Abstract
Background:
Recent literature has suggested that emphysema and chronic bronchitis, traditionally considered to be entities overlapping within chronic obstructive pulmonary disease (COPD), may be distinct disorders. Few studies have examined the differences in patient characteristics and health outcomes between these conditions. This study examined whether COPD phenotypes represent distinct patient populations, in a large nationally representative US sample.
Methods:
Data were obtained from the 2010 US National Health and Wellness Survey (NHWS). NHWS respondents (n = 75,000) were categorized as a COPD phenotype based on their self-reported diagnosis of COPD only (n = 970), emphysema only (n = 399), or chronic bronchitis only (n = 2071). Phenotypes were compared on demographics, health characteristics, treatment patterns, health outcomes, work productivity, and resource use. Variables were compared using Chi-square and analysis of variance tests for categorical and continuous outcomes, respectively. Health outcomes were also examined using regression modeling, controlling for demographic and health characteristic covariates.
Results:
Patients with chronic bronchitis were significantly younger (51.38 years versus 63.24 years for COPD versus 63.30 years for emphysema, P < 0.05) and more likely to be employed (46.98% versus 23.81% for COPD versus 28.33% for emphysema, P < 0.05). Relative to the other phenotypes, patients with chronic bronchitis were also significantly more likely to be female, nonwhite, and to exercise currently (all P < 0.05), and were significantly less likely to be a current or former smoker (P < 0.05). Controlling for demographic and health characteristics, patients self-identified as having COPD only reported significantly worse physical quality of life (adjusted mean 36.69) and health utilities (adjusted mean 0.65) and significantly more absenteeism (adjusted mean 7.08%), presenteeism (adjusted mean 30.73%), overall work impairment (adjusted mean 34.06%), and activity impairment (adjusted mean 46.59%) than the other phenotypes (all P < 0.05).
Conclusion:
These results suggest considerable heterogeneity among different COPD phenotypes with respect to demographics, health characteristics, disease characteristics, treatment patterns, and health outcomes. Research aimed at understanding the differences in patient characteristics and disease presentation of these phenotypes could be used to guide treatment recommendations.
Keywords: chronic obstructive pulmonary disease, emphysema, chronic bronchitis, quality of life, work productivity, health care resource use
Introduction
Chronic obstructive pulmonary disease (COPD) refers to a progressive condition that can result from a number of disease states, most frequently emphysema and chronic bronchitis, along with a subset of asthma sufferers.1 As observed by Soriano et al, there is a substantial, but not complete, overlap among those diagnosed with these disease states, suggesting that a degree of variability exists among patients diagnosed with COPD.2
Recent research has proposed that the heterogeneity associated with the clinical presentation, disease course, and treatment of COPD is sufficiently varied to warrant the identification and classification of phenotypes to guide effective clinical care.3–5 Indeed, the most recent Global Initiative for Obstructive Lung Disease (GOLD) guidelines note the historical imprecision with which the terms “emphysema” and “chronic bronchitis” have been used to describe patients with COPD.6
Several studies have suggested that the presence of COPD is associated with reduced quality of life,7–11 impaired functioning12,13 and greater direct10,11,14,15 and indirect costs.10,11,16,17 However, these studies have assumed a degree of homogeneity among different COPD phenotypes that may not exist. Consequently, potential differences in patient characteristics and outcomes based on phenotype distinction remain unknown.
The few research studies that have examined specific phenotype characteristics and outcomes have reported significant differences, thus establishing a need for further investigation. Hardin et al compared COPD sufferers with and without asthma. They reported that the patients diagnosed with both conditions had significantly worse quality of life.18 Similarly, Mapel et al reported differences in health care utilization by COPD patients based on complexity of pulmonary and nonpulmonary comorbidities.19 Finally, Kanervisto et al examined impairment in activities of daily living between those with COPD and those with chronic bronchitis, reporting that the burden was greater for those with COPD.20
The objective of this study was to extend this prior research to understand better the patient characteristics, treatment patterns, and health outcomes of those diagnosed with chronic bronchitis, emphysema, or COPD. By examining each phenotype in its purest form (ie, excluding those with multiple diagnoses of the above conditions), the true extent of the differences between these phenotypes can be observed.
Methods and materials
Sample source
The current study examined data collected from the 2010 US National Health and Wellness Survey (NHWS). This cross-sectional, self-administered online survey of adults (18+ years; n = 75,000) includes self-reported information on demographics, health care attitudes and behaviors, disease status, and outcomes. A stratified random sample (by gender, age, and ethnicity) was implemented to ensure that the demographic composition of the sample was representative of the corresponding adult population, as reported by the US Census. Respondents are recruited through the Lightspeed Research Internet panel. Informed consent was obtained from respondents prior to survey completion and respondents were compensated for their participation. The NHWS survey and study protocol were approved by Essex IRB Lebanon, NJ. Comparisons between NHWS data and other governmental sources such as the National Health Interview Survey have been made elsewhere.21–23
Study sample
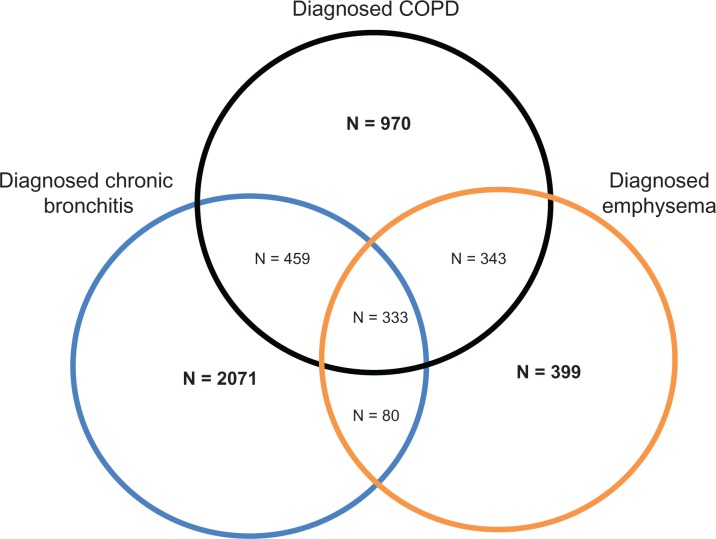
All 75,000 respondents were asked if they have ever experienced COPD, emphysema, or chronic bronchitis (“Which of the following conditions have you ever experienced?”). All respondents who reported experiencing COPD, emphysema, or chronic bronchitis were then asked if their condition has been diagnosed by a physician (“Has your condition been diagnosed by a physician?”, “yes” versus “no”). Patients who did not report a diagnosis of COPD, emphysema, or chronic bronchitis were removed from the analysis (n = 70,345). Additionally, patients with more than one COPD phenotype (those diagnosed with at least two of the following: emphysema, chronic bronchitis, and COPD) were removed from the analysis (n = 1215). The remaining patients were categorized based on their phenotype (COPD only [n = 970], emphysema only [n = 399], or chronic bronchitis only [n = 2071]) and were included in the analysis (Figure 1).
Figure 1.
Venn diagram of sample sizes for patient-reported COPD phenotypes.
Study measures
Demographics and health characteristics
Demographic variables assessed included age, gender, ethnicity, education, household income, and employment type. The following health information was also included: body mass index, smoking status, alcohol use, exercise behavior, and Charlson comorbidity index.24 The Charlson comorbidity index is an index score that is calculated by weighting the presence of specific comorbidities based on their association with future mortality and summing the results. Complete details on the scoring algorithm are provided elsewhere.24
Disease characteristics
Disease characteristics included the number of years since diagnosis of the condition, self-reported disease severity (mild, moderate, or severe), current medication use, and frequency (on a five-point Likert-type scale of “never” to “always”) of dyspnea, fits of coughing, infection, mucus production, and wheezing. The main causes of COPD/chronic bronchitis/emphysema episodes (allergies, exercise, illnesses, occupational hazards, pollutants, smoking, and weather) were also examined.
Quality of life
Quality of life was assessed using the Medical Outcomes Study 12-Item Short Form Survey Instrument.25 Physical component summary and mental component summary scores were examined, both of which have a normed mean of 50 and a standard deviation of 10 for the US population. Health utilities, calculated using the Short-Form-6 Dimensions algorithm, were also included. The Short-Form-6 Dimensions index (health state utilities) is a preference-based single index measure for health.
Work productivity
Work productivity and activity impairment was assessed using the Work Productivity and Activity Impairment questionnaire, a validated instrument that measures work and activity impairment over the past 7 days due to one’s health. It consists of four metrics, ie, absenteeism (the percentage of work time missed), presenteeism (the percentage of impairment experienced while at work), overall work productivity loss (an estimate that is a combination of absenteeism and presenteeism), and activity impairment (the percentage of impairment in daily activities).26 Only patients who reported being employed provided data for absenteeism, presenteeism, and overall work impairment. All patients provided data for activity impairment.
Resource use
Health care utilization was defined by the number of traditional health care provider visits, number of emergency room visits (“how many times have you been to the emergency room for your own medical condition?”), and number of times hospitalized (“how many times have you been hospitalized for your own medical condition?”) in the previous 6 months.
Statistical analyses
Demographic, health characteristic, and disease characteristic variables were compared among the different COPD phenotypes using Chi-square and analysis of variance tests for categorical and continuous variables, respectively. Differences in health outcomes (quality of life, work productivity and activity impairment, and resource use) among the COPD phenotypes were examined using regression modeling. All models controlled for age, gender, ethnicity, education, household income, employment, body mass index, smoking status, alcohol consumption, exercise behavior, and the Charlson comorbidity index. To predict quality of life outcomes, general linear models were used and adjusted means were reported using a least-squares algorithm. To predict work productivity, activity impairment, and resource use, generalized linear models specifying a log link function and a negative binomial distribution were used because of pronounced positive skew. Adjusted means were reported using a maximum-likelihood algorithm and converted back to the original metric. The a priori cutoff for statistical significance was set at P < 0.05 for all statistical tests.
Results
Of the total 2010 US NHWS respondents (n = 75,000) the mean age was 48.17 ± 16.53 years. In total, 48.22% were male, 38.37% were employed full-time, 19.09% were current smokers, 45.15% had a 2009 household income < $50,000, and 58.92% had less than a college degree.
Of patients reporting only one diagnosis, most patients reported being diagnosed with chronic bronchitis (n = 2071; 60.20%), followed by COPD (n = 970; 28.20%) and emphysema (n = 399; 11.60%). Patients with chronic bronchitis were significantly more likely to be female (63.21% versus 42.37% for COPD versus 37.84% for emphysema, P < 0.05) and nonwhite (20.76% versus 10.72% for COPD versus 13.28% for emphysema, P < 0.05) relative to those with COPD and emphysema (Table 1). Patients with chronic bronchitis were also significantly younger relative to those with COPD and emphysema (51.38 years versus 63.24 years for COPD versus 63.30 years for emphysema, P < 0.05) and more likely to be currently employed (46.98% versus 23.81% for COPD versus 28.33% for emphysema, P < 0.05). Patients with chronic bronchitis also had the least comorbidity burden (Charlson comorbidity index 1.70 versus 2.00 for COPD versus 1.82 for emphysema, P < 0.05), were significantly less likely to be a current or former smoker and more likely to exercise (56.74% versus 43.40% for COPD versus 42.11% for emphysema, P < 0.05) relative to the other COPD phenotypes (Table 1).
Table 1.
Patient characteristics and self-reported health behaviors among COPD phenotypes
| Variable |
COPD only (n = 970)
|
Emphysema only (n = 399)
|
Chronic bronchitis only (n = 2071)
|
P | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Male | 559 | 57.63 | 248 | 62.16 | 762 | 36.79 | <0.0001 |
| Female | 411 | 42.37 | 151 | 37.84 | 1309 | 63.21 | <0.0001 |
| White non-hispanic | 866 | 89.28 | 346 | 86.72 | 1641 | 79.24 | <0.0001 |
| Black non-hispanic | 50 | 5.15 | 26 | 6.52 | 189 | 9.13 | 0.0001 |
| Hispanic | 23 | 2.37 | 9 | 2.26 | 93 | 4.49 | 0.0002 |
| Other | 31 | 3.20 | 18 | 4.51 | 148 | 7.15 | <0.0001 |
| High school graduate or less | 245 | 25.26 | 109 | 27.32 | 486 | 23.47 | 0.2140 |
| Some college or higher | 725 | 74.74 | 290 | 72.68 | 1585 | 76.53 | 0.2140 |
| Income: ≤$25,000 | 278 | 28.66 | 120 | 30.08 | 538 | 25.98 | 0.1239 |
| Income: $25,000–$49,999 | 320 | 32.99 | 125 | 31.33 | 631 | 30.47 | 0.3822 |
| Income: $50,000–$74,999 | 152 | 15.67 | 66 | 16.54 | 377 | 18.20 | 0.1982 |
| Income: ≥$75,000 | 158 | 16.29 | 64 | 16.04 | 391 | 18.88 | 0.1284 |
| Income: declined to answer | 62 | 6.39 | 24 | 6.02 | 134 | 6.47 | 0.9412 |
| Full-time employed | 122 | 12.58 | 56 | 14.04 | 633 | 30.56 | <0.0001 |
| Part-time employed | 63 | 6.49 | 32 | 8.02 | 198 | 9.56 | 0.0110 |
| Self-employed | 46 | 4.74 | 25 | 6.27 | 142 | 6.86 | 0.0551 |
| BMI: underweight | 14 | 1.44 | 12 | 3.01 | 28 | 1.35 | 0.1799 |
| BMI: overweight | 286 | 29.48 | 127 | 31.83 | 569 | 27.47 | 0.1676 |
| BMI: normal | 232 | 23.92 | 128 | 32.08 | 440 | 21.25 | <0.0001 |
| BMI: obese | 428 | 44.12 | 130 | 32.58 | 972 | 46.93 | <0.0001 |
| BMI: decline to answer | 10 | 1.03 | 2 | 0.50 | 62 | 2.99 | <0.0001 |
| Never smoked | 111 | 11.44 | 28 | 7.02 | 703 | 33.95 | <0.0001 |
| Former smoker | 544 | 56.08 | 223 | 55.89 | 698 | 33.70 | <0.0001 |
| Current smoker | 315 | 32.47 | 148 | 37.09 | 670 | 32.35 | 0.1875 |
| Currently consume alcohol | 529 | 54.54 | 247 | 61.90 | 1255 | 60.60 | 0.0034 |
| Currently exercise | 421 | 43.40 | 168 | 42.11 | 1175 | 56.74 | <0.0001 |
| Mean | SD | Mean | SD | Mean | SD | ||
|
| |||||||
| Age | 63.24 | 10.90 | 63.30 | 13.32 | 51.38 | 14.80 | <0.0001 |
| Years diagnosed | 7.57 | 7.43 | 9.39 | 9.63 | 16.23 | 14.80 | <0.0001 |
| CCI* | 2.00 | 1.47 | 1.82 | 1.28 | 1.70 | 1.24 | <0.0001 |
Note:
A score calculated by weighting the presence of specific comorbidities based on their association with future mortality and summing the results.
Abbreviations: CCI, Charlson comorbidity index, COPD, chronic obstructive pulmonary disease; BMI, body mass index; SD, standard deviation.
Those with chronic bronchitis have been diagnosed with their condition the longest (16.23 years versus 7.57 years for COPD versus 9.39 for emphysema, P < 0.05) and were the least likely to report the severity of their condition as “moderate” or “severe” relative to patients with COPD or emphysema (65.38% for mild versus 42.47% for COPD versus 49.87% for emphysema, P < 0.05, Table 2). The most frequent symptoms experienced by patients with COPD were dyspnea (mean 3.74 for COPD versus 3.59 for emphysema versus 2.95 for chronic bronchitis, P < 0.05), wheezing (mean 2.97 for COPD versus 2.71 for emphysema versus 2.76 for chronic bronchitis, P < 0.05), and mucus production (mean 2.97 for COPD versus 2.86 for emphysema versus 3.05 for chronic bronchitis, P < 0.05). Patients with emphysema reported a symptom profile similar to that of patients with COPD, but all symptoms were less frequent, albeit not all significantly so. Patients with chronic bronchitis reported the most frequent mucus production, coughing (mean 2.83 for chronic bronchitis versus 2.78 for COPD versus 2.65 for emphysema, P < 0.05), and infections (mean 2.31 for chronic bronchitis versus 1.99 for COPD versus 1.78 for emphysema, P < 0.05) relative to the other phenotypes. The main causes of a COPD/emphysema/chronic bronchitis episode were smoking (58.56% for COPD versus 67.67% for emphysema versus 31.87% for chronic bronchitis, P < 0.05), illnesses, eg, colds and influenza (24.33% for COPD versus 14.79% for emphysema versus 50.27% for chronic bronchitis, P < 0.05), allergies/allergic reactions (21.13% for COPD versus 18.30% for emphysema versus 46.40% for chronic bronchitis, P < 0.05), weather/temperature (30.52% for COPD versus 26.82% for emphysema versus 36.41% for chronic bronchitis, P < 0.05), pollutants/airborne irritants (25.26% for COPD versus 19.05% for emphysema versus 27.23% for chronic bronchitis, P < 0.05), exercise/physical activity (30.41% for COPD versus 27.57% for emphysema versus 19.12% for chronic bronchitis, P < 0.05), and occupational hazards, eg, paint fumes and car exhaust (18.25% for COPD versus 13.03% for emphysema versus 10.96% for chronic bronchitis, P < 0.05).
Table 2.
Disease characteristics and medications used by COPD phenotypes
|
COPD only (n = 970)
|
Emphysema only (n = 399)
|
Chronic bronchitis only (n = 2071)
|
P | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Current medication use (for COPD) | 728 | 75.05 | 211 | 52.88 | 585 | 28.25 | <0.0001 |
| Medication* | |||||||
| SABA only | 89 | 12.92 | 23 | 11.22 | 165 | 33.54 | <0.0001 |
| LAMA-SAMA only | 77 | 11.18 | 20 | 9.76 | 13 | 2.64 | <0.0001 |
| ICS only | 7 | 1.02 | 6 | 2.93 | 29 | 5.89 | 0.3623 |
| LABA only | 5 | 0.73 | 2 | 0.98 | 1 | 0.20 | <0.0001 |
| Oxygen only | 1 | 0.15 | 4 | 1.95 | 0 | 0.00 | 0.0002 |
| ICS + LABA only | 90 | 13.06 | 28 | 13.66 | 67 | 13.62 | 0.5896 |
| LAMA-SAMA + SABA only | 78 | 11.32 | 21 | 10.24 | 36 | 7.32 | 0.9528 |
| ICS + SABA only | 15 | 2.18 | 5 | 2.44 | 39 | 7.93 | <0.0001 |
| LABA + SABA only | 9 | 1.31 | 1 | 0.49 | 2 | 0.41 | 0.3919 |
| ICS + LAMA-SAMA only | 6 | 0.87 | 1 | 0.49 | 2 | 0.41 | 0.0705 |
| LAMA-SAMA + LABA only | 4 | 0.58 | 2 | 0.98 | 1 | 0.20 | 0.2114 |
| ICS + LABA + SABA only | 94 | 13.64 | 14 | 6.83 | 95 | 19.31 | <0.0001 |
| ICS + LAMA-SAMA + LABA only | 58 | 8.42 | 23 | 11.22 | 5 | 1.02 | 0.0213 |
| ICS + LAMA-SAMA + SABA only | 27 | 3.92 | 7 | 3.41 | 6 | 1.22 | <0.0001 |
| LAMA-SAMA + LABA + SABA only | 5 | 0.73 | 2 | 0.98 | 0 | 0.00 | 0.1308 |
| ICS + LAMA-SAMA + LABA + SABA | 116 | 16.84 | 44 | 21.46 | 31 | 6.30 | <0.0001 |
| Oxygen + other treatments | 8 | 1.16 | 2 | 0.98 | 0 | 0.00 | 0.0602 |
| Disease severity | |||||||
| Mild | 412 | 42.47 | 199 | 49.87 | 1354 | 65.38 | <0.0001 |
| Moderate | 434 | 44.74 | 162 | 40.60 | 621 | 29.99 | <0.0001 |
| Severe | 124 | 12.78 | 38 | 9.52 | 96 | 4.64 | <0.0001 |
| Symptom frequency** | |||||||
| Dyspnea (mean, SD) | 3.74 | 1.07 | 3.59 | 1.16 | 2.95 | 1.18 | <0.0001 |
| Coughing (mean, SD) | 2.78 | 1.06 | 2.65 | 1.07 | 2.83 | 1.02 | 0.0040 |
| Infection (mean, SD) | 1.99 | 0.93 | 1.78 | 0.92 | 2.31 | 0.97 | <0.0001 |
| Mucus (mean, SD) | 2.97 | 1.15 | 2.86 | 1.23 | 3.05 | 1.12 | 0.0053 |
| Wheezing (mean, SD) | 2.97 | 1.08 | 2.71 | 1.16 | 2.76 | 1.10 | <0.0001 |
Notes:
Percentages based only on those using a prescription medication;
symptoms were reported on a five-point Likert-type response scale (1 = never to 5 = always).
Abbreviations: LAMA-SAMA, ipratropium, tiotropium; LABA + ICS (fixed-dose combination), budesonide-formoterol, fluticasone-salmeterol; LAMA-SAMA + SABA, albuterol-ipratropium; ICS, flunisolide, mometasone, triamcinolone, fluticasone, fluticasone propionate, budesonide; LABA, arformoterol, formoterol, salmeterol; SABA, albuterol, pirbuterol, albuterol sulfate, levalbuterol; ICS, inhaled corticosteroids; LABA, long-acting beta agonist; LAMA-SAMA, long-acting or short-acting muscarinic antagonist; SABA, short-acting beta-agonist; SD, standard deviation.
The number of patients across all phenotypes who remained untreated varied significantly (24.95% for COPD versus 47.12% for emphysema versus 71.75% for chronic bronchitis, P < 0.05). Short-acting beta-agonist (SABA) use was the most common monotherapy for all phenotypes, but significantly (P < 0.05) more for patients with chronic bronchitis (33.54% versus 11.22% for emphysema versus 12.92% for COPD). Patients with chronic bronchitis were also significantly more likely to use inhaled corticosteroids (ICS) + a long-acting beta agonist (LABA, 13.62%) and ICS + LABA + SABA (19.31%) therapies (P < 0.05). Conversely, triple therapy (ICS + LABA + long-acting or short-acting muscarinic antagonist) was significantly more infrequent for patients with chronic bronchitis whether it was with a SABA (6.30% versus 16.84% for COPD versus 21.46% for emphysema, P < 0.05) or without a SABA (1.02% versus 8.42% for COPD versus 11.22% for emphysema, P < 0.05).
Despite reporting the lowest level of mental component summary scores (chronic bronchitis 43.98 versus 46.95 for COPD versus 48.62 for emphysema, P < 0.05), patients with chronic bronchitis reported the highest levels of physical component summary scores (41.43 for chronic bronchitis versus 34.88 for COPD versus 37.59 for emphysema, P < 0.05), the lowest activity impairment (42.15% for chronic bronchitis versus 50.36% for COPD versus 42.88% for emphysema, P < 0.05), and the fewest hospitalizations (0.22 for chronic bronchitis versus 0.30 for COPD versus 0.30 for emphysema, P < 0.05, Table 3). Patients with COPD reported the lowest physical component summary scores, and the highest levels of activity impairment, hospitalizations, and physician visits (P < 0.05). Patients with emphysema had a worse physical component summary score and more hospitalizations than those with chronic bronchitis. No differences were observed among the phenotypes in terms of work productivity losses or number of emergency room visits.
Table 3.
Unadjusted health outcome differences among COPD phenotypes
|
COPD only (n = 970)
|
Emphysema only (n = 399)
|
Chronic bronchitis only (n = 2071)
|
P | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Mental component score | 46.95 | 12.24 | 48.62 | 12.14 | 43.98 | 12.26 | <0.0001 |
| Physical component score | 34.88 | 11.27 | 37.59 | 10.86 | 41.43 | 12.32 | <0.0001 |
| Health state utilities | 0.65 | 0.14 | 0.68 | 0.14 | 0.66 | 0.14 | 0.0017 |
| Absenteeism (%)a | 6.96 | 19.37 | 5.16 | 15.43 | 7.40 | 19.17 | 0.5136 |
| Presenteeism (%)b | 28.96 | 29.30 | 25.29 | 26.77 | 25.50 | 27.66 | 0.2393 |
| Overall work impairment (%)a | 32.23 | 31.69 | 27.41 | 29.44 | 28.95 | 30.72 | 0.2792 |
| Activity impairment (%) | 50.36 | 31.29 | 42.88 | 31.19 | 42.15 | 32.14 | <0.0001 |
| Number of ER visits | 0.44 | 1.30 | 0.43 | 1.05 | 0.45 | 1.47 | 0.9381 |
| Number of hospitalizations | 0.30 | 0.75 | 0.30 | 1.01 | 0.22 | 0.90 | 0.0257 |
| Number of physician visits | 7.47 | 7.77 | 6.24 | 6.82 | 6.77 | 9.31 | 0.0297 |
Notes:
Because only those currently employed will have absenteeism and overall work impairment data, these results are based on sample sizes of 227, 104, and 941 for COPD only, emphysema only, and chronic bronchitis, respectively;
because only those currently employed and who were at work in the past 7 days will have presenteeism data, these results are based on sample sizes of 222, 103, and 925 for COPD only, emphysema only, and chronic bronchitis respectively.
Abbreviations: COPD, chronic obstructive pulmonary disease; ER, emergency room.
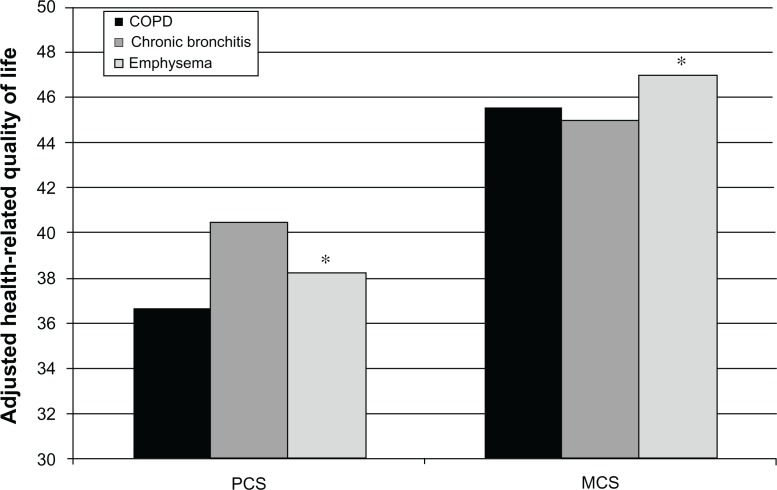
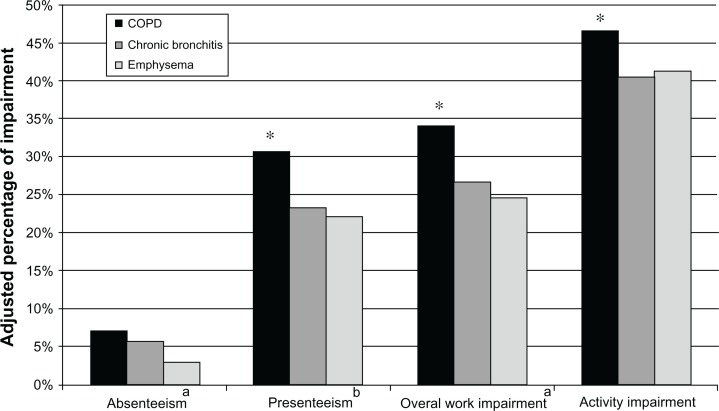
Adjusting for demographics and health characteristics, patients with COPD reported the lowest mean physical component summary scores (36.69) and health utilities (0.65) relative to other phenotypes (Figure 2). Patients with COPD also reported the highest level of work impairment. With the exception of absenteeism, where there were no differences between patients with COPD (7.1%) and chronic bronchitis (5.6%), both were higher than for patients with emphysema (3.0%). Patients with COPD reported greater levels of work and activity impairment relative to those with chronic bronchitis and those with emphysema (Figure 3). No significant differences were observed between the groups with respect to resource utilization.
Figure 2.
Adjusted differences on physical and mental component summary scores among COPD phenotypes.
Note: *P < 0.05 emphysema only vs COPD only and chronic bronchitis only.
Abbreviations: MCS, Mental Component Summar; PCS, Physical Component Summary.
Figure 3.
Adjusted differences on work productivity and activity impairment among COPD phenotypes.
Notes:aBecause only those currently employed will have absenteeism and overall work impairment data, these results are based on sample sizes of 227, 104, and 941 for COPD only, emphysema only, and chronic bronchitis, respectively; bbecause only those currently employed and who were at work in the past seven days will have presenteeism data, these results are based on sample sizes of 222, 103, and 925 for COPD only, emphysema only, and chronic bronchitis respectively; *P < 0.05 emphysema only vs COPD only.
Discussion
Although several studies have reported a considerable burden among patients diagnosed with COPD,7–17 these studies often assume a level of homogeneity across various COPD disease states that may not exist. Indeed, recent literature has suggested that emphysema and chronic bronchitis have sufficient differences in their presentation and disease course that considering them to be separate conditions is appropriate.1–5 Our findings highlight additional differences in health history, disease characteristics, and impact on patient quality of life. Patient-reported medication use in this study reflects a different approach to treatment across the phenotypes, with ICS-LABA use being most prominent among patients with COPD and chronic bronchitis and triple therapy (both with and without a SABA) being most commonly prescribed for patients with emphysema. As emphasized in the most recent GOLD guidelines,6 these patterns highlight chronic bronchitis and emphysema as distinct from COPD, despite their clinical associations.
A number of significant differences between patients reporting only a diagnosis of COPD, emphysema, or chronic bronchitis were found. Differences were most dramatic when comparing patients with chronic bronchitis relative to the other phenotypes. Specifically, those reporting a diagnosis of only chronic bronchitis were younger, had been diagnosed for longer, and were predominantly female. The severity of their condition was more likely to be mild, and the hallmark symptoms were frequent infections and mucus production. These data may reflect the tendency of younger females to visit their physician and report symptoms earlier in the course of the disease. The longer time since diagnosis for patients with chronic bronchitis may also indicate the need for treatment of repeat acute episodes. Epidemiological studies have recently shown that approximately 25% of patients with COPD have never smoked.27 Although smoking history was reported by the majority of patients (>80%) with COPD and emphysema, more than a third of patients with chronic bronchitis had never smoked. Agusti and Vesbo have emphasized the potential role and complexities surrounding COPD at the environmental, clinical, biologic, and genetic levels.3 Continued investigation of such factors and their interactions could reveal stronger links to these lung conditions than smoking history for some patients.
In the current study only 28.3%, 52.9%, and 75.1% of patients with chronic bronchitis only, emphysema only, and COPD only, respectively, reported use of any COPD medications. For all phenotypes, bronchodilator use was prominent, whether used alone or in combination with other medications, along with ICS + LABA regimens. LAMA-SAMA use was generally more frequent than LABA use in our sample, especially among patients with COPD. Recent evidence from the Prevention of Exacerbations with Tiotropium in COPD trial has suggested that LAMA use is superior to LABA use in delaying/reducing exacerbations among patients with moderate-to-severe COPD.28 Other long-term studies of COPD medications have reported no difference in exacerbations for patients using ICS-LABA and LAMAs concurrently compared with ICS-LABA29 or when compared with LAMA alone or LAMA + LABA.30 While the evidence has yielded mixed results for LAMAs, the prominent use of LAMA-SAMAs in this study may reflect the influence of recently published research showing benefits of LAMAs in clinical practice.
To our knowledge, no study has examined the health outcomes among these phenotypes. Therefore, comparisons with existing literature are difficult. Mean scores for each phenotype with respect to quality of life and work productivity are consistent with those observed in prior studies, although these latter studies did not distinguish between phenotypes.8–11 Also consistent with previous studies, patients with COPD reported significantly worse health outcomes (aside from resource use) than chronic bronchitis.19 In addition to these findings, and unstudied in previous research, patients with COPD also reported worse outcomes (aside from resource use) relative to patients with emphysema. Similarities in resource use across these groups may suggest that differences in phenotype presentation do not dramatically influence the frequency and severity of exacerbations or engagement with health care providers. However, these presentations do appear to be related to differences in day-to-day functioning as assessed by quality of life and productivity at work.
Limitations
Data from the NHWS is self-reported, so clinical verification of diagnoses, treatments, and lung function was unavailable. It is possible that patients erroneously recalled the diagnosis they received, and that their perception of disease severity did not coincide with more objective measures, such as forced expiratory volume in one second. Although the GOLD guidelines differentiate between these three phenotypes, the extent to which the clinical community has done so is unclear, which can complicate interpretation of the results. For example, it is possible that a patient could have presented with emphysema and the treating physician could have considered that to be COPD and given that diagnosis to the patient. The same patient may have received a diagnosis of emphysema from another physician. As shown in Figure 1, most patients who reported a diagnosis had been diagnosed with more than one phenotype. As a result, it is unclear the extent to which the differences observed here manifest when multiple diagnoses are present. Of course, the choice to examine pure groups was intentional. Multiple diagnoses may reflect a more complex condition. Further, multiple diagnoses (eg, asthma, heart failure, other causes of dyspnea) may also represent different diagnoses over time, without any clear indication which is the most appropriate given the current disease state of the respondent.
Differences in work productivity were found in the current study; even though employment status was collected for all respondents, the type of occupation was not available. Smoking status (current smoker, former smoker, never smoked) was available for respondents, but the length of time and number of packs of cigarettes used were not collected. Because NHWS is an Internet-based survey, respondents with extremely severe cases of COPD, chronic bronchitis, or emphysema may be under-represented.
Conclusion
In sum, these results suggest considerable heterogeneity among the different COPD phenotypes with respect to demographics, health characteristics, disease characteristics, treatment patterns, and health outcomes. Future research should be cognizant of the phenotypes within COPD samples, because the disease experience and health outcomes may be radically different. Patients self-reporting only COPD, rather than only chronic bronchitis or emphysema, may be more adversely affected by their condition, as indicated by worse health outcomes. Research aimed at understanding the differences in patient characteristics and disease presentation of these phenotypes could be used to guide treatment recommendations.
Footnotes
Disclosure
MDD and SG are employees of Kantar Health, who conducted the National Health and Wellness Survey and were paid consultants to Merck and Co, Inc, in connection with the development of this manuscript and execution of the study. FA-R is an employee of Merck and Co, Inc, which funded and oversaw the study and manuscript development. The authors also wish to acknowledge the background research and editorial assistance of Errol J Philip who is a paid consultant to Kantar Health.
References
- 1.Mannino DM. Chronic obstructive pulmonary disease: epidemiology and evaluation. Hosp Physician. 2001;37(10):22–31. [Google Scholar]
- 2.Soriano JB, Davis KJ, Coleman B, Visick G, Mannino D, Pride NB. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest. 2003;124(2):474–481. doi: 10.1378/chest.124.2.474. [DOI] [PubMed] [Google Scholar]
- 3.Agusti A, Vestbo J. Current controversies and future perspectives in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184(5):507–513. doi: 10.1164/rccm.201103-0405PP. [DOI] [PubMed] [Google Scholar]
- 4.Izquierdo JL, Martin A, de Lucas P, et al. Misdiagnosis of patients receiving inhaled therapies in primary care. Int J Chron Obstruct Pulmon Dis. 2010;5:241–249. doi: 10.2147/copd.s11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2010) Available from: http://www.goldcopd.org/uploads/users/files/globalinitiativeforobstructivelungdiseasereport_april112011.pdf. Accessed October 25, 2011. [Google Scholar]
- 7.Ferrer M, Alonso J, Morera J, et al. Chronic obstructive pulmonary disease stage and health-related quality of life. The Quality of Life of Chronic Obstructive Pulmonary Disease Study Group. Ann Intern Med. 1997;127(12):1072–1079. doi: 10.7326/0003-4819-127-12-199712150-00003. [DOI] [PubMed] [Google Scholar]
- 8.Ståhl E, Lindberg A, Jansson SA, et al. Health-related quality of life is related to COPD disease severity. Health Qual Life Outcomes. 2005;3:56. doi: 10.1186/1477-7525-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutten-van Mölken MP, Oostenbrink JB, Tashkin DP, Burkhart D, Monz BU. Does quality of life of COPD patients as measured by the generic Euroquality of life five-dimension questionnaire differentiate between COPD severity stages? Chest. 2006;130(4):1117–1128. doi: 10.1378/chest.130.4.1117. [DOI] [PubMed] [Google Scholar]
- 10.DiBonaventura MD, Paulose-Ram R, Su J, et al. The impact of COPD on quality of life, productivity loss, and resource use among the elderly United States workforce. COPD. 2012;9(1):46–57. doi: 10.3109/15412555.2011.634863. [DOI] [PubMed] [Google Scholar]
- 11.DiBonaventura MD, Paulose-Ram R, Su J, et al. The burden of chronic obstructive pulmonary disease among employed adults. Int J Chron Obstruct Pulmon Dis. 2012;7:211–219. doi: 10.2147/COPD.S29280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miravitlles M, Alvarez-Sala JL, Lamarca R, et al. Treatment and quality of life in patients with chronic obstructive pulmonary disease. Qual Life Res. 2002;11(4):329–338. doi: 10.1023/a:1015520110663. [DOI] [PubMed] [Google Scholar]
- 13.Jones PW. Activity limitation and quality of life in COPD. COPD. 2007;4(3):273–278. doi: 10.1080/15412550701480265. [DOI] [PubMed] [Google Scholar]
- 14.Faulkner MA, Hilleman DE. The economic impact of chronic obstructive pulmonary disease. Expert Opin Pharmacother. 2002;3(3):219–228. doi: 10.1517/14656566.3.3.219. [DOI] [PubMed] [Google Scholar]
- 15.National Heart Lung and Blood Institute . Morbidity and Mortality: 2009 Chart Book on Cardiovascular, Lung, and Blood Diseases. Bethesda, MS: National Institutes of Health; 2009. [Google Scholar]
- 16.Darkow T, Kadlubek PJ, Shah H, Phillips AL, Marton JP. A retrospective analysis of disability and its related costs among employees with chronic obstructive pulmonary disease. J Occup Environ Med. 2007;49(1):22–30. doi: 10.1097/JOM.0b013e31802db55f. [DOI] [PubMed] [Google Scholar]
- 17.Sin DD, Stafinski T, Ng YC, Bell NR, Jacobs P. The impact of chronic obstructive pulmonary disease on work loss in the United States. Am J Respir Crit Care Med. 2002;165(5):704–707. doi: 10.1164/ajrccm.165.5.2104055. [DOI] [PubMed] [Google Scholar]
- 18.Hardin M, Silverman EK, Barr RG, et al. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12:127. doi: 10.1186/1465-9921-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mapel DW, Dutro MP, Marton JP, Woodruff K, Make B. Identifying and characterizing COPD patients in US managed care. A retrospective, cross-sectional analysis of administrative claims data. BMC Health Serv Res. 2011;11:43. doi: 10.1186/1472-6963-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanervisto M, Saarelainen S, Vasankari T, et al. COPD, chronic bronchitis and capacity for day-to-day activities: negative impact of illness on the health-related quality of life. Chron Respir Dis. 2010;7(4):207–215. doi: 10.1177/1479972310368691. [DOI] [PubMed] [Google Scholar]
- 21.Bolge SC, Doan JF, Kannan H, Baran RW. Association of insomnia with quality of life, work productivity, and activity impairment. Qual Life Res. 2009;18(4):415–422. doi: 10.1007/s11136-009-9462-6. [DOI] [PubMed] [Google Scholar]
- 22.DiBonaventura M, Wagner JS, Yuan Y, L’Italien G, Langley P, Ray Kim W. The impact of hepatitis C on labor force participation, absenteeism, presenteeism and non-work activities. J Med Econ. 2011;14(2):253–261. doi: 10.3111/13696998.2011.566294. [DOI] [PubMed] [Google Scholar]
- 23.Finkelstein EA, Allaire BT, Dibonaventura MD, Burgess SM. Direct and indirect costs and potential cost savings of laparoscopic adjustable gastric banding among obese patients with diabetes. J Occup Environ Med. 2011;53(9):1025–1029. doi: 10.1097/JOM.0b013e318229aae4. [DOI] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Ware J, Jr, Kosinski M, Turner-Bowker DM, Gandek B. How to Score Version 2 of the SF-12v2 Health Survey (with a supplement documenting version 1) Lincoln, RI: Quality Metric Incorporated; 2002. [Google Scholar]
- 26.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 27.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9691):733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 28.Vogelmeier C, Hederer B, Glabb T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093–1103. doi: 10.1056/NEJMoa1008378. [DOI] [PubMed] [Google Scholar]
- 29.Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- 30.Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146(8):545–555. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]