Abstract
The newly excysted juvenile (NEJ) stage of the Fasciola hepatica lifecycle occurs just prior to invasion into the wall of the gut of the host, rendering it an important target for drug development. The cathepsin B enzymes from NEJ flukes have recently been demonstrated to be crucial to invasion and migration by the parasite. Here we characterize one of the cathepsin B enzymes (recombinant FhcatB1) from NEJ flukes. FhcatB1 has biochemical properties distinct from mammalian cathepsin B enzymes, with an atypical preference for Ile over Leu or Arg residues at the P2 substrate position and an inability to act as an exopeptidase. FhcatB1 was active across a broad pH range (optimal activity at pH 5.5–7.0) and resistant to inhibition by cystatin family inhibitors from sheep and humans, suggesting that this enzyme would be able to function in extracellular environments in its mammalian hosts. It appears, however, that the FhcatB1 protease functions largely as a digestive enzyme in the gut of the parasite, due to the localization of a specific, fluorescently labeled inhibitor with an Ile at the P2 position. Molecular modelling and dynamics were used to predict the basis for the unusual substrate specificity: a P2 Ile residue positions the substrate optimally for interaction with catalytic residues of the enzyme, and the enzyme lacks an occluding loop His residue crucial for exopeptidase activity. The unique features of the enzyme, particularly with regard to its specificity and likely importance to a vital stage of the parasite’s life cycle, make it an excellent target for therapeutic inhibitors or vaccination.
Keywords: cathepsin B, Fasciola hepatica, liver fluke, cysteine protease
Introduction
Parasitic Fasciola flatworms (liver flukes) colonise both animal and human hosts, causing the disease fasciolosis which has significant commercial effects on livestock, with over US$3 billion annually in lost agricultural productivity (Spithill et al., 1999; Torgerson and Claxton ,1999). Up to 2.4 million people are infected with liver flukes world-wide, with potentially 18 million undiagnosed and another 180 million people at risk of infection (Anon, 1995; Mas-Coma et al., 1999). In Bolivia, the prevalence of human fasciolosis is as high as 66–100% in certain areas (Mas-Coma et al., 1999). Typically, infected humans and agricultural animals are treated with triclabendazole; however, resistance of parasites toward triclabendazole has been identified (Brennan et al., 2007; Mitchell et al., 1998; Overend and Bowen, 1995; Thomas et al., 2000).
During the life cycle of Fasciola species, cercariae emerge from the intermediate mollusc host, attach to and encyst on semi aquatic surfaces such as plants, forming metacercariae. The infection in humans and animals arises from ingestion of vegetable matter or consumption of water contaminated with metacercariae (Aronja et al., 1995; Hughes et al., 2003), whereupon newly excysted juvenile (NEJ) flukes excyst in the small intestine below the bile duct opening (Dixon, 1966). The NEJ parasites migrate through the duodenal wall, across the peritoneal cavity and through the liver to reach the bile ducts after about 8 weeks, where they continue to grow and develop into adults, reaching full size by 12–14 weeks after infection (Boray, 1969). During in vivo migration and cultivation in vitro, the contents of the lumen of the fluke gut are regularly purged into the parasite’s surroundings, the regurgitants forming part of the constituents of the excretory/secretory products (ESP) of the parasite. The ESP of NEJ and adult F. hepatica flukes has generally been implicated in the acquisition of nutrients, immune evasion and migration (Berasain et al., 1997; Berasain et al., 2000; Chapman and Mitchell, 1982; Dalton et al., 2003; Dowd et al., 1995; Prowse et al., 2002; Smith et al., 1993; Wilson et al., 1998).
Fasciola parasites produce both cathepsin B and L proteases (Cancela et al., 2008; Grams et al., 2001; Harmsen et al., 2004; Meemon et al., 2004; Tkalcevic et al., 1995; Wilson et al., 1998). For up to 5 weeks following excystment, juvenile flukes express at least three cathepsin B proteases, with FhcatB1 (Tkalcevic et al., 1995; Wilson et al., 1998; also recently reported as FhCB2 [Cancela et al., 2008]) and FhCB1 and FhCB3 identified as the major protein components in the ESP of F. hepatica. Cathepsin L-like proteases have also been detected in NEJ flukes (Cancela et al., 2008; Harmsen et al., 2004; Tkalcevic et al., 1995). The fundamental importance of cysteine proteases in the normal functioning of parasites is highlighted by a reduction in Schistosoma mansoni worm burdens in rodent models following treatment with cysteine protease inhibitors (Abdulla et al., 2007) and examples where parasite infections in vivo were eliminated using cysteine protease inhibitors (Engel et al., 1998; Rosenthal et al., 1993; Troeberg et al., 1996). Parasite viability in culture has also been significantly reduced using inhibitors of cysteine proteases (Harth et al., 1993; Mahmoudzadeh-Niknam and McKerrow, 2004; Selzer et al., 1999; Wasilewski et al., 1996). Direct evidence for the importance of cysteine proteases in liver fluke biology is suggested by several observations. A recent study on silencing of F. hepatica FhCatB1 and FhCatL1 by RNA interference in NEJ F. hepatica revealed a significant reduction in gut penetration by this parasite stage in vitro following knock down of the expression of each enzyme (McGonigle et al., 2008). The vaccine potential of juvenile cathepsin B was shown in rats where vaccination with FhCatB1 induced significant reductions in worm burdens, liver damage and parasite mass (Jayaraj et al., 2009). In addition, vaccination studies with cathepsin L proteases of F. hepatica have shown protection against challenge in rodents and ruminants (Dalton et al., 1996; Dalton et al., 2003; Harmsen et al., 2004; Jayaraj et al., 2009; Kesik et al., 2007; Piacenza et al., 1999; Wedrychowicz et al., 2007; Wijffels et al., 1994;).
We have undertaken the first detailed substrate analysis of a NEJ cathepsin B enzyme from Fasciola spp. and our results indicate the importance of cathepsin B enzymes for the viability of NEJ F. hepatica in vitro using an inhibitor that is likely to be cathepsin B-specific. Using a combinatorial peptide inhibitor library, a recombinant version of FhcatB1 was demonstrated to have an unusual specificity for substrates with characteristics commensurate with a broad digestive role in the parasite. Based on the enzyme specificity, a specific fluorescent inhibitor was synthesized, and the localization of the active enzyme and the targets of the cathepsin B inhibitor were characterized in NEJ flukes. The results suggest that FhcatB1 is active in the gut of the parasite and has P2 amino acid preferences with exploitable differences compared to mammalian cathepsin B enzymes.
Materials and Methods
Materials
F. hepatica metacercariae were propagated and harvested from the snail species Lymnaea tormentosa by Dr. Gareth Hutchinson, Elizabeth Macarthur Agricultural Institute, NSW Agriculture, Menangle, New South Wales, Australia. CA-074, CA 074Me, E-64-c, E-64-d were purchased from the Peptide Institute, Inc. (Osaka, Japan). Nickel-NTA agarose was obtained from Qiagen (Melbourne, Australia). Peptide substrates with a 7-amino-4-methylcoumarin leaving group (AMC), E-64 and all other products were purchased from Sigma-Aldrich (Sydney, Australia). Substrate and inhibitor stocks were prepared in dimethyl sulfoxide (DMSO) and stored in aliquots at −20°C. Human cathepsin L was a kind gift from Weiwen Dai, Department of Biochemistry and Molecular Biology, Monash University. The peptide substrates Abz Phe-Arg-Ala-Lys(Dnp)-OH (Exo-OH), Abz-Ala-Pro-Arg-Ala-Ala-Gln-Lys(Dnp)-OH (Endo) and Abz-Phe-Arg-Ala-Lys(Dnp)-NH2 (Exo-NH2) were synthesized at greater than 90% purity by Auspep (Parkville, Australia). Recombinant human cystatins A, B and C were produced as described previously (Jerala et al. 1988, 1994; Cimerman et al. 1999). Sheep cystatin B was purified from liver as described previously (Pike et al. 1992). Enzymes used for profiling of protease specificity were obtained as follows. Recombinant human cathepsins K, S, F and V were kind gifts from Prof Dieter Brömme (University of British Columbia, Vancouver, Canada) and recombinant human cathepsin L was a kind gift of Prof Vito Turk (Josef Stefan Institute, Ljubljana, Slovenia). Cathepsins C and B and papain were obtained from Sigma (St. Louis, MO., USA). Human calpains I and II and cathepsin H were from EMD Biosciences (San Diego, CA., USA). Recombinant cruzain was a kind gift of Prof James McKerrow (University of California, San Francisco, USA) and recombinant falcipain-2 was a kind gift from Dr Phillip Rosenthal (University of California, San Francisco, USA).
Production of recombinant enzymes
Recombinant proFhcatB1 was produced, purified and processed essentially as previously described (Beckham et al., 2006), except that most experiments were performed with enzyme which was of mixed glycosylation as essentially no differences in behaviour between the mixture of forms compared to a defined, 36 kDa form used previously (Beckham et al., 2006) could be discerned. Briefly, recombinant proFhcatB1 was produced in Pichia pastoris and purified via a carboxy-terminal polyhistidine tag on nickel immobilized metal affinity chromatography (IMAC). The recombinant proFhcatB1 eluted from the resin was auto-processed at a concentration of 333 nM at 37°C for 1 h in 100 mM sodium acetate, pH 4.5, 100 mM NaCl, 5 mM EDTA, 10 mM DTT, 50 µg/ml dextran sulfate (500K). The enzyme was diluted 30 fold in 25 mM NaH2PO4.H20, 250 mM NaCl, 10 mM imidazole, pH 7.6, and applied to nickel-nitrilotriacetate (Ni-NTA) washed with the same buffer and the bound protein eluted with 25 mM NaH2PO4, 250 mM NaCl, 100 mM imidazole, pH 7.6. Activity against Z-Phe-Arg-AMC was used to detect FhcatB1 in the eluant. Typically, following autoprocessing and nickel IMAC steps, the active site concentration of the preparation was 40 nM, and the enzyme could be kept on ice for up to 6 hours, without activity being compromised. All kinetic parameters were determined within this time. Human wild-type and H110A cathepsin B glycerol stocks were kindly donated by Dr. John S. Mort (Shriners Hospital for Children, Montreal, Canada). Human wild-type and H110A cathepsin B were expressed in P. pastoris and purified using a method modified from Nägler et al., 1997. The human wild-type and H110A cathepsin B both carried a S115A mutation, eliminating the O-linked glycosylation site, which does not affect the enzymatic activity (Hasnain et al., 1992; Mach et al., 1992). H110A will be the only mutant referred to in the text, while human cathepsin B carrying only the S115A mutation will be referred to as wild type. The P. pastoris cultures were grown, with appropriate methanol induction, for 4 days at 28°C with shaking on a rotary shaker. The supernatant was collected after twice centrifuging at 3000xg for 15 min, concentrated and buffer-exchanged at 4°C into 100 mM sodium acetate, pH 5.0 to allow autoprocessing of human wild-type cathepsin B to occur. The processing of human cathepsin B carrying the H110A mutation was catalyzed with 50 U/ml of pepsin immobilized on agarose beads for 1.5 h at room temperature in 100 mM sodium acetate, pH 4.0. The processed material was purified by ion exchange on a Mono S HR 5/5 cation exchange column, with elution of bound proteins effected using a linear gradient of 0 1 M NaCl in 100 mM sodium acetate, pH 5.0, over 60 ml at a flow rate of 1 ml/min. Cathepsin B was eluted between 400–500 mM NaCl and stored in aliquots at 80°C. FhcatL5 was expressed and purified as previously described (Smooker et al., 2000).
Determination of kinetic constants for cleavage of fluorogenic peptide substrates
Active site molarities of all proteases were estimated by titration with the irreversible cysteine protease inhibitor, E-64 essentially as previously described (Barrett et al., 1982). Assay buffers were pre-warmed prior to enzymatic assays being performed in a 96 or 384 well microtitre plate and the release of the fluorogenic leaving group, AMC, was measured continuously at 37°C at 30 sec intervals for 20 min on a fluorometric microtitre plate reader (Fluostar Galaxy, BMG LabTechnologies). Excitation and emission wavelengths were 370 and 460 nm, respectively. Arbitrary fluorescence units (AFU) were converted to an amount of product using an AMC standard curve. Each experiment was performed in at least duplicate. Initial velocities were determined from the linear portion of the release of fluorescence. Values for initial velocity were plotted against substrate concentration and fitted by non linear regression to the equation below to allow determination of Km and Vmax values using Graphpad Prism 3.
All values determined were derived from linear increases in fluorescence intensities and all errors were below 10%.
Scanning of the S2, S3, and S4 subsites of FhcatB1 with combinatorial libraries
FhcatB1 was autoprocessed and subjected to screening against a positional scanning combinatorial library (PSL). The PSL is composed of peptide sequences spanning the S2, S3 and S4 subsites coupled to an epoxide electrophile warhead previously described (Greenbaum et al., 2000). Briefly, for each library, one of the positions (P2, P3 or P4) was held constant as a single natural amino acid (excluding cysteine and methionine and including norleucine), while the remaining positions were mixtures of the natural amino acids. Autoprocessed FhCatB1 (in the presence of the pro-peptide) was pre-incubated with the libraries at a concentration of 1 µM for 30 min at room temperature followed by labeling of residual active sites with the general cysteine protease probe, I125 DCG-04 for 1 h (Bogyo et al., 2000). The samples were separated by SDS-PAGE and exposed to PhosphorImage screens (Molecular Dynamics). Band intensities were quantified against a background control using the freeware program, NIH Image. The percentage of residual enzyme activity was calculated by dividing the intensity of the inhibitor treated samples by the intensity of the DMSO-treated control sample. An average of two runs for each library was performed to obtain an average percent residual activity for each library member. Percent residual activity values were compared using the hierarchical clustering programs, Cluster and Treeview.
pH-dependent activity and stability of FhcatB1
To measure the pH dependence of the protease activity against a protein substrate, 50 µg/ml of FITC-casein was incubated with 15 nM FhcatB1 in AMT buffer (100 mM acetate, 100 mM MES, 200 mM Tris, 4 mM EDTA [Ellis and Morrison, 1982]), containing 200 mM NaCl and 10 mM DTT at different pH values (4.0–8.0) for 2 h at 37°C. 60 µl of 5% (w/v) TCA was added to 10 µl of the digest and incubated at room temperature for 5 min. The precipitate was collected by centrifugation at 10,000 × g for 10 min at room temperature. 10 µl of the supernatant was removed and added to a 96 well plate containing 190 µl of AMT buffer, pH 8.5. The fluorescence was read at excitation and emission wavelengths of 485 and 520 nm respectively, on a Polarstar plate reader (BMG Lab Technologies, Melbourne, Australia). Controls containing FITC-casein alone were subjected to the same conditions and the values obtained from the fluorescence measurements for the controls were subtracted from the digested samples.
To measure the stability of FhcatB1, the enzyme (20 nM) was autoprocessed and incubated at 37°C in 20 µl AMT buffer of different pH values (4.0–8.0) containing 200 mM NaCl and 10 mM DTT. FhcatB1 was incubated for 0, 5, 10, 20, 40, 60, 90 and 120 min at different pH values and assayed simultaneously for residual activity by the addition of 180 µl of 10 µM Z Phe Arg AMC in FhcatB1 assay buffer, pH 4.5. The final pH of the assay buffer was unaffected by the pH of the AMT buffers used for the initial incubation. The natural log of the residual activity (AFU/minute) was plotted against time (min) and the slope of the linear regression used to calculate the half life (t½) with the following equations (Dennison et al., 1992):
Activity of cathepsin B enzymes against exopeptidase substrates
The activity of FhcatB1, human wild-type cathepsin B and H110A cathepsin B against the substrates used to assess exopeptidase activity (20 µM), was performed in 50 mM NaH2PO4, 200 mM NaCl and 5 mM EDTA, 1 mM DTT, pH 6.0. Since the substrate specificity of the prime subsites of FhcatB1 was not known, the exopeptidase substrates were based on those used for human cathepsin B. These were Abz Phe-Arg-Ala-Lys(Dnp)-OH (called Exo-OH), Abz-Ala-Pro-Arg-Ala-Ala-Gln-Lys(Dnp)-OH (called Endo) and Abz-Phe-Arg-Ala-Lys(Dnp)-NH2 (called Exo-NH2) (Krupa et al., 2002). The substrates were dissolved in 100% dimethyl formamide (DMF) and concentrations determined by the absorbance at 360 nm using an extinction co-efficient of 104 M−1.cm−1. FhcatB1 (15 nM for Endo substrate and 75 nM for Exo substrates) and human wild-type and H110A cathepsin B (both at 0.1 nM) were activated in the above buffer and the activity was determined by recording release of fluorescence at 30 sec intervals for 20 min at excitation and emission wavelengths of 320 and 420 nm, respectively.
Determination of kinetic constants for interaction between cathepsins and cystatins
Cystatins and enzymes were incubated at room temperature for 5 min prior to the addition of Z-Phe-Arg-AMC (final concentration of 10 µM for human cathepsin L, FhcatL5 and FhcatB1 and 20 µM for human cathepsin B). The final concentrations of human cathepsin L, FhcatL5, human cathepsin B and FhcatB1 used were 0.05 nM, 1 nM, 0.05 nM and 1 nM, respectively. The concentrations of cystatins were varied according to the target protease: 0–20nM cystatins against human cathepsin L; 0–50nM against FhCatL5; 0 1000nM against FhCatB1 and human cathepsin B. The Ki values were calculated as previously described (Bieth, 1980).
Determination of kass values for cysteine protease inhibitors
The kass was determined using a discontinuous method as described previously (Bieth, 1984). The proteases (1 nM for FhcatL5 and 5 nM for FhcatB1) were pre-incubated over a period of 10 minutes at increasing inhibitor concentrations (in the range 20–400 nM) and then assayed for residual activity against Z-Phe-Arg-AMC in assay buffer 100 mM sodium acetate, 1.5 mM EDTA, 0.1% (w/v) Brij 35, 1 mM DTT and 0.02% (w/v) sodium azide, pH 5.5. The natural log of the activity was plotted against the pre-incubation time: the slope from these plots equals the observed rate of interaction, kobs. The kobs values were then plotted against the inhibitor concentration, giving a plot with a slope equal to the kass.
Viability of NEJ flukes in the presence of cysteine protease inhibitors
Parasites were excysted as described (Piedrafita et al., 2001). Excysted NEJ parasites were dispensed into the wells of a 96 well tissue culture plate in a final volume of 180 µl RPMI, 10% (v/v) heat inactivated fetal calf serum, 100 U/ml penicillin and 100 µg/ml streptomycin. Inhibitors were added to a final concentration of 50 µM in 0.5% (v/v) DMSO. The controls contained 0.5% (v/v) DMSO without inhibitor. Each well contained 4 parasites and experiments were performed in triplicate for each inhibitor, with 6 wells for the controls. The parasite numbers are presented as the total numbers for the 3 wells. The effect of the inhibitors on the NEJ flukes was determined by microscopic examination and larval killing in vitro was defined as loss of parasite motility, loss of gut peristalsis, grainy internal structures and obvious gross tegumental damage, as previously standardized (Piedrafita et al., 2000; Piedrafita et al., 2001). Conversely, viable parasites in vitro were defined as highly motile with gut peristalsis, clear internal structures and no visible tegumental damage.
Synthesis of the fluorescent inhibitor, TMR-I
The tetramethylrhodamine labeled inhibitor probe (TMR-I), Lys(TMR)-aminohexanoate-Tyr-Ile-epoxysuccinate ethyl ester, was synthesized on a Rink-type resin using Fmoc-based solid phase peptide synthesis essentially as described previously (Greenbaum et al., 2000). Briefly, the resin was elongated using Fmoc-Lys(Boc)-OH, Fmoc-aminohexanoic acid, Fmoc-Tyr(But)-OH, Fmoc-Ile-OH and capped in the last step with the epoxide reactive group. After TFA-mediated cleavage from the solid support and purification, the ε-amine of the lysine was coupled to tetramethylrhodamine succinimide ester. The final product was purified using HPLC and lyophilized before making 10 mM stock solutions in DMSO.
Confocal microscopy of fluorescently labeled inhibitor binding to NEJ flukes
The optimal concentration for confocal images was determined by serially diluting TMR-I from 50 µM to 0.019 nM and incubating with NEJ for 24 h. A concentration of 6.25 µM was found to be optimal; this concentration of CA074Me was also found to be a sub-lethal concentration for NEJ when exposed for 8 days. Consequently, 40 NEJ parasites were either pre-incubated with or without 6.25 µM of CA074Me for up to 7 days followed by incubation with 6.25 µM of TMR-I for 24 h. After incubation, NEJ were washed, fixed with 1% (v/v) paraformaldehyde for 15 min at room temperature and stained with 5 µg/ml DAPI dilactate. NEJ were washed and pipetted onto slides, embedded in Fluorosave mounting medium (Calbiochem, San Diego, CA, U.S.A). Images were collected as a Z-series (0.5 µm step) as individual optical sections by scanning confocal microscopy (LSM 510 Meta). LSM image browser software was used to determine the range of median pixel intensities. Final images from both bright field and fluorescence channels were overlaid. Between 30 and 40 images of individual NEJs were examined for each different treatment to determine a representative view of the distribution of the TMR-I.
Homology modeling and molecular dynamics simulations of FhcatB1 interaction with peptide substrates
Human (pdbid:1CSB), rat (1CPJ) and bovine (1QDQ) cathepsin B structures, in which the occluding loop is in the ‘locked’ conformation, were structurally aligned with MUSTANG (Konagurthu et al., 2006) and used as templates for homology modeling of FhCatB1 (49–51% identity, 4 indels) in MODELLER9v4 (Eswar et al., 2006). Two hundred models were generated with the default ‘automodel’ minimization-simulated annealing protocol, with a gaussian 3 Å distance restraint to maintain a predicted salt bridge between Asp112 and Arg118 of the occluding loop; the models were combined and averaged using MODELLER’s clustering algorithm (cluster_cut=1.5 Å). COOT (Emsley and Cowtan, 2004) was used to confirm that all residues were in allowed regions of the Ramachandran plot, with no unpaired buried charged side-chains. An initial model for a tripeptide substrate docked into the active site, Val-Ile-Arg-AMC, was generated manually based on several inhibitor-bound structures (1GMY, 1BGO and 1KHQ). Conjugate-gradients refinement (CGR) and Langevin molecular dynamics (MD) of the peptide-bound structure was then undertaken using NAMD (Phillips et al., 2005) with the geometry of the molecule maintained by applying a 100 kJ mol−1 harmonic positional restraint to the Cα atoms of the protease (but not the ligand): 1. The model was solvated in a water box of dimensions 65 × 77 × 75 Å. 2. Initially, lateral alignment of the ligand within the active site was enforced by introducing a covalent link between the backbone carbon of P1Arg and the thiol group of Cys29. Simulated annealing was performed by ramping the temperature from 300 K to 1000 K with 100 K per 0.5 ps MD (500 steps), and ramping down to 300 K, followed by 2000-step CGR. 3. The covalent link was removed, 2000-step CGR undertaken, followed by conformational sampling using MD at 300 K for 0.1 ns (100000 steps). 4. The time-averaged structure over the course of the MD was calculated, and subjected to CGR for 2000 steps. 5. Steps 2–4 were repeated for the substrates Val-(Val/Leu/Phe)-Arg-AMC with the homology model of FhcatB1 and the experimental structure of human catB (1CSB). All simulations were performed on the Monash Sun Grid. Visualisation was performed with PyMOL (De Lano Scientific, USA).
Results
An inhibitor selective for F. hepatica cathepsin B enzymes reduces NEJ fluke viability
A number of cysteine protease inhibitors were evaluated for their ability to selectively inhibit one of the two classes of cathepsin enzymes produced by NEJ flukes, using FhcatB1 (a juvenile fluke protease [CB2 of Cancela et al., 2008; Wilson et al., 1998]) and FhcatL5, a cathepsin L protease identified in adult F. hepatica (Smooker et al., 2000), as representative proteases. CA-074 demonstrated the greatest amount of selectivity for FhcatB1 under these conditions, with a 175-fold higher kass value seen with this enzyme over FhcatL5 (Table 1).
Table 1.
Rates of association of cysteine protease inhibitors with FhcatB1 and FhcatL5.
| Inhibitor | FhcatB1 kass (M−1.s−1) |
FhcatL5 kass (M−1.s−1) |
|---|---|---|
| E-64 | 6070.7 ± 412.9 | 14950.0 ± 779.7 |
| E-64-c | 5247 ± 228.8 | 4613.3 ± 183.3 |
| E-64-d | No inhibition | 83.8 ± 1.8 |
| CA-074 | 1940.2 ± 21.9 | 11.1 ± 1.1 |
| CA-074Me | 87.2 ± 4.3 | 143.5 ± 5.8 |
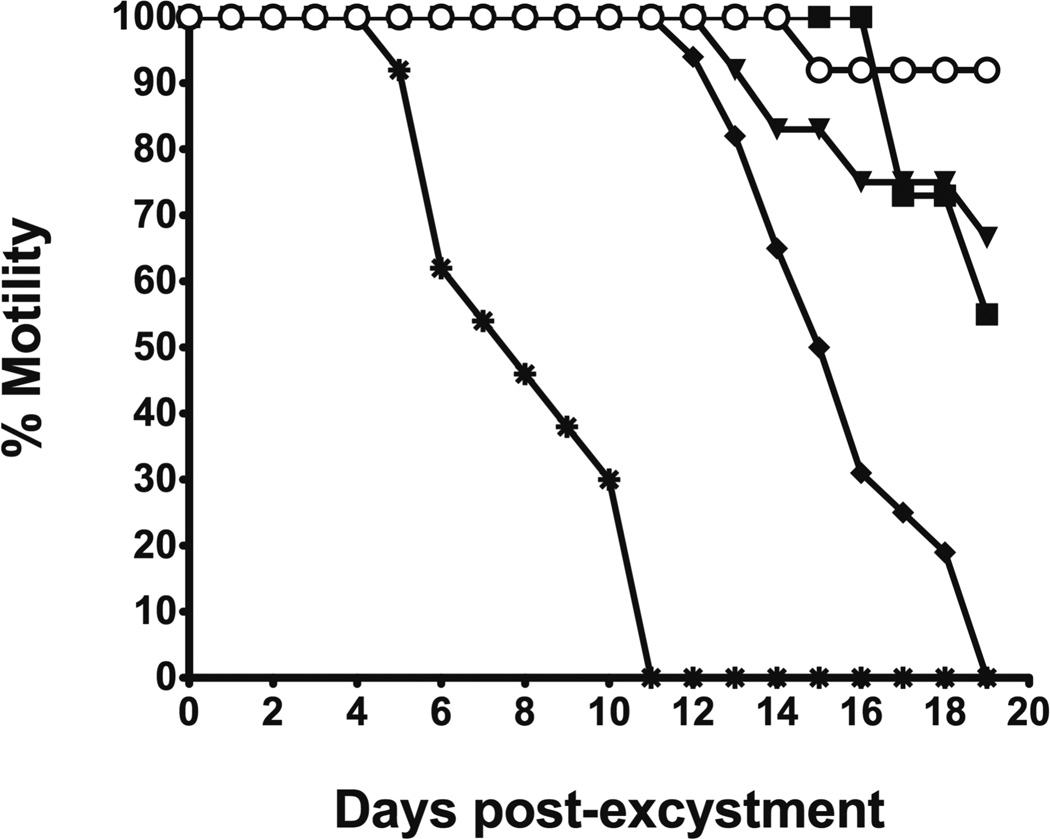
In order to establish if cysteine proteases play a critical role in the viability of NEJ flukes following excystment, the NEJ flukes were maintained in culture in the presence of 50 µM of each inhibitor and examined daily by microscopy for viability, using motility, gut peristalsis, internal structure and tegument damage as indicators of parasite survival. Two separate experiments were performed with different batches of metacercariae and both experiments showed almost identical results. One set of data is shown here using motility as an indicator. Reductions in motility were accompanied by reduced gut peristalsis and damage to both internal structures and the tegument of the NEJ parasites, indicating that motility was most likely a valid guide to the viability of the organisms in culture. A reduction in NEJ fluke viability was observed within 6 days in the presence of the membrane permeable cathepsin B pro inhibitor, CA 074Me (Fig. 1), with the apparent viability completely abrogated after 11 days of culture. CA-074Me is metabolised to CA-074 in cells by esterases (Buttle et al., 1992). Interestingly, only a 45% reduction in viability was seen in the presence of the non-cell permeable inhibitor, CA-074, after 19 days in culture and effects started to appear only after 16 days. The only other inhibitor which completely abrogated the viability of the parasite in culture was the cell permeable form of E-64-c, E-64d: this inhibitor, which affects FhcatL5 but not FhcatB1 (Table 1), started to have effects after 12 days and complete loss of viability was found after 19 days in culture. After 19 days in culture, only a 10% reduction in viability was observed when the NEJ flukes were incubated with the general cysteine protease inhibitor, E 64, while E-64-c only produced a 33% loss in viability.
Fig. 1. Viability of F. hepatica NEJ flukes in the presence of cysteine protease inhibitors.
Following excystment, NEJ flukes were maintained in culture in the presence of 50 µM cysteine protease inhibitors. The NEJ flukes were examined and scored daily for loss of motility. ○, E-64; ▼, E-64-c; ◆ E-64-d; ■, CA-074; ✳, CA-074Me. Parasites incubated in DMSO alone displayed no loss of motility (viability) over the assay period.
FhcatB1 has different substrate specificity to mammalian cathepsin B enzymes
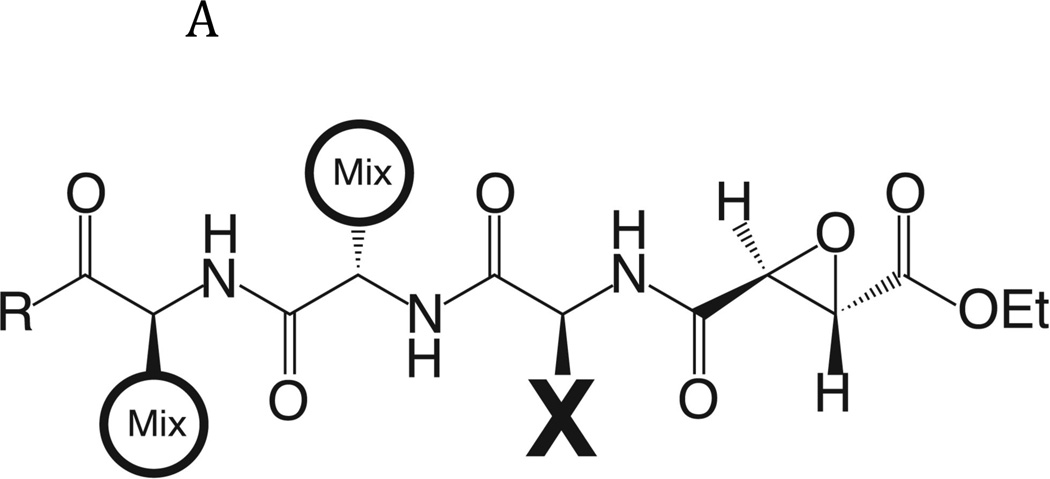
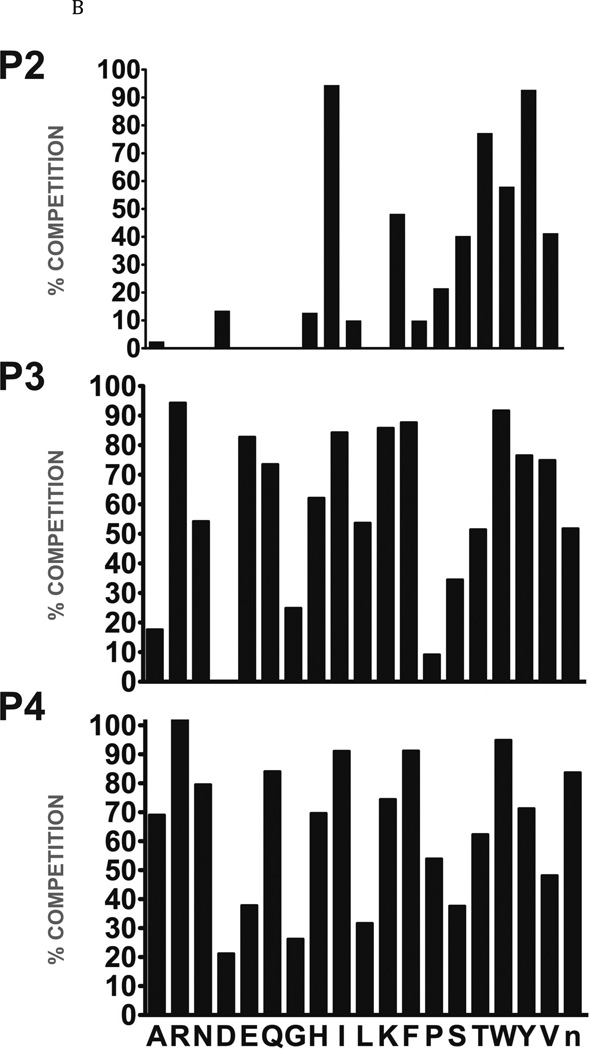
In view of the cytotoxic effect on NEJ of the cathepsin B pro-inhibitor, CA074Me, we decided to further evaluate the specificity of this protease using a positional scanning library of peptide epoxides that has been previously used to profile a range of papain family cysteine proteases (Greenbaum et al., 2000). This tripeptide epoxide library is based on the natural product E-64 scaffold, but contains a single fixed position and two mixed positions (Fig. 2A). By scanning the fixed position through all possible amino acids it is possible to generate overall specificity profiles for a given protease for each of the P2, P3 and P4 positions. To generate this data, purified enzyme was pre-treated with each fixed sub-library and overall covalent binding assessed by measuring residual protease activity using a radiolabeled probe (see methods). Overall, the S2 subsite showed the strictest preferences, with Ile=Val>Trp>Tyr>Phe residues being most preferred at the P2 position (Fig. 2B). Broader substrate preferences were observed at the P3 position, with the least favoured residues being Asp, Pro, Ala and Gly. The P4 position has broader specificity still, with all amino acid classes accepted.
Fig. 2. Determination of the specificity of FhcatB1 using a combinatorial peptide inhibitor library.
A: Inhibitor scaffold used in libraries utilized to determine the specificity of FhcatB1. The scaffold encompasses a fixed position (indicated by ‘X’) which can be changed to each natural amino acid and norleucine, and two mixed positions (indicated by ‘mix’). B: The substrate preferences of FhcatB1 for the P2, P3 and P4 positions were determined using a combinatorial peptide inhibitor library and are displayed as the percentage of competition for each amino acid substituted at the indicated position. ‘n’ represents norleucine (a substitute for methionine). C: Data from positional scanning of the P2 position of FhcatB1 compared with other papain family proteases. Substrate preferences of the S2 subsite of papain family cysteine proteases were determined using combinatorial libraries. The preferences are presented in red for substrates with greater competition, or blue for those with little or no competition, with 50% competition in white. Amino acids, represented as single letters, are above the chart. The enzymes shown other than FhcatB1 are: CalpII, calpain II purified from porcine kidney (EMD Bioscience); CatH, cathepsin H purified from human liver (EMD Bioscience); CalpI, calpain I purified from porcine kidney (EMD Bioscience); CatC, cathepsin C purified from bovine spleen (Sigma); CatB, cathepsin B purified from bovine spleen (Sigma); CatF, recombinant cathepsin F; Papain, purified from Carica papaya (Sigma); CatS, recombinant human cathepsin S; Cruzain, cruzipain from Trypanosoma cruzi; CatL, recombinant human cathepsin L; rFalcipain 2, recombinant falcipain-2 from Plasmodium falciparum; CatK, recombinant human cathepsin K.
The percentage of competition with a radiolabelled inhibitor, presented in Figure 2B, was compared with human cathepsin B and other papain-like cysteine proteases (Fig. 2C). This analysis reveals that FhcatB1 is very different from mammalian cathepsin B with regard to the low preference for both leucine and arginine residues at the P2 position of substrates. In order to validate the results from the specificity scan, the kinetics of cleavage for a number of peptide substrates were examined for FhcatB1 in comparison to human cathepsin B. As may be seen in Table 2, FhcatB1 exhibited the highest activity against Z-Val-Ile-Arg-AMC, with a kcat/Km value approximately 222-fold higher for this substrate compared to an equivalent substrate with a Leu residue at the P2 position (D-Val-Leu-Arg-AMC). It is interesting to note that the human and parasite enzymes also differed markedly in their relative ability to cleave Z-Phe-Arg-AMC and Z-Arg-Arg-AMC. The latter substrate is often used as a diagnostic for cathepsin B-like enzymes and it is apparent that FhcatB1 was far less active against it than the human counterpart.
Table 2. Kinetics of cleavage of peptide substrates by FhcatB1 and human cathepsin B.
The individual kcat and Km values were determined from Michaelis-Menten plots against di- and tri-peptide fluorogenic substrates.
| Substrate | FhcatB1 | Human CatB | ||||
|---|---|---|---|---|---|---|
|
kcat (s−1) |
Km (mM) |
kcat/Km (M−1.s−1) |
kcat (s−1) |
Km (mM) |
kcat/Km (M−1.s−1) |
|
| Z-Phe-Arg-AMC | 0.19* | 0.19* | 1048.1 | 1.71 | 0.047 | 36587.16 |
| Z-Arg-Arg-AMC | 0.16 | 1.22 | 129.3 | 1.09 | 0.10 | 10536.39 |
| Tos-Gly-Pro-Arg-AMC | 0.13 | 0.06* | 2227.1 | 2.26 | 0.88 | 2560.32 |
| Boc-Val-Pro-Arg-AMC | 0.72 | 0.53 | 1348.3 | ND | ND | ND |
| Boc-Asp-Pro-Arg-AMC | 0.69 | 0.35 | 1976.3 | 0.28* | 1.16* | 244.35 |
| D-Val-Leu-Arg-AMC | 0.14* | 4.53* | 32 | ND | ND | ND |
| Boc-Val-Leu-Lys-AMC | -a | -a | - | 3.38 | 1.67* | 2020.32 |
| Z-Val-Ile-Arg-AMC | 0.51 | 0.07 | 7104 | ND | ND | ND |
Indicates above 10% Error
ND Not determined
denotes below detection limit
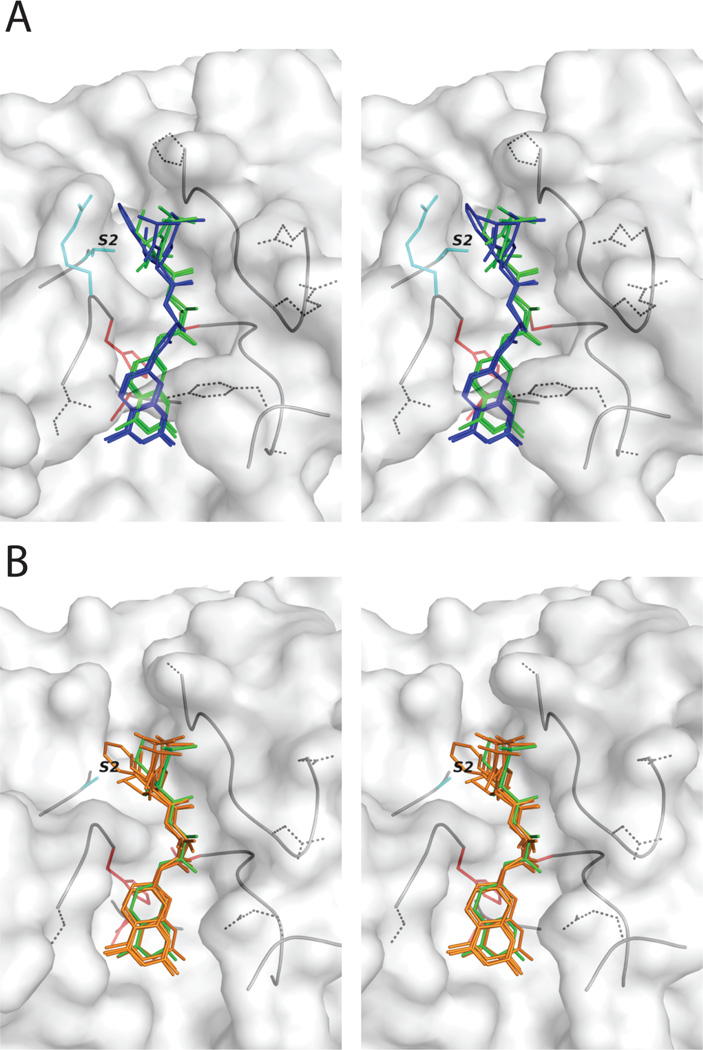
A homology model of FhCatB1 was built using the spatial restraints approach of MODELLER (Eswar et al., 2006) based on structures of mature mammalian cathepsin B structures, two of which were in complex with small molecular weight inhibitors. The model is expected to have a backbone conformation close to that of the true structure due to high sequence identity (~50%) between the model and templates. Energy minimization and molecular dynamics in NAMD (Phillips et al., 2005) were used to predict the mode of binding of tripeptide substrates with differing P2 residues. These simulations suggested a subtle but distinct lateral translation towards the S’ subsites of FhcatB1 for poorer substrates (P2 Phe/Leu) with respect to better substrates (P2 Val/Ile), with a concomitant shift of the P1 carbonyl atom away from the active site cysteine residue (Fig. 3A). This is likely to be the result of an Ala171->Thr174 substitution which partially occludes the S2 pocket, combined with a constriction of the wall of the S2 groove due to a predicted polar interaction between Glu243 (Glu246 in huCatB) and Arg199 (Gly196 in huCatB). The broader S2 subsite of the human homologue results in similar positioning of the peptide chain of each ligand, irrespective of the P2 residue (Val/Ile/Phe/Leu), to that seen with Val/Ile in FhCatB1 (Fig. 3B).
Fig. 3. Homology modeling of the binding of FhcatB1 showing binding of substrates to the active site in comparison to human cathepsin B.
A: A homology model of FhcatB1 showing the predicted positions of tripeptide substrates in the active site. The substrates shown have the general form of Val-P2-Arg-7-amino-4-methylcoumarin, where P2=Val/Ile (green stick) and P2=Leu/Phe (blue stick). The side-chains of the P3 and P1 positions have been omitted for clarity. Residues of the catalytic triad appear in red, the two residues that flank the S2 subsite in FhCatB1 are in cyan, and dotted side-chains indicate substitutions in FhCatB1 with respect to the human enzyme. B: The same procedure was applied, based on the crystal structure of human cathepsin B. All four tripeptide substrates, P2=Ile/Val/Leu/Phe (orange stick), adopt a similar position to P2=Ile in FhCatB1 (shown as green stick for comparison). Stereo figures were prepared using PyMOL (De Lano Scientific).
Characterisation of FhcatB1 enzyme activity
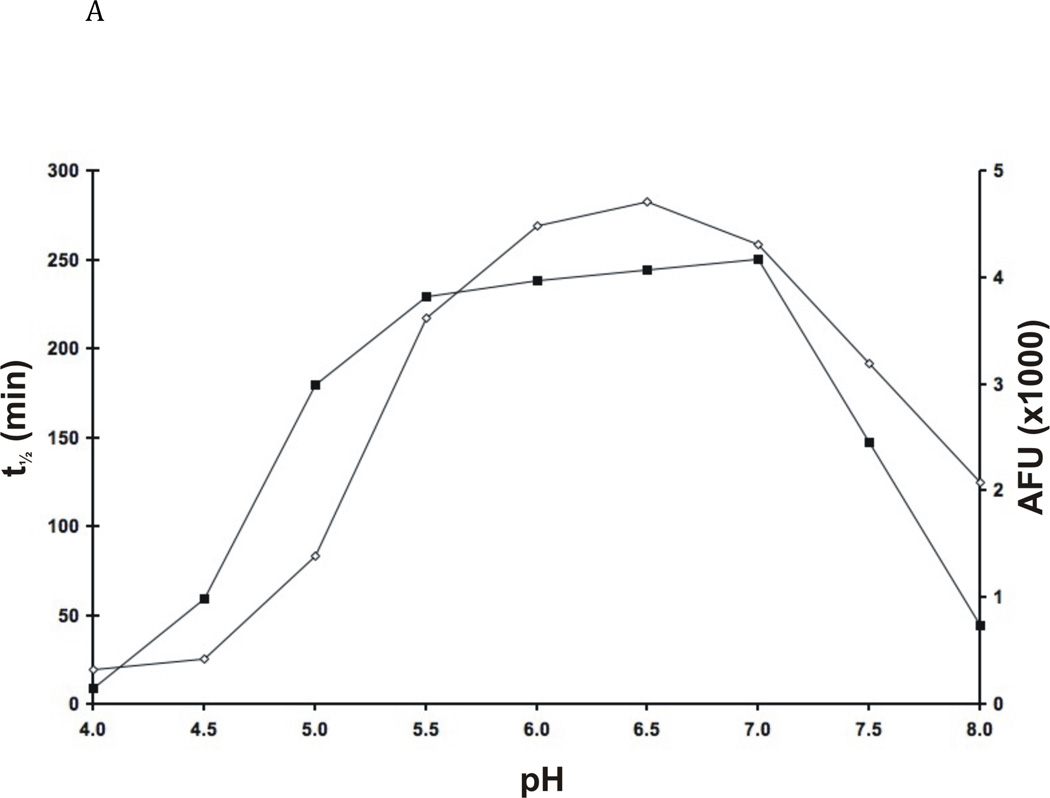
The pH-dependent behaviour of FhcatB1 was characterised in terms of the stability and activity of the enzyme against peptide and protein substrates. The enzyme was stable from pH 5.5–7.0 (half life in assay >200 min) and had moderate stability at pH 4.5 (Fig. 4A). The enzyme was maximally active in the range pH 5.5–7.0, with activity dropping by approximately 30–80% at pH 5.0 and 8.0 (Fig. 4A). These results were seen with both peptide and protein substrates.
Fig. 4. Enzymatic properties of FhcatB1.
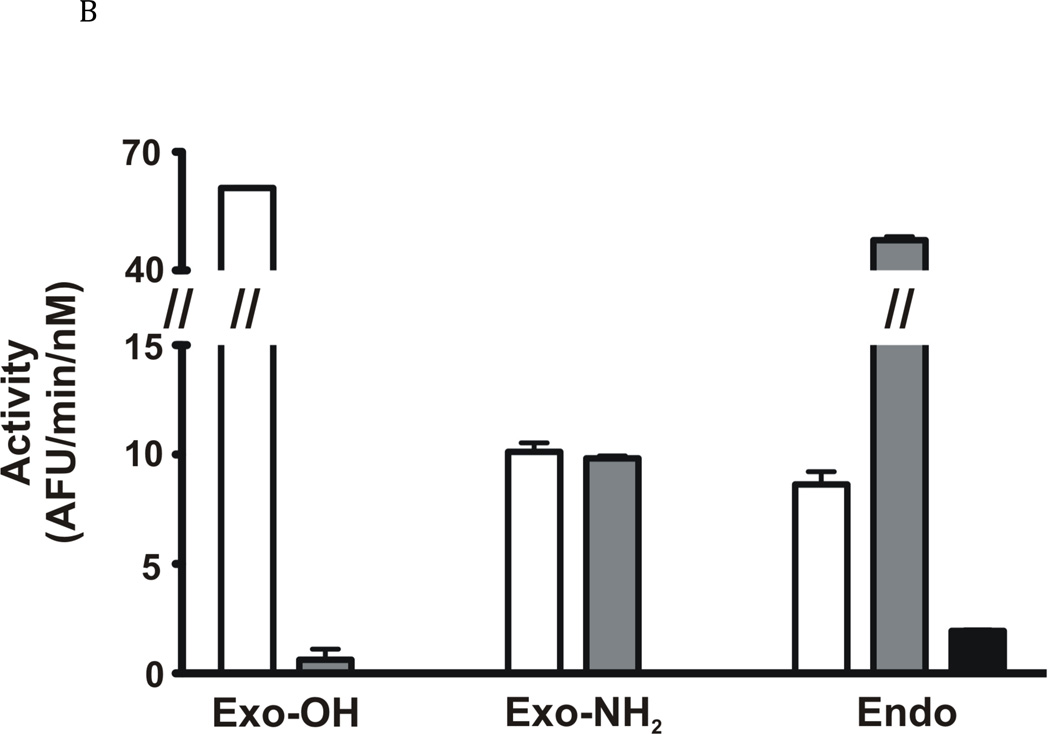
A: FhcatB1 was incubated in AMT buffers at various pH values, following which the residual activity at pH 4.5 was determined against Z Phe Arg AMC (t½ is represented by the closed squares). The activity of FhcatB1 was measured against FITC-casein at different pH values (activity values represented by open diamonds). B: Activity of human cathepsin B enzymes and FhcatB1 against exopeptidase substrates. The initial velocities for human wild-type (open bars), His110Ala cathepsin B (grey bars) and FhcatB1 (dark bars) are plotted as AFU/min/nM enzyme. To aid visualization, a break has been introduced between 15 and 40 AFU/min/nM enzyme.
The ability of FhcatB1 to carry out exopeptidase activity was investigated using three quenched fluorescence substrates, synthesized according to the substrate preferences of human cathepsin B, since at the time of synthesis the substrate preferences of FhcatB1 were not defined. Two substrates were designed to span the S2, S1, S1’ and S2’ subsites. Exo-OH (Abz Phe-Arg-Ala-Lys-(Dnp)-OH) was designed to contain a free carboxyl group at the C-terminus which is thought to interact with His111 on the occluding loop of mammalian cathepsin B, enhancing exopeptidase activity. Exo-NH2 (Abz Phe Arg-Ala-Lys(Dnp)-NH2) was produced with an amido-group at the carboxy terminus, preventing interaction between the substrate and His111. An extended substrate (Abz-Ala-Phe-Arg-Ala-Ala-Gln-Lys(Dnp) OH, called Endo) was synthesized to span the S3-S3’ subsites to examine endopeptidase activity. The initial velocities for cleavage of these substrates by FhcatB1, wild type human cathepsin B and His110A human cathepsin B were determined at pH 6.0. The latter mutant of the human enzyme would be predicted to have no anchoring of the occluding loop to the active site and thus behave as an endopeptidase rather than an exopeptidase. Human wild-type cathepsin B exhibited exopeptidase activity against Exo-OH, but showed much less activity against the amidated (Exo NH2) and extended substrates (Endo) (Fig. 4B). Human cathepsin B carrying the H110A mutation displayed a 60-fold decrease in activity against Exo-OH when compared with the human wild-type cathepsin B, but showed a 5-fold increase against the extended substrate. FhcatB1 showed no activity against either Exo-OH or Exo-NH2, but did hydrolyze the extended substrate (Fig. 4B). These results were seen across a range of pH values for FhcatB1 and thus were not dependent on the pH of the assay. The results strongly indicate that FhcatB1 does not display significant exopeptidase activity.
Human and sheep cystatins inhibit FhcatL5 but not FhcatB1
Inhibition of FhcatB1 by human cystatins A, B and C and sheep cystatin B, in comparison to human cathepsins B and L and FhcatL5, was determined in order to evaluate its susceptibility to these host inhibitors. FhcatB1 was at least 400–14,000-fold less susceptible to inhibition by any cystatin compared to FhcatL5 (Table 3). A similar difference in susceptibility to inhibition by these cystatins could be seen between human cathepsins B and L. The human catB was more susceptible to inhibition by cystatin C than FhcatB1. These results suggest that FhcatB1 is relatively resistant to inhibition by the major class of host inhibitors targeted against cathepsins, while FhcatL5 is highly susceptible to inhibition by the cystatins.
Table 3.
Ki values for interaction between cystatins and human and F. hepatica cathepsins.
| Human cathepsin L |
FhcatL5 | Human cathepsin B |
FhcatB1 | |
|---|---|---|---|---|
| Human cystatin A |
0.174 ± 0.009 |
0.049 ± 0.003 |
370 ± 50 |
225 ± 56 |
| Human cystatin B |
0.176 ± 0.011 |
3.25 ± 0.028 |
No inhibition | 2200 ± 320 |
| Sheep cystatin B |
0.122 ± 0.009 | 0.52 ± 0.041 |
410 ± 25 |
215 ± 32 |
| Human cystatin C |
0.00625 ± 0.0004 |
0.00659 ± 0.0026 |
0.600 ± 0.025 |
93.4 ± 20.4 |
All Ki values shown are expressed in nM.
Localisation of active cathepsin B enzymes in NEJ flukes and identification of the target of the TMR-I active site probe
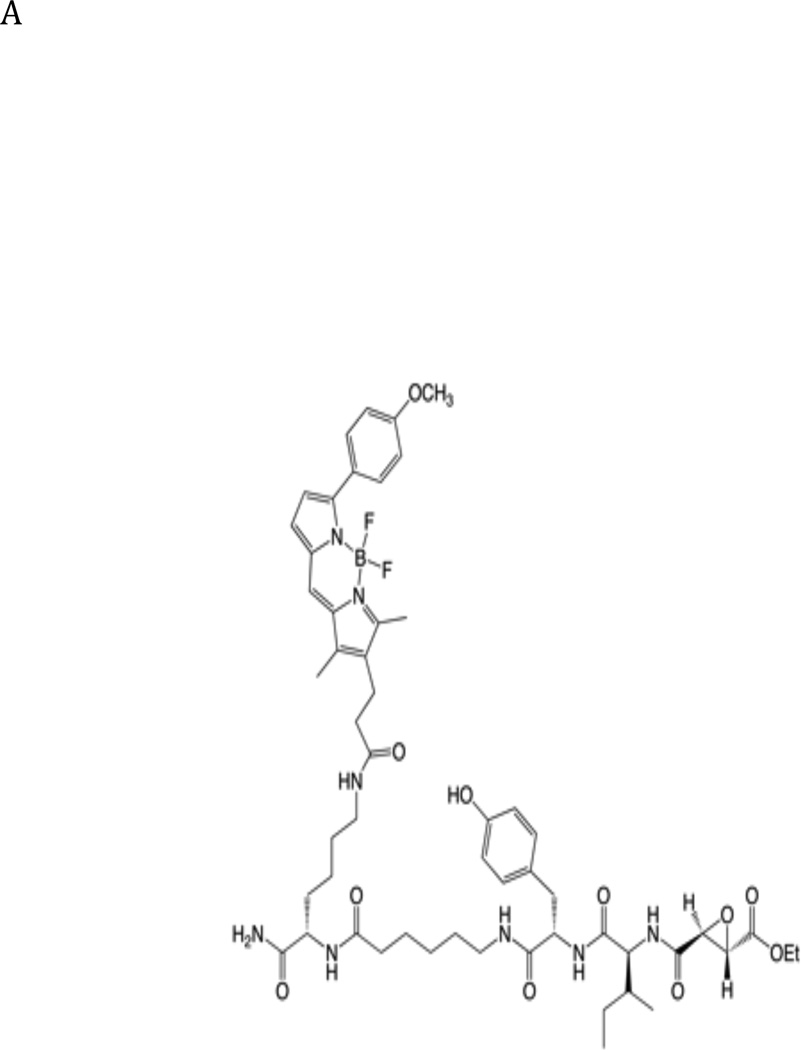
In order to investigate the localization of active FhcatB1 in F. hepatica, we synthesized a fluorescently labeled active site probe (termed TMR-I) based on the general epoxide scaffold used for our library screening. This probe contains an Ile residue at the P2 position that directs selectivity towards FhcatB1 over FhcatL enzymes and a TMR group to provide a fluorescent marker of covalent binding of the inhibitor to active cysteine proteases (Fig. 5A). The association rate constant for binding of fluorescent TMR-I to FhcatB1 was determined in vitro in order to assess whether it was an efficient inhibitor of the enzyme relative to the compounds tested previously. It was found that the kass value was 4 × 105 M−1.s−1, indicating that the newly synthesized probe was in fact a more rapid inhibitor of the enzyme than any other compound tested above (Table 1).
Fig. 5. Incubation of Fasciola hepatica juvenile flukes with a fluorescent active site probe reveals the localisation of active cathepsin B like enzymes.
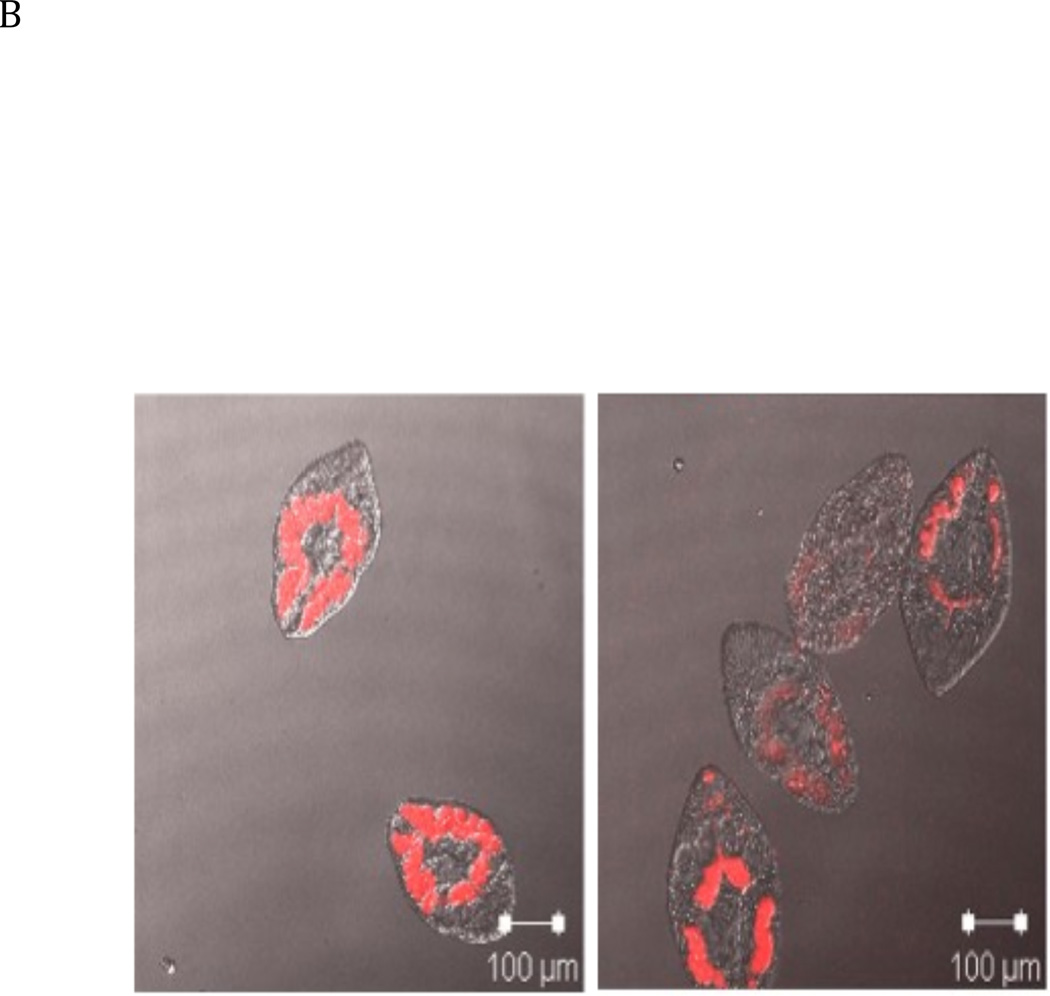
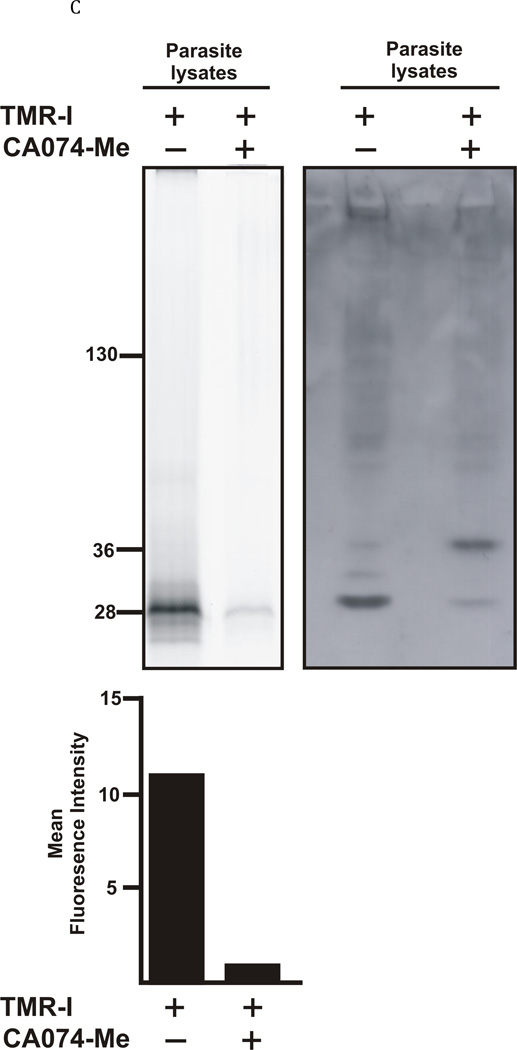
A: Structure of the TMR-I probe used for visualization of the cathepsin B enzymes within the parasites. B: 40 newly excysted juvenile parasites per well were either exposed to the TMR-I (6.25 µM) for 24 hrs (left panel) or exposed to CA074Me (6.25 µM) for 7 days prior to exposure to the TMR-I (6.25 µM) for 24 hrs (right panel). C: Determination of the molecular target of TMR-I and CA074Me. The left hand panel shows a fluorogram of the binding of the TMR-I to proteins in a lysate from F. hepatica NEJ flukes. As indicated, proteins were incubated with the TMR-I in the presence and absence of CA074Me. The intensity of the bands obtained with and without CA074Me is shown below the fluorogram. The right hand panel shows an immunoblot with rabbit anti-FhcatB1 against parasite lysate proteins treated with TMR-I in the presence and absence of CA074Me.
The TMR-I probe was incubated with live juvenile parasites in culture, in the presence and absence of CA074Me, in order to localise the active cathepsin B enzyme/s in the parasite and examine whether the fluorescent active site probe was binding to the same enzyme/s as CA074Me, which had shown marked effects on parasite viability in culture (Fig. 1). It was found that the fluorescent active site probe was strongly localised to the digestive tract of the parasite (Fig. 5B). The localisation was strongly inhibited by pre-incubation of the parasites with a sub-lethal concentration (6.25 µM) of CA074Me for 7 days prior to the addition of the fluorescent probe (Fig. 5B [right hand panel]).
In order to investigate the target/s of the fluorescent probe in the parasite, the flukes that had been incubated with the probe were lysed, electrophoresed on SDS-PAGE and the fluorescence of bands determined (Fig. 5C). It could be shown that the probe was only binding to one band, of approximately 28 kDa (Fig. 5C) in the lysate and that this binding was strongly inhibited (approximately 90%) in lysates from flukes pre-incubated for 7 days with CA074Me; this result provides an initial indication that the probe was specific for an enzyme in the parasite which was competitively targeted by the cathepsin B-selective inhibitor, CA074Me (Fig. 5C). An immunoblot of the same lysates was also carried out using an anti-FhcatB1 antibody raised previously (Kennedy et al., 2006). Interestingly, the anti-FhcatB1 antibody detected a 28 kDa band at the same position as the fluorescent band (Fig. 5C), providing evidence that the band being targeted by the probe was indeed representative of FhcatB1. Of further interest was the finding that the antibody targeted a band of approximately 37 kDa in the lysate which had been pre-incubated with CA074Me, with only a very light band present at the 28 kDa position. These results suggest that the major effect of CA074Me is to prevent processing of the pro-enzyme form of FhcatB1 (37kDa) to a mature, active enzyme with a molecular weight of 28 kDa.
Discussion
The newly excysted juvenile stage of the life cycle of F. hepatica constitutes a prime opportunity for attack by therapeutic drugs or vaccines, since blocking invasion by juvenile flukes would lead to improved health for animals and humans and economic benefits to livestock producers. In order to effectively target this parasite life cycle stage, however, the molecules pivotal to its successful completion need to be elucidated and fully characterized. A recent study (McGonigle et al., 2008) has investigated the role of both cathepsin B and L proteases in the NEJ flukes using gene silencing technology which reduced the expression of each of the protease types by up to 80%. The study showed that the cathepsin B and L proteases, which are found mainly in the gastric compartments of the flukes, were pivotal to invasion through the host gut wall, but the results did not demonstrate whether this effect was due to a critical role of the proteases in parasite homeostasis, invasion mechanisms or another unknown mechanism. Here, we have comprehensively characterized a recombinant form of FhcatB1 (Law et al., 2003; Wilson et al., 1998) and have established that the enzyme shows unique biochemical properties. Data from this study also suggest that maturation and subsequent activity of this enzyme is important to survival of the parasite in vitro.
Our initial experiment showed that the cathepsin B inhibitor CA074 is a highly specific inhibitor of recombinant FhcatB1 relative to its activity against a fluke cathepsin L enzyme, FhcatL5 (Table 1), suggesting that CA074 would be a useful compound to specifically define the role of cathepsin B enzymes compared with cathepsin L enzymes in fluke biology. CA074 and E-64c have been shown to bind to mammalian cathepsin B and papain, respectively, in dissimilar manners (Yamamoto et al., 1997). The CA074 binds with its prolyl-isoleucyl peptidic component in the S1’ binding site of cathepsin B, rather than the S2-S1 region as occurs for the leucyl-leucyl peptidic component of E-64c. The prolyl component of CA074 forms hydrogen bonds with the His110 and His111 amino acids on the occluding loop of human cathepsin B to stabilize this unusual binding mode. It therefore appears that the CA074 is able to bind to FhcatB1 in a similar manner to mammalian cathepsin B (Yamamoto et al., 1997) despite the absence of His111 in the parasite enzyme, indicating that most likely the hydrogen bonds between His110 and the inhibitor are able to compensate for the lack of His111. The cell-permeable CA074 pro-inhibitor, CA074Me, was used to investigate the role of cathepsin B activity in the biology of NEJ flukes under in vitro culture conditions. CA074Me is known to be converted into CA-074 inside cells by the action of esterases (Buttle et al., 1992), although it should be noted that this conversion does not always occur to completion (Montaser et al. 2002). The results showed that CA074Me was able to strikingly reduce the motility and viability of the NEJ parasites in culture. Other inhibitors that were less selective for FhcatB1 in vitro, such as E-64d (a cell permeable form of the general cathepsin inhibitor E-64c; [Towatari et al., 1991]), showed much weaker killing activity against the parasite, taking almost double the time to have any effects compared to CA-074Me. It should be noted that CA074Me was not only non-selective between the cathepsin B and L enzymes, but was also a very poor inhibitor of either of the cathepsin enzymes (23-fold lower kass with FhcatB1 compared to CA074). Thus we reason that the fact that this inhibitor has the strongest effect on NEJ viability of any of the inhibitors suggests that at least some of this compound must be converted to the more potent CA074 form of inhibitor inside the parasite. The lack of effect of non-cell permeable inhibitors on viability suggests that the enzymes are most likely to have an internal rather than external role in parasite homeostasis. These results provide an initial indication that cathepsin B enzymes are important for parasite homeostasis, although further corroboration is required due to the uncertainty surrounding complete removal of the ester group from the CA074Me. We therefore sought to characterize FhcatB1 further in order to provide avenues for selectively examining the role of this enzyme in parasite homeostasis.
Analysis of the substrate preference of FhcatB1 identified Ile as its most preferred reside at the P2 position, which usually dictates cathepsin specificity overall. While Val and Trp residues were almost as preferred as Ile residues at the P2 position, the preference for Ile over Leu residues is unusual compared to other enzymes and in particular is different to FhcatL molecules, which prefer Leu residues at the P2 position (Beckham et al., unpublished data; Stack et al., 2008). The fluorescently TMR-1 labeled probe, which was designed based on the preference of FhcatB1 for Ile at the P2 position, was rapidly taken up by the NEJ flukes and concentrated in the gastric cells, indicating that cathepsin B molecules were likely to be highly active in this compartment and present at high concentrations, a conclusion supported by recent (McGonigle et al., 2008) and previous (Creaney et al., 1996) results using immunolabelling to visualize the localization of the cathepsin B enzymes in the gastric compartment of the NEJ forms of the parasites. The labeling of cathepsin B enzymes with TMR-I in the parasite appeared to be specific due to the apparent inhibition of this labeling by pre-incubation of parasites with sub-lethal doses of CA-074Me, and by exclusive association with a major band at 28 kDa on an SDS-PAGE gel also recognised by an anti-FhcatB1 antibody. Labelling of this 28 kDa band in the NEJ lysate was strongly inhibited by CA-074Me, but interestingly the immunoblotting analysis suggested that this effect was due to a prevention of the maturation of the enzyme, which has been shown to be autocatalytic (Beckham et al., 2006), in the parasite by CA-074Me. It should be noted that the decrease in the labeling with the fluorescent inhibitor upon incubation with CA074Me appeared to be equivalent to the decrease in the intensity of the band at 28 kDa on the immunoblot upon pre-incubation with CA074Me. This supports the specificity of the TMR-I for cathepsin B enzymes, with some likelihood that it is specific for FhcatB1. It also suggests that CA074Me is targeting the cathepsin B enzymes of the NEJ parasites with a potency suggestive of at least some removal of the ester group from CA074Me within the parasite.
Characterization of the recombinant form of FhcatB1 showed that the enzyme was stable and active against both peptide and protein substrates across a wide range of pH values, suggesting that the enzyme is capable of activity in both acidic and neutral pH environments, the former likely to occur in the gastrodermal cells of the parasite and the latter in the host tissues/fluids. We also characterized the susceptibility of the FhcatB1 to inhibition by sheep and human cystatins, since this enzyme would be likely to encounter these inhibitors in the host environment during invasion through the intestinal wall and liver. As might be expected of a cathepsin B enzyme with an intact occluding loop structure, FhcatB1 was much less susceptible to inhibition by cystatins than cathepsin L enzymes, including the FhcatL5 tested here. This suggests that the secreted form of FhcatB1 would be active in the environment through which it was invading and not controlled by host inhibitors, unlike the cathepsin L enzymes. It is known that cystatin-like molecules are expressed by F. hepatica (Khaznadji et al., 2005) and, therefore, these parasite-expressed inhibitors would probably control the activity of cathepsin L molecules over the cathepsin B molecules inside the parasite, possibly limiting the intracellular roles of cathepsin L molecules to specialized compartments devoid of the cystatins. The results were found with all three types of cystatins tested, with cystatins A and B reflective of intracellular forms and cystatin C located extracellularly.
Finally, we characterized the exo- and endo-peptidase activities of FhcatB1 in comparison to human cathepsin B in order to define the activity of the parasite enzyme in this regard. Human cathepsin B, in common with all mammalian versions of the enzyme, has two His residues located on its occluding loop structure, His110 and His111. His110 apparently locks the occluding loop in position via ionic bonds to the body of the enzyme, while His111 docks with the C-terminus of substrates being cleaved by the exopeptidase activity of cathepsin B, where two residues at a time are cleaved from the C-terminus (Krupa et al., 2002). FhcatB1 has the equivalent residue to His110, but has a Val residue at the equivalent 111 position. This should mean that the FhcatB1 occluding loop is in position due to the presence of His110, consistent with the lack of susceptibility of FhcatB1 to inhibition by the cystatins, which has been shown to be due to the presence of the occluding loop in cathepsin B enzymes (Nycander et al., 1998; Pavlova et al., 2000). The lack of a His residue at position 111 in FhcatB1, suggests that interactions with the C-terminus of substrates would be dramatically lowered, however. Indeed, the FhcatB1 was shown to have very little activity against a substrate used to test for exopeptidase activity, relative to its activity against an endopeptidyl substrate, in contrast to human cathepsin B. This effect was not due to the pH value of the assay, as subsequent tests with FhcatB1 revealed the same results across a broad range of pH values (data not shown). The activity against the extended hexapeptide substrate and FITC-casein at pH values at which the occluding loop would be expected to be locked in the closed position in the active site (lower pH values), suggests that the position of the occluding loop might be somewhat flexible and thus facilitate endopeptidase activity by the enzyme.
FhcatB1 has a relatively unique substrate preference at the key P2 position of substrates where the specificity of cathepsins is often dictated (Musil et al., 1991). The preference for Ile over Leu residues in FhcatB1 was particularly different from other cathepsins and from human cathepsin B (Cotrin et al., 2004; St Hilaire et al., 2000). Molecular dynamics modeling studies indicate that very subtle changes to the S2 subsite of FhcatB1 relative to human cathepsin B affect the positioning of the substrate in the active site of the parasite enzyme and this leads to the preference for Val and Ile residues by FhcatB1 at the P2 position. A further marked difference for FhcatB1 was the lack of preference for Arg residues at P2 compared to human cathepsin B, despite the presence of Glu245 in the parasite enzyme. This suggests that despite the presence of the negatively charged residue that normally facilitates the acceptance of an Arg residue at P2 in cathepsin B enzymes (St Hilaire et al., 2000), the altered positioning of the substrate might prevent proper interaction in the parasite enzyme.
This study has shown the importance of the FhcatB1 enzyme to the survival and proper functioning of the NEJ form of the F. hepatica parasite in vitro. This is consistent with data generated via gene silencing technology, which shows that silencing of FhcatB expression affects the ability of NEJ flukes to invade through the gut wall (McGonigle et al., 2008). Thorough characterization of the enzyme has revealed the evolution of unique features with regard to its specificity and mode of catalytic action compared to mammalian counterparts that presumably aid the parasite in its life cycle. It appears that F. hepatica has evolved a novel cathepsin B enzyme that is expressed at high abundance in the invasive juvenile stage, is secreted into host tissues during the first 5 weeks of infection, is antigenic in sheep both during infection (Law et al., 2003) and following vaccination (Kennedy et al., 2006) and is resistant to inhibition by host cystatins. The natural targets of this protease include BSA and IgG (Wilson et al., 1998). These unique features and the apparent importance of the enzyme in parasite biology will allow the future targeting of this enzyme by potential therapeutic inhibitors or by vaccination.
Acknowledgements
This work was supported by the Sandler Center for Basic Research into Parasitic Diseases, Monash University, a program grant from the National Health and Medical Research Council of Australia, the Australian Centre for International Agricultural Research (ACIAR) and by the Slovene Research Agency. JAI is a C.J. Martin Postdoctoral Research Fellow of the National Health and Medical Research Council of Australia. NAMD was developed by the Theoretical and Computational Biophysics Group in the Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign. The authors would like to thank Dr Galia Blum, Stanford University, for helpful discussions and Dr John Mort, Shriners Hospital for Children, Montreal, for providing us with human cathepsin B constructs.
References
- Abdulla MH, Lim KC, Sajid M, McKerrow JH, Caffrey CR. Schistosomiasis mansoni: novel chemotherapy using a cysteine protease inhibitor. PLoS Med. 2007;4:130–138. doi: 10.1371/journal.pmed.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anon . World Health Organization Technical Series No. 849. Geneva, Switzerland: 1995. Control of Foodborne Trematode Infections. [PubMed] [Google Scholar]
- Aronja R, Riancho JA, Aguado JM. Fascioliasis in developed countries: A review of classic and aberrant forms of the disease. Medicine. 1995;74:13–23. doi: 10.1097/00005792-199501000-00002. [DOI] [PubMed] [Google Scholar]
- Barrett AJ, Kembhavi AA, Brown MA, Kirschke H, Knight CG, Tamai M, et al. L-trans-epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins, B. H. and L. Biochem J. 1982;201:189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham SA, Law RHP, Smooker PM, Quinsey NS, Caffrey CR, McKerrow JH, et al. Production and processing of a recombinant Fasciola hepatica cathepsin B-like enzyme (FhcatB1) reveals potential processing mechanisms in the parasite. Biol Chem. 2006;387:1053–1061. doi: 10.1515/BC.2006.130. [DOI] [PubMed] [Google Scholar]
- Berasain P, Goni F, McGonigle S, Dowd AJ, Dalton JP, Frangione B, et al. Proteinases secreted by Fasciola hepatica degrade extracellular matrix and basement membrane components. J Parasitol. 1997;83:1–5. [PubMed] [Google Scholar]
- Berasain P, Carmona C, Frangione B, Dalton JP, Goni F. Fasciola hepatica: Parasite secreted proteinases degrade all human IgG subclasses: Determination of the specific cleavage sites and identification of the immunoglobulin fragments produced. Exp Parasitol. 2000;94:99–110. doi: 10.1006/expr.1999.4479. [DOI] [PubMed] [Google Scholar]
- Bieth JG. Pathophysiological interpretation of kinetic constants of protease inhibitors. B Eur Physiopathol Resp. 1980;16:183–197. doi: 10.1016/b978-0-08-027379-2.50020-x. [DOI] [PubMed] [Google Scholar]
- Bieth JG. In vivo significance of kinetic constants of protein proteinase inhibitors. Biochem Med. 1984;32:387–397. doi: 10.1016/0006-2944(84)90046-2. [DOI] [PubMed] [Google Scholar]
- Bogyo M, Verhelst S, Bellingard-Dubouchaud V, Toba S, Greenbaum D. Selective targeting of lysosomal cysteine proteases with radiolabeled electrophilic substrate analogues. Chem Biol. 2000;7:27–38. doi: 10.1016/s1074-5521(00)00061-2. [DOI] [PubMed] [Google Scholar]
- Boray JC. Experimental fasciolosis in Australia. Adv Parasit. 1969;7:95–210. doi: 10.1016/s0065-308x(08)60435-2. [DOI] [PubMed] [Google Scholar]
- Brennan GP, Fairweather I, Trudgett A, Hoey E, McCoy, McConville M, et al. Understanding triclabendazole resistance. Exp Mol Pathol. 2007;82:104–109. doi: 10.1016/j.yexmp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Buttle DJ, Murata M, Knight CG, Barrett AJ. CA-074 methyl ester: A proinhibitor for intracellular cathepsin B. Arch Biochem Biophys. 1992;299:377–380. doi: 10.1016/0003-9861(92)90290-d. [DOI] [PubMed] [Google Scholar]
- Cancela M, Acosta D, Rinaldi G, Silva E, Duran R, Roche L, et al. A distinctive repertoire of cathepsins is expressed by juvenile invasive Fasciola hepatica. Biochemie. 2008;90:1461–1475. doi: 10.1016/j.biochi.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Chapman H, Mitchell GF. Proteolytic cleavage of immunoglobulin by enzymes released by Fasciola hepatica. Vet Parasitol. 1982;11:165–178. doi: 10.1016/0304-4017(82)90039-5. [DOI] [PubMed] [Google Scholar]
- Cimerman N, Prebanda MT, Turk B, Popovic T, Dolenc I, Turk V. Interaction of cystatin C variants with papain and human cathepsins B, H and L. J Enzyme Inhib. 1999;14:167–174. doi: 10.3109/14756369909036552. [DOI] [PubMed] [Google Scholar]
- Cotrin SS, Puzer L, Souza D, Júdice WA, Juliano L, Carmona AK, et al. Positional-scanning combinatorial libraries of fluorescence resonance energy transfer peptides to define substrate specificity of carboxypeptidases: assays with human cathepsin B. Anal Biochem. 2004;335:244–252. doi: 10.1016/j.ab.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Creaney J, Wilson LR, Dosen M, Sandeman RM, Spithill TW, Parsons JC. Fasciola hepatica: Irradiation-induced alterations in carbohydrate and cathepsin-B protease expression in newly excysted juvenile liver fluke. Exp Parasitol. 1996;83:202–215. doi: 10.1006/expr.1996.0067. [DOI] [PubMed] [Google Scholar]
- Dalton JP, McGonigle S, Rolph TP, Andrews SJ. Induction of protective immunity in cattle against infection with Fasciola hepatica by vaccination with cathepsin L proteinases and with hemoglobin. Infect Immun. 1996;64:5066–5074. doi: 10.1128/iai.64.12.5066-5074.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton JP, O’Neill SO, Stack CM, Collins PR, Walshe A, Sekiya M, et al. Fasciola hepatica cathepsin L-like proteases: biology, function, and potential in the development of first generation liver fluke vaccines. Int J Parasitol. 2003;33:1173–1181. doi: 10.1016/s0020-7519(03)00171-1. [DOI] [PubMed] [Google Scholar]
- Dennison C, Pike RN, Coetzer TH, Kirk K. Characterisation of the activity and stability of single-chain cathepsin L and of proteolytically active cathepsin L/cystatin complexes. Biol Chem Hoppe-Seyler. 1992;373:419–425. doi: 10.1515/bchm3.1992.373.2.419. [DOI] [PubMed] [Google Scholar]
- Dixon KE. The physiology of excystment of the metacercaria of Fasciola hepatica. Parasitology. 1966;56:431–456. doi: 10.1017/s0031182000068931. [DOI] [PubMed] [Google Scholar]
- Dowd AJ, McGonigle S, Dalton JP. Fasciola hepatica cathepsin L proteinase cleaves fibrinogen and produces a novel type of fibrin clot. Eur J Biochem. 1995;232:241–246. doi: 10.1111/j.1432-1033.1995.tb20805.x. [DOI] [PubMed] [Google Scholar]
- Ellis KJ, Morrison JF. Buffers of constant ionic strength for studying pH-dependent processes. Meth Enzymol. 1982;87:405–426. doi: 10.1016/s0076-6879(82)87025-0. [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Engel JC, Doyle P, Hsieh I, McKerrow JH. Cysteine protease inhibitors cure an experimental Trypanosoma cruzi infection. J Exp Med. 1998;188:725–734. doi: 10.1084/jem.188.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswar N, Marti-Renom MA, Webb B, Madhusudhan MS, Eramian D, Shen M, et al. Current Protocols in Bioinformatics. Supplement 15. John Wiley & Sons Inc; 2006. Comparative Protein Structure Modeling With MODELLER. 5.6.1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grams R, Vichasri-Grams S, Sobhon P, Upatham E, Viyanant V. Molecular cloning and characterization of cathepsin L encoding genes from Fasciola gigantica. Parasite Int. 2001;50:105–114. doi: 10.1016/s1383-5769(01)00068-x. [DOI] [PubMed] [Google Scholar]
- Greenbaum D, Medzihradszky KF, Burlingame A, Bogyo M. Epoxide electrophiles as activity-dependent cysteine protease profiling and discovery tools. Chem Biol. 2000;7:569–581. doi: 10.1016/s1074-5521(00)00014-4. [DOI] [PubMed] [Google Scholar]
- Harmsen MM, Cornelissen JB, Buijs HE, Boersma WJ, Jeurissen SH, van Milligen FJ. Identification of a novel Fasciola hepatica cathepsin L protease containing protective epitopes within the propeptide. Int J Parasitol. 2004;34:675–682. doi: 10.1016/j.ijpara.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Harth G, Andrews N, Mills AA, Engel JC, Smith R, McKerrow JH. Peptide-fluoromethyl ketones arrest intracellular replication and intercellular transmission of Trypanosoma cruzi. Mol Biochem Parasit. 1993;58:17–24. doi: 10.1016/0166-6851(93)90086-d. [DOI] [PubMed] [Google Scholar]
- Hasnain S, Hirama T, Tam A, Mort JS. Characterization of recombinant rat cathepsin B and nonglycosylated mutants expressed in yeast. New insights into the pH dependence of cathepsin B-catalyzed hydrolyses. J Biol Chem. 1992;267:4713–4721. [PubMed] [Google Scholar]
- Hughes AJ, Spithill TW, Smith RE, Boutlis CS, Johnson PDR. Human Fasciolosis acquired in an Australian urban setting. Med J Australia. 2003;178:178–179. doi: 10.5694/j.1326-5377.2003.tb05174.x. [DOI] [PubMed] [Google Scholar]
- Jayaraj R, Piedrafita D, Dynon K, Grams R, Spithill TW, Smooker PM. Vaccination against fasciolosis by a multivalent vaccine of stage-specific antigens. Vet Parasitol. 2009 Nov 6; doi: 10.1016/j.vetpar.2008.10.099. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Jerala R, Trstenjak M, Lenarčič B, Turk V. Cloning a synthetic gene for human stefin B and its expression in E. coli. FEBS Lett. 1988;239:41–44. doi: 10.1016/0014-5793(88)80541-6. [DOI] [PubMed] [Google Scholar]
- Jerala R, Zerovnik E, Lohner K, Turk V. Structural basis for the difference in thermodynamic properties between the two cysteine proteinase inhibitors stefins A and B. Prot Eng. 1994;7:977–984. doi: 10.1093/protein/7.8.977. [DOI] [PubMed] [Google Scholar]
- Kennedy NJ, Spithill TW, Tennent J, Wood PR, Piedrafita D. DNA vaccines in sheep: CTLA-4 targeting and CpG motifs enhance immunogenicity in a DNA prime/protein boost strategy. Vaccine. 2006;24:970–979. doi: 10.1016/j.vaccine.2005.08.076. [DOI] [PubMed] [Google Scholar]
- Kesik M, Jedlina-Panasiuk L, Kozak-Cieszczyk M, Płucienniczak A, Wedrychowicz H. Enteral vaccination of rats against Fasciola hepatica using recombinant cysteine proteinase (cathepsin L1) Vaccine. 2007;25:3619–3628. doi: 10.1016/j.vaccine.2007.01.057. [DOI] [PubMed] [Google Scholar]
- Khaznadji E, Collins P, Dalton JP, Bigot Y, Moiré N. A new multi-domain member of the cystatin superfamily expressed by Fasciola hepatica. Int J Parasitol. 2005;35:1115–1125. doi: 10.1016/j.ijpara.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Konagurthu AS, Whisstock JC, Stuckey PJ, Lesk AM. MUSTANG: A multiple structural alignment algorithm. Proteins. 2006;64:559–574. doi: 10.1002/prot.20921. [DOI] [PubMed] [Google Scholar]
- Krupa JC, Hasnain S, Nägler DK, Ménard R, Mort JS. S2' substrate specificity and the role of His110 and His111 in the exopeptidase activity of human cathepsin B. Biochem J. 2002;361:613–619. doi: 10.1042/0264-6021:3610613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law RH, Smooker PM, Irving JA, Piedrafita D, Ponting R, Kennedy NJ, et al. Cloning and expression of the major secreted cathepsin B-like protein from juvenile Fasciola hepatica and analysis of immunogenicity following liver fluke infection. Infect Immun. 2003;71:6921–6932. doi: 10.1128/IAI.71.12.6921-6932.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach L, Stüwe K, Hagen A, Ballaun C, Glössl J. Proteolytic processing and glycosylation of cathepsin B. The role of the primary structure of the latent precursor and of the carbohydrate moiety for the cell-type-specific molecular form of the enzyme. Biochem J. 1992;282:577–582. doi: 10.1042/bj2820577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudzadeh-Niknam H, McKerrow JH. Leishmania tropica: cysteine proteases are essential for growth and pathogenicity. Exp Parasitol. 2004;106:158–163. doi: 10.1016/j.exppara.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Mas-Coma S, Esteban J-G, Bargues MD. Epidemiology of human fascioliasis: a review and proposed new classification. B World Health Organ. 1999;77:340–346. [PMC free article] [PubMed] [Google Scholar]
- McGonigle L, Mousley A, Marks NJ, Brennan GP, Dalton JP, Spithill TW, et al. The silencing of cysteine proteases in Fasciola hepatica newly excysted juveniles using RNA interference reduces gut penetration. Int J Parasitol. 2008;38:149–155. doi: 10.1016/j.ijpara.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Meemon K, Grams R, Grams S, Hofmann A, Korge G, Viyanant V, et al. Molecular cloning and analysis of stage and tissue-specific expression of cathepsin B encoding genes from Fasciola gigantica. Mol Biochem Parasitol. 2004;136:1–10. doi: 10.1016/j.molbiopara.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Mitchell GB, Maris L, Bonniwell MA. Triclabendazole-resistant liver fluke in Scottish sheep. Vet Rec. 1998;143:399. [PubMed] [Google Scholar]
- Montaser M, Lalmanach G, Mach L. CA-074, but not its methyl ester CA-074Me, is a selective inhibitor of cathepsin B within living cells. Biol Chem. 2002;383:1305–1308. doi: 10.1515/BC.2002.147. [DOI] [PubMed] [Google Scholar]
- Musil D, Zucic D, Turk D, Engh RA, Mayr I, Huber R, et al. The refined 2.15 A X-ray crystal structure of human liver cathepsin B: The structural basis for its specificity. EMBO J. 1991;10:2321–2330. doi: 10.1002/j.1460-2075.1991.tb07771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nägler DK, Storer AC, Portaro FC, Carmona E, Juliano L, Ménard R. Major increase in endopeptidase activity of human cathepsin B upon removal of occluding loop contacts. Biochemistry. 1997;36:12608–12615. doi: 10.1021/bi971264+. [DOI] [PubMed] [Google Scholar]
- Nycander M, Estrada S, Mort JS, Abrahamson M, Bjork I. Two-step mechanism of inhibition of cathepsin B by cystatin C due to displacement of the proteinase occluding loop. FEBS Lett. 1998;422:61–64. doi: 10.1016/s0014-5793(97)01604-9. [DOI] [PubMed] [Google Scholar]
- Overend DJ, Bowen FL. Resistance of Fasciola hepatica to triclabendazole. Aust Vet J. 1995;72:275. doi: 10.1111/j.1751-0813.1995.tb03546.x. [DOI] [PubMed] [Google Scholar]
- Pavlova A, Krupa JC, Mort JS, Abrahamson M, Björk I. Cystatin inhibition of cathepsin B requires dislocation of the proteinase occluding loop. Demonstration by release of loop anchoring through mutation of His110. FEBS Lett. 2000;487:156–160. doi: 10.1016/s0014-5793(00)02337-1. [DOI] [PubMed] [Google Scholar]
- Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacenza L, Acosta D, Basmadjian IPDJ, Dalton JP, Carmona C. Vaccination with cathepsin L proteinases and with leucine aminopeptidase induces high levels of protection against fascioliasis in sheep. Infect Immun. 1999;67:1954–1961. doi: 10.1128/iai.67.4.1954-1961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedrafita D, Spithill TW, Dalton JP, Brindley PJ, Sandeman RM, Wood PR, et al. Juvenile Fasciola hepatica are resistant to killing in vitro by free radicals compared with larvae of Schistosoma mansoni. Parasite Immunol. 2000;22:287–295. doi: 10.1046/j.1365-3024.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- Piedrafita D, Parsons JC, Sandeman RM, Wood PR, Estuningsih SE, Partoutomo S, et al. Antibody-dependent cell-mediated cytotoxicity to newly excysted juvenile Fasciola hepatica in vitro is mediated by reactive nitrogen intermediates. Parasite Immunol. 2001;23:473–482. doi: 10.1046/j.1365-3024.2001.00404.x. [DOI] [PubMed] [Google Scholar]
- Pike RN, Coetzer THT, Dennison C. Proteolytically active complexes of cathepsin L and a cysteine proteinase inhibitor; purification and demonstration of their formation in vitro. Arch Biochem Biophys. 1992;294:623–629. doi: 10.1016/0003-9861(92)90734-e. [DOI] [PubMed] [Google Scholar]
- Prowse RK, Chaplin P, Robinson HC, Spithill TW. Fasciola hepatica cathepsin L suppresses sheep lymphocyte proliferation in vitro and modulates surface CD4 expression on human and ovine T cells. Parasite Immunol. 2002;24:57–66. doi: 10.1046/j.0141-9838.2001.00438.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal PJ, Lee GK, Smith RE. Inhibition of a Plasmodium vinckei cysteine proteinase cures murine malaria. J Clin Invest. 1993;91:1052–1056. doi: 10.1172/JCI116262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer PM, Pingel S, Hsieh I, Ugele D, Chan VJ, Engel JC, et al. Cysteine protease inhibitors as chemotherapy: Lessons from a parasite target. P Natl Acad Sci USA. 1999;96:11015–11022. doi: 10.1073/pnas.96.20.11015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Dowd AJ, Heffernan M, Robertson CD, Dalton JP. Fasciola hepatica: a secreted cathepsin L-like proteinase cleaves host immunoglobulin. Int J Parasitol. 1993;23:977–983. doi: 10.1016/0020-7519(93)90117-h. [DOI] [PubMed] [Google Scholar]
- Smooker PM, Whisstock JC, Irving JA, Siyaguna S, Spithill TW, Pike RN. A single amino acid substitution affects substrate specificity in cysteine proteinases from Fasciola hepatica. Protein Sci. 2000;9:2567–2572. doi: 10.1110/ps.9.12.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spithill TW, Smooker PM, Sexton JL, Bozas E, Morrison CA, Creaney J, et al. Development of vaccines against Fasciola hepatica. In: Dalton JP, editor. Fasciolosis. UK: Wallingford: CABI Publishing; 1999. pp. 1–20. [Google Scholar]
- St Hilaire PM, Alves LC, Sanderson SJ, Mottram JC, Juliano MA, Juliano L, et al. The substrate specificity of a recombinant cysteine protease from Leishmania mexicana: application of a combinatorial peptide library approach. Chembiochem. 2000;1:115–122. doi: 10.1002/1439-7633(20000818)1:2<115::aid-cbic115>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- Stack CM, Caffrey CR, Donnelly SE, Seshaadri A, Lowther J, Tort JF, et al. Structural and functional relationships in the virulence-associated cathepsin L proteases of the parasitic live fluke, Fasciola hepatica. J Biol Chem. 2008;283:9896–9908. doi: 10.1074/jbc.M708521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas I, Coles GC, Duffus K. Triclabendazole-resistant Fasciola hepatica in southwest Wales. Vet Rec. 2000;146:200. [PubMed] [Google Scholar]
- Tkalcevic J, Ashman K, Meeusen E. Fasciola hepatica - Rapid identification of newly excysted juvenile proteins. Biochem Biophys Res Commun. 1995;213:169–174. doi: 10.1006/bbrc.1995.2112. [DOI] [PubMed] [Google Scholar]
- Torgerson P, Claxton J. Epidemiology and Control. In: Dalton JP, editor. Fasciolosis. UK: Wallingford: CABI Publishing; 1999. pp. 113–149. [Google Scholar]
- Towatari T, Nikawa T, Murata M, Yokoo C, Tamai M, Hanada K, et al. Novel epoxysuccinyl peptides. A selective inhibitor of cathepsin B, in vivo. FEBS Lett. 1991;280:311–315. doi: 10.1016/0014-5793(91)80319-x. [DOI] [PubMed] [Google Scholar]
- Troeberg L, Pike RN, Morty RE, Berry RK, Coetzer TH, Lonsdale-Eccles JD. Proteases from Trypanosoma brucei brucei. Purification, characterisation and interactions with host regulatory molecules. Eur J Biochem. 1996;238:728–736. doi: 10.1111/j.1432-1033.1996.0728w.x. [DOI] [PubMed] [Google Scholar]
- Wasilewski MM, Lim KC, Phillips J, McKerrow JH. Cysteine protease inhibitors block schistosome hemoglobin degradation in vitro and decrease worm burden and egg production in vivo. Mol Biochem Parasit. 1996;81:179–189. doi: 10.1016/0166-6851(96)02703-x. [DOI] [PubMed] [Google Scholar]
- Wedrychowicz H, Kesik M, Kaliniak M, Kozak-Cieszczyk M, Jedlina-Panasiuk L, Jaros S, et al. Vaccine potential of inclusion bodies containing cysteine proteinase of Fasciola hepatica in calves and lambs experimentally challenged with metacercariae of the fluke. Vet Parasitol. 2007;147:77–88. doi: 10.1016/j.vetpar.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Wijffels GL, Salvatore L, Dosen M, Waddington J, Wilson L, Thompson C, et al. Vaccination of sheep with purified cysteine proteinases of Fasciola hepatica decreases worm fecundity. Exp Parasitol. 1994;78:132–148. doi: 10.1006/expr.1994.1014. [DOI] [PubMed] [Google Scholar]
- Wilson LR, Good RT, Panaccio M, Wijffels GL, Sandeman RM, Spithill TW. Fasciola hepatica: Characterization and cloning of the major cathepsin B protease secreted by newly excysted juvenile liver fluke. Exp Parasitol. 1998;88:85–94. doi: 10.1006/expr.1998.4234. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Hara T, Tomoo K, Ishida T, Fujii T, Hata Y, et al. Binding mode of CA074, a specific irreversible inhibitor, to bovine cathepsin B as determined by X-ray crystal analysis of the complex. J Biochem. 1997;121:974–977. doi: 10.1093/oxfordjournals.jbchem.a021682. [DOI] [PubMed] [Google Scholar]