Abstract
Directional cell migration requires force generation that relies on the coordinated remodeling of interactions with the extracellular matrix (ECM), which is mediated by integrin-based focal adhesions (FAs). Normal FA turnover requires dynamic microtubules, and three members of the diverse group of microtubule plus-end-tracking proteins are principally involved in mediating microtubule interactions with FAs. Microtubules also alter the assembly state of FAs by modulating Rho GTPase signaling, and recent evidence suggests that microtubule-mediated clathrin-dependent and -independent endocytosis regulates FA dynamics. In addition, FA-associated microtubules may provide a polarized microtubule track for localized secretion of matrix metalloproteases (MMPs). Thus, different aspects of the molecular mechanisms by which microtubules control FA turnover in migrating cells are beginning to emerge.
Introduction
Cell migration is essential for development, tissue remodeling, and wound healing, and is abnormal in many pathological states. Cell migration is also a highly complex process. To migrate directionally, cells need to coordinate signaling pathways to control polarity and cytoskeleton rearrangements to generate forces required for directional movement. Force generation relies on the ability of cells to dynamically remodel adhesion sites that connect them to the underlying ECM. Such close contacts with the ECM, now commonly called focal adhesions (FAs), were first described in the 1970s by interference reflection microscopy. Unraveling the relationship between focal adhesion (FA) dynamics and cell migration has been an important aspect of cell biology research in the following decades (Wolfenson et al., 2009). The FA life cycle involves formation of integrin-mediated, nascent adhesions near the cell’s leading edge, which either rapidly turn over or connect to the actin cytoskeleton (Parsons et al., 2010). Actomyosin-mediated pulling forces allow a subset of these nascent FAs to grow and mature, and provide forward traction forces. However, in order for cells to productively move forward, FAs also have to release and disassemble underneath the cell body and in the rear of the cell. Spatial and temporal control of turnover of these mature FAs is important, as they provide a counterbalance to forward traction forces, and regulated FA disassembly is required for forward translocation of the cell body. An important question that we are only beginning to understand is how FA turnover is spatially and temporally regulated to allow cells to appropriately respond to extracellular signals, allowing for coordinated and productive movement. The molecular mechanisms underlying the regulation of FA dynamics are expected to be complex, as the number of potential FA-associated proteins has exploded (Humphries et al., 2009; Kuo et al., 2011), and new ultrastructural approaches are starting to reveal the complexity of FA architecture (Kanchanawong et al., 2010). In addition, FAs in the leading edge and trailing rear differ in biochemistry and function, and thus may require different but coordinated disassembly mechanisms (Broussard et al., 2008). Dynamic microtubules play an important role in controlling FA turnover, and this review focuses on our current understanding of the molecules and mechanisms involved.
Microtubules and FAs: an intimate relationship
It was recognized early on that microtubules are required for cells to migrate directionally (Vasiliev et al., 1970), and that microtubules often appear associated with FAs in migrating cells (Rinnerthaler et al., 1988). Microtubules constitute a highly dynamic cytoskeleton filament system and are characterized by a nonequilibrium polymerization behavior termed dynamic instability. Microtubules stochastically switch between phases of growth and shortening, which allows rapid remodeling of the microtubule cytoskeleton and exploration of the intracellular space (Kirschner and Mitchison, 1986).
In a series of classical experiments using fluorescently labeled proteins in combination with the then fledgling technique of live cell microscopy, it was first demonstrated that microtubules repeatedly target FAs (Kaverina et al., 1998). Although microtubule growth toward FAs at the ventral cell surface was later confirmed by total internal reflection fluorescence microscopy (Krylyshkina et al., 2003), these experiments relied on goldfish fibroblasts with relatively spare microtubule arrays. Whether microtubules grow toward FAs is harder to establish in many mammalian cell types with denser microtubule arrays, and to what extent microtubule targeting of FAs is a general mechanism is thus still controversial. Nevertheless, several proteins have been identified in the meantime that mediate direct and specific microtubule interactions with FAs. In migrating goldfish fibroblasts, microtubule targeting events to FAs are asymmetric, and, remarkably, a higher frequency of microtubule targeting events correlates with zones of FA disassembly (Kaverina et al., 1999; Rid et al., 2005). This demonstrated for the first time that microtubule and FA dynamics are linked, and that microtubules may control FA disassembly. Individual microtubules grow multiple times toward the same or different FAs at which microtubules frequently pause and switch from growth to shortening (Kaverina et al., 1998). Such growth-to-shortening transitions occur five times more frequently at FAs compared with elsewhere in the cytoplasm, and this involves the FA component paxillin (Efimov et al., 2008). Paxillin is a large multidomain scaffolding protein, and although the mechanism by which paxillin influences microtubule dynamics is not understood, it may involve recruitment or local activation of a microtubule catastrophe factor. In addition, paxillin is phosphorylated by FAK, a major modulator of FA dynamics. FAK regulates microtubule stability in the cell periphery (Palazzo et al., 2004) and is also necessary for microtubule-mediated FA disassembly (Ezratty et al., 2005; Schober et al., 2007). It will be interesting to see whether FAK-mediated paxillin phosphorylation plays a role in FA-associated regulation of microtubule dynamics.
Microtubules modulate Rho GTPase signaling
One mechanism by which microtubules could influence FA dynamics is by locally modulating Rho GTPase signaling pathways that control actomyosin-based contractility. Indeed, the effects of global changes in the microtubule polymerization state on FA dynamics have been well established. Pharmacological microtubule depolymerization results in an increase in RhoA activity (Ren et al., 1999), which correlates with increased cell contractility and FA assembly. In contrast, global microtubule polymerization after washout of a microtubule-depolymerizing drug such as nocodazole results in rapid FA disassembly (Ezratty et al., 2005). Although it has been proposed that this is caused by microtubule polymerization–induced activation of Rac1 (Waterman-Storer et al., 1999; Rooney et al., 2010), Rac1 inhibition does not disrupt microtubule regrowth–induced FA disassembly (Ezratty et al., 2005). It has also never been shown unambiguously that such Rac1 activation is not caused by a release from a nocodazole-induced mitotic block, resulting for example from RhoA inactivation after cytokinesis (Piekny et al., 2005). However, although RhoA and Rac1 are generally regarded as antagonistic, spatial and temporal Rho GTPase activation dynamics during cell migration, and therefore Rho GTPase regulation by microtubules, is more complex than previously appreciated (Machacek et al., 2009).
Rho GTPases are mainly activated by guanine nucleotide exchange factors (GEFs) that catalyze GTP loading. GEF-H1 (also known as ARHGEF2) directly binds to microtubules (Ren et al., 1998) and is unable to activate RhoA when it is microtubule-bound (Krendel et al., 2002). Expression of mutated GEF-H1 that cannot bind microtubules results in morphological changes associated with RhoA activation such as increased contractility and stress fiber formation (Krendel et al., 2002). This is similar to what is observed upon global microtubule depolymerization, and it was proposed that GEF-H1 locally activates RhoA by being released from depolymerizing microtubules. Indeed, microtubule depolymerization-induced RhoA activation depends on GEF-H1 (Chang et al., 2008). In addition, FA turnover is perturbed in GEF-H1–depleted cells, and FA-associated tyrosine phosphorylation is decreased, which suggests an FA disassembly defect (Nalbant et al., 2009). However, a direct link between local microtubule-induced FA disassembly and GEF-H1 has not been established, and this simple model is at odds with typical microtubule behavior in migrating cells. It is inconsistent with an increased microtubule catastrophe frequency at FAs (Efimov et al., 2008), as this would be expected to locally release GEF-H1, increase RhoA activity, and promote FA assembly. Similarly, although GEF-H1 is required for RhoA activation in protruding cell edges (Nalbant et al., 2009), it is hard to understand how this could be mediated by GEF-H1 release from depolymerizing microtubules, as microtubules undergo net growth toward the front of migrating cells (Wittmann and Waterman-Storer, 2001; Wittmann et al., 2003; Grigoriev et al., 2006). In contrast, one would expect that GEF-H1 is sequestered by these growing microtubules, which should instead result in decreased RhoA activity and in Rac1 activation. Clearly, microtubule-mediated GEF-H1 regulation is more complex, and may depend on additional mechanisms such as phosphorylation-induced release from microtubules (Callow et al., 2005) and interactions with additional factors such as the dynein light chain Tctex-1 (Meiri et al., 2012). In biochemical experiments, GEF-H1 is also directly recruited to adhesion complexes downstream of tension-mediated integrin activation and is required for subsequent RhoA activation and cellular adaptation to force (Guilluy et al., 2011). Because tension stimulates microtubule growth toward FAs (Kaverina et al., 2002), an untested possibility is that these microtubules sequester GEF-H1 and contribute to local RhoA inhibition. More recently, the Rac1 GEF STEF (also known as Tiam2) was found to be required for microtubule regrowth-induced FA disassembly. STEF may play a role opposing GEF-H1 because in STEF-depleted cells, nocodazole washout fails to activate Rac1 (Rooney et al., 2010). Similarly, MAP1B-mediated microtubule binding of Tiam1 may be involved in Rac1 activation in neuronal cells (Montenegro-Venegas et al., 2010).
Although these data collectively indicate that microtubules can globally influence Rho GTPase signaling, no conclusive model has been proposed for how microtubule polymerization dynamics could locally regulate Rho GTPase activity to control FA turnover. Because individual microtubules stochastically switch between phases of growth and shortening, and microtubules in these different phases coexist side-by-side, it is hard to imagine how GEF activity regulation through simple microtubule binding could result in a meaningful control of intracellular Rho GTPase activity. One possibility is that microtubule interactions of some GEFs are more locally controlled. The Drosophila melanogaster RhoGEF2, for example, binds to growing microtubule ends through interactions with EB1 (Rogers et al., 2004). Thus, RhoGEF2 may be sequestered to microtubules in regions with many growing microtubule ends. However, it is not known whether this controls RhoGEF2 activity, and intracellular diffusion would counteract any hypothetical GEF activity gradient. In addition, RhoGEF2 appears to be insect-specific, and no vertebrate GEF has been identified to date that displays EB1-mediated microtubule plus-end-binding. It is important to note that EB1-mediated plus-end-tracking does not result in net RhoGEF2 transport (Kumar and Wittmann, 2012), and another more plausible possibility is that microtubule-mediated transport of Rho regulators participates in specifying Rho GTPase activity zones. This has not been tested in migrating cells, but in mitotic cells midzone microtubules control concentration and activity of cortical RhoA GEFs and GAPs during cytokinesis, and are required to focus cleavage furrow RhoA activity (Canman, 2009). In any case, more direct interactions of microtubules with FAs are likely important to account for the highly spatially controlled FA dynamics that are observed in migrating cells.
Cortical microtubule adaptors: Adenomatous polyposis coli (APC), MACF1/ACF7, and cytoplasmic linker–associated proteins (CLASPs)
Close interactions between the actin and microtubule cytoskeletons occur in many cell types, including migrating cells and neuronal growth cones (Salmon et al., 2002; Rodriguez et al., 2003; Burnette et al., 2008). The search-and-capture idea that microtubules exploring the intracellular space are stabilized by specific interactions near the leading edge of migrating cells is not new (Kirschner and Mitchison, 1986), and guidance of microtubule growth along F-actin stress fibers toward FAs has been proposed (Kodama et al., 2003; Krylyshkina et al., 2003; Small and Kaverina, 2003). However, only recently have proteins been identified that mediate localized physical interactions of microtubules with FAs. Although very different at first glance, three of these proteins, APC, MACF1/ACF7, and CLASPs, share several noteworthy characteristics (Fig. 1). They all belong to a group of proteins that localize to growing microtubule ends through interactions with EB1 (Akhmanova and Steinmetz, 2008; Kumar and Wittmann, 2012), and are all required for directional cell migration. All three of these proteins have been implicated in microtubule organization and/or stabilization near the leading edge of migrating cells and localize to domains near FAs. Finally, molecular interactions of all three of these proteins are negatively regulated by GSK3β phosphorylation. GSK3β is proposed to be locally inactivated downstream of several different cell polarity pathways (Barth et al., 2008), and GSK3β inactivation is required for peripheral microtubule stabilization (Eng et al., 2006; Kumar et al., 2009). Because GSK3β inactivation at the leading edge of migrating cells would result in dephosphorylation and activation of the microtubule-binding activity of APC, MACF1/ACF7, and CLASPs, these proteins are attractive candidates to mediate local microtubule and FA cross talk.
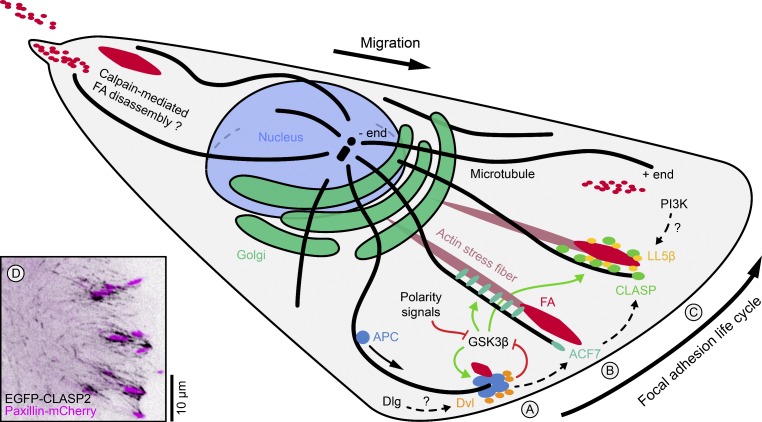
Figure 1.
+TIP-mediated microtubule–FA interactions in the front of a migrating cell. The FA life cycle consists of nascent FA assembly near the cell’s leading edge, actomyosin-mediated maturation, and subsequent disassembly as the cell migrates forward. FA interactions with the microtubule cytoskeleton are important for the regulation of FA dynamics, and three types of +TIPs have been implicated in mediating microtubule–FA interactions. It is unclear how the functions of these proteins overlap, but based on published biochemical interactions and RNAi depletion experiments, we propose a hierarchy of APC, the spectraplakin MACF1/ACF7, and CLASPs, which is indicated by the arrows with broken lines. (A) APC is transported along microtubules to the cell edge and directly interacts with polarity signals such as the Wnt signaling pathway. APC may be involved in stabilizing nascent FAs. It is important to note, however, that only a subset of FAs associate with APC clusters, so other mechanisms must exist. (B) MACF1/ACF7 mediates microtubule interactions with F-actin stress fibers, and is required to guide microtubule growth toward FAs. (C) CLASPs stabilize microtubules in a domain around mature FAs. CLASP accumulation near FAs depends on interactions with the PIP3-binding protein LL5β. Different cell polarity pathways are thought to result in local GSK3β inactivation that in turn stimulates microtubule and/or FA interactions of APC, MACF1/ACF7, and CLASPs. FA disassembly in the retracting rear of the cells differs mechanistically from FA turnover in the front, and it is not known to what extent the same molecules are involved. Disassembling FAs are symbolized by red dots. (D) Contrast-inverted image of CLASP2-decorated microtubules around FAs (labeled with Paxillin-mCherry) near the leading edge of a migrating epithelial cell.
APC is a large multifunctional protein that was first identified as a tumor suppressor mutated in most familial and spontaneous cases of colon cancer. APC plays an important role in the canonical Wnt signaling pathway by regulating β-catenin levels (Aoki and Taketo, 2007). Full-length APC is transported along microtubules and accumulates in dynamic cloud-shaped clusters at the plus ends of a subset of microtubules that converge into protruding cell edges (Fig. 1 A; Näthke et al., 1996; Mimori-Kiyosue et al., 2000; Kita et al., 2006). The heterotrimeric kinesin KIF17 is required for APC cluster formation and itself accumulates in similar clusters at the cell edge (Jaulin and Kreitzer, 2010). APC clusters also closely associate with FAs and may promote FA assembly (Matsumoto et al., 2010). Recent data further indicate that APC, together with the formin mDia, can act as an F-actin filament nucleator (Okada et al., 2010; Breitsprecher et al., 2012). Although the physiological relevance of this activity is not understood, it is tempting to speculate that APC participates in “seeding” new FA sites, and that this may be important for APC’s well-established role as a cell polarity regulator. Cortical APC clusters also colocalize with Dishevelled (Dvl), a central component of the Wnt signaling pathway (Gao and Chen, 2010; Matsumoto et al., 2010). This is an important finding, as the relationship between Wnt signaling and APC localization to microtubule ends is highly controversial and largely unexplored, and APC’s role in cancer progression may be independent of its microtubule-binding activity (Lewis et al., 2012). APC’s role in cell polarity formation and migration may also depend on leading edge interactions with another tumor suppressor protein, Discs Large (Dlg; Etienne-Manneville et al., 2005; Iizuka-Kogo et al., 2005), but the molecular details of this pathway have not been established.
MACF1/ACF7 belongs to the spectraplakin family of cytoskeletal linker proteins that combine multiple cytoskeleton interaction domains (Suozzi et al., 2012). Spectraplakins are F-actin and microtubule cross-linkers that contribute to organization and mechanical resistance of the microtubule cytoskeleton (Karakesisoglou et al., 2000; Applewhite et al., 2010). MACF1/ACF7 localizes to a domain adjacent to FAs (Karakesisoglou et al., 2000; Kodama et al., 2003; Wu et al., 2008b, 2011). The molecular interactions that determine MACF1/ACF7 localization near FAs are not known, although cortical MACF1/ACF7 localization may depend on APC (Zaoui et al., 2010). In addition, cortical MACF1/ACF7 localization, as well as microtubule binding, is inhibited by GSK3β phosphorylation (Wu et al., 2011). MACF1/ACF7 is thought to guide growing microtubules along actin stress fibers toward FAs (Fig. 1 B), and MACF1/ACF7-deficient cells have disorganized peripheral microtubules and display decreased FA turnover (Wu et al., 2008b). MACF1/ACF7 also has F-actin–stimulated ATPase activity, but whether this is involved in FA dynamics regulation has not been established (Wu et al., 2008b). However, an MACF1/ACF7 minigene of only the F-actin and microtubule-binding domains is not sufficient to restore migratory defects in MACF1/ACF7-deficient cells, which suggests that it is more than a simple actin–microtubule cross-linker (Wu et al., 2008b).
CLASPs localize to a domain around FAs that appears very similar to MACF1/ACF7 localization, although colocalization has not been tested in the same cell (Fig. 1 C; Drabek et al., 2006; Lansbergen et al., 2006; Kumar et al., 2009). Similar to the proposed function of MACF1/ACF7, CLASP-deficient cells have a disorganized and less stable peripheral microtubule cytoskeleton (Akhmanova et al., 2001; Mimori-Kiyosue et al., 2005; Drabek et al., 2006; Kumar et al., 2009). CLASPs display a complex regulation of microtubule association. In migrating cells, CLASPs track microtubule plus ends in the cell body, but associate along microtubules in the leading edge (Wittmann and Waterman-Storer, 2005; Kumar et al., 2009). Because GSK3β phosphorylation inhibits CLASP association with microtubules (Wittmann and Waterman-Storer, 2005; Kumar et al., 2009; Watanabe et al., 2009), this gradient of CLASP–microtubule association may result from local GSK3β inactivation near the leading edge. Although direct binding of MACF1/ACF7 to microtubules is also inhibited by GSK3β (Wu et al., 2011), a similar MACF1/ACF7 gradient of microtubule binding in migrating cells has not been demonstrated. Enhanced microtubule binding of nonphosphorylated CLASP and MACF1/ACF7 in the front of the cell may thus contribute to polarized microtubule interactions with FAs. Interactions of CLASP-decorated microtubules with FAs occur along a segment near the microtubule end (Fig. 1 D; Kumar et al., 2009), which one would expect to be more stable compared with “end-on” interactions with only the microtubule tip. In contrast to APC and MACF1/ACF7, CLASP localization around FAs does not depend on microtubules, but on an interaction with the PH-domain containing protein LL5β (Lansbergen et al., 2006). Although LL5β can bind to phosphatidylinositol 3,4,5-trisphosphate (PIP3) as well as the F-actin cross-linker filamin-C (Paranavitane et al., 2003), how LL5β clusters form around FAs is not known. Strikingly, phosphoinositide 3-OH kinase (PI3K) inhibition does not abolish cortical LL5β localization (Lansbergen et al., 2006). Instead, LL5β clusters depend on integrin-mediated adhesion at least at the basal surface of polarized epithelial cells (Hotta et al., 2010), and certain integrins cluster around FAs in a manner similar to LL5β (Carter et al., 1990; Chao et al., 2010). In addition to LL5β, cortical CLASP localization also partially depends on MACF1/ACF7 (Drabek et al., 2006). CLASP-deficient cells also display migration defects (Drabek et al., 2006), and CLASPs are required for FA turnover (unpublished data).
Collectively, these data strongly indicate that the microtubule +TIPs APC, MACF1/ACF7, and CLASPs are involved in similar processes controlling microtubule and FA dynamics during cell migration. However, their functions are not redundant, as inhibition or genetic knockdown of either of these proteins results in cell migration phenotypes. Thus, an important outstanding question is how these proteins function together to control FA turnover. We propose a working model in which a hierarchy of APC, MACF1/ACF7, and CLASPs controls FA dynamics by establishing a microtubule transport pathway that is required for coordinated FA turnover during directed cell migration (Fig. 1). How these +TIPs cooperate with the septin filament system that is also involved in F-actin–guided microtubule growth toward FAs remains to be elucidated (Bowen et al., 2011).
Microtubule-mediated transport from and to FAs
Once microtubules are cortically tethered at adhesion sites, how might they control FA dynamics? Because most intracellular vesicle transport processes occur along microtubules, one possibility is that these microtubules serve as specific tracks for cargo transport from and/or to FAs (Fig. 2). For example, it has long been recognized that coordinated secretion and recycling of integrin adhesion receptors is important for cell migration (Bretscher, 1989). In brief, integrins are internalized and shuttled to either the early endosome (short loop recycling pathway) or to the perinuclear recycling compartment (long loop recycling pathway). Vesicles containing recycled integrins are then re-exocytosed at the leading edge and reused in new FAs (Gu et al., 2011). Transport between different compartments is controlled by Rab GTPases, master regulators of vesicle identity that also mediate vesicle interactions with motor proteins and microtubule-dependent vesicle motility (Horgan and McCaffrey, 2011). The rather complex route of integrins through the endosomal pathway is not a focus of this review and has been described elsewhere (Caswell et al., 2009; Margadant et al., 2011). However, this raises the question of whether microtubule-dependent integrin endocytosis could be a critical step in FA turnover.
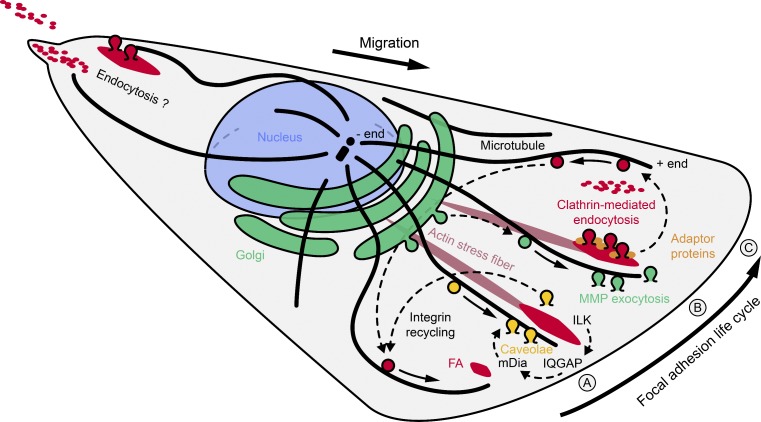
Figure 2.
Microtubule-mediated vesicle trafficking pathways involved in FA dynamics. (A) ILK participates in microtubule stabilization near the leading cell edge, and is required for caveolin transport toward FAs. Caveolae may provide an alternative integrin endocytosis pathway. (B) Exocytosis of MT1-MMP is involved in ECM degradation around FAs. Transport of MT1-MMP vesicles requires microtubules, and ECM proteolysis may initiate FA disassembly. (C) Integrins are internalized by different endocytosis pathways and at least partially recycled back to the cell front. Clathrin-mediated endocytosis is thought to be involved in uptake of FA-associated, activated integrin molecules, whereas clathrin-independent pathways may be more important for endocytosis of inactive integrins. Arrows with broken lines indicate integrin transport and recycling pathways.
Indeed, FA disassembly induced by microtubule regrowth is independent of RhoA and Rac1 activity, but requires several core components of the endocytosis machinery (Ezratty et al., 2005). The GTPase dynamin that is involved in vesicle abscission from the plasma membrane (Ferguson and De Camilli, 2012) is enriched around FAs in nocodazole-treated cells and continues to accumulate during the FA disassembly phase after nocodazole washout (Ezratty et al., 2005). Dynamin is recruited to FAs by FAK and is phosphorylated and activated by FA-associated Src kinase (Wang et al., 2011). Similarly, clathrin heavy chain, a key coat protein of clathrin-mediated endocytosis, also accumulates around FAs, and both dynamin and clathrin are required for microtubule regrowth-induced FA disassembly and integrin internalization (Fig. 2 C; Ezratty et al., 2005, 2009; Chao and Kunz, 2009). In addition, several adaptor proteins that link receptors in the endocytic vesicle membrane to the clathrin coat accumulate around FAs in these assays and are partially required for FA disassembly. Multiple studies found that the clathrin adaptors Dab2 and the closely related autosomal recessive hypercholesterolemia (ARH) are required for FA turnover (Chao and Kunz, 2009; Ezratty et al., 2009). Numb, another clathrin adaptor that was initially identified as a cell fate determinant in neuronal development, also accumulates around FAs and is required for integrin endocytosis (Nishimura and Kaibuchi, 2007). Numb may be recruited to FAs by the core clathrin adaptor AP-2 (Nishimura and Kaibuchi, 2007). AP-2 depletion results in enlarged FAs (Chao and Kunz, 2009), and dominant-negative adaptor proteins that perturb AP-2–clathrin complex formation inhibit integrin internalization (Arjonen et al., 2012). However, other studies have failed to detect localization of AP-2 or Numb near FAs or a requirement for FA disassembly (Ezratty et al., 2009). It is possible that adaptor proteins have endocytosis-independent functions. For example, Dab2 can directly bind the cytoplasmic domain of integrins and may compete with binding to the FA component talin independently of its role in endocytosis (Calderwood et al., 2003). However, such a competition has not been tested directly.
Differences in adaptor protein utilization also suggest that different cell types or different integrins may use different endocytic pathways. Adding to the complexity, integrins also exist in inactive or active, matrix-engaged conformational states (Margadant et al., 2011; Wehrle-Haller, 2012) that may be internalized by different routes, possibly using different clathrin adaptors (Arjonen et al., 2012). Because integrin interactions with Numb and talin are mutually exclusive (Nishimura and Kaibuchi, 2007), Numb-mediated endocytosis may be specific to inactive integrins. In contrast, it has also been proposed that inactive integrins are internalized by clathrin-independent endocytosis mechanisms (Echarri and Del Pozo, 2006), and further experimentation will be required to illuminate the complexity of different endocytosis pathways in integrin recycling and FA turnover.
Although microtubules are almost certainly required for long-range transport of endocytic vesicles, the manner in which microtubules are involved in early steps of endocytosis is less obvious. One possibility is that microtubules target parts of the endocytic machinery to FAs. Microtubules may facilitate transport of clathrin, adaptor proteins, or dynamin to FAs, but these proteins accumulate at FAs largely independent of microtubules (Nishimura and Kaibuchi, 2007; Chao and Kunz, 2009; Ezratty et al., 2009). In contrast, integrin-linked kinase (ILK)-mediated microtubule stabilization is required for caveolin-1 transport and caveolae formation at the cell surface (Fig. 2 A; Wickström et al., 2010a). Although their functions are diverse, caveolae likely represent a clathrin-independent endocytosis pathway (Echarri and Del Pozo, 2006; Kiss, 2012). Despite the name, ILK is thought to not function as a kinase but rather as an adaptor protein that links integrins to the actin cytoskeleton (Wickström et al., 2010b). ILK binds IQGAP1, and at least GFP-tagged IQGAP1 localizes near FAs (Wickström et al., 2010a). IQGAP1 interacts with and possibly recruits the formin mDia1 (Brandt et al., 2007), which has been proposed to stabilize microtubules downstream of RhoA by an unknown mechanism (Wen et al., 2004). Although this microtubule stabilization pathway appears unrelated to the +TIP-mediated mechanisms discussed earlier (Fig. 1), IQGAP1 has been reported to interact with APC and CLASPs (Watanabe et al., 2004, 2009). However, the functional significance of these interactions remains unclear.
FA disassembly by endocytosis would require internalization of active, ECM-engaged integrins. Thus, although endocytosis is evidently important for integrin recycling, it is difficult to envision how this could be the initial step of FA disassembly. FAs rapidly disassemble when actomyosin-mediated contractile forces are released, and kinesin-dependent transport of a relaxation factor along microtubules toward FAs has been postulated early on (Kaverina et al., 1999; Krylyshkina et al., 2002). The identity of such a relaxation factor is still unclear, although signaling molecules such as Src are transported along microtubules and could potentially contribute to FA turnover (Wu et al., 2008a). An alternative mechanism by which pulling forces could be released from FAs is to sever the connection between FAs and the actin cytoskeleton. The calcium-dependent protease calpain cleaves several FA proteins, including talin, that link integrins to the actin cytoskeleton (Bhatt et al., 2002; Bate et al., 2012). Although, inhibition of calpain-mediated talin cleavage perturbs FA disassembly (Franco et al., 2004), it is not by itself sufficient, and further proteolytic processing of the talin cleavage products is required (Huang et al., 2009). In addition, calpain cleavage of other FA components such as paxillin has the opposite effect and stabilizes FAs (Cortesio et al., 2011). It is also unclear how calpain activity could be spatially and temporally controlled in migrating cells, and there is no evidence of microtubule-based calpain transport. Finally, cleavage of an integral FA component such as talin in migrating cells with relatively rapid FA turnover appears to be a highly inefficient method of controlling FA dynamics. Thus, the role of calpain in FA dynamics is complex, and may be more important in the turnover of irreversibly attached FAs in the rear of the cell.
A more efficient way to trigger FA disassembly would be to cut integrin attachment to the ECM. Indeed, new findings indicate that cell-surface proteases are recruited to FAs, and may play a pivotal role in focused degradation of the ECM. The membrane type 1 matrix metalloprotease (MT1-MMP; also known as MMP-14) is targeted to and mediates ECM degradation near FAs (Takino et al., 2007; Wang and McNiven, 2012), and is required for fibronectin and integrin endocytosis (Shi and Sottile, 2011). MT1-MMP also promotes FA turnover (Takino et al., 2006), which would be expected if cleavage of integrin–ECM interactions release FA-associated pulling forces. Although a complex of FAK and p130Cas is required for MT1-MMP recruitment to FAs and for FA disassembly (Meenderink et al., 2010; Wang and McNiven, 2012), it is not known how MT1-MMP is transported to FAs. However, both microtubules and kinesin motors are necessary for MT1-MMP surface localization (Wiesner et al., 2010). Therefore, microtubules stabilized at FAs may provide a localized exocytosis pathway for MMPs that cleave integrin interactions with the ECM and as a result initiate FA disassembly. MMPs play important roles in in vivo cell migration and tissue remodeling, and aberrant extracellular proteolysis is associated with cancer cell invasion and metastasis (Kessenbrock et al., 2010). Despite recent controversy, FAs clearly exist in cells migrating in a 3D matrix (Gierke and Wittmann, 2012; Petrie et al., 2012). In addition, +TIP-mediated microtubule organization and vesicular trafficking are essential for normal protrusive behavior in 3D (Gierke and Wittmann, 2012). It seems probable that FA-targeted vesicular traffic has other important functions in addition to FA turnover. This may partially explain why there are so many different proteins involved in mediating microtubule–FA interactions. One future challenge will be to mechanistically understand how these pathways are linked, and how they function in physiological 3D tissue environments.
Acknowledgments
This work was supported by National Institutes of Health grant R01 GM079139 to T. Wittmann, and American Heart Association postdoctoral fellowship 10POST3870021 to S. Stehbens, and was conducted in a facility constructed with support from the Research Facilities Improvement Program grant C06 RR16490 from the National Center for Research Resources of the National Institutes of Health.
Footnotes
Abbreviations used in this paper:
- APC
- adenomatous polyposis coli
- CLASP
- cytoplasmic linker–associated protein
- FA
- focal adhesion
- GEF
- guanine nucleotide exchange factor
- ILK
- integrin-linked kinase
- MT1-MMP
- membrane type 1 matrix metalloprotease
References
- Akhmanova A., Steinmetz M.O. 2008. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 9:309–322 10.1038/nrm2369 [DOI] [PubMed] [Google Scholar]
- Akhmanova A., Hoogenraad C.C., Drabek K., Stepanova T., Dortland B., Verkerk T., Vermeulen W., Burgering B.M., De Zeeuw C.I., Grosveld F., Galjart N. 2001. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 104:923–935 10.1016/S0092-8674(01)00288-4 [DOI] [PubMed] [Google Scholar]
- Aoki K., Taketo M.M. 2007. Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J. Cell Sci. 120:3327–3335 10.1242/jcs.03485 [DOI] [PubMed] [Google Scholar]
- Applewhite D.A., Grode K.D., Keller D., Zadeh A.D., Slep K.C., Rogers S.L. 2010. The spectraplakin Short stop is an actin-microtubule cross-linker that contributes to organization of the microtubule network. Mol. Biol. Cell. 21:1714–1724 (published erratum appears in Mol. Biol. Cell 2010. 21:2097) 10.1091/mbc.E10-01-0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjonen A., Alanko J., Veltel S., Ivaska J. 2012. Distinct recycling of active and inactive β1 integrins. Traffic. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth A.I., Caro-Gonzalez H.Y., Nelson W.J. 2008. Role of adenomatous polyposis coli (APC) and microtubules in directional cell migration and neuronal polarization. Semin. Cell Dev. Biol. 19:245–251 10.1016/j.semcdb.2008.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate N., Gingras A.R., Bachir A., Horwitz R., Ye F., Patel B., Goult B.T., Critchley D.R. 2012. Talin contains a C-terminal calpain2 cleavage site important in focal adhesion dynamics. PLoS ONE. 7:e34461 10.1371/journal.pone.0034461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt A., Kaverina I., Otey C., Huttenlocher A. 2002. Regulation of focal complex composition and disassembly by the calcium-dependent protease calpain. J. Cell Sci. 115:3415–3425 [DOI] [PubMed] [Google Scholar]
- Bowen J.R., Hwang D., Bai X., Roy D., Spiliotis E.T. 2011. Septin GTPases spatially guide microtubule organization and plus end dynamics in polarizing epithelia. J. Cell Biol. 194:187–197 10.1083/jcb.201102076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt D.T., Marion S., Griffiths G., Watanabe T., Kaibuchi K., Grosse R. 2007. Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J. Cell Biol. 178:193–200 10.1083/jcb.200612071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitsprecher D., Jaiswal R., Bombardier J.P., Gould C.J., Gelles J., Goode B.L. 2012. Rocket launcher mechanism of collaborative actin assembly defined by single-molecule imaging. Science. 336:1164–1168 10.1126/science.1218062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M.S. 1989. Endocytosis and recycling of the fibronectin receptor in CHO cells. EMBO J. 8:1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard J.A., Webb D.J., Kaverina I. 2008. Asymmetric focal adhesion disassembly in motile cells. Curr. Opin. Cell Biol. 20:85–90 10.1016/j.ceb.2007.10.009 [DOI] [PubMed] [Google Scholar]
- Burnette D.T., Ji L., Schaefer A.W., Medeiros N.A., Danuser G., Forscher P. 2008. Myosin II activity facilitates microtubule bundling in the neuronal growth cone neck. Dev. Cell. 15:163–169 10.1016/j.devcel.2008.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood D.A., Fujioka Y., de Pereda J.M., García-Alvarez B., Nakamoto T., Margolis B., McGlade C.J., Liddington R.C., Ginsberg M.H. 2003. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc. Natl. Acad. Sci. USA. 100:2272–2277 10.1073/pnas.262791999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow M.G., Zozulya S., Gishizky M.L., Jallal B., Smeal T. 2005. PAK4 mediates morphological changes through the regulation of GEF-H1. J. Cell Sci. 118:1861–1872 10.1242/jcs.02313 [DOI] [PubMed] [Google Scholar]
- Canman J.C. 2009. Cytokinetic astralogy. J. Cell Biol. 187:757–759 10.1083/jcb.200911084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W.G., Wayner E.A., Bouchard T.S., Kaur P. 1990. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J. Cell Biol. 110:1387–1404 10.1083/jcb.110.4.1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell P.T., Vadrevu S., Norman J.C. 2009. Integrins: masters and slaves of endocytic transport. Nat. Rev. Mol. Cell Biol. 10:843–853 10.1038/nrm2799 [DOI] [PubMed] [Google Scholar]
- Chang Y.C., Nalbant P., Birkenfeld J., Chang Z.F., Bokoch G.M. 2008. GEF-H1 couples nocodazole-induced microtubule disassembly to cell contractility via RhoA. Mol. Biol. Cell. 19:2147–2153 10.1091/mbc.E07-12-1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao W.T., Kunz J. 2009. Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins. FEBS Lett. 583:1337–1343 10.1016/j.febslet.2009.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao W.T., Ashcroft F., Daquinag A.C., Vadakkan T., Wei Z., Zhang P., Dickinson M.E., Kunz J. 2010. Type I phosphatidylinositol phosphate kinase beta regulates focal adhesion disassembly by promoting beta1 integrin endocytosis. Mol. Cell. Biol. 30:4463–4479 10.1128/MCB.01207-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortesio C.L., Boateng L.R., Piazza T.M., Bennin D.A., Huttenlocher A. 2011. Calpain-mediated proteolysis of paxillin negatively regulates focal adhesion dynamics and cell migration. J. Biol. Chem. 286:9998–10006 10.1074/jbc.M110.187294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabek K., van Ham M., Stepanova T., Draegestein K., van Horssen R., Sayas C.L., Akhmanova A., Ten Hagen T., Smits R., Fodde R., et al. 2006. Role of CLASP2 in microtubule stabilization and the regulation of persistent motility. Curr. Biol. 16:2259–2264 10.1016/j.cub.2006.09.065 [DOI] [PubMed] [Google Scholar]
- Echarri A., Del Pozo M.A. 2006. Caveolae internalization regulates integrin-dependent signaling pathways. Cell Cycle. 5:2179–2182 10.4161/cc.5.19.3264 [DOI] [PubMed] [Google Scholar]
- Efimov A., Schiefermeier N., Grigoriev I., Ohi R., Brown M.C., Turner C.E., Small J.V., Kaverina I. 2008. Paxillin-dependent stimulation of microtubule catastrophes at focal adhesion sites. J. Cell Sci. 121:196–204 (published erratum appears in J. Cell Sci. 121:405) 10.1242/jcs.012666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng C.H., Huckaba T.M., Gundersen G.G. 2006. The formin mDia regulates GSK3beta through novel PKCs to promote microtubule stabilization but not MTOC reorientation in migrating fibroblasts. Mol. Biol. Cell. 17:5004–5016 10.1091/mbc.E05-10-0914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S., Manneville J.B., Nicholls S., Ferenczi M.A., Hall A. 2005. Cdc42 and Par6-PKCzeta regulate the spatially localized association of Dlg1 and APC to control cell polarization. J. Cell Biol. 170:895–901 10.1083/jcb.200412172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezratty E.J., Partridge M.A., Gundersen G.G. 2005. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 7:581–590 10.1038/ncb1262 [DOI] [PubMed] [Google Scholar]
- Ezratty E.J., Bertaux C., Marcantonio E.E., Gundersen G.G. 2009. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J. Cell Biol. 187:733–747 10.1083/jcb.200904054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S.M., De Camilli P. 2012. Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 13:75–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco S.J., Rodgers M.A., Perrin B.J., Han J., Bennin D.A., Critchley D.R., Huttenlocher A. 2004. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol. 6:977–983 10.1038/ncb1175 [DOI] [PubMed] [Google Scholar]
- Gao C., Chen Y.G. 2010. Dishevelled: The hub of Wnt signaling. Cell. Signal. 22:717–727 10.1016/j.cellsig.2009.11.021 [DOI] [PubMed] [Google Scholar]
- Gierke S., Wittmann T. 2012. EB1-recruited microtubule +TIP complexes coordinate protrusion dynamics during 3D epithelial remodeling. Curr. Biol. 22:753–762 10.1016/j.cub.2012.02.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev I., Borisy G., Vorobjev I. 2006. Regulation of microtubule dynamics in 3T3 fibroblasts by Rho family GTPases. Cell Motil. Cytoskeleton. 63:29–40 10.1002/cm.20107 [DOI] [PubMed] [Google Scholar]
- Gu Z., Noss E.H., Hsu V.W., Brenner M.B. 2011. Integrins traffic rapidly via circular dorsal ruffles and macropinocytosis during stimulated cell migration. J. Cell Biol. 193:61–70 10.1083/jcb.201007003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilluy C., Swaminathan V., Garcia-Mata R., O’Brien E.T., Superfine R., Burridge K. 2011. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat. Cell Biol. 13:722–727 10.1038/ncb2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan C.P., McCaffrey M.W. 2011. Rab GTPases and microtubule motors. Biochem. Soc. Trans. 39:1202–1206 10.1042/BST0391202 [DOI] [PubMed] [Google Scholar]
- Hotta A., Kawakatsu T., Nakatani T., Sato T., Matsui C., Sukezane T., Akagi T., Hamaji T., Grigoriev I., Akhmanova A., et al. 2010. Laminin-based cell adhesion anchors microtubule plus ends to the epithelial cell basal cortex through LL5alpha/beta. J. Cell Biol. 189:901–917 10.1083/jcb.200910095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Rajfur Z., Yousefi N., Chen Z., Jacobson K., Ginsberg M.H. 2009. Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration. Nat. Cell Biol. 11:624–630 10.1038/ncb1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries J.D., Byron A., Bass M.D., Craig S.E., Pinney J.W., Knight D., Humphries M.J. 2009. Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6. Sci. Signal. 2:ra51 10.1126/scisignal.2000396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka-Kogo A., Shimomura A., Senda T. 2005. Colocalization of APC and DLG at the tips of cellular protrusions in cultured epithelial cells and its dependency on cytoskeletons. Histochem. Cell Biol. 123:67–73 10.1007/s00418-004-0729-2 [DOI] [PubMed] [Google Scholar]
- Jaulin F., Kreitzer G. 2010. KIF17 stabilizes microtubules and contributes to epithelial morphogenesis by acting at MT plus ends with EB1 and APC. J. Cell Biol. 190:443–460 10.1083/jcb.201006044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchanawong P., Shtengel G., Pasapera A.M., Ramko E.B., Davidson M.W., Hess H.F., Waterman C.M. 2010. Nanoscale architecture of integrin-based cell adhesions. Nature. 468:580–584 10.1038/nature09621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakesisoglou I., Yang Y., Fuchs E. 2000. An epidermal plakin that integrates actin and microtubule networks at cellular junctions. J. Cell Biol. 149:195–208 10.1083/jcb.149.1.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I., Rottner K., Small J.V. 1998. Targeting, capture, and stabilization of microtubules at early focal adhesions. J. Cell Biol. 142:181–190 10.1083/jcb.142.1.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I., Krylyshkina O., Small J.V. 1999. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J. Cell Biol. 146:1033–1044 10.1083/jcb.146.5.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I., Krylyshkina O., Beningo K., Anderson K., Wang Y.L., Small J.V. 2002. Tensile stress stimulates microtubule outgrowth in living cells. J. Cell Sci. 115:2283–2291 [DOI] [PubMed] [Google Scholar]
- Kessenbrock K., Plaks V., Werb Z. 2010. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 141:52–67 10.1016/j.cell.2010.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M., Mitchison T. 1986. Beyond self-assembly: from microtubules to morphogenesis. Cell. 45:329–342 10.1016/0092-8674(86)90318-1 [DOI] [PubMed] [Google Scholar]
- Kiss A.L. 2012. Caveolae and the regulation of endocytosis. Adv. Exp. Med. Biol. 729:14–28 10.1007/978-1-4614-1222-9_2 [DOI] [PubMed] [Google Scholar]
- Kita K., Wittmann T., Näthke I.S., Waterman-Storer C.M. 2006. Adenomatous polyposis coli on microtubule plus ends in cell extensions can promote microtubule net growth with or without EB1. Mol. Biol. Cell. 17:2331–2345 10.1091/mbc.E05-06-0498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama A., Karakesisoglou I., Wong E., Vaezi A., Fuchs E. 2003. ACF7: an essential integrator of microtubule dynamics. Cell. 115:343–354 10.1016/S0092-8674(03)00813-4 [DOI] [PubMed] [Google Scholar]
- Krendel M., Zenke F.T., Bokoch G.M. 2002. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat. Cell Biol. 4:294–301 10.1038/ncb773 [DOI] [PubMed] [Google Scholar]
- Krylyshkina O., Kaverina I., Kranewitter W., Steffen W., Alonso M.C., Cross R.A., Small J.V. 2002. Modulation of substrate adhesion dynamics via microtubule targeting requires kinesin-1. J. Cell Biol. 156:349–359 10.1083/jcb.200105051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylyshkina O., Anderson K.I., Kaverina I., Upmann I., Manstein D.J., Small J.V., Toomre D.K. 2003. Nanometer targeting of microtubules to focal adhesions. J. Cell Biol. 161:853–859 10.1083/jcb.200301102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Wittmann T. 2012. +TIPs: SxIPping along microtubule ends. Trends Cell Biol. 22:418–428 10.1016/j.tcb.2012.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Lyle K.S., Gierke S., Matov A., Danuser G., Wittmann T. 2009. GSK3beta phosphorylation modulates CLASP-microtubule association and lamella microtubule attachment. J. Cell Biol. 184:895–908 10.1083/jcb.200901042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J.C., Han X., Hsiao C.T., Yates J.R., III, Waterman C.M. 2011. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol. 13:383–393 10.1038/ncb2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbergen G., Grigoriev I., Mimori-Kiyosue Y., Ohtsuka T., Higa S., Kitajima I., Demmers J., Galjart N., Houtsmuller A.B., Grosveld F., Akhmanova A. 2006. CLASPs attach microtubule plus ends to the cell cortex through a complex with LL5beta. Dev. Cell. 11:21–32 10.1016/j.devcel.2006.05.012 [DOI] [PubMed] [Google Scholar]
- Lewis A., Davis H., Deheragoda M., Pollard P., Nye E., Jeffery R., Segditsas S., East P., Poulsom R., Stamp G., et al. 2012. The C-terminus of Apc does not influence intestinal adenoma development or progression. J. Pathol. 226:73–83 10.1002/path.2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machacek M., Hodgson L., Welch C., Elliott H., Pertz O., Nalbant P., Abell A., Johnson G.L., Hahn K.M., Danuser G. 2009. Coordination of Rho GTPase activities during cell protrusion. Nature. 461:99–103 10.1038/nature08242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant C., Monsuur H.N., Norman J.C., Sonnenberg A. 2011. Mechanisms of integrin activation and trafficking. Curr. Opin. Cell Biol. 23:607–614 10.1016/j.ceb.2011.08.005 [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Fumoto K., Okamoto T., Kaibuchi K., Kikuchi A. 2010. Binding of APC and dishevelled mediates Wnt5a-regulated focal adhesion dynamics in migrating cells. EMBO J. 29:1192–1204 10.1038/emboj.2010.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meenderink L.M., Ryzhova L.M., Donato D.M., Gochberg D.F., Kaverina I., Hanks S.K. 2010. P130Cas Src-binding and substrate domains have distinct roles in sustaining focal adhesion disassembly and promoting cell migration. PLoS ONE. 5:e13412 10.1371/journal.pone.0013412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri D., Marshall C.B., Greeve M.A., Kim B., Balan M., Suarez F., Bakal C., Wu C., Larose J., Fine N., et al. 2012. Mechanistic insight into the microtubule and actin cytoskeleton coupling through dynein-dependent RhoGEF inhibition. Mol. Cell. 45:642–655 (published erratum appears in Mol. Cell 2012. 45:844) 10.1016/j.molcel.2012.01.027 [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y., Shiina N., Tsukita S. 2000. Adenomatous polyposis coli (APC) protein moves along microtubules and concentrates at their growing ends in epithelial cells. J. Cell Biol. 148:505–518 10.1083/jcb.148.3.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y., Grigoriev I., Lansbergen G., Sasaki H., Matsui C., Severin F., Galjart N., Grosveld F., Vorobjev I., Tsukita S., Akhmanova A. 2005. CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J. Cell Biol. 168:141–153 10.1083/jcb.200405094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro-Venegas C., Tortosa E., Rosso S., Peretti D., Bollati F., Bisbal M., Jausoro I., Avila J., Cáceres A., Gonzalez-Billault C. 2010. MAP1B regulates axonal development by modulating Rho-GTPase Rac1 activity. Mol. Biol. Cell. 21:3518–3528 10.1091/mbc.E09-08-0709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbant P., Chang Y.C., Birkenfeld J., Chang Z.F., Bokoch G.M. 2009. Guanine nucleotide exchange factor-H1 regulates cell migration via localized activation of RhoA at the leading edge. Mol. Biol. Cell. 20:4070–4082 10.1091/mbc.E09-01-0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näthke I.S., Adams C.L., Polakis P., Sellin J.H., Nelson W.J. 1996. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J. Cell Biol. 134:165–179 10.1083/jcb.134.1.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Kaibuchi K. 2007. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev. Cell. 13:15–28 10.1016/j.devcel.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Okada K., Bartolini F., Deaconescu A.M., Moseley J.B., Dogic Z., Grigorieff N., Gundersen G.G., Goode B.L. 2010. Adenomatous polyposis coli protein nucleates actin assembly and synergizes with the formin mDia1. J. Cell Biol. 189:1087–1096 10.1083/jcb.201001016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo A.F., Eng C.H., Schlaepfer D.D., Marcantonio E.E., Gundersen G.G. 2004. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science. 303:836–839 10.1126/science.1091325 [DOI] [PubMed] [Google Scholar]
- Paranavitane V., Coadwell W.J., Eguinoa A., Hawkins P.T., Stephens L. 2003. LL5beta is a phosphatidylinositol (3,4,5)-trisphosphate sensor that can bind the cytoskeletal adaptor, gamma-filamin. J. Biol. Chem. 278:1328–1335 10.1074/jbc.M208352200 [DOI] [PubMed] [Google Scholar]
- Parsons J.T., Horwitz A.R., Schwartz M.A. 2010. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 11:633–643 10.1038/nrm2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie R.J., Gavara N., Chadwick R.S., Yamada K.M. 2012. Nonpolarized signaling reveals two distinct modes of 3D cell migration. J. Cell Biol. 197:439–455 10.1083/jcb.201201124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekny A., Werner M., Glotzer M. 2005. Cytokinesis: welcome to the Rho zone. Trends Cell Biol. 15:651–658 10.1016/j.tcb.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Ren Y., Li R., Zheng Y., Busch H. 1998. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J. Biol. Chem. 273:34954–34960 10.1074/jbc.273.52.34954 [DOI] [PubMed] [Google Scholar]
- Ren X.D., Kiosses W.B., Schwartz M.A. 1999. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18:578–585 10.1093/emboj/18.3.578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rid R., Schiefermeier N., Grigoriev I., Small J.V., Kaverina I. 2005. The last but not the least: the origin and significance of trailing adhesions in fibroblastic cells. Cell Motil. Cytoskeleton. 61:161–171 10.1002/cm.20076 [DOI] [PubMed] [Google Scholar]
- Rinnerthaler G., Geiger B., Small J.V. 1988. Contact formation during fibroblast locomotion: involvement of membrane ruffles and microtubules. J. Cell Biol. 106:747–760 10.1083/jcb.106.3.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez O.C., Schaefer A.W., Mandato C.A., Forscher P., Bement W.M., Waterman-Storer C.M. 2003. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat. Cell Biol. 5:599–609 10.1038/ncb0703-599 [DOI] [PubMed] [Google Scholar]
- Rogers S.L., Wiedemann U., Häcker U., Turck C., Vale R.D. 2004. Drosophila RhoGEF2 associates with microtubule plus ends in an EB1-dependent manner. Curr. Biol. 14:1827–1833 10.1016/j.cub.2004.09.078 [DOI] [PubMed] [Google Scholar]
- Rooney C., White G., Nazgiewicz A., Woodcock S.A., Anderson K.I., Ballestrem C., Malliri A. 2010. The Rac activator STEF (Tiam2) regulates cell migration by microtubule-mediated focal adhesion disassembly. EMBO Rep. 11:292–298 10.1038/embor.2010.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon W.C., Adams M.C., Waterman-Storer C.M. 2002. Dual-wavelength fluorescent speckle microscopy reveals coupling of microtubule and actin movements in migrating cells. J. Cell Biol. 158:31–37 10.1083/jcb.200203022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober M., Raghavan S., Nikolova M., Polak L., Pasolli H.A., Beggs H.E., Reichardt L.F., Fuchs E. 2007. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J. Cell Biol. 176:667–680 10.1083/jcb.200608010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Sottile J. 2011. MT1-MMP regulates the turnover and endocytosis of extracellular matrix fibronectin. J. Cell Sci. 124:4039–4050 10.1242/jcs.087858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J.V., Kaverina I. 2003. Microtubules meet substrate adhesions to arrange cell polarity. Curr. Opin. Cell Biol. 15:40–47 10.1016/S0955-0674(02)00008-X [DOI] [PubMed] [Google Scholar]
- Suozzi K.C., Wu X., Fuchs E. 2012. Spectraplakins: Master orchestrators of cytoskeletal dynamics. J. Cell Biol. 197:465–475 10.1083/jcb.201112034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takino T., Watanabe Y., Matsui M., Miyamori H., Kudo T., Seiki M., Sato H. 2006. Membrane-type 1 matrix metalloproteinase modulates focal adhesion stability and cell migration. Exp. Cell Res. 312:1381–1389 10.1016/j.yexcr.2006.01.008 [DOI] [PubMed] [Google Scholar]
- Takino T., Saeki H., Miyamori H., Kudo T., Sato H. 2007. Inhibition of membrane-type 1 matrix metalloproteinase at cell-matrix adhesions. Cancer Res. 67:11621–11629 10.1158/0008-5472.CAN-07-5251 [DOI] [PubMed] [Google Scholar]
- Vasiliev J.M., Gelfand I.M., Domnina L.V., Ivanova O.Y., Komm S.G., Olshevskaja L.V. 1970. Effect of colcemid on the locomotory behaviour of fibroblasts. J. Embryol. Exp. Morphol. 24:625–640 [PubMed] [Google Scholar]
- Wang Y., McNiven M.A. 2012. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J. Cell Biol. 196:375–385 10.1083/jcb.201105153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Cao H., Chen J., McNiven M.A. 2011. A direct interaction between the large GTPase dynamin-2 and FAK regulates focal adhesion dynamics in response to active Src. Mol. Biol. Cell. 22:1529–1538 10.1091/mbc.E10-09-0785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Wang S., Noritake J., Sato K., Fukata M., Takefuji M., Nakagawa M., Izumi N., Akiyama T., Kaibuchi K. 2004. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev. Cell. 7:871–883 10.1016/j.devcel.2004.10.017 [DOI] [PubMed] [Google Scholar]
- Watanabe T., Noritake J., Kakeno M., Matsui T., Harada T., Wang S., Itoh N., Sato K., Matsuzawa K., Iwamatsu A., et al. 2009. Phosphorylation of CLASP2 by GSK-3beta regulates its interaction with IQGAP1, EB1 and microtubules. J. Cell Sci. 122:2969–2979 10.1242/jcs.046649 [DOI] [PubMed] [Google Scholar]
- Waterman-Storer C.M., Worthylake R.A., Liu B.P., Burridge K., Salmon E.D. 1999. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat. Cell Biol. 1:45–50 10.1038/9018 [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B. 2012. Structure and function of focal adhesions. Curr. Opin. Cell Biol. 24:116–124 10.1016/j.ceb.2011.11.001 [DOI] [PubMed] [Google Scholar]
- Wen Y., Eng C.H., Schmoranzer J., Cabrera-Poch N., Morris E.J., Chen M., Wallar B.J., Alberts A.S., Gundersen G.G. 2004. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat. Cell Biol. 6:820–830 10.1038/ncb1160 [DOI] [PubMed] [Google Scholar]
- Wickström S.A., Lange A., Hess M.W., Polleux J., Spatz J.P., Krüger M., Pfaller K., Lambacher A., Bloch W., Mann M., et al. 2010a. Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev. Cell. 19:574–588 10.1016/j.devcel.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickström S.A., Lange A., Montanez E., Fässler R. 2010b. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J. 29:281–291 10.1038/emboj.2009.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner C., Faix J., Himmel M., Bentzien F., Linder S. 2010. KIF5B and KIF3A/KIF3B kinesins drive MT1-MMP surface exposure, CD44 shedding, and extracellular matrix degradation in primary macrophages. Blood. 116:1559–1569 10.1182/blood-2009-12-257089 [DOI] [PubMed] [Google Scholar]
- Wittmann T., Waterman-Storer C.M. 2001. Cell motility: can Rho GTPases and microtubules point the way? J. Cell Sci. 114:3795–3803 [DOI] [PubMed] [Google Scholar]
- Wittmann T., Waterman-Storer C.M. 2005. Spatial regulation of CLASP affinity for microtubules by Rac1 and GSK3beta in migrating epithelial cells. J. Cell Biol. 169:929–939 10.1083/jcb.200412114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann T., Bokoch G.M., Waterman-Storer C.M. 2003. Regulation of leading edge microtubule and actin dynamics downstream of Rac1. J. Cell Biol. 161:845–851 10.1083/jcb.200303082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfenson H., Henis Y.I., Geiger B., Bershadsky A.D. 2009. The heel and toe of the cell’s foot: a multifaceted approach for understanding the structure and dynamics of focal adhesions. Cell Motil. Cytoskeleton. 66:1017–1029 10.1002/cm.20410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Decourt B., Zabidi M.A., Wuethrich L.T., Kim W.H., Zhou Z., MacIsaac K., Suter D.M. 2008a. Microtubule-mediated Src tyrosine kinase trafficking in neuronal growth cones. Mol. Biol. Cell. 19:4611–4627 10.1091/mbc.E08-06-0603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Kodama A., Fuchs E. 2008b. ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell. 135:137–148 10.1016/j.cell.2008.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Shen Q.T., Oristian D.S., Lu C.P., Zheng Q., Wang H.W., Fuchs E. 2011. Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3β. Cell. 144:341–352 10.1016/j.cell.2010.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaoui K., Benseddik K., Daou P., Salaün D., Badache A. 2010. ErbB2 receptor controls microtubule capture by recruiting ACF7 to the plasma membrane of migrating cells. Proc. Natl. Acad. Sci. USA. 107:18517–18522 10.1073/pnas.1000975107 [DOI] [PMC free article] [PubMed] [Google Scholar]