Abstract
The AcrAB-TolC efflux pump is involved in maintaining intrinsic organic solvent tolerance in Escherichia coli. Mutations in regulatory genes such as marR, soxR, and acrR are known to increase the expression level of the AcrAB-TolC pump. To identify these mutations in organic solvent tolerant E. coli, eight cyclohexane-tolerant E. coli JA300 mutants were isolated and examined by DNA sequencing for mutations in marR, soxR, and acrR. Every mutant carried a mutation in either marR or acrR. Among all mutants, strain CH7 carrying a nonsense mutation in marR (named marR109) and an insertion of IS5 in acrR, exhibited the highest organic solvent-tolerance levels. To clarify the involvement of these mutations in improving organic solvent tolerance, they were introduced into the E. coli JA300 chromosome by site-directed mutagenesis using λ red-mediated homologous recombination. Consequently, JA300 mutants carrying acrR::IS5, marR109, or both were constructed and named JA300 acrRIS, JA300 marR, or JA300 acrRIS marR, respectively. The organic solvent tolerance levels of these mutants were increased in the following order: JA300 < JA300 acrRIS < JA300 marR < JA300 acrRIS marR. JA300 acrRIS marR formed colonies on an agar plate overlaid with cyclohexane and p-xylene (6:4 vol/vol mixture). The organic solvent-tolerance level and AcrAB-TolC efflux pump-expression level in JA300 acrRIS marR were similar to those in CH7. Thus, it was shown that the synergistic effects of mutations in only two regulatory genes, acrR and marR, can significantly increase organic solvent tolerance in E. coli.
Keywords: Escherichia coli, Organic solvent tolerance, MarR, AcrR, AcrAB-TolC, Efflux pump
Introduction
Whole-cell biocatalysts are beneficial in the biotransformation involved in their internal cofactor regeneration and in bioconversions requiring multi-step metabolic pathways. Whole-cell biocatalysts are available in two-phase systems consisting of organic solvent and an aqueous medium, that are potentially advantageous for the bioconversion of hydrophobic and/or toxic organic compounds (Schmid et al. 1998 ; Sardessai and Bhosle 2004 ; Heipieper et al. 2007 ). The use of a second organic phase improves productivity levels and product recovery, unlike the case with conventional media whose substrate solubility is poor. One of the main limitations in the application of whole-cell biocatalysts in two-phase systems is the instability of biocatalysts due to the toxicity of organic solvents toward the cells. When microorganisms are incubated in the presence of a large amount of an organic solvent, the extent of growth inhibition is inversely correlated with the log POW of the solvent ( Inoue and Horikoshi 1989 ). Hydrophobic organic solvents with a log POW of 2 to 5 bind to the cells and disrupt the cell membrane (Sikkema et al. 1994 ; Aono et al. 1994 ).
Various mechanisms underlying microbial tolerance and responses to solvents have been revealed by the genetic, physiological and biochemical characterization of organic solvent tolerant bacteria such as Pseudomonas species and E. coli mutants ( Aono 1998 ; Ramos et al. 2002 ; Shimizu et al. 2005 ; Heipieper et al. 2007 ; Okochi et al. 2007 ; Okochi et al. 2008a ; Okochi et al. 2008b ; Torres et al. 2011 ; Segura et al. 2012 ). Until now, more is known about how cells respond to organic solvents, but less about how to develop tolerant strains.
It has been shown that energy-dependent efflux pumps belonging to the resistance/nodulation/cell division (RND) family (Paulsen et al. 1996 ) are important for the maintenance of solvent tolerance in Gram-negative bacteria (White et al. 1997 ; Kieboom et al. 1998 ; Ramos et al. 1998 ; Li et al. 1998 ; Tsukagoshi and Aono 2000 ). In E. coli, the AcrAB-TolC efflux pump belonging to the RND family has been shown to provide intrinsic tolerance to organic solvents ( Tsukagoshi and Aono 2000 ). This pump enhances the release of solvents intracellularly accumulated in E. coli cells. acrAB and tolC are marA/soxS/rob regulon genes ( Barbosa and Levy 2000 ). MarA and SoxS proteins are transcriptional activators belonging to the AraC/XylS family ( Alekshun and Levy 1997 ). These activators control the expression of marA/soxS/rob regulon genes. MarA and SoxS are transcriptionally regulated. Transcription of the marRAB is repressed by MarR, whereas it is autoactivated by MarA. soxS transcription is repressed by SoxR and enhanced by the activated form of SoxR after exposure to superoxides or nitric oxide ( Pomposiello and Demple 2001 ). Mutations in marR or soxR were suggested to enhance the expression level of the AcrAB-TolC efflux pump (Okusu et al. 1996 ; Aono 1998 ; Randall and Woodward 2002 ; Komp Lindgren et al. 2003 ). In addition, acrAB expression is modulated locally by the repressor AcrR (Ma et al. 1996 ). Thus, mutations in acrR can lead to the enhanced expression of AcrAB. Mutations conferring a multidrug resistance phenotype have been found in the genes marR, soxR, and acrR among clinical and veterinary E. coli isolates (Wang et al. 2001 ; Webber and Piddock 2001 ; Komp Lindgren et al. 2003 ; Fernandes et al. 2003 ; Karczmarczyk et al. 2011 ). Some of those studies suggested that organic solvent tolerance is correlated with these mutations in these isolates (Wang et al. 2001 ; Komp Lindgren et al. 2003 ). However, the extent to which these mutations contribute to organic solvent tolerance has not been clarified because E. coli isolates used in these studies had a variety of genetic backgrounds. In addition, the synergistic effects of these mutations on organic solvent tolerance were ambiguous. To clarify the effects of mutations on the tolerance phenotype, it is necessary to reconstruct selected mutations in one type of strains in various combinations.
In this study, we isolated organic solvent-tolerant E. coli mutants and identified mutations in regulatory genes (marR, soxR, acrR). Among these mutants, we selected the one that exhibited the highest organic solvent tolerance and investigated the contributions of each identified mutation to organic solvent tolerance. As a result, we clarified that the E. coli strain can acquire high-level organic solvent tolerance due to mutations in only two regulatory genes (marR and acrR) by reconstructing these mutations in a parent strain. This study provides a new finding for developing an E. coli with high organic solvent tolerance.
Materials and methods
Materials, media, and culture conditions
The organic solvents used were of the highest quality available (Wako Pure Chemical Industries, Osaka, Japan). The purity of these solvents is more than 98%. The organisms were grown aerobically at 30°C in LBG medium consisting of 1% Bacto Tryptone (Difco Laboratories, Detroit, MI), 0.5% Bacto Yeast Extract (Difco), 1% NaCl, and 0.1% glucose. This medium supplemented with 10 mM MgSO4 (LBGMg medium) (Aono et al. 1991 ) was also used. The LBGMg medium was solidified with 1.5% (wt/vol) agar. Ampicillin (50 μg/ml) or kanamycin (50 μg/ml) was added to the medium when necessary. The organisms were also grown in LB medium, which is identical to LBG medium except that glucose is omitted.
Bacterial strains and plasmids
E. coli K-12 derivatives used to evaluate solvent tolerance are summarized in Table 1. Strain JW0452, a BW25113-based acrA::KmR (kanamycin resistant), was supplied by the National Bio-Resource Project (NIG, Mishima, Japan): E. coli (Baba et al. 2006 ).
Table 1.
Bacterial strains and plasmids used
| E. coli strain | Genotype | Reference |
|---|---|---|
| JA300 |
F-thr leuB6 trpC1117 thi rpsL20 hsdS |
Aono et al.
1991 |
| JA300 acrRIS |
Same as JA300, but with acrR::IS5 |
This study |
| JA300 marR |
Same as JA300, but with marR109 |
This study |
| JA300 acrRIS marR |
Same as JA300, but with acrR::IS5 and marR109 |
This study |
| JA300ΔacrA |
Same as JA300, but with acrA::KmR |
This study |
| JA300ΔacrA acrRIS marR |
Same as JA300, but with acrA::KmR, acrR::IS5 and marR109 |
This study |
| BW25113 |
lacIqrrnBT14 lacZWJ16hsdR514 araBADAH33rhaBADLD78 |
Baba et al.
2006 |
| JW0452 | Same as BW25113, but with acrA::KmR | Baba et al. 2006 |
Isolation of cyclohexane-tolerant mutants of E. coli JA300
First, 100 μl of the overnight culture of strain JA300 grown in LBGMg medium was inoculated into 10 ml of fresh LBGMg medium overlaid with 2 ml cyclohexane. The two-phase culture was incubated at 37°C with shaking. Since the growth of cyclohexane-tolerant mutants was observed after 48 h, the aqueous medium phase was spread on LBGMg agar medium. Then, the agar surface was overlaid with a 3-mm-thick layer of cyclohexane. Strains that formed relatively large colonies on the agar after 48 h incubation at 25°C were randomly isolated as cyclohexane-tolerant mutants.
PCR amplification and DNA sequencing of acrR, marR, and soxR
Chromosomal DNA of each bacterial isolate was used as the template for polymerase chain reaction (PCR) amplification. PCR was performed using PrimeSTAR® GXL DNA polymerase (Takara Bio, Kyoto, Japan). The primers used for PCR are listed in Table 2. The primer combinations used are as follows: acrR-F and acrR-R for the entire acrR; marR-F and marR-R for the entire marR and the operator region for marR; and soxR-F and soxR-R for the entire soxR. The PCR products were purified using the QiaQuick PCR purification kit (Qiagen, Hilden, Germany). Direct cycle sequencing in both directions was performed with the same sets of primers.
Table 2.
Primers used in this study
| Primer | Sequence (5′to 3′) | Positionsa |
|---|---|---|
| acrR-F |
AAACCCATTGCTGCGTTTAT |
− 90 to − 71 bp of acrR |
| acrR-R |
AAACCGCAAGAATATCACGA |
+ 711 to + 730 bp of acrR |
| marR-F |
CTGTTCATGTTGCCTGCCAG |
− 321 to − 302 bp of marR |
| marR-R |
CAGTCCAAAATGCTATGAATGG |
+ 482 to + 503 bp of marR |
| soxR-F |
TTTCTGATGGGACATAAATCTGCC |
− 100 to − 77 bp of soxR |
| soxR-R |
TGTGTTGACGTCGGGGGAAA |
+ 537 to + 556 bp of soxR |
| acrA-F |
CCAATTTGAAATCGGACACTCG |
− 32 to − 11 bp of acrA |
| acrA-R |
GCATGTCTTAACGGCTCCTG |
+ 1200 to + 1219 bp of acrA |
| |
|
|
| acrR-rpsL-neo-F |
GCTTCAGGATAATCCCGCTAACTTGAGGA
CGAACTTCTGCGATCCGGTAGGGCCTGGTGATGATGGCGGGATCG |
− 376 to − 327 bp of acrR (indicated by italics),
and − 138 to − 115 bp of rpsL in the rpsL-neo
cassette (underlined) |
| acrR-rpsL-neo-R |
GCGATCGATTTTATCGAGGGTGGCTAATGTATCTGTCAGATCCTGCTG
CATCAGAAGAACTCGTCAAGAAGGCG |
+ 974 to + 1023 bp of acrR (indicated by italics),
and + 750 to + 773 bp of neo in the rpsL-neo
cassette (underlined) |
| marR-rpsL-neo-F |
AAACCGATAAACGCGACGATTAAGCCGCCTGCAATTCGCAGACCGGG
AATGGCCTGGTGATGATGGCGGGATCG |
− 477 to − 428 bp of marR (indicated by italics),
and − 138 to − 115 bp of rpsL in the rpsL-neo
cassette (underlined) |
| marR-rpsL-neo-R |
AAGAGAATAAGCGCAGCTGCTATTGCGGATGAAAGTGGTTTCATGATT
GCTCAGAAGAACTCGTCAAGAAGGCG |
+ 863 to + 912 bp of marR (indicated by italics),
and + 750 to + 773 bp of neo in the rpsL-neo
cassette (underlined) |
| |
|
|
| Repair-acrRIS-F |
TGATCGTACTCTTGCTTACTGAT |
− 575 to − 553 bp of acrR |
| Repair-acrRIS-R |
ATCGTTTTGTGCGTTTTGCAAAT |
+ 1202 to + 1224 bp of acrR |
| Repair-marR-F |
TTTTCGCCTCCGGTGAATCA |
− 527 to − 508 bp of marR |
| Repair-marR-R | AACTGGCTGCGTGGTTTGTT | + 933 to + 952 bp of marR |
aThe positions of the primers are relative to the start codon of each gene.
Site-directed point mutations in E. coli chromosome
For site-directed mutagenesis, the phage λ-based homologous recombination system (Red/ET counterselection Bac modification kit; GeneBridges, Heidelberg, Germany) was used to introduce an rpsL-neo cassette into acrR or marR of strain JA300 and to subsequently replace the cassette with an appropriate DNA fragment. Linear DNA fragments comprising the rpsL-neo cassette for the introduction of the acrR and/or marR region were obtained by PCR using the rpsL-neo template DNA (GeneBridges) as the template. The combinations of primers for the introduction of the rpsL-neo cassette were as follows: acrR-rpsL-neo-F and acrR-rpsL-neo-R for acrR, and marR-rpsL-neo-F and marR-rpsL-neo-R for marR (Table 2). These primers contain homology arms consisting of 50 nucleotides upstream and downstream from the targeted region and 24 nucleotides homologous to the rpsL-neo cassette. For all amplification, PrimeSTAR HS DNA polymerase (Takara Bio) with high fidelity was used. The acrR or marR region into which the rpsL-neo cassette was introduced was replaced with corresponding DNA fragments containing a mutated gene of acrR or marR from strain CH7. The DNA fragments for the replacement of the rpsL-neo cassette were obtained by PCR using chromosomal DNA prepared from strain CH7 as the template. The combinations of primers for replacement of the rpsL-neo cassette are as follows: Repair-acrRIS-F and Repair-acrRIS-R for acrR, and Repair-marR-F and Repair-marR-R for marR.
Transformations of cells by the introduction of linear DNA fragments for recombination were performed by electroporation according to the protocol recommended by the technical manual of the Bac modification kit (Gene Bridges). Briefly, the E. coli strain carrying pRed/ET (Gene Bridges) was cultivated in 1.4 ml of LB medium at 30°C. At an absorbance of 0.3 (600 nm), freshly prepared L-arabinose was added (0.35% wt/vol, final concentration) to the culture inducing redγβα/recA expression, and expression was continued at 37°C. After 1 h, the cells were harvested by centrifugation, washed with 10% (vol/vol) ice-cold glycerol, and resuspended in a final volume of 30 μl in 10% (vol/vol) ice-cold glycerol. DNA fragments (100 to 200 ng) were then added to the sample. Subsequently, the sample was transferred to electroporation cuvettes, and electroporation was carried out with an Electroporator 2510 (Eppendorf, Hamburg, Germany) at 1350 V, 10 μF, and 600 Ohms. The cells were immediately removed from the cuvettes by mixing with 1 ml LB medium, and then were incubated at 37°C for 2 h. The cells were plated on LB agar containing the appropriate antibiotics.
Recombination events were confirmed by PCR and DNA sequencing of the acrR and marR genes. The combinations of primers used are as follows: acrR-F and acrR-R for acrR, and marR-F and marR-R for marR.
Disruption of acrA in strain JA300 and JA300 acrRIS marR
JA300ΔacrA and JA300ΔacrA acrRIS marR were constructed by P1 transduction of kanamycin resistance, with strain JW0452 as the donor. Disruption of the gene was confirmed by PCR analysis using chromosomal DNA prepared from JA300ΔacrA or JA300ΔacrA acrRIS marR as the template. The combination of primers used was acrA-F and acrA-R (Table 2). Consistent with the expected values, the size of the amplified product (acrA) from JA300 was 1,252 bp, and those from kanamycin-resistance cassette transductants were approximately 1.5 kb.
Measurement of the organic solvent tolerance of E. coli
Cultures of E. coli strains in LBGMg medium (optical density at 660 nm [OD660], 0.4 to 0.6) were diluted with 0.8% saline by serial 10-fold dilutions. Each suspension was plated on LBGMg agar. The agar surface was overlaid with a 3-mm-thick layer of an organic solvent. The approximate frequency at which the cells formed colonies on the agar was estimated after 48 h incubation at 25°C.
Quantitation of organic solvent accumulation in E. coli cells
The organic solvent accumulation in E. coli cells was quantitated as described previously (Doukyu et al. 2012 ). An organic solvent was added to a suspension of E. coli cells harvested during the late exponential phase of growth (OD660, 1.5 to 2.0). The suspension was centrifuged after incubation with the solvent for 30 min. After separation from the medium layer, the solvent layer was removed by aspiration. The medium was disposed of by decanting. The cell pellet was recovered and suspended in 1.0 ml of 0.9% NaCl–10 mM MgSO4. A 0.5 ml portion of the suspension was then transferred to an Eppendorf tube, and the remaining portion was kept to measure the protein content. The 0.5 ml cell suspension was extracted with 2.0 ml of CHCl3 by vigorous shaking for 90 min at 25°C in a shaker (Handless shaker SHK-COCK; Asahi Technoglass, Tokyo, Japan). The amount of organic solvent in the CHCl3 extract was measured using a gas chromatography–mass spectrometry apparatus (GCMS-QP2010 Plus; Shimadzu, Kyoto, Japan) with an Rtx®-624 column (30 m by 0.25 mm inside diameter, 1.4 μm film thickness; Restek, PA, USA). The column was eluted with helium gas at a flow rate of 1.69 ml/min. The inlet was set at 150°C, and the oven was programmed as follows: 50°C for 5 min, then increasing by 5°C per minute to 100°C. The total ion chromatogram of an organic solvent was detected with a mass selective detector.
Protein content
Protein content was measured by the method of Lowry et al. (Lowry et al. 1951 ).
Antibodies against AcrA, AcrB, and TolC
Antibodies against AcrA were obtained as described previously (Doukyu et al. 2012 ). Polyclonal antibodies against AcrB and TolC were raised against synthetic peptides corresponding to regions of AcrB and TolC. These antibodies contained an N-terminal cysteine (C + in the peptides shown below) for the conjugation of keyhole limpet hemocyanin. The peptide sequences were as follows: a synthetic peptide for anti-AcrB antibodies, C + KNEDIEHSHTVDHH (corresponding to residues 1036 to 1049) and a peptide for anti-TolC antibodies, C + ARTTTSNGHNPFRN (corresponding to residues 480 to 493). The conjugated peptides were injected into rabbits, and polyclonal antibodies were purified from serum using a Melon gel immunoglobulin G spin purification kit (Thermo Fisher Scientific, Rockford, IL, USA). These antibodies were used to detect AcrA, AcrB, and TolC in the immunoblotting analyses of this study.
Immunoblotting analyses
E. coli was grown in LBGMg medium. The cells were harvested during the exponential phase of growth (OD660, 0.6) by centrifugation (5,000 × g for 10 min at 4°C). The cells were suspended in cold 10 mM Tris–HCl buffer (pH 8.0) and broken by sonication in an icewater bath. Ten micrograms of total cell lysate protein in the supernatant was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in a 12.5% separating gel ( Laemmli 1970 ). The gels were then transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore, Bedford, MA, USA), electrophoretically by the application of 50 V for 30 min in Tris-glycine-methanol buffer (25 mM Tris, 192 mM glycine, 20% [vol/vol] methanol [pH 8.3]). The membrane was blocked overnight at room temperature in Tris-buffered saline (TBS; 0.15% NaCl, 10 mM Tris–HCl, pH 7.4) containing 3% gelatin, washed three times in wash buffer (0.05% Tween 20 in TBS), and hybridized at room temperature with the relevant antibody. The membrane was then probed for 1 h with goat anti-rabbit horseradish peroxidase (Bio-Rad, Hercules, CA, USA). The bands were visualized by use of an alkaline phosphatase color development kit (Bio-Rad). The AcrA, AcrB, and TolC expression levels were quantified by gel analysis software UN-SCAN-IT (Silk Scientific, Orem, UT, USA).
Results
Isolation of cyclohexane-tolerant mutants of E. coli JA300
E. coli K-12 strain JA300 is tolerant to an organic solvent with a logPow value of more than 4 and is highly sensitive to cyclohexane (logPow, 3.4) ( Aono 1998 ). Cyclohexane-tolerant mutants that formed colonies on the LBGMg agar medium overlaid with cyclohexane were isolated from strain JA300. Spontaneous cyclohexane-tolerant mutants from strain JA300 appeared on the LBGMg agar medium overlaid with cyclohexane at a frequency of 1.6 × 10-6. In the present study, to efficiently isolate mutants with high-level organic solvent tolerance, the parent strain JA300 was precultured in the LBGMg liquid medium overlaid with cyclohexane. The viable cell density in the preculture increased to 2.2 × 105 /ml after 48 h incubation. The preculture was spread on the agar medium, and then the agar surface was overlaid with cyclohexane. After 48 h, mutants appeared on the agar medium with cyclohexane at a frequency of 5.0 × 10-3. The organic solvent tolerances of about 100 isolates randomly selected were investigated by measuring colony-forming efficiencies on an agar plate overlaid with cyclohexane. As a result, eight mutants (CH1 to CH8) exhibiting relatively high colony-forming efficiencies on the agar medium with cyclohexane were selected and used in further experiments.
Organic solvent tolerances of the isolated mutants
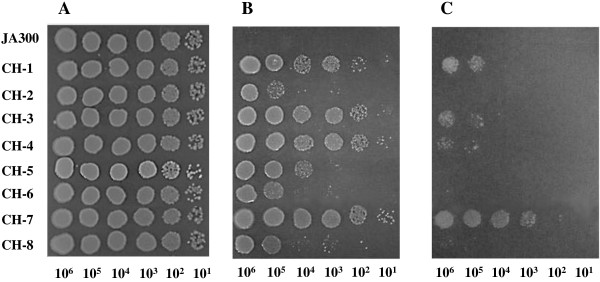
The organic solvent tolerances of the mutants (CH1 to CH8) were investigated by measuring the colony-forming efficiency of each mutant on an agar plate overlaid with pure cyclohexane and a mixture of cyclohexane and p-xylene (7:3 vol/vol mixture) (Figure 1). p-Xylene (logPow, 3.1) shows higher toxicity to cells than cyclohexane. All strains formed colonies in all spots on the plate without any solvent. The parent strain JA300 did not form any colony in the presence of cyclohexane and the solvent mixture. All mutants formed colonies in the presence of cyclohexane, although the colony-forming efficiencies differed among the mutants. Strains CH1, CH3, CH4, and CH7 exhibited relatively high organic solvent tolerance in the presence of cyclohexane. Strains CH2, CH5, CH6, and CH8 did not form any colonies in the presence of the solvent mixture. On the other hand, strain CH7 formed colonies even in spots containing 103 cells in the presence of the solvent mixture and therefore showed the highest colony-forming efficiency among all isolates.
Figure 1.
Colony-forming efficiency of organic solvent-tolerant mutants from E. coli JA300 on LBGMg agar medium in the absence of an organic solvent (A) and in the presence of cyclohexane (B) or cyclohexane and p-xylene (7:3 vol/vol mixture) (C). Each strain was spotted at a tenfold dilution and incubated at 25°C for 48 h.
Identification of mutations in acrR, marR, and soxR
The nucleotide sequences of entire acrR, marR, and soxR genes in organic solvent-tolerant mutants were determined by DNA sequencing to identify mutations in these genes. The DNA sequences were analyzed by comparison with those of the JA300 parent strain. These mutations are summarized in Table 3. The sequences of acrR, marR, and soxR genes in strain JA300 were identical to those present in the E. coli K-12 strain MG1655 genome deposited in GenBank (accession number NC_000913.2). None of the mutants carried a mutation in soxR. Seven of the eight cyclohexane-tolerant mutants carried four different mutations in marR. Among these seven marR mutants, three mutants (CH1, CH3, and CH4) had point mutations causing an amino acid substitution. These mutations were G116C (CH1), L78M (CH3), and R94L (CH4). Four strains (CH5, CH6, CH7, and CH8) had the same mutation, which would cause premature termination of the marR translation. This was caused by a nonsense codon (E109 → TAA stop). This mutation was named marR109.
Table 3.
Mutations inmarRandacrRof cyclohexane-tolerantE. colimutants
| Strain | |||||
|---|---|---|---|---|---|
| |
DNA positiona (Codon substitution) |
Amino acid substitution |
|
DNA positiona (Codon substitution) |
Amino acid substitution
or insertion |
| CH1 |
346 (GGC → TGC) |
G116C |
|
122 (GCT → GAT) |
A41D |
| CH2 |
|
None |
|
3 (ATG → ATA) |
M1I |
| CH3 |
232 (CTG → ATG) |
L78M |
|
|
None |
| CH4 |
281 (CGC → CTC) |
R94L |
|
|
None |
| CH5 |
325 (GAA → TAA) |
E109 → TAA stop |
|
|
None |
| CH6 |
325 (GAA → TAA) |
E109 → TAA stop |
|
|
None |
| CH7 |
325 (GAA → TAA) |
E109 → TAA stop |
|
220 |
Insertion of IS5 |
| CH8 | 325 (GAA → TAA) | E109 → TAA stop | None |
aDNA positions of the mutations are relative to the start codon of each gene.
Three mutants (CH1, CH2, and CH7) carried mutations in acrR. Strain CH2 had a mutation only in acrR. This mutation was found in the translation initiation codon (M1I). The other two mutants (CH1 and CH7) each carried mutations in both marR and acrR. Strain CH1 had a point mutation altering one amino acid residue (A41D) in acrR. Strain CH7 contained an 1195-bp insertion sequence (IS5) ( Sawers 2005 ) integrated within acrR (Figure 2). This sequence was inserted at 220 bp downstream from the initiation codon of the acrR gene, accompanied by the doubling of the tetramer CTAG. The ins5A promoter was oriented in the same direction as the acrAB operon.
Figure 2.
Nucleotide sequence of the acrAB regulatory region. The transcription initiation site (+ 1) of the acrAB operon is capitalized and indicated by a bent arrow. The CTAG IS5 target site, the putative ribosome binding site (RBG) for acrA, and the ATG start codons for acrR and acrAB are shaded. The promoter regions (− 10 and − 35) (Martin et al. 1999 ) and marbox (Su et al. 2007 ) are underlined. The bold arrow above the sequence indicates the insertion site of IS5.
Organic solvent tolerances of mutants carrying mutations in marR and/or acrR derived from strain CH7
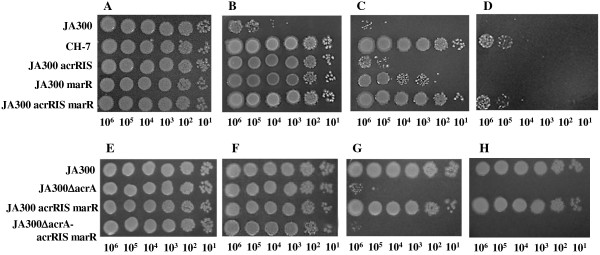
Strain CH7 had a nonsense mutation in marR and an insertion of IS5 in acrR. To clarify the involvement of these mutations in organic solvent tolerance, the marR and/or acrR regions were introduced into the E. coli JA300 chromosome by site-directed mutagenesis using λ red-mediated homologous recombination. Consequently, JA300 mutants carrying acrR::IS5, marR109, or both mutations were constructed and named JA300 acrRIS, JA300 marR, or JA300 acrRIS marR, respectively. The colony-forming efficiencies of these constructed mutants in the presence of organic solvents were compared with those of the parent strain JA300 and strain CH7 (Figure 3). All strains formed colonies in all spots on the plate without any solvent. The parent strain JA300 formed colonies in the spots containing 105-106 cells in the presence of n-hexane (logPow, 3.9). However, strain JA300 hardly formed colonies on the plate overlaid with pure cyclohexane and did not form any colony on the plate with cyclohexane and p-xylene (6:4 mixture). In contrast, the colony-forming efficiencies of the constructed mutants in the presence of the organic solvents were increased in the following order: JA300 acrRIS < JA300 marR < JA300 acrRIS marR. JA300 acrRIS marR exhibited about 102- and 104-fold higher colony-forming efficiencies than those of JA300 acrRIS and JA300 marR, respectively, in the presence of cyclohexane. JA300 acrRIS and JA300 marR did not form any colony on the plate overlaid with the solvent mixture, although JA300 acrRIS marR formed colonies in spots containing 105-106 cells in the presence of the solvent mixture. JA300 acrRIS marR showed similar colony-forming efficiencies as strain CH7 in the presence of the solvents tested.
Figure 3.
Colony-forming efficiency of E. coli JA300 mutants on LBGMg agar medium with or without an organic solvent. Colony-forming efficiency of acrR and/or marR mutants on the agar medium in the absence of an organic solvent (A) and in the presence of n-hexane (B), cyclohexane (C), or cyclohexane and p-xylene (6:4 vol/vol mixture) (D). Colony-forming efficiency of acrA disruptants of JA300 and JA300 acrRIS marR on LBGMg agar medium in the absence of an organic solvent (E) and in the presence of decane (F), nonane (G), or octane (H). Each strain was spotted at a tenfold dilution and incubated at 25°C for 48 h.
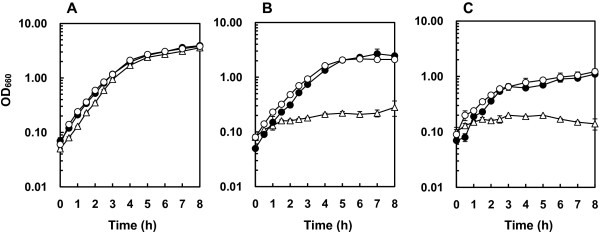
The cell growth of JA300, JA300 acrRIS marR, and CH7 in the LBGMg liquid medium in the presence of n-hexane or cyclohexane was also examined by measuring turbidity (Figure 4). No significant difference was found between the growth of these strains in the absence of organic solvents. The growth of JA300 was highly suppressed by the addition of organic solvents. In contrast, JA300 acrRIS marR and CH7 were able to grow in the presence of these solvents, although the growth rates and yields of these strains were partially reduced by the addition of these solvents as compared to that without any solvent.
Figure 4.
Growth of E. coli JA300, JA300 acrRIS marR, and CH7 in LBGMg liquid medium in the absence of an organic solvent (A) and in the presence of n-hexane (B) and cyclohexane (C). A 100-μl culture of overnight-grown E. coli strain was inoculated to 10 ml of fresh LBGMg liquid medium overlaid with an organic solvent. This two-phase culture was incubated at 30°C. Growth was monitored by measuring turbidity (OD660). Values indicate means of results and standard deviations of results from three independent experiments. Symbols: (△) JA300; (·) JA300 acrRIS marR; (○) CH7.
AcrA, AcrB, and TolC levels in organic solvent-tolerant mutants
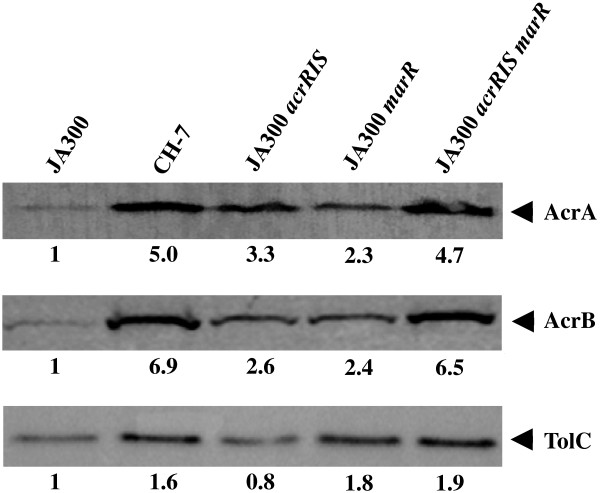
Mutations in marR can increase the expression levels of AcrAB and TolC proteins, which are components of the AcrAB-TolC efflux pump ( Barbosa and Levy 2000 ). In addition, mutations in acrR can enhance the expression of AcrAB (Ma et al. 1996 ; Webber et al. 2005 ). Levels of AcrA, AcrB, and TolC in JA300, CH7, JA300 acrRIS, JA300 marR, and JA300 acrRIS marR were investigated by immunoblotting analysis (Figure 5). Both the AcrA and AcrB levels in JA300 acrRIS were about threefold higher than those in JA300. However, the TolC level in JA300 acrRIS was similar to that in JA300. The levels of AcrA, AcrB, and TolC in JA300 marR were about twice those in JA300. JA300 acrRIS marR exhibited higher expression levels of AcrA and AcrB compared to those of JA300 acrRIS and JA300 marR, but the TolC level in JA300 acrRIS marR was similar to that in JA300 marR. The levels of these three proteins in JA300 acrRIS marR were similar to those in strain CH7.
Figure 5.
Western blot analysis of AcrA, AcrB, and TolC expression. Total cell lysate proteins of JA300 (lane 1), CH7 (lane 2), JA300 acrRIS (lane 3), JA300 marR (lane 4), and JA300 acrRIS marR (lane 5) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with polyclonal anti-AcrA, AcrB, and TolC antibodies, respectively. The expression ratio compared to the AcrA level of JA300 is shown below each lane.
Organic solvent tolerance of acrA-disruptant
Since the marR109 mutation can influence the expression levels of many mar regulon genes, including acrAB and tolC ( Barbosa and Levy 2000 ), it was possible that mar regulon genes other than acrAB and tolC could be involved in the improved organic solvent tolerance in JA300 acrRIS marR. To clarify the extent to which the AcrAB-TolC pump contributed to organic solvent tolerance in JA300 acrRIS marR, a JA300 acrRIS marR-based acrA-disruptant, JA300ΔacrA acrRIS marR, was constructed and then the organic solvent tolerance of this acrA-disruptant was compared to that of JA300ΔacrA (Figure 3). The organic solvent tolerance of JA300ΔacrA was similar to that of the previously reported JA300-based acrAB disruptant ( Tsukagoshi and Aono 2000 ). JA300 was tolerant to nonane (logPow, 5.5) and octane (logPow, 4.9). In contrast, JA300ΔacrA acrRIS marR became sensitive to these solvents. The tolerance level of JA300ΔacrA acrRIS marR was similar to that of JA300ΔacrA.
Accumulation of an organic solvent in E. coli incubated in a two-phase culture system
It has been reported that organic solvent-tolerant E. coli strains in a two-phase culture system maintained low intracellular levels of organic solvents ( Tsukagoshi and Aono 2000 ; Shimizu et al. 2005 ; Doukyu et al. 2012 ). The amounts of n-heptane (logPow, 4.2), n-hexane (logPow, 3.9), or cyclohexane (logPow, 3.4) accumulated in E. coli cells were investigated (Table 4). E. coli cells of JA300, CH7, JA300 acrRIS, JA300 marR, or JA300 acrRIS marR were incubated for 30 min in the presence of organic solvents. The intracellular solvent levels of JA300 acrRIS and JA300 marR were similar to or slightly lower than those of the JA300 parent strain. On the other hand, the intracellular solvent levels of CH7 and JA300 acrRIS marR were remarkably lower than those of the JA300 parent strain. The amounts of n-heptane, n-hexane, and cyclohexane in CH7 were 6%, 20%, and 30% of those for JA300, respectively. On the other hand, the amounts of n-heptane, n-hexane, and cyclohexane in JA300acrRIS marR were 2%, 19%, and 22% of those for JA300, respectively. Thus, the intracellular amounts of organic solvents in JA300 acrRIS marR exhibited levels similar to those in CH7.
Table 4.
Accumulation of organic solvents in E. coli cells in a two-phase system
| Strain | |||
|---|---|---|---|
| |
n-Heptane |
n-Hexane |
Cyclohexane |
| JA300 |
0.060 ± 0.002 |
0.54 ± 0.01 |
1.3 ± 0.1 |
| JA300 acrRIS |
0.051 ± 0.037 |
NDb |
1.2 ± 0.1 |
| JA300 marR |
0.049 ± 0.010 |
NDb |
1.0 ± 0.1 |
| JA300 acrRIS marR |
0.0010 ± 0.0006 |
0.10 ± 0.01 |
0.29 ± 0.01 |
| CH7 | 0.0033 ± 0.0002 | 0.11 ± 0.01 | 0.39 ± 0.01 |
aE. coli strains grown in LBGMg medium were exposed to organic solvents in the two-phase system and incubated for 30 min as described in Materials and Methods. Values indicate means of results and standard deviations of results from three independent experiments.
bND, not determined.
Discussion
In the present study, we isolated cyclohexane-tolerant mutants from cyclohexane-sensitive E. coli K-12 strain JA300 and investigated whether or not these mutants carried mutations in regulatory genes marR, soxR, and acrR. Most of the mutants carried mutations in marR. Three of the seven mutations found in marR caused amino acid substitutions in MarR at the amino acid positions of L78, R94, and G116, and four of the seven mutations led to a translation termination codon at the position of E109 (marR109 mutation). These MarR mutations lie within the region spanning amino acids 61 to 121 in MarR, which are required for DNA binding activity (Alekshun et al. 2001 ). The L78 and R94 residues are highly conserved amino acids in many homologs, although the amino acid at the position of G116 is not conserved (Alekshun et al. 2001 ). The marR109 mutation led to the truncation of 35 C-terminal amino acids in MarR. A previous study showed that the C-terminal region contributes to dimer formation (Notka et al. 2002 ). In any case, the MarR mutations found in the present study are considered to lead to the loss of repressive function. Three mutants isolated in this study carried different mutations in acrR. Two mutations in acrR caused amino acid substitutions in AcrR at the amino acid positions of M1 and A41. The mutation in the translation initiation codon (M1I) will cause the complete inhibition of AcrR translation. The A41 residue positioned within the helical region (α3) forms part of a typical helix-turn-helix motif involved in DNA binding (Li et al. 2007 ). Thus, the A41D mutation will abolish AcrR’s repressive activity. Many IS elements have been shown to activate or inactivate the expression of neighboring genes. In strain CH7, the IS5 inserted within acrR seemed to activate the expression of acrAB through the disruption of transcriptional repression by AcrR. The alteration of organic solvent tolerances of several E. coli mutants by IS integration has been reported. E. coli OST4251 became sensitive to n-hexane because IS2 and IS5 became integrated upstream from the imp/ostA gene, which is involved in organic solvent sensitivity (Abe et al. 2003 ; Ohtsu et al. 2004 ). The hypersensitivity of an E. coli acrB disruptant to organic solvents was suppressed by the integrational activation of the acrEF operon with an IS1 or IS2 element (Kobayashi et al. 2003 ).
In this study, two mutants (CH1 and CH7) carried mutations in both marR and acrR. These mutants exhibited higher organic solvent tolerances than other isolates (Figure 1). In particular, strain CH7 containing marR109 and acrR::IS showed the highest organic solvent tolerance among all isolates. In fluoroquinolone-resistant clinical and veterinary isolates of E. coli, a number of mutations were found in soxR as well as marR and acrR (Wang et al. 2001 ; Webber and Piddock 2001 ; Komp Lindgren et al. 2003 ). In the present study, however, there was no mutation in soxR in cyclohexane-tolerant mutants.
It was possible that unidentified mutations other than marR109 and acrR::IS5 might influence organic solvent tolerance in strain CH7. To clarify the effect of the marR109 and/or acrR::IS5 mutations on organic solvent tolerance in E. coli, we constructed JA300 acrRIS, JA300 marR, and JA300 acrRIS marR. A comparison of the tolerances in these mutants and in strain CH7 revealed that the improved organic solvent tolerance in strain CH7 was caused by a synergistic effect of the double mutations of marR and acrR.
The AcrAB-TolC efflux pump is involved in organic solvent tolerance in E. coli ( Tsukagoshi and Aono 2000 ). The order of organic solvent tolerances of JA300 acrRIS, JA300 marR, and JA300 acrRIS marR was comparable to the order of the expression levels of AcrAB and TolC (Figures 3 and 5). The expression levels of AcrA and AcrB proteins in JA300 acrRIS were similar to, or slightly higher than, the levels in JA300 marR. However, the extent of improvement in organic solvent tolerance in JA300 acrRIS was lower than that in JA300 marR because the disruption of acrR did not influence the expression level of TolC. JA300 acrRIS marR and CH7 equally enhanced the expression levels of AcrAB and TolC compared to JA300 acrRIS and JA300 marR. In addition, the intracellular solvent levels of JA300 acrRIS marR and CH7 were similarly kept lower than those of JA300 acrRIS, JA300 marR, and JA300. These results suggested that the improved organic solvent tolerance in JA300 acrRIS marR and CH7 was a result of enhanced solvent-efflux activity by the overexpressed AcrAB-TolC pump. To clarify the contribution of the AcrAB-TolC pump to organic solvent tolerance in JA300 acrRIS marR, an acrA-disruptant (JA300ΔacrA acrRIS marR) was constructed and its organic solvent tolerance was compared to that of JA300ΔacrA (Figure 3). JA300ΔacrA acrRIS marR became as sensitive to organic solvents as JA300ΔacrA. This result indicated that the AcrAB-TolC pump is essential for JA300 acrRIS marR to acquire high-level organic solvent tolerance. In addition, it suggested that the mar regulon genes other than acrAB and tolC are barely involved in organic solvent tolerance in JA300 acrRIS marR.
Organic solvent-tolerance levels of various mutants and recombinants from strain JA300 have been investigated by measuring the colony-forming efficiencies of mutants on an LBGMg agar plate overlaid with organic solvents. Overexpression of the marA gene has been shown to raise the organic solvent tolerance of E. coli (Asako et al. 1997 ; Shimizu et al. 2005 ). JA300 overexpressing the marA gene formed colonies in spots containing more than 106 cells in the presence of cyclohexane (Shimizu et al. 2005 ). We previously reported that the organic solvent tolerance of strain JA300 significantly improved the double disruptions of marR and proV (Doukyu et al. 2012 ). JA300ΔproVΔmarR formed colonies in spots containing more than 105 cells in the presence of cyclohexane and thus exhibited higher organic solvent-tolerance levels than JA300 overexpressing the marA gene. In the present study, JA300 acrRIS marR showed 104-fold higher colony-forming efficiencies in the presence of cyclohexane than JA300ΔproVΔmarR.
In the present study, we clarified that only two mutations in regulatory genes in acrR and marR confer high-level organic solvent tolerance on E. coli. Owing to the wealth of genetic and metabolic knowledge associated with E. coli, organic solvent-tolerant E. coli can be a convenient and efficient catalyst when it is used as a host expressing enzymes that are useful for producing valuable chemicals in two-phase systems employing organic solvents. The present findings are expected to provide valuable knowledge for increasing organic solvent-tolerance levels in E. coli to improve the usability of whole-cell biocatalysts in two-phase systems.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Rei Watanabe, Email: vn1100032@toyo.jp.
Noriyuki Doukyu, Email: dokyu@toyo.jp.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Young Scientists (B) and by a Grant for the Programme for the Strategic Research Foundation at Private Universities S1101017, organised by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, since April 2011. We would like to thank Shinji Takahashi and Takuma Ise for their technical support.
References
- Abe S, Okutsu T, Nakajima H, Kakuda N, Ohtsu I, Aono R. n-Hexane sensitivity of Escherichia coli due to low expression of imp/ostA encoding an 87 kDa minor protein associated with the outer membrane. Microbiology. 2003;149:1265–1273. doi: 10.1099/mic.0.25927-0. [DOI] [PubMed] [Google Scholar]
- Alekshun MN, Levy SB. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob Agents Chemother. 1997;41:2067–2075. doi: 10.1128/aac.41.10.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekshun MN, Levy SB, Mealy TR, Seaton BA, Head JF. The crystal structure of MarR, a regulator of multiple antibiotic resistance, at 2.3 Å resolution. Nat Struct Biol. 2001;8:710–714. doi: 10.1038/90429. [DOI] [PubMed] [Google Scholar]
- Aono R. Improvement of organic solvent tolerance level of Escherichia coli by overexpression of stress-responsive genes. Extremophiles. 1998;2:239–248. doi: 10.1007/s007920050066. [DOI] [PubMed] [Google Scholar]
- Aono R, Aibe K, Inoue A, Horikoshi K. Preparation of organic solvent tolerant mutants from Escherichia coli K-12. Agric Biol Chem. 1991;55:1935–1938. doi: 10.1271/bbb1961.55.1935. [DOI] [Google Scholar]
- Aono R, Kobayashi H, Joblin KN, Horikoshi K. Effects of organic solvents on growth of Escherichia coli K-12. Biosci Biotechnol Boichem. 1994;58:2009–2014. doi: 10.1271/bbb.58.2009. [DOI] [Google Scholar]
- Asako H, Nakajima H, Kobayashi K, Kobayashi M, Aono R. Organic solvent tolerance and antibiotic resistance increased by overexpression of marA in Escherichia coli. Appl Environ Microbiol. 1997;63:1428–1433. doi: 10.1128/aem.63.4.1428-1433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko K, Tomita M, Wanner B, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2:2006–2008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa TM, Levy SB. Differential expression of over 60 chromosomal genes in Escherichia coli by constitutive expression of MarA. J Bacteriol. 2000;182:3467–3474. doi: 10.1128/JB.182.12.3467-3474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doukyu N, Ishikawa K, Watanabe R, Ogino H. Improvement in organic solvent tolerance by double disruptions of proV and marR genes in Escherichia coli. J Appl Microbiol. 2012;112:464–474. doi: 10.1111/j.1365-2672.2012.05236.x. [DOI] [PubMed] [Google Scholar]
- Fernandes P, Ferreira BS, Cabral JM. Solvent tolerance in bacteria: role of efflux pumps and cross-resistance with antibiotics. Int J Antimicrob Agents. 2003;22:211–216. doi: 10.1016/S0924-8579(03)00209-7. [DOI] [PubMed] [Google Scholar]
- Heipieper HJ, Neumann G, Cornelissen S, Meinhardt F. Solvent-tolerant bacteria for biotransformations in two-phase fermentation systems. Appl Microbiol Biotechnol. 2007;74:961–973. doi: 10.1007/s00253-006-0833-4. [DOI] [PubMed] [Google Scholar]
- Inoue A, Horikoshi K. A Pseudomonas thrives in high concentrations of toluene. Nature. 1989;338:264–265. doi: 10.1038/338264a0. [DOI] [Google Scholar]
- Karczmarczyk M, Martins M, Quinn T, Leonard N, Fanning S. Mechanisms of fluoroquinolone resistance in Escherichia coli isolates from food-producing animals. Appl Environ Microbiol. 2011;77:7113–7120. doi: 10.1128/AEM.00600-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieboom J, Dennis J, de Bont J, Zylstra G. Identification and molecular characterization of an efflux pump involved in Pseudomonas putida S12 solvent tolerance. J Biol Chem. 1998;273:85–91. doi: 10.1074/jbc.273.1.85. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Tsukagoshi N, Aono R. Suppression of hypersensitivity of Escherichia coli acrB mutant to organic solvents by integrational activation of the acrEF operon with the IS1 or IS2 element. J Bacteriol. 2003;183:2646–2653. doi: 10.1128/JB.183.8.2646-2653.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komp Lindgren P, Karlsson A, Hughes D. Mutation rate and evolution of fluoroquinolone resistance in Escherichia coli isolates from patients with urinary tract infections. Antimicrob Agents Chemother. 2003;47:3222–3232. doi: 10.1128/AAC.47.10.3222-3232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li M, Gu R, Su CC, Routh MD, Harris KC, Jewell ES, McDermott G, Yu E. Crystal structure of the transcriptional regulator AcrR from Escherichia coli. J Mol Biol. 2007;374:591–603. doi: 10.1016/j.jmb.2007.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-Z, Li Z, Poole K. Role of the multidrug efflux systems of Pseudomonas aeruginosa in organic solvent tolerance. J Bacteriol. 1998;180:2987–2991. doi: 10.1128/jb.180.11.2987-2991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Ma D, Alberti M, Lynch C, Nikaido H, Hearst JE. The local repressor AcrR plays a modulating role in the regulation of acrAB genes of Escherichia coli by global stress signals. Mol Microbiol. 1996;19:101–112. doi: 10.1046/j.1365-2958.1996.357881.x. [DOI] [PubMed] [Google Scholar]
- Martin RG, Gillette WK, Rhee S, Rosner JL. Structural requirements for marbox function in transcriptional activation of mar/sox/rob regulon promoters in Escherichia coli: sequence, orientation and spatial relationship to the core promoter. Mol Microbiol. 1999;34:431–441. doi: 10.1046/j.1365-2958.1999.01599.x. [DOI] [PubMed] [Google Scholar]
- Notka F, Linde HJ, Dankesreiter A, Niller HH, Lehn N. A C-terminal 18 amino acid deletion in MarR in a clinical isolate of Escherichia coli reduces MarR binding properties and increases the MIC of ciprofloxacin. J Antimicrob Chemother. 2002;49:41–47. doi: 10.1093/jac/49.1.41. [DOI] [PubMed] [Google Scholar]
- Ohtsu I, Kakuda N, Tsukagoshi N, Dokyu N, Takagi H, Wachi M, Aono R. Transcriptional analysis of the ostA/imp gene involved in organic solvent sensitivity in Escherichia coli. Biosci Biotechnol Biochem. 2004;68:458–461. doi: 10.1271/bbb.68.458. [DOI] [PubMed] [Google Scholar]
- Okochi M, Kanie K, Kurimoto M, Yohda M, Honda H. Overexpression of prefoldin from the hyperthermophilic archaeum Pyrococcus horikoshii OT3 endowed Escherichia coli with organic solvent tolerance. Appl Microbiol Biotechnol. 2008;79:443–449. doi: 10.1007/s00253-008-1450-1. [DOI] [PubMed] [Google Scholar]
- Okochi M, Kurimoto M, Shimizu K, Honda H. Increase of organic solvent tolerance by overexpression of manXYZ in Escherichia coli. Appl Microbiol Biotechnol. 2007;73:1394–1399. doi: 10.1007/s00253-006-0624-y. [DOI] [PubMed] [Google Scholar]
- Okochi M, Kurimoto M, Shimizu K, Honda H. Effect of global transcriptional regulators related to carbohydrate metabolism on organic solvent tolerance in Escherichia coli. J Biosci Bioeng. 2008;105:389–394. doi: 10.1263/jbb.105.389. [DOI] [PubMed] [Google Scholar]
- Okusu H, Ma D, Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol. 1996;178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen I, Brown M, Skurray R. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomposiello PJ, Demple B. Redox-operated genetic switches: the SoxR and OxyR transcription factors. Trends Biotechnol. 2001;19:109–114. doi: 10.1016/S0167-7799(00)01542-0. [DOI] [PubMed] [Google Scholar]
- Ramos JL, Duque E, Gallegos MT, Godoy P, Ramos-Gonzalez MI, Rojas A, Teran W, Segura A. Mechanisms of solvent tolerance in gram-negative bacteria. Annu Rev Microbiol. 2002;56:743–768. doi: 10.1146/annurev.micro.56.012302.161038. [DOI] [PubMed] [Google Scholar]
- Ramos JL, Duque E, Godoy P, Segura A. Efflux pumps involved in toluene tolerance in Pseudomonas putida DOT-T1E. J Bacteriol. 1998;180:3323–3329. doi: 10.1128/jb.180.13.3323-3329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall LP, Woodward MJ. The multiple antibiotic resistance (mar) locus and its significance. Res Vet Sci. 2002;72:87–93. doi: 10.1053/rvsc.2001.0537. [DOI] [PubMed] [Google Scholar]
- Sardessai YN, Bhosle S. Industrial potential of organic solvent tolerant bacteria. Biotechnol Prog. 2004;20:655–660. doi: 10.1021/bp0200595. [DOI] [PubMed] [Google Scholar]
- Sawers RG. Transcript analysis of Escherichia coli K-12 insertion element IS5. FEMS Microbiol Lett. 2005;244:397–401. doi: 10.1016/j.femsle.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Schmid A, Kollmer A, Mathys RG, Witholt B. Developments toward large-scale bacterial bioprocesses in the presence of bulk amounts of organic solvents. Extremophiles. 1998;2:249–256. doi: 10.1007/s007920050067. [DOI] [PubMed] [Google Scholar]
- Segura A, Molina L, Fillet S, Krell T, Bernal P, Muñoz-Rojas J, Ramos JL. Solvent tolerance in gram-negative bacteria. Curr Opin Biotechnol. 2012;23:415–421. doi: 10.1016/j.copbio.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Hayashi S, Kako T, Suzuki M, Tsukagoshi N, Doukyu N, Kobayashi T, Honda H. Discovery of glpC, an organic solvent tolerance-related gene in Escherichia coli, using gene expression profiles from DNA microarrays. Appl Environ Microbiol. 2005;71:1093–1096. doi: 10.1128/AEM.71.2.1093-1096.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema J, de Bont JA, Poolman B. Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem. 1994;269:8022–8028. [PubMed] [Google Scholar]
- Su CC, Rutherford DJ, Yu EW. Characterization of the multidrug efflux regulator AcrR from Escherichia coli. Biochem Biophys Res Commun. 2007;361:85–90. doi: 10.1016/j.bbrc.2007.06.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres S, Pandey A, Castro GR. Organic solvent adaptation of gram positive bacteria: applications and biotechnological potentials. Biotechnol Adv. 2011;29:442–452. doi: 10.1016/j.biotechadv.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi N, Aono R. Entry into and release of solvents by Escherichia coli in an organic-aqueous two-liquid-phase system and substrate specificity of the AcrAB-TolC solvent-extruding pump. J Bacteriol. 2000;182:4803–4810. doi: 10.1128/JB.182.17.4803-4810.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Dzink-Fox JL, Chen M, Levy SB. Genetic characterization of highly fluoroquinolone-resistant clinical Escherichia coli strains from China: role of acrR mutations. Antimicrob Agents Chemother. 2001;45:1515–1521. doi: 10.1128/AAC.45.5.1515-1521.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber MA, Piddock LJ. Absence of mutations in marRAB or soxRS in acrB-overexpressing fluoroquinolone-resistant clinical and veterinary isolates of Escherichia coli. Antimicrob Agents Chemother. 2001;45:1550–1552. doi: 10.1128/AAC.45.5.1550-1552.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber MA, Talukder A, Piddock LJ. Contribution of mutation at amino acid 45 of AcrR to acrB expression and ciprofloxacin resistance in clinical and veterinary Escherichia coli isolates. Antimicrob Agents Chemother. 2005;49:4390–4392. doi: 10.1128/AAC.49.10.4390-4392.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DG, Goldman JD, Demple B, Levy SB. Role of the acrAB locus in organic solvent tolerance mediated by expression of marA, soxS, or robA in Escherichia coli. J Bacteriol. 1997;179:6122–6126. doi: 10.1128/jb.179.19.6122-6126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]