Abstract
Objective
To identify and image protein biomarker candidates in the synovial tissue of patients with rheumatoid arthritis (RA) and patients with osteoarthritis (OA).
Methods
A novel matrix-assisted laser desorption/ionization (MALDI) imaging mass spectrometry (IMS) technique was applied to the analysis of synovial tissue. Patients were classified according to the American College of Rheumatology (ACR) criteria for RA. Frozen sections were stained to obtain morphological data. Serial sections were desiccated, and spotted with matrix for MALDI analysis. Ions generated by laser irradiation of the tissue were separated in time, based on their m/z ratio, and were subsequently detected. IMS was used in a ‘profiling’ mode to detect discrete spots for rapid evaluation of proteomic patterns in various tissue compartments. Photomicrographs of the stained tissue images were reviewed by a pathologist. Areas of interest (10 discrete areas/compartment) were marked digitally and the histology-annotated images were merged to form a photomicrograph of the section taken before the MALDI measurement. Pixel coordinates of these areas were transferred to a robotic spotter, the matrix was spotted, and the coordinates of the spots were transferred to a mass spectrometer for spectral acquisition. The data generated were then subjected to biocomputation analysis to reveal the biomarker candidates.
Results
Several peaks (m/z) consistent in mass with calgranulins, defensins, and thymosins were detected and their distribution in various synovial compartments (synovial lining and sublining layer) was demonstrated.
Conclusion
MALDI IMS is a powerful tool for the rapid detection of numerous proteins (in situ proteomics) and was applied here for the analysis of the distribution of proteins in synovial tissue sections.
Rheumatoid arthritis (RA) is a systemic autoimmune disease with a heterogeneous clinical presentation and disease course, ranging from mild disease to severe pathology with bone and cartilage destruction and marked variability in synovial inflammation (1). Although current therapies with biological agents yield significant improvement in the patients, the disease is not yet curable. To provide personalized therapy, preventing irreversible joint damage, a detailed knowledge of synovial tissue subtypes and access to stratification biomarkers is crucial (1). Besides blood and synovial fluid, synovial tissue, which can be obtained easily by closed-needle or arthroscopic biopsy, can assist in the diagnosis of joint disorders.
The currently available biological tests are not sufficiently accurate, leading to delayed diagnosis of the disease. The discovery of new biomarkers is therefore of particular interest for early diagnosis (2). However, as studies progress and become more complex, new enabling approaches are needed that transcend the limitations of current technologies (3).
Imaging mass spectrometry (IMS) is an emerging technology that permits the direct high-throughput analysis and determination of the distribution of molecules (proteins, peptides, lipids, xenobiotics, and metabolites) in tissue sections. Using IMS, biological molecules can be analysed with molecular specificity not readily achievable through other means, and analyses can even be performed on formalin-fixed tissues (3). Because this technology analyses intact tissue, avoiding homogenization and separation steps, the spatial distribution of the molecules within the tissue is preserved (3). To our knowledge, this is the first report combining the application of high-throughput MS with imaging of various molecules in synovial tissue of patients with RA and osteoarthritis (OA).
Materials and methods
Patients
Synovial tissue was acquired from patients with RA and OA undergoing open joint surgery. Tissues were immediately snap frozen in liquid nitrogen. Corresponding tissues were fixed in 4% buffered formalin, embedded in paraffin and investigated by a pathologist. All RA patients fulfilled the criteria of the American College of Rheumatology (ACR) for the diagnosis of RA, had received prior treatment, and had a high synovialitis score (> 6 of 9) (4). Patients with OA were classified according to Altman et al (5) and were not having therapy with anti-rheumatic drugs. Tissue samples were taken according to the Declaration of Helsinki, from individuals treated at Diakonie Hospital, Department of Orthopaedics and the Rheumatology Centre of Rhineland-Palatinate, Bad Kreuznach. Informed consent was given by each patient, and the study design was approved by the local ethics committee.
Histology-directed matrix-assisted laser desorption/ ionization (MALDI) MS and proteomic data analysis
MALDI MS profiling and imaging was performed as described previously (3, 6). For MALDI MS profiling, serial sections from each frozen tissue sample were stained with haematoxylin and eosin (H&E) or thaw-mounted and fixed onto a MALDI plate. Photomicrographs of H&E-stained sections of synovial membranes of RA and OA patients were marked digitally (synovial lining layer and sublining/200 μm, minimum of 10 spots of interest) by a pathologist (JK) for MALDI image correlation. All adipose tissue, potential contamination (e.g. blood), and edges of the tissue were avoided. Histology-annotated optical images were merged to form a photomicrograph of the MALDI section, and pixel coordinates of the annotated areas were obtained for robotic spotting. An acoustic robotic spotter (LabCyte Inc.; Sunnyvale, CA, USA) placed crystalline matrix (20 mg/mL sinapinic acid in 1:1 acetonitrile/0.2% trifluoroacetic acid) spots (180–220 μm in diameter) on each printing coordinate of each tissue. Tissue profile spectra were acquired using an Autoflex Speed (Bruker Daltonics, Bremen, Germany) MALDI mass spectrometer and run using an automated linear mode acquisition method optimized for mass range 2 to 40 kDa, as described previously (5). MALDI MS spectra were baseline corrected, normalized, and aligned using ProTS-Marker (Biodesix Inc., Boulder, CO, USA). Multiple spectra were averaged from the same subject from different experiments for synovial lining layer and sublining.
MALDI MS imaging experiments were performed by spotting the tissue surface with a matrix solution in a defined microspotted array. Spectra were acquired at each position on the sample at a spatial resolution of 250 μm, and spectral files were reconstructed into ion density images for viewing.
Results
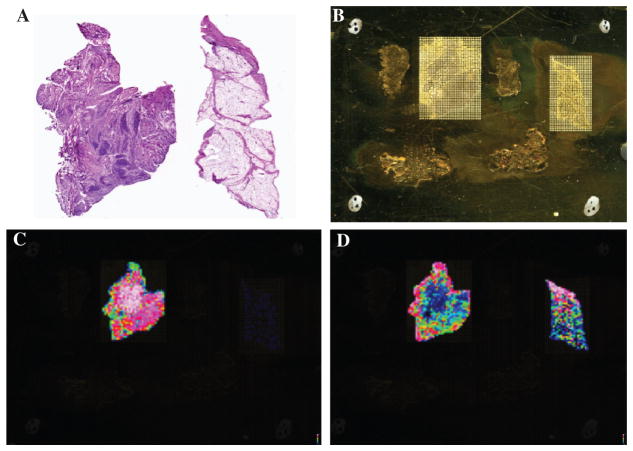
MALDI MS image analysis was applied to synovial tissue of patients with RA and OA. Histological analysis of synovial tissues (H&E) from patients with RA showed a thickened synovial lining layer and heavy chronic inflammation with lymph follicles in the sublining layer, whereas OA synovial tissue was characterized by a mild increase in the thickness of the synovial lining layer and mild lymphocytic infiltration and mild fibrosis (Figure 1A). Parallel sections were desiccated and a matrix was spotted over the surface of the tissue section by specialized sample procedures. Subsequently, molecules present on the spots were ionized by using laser energy and separated in time, based on their m/z ratio (Figure 1B).
Figure 1.
(A) H&E section of synovial tissue (left, RA; right, OA). (B) Numerous matrix spots throughout the synovial tissue (left, RA; right, OA). (C) MALDI MS images at m/z 4747 (thymosin beta-4 truncated with strong expression in the sublining area in RA but not in OA synovial tissue). (D) MALDI MS images at m/z 4964 (thymosin beta-4 with strong expression in the lining area in RA and OA synovial tissue).
Preliminary data when comparing RA and OA synovial tissue showed strong differences in the intensity of known molecules, such as m/z 4737 (probably thymosin beta-10 truncated) (7) and m/z 4747 (probably thymosin beta-4 truncated) (7) (Figure 1C). By contrast, the molecular species at m/z 4964 (probably thymosin beta-4) (Figure 1D) showed an increased in intensity in the synovial lining layer of patients with RA and OA, and to a much lower extent in the synovial sublining layer. Molecules at m/z 5358 with moderate expression in the OA sublining area and RA lining were also detected. MALDI image analysis also revealed molecules at m/z 6226 with moderate expression in areas with lymph follicles in RA synovial tissue but lower expression in other areas or in OA synovial tissue.
IMS was used in a ‘profiling’ mode to detect discrete spots of interest for rapid evaluation of proteomic patterns in various tissue compartments. Photomicrographs of H&E-stained tissue images were reviewed by a pathologist, areas of interest (about 10 discrete areas/ compartments) were marked digitally, and the histology-annotated images were merged to form a photomicrograph of the section on the MALDI target (Figures 2A and 2B). Serial H&E sections were used to guide the placement of the matrix and provided the capability of focusing on areas having a high content of a cell type of interest (synovial lining, synovial sublining). Later, tissues with the spotted matrix were re-evaluated and the spots of interest were marked by red numbers to ensure that only tissue areas with the best preservation and morphology were included in the study (Figure 2). The coordinates of the spots were transferred to the mass spectrometer for spectral acquisition.
Figure 2.
(A) OA and (B) RA synovial tissue with histology-annotated images were merged to form a photomicrograph of the section on the MALDI target. (C) OA and (D) RA synovial tissue with a spotted matrix on the previously marked areas of interest. Only spots with red numbers were evaluated.
We could demonstrate the presence of molecules consistent with masses of peaks identified in previous studies at m/z 3367 (defensin alpha-1-beta) (2), 3439 (defensin alpha-1) (2), 3485 (defensin alpha-3) (2), 10 096 (S100A6, calcyclin) (7, 8), 10 840 (S100A8) (2), 11 656 (S100 A11, calgizzarin) (7, 8), several histones [7005 (histone H2A2 doubly charged), 11 313, 11 354 (histone H4 with one acetylation), 11 396 (histone H4 with two acetylations), 13 781 (histone H2B)] (7), and molecules that had not previously been identified at m/z of 2749, 5357, 5418, 5654, 5675, 9624, 9752, 9768, 11 518, 11 612, 12 352, 12 696, and 13 160.
Most remarkably, MALDI imaging data were obtained from the different regions with peaks at m/z 4737(thymosin beta-10 truncated) (7) and m/z 4747 (thymosin beta-4 truncated) (7) in the synovial sublining and lining layer with strong expression in RA but not in OA tissues, with higher intensity in the sublining layer (Figures 3A and 3B). Additionally, signals at m/z 10840 (probably S100A8) (2) were found in the synovial sublining and lining layer with strong expression in RA but not in OA tissues, with higher intensity in the sublining layer. Peaks at m/z 3367 (defensin alpha-1-beta) (2), 3439 (defensin alpha-1) (2), and 3485 (defensin alpha-3) (2) were located in the synovial sublining and lining layer with strong expression in RA but not in OA tissues (Figures 3C and 3D).
Figure 3.
MALDI MS profile with peaks at m/z 4737 (thymosin beta-10 truncated) and m/z 4747 (thymosin beta-4 truncated) in the synovial sublining (A) and lining (B) layer with strong expression in RA but not in OA tissues, with a higher intensity in the sublining layer. MALDI MS profile with the peaks at m/z 3367 (defensin alpha-1-beta), 3439 (defensin alpha-1), and 3485 (defensin alpha-3) in the synovial sublining (C) and lining (D) layer with strong expression in RA but not in OA tissues.
Discussion
Early diagnosis of RA is essential to provide appropriate therapy to patients with RA. Exact classification of the disease activity by biomarkers is required (1, 2). Recent work has provided proof of principle that biomarkers may be identified to predictive of the response to targeted therapy (9).
Histology, histochemistry, immunohistochemistry, and molecular pathological techniques have been applied to synovial tissue (10). As studies progress, detailed questions are being asked at the molecular level to transcend the limitations of current technologies (3).
Previous studies have identified biomarkers for various arthritides through MS analysis applied to serum, synovial fluid, and synovial tissue (2, 11–14). The consistency of protein patterns in various joints suggests that the biomarker profile truly represents a disease subtype (14) or status of the disease.
To combine morphology with MS, image analysis was applied to synovial tissue of patients with RA and OA. Synovial tissue consists of two major compartments: a lining layer with macrophage-like (type A) and fibroblast-like (type B) synoviocytes and the sublining layer (15). For the first time, applying IMS, we could illustrate various mass spectra generated from proteins expressed in RA and OA synovial tissue (Figures 1C and 1D). Mass spectra were collected predominantly from the synovial lining layer and the subsynovial tissue.
Continued evaluation of the MS images showed interesting preliminary data on comparing RA and OA synovial tissue, with strong differences in intensity of known molecules, for example, at m/z 4737 (thymosin beta-10 truncated) (6) and m/z 4747 (thymosin beta-4 truncated) (7) (Figure 1C). These data are of particular interest because thymosins have been described in plasma of patients with RA (16).
Thymosin beta-4 is the most prominent member of the beta-thymosin family, responsible for T-lymphocyte maturation and actin sequestration. Associated with its role in actin polymerization, thymosin beta-4 has a variety of functions in tumour metastasis; it promotes angiogenesis, and has anti-apoptotic activity (17, 18). Most remarkably, using MALDI MS imaging in a ‘profiling’ mode, peaks at m/z 4737 (thymosin beta-10 truncated) (7) and m/z 4747 (thymosin beta-4 truncated) (7) were found in the synovial sublining and lining layer with strong expression in RA but not in OA tissues (Figures 3A and 3B).
We could detect discrete spots of interest for rapid evaluation (a few minutes) of proteomic patterns with peaks consistent with masses of molecules identified in previous studies. Further experiments are necessary to confirm the identity of these proteins. Preliminary identification of the signals are: m/z 3367 (defensin alpha-1-beta) (2), 3439 (defensin alpha-1) (2), 3485 (defensin alpha-3) (2), 10 096 (calcyclin) (7), 10 840 (S100A8) (2), 11 656 (calgizzarin) (7), and 7005 (histone doubly charged), 11 313, 11 354 (histone with one acetylation), 11 396 (histone with two acetylations), 13781 (histone H2B) (7). Among these proteins, defensins [alpha-1 (HNP-1), alpha-1 beta (HNP-2), and alpha-3 (HNP-3)] are of particular interest because they are potential bio-markers for RA (2). These molecules have two major functions in host defence: direct inhibition of pathogens and modulation of other innate and adaptive immune responses (19). Defensins have been found in neutrophils but also in a variety of leucocytes, including natural killer (NK) cells, T cells, B cells, and monocytes (20). By using IMS in the profiling mode, we could show defensin alpha-1-beta, defensin alpha-1, and defensin alpha-3 in the synovial sublining and lining layer with strong expression in RA but not in OA tissues (Figures 3B and 3C).
Among the potential biomarkers in our profiles, members of the S100 protein family such as S100A8 (myeloid-related protein 8, calgranulin A), S100A9 (myeloid-related protein 14, calgranulin B), and S100A12 (calgranulin C) have prompted particular interest because these biomarker candidates could differentiate patients with arthritides from controls with inflammatory bowel disease (13). Our results are in agreement with findings from previous studies where proteomic analyses identified S100A8, S100A9, and S100A12 in patients with arthritis (11–14), in serum (13), synovial fluid (11), or tissue (12), and have been correlated with many variables associated with disease activity in arthritides, including the number of swollen joints, the Ritchie index (21), the Disease Activity Score using 28 counts (DAS28) (13), C-reactive protein (CRP) concentration (13), erythrocyte sedimentation rate (21), and anti-cyclic citrullinated peptide (13). In accordance with these data, a peak at m/z 10840 (S100A8) has been shown in the synovial sublining and lining layer with strong expression in RA but not in OA tissue.
Further studies are required to apply this technique to formalin-fixed paraffin-embedded tissue and to introduce this method in routine clinical practice. Problems include development of fixatives or demasking procedures to ensure adequate flight characteristics of ions to obtain suitable mass spectra. Biopsy of synovial tissue during arthroscopy is a reliable, safe, and cost-effective technique that is a prerequisite for individual stratification of the disease process and subsequent personalized therapy (9).
In summary, IMS has been successfully applied to synovial tissue of patients with RA and OA, demonstrating the spatial distribution of known biomarker candidates such as defensins and S100 proteins, and the expression of potential biomarkers such as thymosins and other species that could potentially discriminate various disease subtypes, stages of the disease, or alterations in the course of therapy.
Acknowledgments
We thank J Allen for excellent technical assistance. This work received funding from the German Federal Ministry of Education and Research (BMBF, PtJ-Bio, 0313909), the National Institutes of Health (NIH/ NIGMS 5R01 GM58008), and a Vanderbilt Ingram Cancer Centre Core Support Grant (P30 CA68485).
References
- 1.van Baarsen LG, Wijbrandts CA, Timmer TC, van der Pouw Kraan TC, Tak PP, Verweij CL. Synovial tissue heterogeneity in rheumatoid arthritis in relationship to disease activity and biomarkers in peripheral blood. Arthritis Rheum. 2010;62:1602–7. doi: 10.1002/art.27415. [DOI] [PubMed] [Google Scholar]
- 2.Baillet A, Trocme C, Berthier S, Arlotto M, Grange L, Chenau J, et al. Synovial fluid proteomic fingerprint: S100A8, S100A9 and S100A12 proteins discriminate rheumatoid arthritis from other inflammatory joint diseases. Rheumatology (Oxford) 2010;49:671–82. doi: 10.1093/rheumatology/kep452. [DOI] [PubMed] [Google Scholar]
- 3.Seeley EH, Caprioli RM. Molecular imaging of proteins in tissues by mass spectrometry. Proc Natl Acad Sci USA. 2008;105:18126–31. doi: 10.1073/pnas.0801374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krenn V, Morawietz L, Burmester GR, Häupl T. Synovialitis score: histological grading system for chronic rheumatic and non-rheumatic synovialitis. Z Rheumatol. 2005;64:334–42. doi: 10.1007/s00393-005-0704-x. [DOI] [PubMed] [Google Scholar]
- 5.Altman RD. The classification of osteoarthritis. J Rheumatol Suppl. 1995;43:42–3. [PubMed] [Google Scholar]
- 6.Cornett DS, Mobley JA, Dias EC, Andersson M, Arteaga CL, Sanders ME, et al. A novel histology-directed strategy for MALDI-MS tissue profiling that improves throughput and cellular specificity in human breast cancer. Mol Cell Proteomics. 2006;5:1975–83. doi: 10.1074/mcp.M600119-MCP200. [DOI] [PubMed] [Google Scholar]
- 7.Hardesty WM, Kelley MC, Mi D, Low RL, Caprioli RM. Protein signatures for survival and recurrence in metastatic melanoma. J Proteomics. 2011;74:1002–14. doi: 10.1016/j.jprot.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caldwell RL, Gonzalez A, Oppenheimer SR, Schwartz HS, Caprioli RM. Molecular assessment of the tumor protein microenvironment using imaging mass spectrometry. Cancer Genomics Proteomics. 2006;3:279–88. [Google Scholar]
- 9.Gerlag DM, Tak PP. How to perform and analyse synovial biopsies. Best Pract Res Clin Rheumatol. 2009;23:221–32. doi: 10.1016/j.berh.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Kriegsmann J, Berndt A, Hansen T, Borsi L, Zardi L, Bräuer R, et al. Expression of fibronectin splice variants and oncofetal glycosylated fibronectin in the synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Rheumatol Int. 2004;24:25–33. doi: 10.1007/s00296-003-0316-1. [DOI] [PubMed] [Google Scholar]
- 11.Sinz A, Bantscheff M, Mikkat S, Ringel B, Drynda S, Kekow J, et al. Mass spectrometric proteome analyses of synovial fluids and plasmas from patients suffering from rheumatoid arthritis and comparison to reactive arthritis or osteoarthritis. Electrophoresis. 2002;23:3445–56. doi: 10.1002/1522-2683(200210)23:19<3445::AID-ELPS3445>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 12.Tilleman K, Van Beneden K, Dhondt A, Hoffman I, De Keyser F, Veys E, et al. Chronically inflamed synovium from spondyloarthropathy and rheumatoid arthritis investigated by protein expression profiling followed by tandem mass spectrometry. Proteomics. 2005;5:2247–57. doi: 10.1002/pmic.200401109. [DOI] [PubMed] [Google Scholar]
- 13.de Seny D, Fillet M, Ribbens C, Maree R, Meuwis MA, Lutteri L, et al. Monomeric calgranulins measured by SELDI-TOF mass spectrometry and calprotectin measured by ELISA as biomarkers in arthritis. Clin Chem. 2008;54:1066–75. doi: 10.1373/clinchem.2007.099549. [DOI] [PubMed] [Google Scholar]
- 14.Gibson DS, Finnegan S, Jordan G, Scaife C, Brockbank S, Curry J, et al. Stratification and monitoring of juvenile idiopathic arthritis patients by synovial proteome analysis. J Proteome Res. 2009;8:5601–9. doi: 10.1021/pr900680w. [DOI] [PubMed] [Google Scholar]
- 15.Wernicke D, Seyfert C, Gromnica-Ihle E, Stiehl P. The expression of collagenase 3 (MMP-13) mRNA in the synovial tissue is associated with histopathologic type II synovitis in rheumatoid arthritis. Autoimmunity. 2006;39:307–13. doi: 10.1080/08916930600807709. [DOI] [PubMed] [Google Scholar]
- 16.Zheng X, Wu SL, Hincapie M, Hancock WS. Study of the human plasma proteome of rheumatoid arthritis. J Chromatogr A. 2009;1216:3538–45. doi: 10.1016/j.chroma.2009.01.063. [DOI] [PubMed] [Google Scholar]
- 17.Paulussen M, Landuyt B, Schoofs L, Luyten W, Arckens L. Thymosin beta 4 mRNA and peptide expression in phagocytic cells of different mouse tissues. Peptides. 2009;30:1822–32. doi: 10.1016/j.peptides.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Nowak D, Popow-Wozniak A, Raznikiewicz L, Malicka-Blaszkiewicz M. Actin in the wound healing process. Polish Postepy Biochem. 2009;55:138–44. [PubMed] [Google Scholar]
- 19.Doss M, White MR, Tecle T, Hartshorn KL. Human defensins and LL-37 in mucosal immunity. J Leukoc Biol. 2010;87:79–92. doi: 10.1189/jlb.0609382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–93. [PubMed] [Google Scholar]
- 21.Kane D, Roth J, Frosch M, Vogl T, Bresnihan B, FitzGerald O. Increased perivascular synovial membrane expression of myeloid-related proteins in psoriatic arthritis. Arthritis Rheum. 2003;48:1676–85. doi: 10.1002/art.10988. [DOI] [PubMed] [Google Scholar]