Abstract
Background
Diary cards are useful for analyzing exacerbations in chronic obstructive pulmonary disease (COPD), although factors influencing the length and frequency of each episode are poorly understood. This study investigated factors that influence the features of exacerbations in patients with alpha-1 antitrypsin (AAT) deficiency (PiZ phenotype) and COPD.
Methods
Daily diary cards were collected over 2 years. Patients had emphysema visualized and quantified by computed tomography scan, and had at least one documented exacerbation in the previous year.
Results
The patients (n = 23) had a mean age of 52.5 years, forced expiratory volume in one second (FEV1) of 1.2 L (38.4% predicted), corrected gas transfer (KCO) of 0.90 mmol/min/kPa/L (59.7% predicted), and 15th percentile lung density of 44.55 g/L. Two hundred and sixty-three exacerbations (164 treated) were identified. The frequency of treated exacerbations correlated negatively with KCO% predicted (r = −0.432; P = 0.022). Exacerbation length (determined for 17 of the patients for whom diary card data through the episode were available) correlated negatively with baseline 15th percentile lung density (r = −0.361; P = 0.003), and increased the longer treatment was delayed (r = 0.503; P < 0.001). Treatment delay was shorter with higher day 1 symptom score, lower baseline FEV1, FEV1/forced vital capacity, and lower 15th percentile lung density (r = −0.368, 0.272, 0.461, and 0.786; P = 0.004, 0.036, <0.001, and <0.001, respectively). Time to resolution of exacerbation after treatment initiation was not affected by treatment delay, but correlated negatively with KCO% predicted (r = −0.647; P = 0.007).
Conclusion
In alpha-1 antitrypsin deficiency, the frequency and length of resolution of exacerbation were related to baseline gas transfer. Treatment delay adversely affected exacerbation length, and lung density was the best independent predictor of delay in starting treatment.
Keywords: alpha-1 antitrypsin deficiency, antibiotic, exacerbation, gas transfer, lung density, lung function
Introduction
Exacerbations are a recognized feature of chronic obstructive pulmonary disease (COPD) and result in considerable morbidity and increased mortality.1–8 They are also associated with a greater decline in lung function and can adversely affect health status.9–13 For these reasons, exacerbations have become a major outcome measure in a number of clinical trials14–25 and are the subject of numerous translational research studies.26–29 Patients with alpha-1 antitrypsin (AAT) deficiency, a subgroup of COPD, often present in the third or fourth decade with symptoms of chronic bronchitis and exertional breathlessness.30–32 AAT deficiency is associated with development of emphysema, first described in 1963, disproportionate to smoking history at an earlier age than usual COPD.32 Patients with lung disease have symptoms in common with usual COPD with a high prevalence of chronic bronchitis and emphysema. Lung function tests in these patients confirm obstruction with air trapping, although some may have normal gas transfer.33 However, the symptoms, features, and treatment of exacerbations in this group are similar to those of usual COPD.34
Characterization of an exacerbation episode has been based on three major symptoms, ie, increased breathlessness, sputum volume, and sputum purulence, together with the variable contribution of minor symptoms as described by Anthonisen et al.35 These symptoms and their combinations are especially used in guidelines to direct antibiotic therapy. In addition, the treatment required has also been used to grade the severity of the episode.36
Nearly 50% of all COPD exacerbations defined by symptoms do not lead to the patient increasing or changing therapy, and are referred to as “unreported” or “untreated” episodes. These may reflect the patients’ perception of and actual improvement in their well-being,37 but nevertheless may contribute to a decline in lung function and poorer health status.11,12
Exacerbations occur more frequently in subjects with more severe COPD,38 although a linear relationship is difficult to demonstrate and the reporting of episodes does increase with age and lower forced expiratory volume in one second (FEV1).39 Factors known to predict exacerbation frequency are the presence of chronic bronchitis symptoms and frequent exacerbations in the previous year.40 The frequency of exacerbation in AAT-deficient COPD has been shown to correlate with the decline in gas transfer,9 and exacerbations have been shown to be associated with greater inflammation.41 In this group, augmentation therapies have been shown to reduce the frequency and severity of exacerbation and lung function decline.42,43 A delay in treatment initiation has been shown to affect the overall length of the episode adversely,44 although factors influencing the delay other than those mentioned above are largely unknown. There are not enough research data showing the predictors of exacerbation nature, severity, and recovery.27
Although there is a suggestion that exacerbation may be more severe in patients who have AAT deficiency, peak flow changes with therapy did not differ, suggesting that response to therapy is similar in both groups.45 Treatment of the lung manifestations of AAT deficiency does not differ from the standard treatment of COPD.34
The current study was carried out primarily to examine daily diary cards in order to study exacerbation features and outcomes in depth. AAT-deficient COPD patients are a good cohort in which to study the features of exacerbations, because they experience more exacerbations associated with their decline in lung function.9 In a previous study, we were able to show for the first time a clear difference between reported or “treated” exacerbations and unreported or “untreated” exacerbations and to identify potential reasons.37 The aim of this paper is to address further factors that influence resolution of the exacerbation, length of an episode, and delay in starting treatment.
Materials and methods
Subjects
The study design and methods have been previously described in detail.37 In brief, the patients had AAT deficiency of the PiZ type (confirmed by isoelectric focusing) and were attending the ADAPT (Antitrypsin Deficiency Assessment and Programme for Treatment) unit at the University Hospital Birmingham NHS Trust, Birmingham, UK. Patients were participating in an intravenous augmentation therapy trial called EXACTLE (Exacerbations and CT scans as Lung Endpoints; ClinicalTrials.gov identifier: NCT00263887) and recorded and scored their symptoms (breathlessness, sputum color, and volume) on daily diary cards. This patient group has been the subject of a previous publication investigating the characteristics of reported (treated) and unreported (untreated) exacerbations.37 All patients had confirmed airflow obstruction as demonstrated by post bronchodilator spirometry. All had experienced at least one exacerbation in the previous year. Patients were reviewed weekly and diary cards were checked for consistency. Any change in therapy was documented on the diary cards and in the clinical notes. At the end of the study, the cards were collected from the patients and analyzed for exacerbations over 2 years, as described previously.37 Ethical approval for this study was obtained from the local research and ethics committees, and all patients provided their written informed consent before enrolling in the study.
Physiology and computed tomography methods
Baseline full lung function was assessed after administration of nebulized bronchodilators (salbutamol 5 mg and ipratropium bromide 500 μg) in the stable state. All patients had a baseline computed tomography scan at full inspiration and emphysema was visualized and quantified as the 15th percentile lung density (PD15) derived from computed tomographic images, as described previously.46–48
Identification of exacerbations
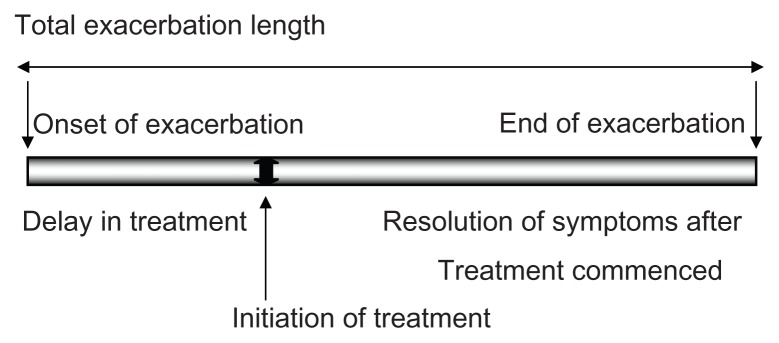
The onset of exacerbation was defined as the first day of an increase in one or more major symptoms, compared with baseline, for at least 2 consecutive days.36 The end of exacerbation was defined as the first day of a return to the patient’s usual clinical state as recorded for at least 2 consecutive days, defining the total length of the episode.
Figure 1 shows a diagrammatic representation of the exacerbation length and its components. Also indicated are the delay in treatment (time taken for treatment to be initiated after the onset of exacerbation) and resolution of symptoms to baseline, defining the end of the exacerbation after commencement of treatment.
Figure 1.
Diagrammatic representation of exacerbation length and components.
Weighting of scores
The scores for the symptoms of breathlessness and well-being were weighted and multiplied by a factor of five as described previously.37 Any increase in instance of cough was given a score of one, and changes in sputum volume and color were scored as indicated on the diary card (Bronkotest®, Middlesex, UK). The change in combined score for any day was used as a marker of severity.
Statistics
Statistical analysis was performed using the Statistical Package for the Social Sciences version 12 for Windows® (SPSS Inc, Cary, NC). Data are presented as the mean (±standard error) or median (interquartile range) depending on the distribution. Statistical comparisons were performed used the Student’s t-test for parametric data and Mann-Whitney U tests for nonparametric data. The Chi-square test was used for discrete variables, and the relationship between exacerbation frequency, length, treatment delay, and outcome to lung function were analyzed using the Spearman correlation. When more than one factor was related to the outcome, multivariate analysis was carried out to determine the best independent predictor.
Results
Baseline characteristics
Diary cards were analyzed for the 23 patients who completed the study for the period of 2 years. Table 1 summarizes their baseline characteristics as the mean ± standard error in parentheses. Of the patients, 16 had chronic sputum production and the mean score for sputum color and volume in the stable state was 1.5 ± 0.3 and 0.9 ± 0.2, respectively. Twenty-one patients were receiving regular, long-acting inhaled bronchodilator therapy (long-acting β2 agonists/long-acting anticholinergics) and 18 patients were also on inhaled corticosteroids. The mean dose of inhaled corticosteroid was 1226.1 ± 211.6 μg per day of beclomethasone dipropionate or equivalent (median/interquartile range 1000 [400–2000]). No patients were receiving long-term systemic corticosteroids. During this trial, 11 of the subjects received active drug.
Table 1.
Patient characteristics
| Patient characteristics | Mean (SE) |
|---|---|
| Total number of patients: female, male | N = 23: 6, 17 |
| Age (years) | 52.5 (2.1) |
| Smoking history | 21 ex-smokers, two never smoked |
| Pack – years | 20.7 (3.1) |
| FEV1 post-BD in L | 1.2 (0.1) |
| FEV1% predicted | 38.4 (3.1) |
| FEV1/VC% | 30 (2.7) |
| KCO (mmol/min/kpa/L) | 0.9 (0.4) |
| KCO (% predicted) | 59.7 (2.9) |
| PD15 baseline (g/L) | 44.55 (3.6) |
Abbreviations: BD, bronchodilator; FEV1, forced expiratory volume in one second; KCO, corrected gas transfer; VC, vital capacity; PD15, 15th percentile lung density
Frequency of exacerbations
A total of 263 episodes (164 treated and 99 untreated) were identified on the 23 diary cards over a period of 24 months. The median (interquartile range) total exacerbation rate was 5.0 (3.5–7.0) per patient per year, and consisted of 3.0 (2.5–4.5) treated episodes and 1.5 (0.5–3.0) episodes where the treatment remained unchanged. Untreated episodes were less likely to be associated with added symptoms suggestive of a coryzal illness, chest pain, or nocturnal symptoms (P < 0.001). The treated episodes were characterized by more symptom scores, a longer duration of breathlessness, and worse well-being, with well-being central to patients seeking medical therapy. We discussed these results in detail in our previous published paper.37
Treatment
Of the 164 treated exacerbations, 31 were treated with increased inhaled bronchodilator therapy alone. Antibiotics were given for 130 exacerbations and, in the case of 50 episodes, patients received systemic steroids. There were 11 hospitalized episodes among five patients. Of the 164 episodes, complete daily diary card data (no missing days) were available for 81 episodes (for 17 of the patients enabling length to be determined) of which 61 were treated with antibiotics (12 episodes were also treated with steroids) and 51 episodes were purulent with a sputum color score of ≥3. Daily data were available for four episodes which required hospital admission.
Factors affecting frequency of treated exacerbations
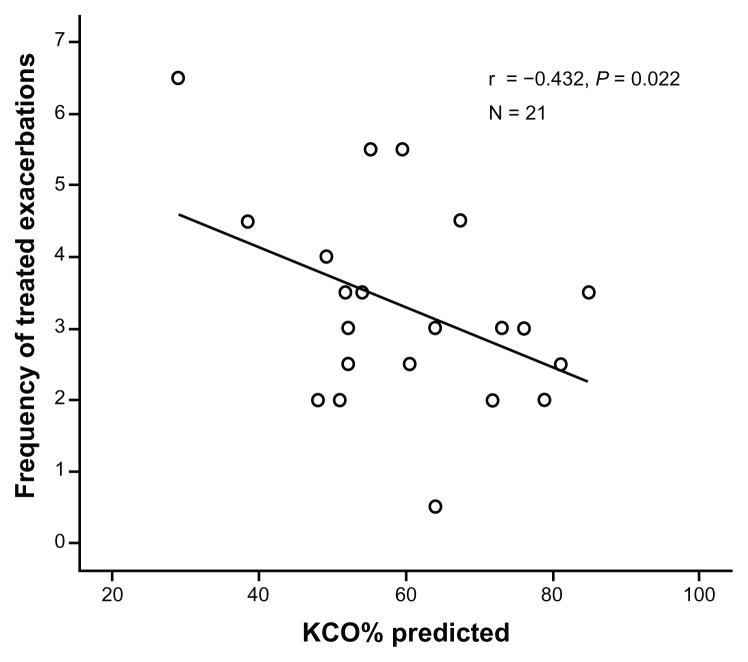
Of the 23 patients, one recorded no treated exacerbation and one had an excessive number (more than 10 in a year), which represented a clear outlier. For the remaining 21 patients, there was a significant negative correlation between corrected gas transfer (KCO% predicted) and frequency of treated episodes (Table 2 and Figure 2). No other baseline characteristic, including baseline PD15, was related to frequency of treated episodes.
Table 2.
Factors that influence exacerbation features
| Factors influencing exacerbation features | Features of the exacerbations | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Treated exacerbation frequency | Likelihood of episode being treated | Exacerbation length in days | Delay in days | Day 1 symptoms score | Resolution of length in days | |
| Spirometry FEV1 | ns | ar = −0.386; P = 0.038 | ns | cr = 0.272; P = 0.036 | cr = −0.234;P= 0.003 | ns |
| FEV1/FVC | br = −0.460; P = 0.013 (N = 21) | br = 0.461; P < 0.001 (N = 17) | br = −0.322;P< 0.001 (N = 81 treated episodes) | |||
| KCO% pred | r= −0.432;P= 0.022 (N = 21) | ns | ns | ns | ns | r= −0.647;P= 0.007 N = 15 |
| Delay in treatment initiation in days | All treated (N = 81 episodes) dr = 0.317; P = 0.006 Antibiotic only treated (N = 49 episodes) er = 0.503; P < 0.001 |
– | r = −0.368; P = 0.004 (N = 81 treated episodes) | ns | ||
| Anthonisen criteria types | Chi-square,P< 0.001 | Chi-square, P = 0.001 (N = 17) | – | Type 1 > Type 2 > Type 3, P < 0.001 (N = 21) | – | |
| Lung densitometry (PD15) | ns | – | r = −0.361; P = 0.003 (N = 81 episodes) | r= 0.786;P< 0.001 (N = 15) | r = −0.170; P = 0.035 (N = 81 episodes) | ns |
| Cold symptom | – | Chi-square,P< 0.001 | Chi-square, P < 0.001 | ns | Chi-square, P < 0.001 (N = 21) | ns |
Notes:
FEV1% predicted;
FEV1/VC ratio;
absolute FEV1;
all episodes treated with antibiotics;
episodes also treated with steroids excluded. The features of the exacerbations are outlined as frequency of treated episodes, the likelihood of the episode being treated, length of the episode, delay in commencing treatment, severity of symptoms on day 1 of the episode, and time to resolution following the start of therapy. The factors that relate to these features are shown (see text for further details for each feature). Correlation coefficients (positive or negative) and P-values of the correlations are reported. The factor that is the best independent predictor of the feature from multivariate analysis is highlighted in bold font.
Abbreviations: FEV1, forced expiratory volume in one second; FVC, forced vital capacity; KCO, corrected gas transfer; PD15, 15th percentile lung density; N, number of patients; ns, not significant.
Figure 2.
Correlation between KCO (% predicted) and frequency of 164 treated exacerbations in 21 patients.
Notes: Each point is the average number of treated episodes/year for each patient. The correlation coefficient (r) and significance of the relationship are shown.
Abbreviation: KCO, corrected gas transfer.
Factors affecting likelihood of an episode being treated
The likelihood of an episode being treated correlated negatively with FEV1% predicted and the FEV1/forced vital capacity (FVC) ratio (Table 2). Other factors shown to affect the rate of treated episodes were the number of major symptoms outlined by Anthonisen et al,35 presence of cold-like symptoms, chest pain, and nocturnal symptoms as previously published37 (Chi-square, P < 0.001 for all). Although all the above-mentioned factors significantly predicted the episodes that required treatment upon univariate analysis, when using the linear regression stepwise analysis, presence of cold-like symptoms was the best independent predictive factor (P < 0.001).
Factors influencing exacerbation length of treated episodes
The mean (±standard error) total length of exacerbation, delay in initiation of antibiotic treatment, and resolution of all symptoms after the start of antibiotic therapy (61 antibiotic-treated episodes in 17 patients) were 17.5 ± 1.9, 4.4 ± 0.5, and 12.9 ± 1.8 days, respectively.
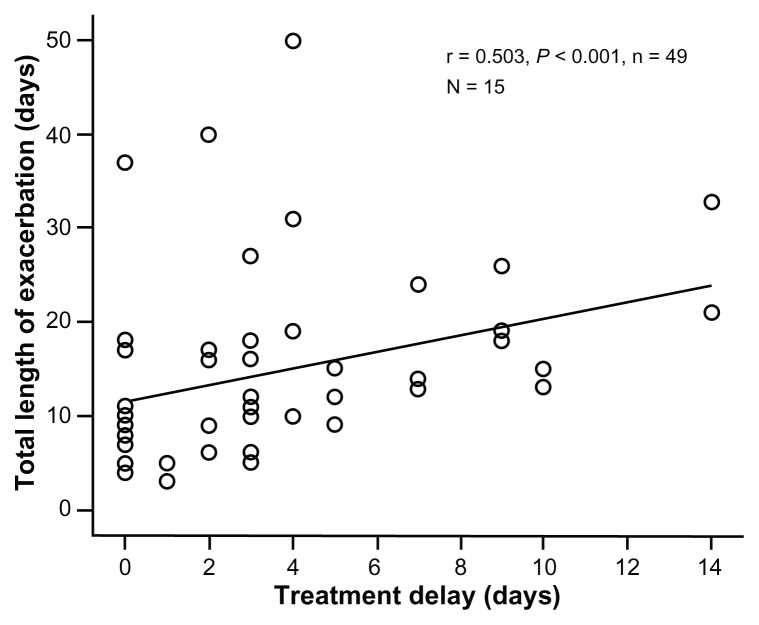
Of the 61 episodes treated with antibiotics, the exacerbation length was greater the longer the delay in initiation of treatment (Table 2). This relationship was stronger when episodes also treated with steroids were excluded (49 episodes in 15 patients; Table 2 and Figure 3).
Figure 3.
Relationship between delay in starting antibiotic treatment and length of exacerbation.
Notes: Each point represents an individual antibiotic-only treated (no systemic steroid) episode (n = 49 in 15 patients). The correlation coefficient (r) and significance are shown.
The length of any treated episode was related to Anthonisen criteria, with type 1 exacerbations lasting longer than type 2, which in turn were longer than type 3 (P = 0.001). However, the average length for all treated episodes correlated negatively with the PD15, with longer episodes relating to lower lung density (Table 2). When analyzed using the linear regression stepwise model, the number of Anthonisen symptoms was the best independent predictor of length of any treated episode. We found no association of length with any of the baseline lung function parameters.
Factors affecting delay in initiation of antibiotic treatment
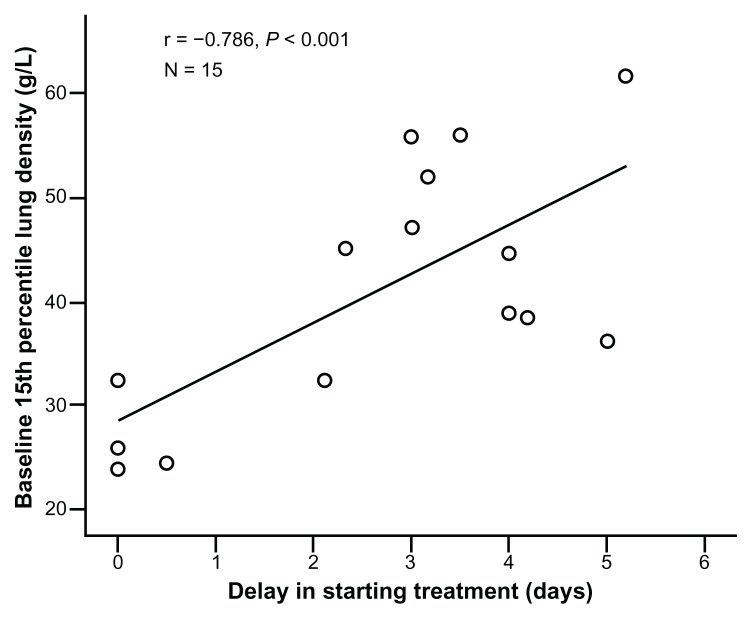
The delay in commencing treatment was shorter in patients with lower absolute FEV1, FEV1/FVC ratio, and PD15 at baseline (r = 0.272, P = 0.036, r = 0.461, P < 0.001, and r = 0.786, P < 0.001; Table 2) and with a higher symptom score on day 1 (r = −0.368, P = 0.004). When analyzed using stepwise linear regression, baseline lung density emerged as the best independent predictor of delay in treatment (P = 0.004), and this association is summarized in Figure 4.
Figure 4.
Association between treatment delay and baseline PD15.
Notes: Each point represents the average delay for each of the 15 patients whose antibiotic-treated episodes were studied in detail. The correlation coefficient (r) is shown, together with the significance of the relationship.
Abbreviation: PD15, 15th percentile lung density.
Factors affecting treated episode symptom score severity
There was a significant negative correlation between symptom score on day 1 and baseline FEV1, FEV1/VC%, and lung density (81 episodes; Table 2). No relationship was found with KCO. When analyzed using stepwise linear regression, FEV1 emerged as the best independent predictor of poorer symptom score on day 1 (P = 0.019).
Factors affecting resolution of exacerbation after treatment initiation
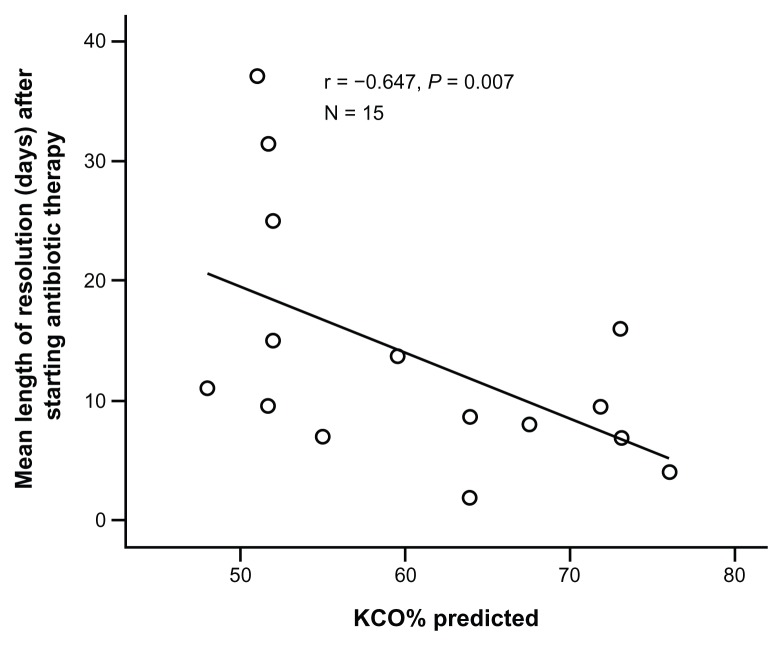
Resolution of symptoms after the start of antibiotic treatment was not affected by delay in the start of therapy. However, there was a significant negative correlation between resolution and KCO% predicted (Table 2 and Figure 5). No other factor affected resolution time following initiation of treatment.
Figure 5.
Relationship between gas transfer and resolution of exacerbation after treatment start.
Notes: Each point represents the average resolution time for each of the 15 patients who experienced and documented antibiotic-treated episodes. The value for the coefficient (r) and the P-value are again shown.
Abbreviation: KCO, corrected gas transfer.
Discussion
Exacerbations of usual COPD have been well described. The current study allows insight into the factors that influence the management and resolution of exacerbations in a more highly characterized group of patients with AAT deficiency. The data show that exacerbations are more likely to lead to therapeutic intervention in subjects with more severe impairment of FEV1 and FEV1/FVC at baseline, and in whom more symptoms show deterioration. These two features are interdependent and are consistent with published studies in usual COPD,39,49,50 indicating that the more severe the underlying functional impairment, the more marked the impact of exacerbations.
The overall length of the episode was related to delay in starting antibiotic treatment, although the time from treatment initiation to resolution was unaltered. These data suggest that the overall length of an exacerbation would therefore be reduced by earlier intervention and supports the aims of patient self-management programs in usual COPD.51,52
The AAT deficiency variant is rare, so precludes the study of large numbers of patients, other than superficially or as part of a formal clinical trial. Nevertheless, these unique data enable some new conclusions to be drawn. We have shown that, in addition to spirometry, other physiologic measures (KCO) and radiologic quantification of emphysema (PD15) are also related to exacerbations and their features. The exacerbation frequency was related to KCO% predicted, as was the length of recovery after initiation of treatment. Furthermore, the delay in start of treatment was also dependent on baseline lung density, and multivariate analysis confirmed that this was the best independent predictor of this feature of the episode.
Although previous studies have explored the relationship between airflow obstruction and the frequency, severity, and resolution of exacerbations in usual COPD,49,50,53 the current study suggests that (at least in AAT deficiency-related COPD) lung density by computed tomography, a more direct measure of emphysema, plays an equal if not more important role in determining several aspects of these episodes. Of particular importance is that the data highlight lung densitometry as a measure that has an impact on yet another feature of COPD, ie, exacerbations, in addition to activity,54 health status,55 and mortality.56 Therefore, assessment of the presence and severity of emphysema should become a routine part of the characterization of COPD patients because these data influence many clinical features, possibly including the frequency and time course of exacerbations, as shown here for AAT deficiency. These COPD features (unlike FEV1 measurement) are less amenable to therapeutic intervention, and this observation may partly explain why accepted therapies only produce a modest effect on exacerbations and their frequency.20–25 It is clear that further studies in a group of usual COPD subjects are warranted.
Limitations of our study include the fact that the number of subjects studied was small (although large for this genetic subset) and not all episodes had daily data recorded before, during, and after the exacerbation. The analysis was also limited to those episodes treated by an intervention because this permitted a delay in treatment and its effect on the time to resolution to be determined. Nevertheless, the detailed physiologic and radiologic characteristics of the patients enabled their role to be investigated, providing insight into factors other than spirometry that may influence the features of the episodes. Apart from the small number of patients, our study could be influenced by involvement in the trial itself, which may influence patient behavior. In addition, 11 of the patients were receiving active AAT therapy. Although this did not influence the frequency of exacerbations in the full trial,57 it is possible that it may have had an effect on characteristics. Nevertheless, the new observations of the relationship to emphysema as determined by gas transfer and densitometry remains of interest and worthy of further study.
In summary, these data build on our previous documentation of features that relate to an exacerbation being treated or untreated in AAT deficiency. The components of the treated exacerbation are dependent upon the severity of the underlying disease and the speed of intervention. The presence of emphysema, and especially lung density, have a greater influence than spirometry on some exacerbation features, and these factors should be included in overall patient assessment for prognosis and determining the correct course of management.
Acknowledgment
The authors would like to thank Anita Pye for assistance with manuscript submission.
Footnotes
Disclosure
This study was supported by an unrestricted grant from Talecris Biotherapeutics Inc, Research Triangle Park, NC. Talecris was acquired by Grifols, effective June 1, 2011. RAS is a member of the Alpha-1 International Registry, director of the UK Alpha-1 Registry, and acts in an advisory role for Talecris and Kamada. He has had travel and lecture fees on behalf of Talecris and is in receipt of an unrestricted research grant from Talecris for studies in AAT deficiency. KV reports no conflicts of interest in this work.
References
- 1.Kendrick S. Emergency admissions: what is driving the increase? Health Serv J. 1995;105:26–28. [PubMed] [Google Scholar]
- 2.Mushlin AI, Black ER, Connolly CA, Buonaccorso KM, Eberly SW. The necessary length of hospital stays for chronic pulmonary disease. JAMA. 1991;266:80–83. [PubMed] [Google Scholar]
- 3.Seneff MG, Wagner DP, Wagner RP, Zimmerman JE, Knaus WA. Hospital and one year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA. 1995;274:1852–1857. [PubMed] [Google Scholar]
- 4.Connors AF, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. Am J Respir Crit Care Med. 1996;154:959–967. doi: 10.1164/ajrccm.154.4.8887592. [DOI] [PubMed] [Google Scholar]
- 5.Hurd S. The impact of COPD on lung health worldwide: epidemiology and incidence. Chest. 2000;117:1S–4S. doi: 10.1378/chest.117.2_suppl.1s. [DOI] [PubMed] [Google Scholar]
- 6.Almagro P, Calbo E, de Echaguen Ochoa, et al. Mortality after hospitalisation for COPD. Chest. 2002;121:1441–1448. doi: 10.1378/chest.121.5.1441. [DOI] [PubMed] [Google Scholar]
- 7.Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality related factors after hospitalisation for acute exacerbations of COPD. Chest. 2003;124:459–467. doi: 10.1378/chest.124.2.459. [DOI] [PubMed] [Google Scholar]
- 8.A Statistics Report from the British Thoracic Society. The Burden of Lung Disease. 2nd ed. 2006. [Accessed October 24, 2012]. Available from: http://www.brit-thoracic.org.uk/Portals/0/Library/BTS%20Publications/burdeon_of_lung_disease2007.pdf.
- 9.Dowson LJ, Guest PJ, Stockley RA. Longitudinal changes in physiological, radiological and health status measurements in alpha (1)-antitrypsin deficiency and factors associated with decline. Am J Respir Crit Care Med. 2001;164:1805–1809. doi: 10.1164/ajrccm.164.10.2106036. [DOI] [PubMed] [Google Scholar]
- 10.Needham M, Stockley RA. Exacerbations in alpha 1 antitrypsin deficiency. Eur Respir J. 2005;25:992–1000. doi: 10.1183/09031936.05.00074704. [DOI] [PubMed] [Google Scholar]
- 11.Donaldson GC, Seemungal TAR, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seemungal TAR, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 13.Doll H, Grey-Amante P, Duprat-Lomon I, et al. Quality of life in acute exacerbation of chronic bronchitis: results from a German population study. Respir Med. 2002;96:39–51. doi: 10.1053/rmed.2001.1208. [DOI] [PubMed] [Google Scholar]
- 14.Pauwels RA, Lofdahl CG, Laitinen LA, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med. 1999;340:1948–1953. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 15.Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J. Multicentre randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. International COPD Study Group. Lancet. 1998;351:773–780. doi: 10.1016/s0140-6736(97)03471-5. [DOI] [PubMed] [Google Scholar]
- 16.Mahler DA, Donohue JF, Barbee RA, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest. 1999;115:957–965. doi: 10.1378/chest.115.4.957. [DOI] [PubMed] [Google Scholar]
- 17.Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217–224. doi: 10.1183/09031936.02.00269802. [DOI] [PubMed] [Google Scholar]
- 18.Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/fluticasone propionate vs salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:144–149. doi: 10.1164/rccm.200602-244OC. [DOI] [PubMed] [Google Scholar]
- 19.Vincken W, van Noord JA, Greefhorst AP, et al. Improved health outcomes in patients with COPD during 1 year’s treatment with tiotropium. Eur Respir J. 2002;19:209–216. doi: 10.1183/09031936.02.00238702. [DOI] [PubMed] [Google Scholar]
- 20.Aaron SD, Sandemheen LK, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol or fluticasone/salmeterol for treatment of chronic obstructive pulmonary disease. Ann Intern Med. 2007;146:545–555. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- 21.Niewoehner DE, Rice K, Cote C, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005;143:317–326. doi: 10.7326/0003-4819-143-5-200509060-00007. [DOI] [PubMed] [Google Scholar]
- 22.Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax. 2003;58:399–404. doi: 10.1136/thorax.58.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celli B, Calverley PM, Anderson JA, et al. The towards a revolution in COPD health (TORCH) study: fluticasone propionate/salmeterol reduces the rate of exacerbations over 3 years. Chest. 2006;130:177s. [Google Scholar]
- 24.Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD009157. doi: 10.1002/14651858.CD009157.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829. doi: 10.1002/14651858.CD006829.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stockley RA, O’Brien C, Pye A, et al. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638–1645. doi: 10.1378/chest.117.6.1638. [DOI] [PubMed] [Google Scholar]
- 27.Bhowmik A, Seemungal TA, Sapsford RJ, Wedzich JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;55:114–120. doi: 10.1136/thorax.55.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel IS, Seemungal TA, Wilks M, Lloyd-Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonization and the frequency, character and severity of COPD exacerbations. Thorax. 2002;57:759–764. doi: 10.1136/thorax.57.9.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seemungal TA, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 30.McElvaney NG, Stoller JK, Buist AS, et al. Baseline characteristics of enrollees in the National Heart, Lung and Blood Institute Registry of alpha 1-antitrypsin deficiency. Alpha 1-Antitrypsin Deficiency Registry Study Group. Chest. 1997;111:394–403. doi: 10.1378/chest.111.2.394. [DOI] [PubMed] [Google Scholar]
- 31.Eden E, Hammel J, Rouhani FN, et al. Asthma features in severe alpha1-antitrypsin deficiency: experience of the National Heart, Lung, and Blood Institute Registry. Chest. 2003;123:765–771. doi: 10.1378/chest.123.3.765. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson S. Pulmonary emphysema and alpha-1-antitrypsin deficiency. Acta Med Scand. 1963;175:197–205. doi: 10.1111/j.0954-6820.1964.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 33.Wilson JS, Galvin JR. Normal diffusing capacity in patients with Pi ZZ alpha(1)-antitrypsin deficiency, severe airflow obstruction, and significant radiographic emphysema. Chest. 2000;118:867–871. doi: 10.1378/chest.118.3.867. [DOI] [PubMed] [Google Scholar]
- 34.Fabbri LM, Luppi F, Beghe B, Rabe KF. Update in chronic obstructive pulmonary disease 2005. Am J Respir Crit Care Med. 2006;173:1056–1065. doi: 10.1164/rccm.2603005. [DOI] [PubMed] [Google Scholar]
- 35.Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Rosin R. Toward a consensus definition for COPD exacerbations. Chest. 2000;117:398S–401S. doi: 10.1378/chest.117.5_suppl_2.398s. [DOI] [PubMed] [Google Scholar]
- 37.Vijayasaratha K, Stockley RA. Reported and unreported exacerbations of COPD – analysis by diary cards. Chest. 2008;133:34–41. doi: 10.1378/chest.07-1692. [DOI] [PubMed] [Google Scholar]
- 38.Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised double blind placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langsetmo L, Platt RW, Ernst P, Bourbeau J. Underreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohort. Am J Respir Crit Care Med. 2008;177:396–401. doi: 10.1164/rccm.200708-1290OC. [DOI] [PubMed] [Google Scholar]
- 40.Ball P, Harris JM, Lowson D, Tilltoson G, Wilson R. Acute infective exacerbations of chronic bronchitis. QJM. 1995;88:61–68. [PubMed] [Google Scholar]
- 41.Hill AT, Campbell EJ, Bayley DL, Hill SL, Stockley RA. Evidence for excessive bronchial inflammation during an acute exacerbation of chronic obstructive pulmonary disease in patients with alpha(1)-antitrypsin deficiency (Pi Z) Am J Respir Crit Care Med. 1999;160:1968–1975. doi: 10.1164/ajrccm.160.6.9904097. [DOI] [PubMed] [Google Scholar]
- 42.The Alpha-1-Antitrypsin Deficiency Registry Study Group. Survival and FEV1 decline in individuals with severe deficiency of alpha1-antitrypsin. Am J Respir Crit Care Med. 1998;158:49–59. doi: 10.1164/ajrccm.158.1.9712017. [DOI] [PubMed] [Google Scholar]
- 43.Lieberman J. Augmentation therapy reduces frequency of lung infections in antitrypsin deficiency: a new hypothesis with supporting data. Chest. 2000;118:1480–1485. doi: 10.1378/chest.118.5.1480. [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson TMA, Donaldson GC, Hurst JR, et al. Early therapy improves outcomes of exacerbations of COPD. Am J Respir Crit Care Med. 2004;169:1298–1304. doi: 10.1164/rccm.200310-1443OC. [DOI] [PubMed] [Google Scholar]
- 45.Wedzicha JA, Donaldson GC. Exacerbations of chronic obstructive pulmonary disease. Respir Care. 2003;48:1204–1213. [PubMed] [Google Scholar]
- 46.Parr DG, Stoel BC, Stolk J, Stockley RA. Validation of computed tomographic lung densitometry for monitoring emphysema in α1-antitrypsin deficiency. Thorax. 2006;61:485–490. doi: 10.1136/thx.2005.054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parr DG, Stoel DC, Stolk J, Stockley RA. Pattern of emphysema distribution in alpha1-antitrypsin deficiency influences lung function impairment. Am J Respir Crit Care Med. 2004;170:1172–1178. doi: 10.1164/rccm.200406-761OC. [DOI] [PubMed] [Google Scholar]
- 48.Parr DG, Sevenoaks M, Deng C, Stockley RA. Detection of emphysema progression in alpha 1-antitrypsin deficiency using CT densitometry; methodological advances. Respir Res. 2008;9:21. doi: 10.1186/1465-9921-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donaldson GC, Seemingul TAR, Patel IS, et al. Longitudinal changes in the nature, severity and frequency of COPD exacerbations. Eur Respir J. 2003;22:931–936. doi: 10.1183/09031936.03.00038303. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Aymerich J, Monsó E, Marrades RM, et al. Risk factors for hospitalization for a chronic obstructive pulmonary disease exacerbation. EFRAM study. Am J Respir Crit Care Med. 2001;164:1002–1007. doi: 10.1164/ajrccm.164.6.2006012. [DOI] [PubMed] [Google Scholar]
- 51.Bourbeau J, Julien M, Mattais F, et al. Reduction of hospital utilisation in patients with COPD: a disease specific self management intervention. Arch Intern Med. 2003;163:585–591. doi: 10.1001/archinte.163.5.585. [DOI] [PubMed] [Google Scholar]
- 52.Gadoury MA, Schwartzman K, Rouleau M, et al. Self-management reduces both short and long term hospitalisation in COPD. Eur Resp J. 2005;26:853–857. doi: 10.1183/09031936.05.00093204. [DOI] [PubMed] [Google Scholar]
- 53.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 54.Stolk J, Putter H, Bakker EM, et al. Progression parameters for emphysema: a clinical investigation. Respir Med. 2007;101:1924–1930. doi: 10.1016/j.rmed.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 55.Stolk J, Ng WH, Bakker EM, et al. Correlation between annual change in health status and computer tomography derived lung density in subjects with α1-antitrypsin deficiency. Thorax. 2003;58:1027–1030. doi: 10.1136/thorax.58.12.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dawkins PA, Dowson LJ, Guest PJ, Stockley RA. Predictors of mortality in alpha 1 antitrypsin deficiency. Thorax. 2003;58:1020–1026. doi: 10.1136/thorax.58.12.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dirksen A, Piitulainen E, Parr DG, et al. Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha-1-antitrypsin deficiency. Eur Respir J. 2009;33:1345–1353. doi: 10.1183/09031936.00159408. [DOI] [PubMed] [Google Scholar]