no ectopic Delta protein is detected. (E,E′) Notch signaling is elevated as assessed by the increase in E(Spl)+ cells (red) in Abl clones. Maximal projection. (F,F′) A basal section of E showing the extra E(spl)+ cells reside at the basal plane. Scale bars: 10 μm.
Increased Notch signaling contributes to loss of neuronal fate in Abl mutant photoreceptors
To confirm the elevated Notch levels observed upon loss of Abl reflect activation of downstream signaling, we first examined expression of the transcriptional target E(spl). Although E(spl) upregulation was not detectable at larval and early pupal stages (data not shown), ectopic E(spl) was evident in Abl clones at 72 hours APF, the stage at which elevated Notch levels appear most prominent (Fig. 3E). Thus in wild-type ommatidia, E(spl) marks the pigment cell lattice surrounding each ommatidium, whereas in Abl mutant clones, in addition to the normal pigment cells, ectopic E(spl)-positive nuclei were observed at the most basal plane where the residual Elav-positive Abl mutant photoreceptor nuclei also reside (Fig. 3F) (Xiong and Rebay, 2011).
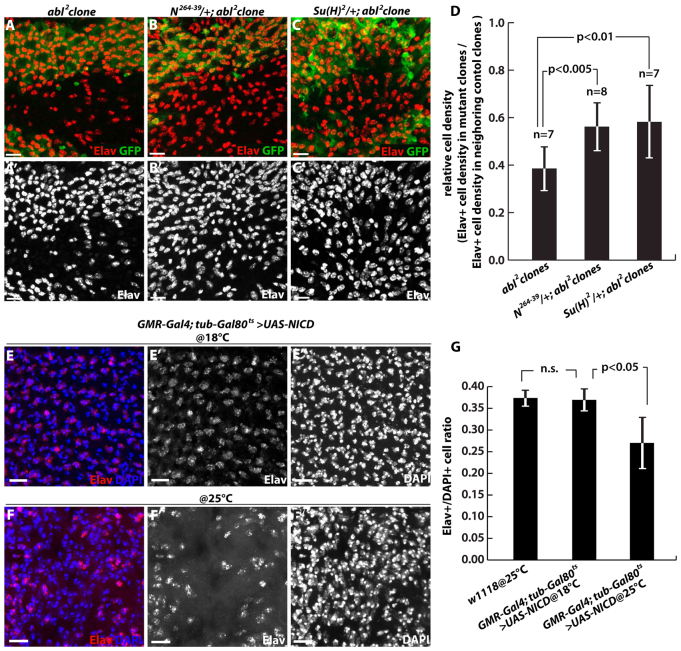
A second prediction of our model is that reduction in dose of either Notch or its downstream effectors should suppress the loss of neuronal fate in Abl mutant photoreceptors. To test this, we looked for restoration of Elav expression in Abl clones in 72 hours APF disks dissected from animals heterozygous for either Notch or its transcriptional effector Suppressor of Hairless [Su(H)]. In both cases, increased numbers of Elav-positive nuclei were apparent relative to Abl mutant clones alone (Fig. 4A-D). Thus although it has been suggested previously that Abl participates in a non-canonical Su(H)-independent Notch signaling pathway during axon guidance in the embryo (Crowner et al., 2003; Giniger, 1998; Le Gall et al., 2008), our genetic interaction results, together with the increased expression of the Su(H) transcriptional target E(spl) observed in Abl mutant clones (Fig. 3E), support the argument that Abl interfaces with the canonical Notch pathway to regulate photoreceptor cell fate maintenance.
Fig. 4.
Reduced Notch signaling restores photoreceptor cell fate in Abl clones. (A-D) Adult eyes carrying Abl clones in wild-type (A), N264-39/+ heterozygous (B) or Su(H)2/+ heterozygous (C) genetic backgrounds were stained with anti-Elav (red) and anti-GFP (green). All images are maximal projections of optical sections spanning the whole disk. (A) Few Elav+ cells remain in Abl2 clones. (B,C) More Elav+ cells are evident in N264-39/+; Abl2 (B) and Su(H)2/+; Abl2 (C) clones. (D) Quantification of Elav+ cell density in N264-39/+; Abl2 and Su(H)2/+; Abl2 clones. Seven to eight clones per genotype were scored, with values plotted as mean ± s.d. (E-G) Dissected eyes from newly eclosed GMR-Gal4; tub-Gal80ts >UAS-NICD adults stained for Elav and DAPI. (E) The 18°C raised control has an organized Elav+ nuclear lattice. (F) Eyes from animals shifted to 25°C at the white prepupal stage have fewer Elav+ cells but normal DAPI-stained cell density. (G) Quantification of Elav+/DAPI+ ratios. Three to five clones per genotype were scored, with values plotted as mean ± s.d. Scale bars: 10 μm.
Finally, if ectopic Notch signaling downstream of loss of Abl is a primary driver of photoreceptor dedifferentiation, then upregulating Notch signaling directly in the pupal photoreceptor cells might be sufficient to induce loss of neuronal marker expression. To minimize the gross disruptions to retinal morphology associated with GMR-Gal4 driven expression of the NICD (Müller et al., 2006), a constitutively active allele of Notch, we employed the temperature-sensitive TARGET gene expression system (McGuire et al., 2004). Thus at 18°C, the permissive condition for the temperature-sensitive allele of the Gal4 repressor protein Gal80, UAS-NICD expression was not induced, and regular lattices of Elav+ cells were observed in adult eyes (Fig. 4E). By contrast, retinal dissections of adults derived from animals shifted to 25°C after completion of photoreceptor specification (∼24 hours APF, see Materials and methods) revealed a reduction in Elav+ cells (Fig. 4F). This phenotype results from induction of NICD expression, as the GMR-Gal4;tub-Gal80ts stock on its own appears phenotypically wild type when cultured continuously at 25°C (data not shown). Although the ommatidial organization of the temperature-shifted animals was greatly improved relative to that of animals cultured constantly at 25°C (data not shown), it was still too irregular to distinguish individual ommatidia. Therefore rather than quantifying the number of Elav+ cells per ommatidium as we did for Abl clones (Fig. 1F), we instead counted the total number of Elav+ nuclei within a field, and then divided by the total number of DAPI-stained nuclei. Using this scoring method, 18°C control eyes had a similar Elav+/DAPI+ ratio as wild-type eyes (∼0.37 versus ∼0.37), whereas the ratio from animals shifted to 25°C was lower (∼0.27) (Fig. 4G). Importantly, the lower ratio resulted from a smaller numerator rather than a larger denominator, indicating that constitutive Notch activation did not significantly alter cell proliferation or survival (supplementary material Fig. S6). Together these results lead us to propose that loss of Abl leads to ectopic activation of Notch signaling in the pupal photoreceptors, and that this ectopic signaling contributes to neuronal dedifferentiation.
Abl mutant photoreceptors may transdifferentiate toward a pigment cell fate
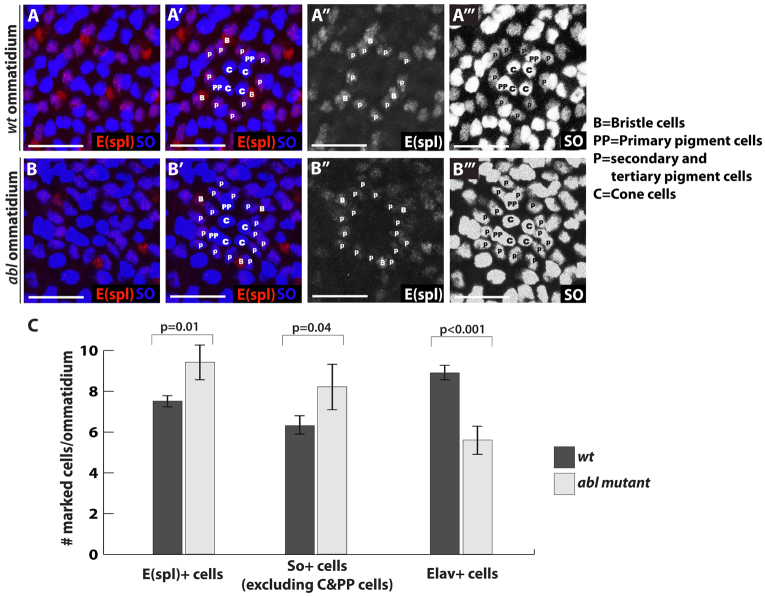
What happens to Abl mutant photoreceptors as they turn off neuronal fate markers? Our results suggest that they neither die nor re-enter the cell cycle. Do they revert to an undifferentiated state or might the increased Notch signaling induce expression of markers characteristic of a different cell type? Given that E(spl) is itself a pigment cell marker (Fig. 3E), we hypothesized they might transdifferentiate toward a pigment cell-like fate. To test this possibility, we examined the expression of a second pigment cell fate marker, Sine oculus (So). Indeed, all the extra E(spl)+ cells in Abl mutant ommatidia also expressed So (Fig. 5A-B″′). We tried to document the transdifferentiation transition by co-staining 72 hours pupal eye disks with anti-Elav and anti-So, but failed to find any Elav/So double-positive cells (supplementary material Fig. S7). This suggests a temporal delay between onset of pigment cell marker expression and loss of neuronal marker expression or fate. This would be consistent with our quantification data in which we find that in 72 hours APF Abl mutant ommatidia, more cells have lost Elav expression (around four cells/ommatidium) than have acquired E(spl) and So expression (around two cells/ommatidium) (Fig. 5C). It is also possible that only half of the dedifferentiating Abl mutant photoreceptors ever turn on pigment cell fate markers, while the rest simply lose neuronal marker expression.
Fig. 5.
Abl mutant photoreceptors may transdifferentiate toward a pigment cell fate. (A-B″′) Magnified views of a wild-type ommatidium (A-A″′, GFP not shown) and an Abl mutant ommatidium (B-B″′, lack of GFP) stained with E(spl) (red, A″,B″) and So (blue, A″′,B″′) antibodies and marked by cell types. E(spl) antibody stains secondary and tertiary pigment cells (marked as P) as well as bristle cells (marked as B), whereas So antibody stains cones (marked as C) and all pigment cells, including primary pigments (marked as PP). Although the numbers of bristle cells and primary pigment cells remain the same, there are more secondary and tertiary pigment cells in the Abl ommatidium. (C) Quantification of E(spl)+, So+ and Elav+ cell density in wild-type and Abl clones at 72 hours APF. Three to five clones per marker were scored, with values plotted as mean ± s.d.
DISCUSSION
In this study we report a novel requirement for the Abl nonreceptor tyrosine kinase in maintaining the terminally differentiated state of Drosophila photoreceptors. The failure to maintain expression of neuronal markers correlates with impaired trafficking and ectopic signaling by the Notch receptor. Consistent with the idea that aberrant Notch activation might drive photoreceptor dedifferentiation, Notch signaling has been shown to inhibit neuronal differentiation in many developmental contexts in all animals (Hansen et al., 2010; Shimizu et al., 2008; Wang et al., 2006), and Notch is normally absent in pupal photoreceptor cells (Fehon et al., 1991). Thus we propose that ectopic activation of Notch signaling provides a molecular mechanism coupling Abl loss to a program of neuronal dedifferentiation in the fly retina.
Dedifferentiation describes a regressive process whereby a differentiated somatic cell loses its mature identity and reverts to an earlier multipotent developmental state. Our observation that some Abl mutant photoreceptors not only lose neuronal marker expression, but also turn on pigment cell marker expression, raises the possibility that the former photoreceptor neurons might transdifferentiate toward a new pigment cell-like state. Genome-wide gene expression analysis of Abl mutant cells should provide a more precise molecular definition of this transition and of the final state of these cells. Experiments to determine whether the dedifferentiated or partially transdifferentiated Abl mutant cells can be redirected toward other cell fates or to re-enter the cell cycle will provide additional insight into the extent of their multipotency and plasticity.
Our previous work has shown that Abl is largely dispensable for photoreceptor cell fate specification, but then plays crucial roles throughout the elaborate morphogenetic programs that lead to rhabdomere formation and determine the spatial organization of ommatidial cells within the epithelium (Xiong and Rebay, 2011). Whether altered Notch signaling and dedifferentiation result directly from failed morphogenesis, or whether they reflect independent requirements for Abl later in development is an open question. The fact that ectopic expression of an activated Notch transgene at mid-pupal stages, after completion of the morphogenetic program, leads to loss of neuronal marker expression, suggests the two can be uncoupled. However Notch trafficking defects are apparent in Abl mutant photoreceptors well before morphogenesis is complete, raising the possibility that these cellular events could be tightly intertwined. Examination of neuronal marker expression and Notch signaling at mid-late pupal stages in apical polarity mutants might help elucidate the extent of molecular coupling between morphogenesis and photoreceptor fate maintenance.
A second open question concerns the molecular mechanisms by which Abl regulates Notch trafficking to activate downstream signaling. Prior work has implicated endocytic trafficking in regulating both ligand-independent and ligand-dependent Notch signaling (Fortini and Bilder, 2009; Lu and Bilder, 2005; Vaccari et al., 2008). Supporting the argument for the former mechanism in Abl mutant photoreceptors, we found that Delta levels are below detection threshold at the mid-pupal stages when Notch accumulation, and presumably signaling, is highest. However, previous studies have reported that ligand-independent activation of Drosophila Notch in mutants affecting late endosome/multivesicular body sorting results in overproliferation rather than dedifferentiation of retinal cells (Lu and Bilder, 2005; Vaccari et al., 2008). One possible explanation to reconcile a model of ligand-independent Notch activation with these observations is that the endocytic pathway genes potentiate early functions of Notch in regulating cell proliferation, whereas loss of Abl affects later roles. Another non-mutually exclusive explanation is that the endocytic defects observed in Abl clones might be highly specific to Notch trafficking, an argument substantiated by lack of effect on two other cell-surface proteins Delta and Egfr, whereas the phenotypes resulting from loss of a general component of the endocytic machinery might reflect a complex disruption of multiple signaling pathways. Alternatively, the ectopic Notch signaling that results from loss of Abl could reflect a ligand-dependent response, either to undetectably low levels of Delta or to an alternate ligand such as Serrate. Analysis of the endocytic route taken by Notch in Abl mutant photoreceptors, genetic exploration of ligand-dependence versus independence, and investigation of Abl interactions with specific Notch regulators like Nedd4, Deltex and Cbl (Jehn et al., 2002; Sakata et al., 2004; Wilkin et al., 2004; Yamada et al., 2011) should help distinguish between the different models.
In conclusion, our results reveal a novel requirement for the Abl tyrosine kinase in preventing Notch activation to maintain the terminally differentiated state of Drosophila photoreceptor cells. The discovery that Abl, a key morphogenetic regulator, is also required for cell fate maintenance, suggests a new molecular strategy for coordinating tissue morphogenesis with differentiation. The extent to which Abl-mediated maintenance of the differentiated cell state might be relevant to other tissues and developmental or pathogenic contexts will be an important direction for future investigation.
Supplementary Material
Acknowledgments
We thank members of the Rebay, Fehon and Horne-Badovinac laboratories for discussions; N. Martin for confocal assistance; E. Bach and R. Fehon for antibodies; R. Fehon for advice on the Notch pulse-chase experiment; and the Bloomington Drosophila Stock Center for flies and the Developmental Studies Hybridoma Bank for antibodies.
Footnotes
Funding
This research was supported by the National Institutes of Health [R01 EY12549 to I.R.], a Women’s Board Fellowship of the University of Chicago and an American Heart Association predoctoral fellowship to W.X. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.088799/-/DC1
References
- Bashaw G. J., Kidd T., Murray D., Pawson T., Goodman C. S. (2000). Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell 101, 703–715 [DOI] [PubMed] [Google Scholar]
- Bennett R. L., Hoffmann F. M. (1992). Increased levels of the Drosophila Abelson tyrosine kinase in nerves and muscles: subcellular localization and mutant phenotypes imply a role in cell-cell interactions. Development 116, 953–966 [DOI] [PubMed] [Google Scholar]
- Cagan R. L., Ready D. F. (1989). Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev. 3, 1099–1112 [DOI] [PubMed] [Google Scholar]
- Crowner D., Le Gall M., Gates M. A., Giniger E. (2003). Notch steers Drosophila ISNb motor axons by regulating the Abl signaling pathway. Curr. Biol. 13, 967–972 [DOI] [PubMed] [Google Scholar]
- Fehon R. G., Johansen K., Rebay I., Artavanis-Tsakonas S. (1991). Complex cellular and subcellular regulation of notch expression during embryonic and imaginal development of Drosophila: implications for notch function. J. Cell Biol. 113, 657–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsthoefel D. J., Liebl E. C., Kolodziej P. A., Seeger M. A. (2005). The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the Netrin receptor Frazzled in Drosophila. Development 132, 1983–1994 [DOI] [PubMed] [Google Scholar]
- Fortini M. E., Bilder D. (2009). Endocytic regulation of Notch signaling. Curr. Opin. Genet. Dev. 19, 323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniger E. (1998). A role for Abl in Notch signaling. Neuron 20, 667–681 [DOI] [PubMed] [Google Scholar]
- Grevengoed E. E., Loureiro J. J., Jesse T. L., Peifer M. (2001). Abelson kinase regulates epithelial morphogenesis in Drosophila. J. Cell Biol. 155, 1185–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevengoed E. E., Fox D. T., Gates J., Peifer M. (2003). Balancing different types of actin polymerization at distinct sites: roles for Abelson kinase and Enabled. J. Cell Biol. 163, 1267–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D. V., Lui J. H., Parker P. R., Kriegstein A. R. (2010). Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554–561 [DOI] [PubMed] [Google Scholar]
- Henkemeyer M. J., Gertler F. B., Goodman W., Hoffmann F. M. (1987). The Drosophila Abelson proto-oncogene homolog: identification of mutant alleles that have pleiotropic effects late in development. Cell 51, 821–828 [DOI] [PubMed] [Google Scholar]
- Izaddoost S., Nam S. C., Bhat M. A., Bellen H. J., Choi K. W. (2002). Drosophila Crumbs is a positional cue in photoreceptor adherens junctions and rhabdomeres. Nature 416, 178–183 [DOI] [PubMed] [Google Scholar]
- Jehn B. M., Dittert I., Beyer S., von der Mark K., Bielke W. (2002). c-Cbl binding and ubiquitin-dependent lysosomal degradation of membrane-associated Notch1. J. Biol. Chem. 277, 8033–8040 [DOI] [PubMed] [Google Scholar]
- Kuzina I., Song J. K., Giniger E. (2011). How Notch establishes longitudinal axon connections between successive segments of the Drosophila CNS. Development 138, 1839–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall M., De Mattei C., Giniger E. (2008). Molecular separation of two signaling pathways for the receptor, Notch. Dev. Biol. 313, 556–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl E. C., Forsthoefel D. J., Franco L. S., Sample S. H., Hess J. E., Cowger J. A., Chandler M. P., Shupert A. M., Seeger M. A. (2000). Dosage-sensitive, reciprocal genetic interactions between the Abl tyrosine kinase and the putative GEF trio reveal trio’s role in axon pathfinding. Neuron 26, 107–118 [DOI] [PubMed] [Google Scholar]
- Longley R. L., Jr, Ready D. F. (1995). Integrins and the development of three-dimensional structure in the Drosophila compound eye. Dev. Biol. 171, 415–433 [DOI] [PubMed] [Google Scholar]
- Lu H., Bilder D. (2005). Endocytic control of epithelial polarity and proliferation in Drosophila. Nat. Cell Biol. 7, 1232–1239 [DOI] [PubMed] [Google Scholar]
- Maurel-Zaffran C., Suzuki T., Gahmon G., Treisman J. E., Dickson B. J. (2001). Cell-autonomous and -nonautonomous functions of LAR in R7 photoreceptor axon targeting. Neuron 32, 225–235 [DOI] [PubMed] [Google Scholar]
- McGuire S. E., Mao Z., Davis R. L. (2004). Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci. STKE 2004, pl6 [DOI] [PubMed] [Google Scholar]
- Müller D., Nagel A. C., Maier D., Preiss A. (2006). A molecular link between Hairless and Pros26.4, a member of the AAA-ATPase subunits of the proteasome 19S regulatory particle in Drosophila. J. Cell Sci. 119, 250–258 [DOI] [PubMed] [Google Scholar]
- Newsome T. P., Schmidt S., Dietzl G., Keleman K., Asling B., Debant A., Dickson B. J. (2000). Trio combines with dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell 101, 283–294 [DOI] [PubMed] [Google Scholar]
- Pellikka M., Tanentzapf G., Pinto M., Smith C., McGlade C. J., Ready D. F., Tepass U. (2002). Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature 416, 143–149 [DOI] [PubMed] [Google Scholar]
- Pendergast A. M. (2002). The Abl family kinases: mechanisms of regulation and signaling. Adv. Cancer Res. 85, 51–100 [DOI] [PubMed] [Google Scholar]
- Ready D. F., Hanson T. E., Benzer S. (1976). Development of the Drosophila retina, a neurocrystalline lattice. Dev. Biol. 53, 217–240 [DOI] [PubMed] [Google Scholar]
- Roignant J.-Y., Treisman J. E. (2009). Pattern formation in the Drosophila eye disc. Int. J. Dev. Biol. 53, 795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata T., Sakaguchi H., Tsuda L., Higashitani A., Aigaki T., Matsuno K., Hayashi S. (2004). Drosophila Nedd4 regulates endocytosis of notch and suppresses its ligand-independent activation. Curr. Biol. 14, 2228–2236 [DOI] [PubMed] [Google Scholar]
- Shimizu T., Kagawa T., Inoue T., Nonaka A., Takada S., Aburatani H., Taga T. (2008). Stabilized beta-catenin functions through TCF/LEF proteins and the Notch/RBP-Jkappa complex to promote proliferation and suppress differentiation of neural precursor cells. Mol. Cell. Biol. 28, 7427–7441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J., Yanfeng W. A., Grumolato L., Aaronson S. A., Mlodzik M. (2010). Abelson family kinases regulate Frizzled planar cell polarity signaling via Dsh phosphorylation. Genes Dev. 24, 2157–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamada M., Farrell D. L., Zallen J. A. (2012). Abl regulates planar polarized junctional dynamics through β-catenin tyrosine phosphorylation. Dev. Cell 22, 309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari T., Lu H., Kanwar R., Fortini M. E., Bilder D. (2008). Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J. Cell Biol. 180, 755–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Somers G. W., Bashirullah A., Heberlein U., Yu F., Chia W. (2006). Aurora-A acts as a tumor suppressor and regulates self-renewal of Drosophila neuroblasts. Genes Dev. 20, 3453–3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkin M. B., Carbery A. M., Fostier M., Aslam H., Mazaleyrat S. L., Higgs J., Myat A., Evans D. A., Cornell M., Baron M. (2004). Regulation of notch endosomal sorting and signaling by Drosophila Nedd4 family proteins. Curr. Biol. 14, 2237–2244 [DOI] [PubMed] [Google Scholar]
- Xiong W., Rebay I. (2011). Abelson tyrosine kinase is required for Drosophila photoreceptor morphogenesis and retinal epithelial patterning. Dev. Dyn. 240, 1745–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W., Dabbouseh N. M., Rebay I. (2009). Interactions with the abelson tyrosine kinase reveal compartmentalization of eyes absent function between nucleus and cytoplasm. Dev. Cell 16, 271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Fuwa T. J., Ayukawa T., Tanaka T., Nakamura A., Wilkin M. B., Baron M., Matsuno K. (2011). Roles of Drosophila deltex in Notch receptor endocytic trafficking and activation. Genes Cells 16, 261–272 [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Charng W. L., Bellen H. J. (2010). Endocytosis and intracellular trafficking of Notch and its ligands. Curr. Top. Dev. Biol. 92, 165–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.