Abstract
γδ T cells are innate lymphocytes that recognize and kill a range of tumor cells and are currently being explored as a target for tumor immunotherapy. However, γδ T cells play a complex role in cancer and can promote, as well as inhibit, tumor growth. In addition to tumor cell killing, γδ T cells express a number of cytokines and other soluble factors in response to tumors. Soluble factors expressed by γδ T cells in these settings include interferon-γ, tumor necrosis factor-α, interleukin (IL)-4, IL-10, transforming growth factor-β, IL-17, and a number of growth factors. These factors have differing and sometimes opposing effects on antitumor immunity and tumor angiogenesis, and likely contribute to the complex role of these cells in cancer. Here, we review studies in both mice and humans that examine differential cytokine secretion by γδ T cells in response to tumors and tumor immunotherapy, and discuss the influence of these γδ T-cell-derived factors on tumor growth.
Introduction
Immunity has long been known to impact cancer development in a diverse manner. Effective immune surveillance of cancerous tumors can suppress tumor growth, but improper and/or prolonged immune activity can actually contribute to its initiation and progression (Vakkila and Lotze 2004, and references cited therein; Chow and others 2012, and references cited therein). Immune responses at tumor sites are a balancing act of inflammatory and regulatory responses that are mediated by many different immune cells and cytokines, many of which display dual functionality by both promoting and inhibiting tumor growth (Chow and others 2012, and references cited therein). Among the immune cells, γδ T cells may be considered important during the establishment of the tumor microenvironment and the development of tumor immunity.
Recently, the role of γδ T cells in tumor immunity has received considerable attention and research. γδ T cells from both humans and mice infiltrate tumor sites, lyse tumor cells, and prevent the growth of a variety of cancers (Fisch and others 1990; Groh and others 1999; Girardi and others 2001; Gao and others 2003; Peng and others 2007; He and others 2010; Bryant and others 2011). Tumor cell recognition by γδ T cells is largely mediated through the recognition of membrane-bound phosphoantigens, such as isopentenyl pyrophosphate (IPP), by the γδ T-cell receptor (TCR) and/or the recognition of stress ligands on the tumor cell through the TCR and NKG2D (Gomes and others 2010, and references cited therein). Due to their antitumor activity, therapeutic strategies aimed at harnessing and enhancing the antitumor properties of these cells have been developed and used in clinics (Gomes and others 2010, and references cited therein; Hannani and others 2012, and references cited therein). These therapies often require interleukin (IL)-2 combined with synthetic phosphoantigens or bisphosphonates, such as zoledronate, which stimulate γδ T cells by enhancing cellular accumulation of IPP (Dieli and others 2007; Benzaïd and others 2011; Hannani and others 2012, and references cited therein). While these therapies show potential, optimal results have not yet been achieved. Several recent reviews have examined the antitumor activity of γδ T cells and their potential for immunotherapy (Table 1).
Table 1.
Select Recent Reviews for γδ T Cells and Cancer
| Topic | Selected article |
|---|---|
| γδ T-cell immunotherapy | Hannani and others (2012); Trends Immunol |
| Castella and others (2011); Cell Mol Life Sci | |
| Kalyan and others (2011); Curr Med Chem | |
| Yoshida and others (2011); Surg Today | |
| Gomes and others (2010); Cancer Res | |
| Tumor escape from γδ T cell attack | Capietto and others (2011); Cell Mol Life Sci |
| γδ T-cell antigen presentation for cancer therapy | Moser and Eberl (2011); Cell Mol Life Sci |
Despite the evidence demonstrating antitumor responses by γδ T cells, the exact role that these cells play in cancer is not entirely clear. In mice, the absence of γδ T cells sometimes leads to enhanced tumor growth, but in some cases, it leads to a reduction in tumor burden (Seo and others 1999; Girardi and others 2001; Gao and others 2003; Ke and others 2003). In human patients, infiltration of γδ T cells into the tumor is associated with better prognosis in some cancers (Bialasiewicz and others 1999), but not in others (Inman and others 2008). These data suggest that, depending on the tumor, γδ T cells can promote, inhibit, or possibly have no significant effect on tumor growth. These differential roles are likely mediated, at least in part, by the diverse repertoire of cytokines and other secreted factors that are induced in these cells, which can be categorized as either inflammatory or regulatory (Bonneville and others 2010, and references cited therein). A better understanding of the diverse roles of γδ T cells and their secreted factors in cancer should allow for a better manipulation of these cells for immunotherapy. In this review, we will summarize the literature with regard to different cytokines and other secreted factors expressed by γδ T cells in response to tumors and examine how these factors could impact tumor immunity and immunotherapy.
γδ T-Cell-Associated Factors That Enhance Antitumor Immunity
γδ T cells are an important early source of the inflammatory cytokines interferon-γ (IFN-γ) and tumor necrosis factor (TNF)-α in many infections and other disease models (Hao and others 2010, and references cited therein). The expression of IFN-γ and TNF-α by γδ T cells is promoted by numerous stimuli, including TCR agonists, ligands to NKG2D, and certain cytokines, such as IL-12 and IL-18 (Groh and others 1999; Wesch and others 2001; Rincon-Orozco and others 2005; Paget and others 2012). IFN-γ and TNF-α are also important cytokines in antitumor responses and inhibit tumor growth through several mechanisms, including the enhancement of antitumor immunity and the inhibition of tumor angiogenesis (Talmadge and others 1987; Lejeune and others 2006; Lu and others 2009). Human γδ T cells express IFN-γ and TNF-α on exposure to tumor cell lines of numerous origins (Groh and others 1999; Poggi and others 2004; Halary and others 2005), suggesting that these cytokines may play a role in γδ T-cell responses to tumors.
In mice, γδ T cells appear to be an important early source of tumor-induced IFN-γ, and the expression of IFN-γ may be essential for optimal antitumor responses by these cells (Gao and others 2003; He and others 2010). The early production of IFN-γ by murine γδ T cells can enhance MHCI expression on tumors, as well as enhance CD8+ T cell responses (Gao and others 2003; Riond and others 2009), suggesting that γδ T cells could be important for augmenting downstream adaptive immune responses to tumors. These data suggest that early IFN-γ secretion by γδ T cells is important for some antitumor responses in mice.
Results in humans suggest a far more complicated story with regard to the role of γδ T-cell-derived IFN-γ and TNF-α in antitumor responses. In cancer patients, the expression of both IFN-γ and TNF-α by γδ T cells is modulated, but not always enhanced. Peripheral γδ T cells from breast cancer patients produce enhanced amounts of TNF-α compared with healthy controls, which is thought to be beneficial (Gaafar and others 2009). However, peripheral γδ T cells from patients with nasopharyngeal cancer and melanoma produce reduced amounts of IFN-γ and TNF-α, which could contribute to defective antitumor immune responses in these patients (Argentati and others 2003; Puan and others 2009). Following the removal of melanoma, IFN-γ and TNF-α expression by γδ T cells is enhanced, suggesting that the reduced expression of these cytokines by γδ T cells is mediated by tumor-associated factors, which benefit the tumor (Provinciali and others 2010). In support of this, mesenchymal stem cells, which are commonly found in tumor microenvironments, were shown to inhibit IFN-γ and TNF-α expression by peripheral γδ T cells through the production of prostaglandin E2, which was induced by γδ T-cell-derived IFN-γ and TNF-α (Martinet and others 2009). In cancer patients undergoing immunotherapy with zoledronate and IL-2, serum levels of IFN-γ increase after treatment (Kunzmann and others 2012). This increase in IFN-γ expression by γδ T cells may be an important factor for successful γδ T-cell immunotherapy, as clinical responses to immunotherapy with zoledronate and IL-2 in one clinical trial correlated with increasing numbers of an effector memory γδ T-cell phenotype, which could produce IFN-γ (Dieli and others 2007). However, in another clinical trial using infusions of zoledronate-activated γδ T cells in multiple myeloma patients, IFN-γ was not believed to be important for the antitumor activity, even though serum levels of IFN-γ increased after treatment (Abe and others 2009). Collectively, these data suggest that the expression of IFN-γ and TNF-α is important in certain cancers for antitumor responses by γδ T cells, and that down-regulation of γδ T-cell-derived IFN-γ and TNF-α may help facilitate immune escape by tumors. However, further studies are needed to better determine their importance in human patients, particularly in response to immunotherapy.
γδ T-Cell-Associated Factors That Suppress Antitumor Immunity
As mentioned earlier, γδ T cells may not always play a beneficial role in antitumor immunity. Instead, in some settings, they likely have a regulatory role, suppressing antitumor responses and enhancing tumor growth. This response is not species specific, in that immunosuppressive γδ T cells have been described in both mouse tumor models and human cancers (Seo and others 1999; Peng and others 2007). Furthermore, their activity appears to be at least partially mediated by certain cytokines.
In a study by Seo and others (1998), murine γδ T cells infiltrating B16 melanoma tumors after 5 days were shown to inhibit Natural Killer (NK) and Natural Killer T (NKT)-cell activity and express large amounts of IL-4 and IL-10, but not IFN-γ. The supernatant fluids from cultures of these cells did not reduce NK and NKT cell cytotoxicity, but reduced their proliferation, suggesting that soluble IL-4 and IL-10 contributed to the inhibition of NK and NKT cell activity by γδ T cells in this model. Additional studies supported this observation and showed that γδ T-cell-derived IL-4 and IL-10, as well as transforming growth factor (TGF)-β, could inhibit antitumor immunity and promote tumor growth in mice. For example, using the B16 melanoma model, Hao and others (2011) showed that the Vγ1 subset of murine γδ T cells promoted tumor growth through the production of IL-4. These Vγ1 γδ T cells reduced the expression of IFN-γ and perforin within the tumor. In addition, IL-4 inhibited the expression of NKG2D and perforin by Vγ4 γδ T cells, which was important for the tumor-promoting activity of these Vγ1 γδ T cells. Seo and others (1999) showed that tumor-infiltrating γδ T cells from MM2 tumor lesions in mice expressed IL-10 and TGF-β, but not IFN-γ or IL-4. γδ T cells isolated from the tumor lesions, as well as the spleens, of these MM2 tumor-bearing mice inhibited the cytotoxic activity of NK cells and CD8+ T cells. Neutralizing IL-10 and TGF-β inhibited some of the suppressive effects of these γδ T cells, suggesting that these cytokines participated in the suppressive activity of these cells. Depletion of these γδ T cells by the use of a specific antibody enhanced antitumor immunity and reduced tumor growth. Finally, a study by Ke and others (2003) also described an immunosuppressive function for γδ T cells in tumor responses, as γδ T cells suppressed responses to an EL4 leukemia tumor cell line modified to express ovalbumin, and IL-10 appeared to play a role in the suppression. Collectively, these data strongly suggest that at least certain subsets of murine γδ T cells can express IL-4, IL-10, and TGF-β in response to certain tumors, inhibiting antitumor immunity.
Immunosuppressive γδ T cells may also play an important role in human cancers. In a study by Peng and others (2007), the Vδ1 subset of tumor-infiltrating γδ T cells from human breast cancer could suppress dendritic cells (DC) maturation and T-cell effector functions, which included proliferation, IL-2 secretion, and CD8+ T-cell antitumor responses in a mouse xenograft model. This suppressive activity was mediated, at least in part, by a soluble factor or factors. The suppressive activity was present in isolated fractions with greater than 100 kDa molecular mass and could be inactivated by heat, but not DNAse or RNAse. However, the factors were not identified. When these cells were stimulated by tumor cells and anti-CD3 antibody, they expressed cytokines that were typically associated with pro-inflammatory responses, including IFN-γ, granulocyte macrophage colony-stimulating factor (GM-CSF), and IL-6, but not IL-1β, TNF-α, IL-12, IL-2, IL-4, IL-10, or TGF-β. These Vδ1 γδ T cells constituted a large percentage of tumor-infiltrating lymphocytes in breast and prostate cancer, suggesting that they may be important in promoting an immunosuppressive microenvironment in these cancers. However, Vδ1 γδ T-cell infiltration into necrotizing melanomas has correlated with increased survival (Bialasiewicz and others 1999), suggesting that the development of suppressive Vδ1 γδ T cells may be specific for certain cancers. Even though the suppressive effects of these cells were not mediated by IL-10 or TGF-β, these results resemble those found in mice by Seo and others (1999), where infiltrating γδ T cells suppressed the activity of CD8+ T cells by secreted factors. Interestingly, stimulation of these suppressive breast cancer Vδ1 γδ T cells by a TLR8 agonist could reverse the suppression of antitumor responses (Peng and others 2007).
Even though human γδ T cells may secrete different soluble factors than murine γδ T cells, which suppress antitumor immunity, certain human peripheral γδ T cells express IL-4, IL-10, and TGF-β on activation (Wesch and others 2001; Kühl and others 2009). In one study, a culture of human γδ T cells with IPP or Daudi lymphoma cells in vitro under Th2-polarizing conditions (rhIL-4, anti-IL-12) resulted in reduced IFN-γ and TNF-α production and enhanced IL-4 production by these γδ T cells (Wesch and others 2001). In the absence of these polarizing conditions, γδ T cells primarily secreted IFN-γ. Furthermore, a study by Gaafar and others (2009) showed that while γδ T cells from breast cancer patients produced very little IL-4, the expansion of these cells by zoledronate and IL-2 led to an increased production of IL-4 by these cells compared with expanded γδ T cells from healthy controls. Therefore, IL-4, IL-10, and TGF-β production by human γδ T cells may also play a role in suppressing antitumor responses, similar to what they do in mice. However, additional studies are needed to confirm this possibility.
Collectively, the results summarized above support the idea that certain human γδ T cells, at least in some cancers, can behave as regulatory cells within the tumor microenvironment, suppress antitumor responses, and promote tumor growth, with secreted factors being considered important for their activity.
Conflicting Role of γδ T-Cell-Derived IL-17 in Tumor Immunity
In addition to their role in tumor responses, a renewed interest in γδ T cells has also emerged due to the discovery that γδ T cells are an important innate source of IL-17, particularly in the mouse. Secretion of IL-17 by murine and human γδ T cells is promoted by TCR and pattern recognition receptor stimulation, along with the cytokines IL-1, IL-6, IL-23, and TGF-β (Ness-Schwickerath and Morita 2011, and references cited therein). Previous studies that describe the role of IL-17 in tumor growth have had conflicting results, suggesting both pro-tumor and antitumor functions for this cytokine (Alshaker and Matalka 2011, and references cited therein). Murine γδ T cells have been identified as a major source of IL-17 in several tumor models, which are summarized next.
In some studies, a detrimental role for γδ T-cell-derived IL-17 in tumor responses has been suggested. Specifically, the expression of IL-17 by tumor-infiltrating γδ T cells in a model of fibrosarcoma in Balb/c mice promoted tumor angiogenesis and, subsequently, enhanced tumor growth (Wakita and others 2010). Consistent with this, others have found that IL-17 enhanced the expression of vascular endothelial growth factor (VEGF), which is an important growth factor in angiogenesis (Liu and others 2011). As such, the promotion of tumor angiogenesis may be considered an important and detrimental function of IL-17+ γδ T cells. Significantly, the local tumor microenvironment was considered important for the expression of IL-17 by these γδ T cells, as cells from the tumor tissue had enhanced IL-17 production compared with normal skin and cells from the spleen and draining lymph nodes of tumor-bearing mice did not increase IL-17 production. Furthermore, IL-6, TGF-β, and IL-23 were involved in the promotion of IL-17 by these γδ T cells. Another study examining lung metastasis showed that the expression of IL-17 enhanced metastasis and reduced survival in experiments involving the Lewis lung carcinoma model (Carmi and others 2011). In these experiments, IL-17 was primarily produced by γδ T cells, and the secretion of IL-17 by γδ T cells was induced by IL-1. Enhanced tumor growth in the lung induced by IL-17 may have been mediated by the reduced potential of antigen-presenting cells to promote Th1 immunity. However, based on the study by Wakita and others (2010), angiogenesis may also have played a role. These data suggest that IL-17 production by γδ T cells clearly promotes tumor growth in some settings.
However, other studies in opposition to the results described earlier demonstrate a beneficial role for IL-17+ γδ T cells in the inhibition of tumor growth. In a mouse model of bladder cancer, treatment with Mycobacterium bovis Bacillus Calmette-Guérin (BCG) enhanced IL-17 expression by γδ T cells, which was essential for optimal neutrophil recruitment into the tumor and a reduction in tumor growth (Takeuchi and others 2011). In another study with a number of different tumor models, the early infiltration of IL-17-producing γδ T cells into the tumor bed of chemotherapy-treated tumors was associated with the subsequent infiltration of IFN-γ–producing CD8+ T cells and the suppression of tumor growth (Ma and others 2011). In these experiments, both IL-17 and IFN-γ were necessary for the inhibition of tumor growth. Based on these results, it has been proposed that immunotherapy aimed at polarizing γδ T cells to express IL-17 might be useful in enhancing the efficacy of chemotherapy (Hannani and others 2012). Interestingly, in both studies where antitumor immunity was enhanced by γδ T-cell-derived IL-17, other cells played an important role for the beneficial response. In the bladder cancer study, neutrophils were important, whereas in the chemotherapy study, IFN-γ-secreting CD8+ T cells were important. Therefore, it is possible that in the absence of these other responses, IL-17 production by γδ T cells could lose its benefit and, therefore, enhance tumor growth as described earlier. Further studies are needed to better clarify the role of γδ T-cell-derived IL-17 on tumor growth and determine whether γδ T cell production of IL-17 has relevance to human cancers.
Potentially Underappreciated Role of γδ T-Cell-Derived Growth Factors in Tumor Immunity
Tumors have been described as wounds that do not heal, and numerous growth factors, including keratinocyte growth factor (KGF), play a role in their progression (Ceccarelli and others 2012, and references cited therein). In addition to pro- and anti-inflammatory cytokines, γδ T cells are a source of a number of growth factors. This has been well defined in the mouse, where skin-associated γδ T cells are a major source of KGF and are essential for optimal wound healing (Jameson and others 2002). In humans, γδ T cells produce transcripts and/or proteins for a number of growth factors, including KGF, insulin-like growth factor (IGF)-1, epidermal growth factor (EGF), fibroblast growth factor (FGF)-9, angiogenin (ANG), platelet-derived growth factor (PDGF), and VEGF (Workalemahu and others 2004; Schilbach and others 2008). Furthermore, in human peripheral Vδ2 γδ T cells, the expression of FGF-9 is enhanced by IPP (Workalemahu and others 2004). As such, the expression of growth factors by tumor-infiltrating γδ T cells could potentially represent a significant response that promotes tumor growth in some settings.
Expression of growth factors in human γδ T cells
In a study by Schilbach and others (2008), human Vδ2 and Vδ1 T cells were expanded and found to produce a number of growth factors, including IGF-1, EGF, PDGF, ANG, and VEGF. When these cells were cultured with a neuroblastoma cell line, the Vδ1 cells produced reduced amounts of these growth factors, while Vδ2 cells produced slightly increased amounts. These data prompted the authors to suggest that Vδ1 γδ T cells may be better at promoting antitumor responses to this type of tumor, partially due to their reduced expression of growth factors. The expression of VEGF by γδ T cells, particularly in response to a tumor cell, is intriguing, as VEGF is vital for tumor angiogenesis, growth, and metastasis (Saharinen and others 2011, and references cited therein). In addition to direct VEGF expression by γδ T cells, KGF and FGF-9 are capable of promoting VEGF expression in other cells in a paracrine manner (Niu and others 2007; Behr and others 2010). Therefore, γδ T cells may also stimulate VEGF expression indirectly by the expression of other growth factors. These data suggest that γδ T cells may participate in the production of growth factors within the tumor microenvironment, functions that have not yet been attributed to γδ T cells.
A recent clinical study examining the treatment of patients with zoledronate and IL-2 observed an increase in VEGF levels in these patients, in addition to an expansion of γδ T cells and other immune cells (Kunzmann and others 2012), supporting the possible role of γδ T-cell-derived growth factors in human cancer. Interestingly, the increase in VEGF was more pronounced in patients with solid tumors compared with those with leukemia. It is unknown whether γδ T cells played a direct role in this increase of VEGF production. However, these data would be consistent with the previously discussed studies which demonstrated that activated γδ T cells express VEGF, as well as factors which can indirectly promote the expression of VEGF. Significantly, elevated VEGF levels in these patients correlated with a lack of success of the therapy. Even if γδ T cells were not important for this enhanced VEGF expression, it appears to be an important obstacle to be overcome in optimizing γδ T-cell immunotherapy. Further studies are warranted to determine whether γδ T cells are an important source of tumor-promoting growth factors in mice or humans.
Influences on Differential Cytokine Secretion by γδ T Cells in Tumor Studies
Differential cytokine production and behavior by γδ T cells is obviously an important variable in mouse studies that examine the role of γδ T cells in cancer, but there are important caveats to be considered in defining these roles. Differences in mouse strain, age, and other factors (source, housing, etc.) in these studies may influence γδ T-cell cytokine secretion and subset distribution, which could influence the effect of γδ T cells on tumor growth in these experiments. For example, a study on West Nile Virus demonstrated that the numbers and behavior of Vγ1 and Vγ4 γδ T cells in mice could vary with age (Welte and others 2008). In addition, epidermal γδ T cells from Balb/c mice were shown to produce less IFN-γ in response to IL-12 and IL-18 than those from C57BL/6 mice (Sugaya and others 1999). Therefore, in mouse studies examining the role of γδ T cells in cancer, it is likely important to further examine γδ T-cell responses and subsets within the specific mice used for the study in the absence of tumor cells, as variations in these factors would likely lead to variable tumor responses by the γδ T cells.
Conclusions
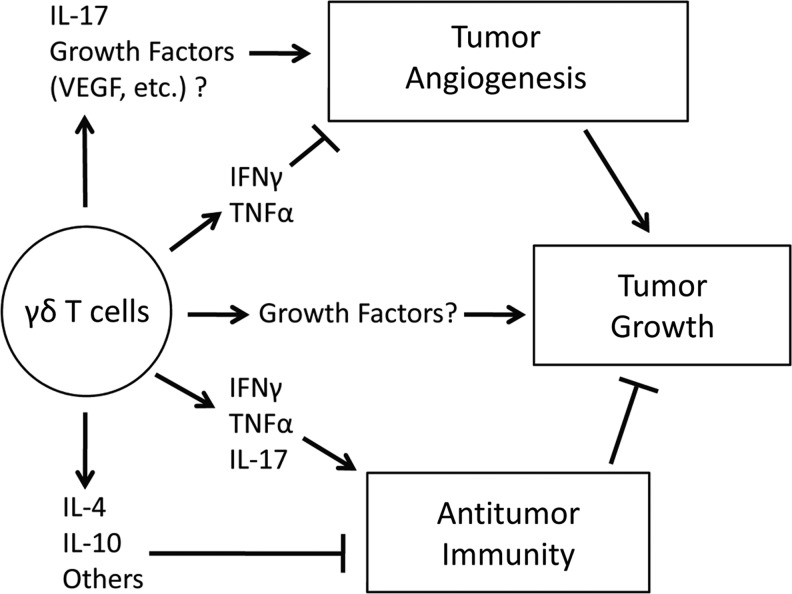
In response to tumor cells, γδ T cells produce a variety of cytokines that both inhibit and enhance antitumor immune responses, which likely accounts for some of the conflicting reports about the role of these cells in antitumor immunity (Fig. 1). Among these cytokines, IFN-γ, and possibly TNF-α, contribute to the ability of γδ T cells to inhibit tumor growth. In contrast, the expression of IL-4, IL-10, TGF-β, other unknown factors, and possibly growth factors, by γδ T cells suppress antitumor immunity and enhance tumor growth. The expression of IL-17 by γδ T cells appears to have conflicting effects on tumor growth, which could be dependent on the type of cancer or other factors, such as tumor infiltration of other cell types or the use of chemotherapy. The expression of these cytokines by γδ T cells influences downstream adaptive immune responses to tumors, which are consistent with the described ability of γδ T cells to link innate and adaptive immunity (Holtmeier and Kabelitz 2005, and references cited therein). γδ T-cell-derived IFN-γ and IL-17 enhance CD8+ T-cell responses, while IL-10, TGF-β, and other γδ T-cell-derived soluble factors inhibit them. Therefore, in addition to their lytic activity, several studies suggest that the influence of γδ T cells on adaptive immune responses to tumors is an important part of their role in antitumor immunity.
FIG. 1.
Summary of the influence of γδ T-cell-derived cytokines and growth factors on tumor growth.
Differential cytokine production by γδ T cells may also be considered important in γδ T-cell immunotherapy. The stimulation of γδ T cells with synthetic phosphoantigens or bisphosphonates may only enhance γδ T-cell responses that are already influenced by the tumor environment, beneficial or not, which could account for the variable effectiveness of these therapies. Therefore, the identification of therapeutic options that enhance and favor the production of beneficial antitumor cytokines and soluble factors by γδ T cells, while minimizing or removing detrimental factors, may be key to unlocking the maximum potential of γδ T-cell immunotherapy. A good example of this concept can be found in the study by Peng and others (2007), where they were able to reverse the immunosuppressive phenotype of tumor-infiltrating γδ T cells by stimulating them with a TLR8 agonist. Other options may include the use of additional cytokines to further enhance the antitumor activity of γδ T cells. For example, the addition of IL-18 to zoledronate and IL-2 enhances IFN-γ and TNF-α expression by γδ T cells compared with zoledronate and IL-2 alone (Li and others 2010). The use of anti-VEGF and other antiangiogenesis therapies may inhibit any pro-angiogenesis responses induced by γδ T cells or γδ T-cell immunotherapy. Furthermore, chemotherapy might also have the potential to enhance the effectiveness of γδ T-cell immunotherapy, as discussed by Hannani and others (2012). In conclusion, in order to better understand the complex role of γδ T cells in cancer and improve the effectiveness of γδ T-cell immunotherapy, additional studies are needed that examine the cytokine profiles of γδ T cells in response to tumors and immunotherapy, as well as identify ways to best manipulate this profile for the benefit of the patient.
Acknowledgments
Our studies are supported by grants from the National Institutes of Health (NIH) (NCCAM AT0004986-01), NIH COBRE (P20 RR020185), M.J. Murdock Charitable Trust, and The Montana State University Agricultural Experiment Station. The authors would like to thank Dana Doney, Amanda Robison, and Dr. Jeff Holderness for a critical review of the article.
Author Disclosure Statement
No competing financial interests exist.
References
- Abe Y. Muto M. Nieda M. Nakagawa Y. Nicol A. Kaneko T. Goto S. Yokokawa K. Suzuki K. Clinical and immunological evaluation of zoledronate-activated Vgamma9gammadelta T-cell-based immunotherapy for patients with multiple myeloma. Exp Hematol. 2009;37(8):956–968. doi: 10.1016/j.exphem.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Alshaker HA. Matalka KZ. IFN-γ, IL-17 and TGF-β involvement in shaping the tumor microenvironment: the significance of modulating such cytokines in treating malignant solid tumors. Cancer Cell Int. 2011;11:33. doi: 10.1186/1475-2867-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argentati K. Re F. Serresi S. Tucci MG. Bartozzi B. Bernardini G. Provinciali M. Reduced number and impaired function of circulating gamma delta T cells in patients with cutaneous primary melanoma. J Invest Dermatol. 2003;120(5):829–834. doi: 10.1046/j.1523-1747.2003.12141.x. [DOI] [PubMed] [Google Scholar]
- Behr B. Leucht P. Longaker MT. Quarto N. Fgf-9 is required for angiogenesis and osteogenesis in long bone repair. Proc Natl Acad Sci U S A. 2010;107(26):11853–11858. doi: 10.1073/pnas.1003317107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzaïd I. Mönkkönen H. Stresing V. Bonnelye E. Green J. Mönkkönen J. Touraine JL. Clézardin P. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vgamma9Vdelta2 T-cell chemotaxis and cytotoxicity in vivo. Cancer Res. 2011;71(13):4562–4572. doi: 10.1158/0008-5472.CAN-10-3862. [DOI] [PubMed] [Google Scholar]
- Bialasiewicz AA. Ma JX. Richard G. Alpha/beta- and gamma/delta TCR(+) lymphocyte infiltration in necrotising choroidal melanomas. Br J Ophthalmol. 1999;83(9):1069–1073. doi: 10.1136/bjo.83.9.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville M. O'Brien RL. Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10(7):467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- Bryant NL. Gillespie GY. Lopez RD. Markert JM. Cloud GA. Langford CP. Arnouk H. Su Y. Haines HL. Suarez-Cuervo C. Lamb LS., Jr Preclinical evaluation of ex vivo expanded/activated γδ T cells for immunotherapy of glioblastoma multiforme. J Neurooncol. 2011;101(2):179–188. doi: 10.1007/s11060-010-0245-2. [DOI] [PubMed] [Google Scholar]
- Capietto AH. Martinet L. Fournié JJ. How tumors might withstand γδ T-cell attack. Cell Mol Life Sci. 2011;68(14):2433–2442. doi: 10.1007/s00018-011-0705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmi Y. Rinott G. Dotan S. Elkabets M. Rider P. Voronov E. Apte RN. Microenvironment-derived IL-1 and IL-17 interact in the control of lung metastasis. J Immunol. 2011;186(6):3462–3471. doi: 10.4049/jimmunol.1002901. [DOI] [PubMed] [Google Scholar]
- Castella B. Vitale C. Coscia M. Massaia M. Vγ9Vδ2 T cell-based immunotherapy in hematological malignancies: from bench to bedside. Cell Mol Life Sci. 2011;68(14):2419–2432. doi: 10.1007/s00018-011-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli S. Romano F. Angeloni A. Marchese C. Potential dual role of KGF/KGFR as a target option in novel therapeutic strategies for the treatment of cancers and mucosal damages. Expert Opin Ther Targets. 2012;16(4):377–393. doi: 10.1517/14728222.2012.671813. [DOI] [PubMed] [Google Scholar]
- Chow MT. Möller A. Smyth MJ. Inflammation and immune surveillance in cancer. Semin Cancer Biol. 2012;22(1):23–32. doi: 10.1016/j.semcancer.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Dieli F. Vermijlen D. Fulfaro F. Caccamo N. Meraviglia S. Cicero G. Roberts A. Buccheri S. D'Asaro M. Gebbia N. Salerno A. Eberl M. Hayday AC. Targeting human gamma delta T cells with zoledronate and interleukin-2 for immunotherapy of hormone- refractory prostate cancer. Cancer Res. 2007;67(15):7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch P. Malkovsky M. Braakman E. Sturm E. Bolhuis RL. Prieve A. Sosman JA. Lam VA. Sondel PM. Gamma/delta T cell clones and natural killer cell clones mediate distinct patterns of non-major histocompatibility complex-restricted cytolysis. J Exp Med. 1990;171(5):1567–1579. doi: 10.1084/jem.171.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaafar A. Aljurf MD. Al-Sulaiman A. Iqniebi A. Manogaran PS. Mohamed GE. Al-Sayed A. Alzahrani H. Alsharif F. Mohareb F. Ajarim D. Tabakhi A. Al-Hussein K. Defective gammadelta T-cell function and granzyme B gene polymorphism in a cohort of newly diagnosed breast cancer patients. Exp Hematol. 2009;37(7):838–848. doi: 10.1016/j.exphem.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Gao Y. Yang W. Pan M. Scully E. Girardi M. Augenlicht LH. Craft J. Yin Z. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198(3):433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi M. Oppenheim DE. Steele CR. Lewis JM. Glusac E. Filler R. Hobby P. Sutton B. Tigelaar RE. Hayday AC. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294(5542):605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- Gomes AQ. Martins DS. Silva-Santos B. Targeting γδ T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Res. 2010;70(24):10024–10027. doi: 10.1158/0008-5472.CAN-10-3236. [DOI] [PubMed] [Google Scholar]
- Groh V. Rhinehart R. Secrist H. Bauer S. Grabstein KH. Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci U S A. 1999;96(12):6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halary F. Pitard V. Dlubek D. Krzysiek R. de la Salle H. Merville P. Dromer C. Emilie D. Moreau JF. Déchanet-Merville J. Shared reactivity of Vdelta2neg gamma delta T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med. 2005;201(10):1567–1578. doi: 10.1084/jem.20041851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannani D. Ma Y. Yamazaki T. Déchanet-Merville J. Kroemer G. Zitvogel L. Harnessing γδ T cells in anticancer immunotherapy. Trends Immunol. 2012;33(5):199–206. doi: 10.1016/j.it.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Hao J. Dong S. Xia S. He W. Jia H. Zhang S. Wei J. O'Brien RL. Born WK. Wu Z. Wang P. Han J. Hong Z. Zhao L. Yin Z. Regulatory role of Vγ1 γδ T cells in tumor immunity through IL-4 production. J Immunol. 2011;187(10):4979–4986. doi: 10.4049/jimmunol.1101389. [DOI] [PubMed] [Google Scholar]
- Hao J. Wu X. Xia S. Li Z. Wen T. Zhao N. Wu Z. Wang P. Zhao L. Yin Z. Current progress in γδ T-cell biology. Cell Mol Immunol. 2010;7(6):409–413. doi: 10.1038/cmi.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W. Hao J. Dong S. Gao Y. Tao J. Chi H. Flavell R. O'Brien RL. Born WK. Craft J. Han J. Wang P. Zhao L. Wu J. Yin Z. Naturally activated V gamma 4 gamma delta T cells play a protective role in tumor immunity through expression of eomesodermin. J Immunol. 2010;185(1):126–133. doi: 10.4049/jimmunol.0903767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmeier W. Kabelitz D. Gammadelta T cells link innate and adaptive immune responses. Chem Immunol Allergy. 2005;86:151–183. doi: 10.1159/000086659. [DOI] [PubMed] [Google Scholar]
- Inman BA. Frigola X. Harris KJ. Kuntz SM. Lohse CM. Leibovich BC. Kwon ED. Questionable relevance of gamma delta T lymphocytes in renal cell carcinoma. J Immunol. 2008;180(5):3578–3584. doi: 10.4049/jimmunol.180.5.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson J. Ugarte K. Chen N. Yachi P. Fuchs E. Boismenu R. Havran WL. A role for skin gammadelta T cells in wound repair. Science. 2002;296(5568):747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- Kalyan S. Wesch D. Kabelitz D. Aminobisphosphonates and toll-like receptor ligands: recruiting Vγ9Vδ2 T cells for the treatment of hematologic malignancy. Curr Med Chem. 2011;18(34):5206–5216. doi: 10.2174/092986711798184280. [DOI] [PubMed] [Google Scholar]
- Ke Y. Kapp LM. Kapp JA. Inhibition of tumor rejection by gammadelta T cells and IL-10. Cell Immunol. 2003;221(2):107–114. doi: 10.1016/s0008-8749(03)00066-2. [DOI] [PubMed] [Google Scholar]
- Kühl AA. Pawlowski NN. Grollich K. Blessenohl M. Westermann J. Zeitz M. Loddenkemper C. Hoffmann JC. Human peripheral gammadelta T cells possess regulatory potential. Immunology. 2009;128(4):580–588. doi: 10.1111/j.1365-2567.2009.03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzmann V. Smetak M. Kimmel B. Weigang-Koehler K. Goebeler M. Birkmann J. Becker J. Schmidt-Wolf IG. Einsele H. Wilhelm M. Tumor-promoting versus tumor- antagonizing roles of γδ T cells in cancer immunotherapy: results from a prospective phase I/II trial. J Immunother. 2012;35(2):205–213. doi: 10.1097/CJI.0b013e318245bb1e. [DOI] [PubMed] [Google Scholar]
- Lejeune FJ. Liénard D. Matter M. Rüegg C. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun. 2006;6:6. [PubMed] [Google Scholar]
- Li W. Kubo S. Okuda A. Yamamoto H. Ueda H. Tanaka T. Nakamura H. Yamanishi H. Terada N. Okamura H. Effect of IL-18 on expansion of gammadelta T cells stimulated by zoledronate and IL-2. J Immunother. 2010;33(3):287–296. doi: 10.1097/CJI.0b013e3181c80ffa. [DOI] [PubMed] [Google Scholar]
- Liu J. Duan Y. Cheng X. Chen X. Xie W. Long H. Lin Z. Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407(2):348–354. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Lu Y. Yang W. Qin C. Zhang L. Deng J. Liu S. Qin Z. Responsiveness of stromal fibroblasts to IFN-gamma blocks tumor growth via angiostasis. J Immunol. 2009;183(10):6413–6421. doi: 10.4049/jimmunol.0901073. [DOI] [PubMed] [Google Scholar]
- Ma Y. Aymeric L. Locher C. Mattarollo SR. Delahaye NF. Pereira P. Boucontet L. Apetoh L. Ghiringhelli F. Casares N. Lasarte JJ. Matsuzaki G. Ikuta K. Ryffel B. Benlagha K. Tesnière A. Ibrahim N. Déchanet-Merville J. Chaput N. Smyth MJ. Kroemer G. Zitvogel L. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med. 2011;208(3):491–503. doi: 10.1084/jem.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet L. Fleury-Cappellesso S. Gadelorge M. Dietrich G. Bourin P. Fournié JJ. Poupot R. A regulatory cross-talk between Vgamma9Vdelta2 T lymphocytes and mesenchymal stem cells. Eur J Immunol. 2009;39(3):752–762. doi: 10.1002/eji.200838812. [DOI] [PubMed] [Google Scholar]
- Moser B. Eberl M. γδ T-APCs: a novel tool for immunotherapy? Cell Mol Life Sci. 2011;68(14):2443–2452. doi: 10.1007/s00018-011-0706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness-Schwickerath KJ. Morita CT. Regulation and function of IL-17A- and IL-22- producing γδ T cells. Cell Mol Life Sci. 2011;68(14):2371–2390. doi: 10.1007/s00018-011-0700-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J. Chang Z. Peng B. Xia Q. Lu W. Huang P. Tsao MS. Chiao PJ. Keratinocyte growth factor/fibroblast growth factor-7-regulated cell migration and invasion through activation of NF-kappaB transcription factors. J Biol Chem. 2007;282(9):6001–6011. doi: 10.1074/jbc.M606878200. [DOI] [PubMed] [Google Scholar]
- Paget C. Chow MT. Duret H. Mattarollo SR. Smyth MJ. Role of γδ T cells in α- galactosylceramide-mediated immunity. J Immunol. 2012;188(8):3928–3939. doi: 10.4049/jimmunol.1103582. [DOI] [PubMed] [Google Scholar]
- Peng G. Wang HY. Peng W. Kiniwa Y. Seo KH. Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27(2):334–348. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Poggi A. Venturino C. Catellani S. Clavio M. Miglino M. Gobbi M. Steinle A. Ghia P. Stella S. Caligaris-Cappio F. Zocchi MR. Vdelta1 T lymphocytes from B-CLL patients recognize ULBP3 expressed on leukemic B cells and up-regulated by trans-retinoic acid. Cancer Res. 2004;64(24):9172–9179. doi: 10.1158/0008-5472.CAN-04-2417. [DOI] [PubMed] [Google Scholar]
- Provinciali M. Re F. Tucci MG. Ricotti F. Lattanzio F. Persistent ex vivo low number and functional in vitro recovery of circulating gammadelta T cells after removal of a cutaneous primary melanoma. Scand J Immunol. 2010;72(2):142–149. doi: 10.1111/j.1365-3083.2010.02413.x. [DOI] [PubMed] [Google Scholar]
- Puan KJ. Low JS. Tan TW. Wee JT. Tan EH. Fong KW. Chua ET. Jin C. Giner JL. Morita CT. Goh CH. Hui KM. Phenotypic and functional alterations of Vgamma2Vdelta2 T cell subsets in patients with active nasopharyngeal carcinoma. Cancer Immunol Immunother. 2009;58(7):1095–1107. doi: 10.1007/s00262-008-0629-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon-Orozco B. Kunzmann V. Wrobel P. Kabelitz D. Steinle A. Herrmann T. Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol. 2005;175(4):2144–2151. doi: 10.4049/jimmunol.175.4.2144. [DOI] [PubMed] [Google Scholar]
- Riond J. Rodriguez S. Nicolau ML. al Saati T. Gairin JE. In vivo major histocompatibility complex class I (MHCI) expression on MHCI low tumor cells is regulated by gammadelta T and NK cells during the early steps of tumor growth. Cancer Immun. 2009;9:10. [PMC free article] [PubMed] [Google Scholar]
- Saharinen P. Eklund L. Pulkki K. Bono P. Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med. 2011;17(7):347–362. doi: 10.1016/j.molmed.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Schilbach K. Frommer K. Meier S. Handgretinger R. Eyrich M. Immune response of human propagated gammadelta-T-cells to neuroblastoma recommend the Vdelta1+ subset for gammadelta-T-cell-based immunotherapy. J Immunother. 2008;31(9):896–905. doi: 10.1097/CJI.0b013e31818955ad. [DOI] [PubMed] [Google Scholar]
- Seo N. Tokura Y. Furukawa F. Takigawa M. Down-regulation of tumoricidal NK and NK T cell activities by MHC Kb molecules expressed on Th2-type gammadelta T and alphabeta T cells coinfiltrating in early B16 melanoma lesions. J Immunol. 1998;161(8):4138–4145. [PubMed] [Google Scholar]
- Seo N. Tokura Y. Takigawa M. Egawa K. Depletion of IL-10- and TGF-beta-producing regulatory gamma delta T cells by administering a daunomycin-conjugated specific monoclonal antibody in early tumor lesions augments the activity of CTLs and NK cells. J Immunol. 1999;163(1):242–249. [PubMed] [Google Scholar]
- Sugaya M. Nakamura K. Tamaki K. Interleukins 18 and 12 synergistically upregulate interferon-gamma production by murine dendritic epidermal T cells. J Invest Dermatol. 1999;113(3):350–354. doi: 10.1046/j.1523-1747.1999.00697.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi A. Dejima T. Yamada H. Shibata K. Nakamura R. Eto M. Nakatani T. Naito S. Yoshikai Y. IL-17 production by γδ T cells is important for the anti-tumor effect of Mycobacterium bovis bacillus Calmette-Guérin treatment against bladder cancer. Eur J Immunol. 2011;41(1):246–251. doi: 10.1002/eji.201040773. [DOI] [PubMed] [Google Scholar]
- Talmadge JE. Tribble HR. Pennington RW. Phillips H. Wiltrout RH. Immunomodulatory and immunotherapeutic properties of recombinant gamma-interferon and recombinant tumor necrosis factor in mice. Cancer Res. 1987;47(10):2563–2570. [PubMed] [Google Scholar]
- Vakkila J. Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004;4(8):641–648. doi: 10.1038/nri1415. [DOI] [PubMed] [Google Scholar]
- Wakita D. Sumida K. Iwakura Y. Nishikawa H. Ohkuri T. Chamoto K. Kitamura H. Nishimura T. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol. 2010;40(7):1927–1937. doi: 10.1002/eji.200940157. [DOI] [PubMed] [Google Scholar]
- Welte T. Lamb J. Anderson JF. Born WK. O'Brien RL. Wang T. Role of two distinct gammadelta T cell subsets during West Nile virus infection. FEMS Immunol Med Microbiol. 2008;53(2):275–283. doi: 10.1111/j.1574-695X.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesch D. Glatzel A. Kabelitz D. Differentiation of resting human peripheral blood gamma delta T cells toward Th1- or Th2-phenotype. Cell Immunol. 2001;212(2):110–117. doi: 10.1006/cimm.2001.1850. [DOI] [PubMed] [Google Scholar]
- Workalemahu G. Foerster M. Kroegel C. Expression and synthesis of fibroblast growth factor-9 in human gammadelta T-lymphocytes. Response to isopentenyl pyrophosphate and TGF-beta1/IL-15. J Leukoc Biol. 2004;75(4):657–663. doi: 10.1189/jlb.0902471. [DOI] [PubMed] [Google Scholar]
- Yoshida Y. Nakajima J. Wada H. Kakimi K. γδ T-cell immunotherapy for lung cancer. Surg Today. 2011;41(5):606–611. doi: 10.1007/s00595-010-4478-7. [DOI] [PubMed] [Google Scholar]