Abstract
Objective To assess the long term effects of two different modes of disease management (comprehensive self management and routine monitoring) on quality of life (primary objective), frequency and patients’ management of exacerbations, and self efficacy (secondary objectives) in patients with chronic obstructive pulmonary disease (COPD) in general practice.
Design 24 month, multicentre, investigator blinded, three arm, pragmatic, randomised controlled trial.
Setting 15 general practices in the eastern part of the Netherlands.
Participants Patients with COPD confirmed by spirometry and treated in general practice. Patients with very severe COPD or treated by a respiratory physician were excluded.
Interventions A comprehensive self management programme as an adjunct to usual care, consisting of four tailored sessions with ongoing telephone support by a practice nurse; routine monitoring as an adjunct to usual care, consisting of 2-4 structured consultations a year with a practice nurse; or usual care alone (contacts with the general practitioner at the patients’ own initiative).
Outcome measures The primary outcome was the change in COPD specific quality of life at 24 months as measured with the chronic respiratory questionnaire total score. Secondary outcomes were chronic respiratory questionnaire domain scores, frequency and patients’ management of exacerbations measured with the Nijmegen telephonic exacerbation assessment system, and self efficacy measured with the COPD self-efficacy scale.
Results 165 patients were allocated to self management (n=55), routine monitoring (n=55), or usual care alone (n=55). At 24 months, adjusted treatment differences between the three groups in mean chronic respiratory questionnaire total score were not significant. Secondary outcomes did not differ, except for exacerbation management. Compared with usual care, more exacerbations in the self management group were managed with bronchodilators (odds ratio 2.81, 95% confidence interval 1.16 to 6.82) and with prednisolone, antibiotics, or both (3.98, 1.10 to 15.58).
Conclusions Comprehensive self management or routine monitoring did not show long term benefits in terms of quality of life or self efficacy over usual care alone in COPD patients in general practice. Patients in the self management group seemed to be more capable of appropriately managing exacerbations than did those in the usual care group.
Trial registration Clinical trials NCT00128765.
Introduction
Chronic obstructive pulmonary disease (COPD) is a slowly progressive lung disease characterised by deterioration of lung function and quality of life and periods of acute exacerbation. Its substantial prevalence— in 2005 one in 59 people in England were recorded as having COPD diagnosed by a physician1—and its huge social and economic impact make COPD a major health problem.2 Most patients with COPD have mild to moderate disease and are treated in general practice.3 To face the burden of COPD, well studied and effective management strategies are essential, particularly in primary care.
According to current COPD guidelines, symptoms and airflow obstruction should be monitored regularly to guide modification of treatment and to identify complications early.2 3 Routine monitoring should contribute to achieving management goals in COPD—that is, to delay disease progression and to alleviate its manifestations.4 The responsibility for this management strategy is largely with the healthcare professional, who relies on protocols and acts as an expert. The effects of monitoring routines on clinical outcomes such as health status seem to be doubtful.4 In addition, whether care should be based on uniform routine consultations or should be more tailored to individual patient’s needs with a shared responsibility between physician and patient has been questioned.5
Comprehensive self management programmes focus on the needs of the individual patient. These programmes are based on the presumption that effective modification of behaviour can be attained only if patients’ self efficacy has been improved.6 Patients who have enough confidence in their ability to successfully respond to certain events, such as at the time of an exacerbation, can more easily modify and maintain the desired behaviour. The behavioural modification should ultimately result in improved clinical outcomes.7 COPD self management programmes have shown positive effects on patients’ quality of life and healthcare use in secondary care settings, but the benefits in general practice are still inconclusive.8 9
Our primary objective was to assess the long term effects of two different modes of COPD disease management—comprehensive self management and routine monitoring—on quality of life in COPD patients in general practice. As secondary objectives, we assessed the effects on frequency and patients’ management of exacerbations and on self efficacy.
Methods
Study design
This was a 24 month, multicentre, investigator blinded, three arm, parallel group, randomised controlled trial. After having obtained signed informed consent, we randomly allocated participating patients with COPD to comprehensive self management as an adjunct to usual care, routine monitoring through scheduled periodic monitoring visits as an adjunct to usual care, or usual care alone.
Setting and participants
Fifteen general practices in the Netherlands (Nijmegen region) recruited patients between June 2004 and September 2006. The practices invited patients for an eligibility assessment by following a standardised procedure in which they started inviting patients on the basis of a list of all patients who (according to the diagnostic codes in their electronic medical record system) had been diagnosed as having COPD. The investigators randomised the order in which the patients appeared on the list. This assessment consisted of pre-bronchodilator and post-bronchodilator spirometry,10 as well as collection of data on sociodemographic characteristics, smoking habits, current comorbid conditions, and current use of respiratory drugs.
Patients were eligible for the study if they were aged at least 35 years and their post-bronchodilator ratio of forced expiratory volume in one second to forced vital capacity was less than 0.70. Exclusion criteria were post-bronchodilator forced expiratory volume in one second below 30% predicted, treatment by a respiratory physician, severe comorbid conditions with a reduced life expectancy, inability to communicate in the Dutch language, and objections to one or more of the modes of disease management used in the study.
Randomisation and interventions
We randomised participants by using a computer generated two block randomisation procedure with stratification on severity of COPD (mild or moderate v severe airflow obstruction),2 smoking status (current v former smoker), and frequency of exacerbations in the previous 24 months (<2 v ≥2 exacerbations). To ensure that the investigators were blinded to individual treatment allocation, practice nurses informed the patients of their allocation. We prevented potential treatment contamination caused by provision of self management, routine monitoring, and usual care within the same practice by using strict protocols and registration forms and by providing the required self management materials only for the patients randomised to the self management group. At the end of the study we reviewed the registration forms and the patients’ electronic medical files.
We randomly allocated patients to usual care, self management, or routine monitoring. Usual care reflected the care for COPD patients as provided by most general practices in the Netherlands in 2005. Patients received care from their general practitioner at their own initiative when they consulted with aggravation of symptoms. Patients in the usual care group did not receive any care from the practice nurse—that is, they were not monitored on a routine base and did not receive (parts of) a self management programme.
Patients in the self management group received a translated and modified version of the Canadian self management programme “Living well with COPD.”11 Four Dutch general practitioners and four patients with mild to moderate COPD who did not participate in the trial evaluated the version used in the trial. Unlike the original Canadian programme, our final version did not include an exercise programme (table A of the web appendix shows the differences). The self management programme consisted of paper modules and a written exacerbation action plan. Topics covered in the modules were COPD disease knowledge, respiratory drugs, breathing techniques, managing exacerbations, maintaining a healthy lifestyle, managing stress and anxiety (optional), and home exercise (optional). The individualised written exacerbation action plan covered early recognition of and prompt action in the course of an exacerbation. Actions included increase in bronchodilator use; initiation of standing prescriptions for prednisolone, antibiotics (if applicable), or both; or contacting the practice nurse or general practitioner. The practice nurse of each participating practice acted as case manager and applied the programme to the individual patient in two to four sessions of approximately one hour each, scheduled in four to six consecutive weeks. The sessions took place in the general practice. The number of sessions depended on the patient’s needs, but it was at least two. Subsequently, the nurse called each patient six times during the rest of the study period to reinforce self management skills. The nurse was available for advice during business hours. Before the study, all nurses were trained in how to apply the self management programme. In addition, all nurses were observed at least once by a respiratory nurse who was a member of the study group and experienced in the self management programme. The respiratory nurse also coached the practice nurses by using a message board on a secured web based application during the rest of the follow-up.
For participants in the routine monitoring group, practice nurses scheduled routine monitoring visits in the general practice, on top of usual care. The contents of the monitoring visits were based on the national and international COPD guidelines at time of the study.12 13 At each consultation, the practice nurse evaluated the severity of symptoms and limitations, health status, adverse effects of and compliance with respiratory drugs, the use of inhaler devices, and frequency of exacerbations; weight and lung function were measured once a year. The contents of the routine visits were not tailored to individual patient’s needs, nor were self management elements such as the use of a written exacerbation action plan included. The general practitioner determined the individual frequency of monitoring, depending on the severity of airflow obstruction and level of dyspnoea (Medical Research Council dyspnoea score14), but it was at least once a year with a maximum of four times a year.
Outcomes and follow-up
Our primary pre-specified outcome was the change from baseline in health related quality of life after 24 months as measured by the self administered chronic respiratory questionnaire.15 16 This consists of 20 questions on a seven point Likert-type scale (a higher score indicating better quality of life), comprising a total score and four domain scores for dyspnoea, mastery, fatigue, and emotion. The minimal clinically important difference for the questionnaire has been established at 0.5 points.17 Secondary pre-specified outcomes were the change in chronic respiratory questionnaire domain scores, exacerbation frequency and management as recorded with an automated call system, and total and five domain scores for self efficacy as measured with the COPD self-efficacy scale.18 To assess short term effects of the interventions, we analysed differences in chronic respiratory questionnaire and COPD self-efficacy scale total and domain scores at six months.
All participants visited the pulmonary function laboratory of the Radboud University Nijmegen Medical Centre at baseline, 12 months, and 24 months. At six months and 18 months, a trained lung function technician visited patients at home. During all study visits, data were collected on smoking habits, respiratory drugs, spirometry,10 health related quality of life, and self efficacy. The data collected were not provided to the practices.
We assessed frequency and patients’ management of exacerbations with the Nijmegen telephonic exacerbation assessment system (TEXAS).19 Patients were called once every two weeks on the day and at the time of their preference. Patients answered (yes or no) to questions from an automated voice on changes in respiratory symptoms and management of exacerbations—that is, increase in bronchodilator use; initiation of oral prednisolone, antibiotics, or both; and unscheduled healthcare use in the two weeks before the call. TEXAS was not part of any intervention and did not alert the patient when an exacerbation was imminent. The validity of TEXAS as a research tool has been shown previously.19 We defined exacerbations as a change for at least two consecutive days in either two or more major symptoms (dyspnoea, sputum purulence, sputum amount) or any one major symptom plus at least one minor symptom (colds, wheeze, sore throat, cough).20 21
Sample size calculation and statistical analyses
Sample size calculation using analysis of variance showed that we needed 55 patients in each treatment arm for 80% power (α=0.05, two sided) to detect a minimal clinically important difference in the change in the mean chronic respiratory questionnaire total score of 0.5 points at 24 months with a standard deviation of 0.8, a mean total score of 4.8 under the null hypothesis,22 and an anticipated dropout rate of 25%. Our primary analysis was based on the intention to treat principle and included all available data for all participants. We compared self management with usual care and routine monitoring with usual care. We did not impute any missing data.
We show baseline characteristics as number (percentage), mean (SD), or median (interquartile range). Because of repeated measurements for each patient, we used generalised estimating equations analyses with a compound symmetry structure and including the data at all time points (including baseline) to analyse differences within and between groups for the outcomes chronic respiratory questionnaire total and domain scores and COPD self-efficacy scale total and domain scores. We show only the changes at six months (short term effects) and 24 months (long term effects). We used a generalised estimating equations logistic regression model with compound symmetry to estimate differences in clinically important improvements (minimal clinically important difference ≥0.5) of chronic respiratory questionnaire total scores between treatment arms. We counted the number of exacerbations recorded by TEXAS. A new exacerbation was distinguished from a previous one if it was preceded by two weeks in which symptoms had not worsened. We expressed exacerbation rates as number of exacerbations per patient per year and compared them by using weighted rate ratios.23 We tested statistical significance by using a negative binomial regression analysis with the patient as the unit of analysis.24 We estimated differences in exacerbation management by using generalised estimating equations logistic regression models with compound symmetry and exacerbation as the unit of analysis. To all models we added sex, age, education level, long acting bronchodilator use, and inhaled corticosteroid use as covariates. We used the statistical package SAS version 9.2 for Windows for all analyses.
Results
Recruitment and patients’ characteristics
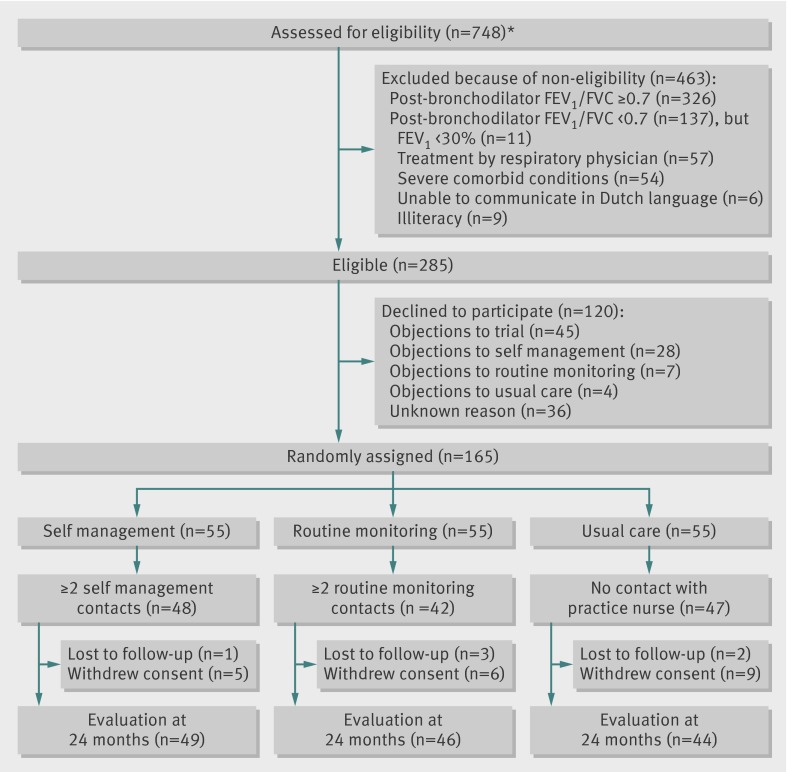
Figure 1 shows the flow of patients through the study. Of the 748 patients whose general practitioner considered them to have COPD, 326 (43.6%) did not meet our inclusion criterion of post-bronchodilator ratio of forced expiratory volume in one second to forced vital capacity of less than 0.70. No differences were apparent between eligible patients who declined to participate (n=120) and those who were randomised (n=165) in terms of age (66.8 v 65.1 years, P=0.15), sex (61% v 63% males, P=0.65), and post-bronchodilator forced expiratory volume in one second as per cent predicted (70% v 68%, P=0.38). Almost 16% (n=26) of the patients dropped out during follow-up. Baseline characteristics did not differ between dropouts and participants who completed follow-up. Table 1 shows that at inclusion in the study, patients’ characteristics were well balanced between the three study groups, except for sex. Overall, more than half of the patients were male, most patients had mild to moderate airflow obstruction, and a median of 1.0 exacerbation was reported to the general practitioner in the two years before the study.
Fig 1 Flow diagram of study. *Eligibility was assessed in general practice by measurement of pre-bronchodilator and post-bronchodilator lung function10 and collection of data on sociodemographic characteristics, smoking habits, current medical conditions, and current use of respiratory drugs. FEV1= forced expiratory volume in one second; FVC=forced vital capacity
Table 1.
Patients’ characteristics at study inclusion. Values are numbers (percentages) unless stated otherwise
| Characteristics | Self management (n=55) | Routine monitoring (n=55) | Usual care (n=55) |
|---|---|---|---|
| Mean (SD) age (years) | 65.5 (11.5) | 65.8 (8.3) | 63.5 (10.3) |
| Male sex | 37 (67) | 42 (76) | 28 (51) |
| Median (interquartile range) COPD duration (years) | 7.0 (3.8-13.0) | 6.0 (3.0-11.0) | 8.0 (4.0-13.0) |
| Low educational level | 30 (55) | 35 (64) | 27 (49) |
| Current smoker | 16 (29) | 15 (27) | 18 (33) |
| Post-bronchodilator FEV1/FVC < lower limit of normal | 43 (78) | 42 (76) | 38 (69) |
| Mean (SD) post-bronchodilator FEV1 (% predicted) | 66.3 (16.5) | 62.9 (14.4) | 67.0 (18.0) |
| No (interquartile range) of previous GP diagnosed exacerbations* | 1.0 (0-2.0) | 1.0 (0-2.0) | 0.5 (0-2.0) |
| Mean (SD) MRC dyspnoea score | 2.02 (0.94) | 1.87 (0.72) | 1.73 (0.76) |
| Respiratory drug treatment: | |||
| None | 11 (20) | 16 (29) | 19 (35) |
| Long acting bronchodilators | 29 (53) | 14 (25) | 10 (21) |
| Inhaled corticosteroids | 35 (64) | 21 (38) | 17 (31) |
| Relevant comorbidities: | |||
| Depression or anxiety | 4 (7) | 2 (4) | 2 (4) |
| Heart failure | 7 (13) | 7 (13) | 4 (7) |
| Cancer | 4 (7) | 3 (5) | 1 (2) |
COPD=chronic obstructive pulmonary disease; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; GP=general practitioner; MRC=Medical Research Council.
*Unscheduled contacts with GP for worsening of respiratory symptoms in previous 24 months.
Primary outcome
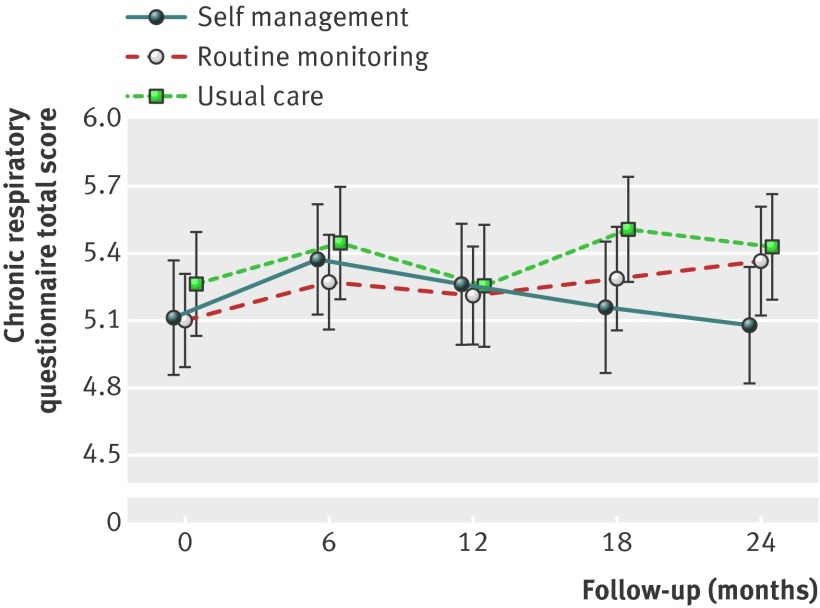
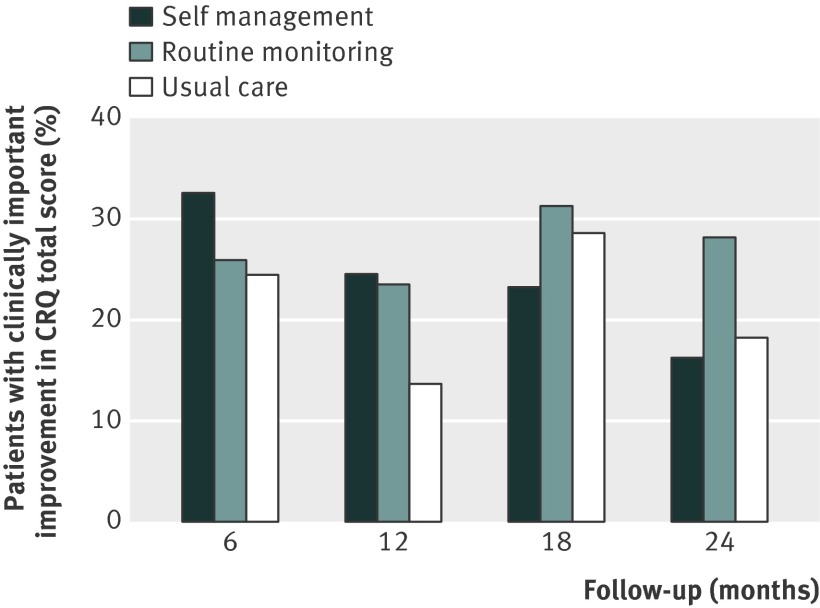
Figure 2 shows the changes in COPD specific quality of life (chronic respiratory questionnaire total score) for the self management, routine monitoring, and usual care groups. At 24 months, the mean treatment differences between self management and usual care and between routine monitoring and usual care were not statistically significant (table 2). More patients in the routine monitoring group than in the usual care group showed a clinically important improvement (13/46 (28%) v 8/44 (18%)) (fig 3 ), but this difference was not statistically significant (adjusted odds ratio 1.44, 95% confidence interval 0.61 to 3.38).
Fig 2 Changes in unadjusted means of chronic respiratory questionnaire total score during 24 months of follow-up for the self management, routine monitoring, and usual care groups. Bars indicate 95% CIs
Table 2.
Primary and secondary clinical outcomes: within and between group differences at 24 months*. Values are mean (SD) unless stated otherwise
| Self management (SM) (n=55) | Routine monitoring (RM) (n=55) | Usual care (UC) (n=55) | Treatment difference at 24 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Change at 24 months (95% CI) | Baseline | Change at 24 months (95% CI) | Baseline | Change at 24 months (95% CI) | SM v UC | RM v UC | ||||
| Primary outcome | |||||||||||
| CRQ total | 5.11 (0.94) | −0.10 (−0.28 to 0.084) | 5.10 (0.77) | 0.28 (0.088 to 0.47) | 5.26 (0.81) | 0.12 (−0.042 to 0.42) | −0.22 (−0.49 to 0.042) | 0.16 (−0.11 to 0.42) | |||
| Secondary outcomes | |||||||||||
| CRQ emotions | 5.13 (1.02) | 0.0026 (−0.24 to 0.24) | 5.18 (0.91) | 0.34 (0.088 to 0.59) | 5.2 (1.08) | 0.31 (0.062 to 0.56) | −0.31 (−0.66 to 0.039) | 0.027 (−0.33 to 0.38) | |||
| CRQ mastery | 4.75 (0.89) | −0.13 (−0.37 to 0.10) | 4.85 (0.77) | −0.017 (−0.26 to 0.23) | 4.91 (0.65) | 0.071 (−0.17 to 0.32) | −0.20 (−0.55 to 0.14) | −0.088 (−0.44 to 0.26) | |||
| CRQ fatigue | 4.79 (1.34) | −0.18 (−0.49 to 0.13) | 4.75 (1.18) | 0.34 (0.019 to 0.65) | 5.04 (1.18) | −0.007 (−0.32 to 0.31) | −0.17 (−0.62 to 0.27) | 0.34 (−0.11 to 0.79) | |||
| CRQ dyspnoea | 5.68 (1.21) | −0.16 (−0.42 to 0.11) | 5.47 (1.29) | 0.40 (0.13 to 0.67) | 5.82 (1.08) | 0.063 (−0.26 to 0.28) | −0.16 (−0.54 to 0.21) | 0.40 (0.014 to 0.78)† | |||
| CSES total | 3.53 (1.0) | −0.15 (−0.47 to 0.18) | 3.67 (0.86) | −0.12 (−0.45 to 0.20) | 3.6 (0.92) | 0.025 (−0.31 to 0.36) | −0.17 (−0.64 to 0.30) | −0.15 (−0.62 to 0.32) | |||
| CSES negative affect | 3.6 (0.92) | −0.27 (−0.57 to 0.025) | 3.51 (1.42) | −0.091 (−0.39 to 0.20) | 3.55 (0.84) | −0.22 (−0.53 to 0.09) | −0.053 (−0.48 to 0.38) | 0.13 (−0.30 to 0.56) | |||
| CSES emotional arousal | 3.67 (0.86) | −0.24 (−0.56 to 0.067) | 3.75 (0.8) | −0.24 (−0.55 to 0.07) | 3.69 (0.82) | −0.15 (−0.47 to 0.17) | −0.094 (−0.54 to 0.36) | −0.091 (−0.54 to 0.36) | |||
| CSES physical exertion | 3.01 (1.11) | −0.053 (−0.36 to 0.26) | 3.03 (1.01) | 0.16 (−0.15 to 0.47) | 2.93 (0.84) | 0.12 (−0.20 to 0.45) | −0.17 (−0.62 to 0.27) | 0.038 (−0.41 to 0.48) | |||
| CSES weather/ environment | 3.26 (1.0) | −0.18 (−0.49 to 0.12) | 3.36 (0.79) | −0.079 (−0.39 to 0.23) | 3.26 (0.9) | 0.13 (−0.19 to 0.45) | −0.32 (−0.76 to 0.13) | −0.21 (−0.65 to 0.23) | |||
| CSES behavioural risk factors | 3.34 (1.02) | −0.17 (−0.49 to 0.15) | 3.35 (0.91) | 0.019 (−0.30 to 0.34) | 3.47 (0.92) | −0.084 ( −0.42 to 0.25) | −0.086 (−0.55 to 0.38) | 0.10 (−0.36 to 0.57) | |||
CRQ=chronic respiratory questionnaire; CSES=COPD self-efficacy scale.
*Generalised estimating equations analysis with compound symmetry structure and covariates of sex, age, educational level, long acting bronchodilator use, and inhaled corticosteroid use, and including data at all time points.
†P=0.042.
Fig 3 Percentages of patients with clinically important improvements (≥0.5 improvement from baseline) in chronic respiratory questionnaire (CRQ) total score during 24 months of follow-up
Secondary outcomes
Chronic respiratory questionnaire domain scores—Changes at 24 months in the chronic respiratory questionnaire domain scores were not statistically significant (table 2), except for the dyspnoea domain which showed improvement in the routine monitoring group compared with the usual care group.
Exacerbation frequency and management—153 patients reported a total of 829 exacerbations. Frequency of exacerbations did not differ between the three groups (table 3). In the second year of follow-up, more exacerbations in the self management group than in the usual care group were managed by an increase in bronchodilator use (odds ratio 2.81, 1.16 to 6.82) and by starting prednisolone, antibiotics, or both (3.98, 1.10 to 15.58) (table 4). Also, in the second study year, more exacerbations in the self management group than in the other two groups tended to be reported to the general practitioner or nurse (not statistically significant) (table 4).
Table 3.
Differences in exacerbation rate* per patient between groups
| Time | Self management (SM) (n=53) | Routine monitoring (RM) (n=55) | Usual care (UC) (n=48) | Rate ratio (95% CI)† | |
|---|---|---|---|---|---|
| SM v UC | RM v UC | ||||
| Baseline to 12 months | 2.83 | 3.25 | 2.73 | 1.10 (0.86 to 1.40) | 1.25 (0.98 to 1.58) |
| 12-24 months | 2.45 | 2.38 | 2.17 | 1.16 (0.81 to 1.67) | 1.15 (0.80 to 1.65) |
*Measured by automated telephonic exacerbation assessment system.
†Weighted rate ratios were tested for statistical significance by using negative binomial regression analyses.
Table 4.
Differences in patients’ management of exacerbations between groups. Values are numbers (percentages) unless stated otherwise
| Exacerbation management | Self management (SM) (n=53) | Routine monitoring (RM) (n=55) | Usual care (UC) (n=48) | Odds ratio (95% CI)* | |
|---|---|---|---|---|---|
| SM v UC | RM v UC | ||||
| Baseline to 12 months: | (n=150)† | (n=179)† | (n=131)† | ||
| Increased bronchodilator | 65 (43) | 65 (36) | 40 (31) | 1.70 (0.81 to 3.54) | 1.22 (0.60 to 2.48) |
| Prednisolone and/or antibiotics | 16 (11) | 22 (12) | 13 (10) | 1.24 (0.43 to 3.57) | 1.28 (0.57 to 2.87) |
| Unscheduled medical contact | 20 (13) | 27 (15) | 18 (14) | 1.09 (0.42 to 2.81) | 1.08 (0.52 to 2.23) |
| 12-24 months: | (n=130)† | (n=131)† | (n=104)† | ||
| Increased bronchodilator | 60 (46) | 56 (43) | 28 (27) | 2.81 (1.16 to 6.82) | 2.17 (0.95 to 4.95) |
| Prednisolone and/or antibiotics | 20 (15) | 9 (7) | 5 (5) | 3.98 (1.10 to 15.58) | 1.71 (0.44 to 5.94) |
| Unscheduled medical contact | 24 (18) | 12 (9) | 12 (12) | 2.07 (0.60 to 7.15) | 0.93 (0.30 to 2.95) |
*Estimated by using generalised estimating equations logistic regression models with compound symmetry with sex, age, educational level, long acting bronchodilator use, and inhaled corticosteroid use as covariates.
†Number of exacerbations.
COPD self-efficacy scale total and domain scores—We found no statistically significant changes or differences in patients’ self efficacy according to the COPD self-efficacy scale total and domain scores at 24 months (table 2).
Differences at six months—At six months, differences in chronic respiratory questionnaire domain scores and self efficacy total and domain scores were not statistically significant (table B in web appendix). More patients in the self management group than in the usual care group achieved a clinically important improvement in the chronic respiratory questionnaire total score (16/49 (33%) v 11/45 (24%)) (fig 3 ), but this was not statistically significant (adjusted odds ratio 1.33, 0.52 to 3.4).
Process evaluation
In the self management group, patients received a mean of 3.4 (SD 1.5) sessions with the practice nurse with a mean duration of 50.1 (12.8) minutes per session. Practice nurses had 190 telephone contacts with 44 patients (mean contacts 4.5 (1.6); mean duration 15.3 (4.5) minutes). Seven (13%) patients did not receive any session or telephone contact, and 48 (87%) received two or more sessions. In the routine monitoring group, patients received a mean of 3.4 (2.5) nurse consultations with a mean duration of 27.4 (13.7) minutes per contact. Six (11%) patients did not receive any consultation, and 42 (76%) patients received two or more consultations. In the usual care group, eight (15%) patients had one or more COPD related scheduled contacts with the practice nurse and, therefore, deviated from the study protocol.
Discussion
At 24 months, neither self management nor routine monitoring showed significant benefits over usual care alone in terms of disease specific quality of life, exacerbation frequency, or self efficacy in patients with chronic obstructive pulmonary disease in general practice. Compared with usual care, patients in the self management group seemed to be more capable of taking appropriate actions to manage their exacerbations—that is, increasing their bronchodilator use (odds ratio 2.81, 95% confidence interval 1.16 to 6.82) and starting prednisolone, antibiotics, or both (3.98, 1.10 to 15.58).
Strengths and weaknesses of study
We used an existing, well studied (in secondary care), and effective self management programme, Living Well with COPD,11 instead of developing and testing a new one. This answers the previous criticism on the use of different self management programmes resulting in insufficient data for meta-analysis and difficulties in formulating clear recommendations.8 Our study has a long follow-up (24 months), which follows the hypothesis that time is needed to change behaviour and gain effects caused by self management in patients with COPD.25
We should be careful with generalising our results to general practice as a whole. Firstly, we excluded more than 60% of the patients who had COPD according to their general practitioner and had a recruitment assessment. In most cases, this was owing to a post-bronchodilator ratio of forced expiratory volume in one second to forced vital capacity of 0.70 or above. This suggests that although treated as patients with COPD, a substantial proportion of the COPD population in general practice has been misdiagnosed according to current guidelines. The recent awareness that spirometry has been underused in general practice might improve its use and diminish the number of patients misdiagnosed as having COPD.26 Secondly, of the 285 eligible patients, less than 60% were willing to participate. Although these patients were comparable to those who declined participation as regards sex, age, and disease severity (forced expiratory volume in one second), we do not have information on how representative the trial population was in terms of other relevant factors such as baseline quality of life and exacerbation history. Thirdly, almost 16% of the participants dropped out during follow-up. However, baseline characteristics did not differ between dropouts and participants who finished follow-up. Also, we anticipated a dropout rate of 25% in our sample size calculation. The dropout rate was lowest in the self management group, which may suggest that patients in this group were more motivated to adhere to COPD treatment because they were more “involved” in the long term management of their disease.
Provision of self management, routine monitoring, and usual care within the same general practice leads to a risk of treatment contamination. Therefore, we used strict protocols and registration forms for the practice nurses to minimise the risk of treatment contamination, and we checked practices’ compliance with the protocols during and after the study. The risk of contamination would have been smaller with a clustered randomised trial design, but cluster randomised trials are more complex to implement and require more participants and practices to obtain equivalent statistical power.27
Interpretation with reference to other studies
To our knowledge, this is the first study that compares two different methods of COPD management in patients in general practice. In the self management group, care was tailored to individual needs and close collaboration took place between the patient and the healthcare professional. In the routine monitoring group, care was based on uniform planned contacts and the healthcare provider (practice nurse) acted as an expert and strictly followed the contents of the COPD monitoring protocol.
Routine monitoring did not affect quality of life, which confirms the results of a previous trial on the effects of a COPD monitoring routine in general practice.28 We could not confirm the effects of the self management programme on disease specific quality of life as previously observed in the Canadian trial.11 Several possible explanations for this exist. Firstly, our version of the programme was provided at a lower intensity than the original Canadian version—it had a maximum of four individual sessions of 50 minutes each compared with weekly one hour sessions for seven to eight weeks.11 Thus, less time was available to spend on motivating patients to change their behaviour. Secondly, the original Canadian programme also included an exercise programme, which may have been an important element of the programme.29 On the other hand, no evidence exists to show that a hospital based extensive exercise programme is effective for COPD patients in primary care; also, we used the original modules that covered the importance of physical activity and exercise at home. Thirdly, differences existed between the study populations—the Canadian patients had more severe COPD, were managed by respiratory specialists in secondary care, and had been admitted to hospital for an acute exacerbation at least once in the preceding year.11
The mean chronic respiratory questionnaire total score at baseline in our study was high and comparable to those in other primary care COPD studies.28 30 This limits the room for improvement in our primary outcome (“ceiling effect”). Exploration of whether patients with low baseline quality of life scores had more benefit from the programme would be interesting. However, the size of our study population limits our ability to do meaningful subgroup analyses. In contrast with self management trials that showed positive effects,11 31 we did not use specialist respiratory nurses but practice nurses who were (before the study) not familiar with the self management programme. We noticed a wide heterogeneity among the nurses in COPD education, experiences, and attitude, and we believe that—despite our training and coaching—individual differences may have influenced the ability of patients to adopt self management behaviour.
In the process of self management, self efficacy and behavioural change are important factors in the causal chain towards potential health gains.7 In both the self management and routine monitoring groups, patients did not show an improvement in perceived self efficacy as measured with the COPD self-efficacy scale.18 This is in line with a recent study.32 Compared with a secondary care COPD population,33 baseline levels of COPD self-efficacy scale total and domain scores in our study were high, indicating a high level of confidence. As with quality of life, this could have limited the room for improvement. In our study, self management behaviour was reflected by exacerbation management. In contrast with the routine monitoring group, patients in the self management group received a tailored written action plan for exacerbation management. Equipping COPD patients with a written action plan has previously shown positive effects on duration of exacerbation.34 Our finding that, in the second year of the study, patients in the self management group compared with patients in the usual care group showed improved exacerbation management in terms of increasing their bronchodilator use and particularly in terms of starting prednisolone, antibiotics, or both suggests that an individualised action plan and a long timeframe are both needed to establish the effects of COPD self management programmes. Most exacerbations remained unreported to the healthcare professional. This is in line with other studies on exacerbation management.35 36 Given the importance of timely management of exacerbations to prevent complications and expedite recovery, we propose that further studies on self management in general practice should focus on effective exacerbation management as the primary outcome.
Conclusions and recommendations for practice
Patients with COPD who were treated in general practice did not benefit from self management or routine monitoring over usual care alone, except that patients who were enrolled in the self management programme seemed to be more capable of appropriately managing exacerbations. The chronic care structure in Dutch general practice has significantly evolved since the start of our study. More attention is now paid to the other components of chronic care that increase the chance of self management success.37 Nowadays, there is a better prepared delivery system with structured collaboration between healthcare professionals, more and better equipped practice nurses, a continuum of care to enhance self management behaviour, more decision support from secondary care for the diagnosis and management of COPD, and the development of clinical information systems to support both the professional and the patient. Policy makers and healthcare professionals should consider this when interpreting the findings of our study.
What is already known on this topic
Well studied and effective management strategies are needed to face the burden of chronic obstructive pulmonary disease (COPD), particularly in general practice
The effects of routine monitoring according to COPD guidelines seem to be doubtful
Comprehensive self management programmes have shown benefits, but the effects on COPD patients in general practice are inconclusive
What this study adds
Comprehensive self management or routine monitoring did not show long term benefits in terms of quality of life or self efficacy in COPD patients in general practice
Patients in the self management group seemed more capable than those in the usual care group of appropriately managing exacerbations by increasing the use of bronchodilators and initiating prednisolone and/or antibiotics
We acknowledge the contribution of Ellen Erren, respiratory nurse at the Department of Pulmonary Diseases, Radboud University Nijmegen Medical Centre, the Netherlands, who supervised the training for the practice nurses and coached them thereafter. We are grateful to all study personnel of each participating general practice and to all participating patients for their dedication in this study.
Contributors: EWMAB, JHV, CvW, and TRJS participated in the original design of the study. EWMAB, JHV, and TRJS supervised the collection of data. EWMAB, RA, and TRJS did the statistical analyses. EWMAB led the writing of the report, which was co-led by JB and TRJS and assisted by all other authors. All authors had full access to all of the study data, assisted in the interpretation of the data, and have seen and approved the final version of the report. EWMAB is the guarantor.
Funding: This study was funded by the Netherlands Organisation for Health Research and Development (ZonMw) and Partners in Care Solutions for COPD (PICASSO). The funding sources had no role in the design, conduct, or reporting of the study.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no authors received any support from any company for the submitted work; no authors have any relationship with any company that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The study was approved by the Medical Ethics Committee of the Radboud University Nijmegen Medical Centre (number 2004/249). All patients gave written informed consent.
Data sharing: Technical appendix, statistical code, and dataset are available from the corresponding author.
Cite this as: BMJ 2012;345:e7642
Web Extra. Extra material supplied by the author
References
- 1.Simpson CR, Hippisley-Cox J, Sheikh A. Trends in the epidemiology of chronic obstructive pulmonary disease in England: a national study of 51,804 patients. Br J Gen Pract 2010;60:277-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: revised 2011. GOLD, 2011 (available from www.goldcopd.org).
- 3.Bellamy D, Bouchard J, Henrichsen S, Johansson G, Langhammer A, Reid J, et al. International Primary Care Respiratory Group (IPCRG) guidelines: management of chronic obstructive pulmonary disease (COPD). Prim Care Respir J 2006;15:48-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van den Bemt L, Schermer T, Smeele I, Bischoff E, Jacobs A, Grol R, et al. Monitoring of patients with COPD: a review of current guidelines’ recommendations. Respir Med 2008;102:633-41. [DOI] [PubMed] [Google Scholar]
- 5.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA 2002;288:2469-75. [DOI] [PubMed] [Google Scholar]
- 6.Bandura A. The assessment and predictive generality of self-percepts of efficacy. J Behav Ther Exp Psychiatry 1982;13:195-9. [DOI] [PubMed] [Google Scholar]
- 7.Bourbeau J, Bischoff E, Sedeno MF. Self-management in prevention and early intervention of exacerbations. In: Wedzicha J, Martinez FJ, eds. Chronic obstructive pulmonary disease exacerbations. 1st ed. Informa Healthcare, 2008:357-68.
- 8.Effing T, Monninkhof EM, Van der Valk PD, van der Palen J, van Herwaarden CL, Partridge MR, et al. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2007;4:CD002990. [DOI] [PubMed] [Google Scholar]
- 9.Cranston JM, Crockett AJ, Moss JR, Pegram RW, Stocks NP. Models of chronic disease management in primary care for patients with mild-to-moderate asthma or COPD: a narrative review. Med J Aust 2008;188:S50-2. [DOI] [PubMed] [Google Scholar]
- 10.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [DOI] [PubMed] [Google Scholar]
- 11.Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupre A, Begin R, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med 2003;163:585-91. [DOI] [PubMed] [Google Scholar]
- 12.Geijer RM, van Schayck CP, van Weel C, Sachs AP, Bottema BJ, Smeele IJ, et al. NHG-Standaard COPD: behandeling. Huisarts Wet 2001;44:207-19. [Google Scholar]
- 13.National Institute for Clinical Excellence (NICE). Chronic obstructive pulmonary disease: national clinical guideline for management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004;59(suppl I):i1-232. [Google Scholar]
- 14.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puhan MA, Guyatt GH, Goldstein R, Mador J, McKim D, Stahl E, et al. Relative responsiveness of the chronic respiratory questionnaire, St. Georges respiratory questionnaire and four other health-related quality of life instruments for patients with chronic lung disease. Respir Med 2007;101:308-16. [DOI] [PubMed] [Google Scholar]
- 16.Schunemann HJ, Goldstein R, Mador MJ, McKim D, Stahl E, Puhan M, et al. A randomised trial to evaluate the self-administered standardised chronic respiratory questionnaire. Eur Respir J 2005;25:31-40. [DOI] [PubMed] [Google Scholar]
- 17.Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. J Clin Epidemiol 1996;49:1215-9. [DOI] [PubMed] [Google Scholar]
- 18.Wigal JK, Creer TJ, Kotses H. The COPD self-efficacy scale. Chest 1991;99:1193-6. [DOI] [PubMed] [Google Scholar]
- 19.Bischoff EW, Boer LW, Molema J, Akkermans R, van Weel C, Vercoulen JH, et al. Validity of an automated telephonic system to assess COPD exacerbation rates. Eur Respir J 2012;39:1090-6. [DOI] [PubMed] [Google Scholar]
- 20.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;161:1608-13. [DOI] [PubMed] [Google Scholar]
- 22.Schermer T, Chavannes N, Dekhuijzen R, Wouters E, Muris J, Akkermans R, et al. Fluticasone and N-acetylcysteine in primary care patients with COPD or chronic bronchitis. Respir Med 2009;103:542-51. [DOI] [PubMed] [Google Scholar]
- 23.Suissa S. Statistical treatment of exacerbations in therapeutic trials of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;173:842-6. [DOI] [PubMed] [Google Scholar]
- 24.Aaron SD, Fergusson D, Marks GB, Suissa S, Vandemheen K, Doucette S, et al. Counting, analyzing and reporting exacerbations of COPD in randomized, controlled trials. Thorax 2008;63:122-8. [DOI] [PubMed] [Google Scholar]
- 25.Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns 2004;52:271-7. [DOI] [PubMed] [Google Scholar]
- 26.Poels PJ, Schermer TR, van Weel C, Calverley PM. Spirometry in chronic obstructive pulmonary disease. BMJ 2006;333:870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell MK, Elbourne DR, Altman DG, for the CONSORT Group. CONSORT statement: extension to cluster randomised trials. BMJ 2004;328:702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van den Bemt L, Schermer T, Smeele I, Boonman-de Winter L, van Boxem TJ, Denis J, et al. An expert-supported monitoring system for patients with chronic obstructive pulmonary disease in general practice: results of a cluster randomised controlled trial. Med J Aust 2009;191:249-54. [PubMed] [Google Scholar]
- 29.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006;4:CD003793. [DOI] [PubMed] [Google Scholar]
- 30.Schermer T, Chavannes N, Dekhuijzen R, Wouters E, Muris J, Akkermans R, et al. Fluticasone and N-acetylcysteine in primary care patients with COPD or chronic bronchitis. Respir Med 2009;103:542-51. [DOI] [PubMed] [Google Scholar]
- 31.Rice K, Dewan N, Bloomfield H, Grill J, Schult T, Nelson D, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2010;182:890-6. [DOI] [PubMed] [Google Scholar]
- 32.Bucknall C, Miller G, Lloyd S, Cleland J, McCluskey S, Cotton M, et al. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: randomised controlled trial. BMJ 2012;344:e1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kara M, Asti T. Effect of education on self-efficacy of Turkish patients with chronic obstructive pulmonary disease. Patient Educ Couns 2004;55:114-20. [DOI] [PubMed] [Google Scholar]
- 34.Bischoff EW, Hamd DH, Sedeno MF, Benedetti A, Schermer TR, Bernard S, et al. Effects of written action plan adherence on COPD exacerbation recovery. Thorax 2011;66:26-31. [DOI] [PubMed] [Google Scholar]
- 35.Langsetmo L, Platt RW, Ernst P, Bourbeau J. Underreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohort. Am J Respir Crit Care Med 2008;177:396-401. [DOI] [PubMed] [Google Scholar]
- 36.Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;169:1298-303 [DOI] [PubMed] [Google Scholar]
- 37.Adams SG, Smith PK, Allan PF, Anzueto A, Pugh JA, Cornell JE. Systematic review of the chronic care model in chronic obstructive pulmonary disease. Arch Intern Med 2007;167:551-61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.