Abstract
Purpose
To assess the outcomes of pars plana vitrectomy for the treatment of malignant glaucoma in patients with and without previous filtration surgery.
Patients and methods
Data of 15 patients developing malignant glaucoma after trabeculectomy (60%) or following ophthalmic interventions other than filtration surgery (40%) were recorded retrospectively. Pars plana vitrectomy was performed in case of failed medical or laser treatment recreating the normal pathway of aqueous humor. The main outcome measures were the postoperative intraocular pressure (IOP), the frequency of complications, and success rate based on the following criteria: IOP reduction by ≥20% and to ≤21 mmHg (definition one) or an IOP < 18 mmHg (definition two) with (qualified success) and without (complete success) glaucoma medication.
Results
Vitrectomy reduced IOP from baseline in eyes with and without previous trabeculectomy during a median follow-up of 16.4 months (range 7 days to 58 months); although the majority of patients required glaucoma medication to reach desired IOP. The complete success rates were 11% (both definitions) for patients with filtering blebs and none of the patients without previous trabeculectomy had complete success at the 12-month visit. Complications were few and included transient shallowing of the anterior chamber, choroidal detachment, corneal decompensation, filtering bleb failure, and need for further IOP-lowering procedures.
Conclusion
Pars plana vitrectomy is equally effective for malignant glaucoma caused by trabeculectomy or interventions other than filtration surgery, although IOP-lowering medication is necessary in nearly all cases to maintain target IOP.
Keywords: ciliolenticular block glaucoma, malignant glaucoma, pars plana vitrectomy, trabeculectomy
Introduction
Malignant glaucoma is a rare but serious condition of secondary angle closure mostly seen after filtration surgery, but it may also occur spontaneously.1 It has also been described after laser iridotomy,2 laser capsulotomy,3 cataract surgery,4 topical miotic therapy,5 and blunt trauma.6 Some basic mechanisms of ciliolenticular block glaucoma are poorly understood. Lens disproportion and lens–ciliary body apposition in small eyes and anterior hyaloid changes with increased hydraulic resistance are supposed to be major pathophysiological factors.7 Besides these factors, poor vitreous flow and a tendency towards expansion of the choroidal volume in small eyes with angle closure refractory to iridotomy are further possible mechanisms of malignant glaucoma as proposed by Quigley et al.8 These features lead to aqueous misdirection into the vitreous cavity restricted by the anterior hyaloid membrane and additional forward movement of the lens–iris diaphragm.9,10 It is clinically characterized by an axial shallowing of the anterior chamber in the absence of a pupillary block mechanism, choroidal effusion, or hemorrhage, despite the presence of a patent iridectomy.11 Intraocular pressure (IOP) is usually dramatically increased, but may also be within the normal range.12 Treatment of malignant glaucoma should be done stepwise. Medical treatment includes topical antiglaucomatous medication, cycloplegic agents, aqueous suppressants, systemic carbonic anhydrase inhibitors, and hyperosmotic drugs inducing a posterior movement of the lens–iris diaphragm, reducing production of aqueous humor, and dehydrating the vitreous volume.13 The performance of laser capsulotomy,14 or laser anterior hyaloidectomy,15 and argon laser of ciliary processes through a peripheral iridectomy16 to relieve the ciliolenticular block are recommended for unsuccessful medical treatment. When medical and laser treatment failed, surgery should be performed to interrupt the ciliolenticular block. Pars plana vitrectomy in pseudophakic eyes or combined vitrectomy and cataract surgery in phakic eyes are effective methods to allow aqueous outflow into the anterior chamber.17,18 Apart from anterior vitrectomy alone or in combination with phacoemulsification17–19 or trabeculectomy20 via a posterior approach (pars plana), a procedure called zonulo-hyaloido-vitrectomy through a peripheral iridectomy or iridotomy via the anterior chamber was described by Lois et al in 2001.21 Due to the difficulty in visualization of the anterior hyaloid membrane in phakic eyes and the risk of damaging the lens, Chen et al published a new technique of videoendoscope-guided fluorescein-assisted vitrectomy in phakic eyes.22
In this study, a series of patients diagnosed with malignant glaucoma, who underwent pars plana vitrectomy to interrupt the ciliolenticular block mechanism, were reviewed. Major outcome measures were the event preceding malignant glaucoma, the efficacy of vitrectomy including success rate based on IOP reduction and need for medical treatment, and complications following vitrectomy in eyes with and without previous trabeculectomy.
Material and methods
Patients
Clinical records of 15 patients were reviewed retrospectively undergoing pars plana vitrectomy for treatment of malignant glaucoma at the University Eye Hospital of Wuerzburg, Germany between 1995 and 2010. The following parameters were recorded: age, sex, lens status, primary ophthalmic diagnosis, past medical history, glaucoma medication, and the event preceding malignant glaucoma. Preoperatively, all patients received a standard ophthalmic examination including best corrected visual acuity measurement, applanation tonometry, slit lamp biomicroscopy, and indirect ophthalmoscopy.
Clinical inclusion criteria of malignant glaucoma were as follows: shallow anterior chamber in the presence of a patent iridectomy or iridotomy after a choroidal hemorrhage or effusion have been ruled out. Most of the patients had an increased IOP at the onset of malignant glaucoma. However, three patients were included according to the inclusion criteria despite having an IOP < 20 mmHg.
Patients were divided into a group with (Trab group) and one without (nonTrab group) previous trabeculectomy to evaluate the influence of vitrectomy on success of patients who had undergone filtration surgery before.
Success was defined as an IOP ≤ 21 mmHg and IOP reduction by ≥20% (definition one) or an IOP < 18 mmHg to baseline measures (definition two) with (qualified success) and without (complete success) IOP-lowering medication.
Surgical technique
Prior to surgical treatment, neodymium:yttrium-aluminium-garnet (Nd:YAG) laser iridotomy was performed in all patients of the nonTrab group to rule out a pupillary block. Of these, three patients required additional surgical iridectomy for inconsistent iridotomy. As intensive medical treatment (including cycloplegic agents and topical and systemic IOP-lowering medication) remained unsuccessful and IOP was persistently elevated, pars plana vitrectomy became a prerequisite. A 20-gauge port was used in six patients of the Trab group and in all six patients of the nonTrab group and a 23-gauge port in three patients of the Trab group. Anterior vitrectomy via pars plana was performed using a three-port technique to disrupt the anterior hyaloid membrane ensuring aqueous outflow from the vitreous cavity into the anterior chamber in order to interrupt the ciliolenticular block mechanism. Existing adhesions and remnants of the vitreous body around the peripheral iridectomy were removed to allow patency for anterior aqueous flow. Vitrectomy was continued until the anterior chamber deepened. In phakic eyes with significant cataract, combined vitrectomy and cataract extraction were performed.
Data analysis
SPSS® version 19.0 for Windows (IBM Corporation, Armonk, NY, USA) was used for statistical analyses and to create figures. Categorical variables were evaluated with Pearson’s chi-squared test or Fisher’s exact test. Differences between groups in single discrete variables of nonnormal distribution were tested for significance using the Mann–Whitney U test for small sample sizes. A maximum two-tailed P-value of <0.05 was considered an indication of statistical significance.
Results
The results of 15 patients undergoing anterior vitrectomy for treatment of malignant glaucoma during a 12-month follow-up are described.
Baseline data
Nine patients (Trab group) developed a ciliolenticular block following filtration surgery with a patent iridectomy in the absence of a pupillary block, hypotony, choroidal effusion or hemorrhage (Table 1). In six patients (nonTrab group), several intraocular surgeries, laser, or cyclodestructive treatments were performed prior to malignant glaucoma (Table 2). Malignant glaucoma occurred in seven patients after trabeculectomy and in two patients after combined trabeculectomy and cataract extraction. The median time between filtration surgery and malignant glaucoma was 41.4 days (range: 5–205 days). Of the patients in the nonTrab group, one patient developed a ciliolenticular block after neodymium:yttrium-aluminium-garnet (Nd:YAG) laser peripheral iridotomy five patients after cataract surgery. Mean time to malignant glaucoma was 14.0 days (range: 1–41 days) with one patient, who had a cataract extraction 9 years prior, excluded.
Table 1.
Characteristics of nine patients with previous trabeculectomy (Trab group) undergoing vitrectomy for malignant glaucoma
| Patient/ sex/ age/ eye | Axial length (mm) | Pseudo-phakic | Procedure prior to malignant glaucoma | Time to malignant glaucoma (days) | Baseline | Last visit | Follow-up (months) | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| IOP (mmHg) | BCVA (logMAR) | IOP (mmHg) | BCVA (logMAR) | ||||||
| 1/f/75/R | – | Yes | Trab | 6 | 16 | 3.00 | 13 | 3.00 | 7 days |
| 2/f/60/L | 21.16 | Yes | Phaco-Trab | 13 | 24 | 0.40 | 18 | 0.10 | 37 |
| 3/f/58/R | 21.07 | Yes | Phaco-Trab | 17 | 23 | 0.70 | 14 | 0.20 | 13 |
| 4/f/73/R | 21.20 | Yes | Trab | 31 | 20 | 1.30 | 12 | 0.50 | 6 |
| 5/f/41/L | 21.31 | No | Trab | 205 | 23 | 0.70 | 22 | 0.70 | 11 |
| 6/m/49/L | 23.32 | No | Trab | 18 | 13 | 2.00 | 15 | 1.30 | 12 |
| 7/m/64/L | 22.63 | No | Trab | 5 | 44 | 1.30 | 11 | 0.40 | 58 |
| 8/f/88/L | – | Yes | Trab | 5 | 30 | 3.00 | 22 | 3.00 | 14 days |
| 9/m/73/L | 22.95 | No | Trab | 36 | 32 | 3.00 | 21 | 1.20 | 25 |
Abbreviations: Trab, trabeculectomy; Phaco-Trab, combined trabeculectomy and phacoemulsification with posterior chamber intraocular lens implantation.
Table 2.
Characteristics of six patients without previous trabeculectomy (nonTrab group) undergoing vitrectomy for malignant glaucoma
| Patient/ sex/age/ eye | Axial length (mm) | Pseudophakic | Procedure prior to malignant glaucoma | Time to malignant glaucoma (days) | Baseline | Last visit | Follow-up (months) | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| IOP (mmHg) | BCVA (logMAR) | IOP (mmHg) | BCVA (logMAR) | ||||||
| 1/f/83/R | 21.93 | Yes | Phaco† | –† | 40 | 3.00 | 21 | 3.00 | 12 days |
| 2/f/77/L | – | Yes | Phaco | 9 | 30 | 1.30 | 10 | 1.30 | 13 |
| 3/f/61/R | 21.87 | Yes | Nd:YAG I | 6 | 16 | 0.70 | 19 | 0.50 | 15 |
| 4/f/81/R | 23.55 | Yes | Phaco | 41 | 31 | 1.50 | 25 | 1.00 | 31 |
| 5/m/80/L | – | Yes | Phaco | 1 | 78 | 3.00 | 8 | 1.30 | 1 |
| 6/m/85/R | – | Yes | Phaco | 13 | 38 | 1.30 | 20 | 3.00 | 23 |
Note:
Cataract extraction was performed 9 years prior to malignant glaucoma.
Abbreviations: Phaco, phacoemulsification with posterior chamber intraocular lens implantation; Nd:YAG I, neodymium:yttrium-aluminium-garnet laser iridotomy.
The mean age of six female and three male patients in the Trab group was 65 years (range: 41–88 years). Four women and two men with a mean age of 78 years (range: 61–85 years) were included in the nonTrab group and were significantly older than patients with filtering blebs (P = 0.046). Patients in both groups had a median axial length of 22.0 ± 1.0 mm and 22.5 ± 1.0 mm (P = 0.383) with a median lens thickness of 4.8 ± 0.9 mm and 4.6 ± 0.7 mm (P = 0.800), respectively.
Five patients in the Trab group and all the patients in the nonTrab group were pseudophakic at the time of diagnosing malignant glaucoma. Two phakic patients of the Trab group had combined cataract extraction while performing vitrectomy. Indications for combined surgery were a significant cataract and failed deepening of the anterior chamber during vitrectomy.
IOP
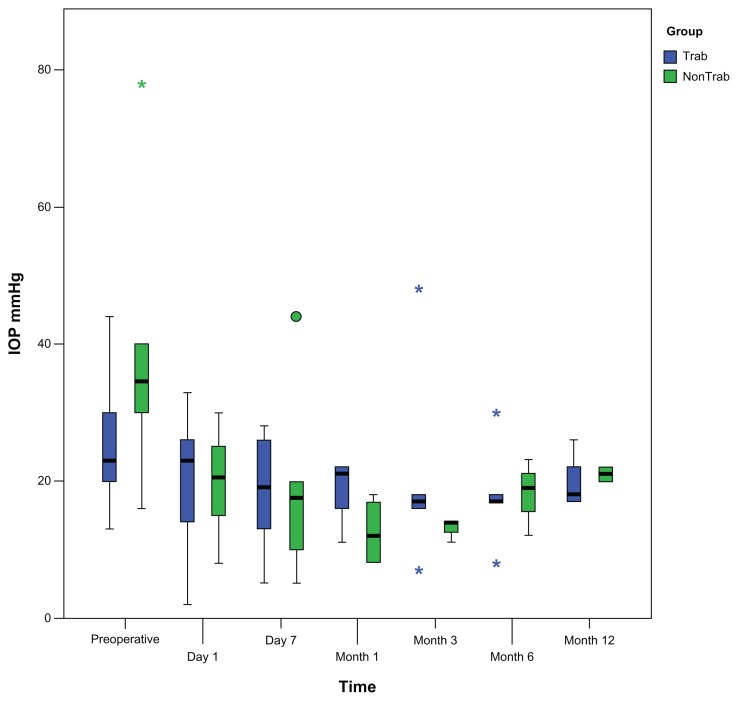
The mean IOP of patients in the Trab group was 25.0 ± 9.3 mmHg at the time of malignant glaucoma and was lower than in patients of the nonTrab group (38.8 ± 21.0 mmHg), although no statistical difference was found (P = 0.135). Figure 1 illustrates IOP development during follow-up for both groups. A reduction in IOP was observed after 12 months compared to baseline IOP for both groups. IOP was 20.0 ± 3.9 mmHg in the Trab group and 20.3 ± 3.3 mmHg in the nonTrab group at the 12-month visit. No statistical difference in IOP was found between the two groups (P = 0.571).
Figure 1.
Development of intraocular pressure in patients with and without trabeculectomy prior to malignant glaucoma. Reduction of postoperative intraocular pressure was obtained during follow-up. Intraocular pressure was not statistically significant different between the two groups during follow-up.
Notes: Box plots illustrate the median (50th percentile) as a black center line and the 25th and 75th percentile as the lower and upper hinges of the box; circles represent minor outliers; stars represent major outliers.
Abbreviations: IOP, intraocular pressure; Trab, trabeculectomy.
Medication
Preoperatively, patients of the Trab group received a total number of 2.0 ± 1.3 (range one to four) topical agents. Preoperatively, patients of the nonTrab group needed 3.0 ± 1.3 (range two to five) topical IOP-lowering drugs. Additional systemic medication (acetazolamide, mannitol) was necessary in all patients of both groups to lower IOP. The number of patients with IOP-lowering medication dropped to two patients in the Trab group needing 2.0 ± 1.4 medication after 1 month. One patient in the nonTrab group was on 4.0 topical medications at the 1-month visit. However, seven patients in the Trab group received a total of 2.3 ± 0.8 medications and four patients in the nonTrab group received 2.5 ± 1.0 medications at the last available visit of each patient (range: 7 days to 58 months). No statistical difference concerning pre- and postoperative IOP-lowering medication was found between the two groups during follow-up (P = 0.781).
Success
According to the criteria for qualified success, two (40.0%) patients in the Trab group fulfilled both criteria of qualified success after 12 months (follow-up rate 55.6%). None of the patients in the nonTrab group had an IOP ≤ 21 mmHg and ≥20% reduction of IOP from baseline or an IOP < 18 mmHg with or without IOP-lowering medication (follow-up rate 33.3%). Statistical analyses revealed no significant differences between the two groups (Table 3).
Table 3.
Baseline data and outcomes of success at 6 and 12 months after pars plana vitrectomy
| Trab group | nonTrab group | P-value | |
|---|---|---|---|
| Preoperative | |||
| IOP (mmHg) | 25.0 ± 9.3 | 38.8 ± 21.0 | 0.135‡ |
| Number of IOP-lowering medication | 2.0 ± 1.3 | 3.0 ± 1.3 | 0.130‡ |
| Number of patients with medication (%) | 9 (100.0) | 6 (100.0) | |
| At 6 months | |||
| Number of patients (%) | 5 (55.6) | 3 (50.0) | |
| IOP (mmHg) | 18.0 ± 7.8 | 18.0 ± 5.6 | 0.732‡ |
| Qualified success [n (%)] | |||
| ≤ 21 mmHg + 20% IOP ↓ | 3 (60.0) | 2 (66.7) | 1.000† |
| < 18 mmHg | 3 (60.0) | 1 (33.3) | 0.604† |
| Number of IOP-lowering medication | 3.3 ± 1.5 | 3.5 ± 2.1 | 1.000‡ |
| Number of patients with medication (%) | 3 (60.0) | 2 (66.7) | |
| Complete success [n (%)] | |||
| ≤ 21 mmHg + 20% IOP ↓ | 2 (40.0) | 1 (33.3) | 1.000† |
| < 18 mmHg | 2 (40.0) | 0 (0.0) | 0.486† |
| At 12 months | |||
| Number of patients (%) | 5 (55.6) | 2 (33.3) | |
| IOP (mmHg) | 20.0 ± 3.9 | 20.3 ± 3.3 | 0.571† |
| Qualified success [n (%)] | |||
| ≤ 21 mmHg + 20% IOP ↓ | 2 (40.0) | 0 (0) | 0.486† |
| < 18 mmHg | 2 (40.0) | 0 (0) | 0.486† |
| Number of IOP-lowering medication | 2.8 ± 1.3 | 4.0 ± 2.8 | 0.800‡ |
| Number of patients with medication (%) | 4 (80.0) | 2 (100.0) | |
| Complete success [n (%)] | |||
| ≤ 21 mmHg + 20% IOP ↓ | 1 (20.0) | 0 (0) | 1.000† |
| < 18 mmHg | 1 (20.0) | 0 (0) | 1.000† |
Notes:
Pearson’s chi-squared test (Fisher’s exact test);
Mann-Whitney U test; values are means ± standard deviation unless otherwise stated.
Abbreviations: IOP, intraocular pressure; Trab, trabeculectomy.
Complete success rate was 20.0% (one patient) according to both definitions (IOP ≤ 21 mmHg and ≥20% IOP reduction; IOP < 18 mmHg) in the Trab group at the 12-month visit (follow-up rate 55.6%). In the nonTrab group, none of the patients had complete success (follow-up rate 33.3%). There was no statistical difference in complete success found between the two groups during follow-up (Table 3).
Complications and further interventional management
Pars plana vitrectomy was successful in terminating malignant glaucoma and facilitated intraoperative deepening of the anterior chamber in all cases. The incidence of complications and surgical revisions are shown in Table 4.
Table 4.
Incidence of postoperative complications and further interventions
| Trab group | nonTrab group | P-value† | |
|---|---|---|---|
| Hypotony (<5 mmHg) | 3 (33.3) | 1 (16.7) | 0.604 |
| Duration (range) | 1–7 days | 10 days | 0.500 |
| Shallow anterior chamber | 4 (44.4) | 2 (33.3) | 1.000 |
| Choroidal detachment | 3 (33.3) | – | 0.229 |
| Duration (range) | 3–9 days | – | |
| Corneal decompensation | 3 (33.3) | 2 (33.3) | 1.000 |
| Progression of cataract | 2 (22.2)# | –‡ | 0.486 |
| Conjunctival leakage | 1 (11.1) | – | 1.000 |
| Choroidal effusion | – | – | – |
| Iris incarceration | – | – | – |
| Blebitis/endophthalmitis | – | – | – |
| Additional procedures | |||
| Diode cyclophotocoagulation | 4 (44.4) | 2 (33.3) | 1.000 |
| Cyclocryocoagulation | 4 (44.4) | 3 (50.0) | 1.000 |
| Revitrectomy | 1 (11.1) | – | 1.000 |
Notes:
Pearson’s chi-squared test (Fisher’s exact test);
all patients were pseudophakic at the time of vitrectomy;
two patients remained phakic after initial vitrectomy; data represents absolute values (percentage).
Abbreviation: Trab, trabeculectomy.
Three of nine patients (33.3%) in the Trab group had transient hypotony (defined as an IOP < 5 mmHg) for a mean duration of 3.3 ± 3.2 days (range: 1–7 days). Of these, the 23-gauge approach was used for vitrectomy in two patients. All three patients with filtering blebs suffering from hypotony had a choroidal detachment for a duration ranging 3–9 days. Postoperative hypotony lasting > 10 days without choroidal detachment was seen in one patient (16.7%) of the nonTrab group. There was no statistical difference in length of hypotony between both groups (P = 0.500). In four patients (44.4%) of the Trab group, anterior chamber flattened again for 1–7 days postoperatively and deepened spontaneously thereafter without further interventions. In contrast, in two patients (33.3%) of the nonTrab group, the anterior chamber was shallow for 3 days after vitrectomy, deepened spontaneously, and remained deep thereafter. Further adverse events were failure of filtering blebs in four patients and progression of cataract in two patients of the Trab group. Neither choroidal effusion, blebitis, endophthalmitis, nor iris incarceration was seen in either group during follow-up.
Incidence of further surgical interventions including cyclodestructive procedures and revitrectomy is summarized in Table 4. In five patients (55.6%) of the Trab group and in three patients (50%) of the nonTrab group, one or more additional IOP-lowering procedures were necessary. In three patients of the Trab group, four diode cyclophotocoagulations and cyclocryocoagulations were performed, respectively. Two patients in the nonTrab group needed diode cyclophotocoagulation and three patients received cyclocryocoagulation. No statistical difference regarding complications or surgical revisions was found between the two groups.
Discussion
Ciliolenticular block glaucoma remains one of the most challenging serious adverse events necessitating immediate treatment to reduce aqueous misdirection into the vitreous and consecutive displacement of the lens–iris diaphragm. In a recent review by Ng and Morgan, different pathophysiological features are described to play an important role for development of ciliolenticular block glaucoma, although not every part is clearly understood.7 Lens disproportion and lens–ciliary body apposition in at-risk eyes (hyperopic, nanophthalmic) and anterior hyaloid changes with increased hydraulic resistance due to a strong adhesion between vitreous, lens, and ciliary body are major pathogenic factors.7 In angle closure eyes, the lens is disproportionally large and increases in size, thereby pushing the iris and ciliary body forwards. In addition, as the lens–ciliary body distance decreases, the fluid resistance increases and aqueous humor passes through the anterior vitreous restricted by the anterior hyaloid. Moreover, lens apposition to the ciliary body may lead to reduced aqueous flow into the anterior chamber. Both lens disproportion and lens–ciliary body apposition result in forward movement of the lens–iris diaphragm and a shallow anterior chamber.7 Since malignant glaucoma also occurs in aphakic eyes, the lens seems not to be the only cause. Flow resistance increases as the aqueous humor passes through the vitreous body and compresses the anterior hyaloid face. The pressure differential between the anterior and posterior chamber leads to an anterior displacement of iris and ciliary body. Quigley et al described poor vitreous flow and a tendency towards expansion of the choroidal volume in small eyes with angle closure refractory to iridotomy as possible mechanisms of malignant glaucoma.8
Anterior vitrectomy for malignant glaucoma refractory to medical and laser treatment facilitates aqueous humor outflow into the anterior chamber and reflects one recognizing pathophysiologic role of the vitreous in the mechanism of malignant glaucoma.7,23,24 Results of vitrectomy in pseudophakic and aphakic eyes developing malignant glaucoma have been published in several previous studies.18–22,25,26 Nevertheless, vitrectomy alone in phakic eyes may be disappointing due to the difficulty in visualization and removal of the anterior hyaloid membrane without lens damaging.18,25,26 Therefore, combined cataract extraction and vitrectomy even in eyes without lens opacities or mild lens opacities are strongly recommended.
In this study of 15 patients diagnosed with malignant glaucoma, the efficacy and safety of pars plana vitrectomy for treatment of the ciliolenticular block was evaluated. The results were based on data of patients with and without previous filtration surgery during a median follow-up of 16.4 months. It was found that vitrectomy for malignant glaucoma was successful in IOP reduction after medical treatment failed. Vitrectomy seems to be effective in patients with and without previous trabeculectomy with adequate reduction of IOP, although IOP-lowering medication is necessary in nearly all cases to maintain desired IOP.
Byrnes et al reported on a case series report of 20 patients with malignant glaucoma and success of vitrectomy in 50% of phakic patients and 90% of pseudophakic eyes.18 They recommended combined vitrectomy and crystalline lens extraction to allow removal of the entire anterior vitreous without damaging the lens. They defined success as immediate backward movement of the iris–lens diaphragm during vitrectomy recovering the aqueous outflow, and deepening of the anterior chamber. This is in contrast to the current study in which success was based on criteria of IOP reduction and medication use. Although the anterior chamber deepened after vitrectomy in all cases of the current study, medication or subsequent IOP-lowering procedures became necessary in the majority of patients to reach target IOP.
A study by Harbour et al, in which surgical outcomes of vitrectomy of ten pseudophakic and 14 phakic eyes were compared, revealed a successful initial vitrectomy in 100% of seven phakic eyes undergoing concurrent lens extraction and in 71% of seven phakic eyes without cataract extraction.26 In pseudophakic patients, the initial vitrectomy was successful in 90% of patients. Therefore, lensectomy during vitrectomy may be considered in cases of dense cataract or inability to deepen the anterior chamber. In the current study, more than two-thirds of patients were pseudophakic at the time of vitrectomy, whereas two patients received cataract extraction during vitrectomy. Thus, two patients remained phakic and also had a successful IOP reduction after deepening of the anterior chamber, but showed a progression of cataract during follow-up.
In the current study, the 23-gauge transconjunctival sutureless vitrectomy was equally effective compared to the standard three-port 20-gauge system regarding reduction of IOP and success rate. While the 23-gauge approach is frequently associated with a higher incidence of postoperative hypotony, no differences were found in complication rates between both systems. Furthermore, complications were few in both groups. This is in line with previously published data on complications after vitrectomy for malignant glaucoma mostly encountering transient shallowing of the anterior chamber, hypotony, retinal or choroidal detachment, corneal decompensation, and bleb failure needing surgical revision.18,26
Lois et al reported a novel technique of zonulo-hyaloido-vitrectomy using a vitreous cutter through a peripheral iridectomy or iridotomy via the anterior chamber in five patients.21 Resolution of malignant glaucoma was achieved in all cases during a follow-up of 5.5 months. Lois et al stated that zonulo-hyaloido-vitrectomy via an anterior approach appears to be an option in the treatment of pseudophakic malignant glaucoma and can be safely done by an anterior segment surgeon.21
Due to the difficulty in visualization of the anterior hyaloid membrane in phakic eyes, Chen et al introduced a novel technique of videoendoscope-guided fluorescein-assisted vitrectomy without cataract extraction in selected cases of prepresbyopic patients to avoid the need for clear lens extraction.22 However, they stated that the limited illumination field of the videoendoscope, when simultaneously used with the vitreous cutter, may contribute to an unintentional touch of the lens during vitrectomy. Previous studies have addressed the importance of lensectomy to achieve desired IOP reduction after the initial vitrectomy since pseudophakic eyes compared to phakic eyes show a higher success rate in terminating malignant glaucoma.18,19,25,26
The current study has some limitations. First, retrospective data commonly has more sources of error due to confounding and bias. Second, the sample size was too small to detect small differences between the two groups. It is difficult to collect a large number of cases since malignant glaucoma is a very rare disease. Prospective trials with greater statistical power will be needed to establish surgical methods of malignant glaucoma.
Conclusion
In summary, pars plana vitrectomy using the 20- and 23-gauge approach is effective for treatment of malignant glaucoma in patients with and without previous filtration surgery. Efficacy regarding reduction of IOP, success, and complications rates was similar in eyes with and without previous trabeculectomy.
Acknowledgments
This publication was funded by the German Research Foundation (DFG) and the University of Wuerzburg in the funding program Open Access Publishing.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Schwartz AL, Anderson DR. Malignant glaucoma in an eye with no antecedent operation or miotics. Arch Ophthalmol. 1975;93(5):379–381. doi: 10.1001/archopht.1975.01010020391015. [DOI] [PubMed] [Google Scholar]
- 2.Cashwell LF, Martin TJ. Malignant glaucoma after laser iridotomy. Ophthalmology. 1992;99(5):651–658. doi: 10.1016/s0161-6420(92)31913-x. [DOI] [PubMed] [Google Scholar]
- 3.Arya SK, Sonika, Kochhar S, Kumar S, Kang M, Sood S. Malignant glaucoma as a complication of Nd:YAG laser posterior capsulotomy. Ophthalmic Surg Lasers Imaging. 2004;35(3):248–250. [PubMed] [Google Scholar]
- 4.Hanish SJ, Lamberg RL, Gordon JM. Malignant glaucoma following cataract extraction and intraocular lens implant. Ophthalmic Surg. 1982;13(9):713–714. [PubMed] [Google Scholar]
- 5.Rieser JC, Schwartz B. Miotic-induced malignant glaucoma. Arch Ophthalmol. 1972;87(6):706–712. doi: 10.1001/archopht.1972.01000020708018. [DOI] [PubMed] [Google Scholar]
- 6.Theelen T, Klevering BJ. Malignant glaucoma following blunt trauma of the eye. Ophthalmologe. 2005;102(1):77–81. doi: 10.1007/s00347-004-1038-9. German. [DOI] [PubMed] [Google Scholar]
- 7.Ng WT, Morgan W. Mechanisms and treatment of primary angle closure: a review. Clin Experiment Ophthalmol. 2012;40(4):e218–e228. doi: 10.1111/j.1442-9071.2011.02604.x. [DOI] [PubMed] [Google Scholar]
- 8.Quigley HA, Friedman DS, Congdon NG. Possible mechanisms of primary angle-closure and malignant glaucoma. J Glaucoma. 2003;12(2):167–180. doi: 10.1097/00061198-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Luntz MH, Rosenblatt M. Malignant glaucoma. Surv Ophthalmol. 1987;32(2):73–93. doi: 10.1016/0039-6257(87)90101-9. [DOI] [PubMed] [Google Scholar]
- 10.Simmons RJ. Malignant glaucoma. Br J Ophthalmol. 1972;56(3):263–272. doi: 10.1136/bjo.56.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lippas J. Mechanics and the treatment of malignant glaucoma and the problem of a flat anterior chamber. Am J Ophthalmol. 1964;57:620–627. doi: 10.1016/0002-9394(64)92508-5. [DOI] [PubMed] [Google Scholar]
- 12.Burgansky-Eliash Z, Ishikawa H, Schuman JS. Hypotonous malignant glaucoma: aqueous misdirection with low intraocular pressure. Ophthalmic Surg Lasers Imaging. 2008;39(2):155–159. doi: 10.3928/15428877-20080301-03. [DOI] [PubMed] [Google Scholar]
- 13.Ruben S, Tsai J, Hitchings RA. Malignant glaucoma and its management. Br J Ophthalmol. 1997;81(2):163–167. doi: 10.1136/bjo.81.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Little BC, Hitchings RA. Pseudophakic malignant glaucoma: Nd:YAG capsulotomy as a primary treatment. Eye (Lond) 1993;7(Pt 1):102–104. doi: 10.1038/eye.1993.21. [DOI] [PubMed] [Google Scholar]
- 15.Halkias A, Magauran DM, Joyce M. Ciliary block (malignant) glaucoma after cataract extraction with lens implant treated with YAG laser capsulotomy and anterior hyaloidotomy. Br J Ophthalmol. 1992;76(9):569–570. doi: 10.1136/bjo.76.9.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herschler J. Laser shrinkage of the ciliary processes. A treatment for malignant (ciliary block) glaucoma. Ophthalmology. 1980;87(11):1155–1159. doi: 10.1016/s0161-6420(80)35117-8. [DOI] [PubMed] [Google Scholar]
- 17.Momoeda S, Hayashi H, Oshima K. Anterior pars plana vitrectomy for phakic malignant glaucoma. Jpn J Ophthalmol. 1983;27(1):73–79. [PubMed] [Google Scholar]
- 18.Byrnes GA, Leen MM, Wong TP, Benson WE. Vitrectomy for ciliary block (malignant) glaucoma. Ophthalmology. 1995;102(9):1308–1311. doi: 10.1016/s0161-6420(95)30870-6. [DOI] [PubMed] [Google Scholar]
- 19.Sharma A, Sii F, Shah P, Kirkby GR. Vitrectomy-phacoemulsification-vitrectomy for the management of aqueous misdirection syndromes in phakic eyes. Ophthalmology. 2006;113(11):1968–1973. doi: 10.1016/j.ophtha.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 20.Tsai YY, Tseng SH. Combined trabeculectomy and vitrectomy for pseudophakic malignant glaucoma and extensive peripheral anterior synechia-induced secondary glaucoma. J Cataract Refract Surg. 2004;30(3):715–717. doi: 10.1016/S0886-3350(03)00665-5. [DOI] [PubMed] [Google Scholar]
- 21.Lois N, Wong D, Groenewald C. New surgical approach in the management of pseudophakic malignant glaucoma. Ophthalmology. 2001;108(4):780–783. doi: 10.1016/s0161-6420(00)00642-4. [DOI] [PubMed] [Google Scholar]
- 22.Chen SD, Salmon JF, Patel CK. Videoendoscope-guided fluorescein-assisted vitrectomy for phakic malignant glaucoma. Arch Ophthalmol. 2005;123(10):1419–1421. doi: 10.1001/archopht.123.10.1419. [DOI] [PubMed] [Google Scholar]
- 23.Shaffer RN. The role of vitreous detachment in aphakic and malignant glaucoma. Trans Am Acad Ophthalmol Otolaryngol. 1954;58(2):217–231. [PubMed] [Google Scholar]
- 24.Chandler PA. A new operation for malignant glaucoma: a preliminary report. Trans Am Ophthalmol Soc. 1964;62:408–424. [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai JC, Barton KA, Miller MH, Khaw PT, Hitchings RA. Surgical results in malignant glaucoma refractory to medical or laser therapy. Eye (Lond) 1997;11(Pt 5):677–681. doi: 10.1038/eye.1997.176. [DOI] [PubMed] [Google Scholar]
- 26.Harbour JW, Rubsamen PE, Palmberg P. Pars plana vitrectomy in the management of phakic and pseudophakic malignant glaucoma. Arch Ophthalmol. 1996;114(9):1073–1078. doi: 10.1001/archopht.1996.01100140275003. [DOI] [PubMed] [Google Scholar]