Abstract
Age-related changes in lower limb joint position sense and their contributions to postural stability are well documented. In contrast, only a few studies have investigated the effect of age on proprioceptive hand function. Here, we introduce a novel test for measuring joint position sense in the fingers of the human hand. In a concurrent matching task, subjects had to detect volume differences between polystyrene balls grasped with their dominant (seven test stimuli: 126–505 cm3) and their nondominant hand (three reference stimuli: 210, 294, and 505 cm3). A total of 21 comparisons were performed to assess the number of errors, the weight of errors (ie, the volume difference between test and reference stimuli), and the direction of errors (ie, over- or underestimation of test stimulus). The test was applied to 45 healthy subjects aged 21 to 79 years. Our results revealed that all variables changed significantly with age, with the number of errors showing the strongest increase. We also assessed tactile acuity (two-point discrimination thresholds) and sensorimotor performance (pegboard performance) in a subset of subjects, but these scores did not correlate with joint position sense performance, indicating that the test reveals specific information about joint position sense that is not captured with pure sensory or motor tests. The average test–retest reliability assessed on 3 consecutive days was 0.8 (Cronbach’s alpha). Our results demonstrate that this novel test reveals age-related decline in joint position sense acuity that is independent from sensorimotor performance.
Keywords: aging, hand functions, joint position sense
Introduction
Proprioception refers to the sense of knowing where one’s body is located in space, and consists of joint position sense (static information) and kinesthetic movement sense (dynamic information).1 Both components are important for generating smooth, coordinated movements, as well as for the maintenance of body posture and balance.2
There are three major sources of proprioceptive information that mediate the conscious perception of limb movement and position. Muscle spindles,3–5 cutaneous receptors, and joint mechanoreceptors6,7 provide feedback to the central nervous system that is essential for determining distal body segment positions (for a review on the contributions of different afferents to the movement sense in the human hand, see Cordo et al8). Data acquired in deafferented patients demonstrate that movement onset is delayed and trajectory formation is highly inaccurate without proprioception.9,10
Age-related changes in the joint position sense
A number of groups have investigated the effects of healthy aging on proprioceptive performance. Although some early studies were not able to demonstrate an age-related change,11,12 more recent investigations have provided evidence that proprioception is affected by age.2,13–16 When the performance of older subjects was compared with that of younger subjects in cross-sectional studies, there was a decrease in joint position sense and an increase in the movement detection threshold. For the lower limb, these results were shown for both knee joint position sense17 and ankle joint position sense.16 Similarly, for the upper limb, decreases in the joint position sense in the elbow and fingers were observed.18,19 Movement detection thresholds increased with advancing age, as shown in the knee,20 the ankle,21 and the metatarsophalangeal joints of the lower limbs and the metacarpophalangeal joints of the upper limbs.11
Decline in lower-limb proprioception has been associated with balance problems in the elderly,22 and a consequent higher incidence of falls.23 Hurley et al17 investigated possible relations between proprioceptive acuity and activities of daily living in the elderly. They found a significant correlation between joint position sense acuity of the knee as assessed with an ipsilateral remembered matching task and individual walking performance.17 By contrast, the impact of age-related changes on the joint position sense of the hand is less clear. Joint position sense is usually assessed in stroke patients24,25 or in those with age-associated diseases such as arthritis.26 Despite the demonstrated importance of proprioceptive feedback for coordinated hand and arm control, few studies have addressed the effects of healthy aging on human upper-limb proprioception,13 which could be due to the lack of simple tests for assessing proprioceptive hand functions in healthy subjects.27
Quantitative assessment of limb position sense
Recent studies focusing on age-related changes of position sense use matching tasks for a single joint or limb segment, such as for the elbow, arm, hip, knee, ankle, and toe (for a review see Goble et al28). To our knowledge, there are only a few tests designed to assess the position sense in finger joints.18,27,29,30
Proprioceptive abilities of patients are commonly tested on the basis of the ability to accurately discriminate the upward or downward position of a passively moved finger, toe, or more proximal single joint.31 Some clinicians use the thumb localization test.32 These clinical tools have poor interrater reliability and sensitivity, and poor or absent normal value criteria.24,31 Although more quantitative measures for joint position sense exist,25,33,34 they often examine a single joint and require manual repositioning of the limb.24 New methods are needed to gain insight into hand proprioception in both normal and clinical populations. An intact joint position sense is an essential prerequisite for a number of higher sensorimotor skills, such as haptic object exploration,35 which may be affected in cerebrovascular diseases36 and other pathological states.37
Here, we report age-related changes of the joint position sense of the human hand in a group of healthy adults and elderly individuals using a novel paradigm. The requirements that had to be complied by the presented test were threefold. On the one side, we aimed to develop a test, which can be used quickly and without sophisticated technical equipment. This might be of special interest for future use in clinical settings. On the other side, we wanted to assure that the fingers maintained defined positions during testing, which suggested the use of an object-related testing. Using spherical objects of different sizes that have to be grasped by the subjects allows for a comparison of the perception of different, clearly defined finger positions. Finally, the tested joint positions should be related to sensorimotor, everyday life performance. In this context, the enclosure of objects is a highly stereotypic hand movement that is used to explore different object features, thereby supporting haptic object recognition.35,38
By using an object-based spherical hand grasp-matching task, all the above mentioned requirements could be fulfilled.
Methods
Participants
We recruited 45 healthy volunteers with no history of hand injury or pathology. The measurements were carried out in three groups of adults: 13 young subjects (average age: 25.1 ± 3.6 years, range: 20–30 years, six males), 17 mid-aged subjects (average age: 48.1 ± 9.4 years, range: 33–63 years, eight males), and 15 older subjects (average age: 71.2 ± 3.9 years, range: 66–79 years, eight males). All subjects were right-handed according to the Edinburgh Handedness Inventory39 (young subjects: 88.5 ± 8.0; mid-aged subjects: 82.9 ± 7.7; older subjects: 85.0 ± 7.3). The local ethics committee approved the study, and all subjects provided written informed consent before participating.
Joint position sense assessment using a spherical hand grasp-matching task
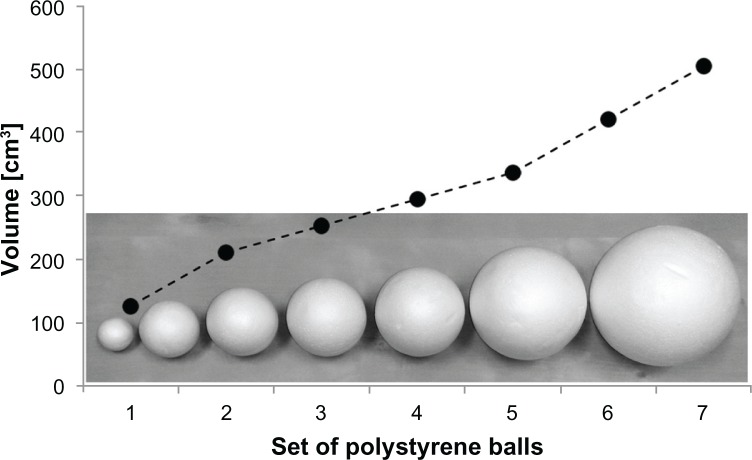
All subjects were asked to compare lightweight polystyrene balls of different diameters to a reference ball in their nondominant hand without visual information. The subjects had to place their hands with palms upward on a cushion covered by a screen. Each set of polystyrene balls (test and reference ball) was placed in the subject’s opened hands by the experimenter. The subjects were instructed to enclose the balls with their fingers and to maintain the hand in this position. Exploring the balls by repeated opening and closing of the fingers was not permitted. The subjects had to indicate if they perceived the tested object to be larger, smaller, or equal in volume to the reference within 5 seconds. In three consecutive subtests, the complete set of polystyrene balls (126 cm3, 0.43 g; 210 cm3, 1.48 g; 252 cm3, 1.86 g; 294 cm3, 2.4 g; 337 cm3, 6.7 g; 421 cm3, 10.5 g; 505 cm3, 18.4 g) was compared to a small reference (210 cm3), to a midsized reference (294 cm3), and to a large reference (505 cm3) (Figure 1). Thereby, the order of the references and the test balls was randomized.
Figure 1.
Set of polystyrene balls used for the assessment of joint position sense.
Notes: A set of seven polystyrene balls (126, 210, 252, 294, 337, 421, and 505 cm3) was used to quantify the performance of the joint position sense of the subjects. One ball was enclosed in each hand simultaneously to detect size differences. As the enclosure is a typical and highly stereotypical explorative procedure by which to gather information about an object’s volume,38,40,41 all calculations were carried out in units of cubic centimeters (cm3).
We calculated the number of errors (NE, total of 21 decisions), the weight of errors (WE, volume difference of reference and test object), and the direction of errors (DE). The DE was calculated by summing up all errors where the size of the reference ball was overestimated (+1 for every error) or underestimated (−1 for every error). The entire test took approximately 5 minutes.
Tactile acuity assessment
We assessed tactile acuity by measuring spatial stationary two-point discrimination thresholds on the tip of the index finger of the dominant hand with the method of constant stimuli.42–47 The stimuli consisted of seven pairs of brass needles with distances ranging from 0.7 to 7 mm, as well as a single needle. All stimuli were applied for approximately 1 second with an application force of approximately 150 mN. The presentation of all stimuli was repeated ten times in a randomized order resulting in 80 trials per session. All subjects had to complete one training session to become familiar with the testing procedure. The summed responses of every session were plotted against the needle distances resulting in a psychometric function plot, which was fitted using binary logistic regression. The threshold was taken from the fit at the value where 50% correct responses were obtained.
Dexterity assessment
A standardized pegboard test was used to investigate fine and gross motor dexterity and hand, finger, and arm coordination.48 A 5 × 30 cm ledge with 25 drilled holes was located to the right of the subject. A container with 25 metal pins (1 × 0.25 cm) was placed 30 cm from the ledge. The subjects were instructed to pick up the pins with their dominant hand one by one from the container and insert them into the holes on the ledge as quickly as possible. If one of the metal pins was dropped, subjects were instructed to go on with the next pin. The time required to complete the test was recorded. All subjects had to accomplish two training sessions to become familiar with the testing procedure.
Statistical analysis
The analysis of between-subtest differences was carried out using repeated measures analysis of variance (ANOVA) and subsequent post hoc tests. To examine between-group differences in performance, we used a least significant difference post hoc test for the variables NE, WE, and DE. Correlation analyses were carried out using Spearman’s rank correlation tests (NE and WE), Pearson’s correlation tests (DE), and partial correlations (corrected for age). All data are given as mean values (M) and standard error of means (SEM), or standard deviations (SD). Statistical analyses were carried out using the Statistical Package for the Social Sciences (SPSS) statistics software (v 20; IBM Corporation, Armonk, NY).
Results
Between-subtest differences
We investigated differences between the three joint position sense subtests (Figure 2). There was no significant interaction between the factors of “group” (young, adult, older adults) and “session” (large reference, midsized reference, small reference) in repeated measures ANOVA for the NE (F(4,84) = 0.666; P = 0.618), WE (F(4,84) = 0.073; P = 0.990), or DE (F(4,84) = 1.925; P = 0.114). For that reason, the data from all age groups were pooled for further analysis.
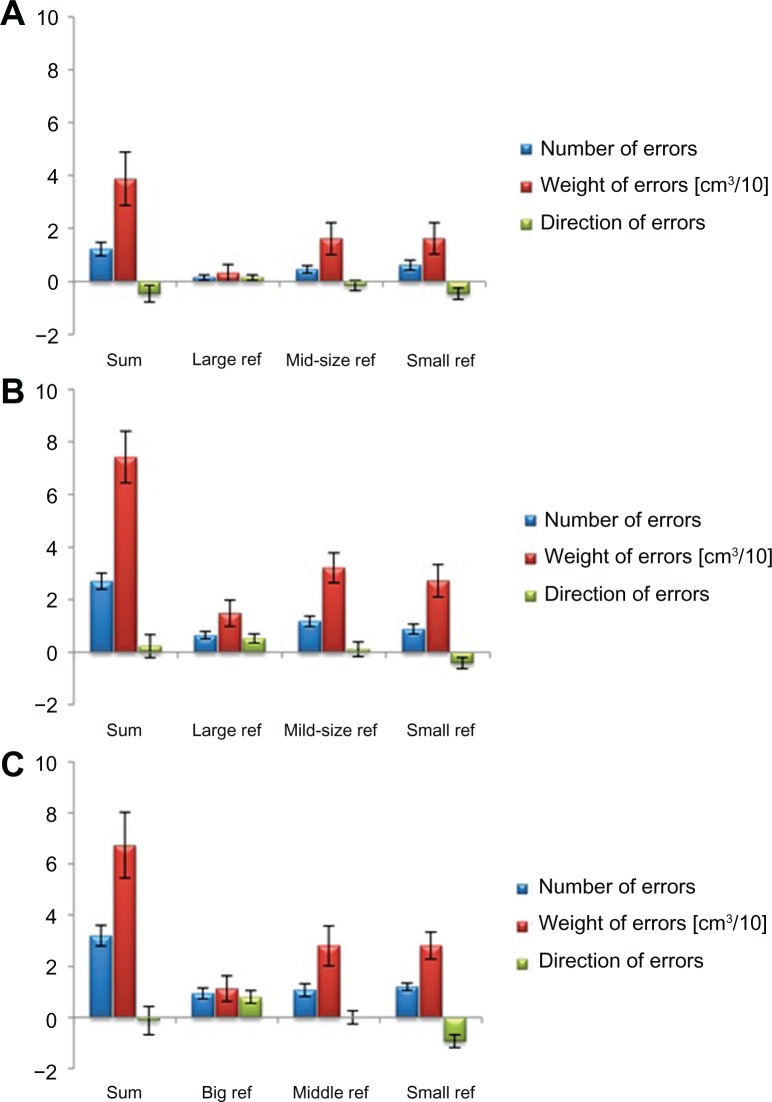
Figure 2.
Joint position sense performance in the three subtests (large reference, mid-size reference, and small reference). The performance was assessed in young subjects (A), adult subjects (B), and older adults (C).
Notes: There was no significant interaction of the factors “group” (young, mid-aged adults, older adults) and “session” (big reference, mid-size reference, small reference) in repeated measures ANOVA for the number of errors (F(4,84) = 0.666; P = 0.618), the weight of errors (F(4,84) = 0.073; P = 0.990), and the direction of errors (F(4,84) = 1.925; P = 0.114). Data is given as mean ± standard error for all age groups and subtests. The results of all subtests were summed up per group (sum). The variable weight of errors was adjusted to fit into the diagrams [cm3/10].
Abbreviation: ANOVA, analysis of variance.
Post hoc testing did not reveal any significant difference between the NE measured in the subtest using a large reference (0.58 ± 0.15), a midsized reference (0.90 ± 0.20), or a small reference (0.90 ± 0.66) (P ≥ 0.149). For the WE, different results were found. In the subtest using a large reference, the WE was significantly lower (9.77 ± 4.41 cm3) as compared to the subtest using a midsized reference (25.45 ± 6.55 cm3; P = 0.002) or a small reference (23.83 ± 5.80 cm3; P = 0.011). There were no significant differences between the last two subtests (P = 1.000).
On the basis of the design of the joint position sense test, we expected significant results for DE between-subtest differences. Using a large reference in the first subtest, errors can only occur if a subject rates the test ball to be larger than the reference (positive DE). When a small reference ball is used (last subtest), errors can only occur if a subject rates the test ball to be smaller than the reference (negative DE). In the second subtest (midsized reference), errors can occur in both directions. Therefore, the sum of all subtests was expected to be approximately 0. These expectations were fulfilled, as the post hoc test revealed significant differences (P ≤ 0.020) between the DE in the first (0.49 ± 0.17), second (−0.01 ± 0.24), and third subtests (−0.60 ± 0.22).
Between-subject differences
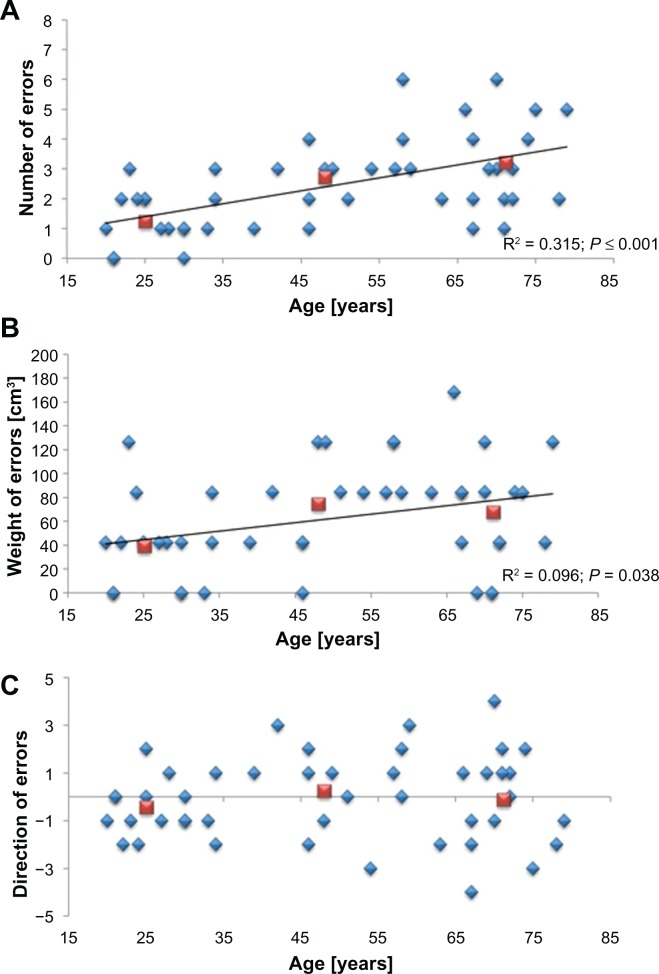
For the analysis of between-subject differences, the sum of every variable of the three subtests (large reference, midsized reference, small reference) was used (Figure 2). In general, we found a significant age-related decrease in joint position sense acuity (Figure 3). The NE increased significantly from 1.23 ± 0.26 in young subjects, to 2.71 ± 1.26 in mid-aged subjects, and to 3.20 ± 1.57 in older adults (n = 45, r = 0.543, P ≤ 0.001). Post hoc testing revealed significant differences in NE between young and mid-aged subjects (P = 0.003), and between young subjects and older adults (P = 0.001). There was no significant difference between the NE of mid-aged subjects and older adults (P = 0.563) (Figure 3A).
Figure 3.
Joint position sense performance across the lifespan. There was a significant age-related change across all variables acquired by the joint position sense test. The number of errors increased with age (N = 45; r = 0.543, P ≤ 0.001) (A) Similarly, the weight of errors increased with age (N = 45; r = 0.311, P = 0.038) (B). There was no significant difference in the average direction of errors between the subjects of the three age groups (N = 45; r = 0.072, P = 0.637), but the scatter of these data increased with age (N = 9 (ie, standard deviation obtained in the three subtests per age group); r = 0.543, P ≤ 0.001) (C).
Note: Data is given as single subject data (blue diamonds) and mean for each age group (red squares).
In young subjects, the WE was 38.83 ± 1.01 cm3. This value increased to 74.24 ± 0.99 cm3 in mid-aged and 67.32 ± 1.29 cm3 in older adults (n = 45, r = 0.311, P = 0.038). There was a significant difference between the performance of young and mid-aged subjects (P = 0.030), but there was no significant difference between the performance of older adults and all other subjects (P ≥ 0.087) (Figure 3B).
The DE was balanced across all age groups (P ≥ 0.292). For young subjects, we calculated an average DE of 1.23 ± 0.26, for mid-aged adults 0.24 ± 0.44, and for older adults 0.13 ± 0.55 (Figure 3C). There was a significant age-related increase in scatter for the variable DE (n = 9, r = 0.543, P ≤ 0.001) that was not found for the variables NE or WE (n = 9, r ≤ 0.622, P ≥ 0.074). These results demonstrate that the between-subject differences increase with age.
Correlation analyses of proprioceptive parameters
For correlation analyses (partial correlation, corrected for subject age) between all parameters of proprioceptive performance that were assessed during the test, we used the absolute values of the DE. We found a significant correlation between the NE and the WE (n = 45, r = 0.737, P ≤ 0.001). Furthermore, the absolute values of the DE and the NE were significantly correlated (n = 45, r = 0.418, P = 0.005). There was no correlation between the absolute values of the DE and the WE (n = 45, r = 226, P = 0.140).
Test–retest reliability
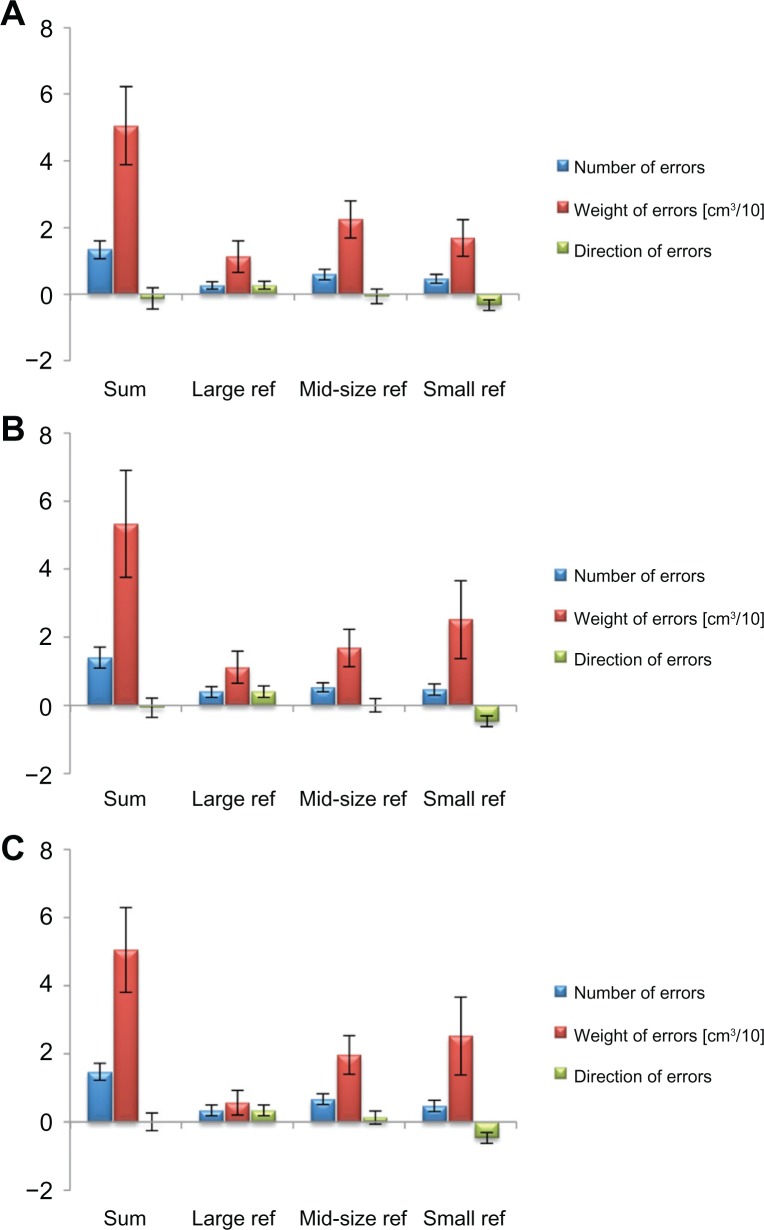
The reproducibility of the proprioceptive assessment was analyzed on an individual subject level. Therefore, we investigated test–retest reliability in a subset of 15 mid-aged subjects (41.5 ± 12.2 years) who performed the joint position sense test on 3 consecutive days. The subjects’ performance on a given day was compared with the equivalent values on the other days. All calculations were carried out using the summed variables NE, WE, and DE that were measured in the three consecutive assessments (large reference, mid-size reference, small reference; Figure 5). Concerning the NE, we found a test–retest reliability of 0.787 (Cronbach’s alpha). The average intraclass correlation coefficient (95% confidence interval [CI]) between all days was 0.799 (0.531–0.927). The reliability of the WE was 0.761 with an average intraclass correlation coefficient (95% CI) between all days of 0.776 (0.477–0.918). Finally, the test–retest reliability for the DE in the joint position sense test was 0.833, with an average intraclass correlation coefficient (95% CI) between all days of 0.842 (0.631–0.942).
Figure 5.
Test–retest reliability of the joint position sense assessment. The test–retest reliability was investigated in a subset of 15 mid-aged subjects, who performed the joint position sense test on 3 consecutive days (day one (A); day two (B); day three (C)) within 1 week.
Notes: All calculations were based on the summed data of the three subtests (sum). Test–retest reliability was acceptable for the number of errors (Cronbach’s alpha = 0.787) and the weight of errors (Cronbach’s alpha = 0.761); moreover, the reliability of the direction of errors was good (Cronbach’s alpha 0.833).
Relation between joint position sense, tactile acuity, and dexterity of the hand
To investigate the specificity of the joint position sense assessment, we additionally performed correlation analyses (partial correlation corrected for age) between all joint position parameters and individual tactile acuity (ie, two-point discrimination thresholds), as well as individual dexterity (ie, pin-plugging performance). In summary, there were no significant correlations between NE, WE, or DE and individual two-point discrimination thresholds (n = 35, r ≤ 0.192, P ≥ 0.256). Furthermore, we found no significant correlations between NE, WE, or DE and individual pin-plugging performance (n = 13, r ≤ 0.414, P ≥ 0.125).
Discussion
The aim of the present study was to evaluate age-related changes in the joint position sense of the healthy human hand by means of a novel assessment. Our results from 45 individuals aged 20–79 years revealed significant age-related declines of the joint position sense. Comparison with additional assessments of tactile acuity and manual dexterity revealed no correlation, indicating that the assessment captures joint position sense performance that is not evaluated with sensory or motor tests.
Age-related changes affecting proprioception
Peripheral and central level neurophysiological factors are responsible for age-related proprioception declines (for a review see Goble et al28), which have been reported in studies investigating dynamic position sense at the ankle,16 upper-limb proprioceptive acuity,14,15 and passive wrist movement.13 Capsular thickness increases in aged muscle spindles, whereas spindle diameter and the total number of intrafusal fibers decreases. Collectively, these changes result in a general loss of sensitivity that affects stretch-sensitive mechanoreceptors.49 Cutaneous receptors, which also contribute to proprioception, also show age-related alterations. The number of Meissner and Pacinian corpuscles decrease, thereby leading to reduced mean density per unit of skin area.49,50 Finally, there is a decline in the number of joint mechanoreceptors, including Ruffini, Pacinian, and Golgi tendon type receptors.51 In addition to these peripheral alterations, there are changes in the aged central nervous system that affect proprioception. Many studies revealed a direct proportionality between individual experience or use of special skills and the amount of cortical reorganization in humans.52–54 Studies in patients and aged subjects showed that reduced use might dramatically change cortical representational areas not only in the motor domain,55 but also in the sensory domain.56,57
Behavioral investigations utilizing position matching tasks of different processing demands have shown that proprioceptive feedback processing in the human brain shows age-related impairment depending on the difficulty of the task.15 Those that involve either memory (eg, ipsilateral remembered matching tasks) or interhemispheric transfer (eg, contralateral remembered matching tasks) of proprioceptive information do not show significant differences in elderly subjects. In contrast, when both memory and interhemispheric transfer are necessary for a given task, the increased proprioceptive processing demands significantly affected acuity in the elderly.15 Further evidence for diminished proprioceptive abilities in the elderly comes from studies investigating postural stability.58 When older adults are faced with dual-task situations (eg, upright stance and a concurrent cognitive task), postural sway increases.59 The results demonstrate that older adults might need to recruit greater attentional resources for maintaining balance in the feedback mode of motor control, and this may be at the expense of other cognitive processes.
A novel method for assessing proprioceptive hand function
The ability to sense the static position of a joint or limb segment is by far the most common assessment of proprioceptive acuity. Tests of position sense typically focus on the accuracy by which an individual can identify or match a target joint angle in the absence of vision, and are conducted as either ipsilateral remembered matching tasks or contralateral concurrent matching tasks (for a review see Goble et al28). Here, we employed a novel joint position sense test as a bilateral concurrent matching task to avoid the potential confound of decreased memory abilities in older adults.60 On the other hand, bilateral concurrent matching is also subject to limitations of its own in that this task relies on interhemispheric communication.61 Nevertheless, in the context of the presented joint position sense test, the contralateral concurrent matching design has a great advantage, as the subjects can rely on online feedback to make their decisions.
The testing procedure utilizes enclosure, a highly stereotypical posture of the hand. Enclosure belongs to the so-called exploratory procedures involved in human haptic processing,38,40,41 and it is the first step in gathering basic information about an object, such as shape and volume (see Lederman and Klatzky for details62). Exploratory procedures are crucial for successful interaction with the environment and strongly rely on joint position sense. The test of proprioceptive hand function described in the present work was designed with the intention to rule out other sources of information that might interfere with the assessment of finger joint position sense. As the subjects were instructed to avoid further finger movements (ie, exploration) after the enclosure of the polystyrene balls, they had to focus their attention to the perceived size differences, which is directly linked to the degree of flexion of the finger joints. By placing the hands on a supporting cushion and using lightweight polystyrene balls, the subjects’ ability to differentiate the balls by means of weight was extremely limited. To some extent, weight can be perceived when an object simply rests on a stationary hand; however, active exploration, particularly lifting and wielding the object, is crucial to judging weight exactly.63
Nevertheless, future experiments using the joint position test will be improved in two aspects. First, the subjects will be trained to enclose the polystyrene balls with defined pressure by implementing training sessions with a dynamometer grip ball. By means of this training, all subjects should apply the same grip force during joint position sense testing. Second, the subjects will perform the task while they are wearing gloves to veil the size differences of skin portions that are in contact with the test object. This approach should force subjects to primarily rely on information from the joint position sense and rule out other sources of information as far as possible.
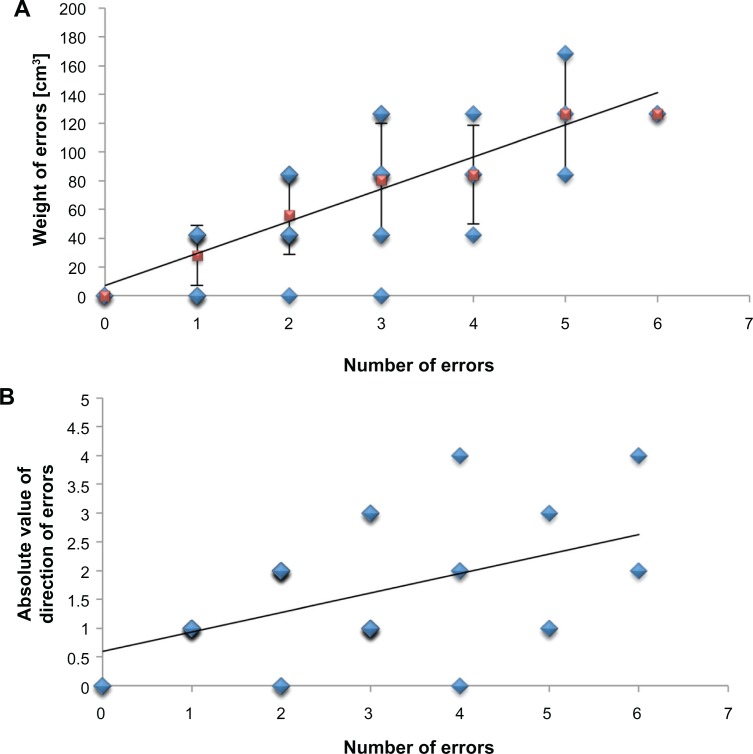
From the data collected with this joint position sense test, we concluded that the NE is the main variable describing age-related changes in healthy joint position sense of the hand (increase of 0.4 errors/decade; range: 0–21 errors). The variable WE is also affected by age, but to a much lesser extent (increase of 7.1 cm3/decade, range: 0–252.4 cm3). Given that there was no significant difference between the WE of mid-aged and older adults (Figure 3B) points toward a saturation of this variable during late adulthood. Further tests in clinical subpopulations (eg, stroke patients) will determine if this variable might be a useful indicator of pathological impairment of proprioceptive hand function. Thereby, the linear relation of the NE and the WE (Figure 4A; ie, 1:22) might be used as normative data to detect pathological changes. Accordingly, a small NE with high weight, or vice versa, might be considered abnormal. The direction of errors does not seem to be a useful indicator for age-related changes in proprioceptive hand function because the differences are highly variable among subjects. Nevertheless, the absolute values of DE increase with age. This parameter might be used for monitoring individual treatment-induced alterations in patients, as it is very reliable in healthy subjects (Figure 5). The fact that there were no significant correlations between individual joint position sense performance and tactile acuity or dexterity provides evidence that the test specifically measures joint position sense performance that is not assessed with sensory or motor tasks. On the other hand, more experiments are necessary to investigate the possible correlation between individual performance in the presented joint position test and performance in tests of haptic object exploration, which also rely on joint position sense to some extent. In this context, it would be of special interest to further reveal underlying age-related changes that account for declining haptic performance in later life.35
Figure 4.
Correlation-analyses of joint position sense parameters. We calculated significant correlations between the number of errors and both other parameters of the proprioceptive hand function test (weight of errors; partial correlation corrected for age; N = 45; r = 0.737; P ≤ 0.001 (A), and absolute value of direction of errors; partial correlation corrected for age; N = 45; r = 0.418; P = 0.005 (B)).
Notes: The analyses were based on the summed values of the three subtests. Blue diamonds give individual data; red squares give mean ± SD.
There are some noteworthy advantages of the test described here. First, it does not require expensive or sophisticated equipment that is often necessary for apparatus-based joint position sense assessment.24,26,34 Second, the test can be rapidly administered, which is appropriate for subjects who are not able to participate in longer assessments. Blinding the subject to the testing procedure eliminates negative feedback that occurs in other tests, where subjects realize their own inaccuracies (eg, in the thumb localization test). Finally, the simple application makes the test robust against rater-dependent error.
Conclusion
The results of this novel assessment of proprioceptive hand function demonstrate a clear age-related decline of joint position sense performance in a cohort of healthy individuals. This observation is based on an object-based quantitative approach that records three different parameters describing the accuracy of estimating object volume without visual inspection, and thereby the perception of joint positions in the hand. Further investigation is necessary to prove the possible relations between age-related changes in joint position sense and activities of daily living that rely on proper hand function. In addition, the presented test might also be applicable in pathological settings to help quantify changes of joint position sense after stroke or hand injury, as well as to determine the effects of rehabilitation measures.
Acknowledgments
This research was supported by grants from the German Research Foundation (Deutsche Forschungsgemeinschaft [DFG]) to HRD (Di 334/19-1, SFB 874) and MT (Te 315/4-1, SFB 874), as well as from a grant from FoRUM (F-637-08) to TK, and a grant from ADTV to JCK.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gandevia SC, Refshauge KM, Collins DF. Proprioception: peripheral inputs and perceptual interactions. Adv Exp Med Biol. 2002;508:61–68. doi: 10.1007/978-1-4615-0713-0_8. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro F, Oliveira J. Aging effects on joint proprioception: the role of physical activity in proprioception preservation. Eur Rev Aging Phys Act. 2007;4(2):71–76. [Google Scholar]
- 3.Proske U. Kinesthesia: the role of muscle receptors. Muscle Nerve. 2006;34(5):545–558. doi: 10.1002/mus.20627. [DOI] [PubMed] [Google Scholar]
- 4.Proske U, Wise AK, Gregory JE. The role of muscle receptors in the detection of movements. Prog Neurobiol. 2000;60(1):85–96. doi: 10.1016/s0301-0082(99)00022-2. [DOI] [PubMed] [Google Scholar]
- 5.Proske U. What is the role of muscle receptors in proprioception? Muscle Nerve. 2005;31(6):780–787. doi: 10.1002/mus.20330. [DOI] [PubMed] [Google Scholar]
- 6.Edin B. Cutaneous afferents provide information about knee joint movements in humans. J Physiol. 2001;531(Pt 1):289–297. doi: 10.1111/j.1469-7793.2001.0289j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edin BB. Finger joint movement sensitivity of non-cutaneous mechanoreceptor afferents in the human radial nerve. Exp Brain Res. 1990;82(2):417–422. doi: 10.1007/BF00231261. [DOI] [PubMed] [Google Scholar]
- 8.Cordo PJ, Horn JL, Künster D, Cherry A, Bratt A, Gurfinkel V. Contributions of skin and muscle afferent input to movement sense in the human hand. J Neurophysiol. 2011;105(4):1879–1888. doi: 10.1152/jn.00201.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon J, Ghilardi MF, Ghez C. Impairments of reaching movements in patients without proprioception. I. Spatial errors. J Neurophysiol. 1995;73(1):347–360. doi: 10.1152/jn.1995.73.1.347. [DOI] [PubMed] [Google Scholar]
- 10.Ghez C, Gordon J, Ghilardi MF. Impairments of reaching movements in patients without proprioception. II. Effects of visual information on accuracy. J Neurophysiol. 1995;73(1):361–372. doi: 10.1152/jn.1995.73.1.361. [DOI] [PubMed] [Google Scholar]
- 11.Kokmen E, Bossemeyer RW, Jr, Williams WJ. Quantitative evaluation of joint motion sensation in an aging population. J Gerontol. 1978;33(1):62–67. doi: 10.1093/geronj/33.1.62. [DOI] [PubMed] [Google Scholar]
- 12.Lovelace EA, Aikens JE. Vision, kinesthesis, and control of hand movement by young and old adults. Percept Mot Skills. 1990;70(3 Pt 2):1131–1137. doi: 10.2466/pms.1990.70.3c.1131. [DOI] [PubMed] [Google Scholar]
- 13.Wright ML, Adamo DE, Brown SH. Age-related declines in the detection of passive wrist movement. Neurosci Lett. 2011;500(2):108–112. doi: 10.1016/j.neulet.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamo DE, Alexander NB, Brown SH. The influence of age and physical activity on upper limb proprioceptive ability. J Aging Phys Act. 2009;17(3):272–293. doi: 10.1123/japa.17.3.272. [DOI] [PubMed] [Google Scholar]
- 15.Adamo DE, Martin BJ, Brown SH. Age-related differences in upper limb proprioceptive acuity. Percept Mot Skills. 2007;104(3 Pt 2):1297–1309. doi: 10.2466/pms.104.4.1297-1309. [DOI] [PubMed] [Google Scholar]
- 16.Verschueren SM, Brumagne S, Swinnen SP, Cordo PJ. The effect of aging on dynamic position sense at the ankle. Behav Brain Res. 2002;136(2):593–603. doi: 10.1016/s0166-4328(02)00224-3. [DOI] [PubMed] [Google Scholar]
- 17.Hurley MV, Rees J, Newham DJ. Quadriceps function, proprioceptive acuity and functional performance in healthy young, middle-aged and elderly subjects. Age Ageing. 1998;27(1):55–62. doi: 10.1093/ageing/27.1.55. [DOI] [PubMed] [Google Scholar]
- 18.Ferrell WR, Crighton A, Sturrock RD. Position sense at the proximal interphalangeal joint is distorted in patients with rheumatoid arthritis of finger joints. Exp Physiol. 1992;77(5):675–680. doi: 10.1113/expphysiol.1992.sp003633. [DOI] [PubMed] [Google Scholar]
- 19.Ferrell WR, Crighton A, Sturrock RD. Age-dependent changes in position sense in human proximal interphalangeal joints. Neuroreport. 1992;3(3):259–261. doi: 10.1097/00001756-199203000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Yan T, Hui-Chan CW. The ability to detect movement of the knee joint is decreased with aging. Arch Phys Med Rehabil. 2000;81:1274. [Google Scholar]
- 21.Gilsing MG, Van den Bosch CG, Lee SG, et al. Association of age with the threshold for detecting ankle inversion and eversion in upright stance. Age Ageing. 1995;24(1):58–66. doi: 10.1093/ageing/24.1.58. [DOI] [PubMed] [Google Scholar]
- 22.Woollacott MH, Shumway-Cook A, Nashner LM. Aging and posture control: changes in sensory organization and muscular coordination. Int J Aging Hum Dev. 1986;23(2):97–114. doi: 10.2190/VXN3-N3RT-54JB-X16X. [DOI] [PubMed] [Google Scholar]
- 23.Sorock GS, Labiner DM. Peripheral neuromuscular dysfunction and falls in an elderly cohort. Am J Epidemiol. 1992;136(5):584–591. doi: 10.1093/oxfordjournals.aje.a116536. [DOI] [PubMed] [Google Scholar]
- 24.Dukelow SP, Herter TM, Moore KD, et al. Quantitative assessment of limb position sense following stroke. Neurorehabil Neural Repair. 2010;24(2):178–187. doi: 10.1177/1545968309345267. [DOI] [PubMed] [Google Scholar]
- 25.Carey LM, Oke LE, Matyas TA. Impaired limb position sense after stroke: a quantitative test for clinical use. Arch Phys Med Rehabil. 1996;77(12):1271–1278. doi: 10.1016/s0003-9993(96)90192-6. [DOI] [PubMed] [Google Scholar]
- 26.Peixoto JG, Dias JM, Dias RC, da Fonseca ST, Teixeira-Salmela LF. Relationships between measures of muscular performance, proprioceptive acuity, and aging in elderly women with knee osteoarthritis. Arch Gerontol Geriatr. 2011;53(2):e253–e257. doi: 10.1016/j.archger.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Wycherley AS, Helliwell PS, Bird HA. A novel device for the measurement of proprioception in the hand. Rheumatology (Oxford) 2005;44(5):638–641. doi: 10.1093/rheumatology/keh568. [DOI] [PubMed] [Google Scholar]
- 28.Goble DJ, Coxon JP, Wenderoth N, Van Impe A, Swinnen SP. Proprioceptive sensibility in the elderly: degeneration, functional consequences and plastic-adaptive processes. Neurosci Biobehav Rev. 2009;33(3):271–278. doi: 10.1016/j.neubiorev.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Moberg E. The role of cutaneous afferents in position sense, kinaesthesia, and motor function of the hand. Brain. 1983;106(Pt 1):1–19. doi: 10.1093/brain/106.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Clark FJ, Burgess RC, Chapin JW, Lipscomb WT. Role of intramuscular receptors in the awareness of limb position. J Neurophysiol. 1985;54(6):1529–1540. doi: 10.1152/jn.1985.54.6.1529. [DOI] [PubMed] [Google Scholar]
- 31.Lincoln NB, Crow JL, Jackson JM, Waters GR, Adams SA, Hodgson P. The unreliability of sensory assessments. Clin Rehabil. 1991;5:273–282. [Google Scholar]
- 32.Hirayama K, Fukutake T, Kawamura M. ‘Thumb localizing test’ for detecting a lesion in the posterior column-medial lemniscal system. J Neurol Sci. 1999;167(1):45–49. doi: 10.1016/s0022-510x(99)00136-7. [DOI] [PubMed] [Google Scholar]
- 33.Goble DJ, Lewis CA, Brown SH. Upper limb asymmetries in the utilization of proprioceptive feedback. Exp Brain Res. 2006;168(1–2):307–311. doi: 10.1007/s00221-005-0280-y. [DOI] [PubMed] [Google Scholar]
- 34.Leibowitz N, Levy N, Weingarten S, et al. Automated measurement of proprioception following stroke. Disabil Rehabil. 2008;30(24):1829–1836. doi: 10.1080/09638280701640145. [DOI] [PubMed] [Google Scholar]
- 35.Kalisch T, Kattenstroth JC, Kowalewski R, Tegenthoff M, Dinse HR. Cognitive and tactile factors affecting human haptic performance in later life. PloS One. 2012;7(1):e30420. doi: 10.1371/journal.pone.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith PS, Dinse HR, Kalisch T, Johnson M, Walker-Batson D. Effects of repetitive electrical stimulation to treat sensory loss in persons poststroke. Arch Phys Med Rehabil. 2009;90(12):2108–2111. doi: 10.1016/j.apmr.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Grunwald M, Ettrich C, Busse F, Assmann B, Dähne A, Gertz HJ. Angle paradigm: a new method to measure right parietal dysfunctions in anorexia nervosa. Arch Clin Neuropsychol. 2002;17(5):485–496. [PubMed] [Google Scholar]
- 38.Lederman SJ, Klatzky RL. Hand movements: a window into haptic object recognition. Cogn Psychol. 1987;19(3):342–368. doi: 10.1016/0010-0285(87)90008-9. [DOI] [PubMed] [Google Scholar]
- 39.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 40.Klatzky RL, Lederman SJ, Metzger VA. Identifying objects by touch: an “expert system”. Percept Psychophys. 1985;37(4):299–302. doi: 10.3758/bf03211351. [DOI] [PubMed] [Google Scholar]
- 41.Lederman SJ, Klatzky RL. Haptic classification of common objects: knowledgedriven exploration. Cogn Psychol. 1990;22(4):421–459. doi: 10.1016/0010-0285(90)90009-s. [DOI] [PubMed] [Google Scholar]
- 42.Kalisch T, Tegenthoff M, Dinse HR. Improvement of sensorimotor functions in old age by passive sensory stimulation. Clin Interv Aging. 2008;3(4):673–690. doi: 10.2147/cia.s3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dinse HR, Kalisch T, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M. Improving human haptic performance in normal and impaired human populations through unattended activation-based learning. ACM Trans Appl Percept. 2005;2(2):71–88. [Google Scholar]
- 44.Godde B, Stauffenberg B, Spengler F, Dinse HR. Tactile coactivation-induced changes in spatial discrimination performance. J Neurosci. 2000;20(4):1597–1604. doi: 10.1523/JNEUROSCI.20-04-01597.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pleger B, Dinse HR, Ragert P, Schwenkreis P, Malin JP, Tegenthoff M. Shifts in cortical representations predict human discrimination improvement. Proc Natl Acad Sci U S A. 2001;98(21):12255–12260. doi: 10.1073/pnas.191176298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dinse HR, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M. Pharmacological modulation of perceptual learning and associated cortical reorganization. Science. 2003;301(5629):91–94. doi: 10.1126/science.1085423. [DOI] [PubMed] [Google Scholar]
- 47.Kalisch T, Tegenthoff M, Dinse HR. Differential effects of synchronous and asynchronous multifinger coactivation on human tactile performance. BMC Neurosci. 2007;8:58. doi: 10.1186/1471-2202-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalisch T, Wilimzig C, Kleibel N, Tegenthoff M, Dinse HR. Age-related attenuation of dominant hand superiority. PLoS One. 2006;1:e90. doi: 10.1371/journal.pone.0000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaffer SW, Harrison AL. Aging of the somatosensory system: a translational perspective. Phys Ther. 2007;87(2):193–207. doi: 10.2522/ptj.20060083. [DOI] [PubMed] [Google Scholar]
- 50.Iwasaki T, Goto N, Goto J, Ezure H, Moriyama H. The aging of human Meissner’s corpuscles as evidenced by parallel sectioning. Okajimas Folia Anat Jpn. 2003;79(6):185–189. doi: 10.2535/ofaj.79.185. [DOI] [PubMed] [Google Scholar]
- 51.Morisawa Y. Morphological study of mechanoreceptors on the coracoacromial ligament. J Orthop Sci. 1998;3(2):102–110. doi: 10.1007/s007760050029. [DOI] [PubMed] [Google Scholar]
- 52.Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270(5234):305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- 53.Schwenkreis P, El Tom S, Ragert P, Pleger B, Tegenthoff M, Dinse HR. Assessment of sensorimotor cortical representation asymmetries and motor skills in violin players. Eur J Neurosci. 2007;26(11):3291–3302. doi: 10.1111/j.1460-9568.2007.05894.x. [DOI] [PubMed] [Google Scholar]
- 54.Pascual-Leone A, Torres F. Plasticity of the sensorimotor cortex representation of the reading finger in Braille readers. Brain. 1993;16(Pt 1):39–52. doi: 10.1093/brain/116.1.39. [DOI] [PubMed] [Google Scholar]
- 55.Liepert J, Tegenthoff M, Malin JP. Changes of cortical motor area size during immobilization. Electroencephalogr Clin Neurophysiol. 1995;97(6):382–386. doi: 10.1016/0924-980x(95)00194-p. [DOI] [PubMed] [Google Scholar]
- 56.Lissek S, Wilimzig C, Stude P, et al. Immobilization impairs tactile perception and shrinks somatosensory cortical maps. Curr Biol. 2009;19(10):837–842. doi: 10.1016/j.cub.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 57.Kalisch T, Ragert P, Schwenkreis P, Dinse HR, Tegenthoff M. Impaired tactile acuity in old age is accompanied by enlarged hand representations in somatosensory cortex. Cereb Cortex. 2009;19(7):1530–1538. doi: 10.1093/cercor/bhn190. [DOI] [PubMed] [Google Scholar]
- 58.Kalisch T, Kattenstroth JC, Noth S, Tegenthoff M, Dinse HR. Rapid assessment of age-related differences in standing balance. J Aging Res. 2011;2011:160490. doi: 10.4061/2011/160490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doumas M, Smolders C, Krampe RT. Task prioritization in aging: effects of sensory information on concurrent posture and memory performance. Exp Brain Res. 2008;187(2):275–281. doi: 10.1007/s00221-008-1302-3. [DOI] [PubMed] [Google Scholar]
- 60.Grady CL. Cognitive neuroscience of aging. Ann N Y Acad Sci. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- 61.Ota M, Obata T, Akine Y, et al. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. Neuroimage. 2006;31(4):1445–1452. doi: 10.1016/j.neuroimage.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 62.Lederman SJ, Klatzky RL. Extracting object properties through haptic exploration. Acta Psychol (Amst) 1993;84(1):29–40. doi: 10.1016/0001-6918(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 63.Brodie EE, Ross HE. Sensorimotor mechanisms in weight discrimination. Percept Psychophys. 1984;36(5):477–481. doi: 10.3758/bf03207502. [DOI] [PubMed] [Google Scholar]