Abstract
Background
Arteriogenesis and collateral formation are complex processes requiring integration of multiple inputs to coordinate vessel branching, growth, maturation and network size. Factors regulating these processes have not been determined.
Methods and Results
We used a dominant-negative IκBαSR construct under control of an endothelial-specific inducible promoter to selectively suppress endothelial NFκB activation during development or in the adult vasculature or in vitro. Inhibition of NFκB activation resulted in formation of an excessively branched arterial network that was composed of immature vessels and provided poor distal tissue perfusion. Molecular analysis demonstrated reduced adhesion molecules expression leading to decreased monocyte influx, reduced HIF-1α levels and a marked decrease in Dll4 expression with a consequent decrease in Notch signaling. The latter was the principal cause of increased vascular branching, as treatment with Jagged-1 peptide reduced the size of arterial network to baseline levels.
Conclusions
These findings identify NFκB as a key regulator of adult and developmental arteriogenesis and collateral formation. NFkB achieves this by regulating HIF1α-dependent expression of VEGF-A and PDGF-BB that are necessary for development and maturation of the arterial collateral network and by regulating Dll4 expression that in turn determines the network’s size and complexity.
Keywords: arteriogenesis, NFκB, HIF, Dll4
INTRODUCTION
Development of arterial circulation (arteriogenesis) in general and arterial collateral circulation in particular is poorly understood. During embryogenesis formation of arteries is driven by little known molecular processes that specify arterial fate and determine physical extent and branching pattern of the newly forming vasculature as well as by complex spatial guidance clues1–4. In adult settings arteriogenesis occurs in a limited set of circumstances with shear stress5, 6 and inflammation7, 8 thought to be key drivers. Collaterals represent a special case of arterial circulation9. By definition, these are arterial vessels connecting two arteries or artery branches and for this reason have been thought to play a protective role, allowing continuous arterial blood supply in cases of individual arterial trunk occlusions10. Indeed, there is a substantial body of literature connecting extensive collateral circulation to improved clinical and functional outcomes11, 12.
Similar to arteriogenesis, drivers of collateral development and factors specifying their location and extent are largely unknown. The extent of arterial branching during development is one of the key factors determining overall arterial density and the frequency of arterial connections, thereby directly affecting collateral formation. Notch signaling plays a central role in regulation of vascular branching morphogenesis2. Alterations in expression of Notch receptors (Notch-1 and Notch-4) and their ligands delta-like (Dll) 1 and Dll4 have been linked to alterations in collateral extent. In particular, Dll4 is required for normal vascular development. Loss of even a single Dll4 allele results in early embryonic lethality due to lack of well-defined arteries and increased number of vessel branches and sprouts13, 14. A similar phenotype is observed in Notch1 knockout mice15. In adult tissues, Dll4 expression is observed in newly forming arteries and capillary sprouts after ischemia16.
Dll1 is also essential for arterial development with Dll1-Notch signaling required for arterial expression of VEGFR2 and neuropilin-1 with the absence of Dll1 resulting in increased expression of a vein-specific transcription factor COUP-TFII17. Mice heterozygous for Dll1 have fewer tip cells during angiogenic sprouting in the developing retinal circulation and impaired vessel branching18. Furthermore, adult heterozygous Dll1 mice demonstrate impaired collateral growth and reduced endothelial Notch activation in the hindlimb ischemia model leading to impaired blood flow recovery19. While Delta/Notch signaling determines arterial vasculature patterning, VEGF-A plays a central role in directing developmental and adult arteriogenesis20 and it is equally critical to collateral formation21, 22. A decrease in VEGF signaling impairs arterial developmental and adult arterial branching morphogenesis23, 24.
Whereas VEGF plays a central role in development and postnatal arteriogenesis, the source of the growth factor and the stimulus for its production in arteriogenesis in adult tissues are subject to debate. Principal possibilities include ischemic tissues, infiltrating blood-derived monocytes/macrophages and the blood vessels themselves. The key factor regulating VEGF production in ischemic tissues is HIF1α. HIF1α levels in turn are controlled in a highly complex manner by several regulators including VHL and PHD proteins25. To add to the complexity, recent studies have suggested that HIF levels are also controlled by the transcriptional factor nuclear factor kappa-B (NFκB) 26 with NFκB subunits RelA (p65) and p50 directly interacting with HIF-1α promoter27. Importantly, unlike other HIF1α regulators, NFκB can stabilize HIF1α levels not just under hypoxic but also normoxic conditions28. The latter is critically important to arteriogenesis as growth of collateral arteries occurs in tissues demonstrating minimal ischemia29.
Monocyte-derived macrophages are another source of VEGF during adult arteriogenesis. Indeed, monocytes have long been considered to be crucial to arteriogenesis7, 30 due in part to their production of VEGF-A and FGF229. A number of signals have been proposed to trigger monocyte recruitment at arteriogenesis sites including shear-stress-induced activation of adhesion molecules and local production of VEGF that in, turn, trigger synthesis of SDF-131.
In settings of adult arteriogenesis, where hypoxia is not a major driver, NFκB in the endothelium can be activated by increased shear stress caused by an arterial occlusion. This activation of the endothelial NFκB cascade can lead not only to local production of VEGF but also to accumulation of blood-derived monocytes/macrophages due to increased expression of adhesion molecules leading to further increase in local accumulation of VEGF. NFκB cascade, therefore, appears central to two important events in arteriogenesis- production of VEGF and accumulation of monocytes/macrophages. Despite the seeming importance of this role, NFκB function in arteriogenesis has not been fully addressed and there is no known link between NFκB and Delta/Notch signaling pathway.
In the present study we thought to examine the role of endothelial NFκB in adult arteriogenesis by expressing a dominant negative IκBα construct that fully blocks NFκB activation. This resulted in decreased activation of adhesion molecules, a marked reduction in monocytes influx into tissues and decreased HIF1α activation. Surprisingly, this was also associated with a massive increase in arterial collaterals and a very poor restoration of flow that was due to decreased expression of Dll4. Furthermore, the newly formed vasculature was very immature with poor mural cell coverage. Thus, in addition to controlling HIF-dependent activation of VEGF production and monocyte accumulation, NFκB also regulates Dll4/Notch-dependent regulation of branching morphogenesis and extent of collateral formation. These data place NFκB at the center of arteriogenic response.
Materials and Methods
Conditional transgenic mice: IkBαSR
To generate conditional transgenic mice, IkBαSR-HA cDNA (kindly provided by Dr. J.A. DiDonato) 32 was subcloned in pBI-G vector (Clonetch) that is sensitive to tetracycline transactivator (tTA) and allows simultaneous expression of LacZ and IkBαSR-HA under the control of a tetracycline response promoter (TRE). Endothelial specific expression of IkBαSR was achieved by crossing TRE-IkBαSR with Tie2-tTA33 or VEC-tTA34 transgenic mouse lines. For hindlimb ischemia model the transgene expression of IkBαSR, repressed during embryonic and postnatal development by doxycycline in diet 200 mg/kg (Bio-Serv) was induced on normal chow diet, at 10–12 weeks of age, 3 days before femoral artery ligation. In the case of retinal vasculature assessment, the transgene expression of VEC- IkBαSR was continuously induced during embryonic and postnatal development. Littermates inheriting only IkBαSR or tTA transgene were used as controls. All animal experiments were performed under a protocol approved by the Institutional Animal Care and Use Committee of Yale University.
Hindlimb ischemia model, Laser Doppler Imaging (LDI) and High Resolution computed Tomography (micro-CT)
were performed as described previously 35. Flow images were analyzed with Moor LDI processing software and reported as the ratio of flow in the right (ischemic) to left (nonischemic) hindlimb (R/L). Quantitative micro-CT data were expressed as a vascular segment number, representing total number of vessels, of specified diameter, counted in 500 Z-stack sections for thigh or 250 Z-stack sections for calf images.
Immunohistochemistry
Frozen sections of adductor or gastrocnemius muscle samples were immunostained with antibodies specific for CD45 Ab (eBioscience), CD31 (Chemicon), HA-tag (Roche), NG2 chondroitin sulphate proteoglycan (Millipore). Nuclei were visualized with DAPI staining. Cell coverage area was determined in captured images with Zeiss confocal microscope and analyzed using Image J software.
Flow cytometry (FSC/SSC)
of mechanically dissociated muscle specimens was performed on a FACSCanto (BD Biosciences, San Jose, CA), gating on CD45+ leukocytes. Antibodies were specific for CD45 (30-F11), CD11c (HL3), from BD Biosciences; F4/80 (BM8), CD11b (M1/70), Gr1 (RB6-8C5), B220 (RA3-6B2) and CD3 (145-2C11), from eBioscence.
Cell culture experiments
Confluent HUVECs (Lonza), passage 3, cultured in complete medium (Lonza) at 37°C, 5%CO2, were transduced with Ad-Null, Ad-GFP or Ad- IkBαSR (UNC Gene Therapy Vector Core) (100 MOI) for 24–48 hr. The treatment with TNFα (10ng/ml) or VEGFA (50ng/ml) was carried out for 24 hr in starvation medium (EBM-2 medium (Lonza) supplemented with 0.5% FBS, 0.25% BSA and antibiotics. Cells were starved overnight prior treatment.
Hypoxia experiments
HUVECs were transduced with Ad- GFP or Ad-IkBαSR and cultured under either normoxic (room air) or hypoxic (0.4% O2) conditions. After 24 hr, endothelial cells were harvested and nuclear extract isolated using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific).
Shear stress experiments
Flow experiments were performed using a parallel plate flow chamber. HUVECs transduced with Ad-GFP or Ad-IkBαSR were plated on 10 µg/ml fibronectin coated slides. After overnight starvation in media containing 2% FBS and 0.1% ECGS cells were subjected to oscillatory flow for 18 hr as described.36
Endothelial cell sprouting assay
HUVEC were transduced with 2X106 pfu Ad-IkBαSR or Ad-Null. 24 hours after transduction, cells were harvested and resuspended in 300µl fibrinogen solution (2.5mg/ml fibrinogen (Sigma) in EBM-2 supplemented with 2%FBS and 50µg/ml aprotinin (Sigma)), and plated on top of a pre-coated fibrin layer (400µl fibrinogen solution clotted with 1U thrombin (Sigma) for 20min at 37C). The second layer of fibrin was added and allowed to clot for 1 hour at 37°C. Human fibroblasts, WI-38 cells (250,000 cells/well), in EBM-2 supplemented with 2%FBS and 25ng/ml VEGF-A, were then plated on top of the second fibrin layer. Cultures were then incubated at 37°C, 5%CO2. After 48 hours, the 17-mer JAG1 peptide (CDDYYYGFGCNKFCRPR) or control scrambled peptide (RCGPDCFDNYGRYKYCF) 37 was added to the cultures. After 3–5 days, the cultures were labeled with 4µg/ml Calcein AM for 1 hour, and imaged by fluorescence using a standard FITC filter.
Whole mount retina staining
Eyeballs were removed from neonates at P4 and processed as described 24. Quantification of retinal vasculature was done using the Biologic CMM Analyser Software 38. The data are presented as a number of vessel branchpoints, vessel length and number of segments per 10X magnification fields.
Whole mount brain staining
Brains were harvested at P4, fixed in 1:4 DMSO/methanol overnight at 4°C, washed 3 times in methanol and stored in methanol at −20°. Brains were then re-hydrated, washed in PBS and blocked overnight in TNB with 0.5% Triton-X. After overnight incubation with Cy3-SMA (Sigma), brains were washed and imaged using a fluorescent dissecting microscope.
Jagged peptide treatment in neonates
Jag1 peptide or scrambled peptide were resuspended in 50% DMSO and 50% H2O at a concentration of 10 mg/ml. The peptides were injected (25 μl/injection) subcutaneously 3 times every 12 hours starting at P2. Mice were sacrificed at P4 and retinas harvested.
Western blotting
Immunoblotting
Proteins, tissue or cell homogenates, were extracted in RIPA lysis buffer supplemented with protease inhibitor cocktail (Roche). The lysates were clarified by ultracentrifugation (14,000 rpm, 15 min at 4°C), and 30 µg protein extract, determined by BCA assay, was subjected to SDS-PAGE. Following transfer to Immobilon membranes (Millipore), the membranes were blocked with 5% nonfat dried milk in TBS, pH 7.4, containing 0.1% Tween 20, and immunoblotted using specific antibodies against: IkBα, VEGF-A, VECadherin, PDGFb, ICAM-1, actin (Santa Cruz Biotechnology); HA tag (Roche); Jagged1, Dll4 , Notch1, Cleaved Notch (Val 1744), VEGFR2, PDGFRb, FGFR1, IGF-IRβ, GFP (Cell Signaling);HIF1α (BD Transduction Laboratories); HIF2α (Novus Biological); TBN (Abcam). Immunoreactive bands were visualized using the horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescent substrate (Pierce). Images were captured with G:Box gel imaging system (Syngene).
Statistical analysis
Data are presented as mean ± SEM. Differences between multiple groups were assessed using one-way ANOVA followed by Bonferonni post-hoc test for multiple comparisons. Comparisons between 2 independent groups were performed using a two-sample t test. Flow measurements were analyzed using a linear mixed effect model for repeated measurements as well as t tests. All p values were calculated using two-tailed statistical tests. Differences were considered significant whe p<0.05. Data were analyzed using IBM SPSS Statistics 19.00.
Results
NFkB suppression in endothelial cells impairs effective arteriogenesis
To study the role of endothelial NFkB in angiogenesis and arteriogenesis, we generated a transgenic mouse expressing a mutant form of IkBα under control of ischemia-sensitive Tie2 or VE-cadherin (VEC) promoter using a “tet-off” approach. The double transgenic mice (Tie2-IkBαSR, VEC-IkBαSR) were kept on doxycycline throughout development and postnatal life with the drug being discontinued 3 days prior to initiation of experiments.
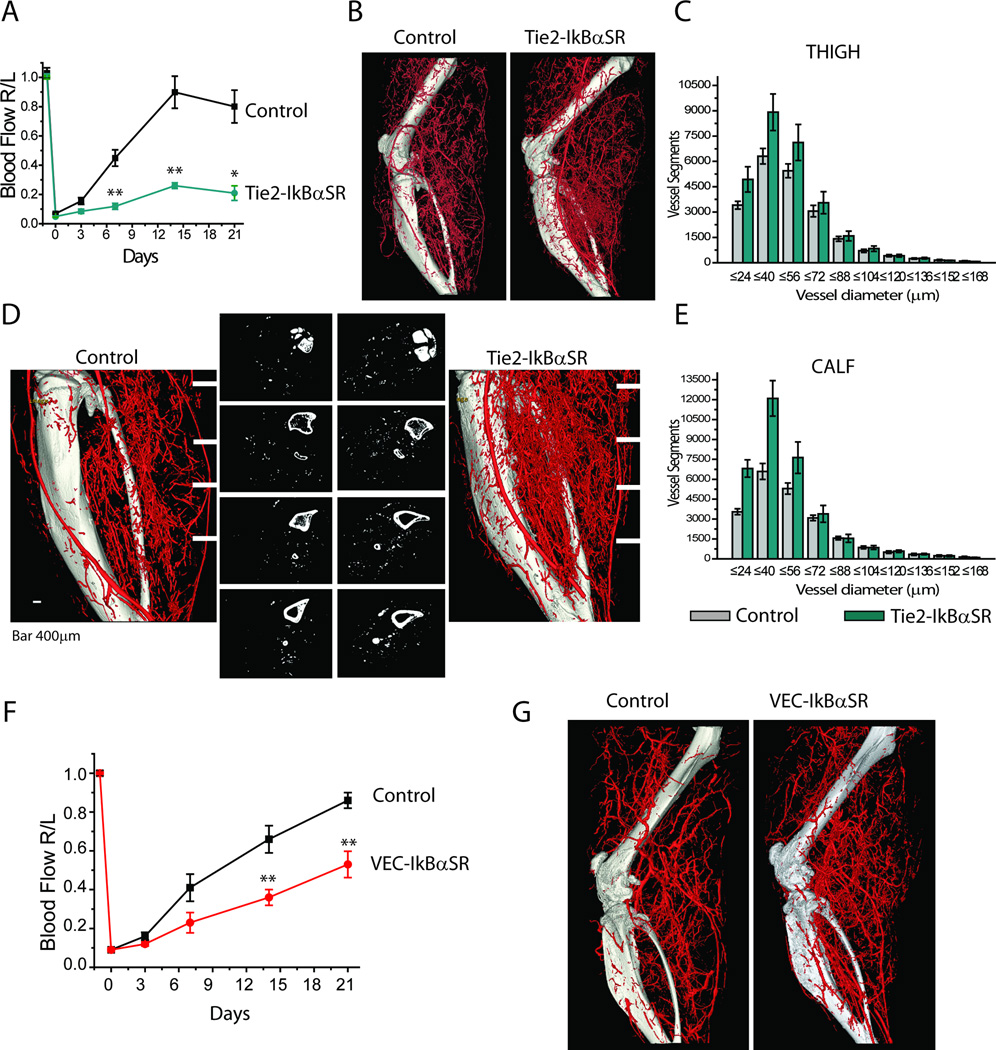
Serial measurements of blood flow in the distal hindlimb following ligation of the common femoral artery using serial deep penetrating laser-Doppler demonstrated nearly complete recovery in control mice while perfusion in Tie2-IkBαSR mice never recovered (Fig 1A). Surprisingly, despite this profound reduction in blood flow recovery, micro-CT (mCT) imaging of the hindlimb vasculature demonstrated extensive development of the above-the-knee arterial collateral circulation both in control and Tie2- IkBαSR mice groups (Fig 1B). Quantitative analysis demonstrated higher numbers of smaller arteries in Tie2-IkBαSR transgenics (Fig 1C). Furthermore, below the knee mCT images of arterial vasculature showed very extensive neovascularization in Tie2-IkBαSR mice with a markedly abnormal vascular branching pattern reminiscent of a tumor circulation (Fig 1D,E).
Figure 1.
Impaired blood flow recovery and increased arteriolar branches in Tie2-IkBαSR and VEC-IkBαSR transgenic mice following ligation of femoral artery. (A) Blood flow perfusion in Tie2-IkBαSR shown as ratio of right to left hindlimb (R/L) at various time points after femoral artery ligation. (B) Representative micro-CT angiograms of entire hindlimb 21 days after femoral artery ligation. (C) Quantitative analysis of micro-CT angiograms presented as a total number of vascular structures of specified diameters counted in thigh. (D) Distribution of vascular structures below the knee. Representative 2D micro-CT images at 4 comparable levels in Tie2-IkBαSR transgenic mice compared with controls. (E) Quantitative analysis of micro-CT angiograms presented as total number of vascular structures of specified diameter counted in calf. (F) Blood flow perfusion in VEC-IkBαSR transgenic mice as a ratio R/L hindlimb at various time points after femoral artery ligation. (G) Representative micro-CT angiograms of entire hindlimb 21 days after femoral artery ligation. *p<0.05, ** p<0.01, n=3-8mice/group. Levene’s test for equality of variance was applied; it was found that measurements at days 7 and 14 had heterogeneous variances, so the unequal-variance version of the t-test was used (Fig. 1A).
To exclude the possibility that Tie-2 expression in cells other than endothelium was responsible for the observed phenotype, we used VE-cadherin promoter as a driver for IkBαSR expression. Similar to Tie2-IkBαSR mice, VEC-IkBαSR mice demonstrated a significant decline in blood flow recovery following the common femoral artery ligation (Fig 1F) that was associated with an increase in the number of small arteries in ischemic hindlimb (Fig 1G).
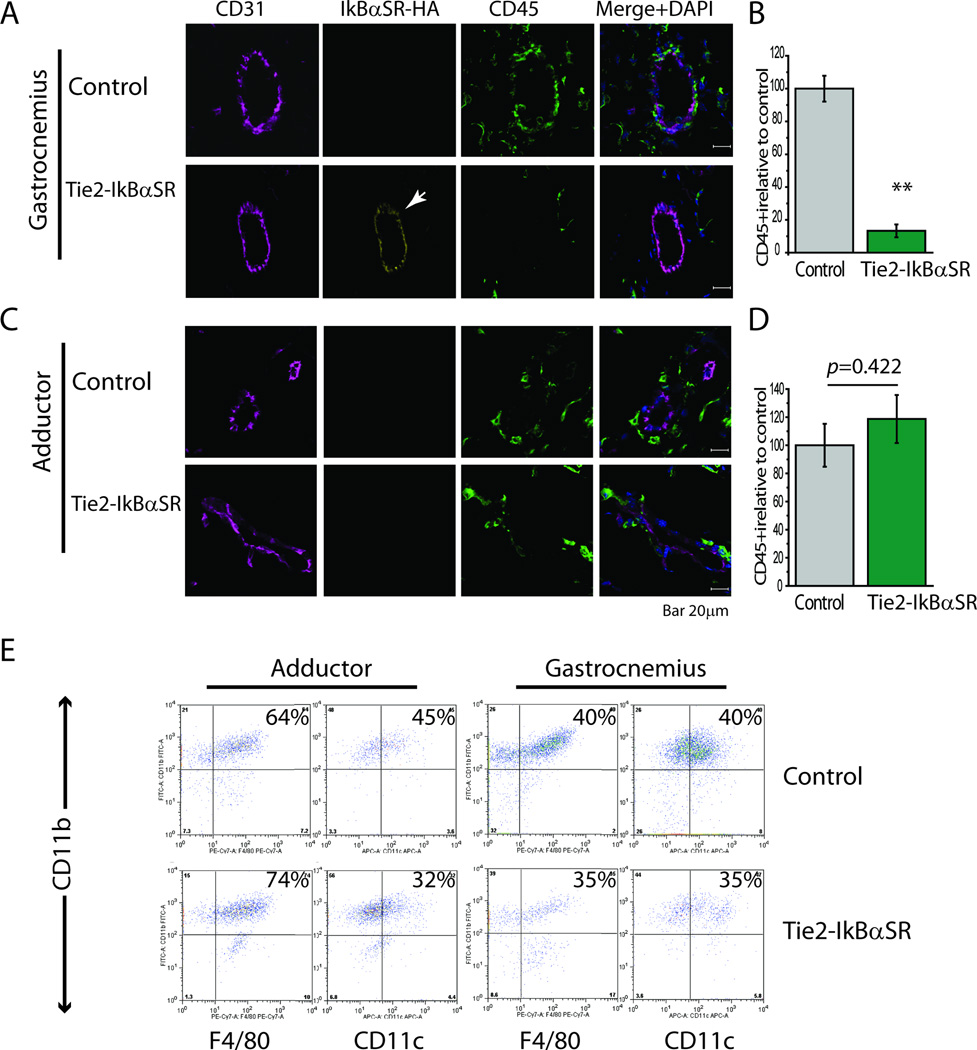
Arteriogenesis is thought to be to a large extent driven by accumulation of monocyte-derived macrophages around the remodeling vasculature. To explore the effect of shutdown of NFkB signaling on this process, we examined accumulation of various mononuclear cell subsets in above- and below-the-knee tissues of Tie2-IkBαSR and control mice following induction of ischemia. Immunocytochemical analysis demonstrated extensive transgene expression in endothelial cells in tissue sections from ischemic gastrocnemius muscle while its expression in the relative non-ischemic adductor muscle was quite low (Fig 2A,B). In agreement with the shutdown in endothelial NFκB activation, there was a marked decrease in the number of CD45+ mononuclear cells in gastrocnemius of Tie2-IkBαSR compared to control mice (Fig 2C), while there were no differences in the presence of these cells in the adductor region (Fig 2D). To confirm that ischemia activation of Tie2-driven IkBαSR construct expression reduces equally influx of various subtypes blood-derived mononuclear cells, FAC sorting was used to determine the presence of Cd11b, CD11c and F4/80 macrophages. While absolute numbers were markedly lower in tissues from Tie2-IkBαSR, the relative cellular subtypes’ composition was the same in both groups (Fig 2E).
Figure 2.
Decreased accumulation of blood-derived mononuclear cells in Tie2-IkBαSR transgenic mice. (A) Representative immunostaining of gastrocnemius muscle sections with antibodies against CD45, CD31 and HA-tag at day 3. (B) Quantification of CD45+ cells in gastrocnemius muscle in Tie2-IkBαSR transgenic mice as compared with controls. (C) Representative immunostaining of adductor muscle sections with antibodies against CD45, CD31 and HA-tag at day 3. (D) Quantification of CD45+ cells in adductor muscle in Tie2-IkBαSR transgenic mice as compared with controls. (E) Representative flow cytometry (FSC/SSC) analysis of monocytes (CD11b+F4/80−), macrophages (CD11b+F4/80+) and dendritic cells (CD11b+CD11c+) in adductor and gastrocnemius muscles 3 days after femoral artery ligation.
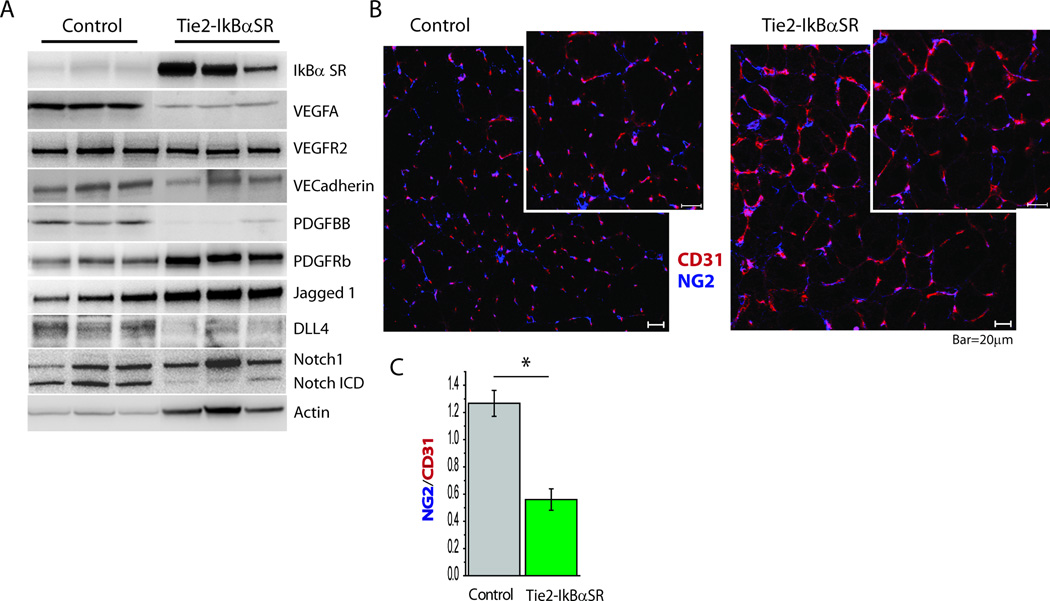
To establish the molecular mechanism responsible for the marked increased in vascular branching in Tie2-IkBαSR mice, we next set out to examine key potential regulators. Western blotting of gastrocnemius tissues confirmed expression of IkBαSR construct inTie2-IkBαSR mice (Fig. 3A). This endothelial shutdown of NFκB activation was associated with a marked reduction in tissue expression of HIF-1α-dependent genes VEGF-A and PDGF-BB and a moderate reduction in VEGFR2. Examination of the Notch signaling cascade demonstrated somewhat increased Jag-1 and markedly decreased Dll4 and Notch ICD levels suggesting reduced Notch activation (Fig 3A). Since PDGF-BB is a key mediator of vascular smooth muscle coverage, we examined newly formed blood vessels from the ischemic gastrocnemius region in control and Tie2-IkBαSR mice. In agreement with the reduction in PDGF-BB expression, there was a significant decrease in pericyte coverage of the neovasculature of Tie2-IkBαSR mice (Fig 3B,C).
Figure 3.
Molecular signature of increased vascular branching in Tie2-IkBαSR transgenic mice. (A) Western blot analysis of IkBαSR , VEGFA, VEGFR2, VECadherin, PDGF-BB, PDGFRb, Jagged1, Dll4, Notch1-ICD in gastrocnemius tissue lysates in Tie2-IkBαSR transgenic mice compared with controls 7 days after femoral artery ligation. (B) Representative immunostaining of gastrocnemius muscle section with antibodies specific for CD31 and NG2 chondroitin sulphate proteoglycan at day 7. (C) Mural cell coverage determined as ratio of NG2 to CD31 staining. *p<0.05.
Endothelial NFkB suppression affects Delta-Notch signaling
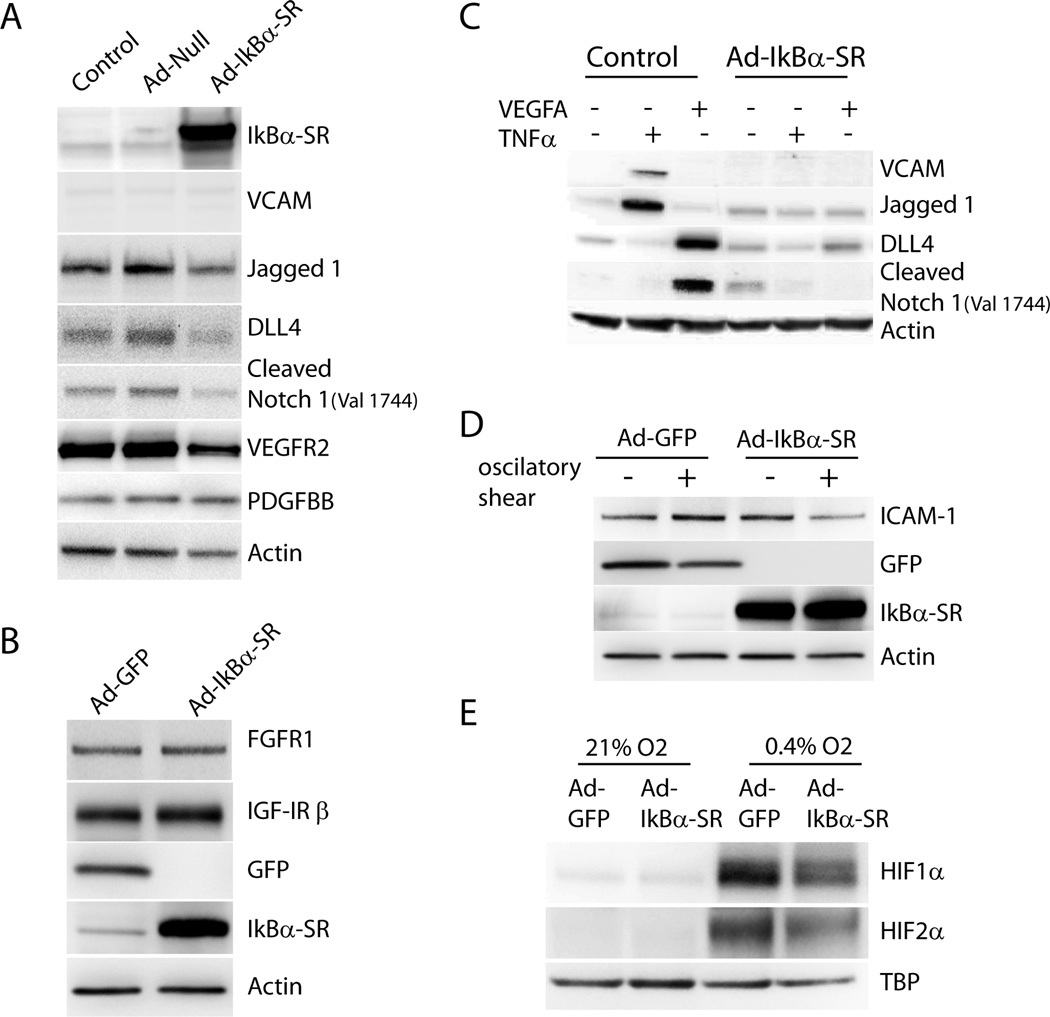
Reduction in Notch signaling suggested by decreased tissue Dll4 and Notch ICD levels may well account for increased vascular branching seen in Tie2-IkBαSR mice. To confirm the effect of a shutdown in NFkB activity on Notch/Delta signaling in endothelial cells in vitro, Ad-IkBαSR or control viruses (Ad-Null) were expressed in HUVEC. Western blotting demonstrated a reduction in baseline DLL4, Jagged-1 and Notch-ICD levels as well as a decrease in VEGFR2 expression (Fig 4A). To determine whether IkBαSR expression in HUVEC affect other angiogenic signaling we assessed the expression of FGFR1 and IGFR1 in HUVEC transduced with Ad- IkBαSR. Western blotting demonstrated no changes in expression of either receptor (Fig 4B) following transduction with Ad- IkBαSR compared to Ad-GFP virus.
Figure 4.
NFkB suppression in endothelial cells affects Delta-Notch signaling. (A) Western blot analysis of IkBαSR, VCAM, Jagged1, Dll4, Cleaved Notch (Val 1744), VEGFR2 and PDGF-BB in HUVEC transduced with Ad-IkBαSR or Ad-Null compared with control (untreated cells). (B) Western blot analysis of FGFR1 and IGF-IR β in HUVEC transduced with Ad-IkBαSR or Ad-GFP. (C) Control and Ad-IkBαSR transduced HUVEC were treated with VEGFA (50ng/ml) or TNFα (10ng/ml) for 24 hr. Western blot analysis of VCAM, Jagged1, Dll4, Cleaved Notch1 (Val 1744).(D) ICAM-1 expression in HUVEC transduced with Ad-IkBαSR or Ad-GFP subjected to oscillatory shear stress for 18 hr. (E) Western blot analysis of HIF1α and HIF2α expression in Ad-IkBαSR or Ad-GFP transduced cells subjected to 0.4% O2(Hypoxia) or 21% O2 (Normoxia) for 24hr.
Induction of arteriogenesis following arterial ligation in vivo is accompanied by an influx of mononuclear cells secreting, among other factors, VEGF and TNFα. To evaluate the effect of NFkB suppression on endothelial cells responses to these growth factors, control and Ad-IkBαSR transduced HUVEC were treated with either VEGF-A or TNFα. As expected, TNFα induced VCAM-1 that was suppressed by IkBαSR expression. TNFα also stimulated expression of Jagged-1 while suppressing Dll4. Both of these actions were suppressed by IkBαSR expression (Fig 4C). In agreement with previous observations, VEGF-A induced expression of DLL4 expression leading to Notch-1 cleavage. Remarkably, this action of VEGF turned out to be NFκB dependent as IκBαSR fully blocked both an increase in Dll4 expression and Notch activation (Fig 4C).
Since NFkB plays a central role in activation of adhesion molecule expression in response to shear stress39, 40, we next determined the effect of Ad- IkBαSR transduction on the expression of ICAM1 in response to shear stress. As expected, the induction of ICAM-1 expression in response to oscillatory shear stress was impaired in HUVEC expressing IkBαSR (Fig 4D).
To investigate whether NFkB suppression results in decreased hypoxia-driven induction of HIF levels, we assessed HIF1α/2α expression in Ad-IkBαSR-transduced HUVECs. Twenty four hours of culture under hypoxia (0.4% O2) conditions resulted in a substantial increase in HIF1α and HIF2α levels in GFP-transduced HUVECs. This increase was substantially attenuated in Ad- IkBαSR expressing cells (Fig 4E).
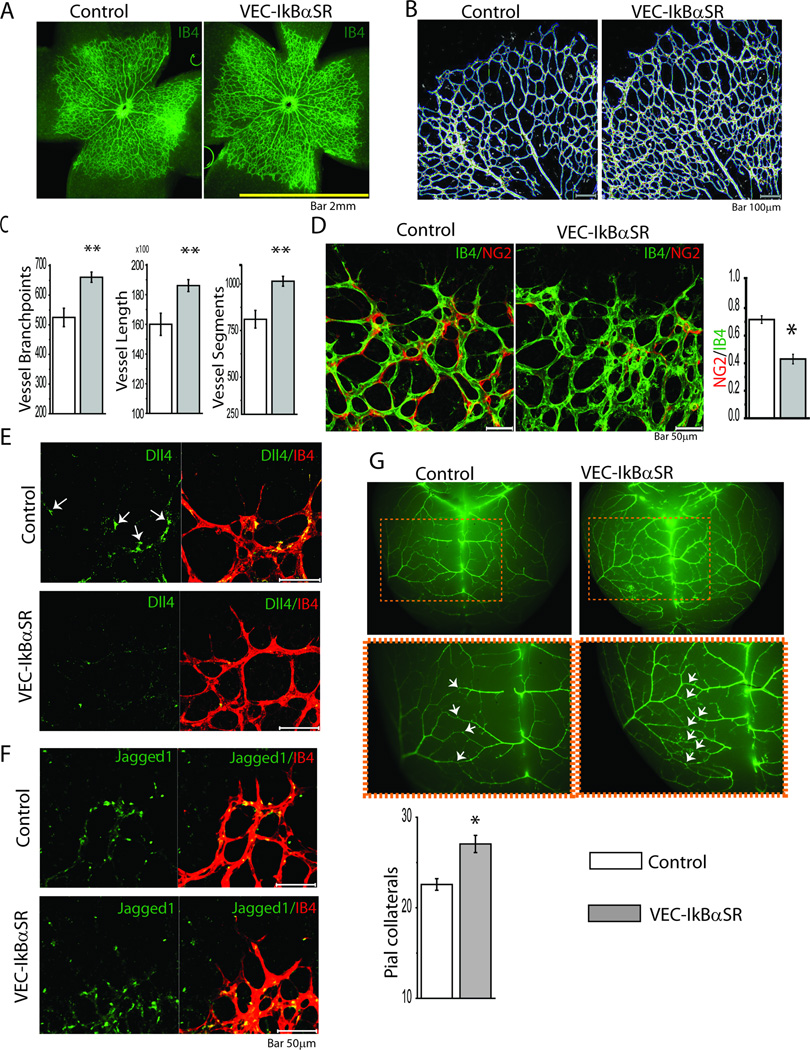
To further study the significance of shutdown of NFκB-dependent Delta-Notch signaling, we examined retinal vasculature in newborn VEC-IkBαSR mice. Whole mounts of retinal vasculature on postnatal day 4 demonstrated excessive branching in VEC-IkBαSR mice with an overall increase in vessel length and the number of vessel segments (Fig 5A,B). Lectin staining of P4 retinal vasculature confirmed increased vessel sprouting (Fig 5B,C). Similar to the hindlimb vasculature, retinal vessels in VEC-IkBαSR mice demonstrated decreased pericyte coverage as demonstrated by NG2 staining (Fig 5D). Staining with anti-Dll4 antibody demonstrated reduced Dll4 expression in tip cells in VEC- IkBαSR compared to control mice (Fig 5E) while there was no change in Jagged-1 expression (Fig 5F). To evaluate whether suppression of NFkB signaling increase the number of pre-existing collaterals, we examined the arteries and pial arteriolar-arteriolar collateral anastomoses between the MCA and the ACA in control and VEC-IκBαSR mice four days after birth. Immunofluorescent staining demonstrated a significant increase in artery-to-artery connections in the pial circulation in VEC- IkBαSR mice (Fig 5G).
Figure 5.
Retinal vasculature and pial collateral in VEC-IkBαSR transgenic mice at P4. (A) Representative isolectin B4 (IB4) staining of whole-mounted retina. (B) Retinal vasculature and (C) quantitative analysis of vessel branchpoints, length and segments in VEC-IkBαSR transgenic mice compared to controls. (D) Representative IB4 staining and immunostaining with anti NG2 Ab of vascular front and mural cell coverage determined as ratio of NG2/CD31 staining in VEC-IkBαSR transgenic mice compared to controls. (E) Representative immunostaining of retinal vasculature with anti Dll4 Ab in VEC-IkBαSR transgenic mice compared to control mice. Note Dll4 expression in tip cells in controls (arrows) and reduced Dll4 expression in tip cells in VEC-IkBαSR mice. (F) Representative immunostaining of retinal vasculature with anti Jagged1 Ab in VEC-IkBαSR transgenic mice compared to control mice. Note no difference in Jagged1 expression in stalk cells between VEC-IkBαSR mice and controls. (G) Representative immunostaining with anti SMA Ab and pial collateral quantification in VEC-IkBαSR transgenic mice compared with control mice. Note increased collateral formation in VEC-IkBαSR transgenic mice (arrows) *p<0.05, ** p<0.01. n=9 mice/group.
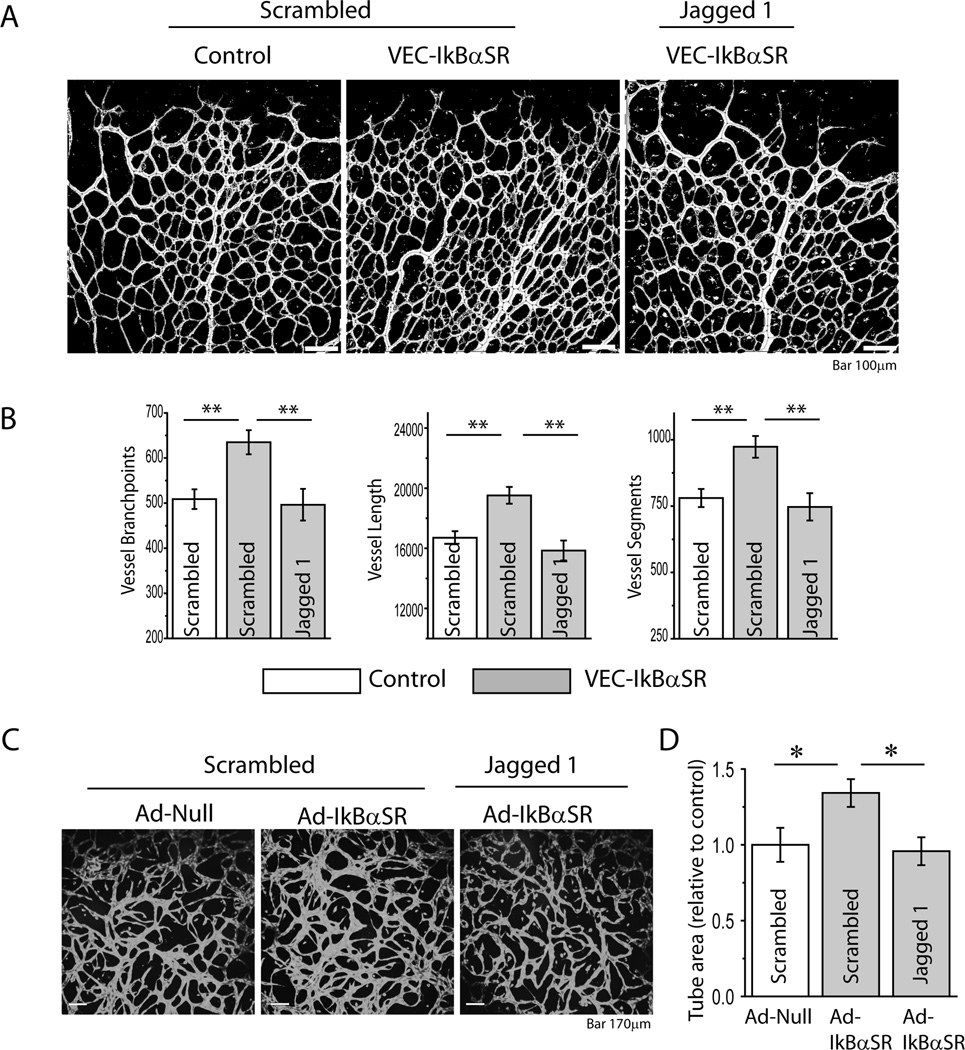
Taken together, these observations suggest that decreased Notch-1 activation due to reduced Dll4 expression is the cause of abnormally increased vascular density in these mice. To confirm this hypothesis, VEC-IkBαSR mice were injected with Jagged-1or scrambled peptide shortly after birth and the retinal vasculature was examined at P4. Treatment with Jag-1 but not scrambled peptide reduced retinal vessel branching to control levels (Fig 6A,B). As an additional confirmation, we examined endothelial cell sprouting in vitro. Transduction of HUVEC with an Ad-IκBαSR but not Ad-Null construct increased sprouting that was reduced by Jagged-1 peptide treatment to baseline level (Fig 6C, D).
Figure 6.
Treatment with Jagged1 reduces vessel branching in VEC-IkBαSR transgenic mice and regulates vessel sprouting in HUVEC expressing IkBαSR. (A) Retinal vasculature and (B) quantitative analysis of vessel branchpoints, length and segments in VEC-IkBαSR transgenic mice treated with Jagged 1 or scrambled peptide compared to controls. (C) Representative Calcein AM staining of HUVEC sprouting. HUVEC were transduced with Ad- IkBαSR or Ad-Null and treated with Jagged 1 or scrambled peptide. (D) Quantitative analysis of tube area. *p<0.05, ** p<0.01, n=3 mice/group, n=5 sprouting assays/group.
DISCUSSION
The present study demonstrates that a suppression of endothelial NFκB activation results in decreased HIF1α expression that is accompanied by decreased VEGF-A and PDGF-BB levels, and a reduction in Dll4 expression that, in turn, leads to decreased Notch activation. In vitro this results in increased sprouting and in vivo in markedly increased arterial density and a larger number of artery-to-artery connections both during development (pial collaterals and the retinal vasculature) and in adult arteriogenesis settings (hindlimb ischemia model) despite markedly reduced levels of blood-derived tissue macrophages.
Thus formed, the arterial circulation is disorganized with multiple inter-connections and poor mural cell coverage. Remarkably, despite a massive increase in arterial collateral density following common femoral artery ligation both in Tie2-IκBαSR and VEC-IkBαSR mice, the distal blood flow was profoundly impaired leading to a poor recovery and extensive tissue injury. This increase in collateral density can be traced to decreased expression of Dll4 and subsequent reduced activation of Notch signaling as activation of Notch with systemically administered Jagged-1 peptide restores the arterial circulation to normal. Thus NFκB-dependent activation of Delta-4/Notch signaling and HIF-1α/2α expression is critical to proper formation of arterial circulation and arterial collaterals.
Inhibition of endothelial NFκB signaling was achieved using an IκBα mutant construct (IκBαSR) that has been previously validated 41. For the hindlimb arteriogenesis studies we have chosen a Tie2 promoter construct that is expressed predominantly under ischemic conditions and is not expressed during development33. This has allowed us to compare the extent of arteriogenesis and collateral formation after the common femoral artery ligation without worrying about different levels of pre-existing collaterals. As Tie2 is expressed to some extent in certain mononuclear cell lineages, and to examine arteriogenesis and collateral formation during development, we have also studied an IkBαSR mouse line under the control of VE-cadherin promoter that is much more endothelial-restricted. In both cases, inhibition of endothelial NFκB activation led to increased arterial branching (retina, hindlimb vasculature) and collateral formation (pial circulation, hindlimb vasculature) and similar effects on endothelial gene expression in vitro.
As expected, dominant negative IkBαSR construct fully suppressed NFκB activation in the endothelium, as demonstrated for example, by decreased expression of VCAM and ICAM-1. This decrease in adhesion receptors expression likely explains a marked decrease in the influx of monocytes into the tissue surrounding the ligated common femoral artery as demonstrated by both histological analysis and FACS analysis of tissue extracts. Among the monocytes subtypes examined all were equally decreased suggesting that reduced endothelial adhesion was the primary cause. In agreement with prior studies, this suppression of NFκB activation resulted in decreased stabilization of HIF1α26 and HIF-2α42 and reduced expression of HIF-dependent genes VEGF-A and PDGF-BB.
The unexpected increase in arterial branching following suppression of endothelial NFκB activation appears to be due to decreased Dll4 expression and a consequent reduction in Notch activation. Dll4 levels were dramatically reduced in the ischemic hindlimb of Tie2-IκBαSR mice as well as in the developing retina vasculature of VEC-IkBαSR mice. This decrease in Dll4 levels is a direct consequence of suppressed NFκB activation as transduction of HUVEC with Ad-IkBαSR in HUVEC results in decrease in basal as well as VEGF-induced Dll4 expression. Finally, increased arterial branching observed in these settings could be reversed by activation of Notch by Jag-1 peptide. These findings are similar to the recently reported endothelial deletion of HIF2α that also results in decreased expression of Dll4, poor recovery from common femoral artery ligation and excessive arterial branching42.
Similarly to Dll4, Jagged-1 expression is also directly NFκB-dependent both in terms of its baseline level and TNFα-dependent induction of expression. However, while in the case of Dll4 NFκB was required for VEGF-driven induction of expression, in the case of Jag-1 TNFα was the major driver. Ischemic tissues in Tie2-IκBαSR mice had higher expression of TNFα than controls, likely reflecting more severe tissue damage. This excess of Jag-1 expression was not sufficient to compensate for reduced Dll4 expression in terms of suppression of excessive endothelial Notch signaling.
In addition to excessive branching, newly formed vasculature in our study appeared immature with poor pericyte coverage. The latter can be attributed to decreased HIF-dependent endothelial secretion of PDGF-BB, the main pericyte attractor. In addition, Notch signaling has been reported to regulate PDGFβ receptor expression in SMC43. Decreased Notch activation in these mice may have made developing vasculature less sensitive to PDGF stimulation as its levels were also reduced.
This link between NFκB activation and Dll4 expression has not, to our knowledge, been reported. An indirect support for this concept is the observation of increased mammary duct branching in IκBα knockout mice44. Little is known about regulation of Dll4 expression and the mechanism of NFκB-dependent regulation of its activity is not certain. The effect can be direct, given a RelA binding site in the Dll4 promoter.
An increase in tumor vascularization due to increased sprouting and numerous vascular interconnections has been observed following suppression of Dll4 activity or expression. At the same time tumors themselves appeared more ischemic and poorly perfused while tumor growth was actually decreased45. In addition, similar to findings in the present study, the excessive vasculature forming following the loss of a Dll4 allele or inhibition of its signaling showed decreased maturation and markedly decreased pericyte coverage46. Furthermore, inhibition of Notch signaling with Ad-driven soluble Dll4 in the setting of hindlimb ischemia in mice decreased blood flow recovery and led to chaotic capillary sprouting resulting in disorganized, low-perfused vascular networks16.
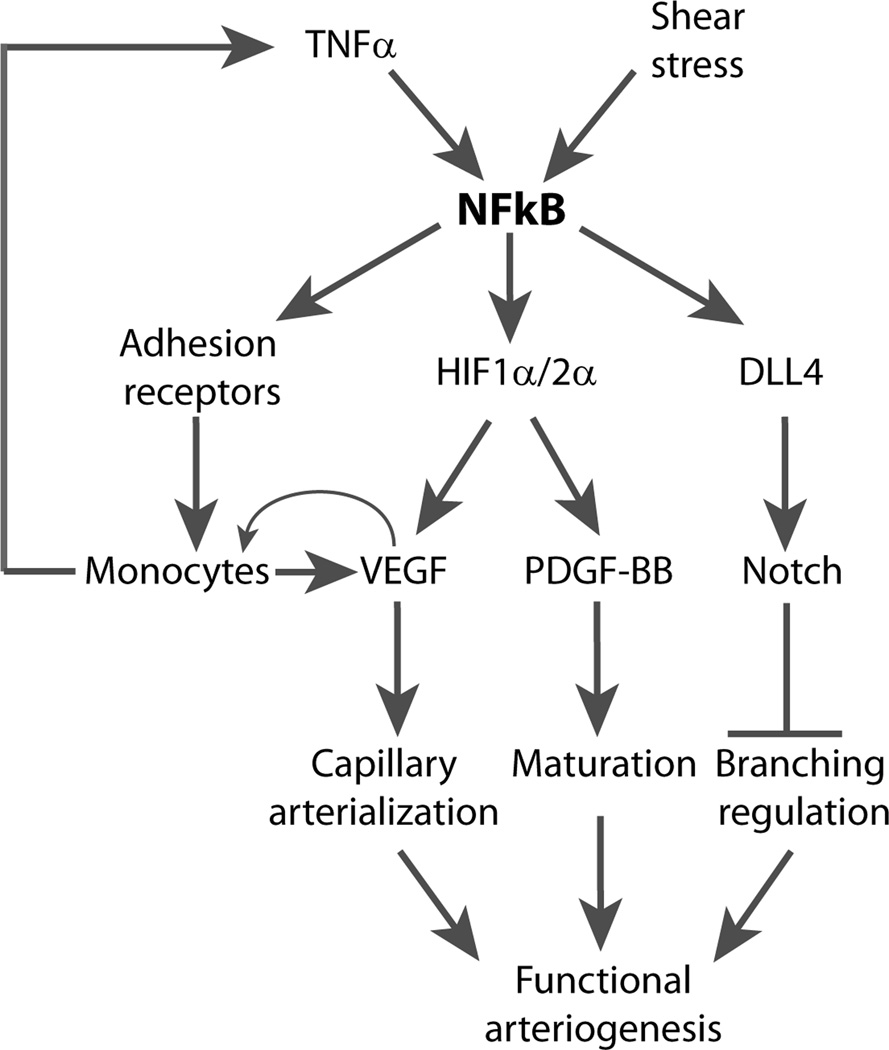
These results place endothelial NFκB squarely at the center of both developmental and adult arteriogenesis and provide new insight into its biology (Fig 7). While numerous factors including TNFα, inflammatory cytokines and LPS among others can activate NFκB, the key activator involved in arteriogenic settings is probably shear stress. Increased shear stress would certainly be expected following common femoral artery ligation and blood flow in the developing retina may be sufficient to activate NFκB in that setting as well. Once activated, NFkB directly regulates expression of adhesion receptors thereby leading to tissue accumulation of monocytes and stabilization of HIF1α and HIF2α which in turn activates expression of VEGF-A and PDGF-BB. Increased local VEGF levels further recruit blood-derived monocytes via SDF131 that in turn contribute to tissue VEGF levels setting up a positive feedback loop. This leads to active remodeling of capillary into the new arterial circulation47 while PDGF-BB-driven pericyte recruitment leads to its maturation. In parallel, NFκB activates DLL4 expression leading to activation of Delta/Notch signaling and effective control of the forming network’s branching.
Figure 7.
Schema of NFκB role in arteriogenesis and collateral formation. NFkB activation leads to stabilization of HIF1a expression with a subsequent induction of VEGF-A and PDGF-BB expression, capillary proliferation and maturation into a new arterial collateral network. The size of the network is controlled by NFκB-dependent activation of Dll4 expression and Notch signaling. In addition, NFκB induces monocytes influx that further promotes collateral growth.
All three process regulated by NFκB are crucial to effective arteriogenesis and all three need to operate in tandem to ensure formation of the functional vasculature. Lack of shear stress sensing, impaired monocyte recruitment or lack of monocytes themselves have all been linked to impaired arteriogenesis8, 48. Similarly, HIF-dependent factors VEGF-A and PDGF-BB are critical to arteriogenesis21, 22 and vascular maturation respectively49. Finally, Dll4 signaling input is required for proper trimming of the vascular network thereby preventing excessive interconnections that impair effective distal perfusion45.
Another important finding is that excessive arteriogenesis leading to formation of excessive collateral network is not always a good thing. Our findings in this regard confirm observations in cancer studies and emphasize the need for balance in induction of collateral growth. They also suggest that manipulation of Delta/Notch signaling may not be the best way to induce therapeutic arteriogenesis.
In summary, we find that endothelial NFkB signaling plays a key role in regulation of adult and developmental arteriogenesis and formation of collateral circulation by regulation monocyte entry, production of VEGF and PDGF and, via Dll4, the size and complexity of the collateral network.
Collateral circulation plays a protective role in case of an ischemic insult precipitated by the occlusion of an arterial vessel. Despite their presumed physiological and clinical importance, little is known about how collaterals develop. The thorough understanding of this process is important to any attempts at therapeutic arteriogenesis. Collaterals are thought to arise either by expansion from the pre-existing vessels or by de novo growth. In either case, the key stimulus is probably mechanical in nature, such as shear stress, and not tissue ischemia per se. One well understood signaling mechanism that controls the size and density of a vascular network is the Delta/Notch pathway. A number of studies have demonstrated that activation of Notch-1 by its ligand delta-like-4 (Dll4) leads to a decrease in vascular branching. However, the link between Dll4/Notch signaling and shear stress has not been established. Endothelial shear stress activates transcription factor NFkB that controls expression of a broad array of genes involved in cell adhesion and migration. In this study we unexpectedly show that Dll4 expression is critically dependent on NFkB, likely via NFkB-dependents stabilization of hypoxia-inducible factor (HIF) 1 and 2 genes. In the absence of endothelial NFkB activation reduced Dll4 levels lead to a much greater expansion in collateral growth than seen under the normal circumstances. Paradoxically, that led to a severe impairment in tissue blood flow. Thus, while increasing collateral density is a worthy therapeutic goal, the quality of the collateral network is just as important as its size.
Acknowledgements
We thank Karen Moodie (Dartmouth Medical School) and Jiasheng Zhang (Yale University) for their expert surgical assistance and Jose R. Conejo-Garcia for his help with tissue macrophages FACS.
Funding Sources: This study was supported, in part by, NIH grant HL53793 (MS), the Leducq Foundation ARTEMIS Transatlantic network grant (AE, MS) and AHA SDG grant 0635107N (DT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None.
References
- 1.le Noble F, Fleury V, Pries A, Corvol P, Eichmann A, Reneman RS. Control of arterial branching morphogenesis in embryogenesis: Go with the flow. Cardiovasc Res. 2005;65:619–628. doi: 10.1016/j.cardiores.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Horowitz A, Simons M. Branching morphogenesis. Circ Res. 2008;103:784–795. doi: 10.1161/CIRCRESAHA.108.181818. [DOI] [PubMed] [Google Scholar]
- 3.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104:576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 4.Chappell JC, Bautch VL. Vascular development: Genetic mechanisms and links to vascular disease. Curr Top Dev Biol. 2010;90:43–72. doi: 10.1016/S0070-2153(10)90002-1. [DOI] [PubMed] [Google Scholar]
- 5.Eitenmuller I, Volger O, Kluge A, Troidl K, Barancik M, Cai WJ, Heil M, Pipp F, Fischer S, Horrevoets AJ, Schmitz-Rixen T, Schaper W. The range of adaptation by collateral vessels after femoral artery occlusion. Circ Res. 2006;99:656–662. doi: 10.1161/01.RES.0000242560.77512.dd. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Rubin J, Tzima E. Role of pecam-1 in arteriogenesis and specification of preexisting collaterals. Cir Res. 2010;107:1355–1363. doi: 10.1161/CIRCRESAHA.110.229955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeda Y, Costa S, Delamarre E, Roncal C, Leite de Oliveira R, Squadrito ML, Finisguerra V, Deschoemaeker S, Bruyere F, Wenes M, Hamm A, Serneels J, Magat J, Bhattacharyya T, Anisimov A, Jordan BF, Alitalo K, Maxwell P, Gallez B, Zhuang ZW, Saito Y, Simons M, De Palma M, Mazzone M. Macrophage skewing by phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature. 2011;479:122–126. doi: 10.1038/nature10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalothorn D, Faber JE. Formation and maturation of the native cerebral collateral circulation. J Mol Cell Cardiol. 2010;49:251–259. doi: 10.1016/j.yjmcc.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seiler C. The human coronary collateral circulation. Eur J Clin Invest. 2010;40:465–476. doi: 10.1111/j.1365-2362.2010.02282.x. [DOI] [PubMed] [Google Scholar]
- 11.Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi SF, Zbinden S, Wustmann K, Billinger M, Vogel R, Cook S, Wenaweser P, Togni M, Windecker S, Meier B, Seiler C. Beneficial effect of recruitable collaterals: A 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116:975–983. doi: 10.1161/CIRCULATIONAHA.107.703959. [DOI] [PubMed] [Google Scholar]
- 12.Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: A meta-analysis. Eur Heart J. 2012;33:614–621. doi: 10.1093/eurheartj/ehr308. [DOI] [PubMed] [Google Scholar]
- 13.Duarte A, Hirashima M, Benedito R, Trindade A, Diniz P, Bekman E, Costa L, Henrique D, Rossant J. Dosage-sensitive requirement for mouse dll4 in artery development. Genes Dev. 2004;18:2474–2478. doi: 10.1101/gad.1239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A. 2004;101:15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 16.Al Haj Zen A, Oikawa A, Bazan-Peregrino M, Meloni M, Emanueli C, Madeddu P. Inhibition of delta-like-4-mediated signaling impairs reparative angiogenesis after ischemia. Circ Res. 2010;107:283–293. doi: 10.1161/CIRCRESAHA.110.221663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorensen I, Adams RH, Gossler A. Dll1-mediated notch activation regulates endothelial identity in mouse fetal arteries. Blood. 2009;113:5680–5688. doi: 10.1182/blood-2008-08-174508. [DOI] [PubMed] [Google Scholar]
- 18.Napp LC, Augustynik M, Paesler F, Krishnasamy K, Woiterski J, Limbourg A, Bauersachs J, Drexler H, Le Noble F, Limbourg FP. Extrinsic notch ligand delta-like 1 regulates tip cell selection and vascular branching morphogenesis. Circ Res. 2012;110:530–535. doi: 10.1161/CIRCRESAHA.111.263319. [DOI] [PubMed] [Google Scholar]
- 19.Limbourg A, Ploom M, Elligsen D, Sorensen I, Ziegelhoeffer T, Gossler A, Drexler H, Limbourg FP. Notch ligand delta-like 1 is essential for postnatal arteriogenesis. Circ Res. 2007;100:363–371. doi: 10.1161/01.RES.0000258174.77370.2c. [DOI] [PubMed] [Google Scholar]
- 20.Eichmann A, Simons M. Vegf signaling inside vascular endothelial cells and beyond. Curr Opin Cell Biol. 2012;24:188–193. doi: 10.1016/j.ceb.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-a specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res. 2008;103:1027–1036. doi: 10.1161/CIRCRESAHA.108.181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toyota E, Warltier DC, Brock T, Ritman E, Kolz C, O'Malley P, Rocic P, Focardi M, Chilian WM. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation. 2005;112:2108–2113. doi: 10.1161/CIRCULATIONAHA.104.526954. [DOI] [PubMed] [Google Scholar]
- 23.Chittenden TW, Claes F, Lanahan AA, Autiero M, Palac RT, Tkachenko EV, Elfenbein A, Ruiz de Almodovar C, Dedkov E, Tomanek R, Li W, Westmore M, Singh JP, Horowitz A, Mulligan-Kehoe MJ, Moodie KL, Zhuang ZW, Carmeliet P, Simons M. Selective regulation of arterial branching morphogenesis by synectin. Dev Cell. 2006;10:783–795. doi: 10.1016/j.devcel.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Lanahan AA, Hermans K, Claes F, Kerley-Hamilton JS, Zhuang ZW, Giordano FJ, Carmeliet P, Simons M. Vegf receptor 2 endocytic trafficking regulates arterial morphogenesis. Dev Cell. 2010;18:713–724. doi: 10.1016/j.devcel.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1alpha by nf-kappab. Biochem J. 2008;412:477–484. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonello S, Zahringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Gorlach A. Reactive oxygen species activate the hif-1alpha promoter via a functional nfkappab site. Arterioscler Thromb Vasc Biol. 2007;27:755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- 29.Simons M. Angiogenesis: Where do we stand now? Circulation. 2005;111:1556–1566. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- 30.Buschmann IR, Hoefer IE, van Royen N, Katzer E, Braun-Dulleaus R, Heil M, Kostin S, Bode C, Schaper W. Gm-csf: A strong arteriogenic factor acting by amplification of monocyte function. Atherosclerosis. 2001;159:343–356. doi: 10.1016/s0021-9150(01)00637-2. [DOI] [PubMed] [Google Scholar]
- 31.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. Vegf-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 32.Jobin C, Panja A, Hellerbrand C, Iimuro Y, Didonato J, Brenner DA, Sartor RB. Inhibition of proinflammatory molecule production by adenovirus-mediated expression of a nuclear factor kappab super-repressor in human intestinal epithelial cells. J Immunol. 1998;160:410–418. [PubMed] [Google Scholar]
- 33.Deutsch U, Schlaeger TM, Dehouck B, Doring A, Tauber S, Risau W, Engelhardt B. Inducible endothelial cell-specific gene expression in transgenic mouse embryos and adult mice. Exp Cell Res. 2008;314:1202–1216. doi: 10.1016/j.yexcr.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 34.Sun JF, Phung T, Shiojima I, Felske T, Upalakalin JN, Feng D, Kornaga T, Dor T, Dvorak AM, Walsh K, Benjamin LE. Microvascular patterning is controlled by fine-tuning the akt signal. Proc Natl Acad Sci U S A. 2005;102:128–133. doi: 10.1073/pnas.0403198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tirziu D, Moodie KL, Zhuang ZW, Singer K, Helisch A, Dunn JF, Li W, Singh J, Simons M. Delayed arteriogenesis in hypercholesterolemic mice. Circulation. 2005;112:2501–2509. doi: 10.1161/CIRCULATIONAHA.105.542829. [DOI] [PubMed] [Google Scholar]
- 36.Funk SD, Yurdagul A, Jr, Green JM, Jhaveri KA, Schwartz MA, Orr AW. Matrix-specific protein kinase a signaling regulates p21-activated kinase activation by flow in endothelial cells. Circ Res. 2010;106:1394–1403. doi: 10.1161/CIRCRESAHA.109.210286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 38.Jones EA, Yuan L, Breant C, Watts RJ, Eichmann A. Separating genetic and hemodynamic defects in neuropilin 1 knockout embryos. Development. 2008;135:2479–2488. doi: 10.1242/dev.014902. [DOI] [PubMed] [Google Scholar]
- 39.Weber KS, Draude G, Erl W, de Martin R, Weber C. Monocyte arrest and transmigration on inflamed endothelium in shear flow is inhibited by adenovirus-mediated gene transfer of ikappab-alpha. Blood. 1999;93:3685–3693. [PubMed] [Google Scholar]
- 40.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 41.Hasegawa Y, Saito T, Ogihara T, Ishigaki Y, Yamada T, Imai J, Uno K, Gao J, Kaneko K, Shimosawa T, Asano T, Fujita T, Oka Y, Katagiri H. Blockade of the nuclear factor-kappab pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation. 2012;125:1122–1133. doi: 10.1161/CIRCULATIONAHA.111.054346. [DOI] [PubMed] [Google Scholar]
- 42.Skuli N, Majmundar AJ, Krock BL, Mesquita RC, Mathew LK, Quinn ZL, Runge A, Liu L, Kim MN, Liang J, Schenkel S, Yodh AG, Keith B, Simon MC. Endothelial hif-2alpha regulates murine pathological angiogenesis and revascularization processes. J Clin Invest. 2012;122:1427–1443. doi: 10.1172/JCI57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin S, Hansson EM, Tikka S, Lanner F, Sahlgren C, Farnebo F, Baumann M, Kalimo H, Lendahl U. Notch signaling regulates platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Circ Res. 2008;102:1483–1491. doi: 10.1161/CIRCRESAHA.107.167965. [DOI] [PubMed] [Google Scholar]
- 44.Brantley DM, Chen CL, Muraoka RS, Bushdid PB, Bradberry JL, Kittrell F, Medina D, Matrisian LM, Kerr LD, Yull FE. Nuclear factor-kappab (nf-kappab) regulates proliferation and branching in mouse mammary epithelium. Mol Biol Cell. 2001;12:1445–1455. doi: 10.1091/mbc.12.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thurston G, Noguera-Troise I, Yancopoulos GD. The delta paradox: Dll4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer. 2007;7:327–331. doi: 10.1038/nrc2130. [DOI] [PubMed] [Google Scholar]
- 46.Scehnet JS, Jiang W, Kumar SR, Krasnoperov V, Trindade A, Benedito R, Djokovic D, Borges C, Ley EJ, Duarte A, Gill PS. Inhibition of dll4-mediated signaling induces proliferation of immature vessels and results in poor tissue perfusion. Blood. 2007;109:4753–4760. doi: 10.1182/blood-2006-12-063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bailey AM, O'Neill TJt, Morris CE, Peirce SM. Arteriolar remodeling following ischemic injury extends from capillary to large arteriole in the microcirculation. Microcirculation. 2008;15:389–404. doi: 10.1080/10739680701708436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Royen N, Hoefer I, Bottinger M, Hua J, Grundmann S, Voskuil M, Bode C, Schaper W, Buschmann I, Piek JJ. Local monocyte chemoattractant protein-1 therapy increases collateral artery formation in apolipoprotein e-deficient mice but induces systemic monocytic cd11b expression, neointimal formation, and plaque progression. Circ Res. 2003;92:218–225. doi: 10.1161/01.res.0000052313.23087.3f. [DOI] [PubMed] [Google Scholar]
- 49.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]