Abstract
We have examined the transport of the precursor of the 17-kD subunit of the photosynthetic O2-evolving complex (OE17) in intact chloroplasts in the presence of inhibitors that block two protein-translocation pathways in the thylakoid membrane. This precursor uses the transmembrane pH gradient-dependent pathway into the thylakoid lumen, and its transport across the thylakoid membrane is thought to be independent of ATP and the chloroplast SecA homolog, cpSecA. We unexpectedly found that azide, widely considered to be an inhibitor of cpSecA, had a profound effect on the targeting of the photosynthetic OE17 to the thylakoid lumen. By itself, azide caused a significant fraction of mature OE17 to accumulate in the stroma of intact chloroplasts. When added in conjunction with the protonophore nigericin, azide caused the maturation of a fraction of the stromal intermediate form of OE17, and this mature protein was found only in the stroma. Our data suggest that OE17 may use the sec-dependent pathway, especially when the transmembrane pH gradient-dependent pathway is inhibited. Under certain conditions, OE17 may be inserted across the thylakoid membrane far enough to allow removal of the transit peptide, but then may slip back out of the translocation machinery into the stromal compartment.
Although the chloroplast possesses its own genome, the majority of proteins found in this organelle are nuclear encoded, synthesized on cytoplasmic ribosomes, and posttranslationally imported. The process by which these proteins are directed to their final destination is complicated by the fact that chloroplasts possess three membranes that define six distinct destinations for the newly imported protein.
Nuclear-encoded chloroplast proteins are generally synthesized as higher-molecular-mass precursors that possess a cleavable, amino-terminal extension called a transit peptide. This topogenic sequence acts to direct the polypeptide from the cytoplasm to its final location within the chloroplast. Proteins residing in the thylakoid lumen are required to cross the envelope and thylakoid membranes, and generally possess a bipartite transit peptide. The first region of the targeting sequence directs the protein across the envelope membranes into the stroma, where it is cleaved by the stromal-processing protease, resulting in an intermediate-sized protein species. The remaining region of the transit peptide then targets this stromal intermediate to the thylakoid lumen, where it is removed by the membrane-bound lumenal processing protease, generating the mature-sized protein (for review, see Theg and Scott, 1993; Cline and Henry, 1996; Haucke and Schatz, 1997).
There have been extensive efforts to determine the mechanism by which proteins are transported into or across the thylakoid membrane. Currently, four pathways have been defined by their energy requirements, and some of their components have been identified. The first pathway appears to be spontaneous; no energy sources or protease-sensitive membrane factors seem to be required (Michl et al., 1994; Lorkovic et al., 1995). The second pathway shares some features with protein translocation across the ER membrane. A chloroplast homolog of the GTP-requiring signal-recognition particle 54-kD subunit has been cloned and sequenced from Arabidopsis thaliana (Franklin and Hoffman, 1993), and its involvement in the thylakoid targeting of the light-harvesting, chlorophyll-binding protein has been demonstrated (Li et al., 1995). The third pathway is homologous to the sec pathway in Escherichia coli. Recently, chloroplast homologs of SecA (Nakai et al., 1994; Yuan et al., 1994; Berghofer et al., 1995) and SecY (Laidler et al., 1995) have been identified, and the involvement of cpSecA in thylakoid protein translocation has been confirmed (Yuan et al., 1994). Transport of thylakoid proteins by the sec-dependent pathway is inhibited by the action of azide on SecA (Yuan et al., 1994). As a result, azide is used diagnostically in chloroplast import studies to identify proteins that are translocated by this pathway (Knott and Robinson, 1994). Proteins known to be transported in an azide-sensitive manner include OE33, plastocyanin, and PSI-3 (Cline et al., 1992; Karnauchov et al., 1994; Knott and Robinson, 1994; Yuan and Cline, 1994). As in E. coli, translocation of these proteins has been demonstrated to require ATP. In addition, a transmembrane pH gradient has been shown to stimulate their transport (Cline et al., 1992).
The fourth pathway for thylakoid protein transport appears to be truly unique in that it is dependent only on a transthylakoidal ΔpH, making it the only known energy-requiring protein transport process that can drive translocation in the absence of nucleotide triphosphates. Cline et al. (1992) demonstrated that ATP-depleting enzymes, as well as compounds such as valinomycin, which dissipate the membrane potential, had no discernible effect on protein transport by this pathway. However, if the protonophore nigericin was used to collapse the ΔpH across the thylakoid membrane, protein transport into the thylakoids was abolished, even when ATP was included in the assay medium. Because these experiments were conducted in isolated thylakoids, an essential role for stromal factors in translocation could also be eliminated. Additional experiments have also demonstrated a requirement for the ΔpH throughout the entire import process, thus excluding the possibility that it is used only to unfold proteins or to initiate transport (Brock et al., 1995). Four lumenal proteins that use this pathway have been identified: OE17, OE23, PSI-N, and PSII-T (Mould and Robinson, 1991; Cline et al., 1992; Ikeuchi, 1992; Nielsen et al., 1994).
Competition experiments using overexpressed precursor proteins from the ΔpH- and sec-dependent pathways revealed that proteins from one pathway do not compete with those from the other pathway during transport across the thylakoid membrane (Cline et al., 1993). Thus, it has been suggested that each pathway displays substrate specificity and possesses distinct components. A number of our own experiments have led us to consider the possibility that there might be shared components used by both pathways that had gone undetected in previous studies. To address this question more completely, we examined the effect of azide and nigericin on the thylakoid import of OE17, a protein transported by the ΔpH-dependent pathway. In these experiments we have detected the involvement of an azide-sensitive factor that results in the maturation, but not the compete transport, of this protein in the presence of nigericin.
MATERIALS AND METHODS
Chloroplast Isolation and Preparation
Intact chloroplasts were isolated from 12- to 14-d-old pea (Pisum sativum cv Progress 9) seedlings, as described by Theg and Geske (1992). Chloroplasts were resuspended in IB that contained 330 mm sorbitol and 50 mm K-Hepes, pH 8.0 (1× IB). Thylakoids were prepared from intact chloroplasts by osmotic lysis. Chloroplasts were pelleted, resuspended in 10 mm K-Hepes, pH 8.0, with 5 mm MgCl2 (lysis buffer), and incubated on ice for 5 min. Membranes were collected by pelleting at full speed in a microfuge for 5 min at 4°C. These membranes were washed twice in lysis buffer and their final concentration was adjusted to 1 mg/mL chlorophyll. Stroma was prepared by lysing chloroplasts at a high chlorophyll concentration with lysis buffer, incubating on ice for 5 min, pelleting the membranes at full speed in a microfuge, and collecting the supernatant (stroma). The stroma was then further centrifuged for 15 min at 4°C in a microfuge and transferred to a new tube for use. Where indicated, thylakoids were resuspended at 1 mg/mL chlorophyll in this stromal extract.
Protein Import Reaction Conditions
Cloned genes for OE17 and OE23 were transcribed and translated with [3H]Leu (NEN-DuPont) in vitro, as described previously (Cline et al., 1992). A typical import reaction was performed in a volume of 60 μL and contained approximately 500,000 dpm of 3H-labeled precursor protein, 5 mm MgCl2, 5 mm ATP, and 0.33 mg/mL chlorophyll in 1× IB. Import reactions were conducted at room temperature in the light (150 μE m−2 s−1) for 30 min. Protease treatment of chloroplast membranes was achieved by the addition of 200 μg thermolysin/mL with 5 mm CaCl2. After incubating for 15 min on ice, protease digestion was terminated by washing the membranes twice in IB with 25 mm EDTA. Samples not digested with protease were treated similarly without the addition of thermolysin. Unless otherwise noted, chloroplast import assays were terminated by repurifying the chloroplasts through silicon oil into 1.5 m PCA (Leheny and Theg, 1994). With the exception of the experiment presented in Figure 9, experiments performed with isolated thylakoids were terminated by the addition of 1 mL of cold IB, followed by two washes. The samples in Figure 1 were terminated by repurifying the thylakoid membranes through silicon oil (a 50:50 [v/v] mixture of Wacker AR20 and AR200 [Stauffer-Wacker Silicones Corp., Adrian, MI]) into PCA. Reactions that contained thylakoids with stroma were terminated by the addition of an equal volume of 2× sample buffer (0.125 m Tris, pH 6.8, 0.4% SDS, 20% glycerol, and 10% β-mercaptoethanol). Samples were separated on a 15% acrylamide gel and visualized by fluorography. Quantitation of fluorograms for Figure 8 was performed by densitometry (Bio Image, Ann Arbor, MI), as described by Ettinger and Theg (1991), with individual calibrations performed for each fluorogram.
Figure 9.
A, Reactions run in the absence of inhibitor; B, reactions run in the presence of azide; C, reactions run in the presence of nigericin; and D, reactions run in the presence of azide and nigericin. Lanes 1, Intact chloroplasts; lanes 2, isolated thylakoids; lanes 3, stromal fraction; lanes 4, mock protease-treated thylakoids; and lanes 5, protease-treated thylakoids. Intact chloroplasts were preincubated with nigericin (6 μm) and azide (30 mm) (or equivalent volumes of ethanol and water) for 10 min in the dark on ice. Import reactions with precursor OE23 and subsequent fractionations were performed as described in Figure 4, except that an aliquot of thylakoids was removed before the protease treatment. The standard (std) represents 20% of the precursor added to each reaction; the positions of the precursor (p), intermediate (i), and mature (m) proteins are noted.
Figure 1.
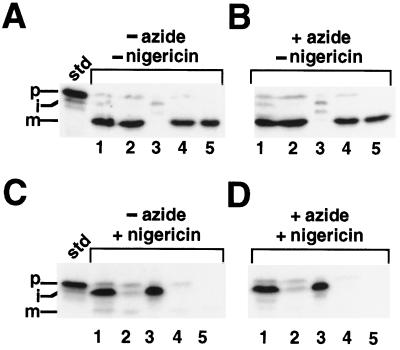
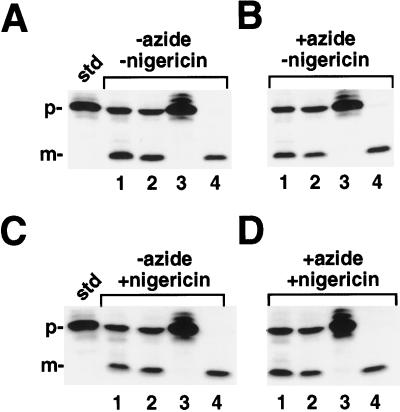
Azide restores OE17 processing in the presence of uncouplers. Where indicated, isolated intact chloroplasts were preincubated with 30 mm azide, 15 μm CCCP, and 6 μm nigericin (or equivalent volumes of water or ethanol) for 10 min in the dark on ice. To start the reactions, the chloroplasts were added to the import reaction mixture (final concentrations of inhibitors were 10 mm azide, 5 μm CCCP, and 2 μm nigericin) and placed in light for 30 min. Reactions were terminated by centrifuging into PCA as described in Methods. The standard (std) represents 20% of the prOE17 added to each reaction; the positions of the precursor (p), intermediate (i), and mature (m) proteins are noted.
Figure 8.
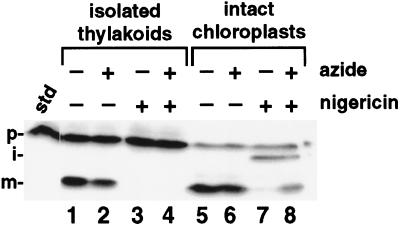
Intact chloroplasts (lanes 5–8) and washed thylakoids isolated from intact chloroplasts (lanes 1–4) were preincubated for 10 min in the dark with 6 μm nigericin, 30 mm azide, or equivalent volumes of ethanol and water. Reactions were initiated by the 3-fold dilution of chloroplasts or thylakoids into the import mixture and proceeded for 30 min in the light. Chloroplasts and thylakoids were reisolated by centrifugation into PCA through Wacker AR200 and a 50:50 (v/v) ratio of Wacker AR20 and AR200 silicon oils, respectively. The standard (std) represents 20% of the precursor added to each reaction; the positions of the precursor (p), intermediate (i), and mature (m) proteins are noted.
Preincubation of Chloroplasts with Inhibitors and ATP-Regenerating Compounds
Where indicated, chloroplasts or thylakoids were preincubated at 1 mg/mL chlorophyll for 10 min on ice with 6 μm nigericin (ethanolic stock), 30 mm sodium azide (aqueous stock), 15 μm CCCP (ethanolic stock), or an equivalent volume of water or ethanol. In the experiment presented in Figure 8, 1 mm PMSF was included in the chloroplast preincubation reactions. In the experiment presented in Figure 7, chloroplasts were preincubated with 3 mm OAA and 3 mm DHAP or an equivalent volume of IB for 10 min on ice in the dark.
Figure 7.
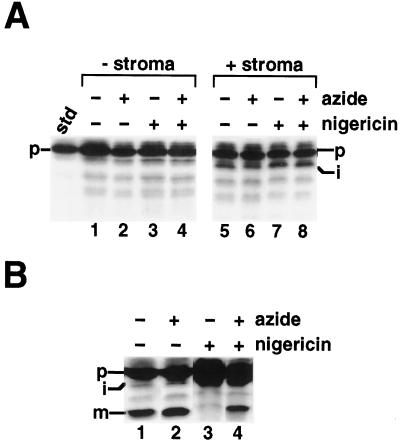
A, Stromal extract does not process prOE17 to its mature form. B, Thylakoid membranes are required for maturation. Lysis buffer or concentrated stromal extract collected from the lysis of chloroplasts at 6 mg chlorophyll/mL were preincubated with 6 μm nigericin (or ethanol) and 30 mm azide (or water) for 10 min on ice in the dark. These solutions were added to 1× IB containing radiolabeled prOE17, 5 mm MgCl2, and 5 mm ATP, with no chloroplast membranes (A) or in the presence of thylakoid membranes (B). The samples were incubated in the light for 30 min and terminated by the addition of 2× sample buffer without further purification. The standard (std) represents 20% of the precursor added to each reaction; the positions of the precursor (p), intermediate (i), and mature (m) proteins are noted.
9-Aminoacridine Measurements
Measurements of the thylakoid ΔpH presented in Figure 3 were made using the method described by Schuldiner et al. (1972). 9-Aminoacridine was purchased from Sigma, and its fluorescence and the quenching thereof, in response to ΔpH-generating illumination of isolated thylakoids, was measured in an AB-2 fluorometer (SLM-Aminco, Rochester, NY).
Figure 3.
Import reactions were initiated by the addition of chloroplasts to 1× IB (final concentration in the reaction was 0.33 mg/mL chlorophyll) containing radiolabeled prOE17, 5 mm MgCl2 and 5 mm ATP. A, Reactions incubated in the absence of both inhibitors; B, reactions incubated with azide alone; C, reactions incubated with nigericin alone; and D, reactions incubated with both inhibitors. Lanes 1, Intact chloroplasts; lanes 2, washed thylakoids (containing contaminating envelope membranes); lanes 3, supernatant fraction after envelope lysis of sample in lanes 2 containing stromal components and envelope membranes; and lanes 4, protease-treated thylakoids. The reactions were treated as in Figure 1. After this incubation, the samples were placed back in the light at 25°C for an additional 15 min. The chloroplast envelopes in lanes 2 and 4 were osmotically lysed in lysis buffer (see Methods) containing 2 μm nigericin, whereas the samples in lanes 1 were maintained in isotonic buffer (1× IB with 2 μm nigericin). Reactions were incubated on ice in the dark for 5 min. The osmoticum was then restored to samples in lanes 2 and 4 with the addition of 2× IB containing 2 μm nigericin (an equivalent volume of 1× IB with 2 μm nigericin was added to the samples in lanes 1). Samples in lanes 4 were protease treated with thermolysin (200 μg/mL with 5 mm CaCl2); samples in lanes 1 and 2 received 5 mm CaCl2 only. After inactivating thermolysin with the addition of 25 mm EDTA, membranes were pelleted and the supernatant collected from the samples in lanes 2. This supernatant was precipitated with 1.5 m PCA and loaded in lane 3. The standard (std) represents 20% of the precursor added to each reaction; the positions of the precursor (p) and mature (m) proteins are noted.
RESULTS
The import of OE17 into thylakoids has been shown to occur via the ΔpH-dependent pathway, thus making its translocation sensitive to protonophores that collapse transmembrane pH gradients (Cline et al., 1992; Klosgen et al., 1992). Figure 1 shows the import of OE17 into intact chloroplasts in the presence and absence of azide and the ionophores nigericin and CCCP. As expected, OE17 was transported into the thylakoid lumen and processed to its final, mature size in the presence or absence of azide, and the inclusion of nigericin or CCCP resulted in the inhibition of transport across the thylakoid membrane, as demonstrated by the accumulation of the stromal intermediate. We were surprised to discover, however, that when azide was included with these uncouplers in the preincubation, the amount of stromal intermediate appeared to decrease with a corresponding increase in the abundance of mature-sized protein. Inclusion of the electrogenic ionophore valinomycin had no effect on these experiments (data not shown). To our knowledge, this is the first demonstration of a protein transported by the ΔpH-dependent pathway that is processed in the apparent absence of a transmembrane pH gradient. Because the combined effect of azide occurred equally well with nigericin and CCCP, we used only nigericin in the subsequent experiments.
There are two simple explanations for the effect of azide on OE17 maturation in the absence of a thylakoid pH gradient. The first is that azide somehow inactivated, or at least decreased, the efficacy of nigericin in collapsing the ΔpH across the membrane. The second possibility is that the combination of the two inhibitors lysed the thylakoids and released the thylakoid-processing protease into the stroma. These possibilities are addressed by the experiments reported below.
Nigericin Is Still Active in the Presence of Azide
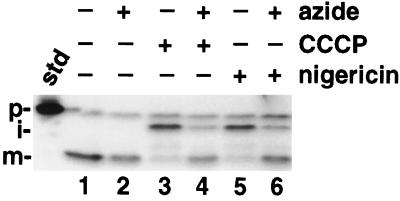
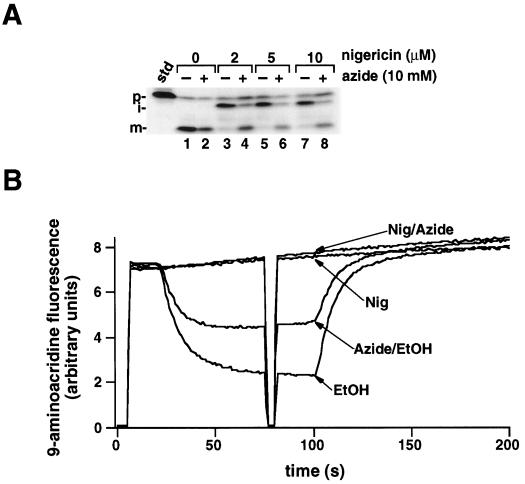
In the experiment presented in Figure 1, 2 μm nigericin was used to dissipate the ΔpH across the thylakoid membrane, a concentration in excess of that required to inhibit OE17 transport (Mould and Robinson, 1991; Cline et al., 1992). We considered the possibility that azide might necessitate the inclusion of a higher concentration of nigericin to achieve the same result. Figure 2A demonstrates that concentrations as high as 10 μm nigericin still resulted in the maturation of OE17 when azide was present. To directly measure the membrane pH gradient in the presence of these inhibitors, we used 9-aminoacridine, a self-quenching indicator of the ΔpH, with the acidification of the lumen manifested as a decrease in fluorescence (Schuldiner et al., 1972). As shown by the trace of relative fluorescence presented in Figure 2B, no pH gradient was formed when the samples contained nigericin, regardless of the presence of azide. Similar results were observed with CCCP. This demonstrates that the OE17 maturation that was observed in the presence of nigericin and azide (Fig. 1) was not the result of the reestablishment of a ΔpH across the thylakoid membrane. Measurements of the H+-pumping activities of thylakoids (Dilley, 1970) independently confirmed that a ΔpH was not formed when nigericin and azide were present together (data not shown). At this time, we cannot explain why a smaller pH gradient was established in the presence of azide alone relative to the azide-free control.
Figure 2.
A, Increasing concentrations of nigericin do not abolish OE17 processing in the presence of azide. Intact chloroplasts were preincubated with 0, 6, 15, or 30 μm nigericin (always with the same volume of ethanol) in the dark for 10 min on ice in the presence or absence of 30 mm azide (or an equivalent volume of water). Chloroplasts were diluted 3-fold into the import mixture to start the assay, and reactions were allowed to continue for 30 min in the light. The import assay was terminated and the samples analyzed as described for Figure 1. The standard (std) represents 20% of the precursor added to each reaction; the positions of the precursor (p), intermediate (i), and mature (m) proteins are noted. B, Azide does not inactivate the uncoupling activity of nigericin. Isolated thylakoids were preincubated with 6 μm nigericin (or an equivalent volume of ethanol) and 30 mm azide (or an equivalent volume of water) for 10 min on ice in the dark. Thylakoids were then diluted 3-fold into import buffer containing 20 μm methyl viologen and 3 mm MgCl2 to a concentration of 20 μg/mL. 9-Aminoacridine (5 μm) was added and the fluorescence was measured. Saturating red actinic light was turned on and off at 20 and 100 s; the shutter in front of the measuring beam was briefly shut at 75 s to determine if the sample chamber was light tight.
The Combination of Nigericin and Azide Does Not Cause Chloroplast Lysis
Although each inhibitor alone does not compromise thylakoid membrane integrity, we investigated whether they might result in vesicle lysis when present in combination, thereby exposing the stromal intermediate to the thylakoid processing peptidase located on the lumenal face of the membrane (Kirwin et al., 1989). To address this question we allowed the mature protein to accumulate in the lumen before the addition of nigericin and azide. If these compounds acted to release the processing protease from the thylakoid vesicles, the mature protein should also be released and become accessible to exogenous protease. We saw no decrease in the amount of mature-sized protein in the presence of nigericin and azide after protease treatment (Fig. 3D, lane 4) compared with the protected mature protein seen in the absence of this combination of inhibitors (Fig. 3, A–C, lanes 4). Furthermore, the mature protein was not precipitated from the supernatant after lysis of the envelope membranes (Fig. 3D, lane 3) but, rather, pelleted with the thylakoids (Fig. 3D, lane 2). These data indicate that the thylakoids were not lysed by the combination of azide and nigericin.
mOE17 Formed in the Presence of Nigericin and Azide Is in the Stroma
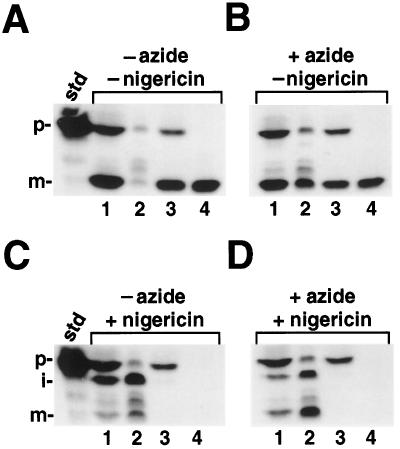
Having ruled out the more trivial explanations for the appearance of mOE17 under our experimental conditions, we asked whether this protein had been fully transported into the thylakoid lumen. To this end, we conducted import experiments for 30 min, followed by chloroplast fractionation and thermolysin treatment of the thylakoids (Fig. 4). As expected, when inhibitors were absent from the import assay, mOE17 fractionated with the thylakoids and was protease resistant, confirming its location in the thylakoid lumen (Fig. 4, A–C, lanes 4). Surprisingly, when the sample that had been incubated with both nigericin and azide was fractionated, the mOE17 was found to be soluble in the stromal fraction (Fig. 4D, lane 2), rather than in the thylakoid lumen (Fig. 4D, lane 4). A similar appearance of mOE17 in the stroma was previously noted when the targeting sequence had been replaced by that for the precursor of OE33, and was attributed to inefficient and aberrant targeting of an artificial chimeric protein (Clausmeyer et al., 1993; Henry et al., 1994). However, our experiments were performed with authentic prOE17, and so this cannot be the explanation for our observations.
Figure 4.
A, Reactions incubated in the absence of both inhibitors; B, reactions incubated with azide alone; C, reactions incubated with nigericin alone; and D, reactions incubated with both inhibitors. Lanes 1, Intact chloroplasts; lanes 2, stromal fractions; lanes 3, washed thylakoids; and lanes 4, protease-treated thylakoids. A 4× import reaction was initiated by the addition of 80 μg of chlorophyll and a 30-min import reaction was conducted in the light. At this time, one aliquot was removed and the sample was centrifuged through silicon oil into PCA; these intact chloroplasts were run in lanes 1. The remaining chloroplasts were pelleted for 30 s in a microfuge, the supernatant was removed, and the chloroplasts were resuspended in 0.5 volume of envelope lysis buffer containing 4 μg of aprotinin, 4 μg of leupeptin, and 1 mm PMSF. To distinguish between mOE17 bound to the lumenal face of the membrane and proteins that were fully translocated, we protease treated one-half of the thylakoids with thermolysin (lanes 4), whereas the other half was mock-protease treated (lanes 3). The thylakoid fraction also contained fragmented envelope membranes; thus, some prOE17 is seen in the samples that are not protease treated (lanes 3). The standard (std) represents 20% of the precursor added to each reaction; the positions of the precursor (p), intermediate (i), and mature (m) proteins are noted.
The results of the experiment shown in Figure 4 suggested two possibilities for the mechanism by which OE17 is processed to the mature size in the presence of nigericin and azide. First, it is possible that iOE17 was cleaved by an uncharacterized stromal protease, and did not engage the thylakoid-targeting apparatus. Alternatively, some portion of the protein may have actually crossed the thylakoid membrane far enough to be processed by the lumenal-processing protease, but then slipped out of the transport pore back into the stroma. In either case, this activity was manifested only in the presence of both azide and uncoupler. In the reactions containing azide alone (no uncoupler), a significant fraction of the mature polypeptide also fractionated with the stroma (Fig. 4B, lane 2). These results suggest that azide plays a previously undetected role in either OE17 processing or transport.
Stromal Proteases Do Not Appear to Be Responsible for the Generation of mOE17 That Accumulates in the Presence of Nigericin and Azide
There are several proteases present in the stroma that have been shown to digest imported proteins (Musgrove et al., 1989; Lindahl et al., 1996). We explored the possibility that azide might favor a nonnative conformation of iOE17, which would make it more susceptible to these proteases, thereby leading to a mature-sized proteolytic product. This postulate suggests that, although stromal factors are thought not to be involved in OE17 transport (Cline et al., 1992), iOE17 may in fact be complexed with another component, such as a stromal chaperone.
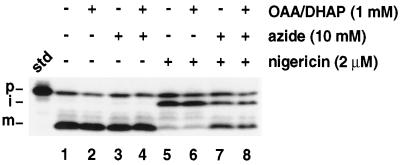
Hsp70 chaperones have been identified as components of the chloroplast envelope machinery (Marshall et al., 1990; Ko et al., 1992; Wu et al., 1994) and as soluble factors in the stroma (Marshall and Keegstra, 1992). Presumably, these chaperones bind to polypeptides as they are being translocated across the envelope membranes, perhaps to maintain the proteins in a loosely folded conformation and to prevent their premature folding. Upon complete translocation of the protein, ATP would be expected to be hydrolyzed and the chaperones released. The necessity for ATP hydrolysis by the chaperone suggests two possibilities for a chaperone-mediated effect of azide on OE17 processing. First, through its effect on the chloroplast coupling factor (Murataliev et al., 1991), azide might restrict ATP synthesis in the plastid, which could inhibit the release of the chaperone from the partially folded protein. Alternatively, azide might bind to the hsp70 ATP-binding site directly, again resulting in an extended interaction between the chaperone and iOE17. In either case, maintenance of the polypeptide in a loose conformation might result in the exposure of a proteolytic site that is normally not accessible to stromal proteases when the protein is tightly folded. We tested the first of these postulates by including the Calvin-Bensen cycle intermediates OAA and DHAP in our reactions. These intermediates allow high stromal ATP concentrations to be maintained independently of photophosphorylation (see Olsen and Keegstra, 1992). Figure 5 demonstrates that the inclusion of these compounds had no effect on the appearance of the mature-sized protein when azide was included with nigericin (Fig. 5, compare lane 7 with lane 8). This suggests that azide does not act indirectly in our experiments through an effect on stromal ATP concentrations. These data, however, do not exclude the possibility that azide occupies a chaperone ATP-binding site.
Figure 5.
Intact chloroplasts were preincubated with OAA/DHAP (3 mm), azide (30 mm), nigericin (6 μm), or equivalent volumes of 1× IB, water, or ethanol, respectively, for 10 min on ice in the dark. Reactions were initiated by the 3-fold dilution of chloroplasts into the import mixture and continued for 30 min in the light. The assay was terminated and samples were analyzed as described in Figure 1. The standard (std) represents 20% of the precursor added to each reaction; the positions of the precursor (p), intermediate (i), and mature (m) proteins are noted.
In an attempt to inhibit stromal proteases directly, import reactions were performed with PMSF, a membrane-permeable Ser protease inhibitor. Figure 6 shows that the inclusion of PMSF in the import reactions did not result in a decrease in the maturation of iOE17 in the presence of azide and nigericin, indicating that this phenomenon is not caused by a stromal Ser protease.
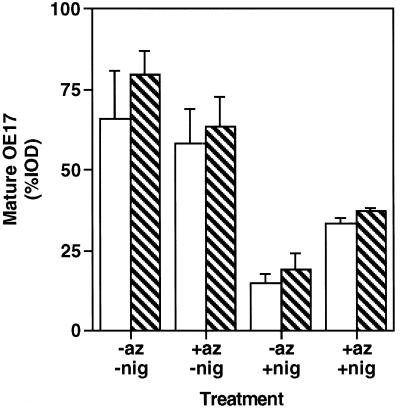
Figure 6.
Chloroplasts were preincubated with 1 mm PMSF (striped bars) or an equivalent volume of isopropanol (open bars) in the presence of nigericin (6 μm) and azide (30 mm) (or equivalent volumes of ethanol and water) for 10 min on ice in the dark. These chloroplasts were then diluted 3-fold into an import mixture containing 1 mm PMSF to initiate the protein-transport assay. Reactions proceeded for 30 min in the light and were terminated by repurifying the chloroplasts through silicon oil. Samples were separated by SDS-PAGE, visualized by fluorography, and the fluorographs were quantitated by densitometry. %IOD represents mOE17 as a fraction of the total amount of radiolabeled protein found in the sample. The data shown represent the average from three experiments.
To test directly for involvement of a stromal protease in OE17 maturation, we incubated the precursor in a concentrated stromal extract that had itself been preincubated with azide and nigericin, and compared it with precursors that had been incubated in chloroplast lysis buffer alone (control). Figure 7A reveals that stromal extract did not cause cleavage of the precursor to the mature size, regardless of whether inhibitors were present. This lack of processing was not attributable to a general decline in stromal enzymatic activity because the precursor was processed to the intermediate form by the soluble stromal processing protease. When thylakoid membranes were included in the assay, however, maturation of the protein in the presence of nigericin and azide was restored (Fig. 7B, lane 4). Furthermore, processing in the presence of membranes was found to be inhibited by HgCl2 (data not shown), a compound that modifies thiol groups and is often used to terminate protein transport across the thylakoid membrane (Reed et al., 1990; Yuan and Cline, 1994). These results, in conjunction with the fact that PMSF had no effect on OE17 maturation in the presence of azide and nigericin (Fig. 6), suggest that a stromal protease is not responsible for the mature-sized product generated in our experiments, and that iOE17 may interact with the thylakoid membrane during the cleavage event.
Role of a Stromal Factor in OE17 Maturation
Because thylakoid membranes appeared to be necessary for maturation of OE17 in the presence of nigericin and azide (Fig. 7B), we asked whether a membrane-bound protease, exposed on the stromal surface, might be responsible for digesting the protein to a mature-sized proteolytic fragment. To address this possibility, we performed import experiments using isolated thylakoids that had been washed several times to purify the membranes away from loosely bound stromal factors (Fig. 8). As expected, OE17 import was prevented when only nigericin was present (Fig. 8, lane 3). However, in contrast to the situation found in whole chloroplasts, the addition of azide to the nigericin-treated samples did not result in maturation of the precursor (Fig. 8, lane 4). This was also true if CCCP was used in place of nigericin (data not shown). Thus, it appears that a stromal-facing, membrane-bound protease is not solely responsible for cleaving OE17 to a mature-sized protein. Furthermore, this experiment suggests that a stromal factor may be required for this process because maturation was seen only when thylakoids were incubated with additional stroma (Fig. 7B). Unfortunately, reconstitution of azide-/nigericin-sensitive maturation of OE17 in isolated thylakoids was not reliably reproducible, and when achieved, occurred with low efficiency. This precluded our investigation of the effects of a broader range of exogenously added protease inhibitors on this process, and of a potential requirement for nucleotide triphosphate hydrolysis.
OE23 Processing Is Not Affected by Azide in the Absence of a ΔpH
To determine if the nigericin-/azide-dependent precursor processing event is a general phenomenon, we carried out chloroplast fractionation after import of OE23, another polypeptide that uses the ΔpH-dependent pathway (Fig. 9). We found that OE23 accumulated as a stromal intermediate in the absence of a pH gradient (Fig. 9C, lane 3), even when azide was present (Fig. 9D, lane 3). Thus, it appears that there is some difference between the mechanism of OE17 and OE23 import that still allows the former, but not the latter, to be processed under conditions in which its complete translocation is inhibited.
DISCUSSION
We undertook the experiments presented here in an effort to determine if there are common components used by the ΔpH- and sec-dependent pathways that had previously gone undetected. Using nigericin to inhibit transport by the ΔpH-dependent pathway, and azide to inhibit the sec-dependent pathway, we have discovered what appears to be a role for an azide-sensitive factor in the transport of a protein that follows the ΔpH-dependent transport pathway into the thylakoid lumen. Our initial data demonstrated that the addition of azide to chloroplasts that could not maintain a thylakoid transmembrane ΔpH resulted in restoration of prOE17 processing to the mature-sized protein (Fig. 1). Direct measurements of the ΔpH under these conditions indicated that the restoration of processing was not simply the result of reduced efficacy of the protonophore in the presence of azide (Fig. 2B). Furthermore, we showed that processing was not simply a result of induced thylakoid lysis (Fig. 3).
Although azide restored OE17 processing in the absence of a transmembrane pH gradient, we found that protein targeting was still aberrant because the mOE17 protein fractionated with the chloroplast stoma instead of with the thylakoid lumen, its correct destination (Fig. 4). This azide-induced mislocalization could also be observed to a lesser extent when a ΔpH was present across the thylakoid membrane, indicating that azide plays an inhibitory role in OE17 import under conditions in which the primary translocation pathway for this substrate is still operative. In the experiment presented in Figure 2B, we measured a smaller pH gradient in the presence of azide relative to control thylakoids. However, because the ΔpH generated under these conditions was still well above levels required for efficient transport via the ΔpH-dependent pathway (Brock et al., 1995), it is unlikely that this lower pH gradient accounts for the presence of mOE17 in the stroma.
Further characterization of the effect of azide in restoring OE17 processing in the presence of protonophores suggested the involvement of a stromal factor, since azide was ineffective at restoring OE17 maturation when isolated thylakoids had been purified away from stroma. This was surprising because it has been amply demonstrated that stromal factors are not required for efficient transport of proteins into the thylakoid lumen via the ΔpH-dependent pathway (Mould et al., 1991; Cline et al., 1992). We explored the possibility that this newly detected stromal factor might be the actual protease that is responsible for OE17 maturation. The membrane-permeable protease inhibitor PMSF had no effect on azide-induced restoration of processing in nigericin-treated plastids (Fig. 6). Other protease inhibitors could not be investigated because we were unable to consistently reconstitute this assay using washed thylakoids and concentrated stroma. However, the fact that we were unable to generate mOE17 by incubating it with stromal extract in the absence of thylakoid membranes (Fig. 7) makes it unlikely that the processing event is mediated by a soluble stromal protease.
We have also considered a model in which a stromal factor maintains OE17 in a protease-sensitive conformation. In this view, a protease that is associated with the stromal face of the thylakoid membrane would cleave prOE17 polypeptides only in the presence of the azide-sensitive stromal factor. A number of chaperones have been identified in the stromal compartment (Hemmingsen et al., 1988; Marshall and Keegstra, 1992; Moore and Keegstra, 1993). Although we are unaware of any reports demonstrating an association of OE17 with any of these stromal chaperones, it is possible that trapping this substrate in the stromal compartment by inactivating its normal thylakoid translocation pathway could allow it to form an association with chaperones that had gone previously undetected. The azide effect observed in our experiments could be explained by an azide-dependent inhibition of ATP hydrolysis by the chaperone, which could result in a prolonged chaperone-substrate interaction and an increased protease susceptibility of the loosely folded substrate. It is noteworthy, however, that we were unable to detect an association of OE17 with other components present in the stroma (E.A. Leheny and S.M. Theg, unpublished results).
Our data suggest that the proteolytic processing activity resides either within the thylakoids or on the external thylakoid membrane surface because processing does not occur during incubation of prOE17 with the stroma alone (Fig. 7). Recently, a homolog of bacterial FtsH, an ATP-dependent metalloprotease, has been cloned and its protein product has been localized to the thylakoid membrane (Lindahl et al., 1996). Might this integral membrane protein be responsible for the processing of OE17 in our experiments? In support of this notion, the deduced amino acid sequence and topology of the plastid FtsH indicate that the ATP- and Zn-binding sites required for proteolytic activity of the related bacterial enzyme are both exposed to the stromal compartment (Lindahl et al., 1996). However, azide does not affect either proteolytic activity or in vitro ATP hydrolysis in the bacterial FtsH homolog (Tomoyasu et al., 1995). Furthermore, FtsH cleaves target proteins at many sites (Tomoyasu et al., 1995), whereas the cleavage of OE17 that we observed in our experiments was specific, resulting in mOE17. If the chloroplast FtsH protease is responsible for this processing, one would have to hypothesize that a stromal factor stimulates its activity in the presence of azide, and that its specificity is such that degradation products are the same size as those created by the lumenal thylakoid processing protease.
Although we cannot rule out the possibility that a nonspecific protease is responsible for the azide-/nigericin-dependent maturation of OE17, we favor a third model, which posits that this protein is partially translocated across the membrane in the presence of azide and protonophores. Translocation of the amino-terminal portion would allow processing by the thylakoid processing peptidase, but in the absence of a ΔpH to complete translocation, the transporting protein would then be released from the translocation pore back into the stroma. A similar retrograde transport event has been invoked by Roberts et al. (1991) to explain the presence of mature-sized ricin in the stroma after its import into chloroplasts behind lumen-directed transit peptides. Retrograde movement of partially translocated precursors was also observed in mitochondria in the absence of the transmembrane potential difference (Ungermann et al., 1996). This model is consistent with all of our experiments, and is uniquely supported by our observation that processing is sensitive to the translocation inhibitor HgCl2. We recognize, however, that HgCl2 may also inhibit stromal or membrane-associated proteases in addition to the transport machinery.
The involvement of an azide-sensitive stromal factor in the maturation of OE17 in the presence of ionophores suggests (although it does not prove) a role for cpSecA in this process. Assuming that, as in bacterial SecA, cpSecA undergoes repeated rounds of membrane insertion and deinsertion (Economou and Wickner, 1994; Kim et al., 1994), and that the deinsertion step is inhibited by azide (Nishiyama et al., 1996), one can construct a reaction scheme in which azide-bound cpSecA presents the amino terminus of OE17 in the lumen in such a way as to allow cleavage of the transit peptide. In the absence of the ΔpH driving force, the protein would be unable to continue into the lumen and would then slip back out into the stroma.
The postulate that SecA plays a role in the transport of certain proteins that use the ΔpH-dependent pathway has interesting implications. “Back-up” transport systems have been identified for other translocation processes (Nunnari and Walter, 1996; Schatz and Dobberstein, 1996), and our data suggest the possibility of pathway redundancy in chloroplast protein transport as well. In this respect, it is interesting to note that two nuclear mutations in maize that cause defective targeting to the thylakoid lumen have been characterized (Voelker and Barkan, 1995). The first, hcf106, specifically affects proteins that are transported by the ΔpH-dependent pathway, whereas the second, tha1, affects proteins on the sec-dependent pathway. The phenotype associated with these mutations is that proteins accumulate as stromal intermediates because their transport into the thylakoids is inhibited. The mutation in tha1 has recently been identified as a null mutation of the SecA gene (Voelker et al., 1997). In vivo data from tha1 indicate that a certain fraction of the proteins transported by the sec-dependent pathway are still correctly transported to the lumen (Voelker and Barkan, 1995). This raises the possibility that some components of the different translocation machineries may be redundant and called into use if the pathway normally used by a protein is inhibited.
This view is further supported by the recent study of Henry et al. (1997) in which a “dual-targeting” transit peptide was constructed that was capable of directing passenger proteins to either the sec- or ΔpH-dependent pathways. In these experiments, however, proteins normally on the ΔpH-dependent pathway were found to be incompatible with the sec pathway, such that dual-targeting OE17 was transported only on the former pathway. The experiments presented here suggest that under some conditions authentic prOE17 might traverse the sec pathway, albeit inefficiently, suggesting that our understanding of the structural requirements for pathway-specific targeting remains incomplete.
At this time, we do not know the identity of the azide-sensitive factor involved in the OE17 processing described here, and the model involving cpSecA is speculative. It is consistent, however, with both our observations and the known mode of action of azide on the SecA cycle. Experiments are currently under way to test predictions generated by this view. It will be interesting to determine if thylakoid protein import will be another example of a cellular protein translocation system that has developed redundancies as a fail-safe mechanism for function.
ACKNOWLEDGMENT
We thank Marc Tormey for assistance with preparation of the figures.
Abbreviations:
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- cpSecA
the chloroplast homolog of bacterial SecA
- ΔpH
transmembrane pH gradient
- DHAP
dihydroxyacetone phosphate
- IB
import buffer
- OAA
oxaloacetic acid
- OE33
OE23, and OE17, the 33-, 23-, and 17-kD subunits of the O2-evolving complex, respectively
- PCA
perchloric acid
- prOE17
iOE17, and mOE17, the precursor, intermediate, and mature forms of OE17, respectively
Footnotes
This work was supported in part by the U.S. Department of Agriculture (grant no. 95-37304-2325 to S.M.T.) and by a National Science Foundation training grant fellowship to S.A.T.
LITERATURE CITED
- Berghofer J, Karnauchov I, Herrmann RG, Klosgen RB. Isolation and characterization of a cDNA encoding the SecA protein from spinach chloroplasts: evidence for azide resistance of sec-dependent protein translocation across thylakoid membranes in spinach. J Biol Chem. 1995;270:18341–18346. doi: 10.1074/jbc.270.31.18341. [DOI] [PubMed] [Google Scholar]
- Brock IW, Mills JD, Robinson D, Robinson C. The ΔpH-driven, ATP-independent protein translocation mechanism in the chloroplast thylakoid membrane. J Biol Chem. 1995;270:1657–1662. doi: 10.1074/jbc.270.4.1657. [DOI] [PubMed] [Google Scholar]
- Clausmeyer S, Klosgen RB, Herrmann RG. Protein import into chloroplasts: the hydrophilic lumenal proteins exhibit unexpected import and sorting specificities in spite of structurally conserved transit peptides. J Biol Chem. 1993;268:13869–13876. [PubMed] [Google Scholar]
- Cline K, Ettinger WF, Theg SM. Protein-specific energy requirements for protein transport across or into thylakoid membranes: two lumenal proteins are transported in the absence of ATP. J Biol Chem. 1992;267:2688–2696. [PubMed] [Google Scholar]
- Cline K, Henry R. Import and routing of nucleus-encoded chloroplast proteins. Annu Rev Cell Biol. 1996;12:1–26. doi: 10.1146/annurev.cellbio.12.1.1. [DOI] [PubMed] [Google Scholar]
- Cline K, Henry R, Li C, Yuan J. Multiple pathways for protein transport into or across the thylakoid membrane. EMBO J. 1993;12:4105–4114. doi: 10.1002/j.1460-2075.1993.tb06094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilley RA. The effect of various energy-conversion states of chloroplast on proton and electron transport. Arch Biochem Biophys. 1970;137:270–283. doi: 10.1016/0003-9861(70)90434-0. [DOI] [PubMed] [Google Scholar]
- Economou A, Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- Ettinger WF, Theg SM. Physiologically active chloroplasts contain pools of unassembled extrinsic proteins of the photosynthetic oxygen-evolving enzyme complex in the thylakoid lumen. J Cell Biol. 1991;115:321–328. doi: 10.1083/jcb.115.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin AE, Hoffman NE. Characterization of a chloroplast homologue of the 54-kDa subunit of the signal recognition particle. J Biol Chem. 1993;268:22175–22180. [PubMed] [Google Scholar]
- Haucke V, Schatz G. Import of proteins into mitochondria and chloroplasts. Trends Cell Biol. 1997;7:103–106. doi: 10.1016/S0962-8924(96)10052-0. [DOI] [PubMed] [Google Scholar]
- Hemmingsen SM, Woolford C, van der Vies SM, Tilly K, Dennis DT, Georgepolous C, Hendrix RW, Ellis RJ. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988;333:330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Henry R, Carrigan M, McCaffery M, Ma X, Cline K. Targeting determinants and proposed evolutionary basis for the Sec and delta pH protein import systems in chloroplast thylakoid membranes. J Cell Biol. 1997;136:823–832. doi: 10.1083/jcb.136.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R, Kapazoglou A, McCaffery M, Cline K. Differences between lumen targeting domains of chloroplast transit peptides determine pathway specificity for thylakoid transport. J Biol Chem. 1994;269:10189–10192. [PubMed] [Google Scholar]
- Ikeuchi M. Subunit proteins of photosystem II. Bot Mag Tokyo. 1992;105:327–373. [Google Scholar]
- Karnauchov I, Cai DG, Schmidt I, Herrmann RG, Klosgen RB. The thylakoid translocation of subunit 3 of photosystem I, the psaf gene product, depends on a bipartite transit peptide and proceeds along an azide-sensitive pathway. J Biol Chem. 1994;269:32871–32878. [PubMed] [Google Scholar]
- Kim Y, Rajapandi T, Oliver D. SecA protein is exposed to the periplasmic surface of the E. coli inner membrane in its active state. Cell. 1994;78:845–853. doi: 10.1016/s0092-8674(94)90602-5. [DOI] [PubMed] [Google Scholar]
- Kirwin PM, Meadow JW, Shackleton JB, Musgrove JE, Elderfield PD, Mould R, Hay NA, Robinson C. ATP-dependent import of a lumenal protein by isolated thylakoid vesicles. EMBO J. 1989;8:2251–2255. doi: 10.1002/j.1460-2075.1989.tb08349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosgen RB, Brock IW, Herrmann RG, Robinson C. Proton gradient-driven import of the 16 kDa oxygen-evolving complex protein as the full precursor protein by isolated thylakoids. Plant Mol Biol. 1992;18:1031–1034. doi: 10.1007/BF00019226. [DOI] [PubMed] [Google Scholar]
- Knott TG, Robinson C. The SecA inhibitor, azide, reversibly blocks the translocation of a subset of proteins across the chloroplast thylakoid membrane. J Biol Chem. 1994;269:7843–7846. [PubMed] [Google Scholar]
- Ko K, Bornemisza O, Kourtz L, Ko ZW, Plaxton WC, Cashmore AR. Isolation and characterization of a cDNA clone encoding a cognate 70-kDa heat shock protein of the chloroplast envelope. J Biol Chem. 1992;267:2986–2993. [PubMed] [Google Scholar]
- Laidler V, Chaddock AM, Knott TG, Walker D, Robinson C. A SecY homolog in Arabidopsis thaliana: sequence of a full-length cDNA clone and import of the precursor protein into chloroplasts. J Biol Chem. 1995;270:17664–17667. doi: 10.1074/jbc.270.30.17664. [DOI] [PubMed] [Google Scholar]
- Leheny EA, Theg SM. Apparent inhibition of chloroplast protein import by cold temperatures is due to energetic considerations, not by membrane fluidity. Plant Cell. 1994;6:427–437. doi: 10.1105/tpc.6.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Henry R, Yuan J, Cline K, Hoffman NE. A chloroplast homologue of the signal recognition particle subunit SRP54 is involved in the posttranslational integration of a protein into thylakoid membranes. Proc Natl Acad Sci USA. 1995;92:3789–3793. doi: 10.1073/pnas.92.9.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl M, Tabak S, Cseke L, Pichersky E, Andersson B, Adam Z. Identification, characterization, and molecular cloning of a homologue of the bacterial FtsH protease in chloroplasts of higher plants. J Biol Chem. 1996;271:29329–29334. doi: 10.1074/jbc.271.46.29329. [DOI] [PubMed] [Google Scholar]
- Lorkovic ZJ, Schroder WP, Pakrasi HB, Irrgang KD, Herrmann RG, Oelmuller R. Molecular characterization of psbw, a nuclear-encoded component of the photosystem II reaction center complex in spinach. Proc Natl Acad Sci USA. 1995;92:8930–8934. doi: 10.1073/pnas.92.19.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JS, DeRocher AE, Keegstra K, Vierling E. Identification of heat shock protein hsp70 homologues in chloroplasts. Proc Natl Acad Sci USA. 1990;87:374–378. doi: 10.1073/pnas.87.1.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JS, Keegstra K. Isolation and characterization of a cDNA clone encoding the major hsp70 of the pea chloroplastic stroma. Plant Physiol. 1992;100:1048–1054. doi: 10.1104/pp.100.2.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl D, Robinson C, Shackleton JB, Herrmann RG, Klosgen RB. Targeting of proteins to the thylakoids by bipartite presequences: CFoII is imported by a novel, third pathway. EMBO J. 1994;13:1310–1317. doi: 10.1002/j.1460-2075.1994.tb06383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Keegstra K. Characterization of a cDNA clone encoding a chloroplast-targeted Clp homologue. Plant Mol Biol. 1993;21:525–537. doi: 10.1007/BF00028809. [DOI] [PubMed] [Google Scholar]
- Mould RM, Robinson C. A proton gradient is required for the transport of 2 lumenal oxygen-evolving proteins across the thylakoid membrane. J Biol Chem. 1991;266:12189–12193. [PubMed] [Google Scholar]
- Mould RM, Shackleton JB, Robinson C. Transport of proteins into chloroplasts: requirements for the efficient import of two lumenal oxygen-evolving complex proteins into isolated thylakoids. J Biol Chem. 1991;266:17286–17289. [PubMed] [Google Scholar]
- Murataliev MB, Milgrom YM, Boyer PD. Characteristics of the combination of inhibitory Mg2+ and azide with the F1 ATPase from chloroplasts. Biochemistry. 1991;30:8305–8310. doi: 10.1021/bi00098a004. [DOI] [PubMed] [Google Scholar]
- Musgrove JE, Elderfield PD, Robinson C. Endopeptidases in the stroma and thylakoids of pea chloroplasts. Plant Physiol. 1989;90:1616–1621. doi: 10.1104/pp.90.4.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai M, Nohara T, Sugita D, Endo T. Identification and characterization of the Sec-A protein homologue in the cyanobacterium Synechococcus PCC7942. Biochem Biophys Res Commun. 1994;200:844–851. doi: 10.1006/bbrc.1994.1528. [DOI] [PubMed] [Google Scholar]
- Nielsen VS, Mant A, Knoetzel J, Moller BL, Robinson C. Import of barley photosystem-I subunit-N into the thylakoid lumen is mediated by a bipartite presequence lacking an intermediate processing site: role of the ΔpH in translocation across the thylakoid membrane. J Biol Chem. 1994;269:3762–3766. [PubMed] [Google Scholar]
- Nishiyama K-I, Suzuki T, Tokuda H. Inversion of the membrane topology of SecG coupled with SecA-dependent preprotein translocation. Cell. 1996;85:71–81. doi: 10.1016/s0092-8674(00)81083-1. [DOI] [PubMed] [Google Scholar]
- Nunnari J, Walter P. Regulation of organelle biogenesis. Cell. 1996;84:389–394. doi: 10.1016/s0092-8674(00)81283-0. [DOI] [PubMed] [Google Scholar]
- Olsen LJ, Keegstra K. The binding of precursor proteins to chloroplasts requires nucleoside triphosphates in the intermembrane space. J Biol Chem. 1992;267:433–439. [PubMed] [Google Scholar]
- Reed JE, Cline K, Stephens LC, Bacot KO, Viitanen PV. Early events in the import/assembly pathway of an integral thylakoid protein. Eur J Biochem. 1990;194:33–42. doi: 10.1111/j.1432-1033.1990.tb19423.x. [DOI] [PubMed] [Google Scholar]
- Roberts LM, Meadows JW, Robinson C. Targeting of ricin A chain into pea chloroplasts. FEBS Lett. 1991;293:134–136. doi: 10.1016/0014-5793(91)81169-9. [DOI] [PubMed] [Google Scholar]
- Schatz G, Dobberstein B. Common principles of protein translocation across membranes. Science. 1996;271:1519–1525. doi: 10.1126/science.271.5255.1519. [DOI] [PubMed] [Google Scholar]
- Schuldiner S, Rottenberg H, Avron M. Determination of ΔpH in chloroplasts. 2. Fluorescent amines as a probe for the determination of ΔpH in chloroplasts. Eur J Biochem. 1972;25:64–70. doi: 10.1111/j.1432-1033.1972.tb01667.x. [DOI] [PubMed] [Google Scholar]
- Theg SM, Geske FJ. Biophysical characterization of a transit peptide directing chloroplast protein import. Biochemistry. 1992;31:5053–5060. doi: 10.1021/bi00136a018. [DOI] [PubMed] [Google Scholar]
- Theg SM, Scott SV. Protein import into chloroplasts. Trends Cell Biol. 1993;3:186–190. doi: 10.1016/0962-8924(93)90212-j. [DOI] [PubMed] [Google Scholar]
- Tomoyasu T, Gamer J, Bukau B, Kanemori M, Mori H, Rutman AJ, Oppenheim AB, Yura T, Yamanaka K, Niki H and others. Escherichia coli FtsH is a membrane-bound, ATP-dependent protease which degrades the heat-shock transcription factor ς32. EMBO J. 1995;14:2551–2560. doi: 10.1002/j.1460-2075.1995.tb07253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Guiard B, Neupert W, Cyr DM. The Δψ and hsp70/MIM44-dependent reaction cycle driving early steps of protein import into mitochondria. EMBO J. 1996;15:735–744. [PMC free article] [PubMed] [Google Scholar]
- Voelker R, Barkan A. Two nuclear mutations disrupt distinct pathways for targeting proteins to the chloroplast thylakoid. EMBO J. 1995;14:3905–3914. doi: 10.1002/j.1460-2075.1995.tb00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker R, Mendel-Hartvig J, Barkan A. Transposon-disruption of a maize nuclear gene, tha1, encoding a chloroplast SecA homolog: in vivo role of SecA in thylakoid protein targeting. Genetics. 1997;145:467–478. doi: 10.1093/genetics/145.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Seibert SS, Ko K. Identification of chloroplast envelope proteins in close physical proximity to a partially translocated chimeric precursor. J Biol Chem. 1994;269:32264–32271. [PubMed] [Google Scholar]
- Yuan J, Cline K. Plastocyanin and the 33-kDa subunit of the oxygen-evolving complex are transported into thylakoids with similar requirements as predicted from pathway specificity. J Biol Chem. 1994;269:18463–18467. [PubMed] [Google Scholar]
- Yuan J, Henry R, McCaffery M, Cline K. SecA homolog in protein transport within chloroplasts: evidence for endosymbiont derived sorting. Science. 1994;266:796–798. doi: 10.1126/science.7973633. [DOI] [PubMed] [Google Scholar]