Abstract
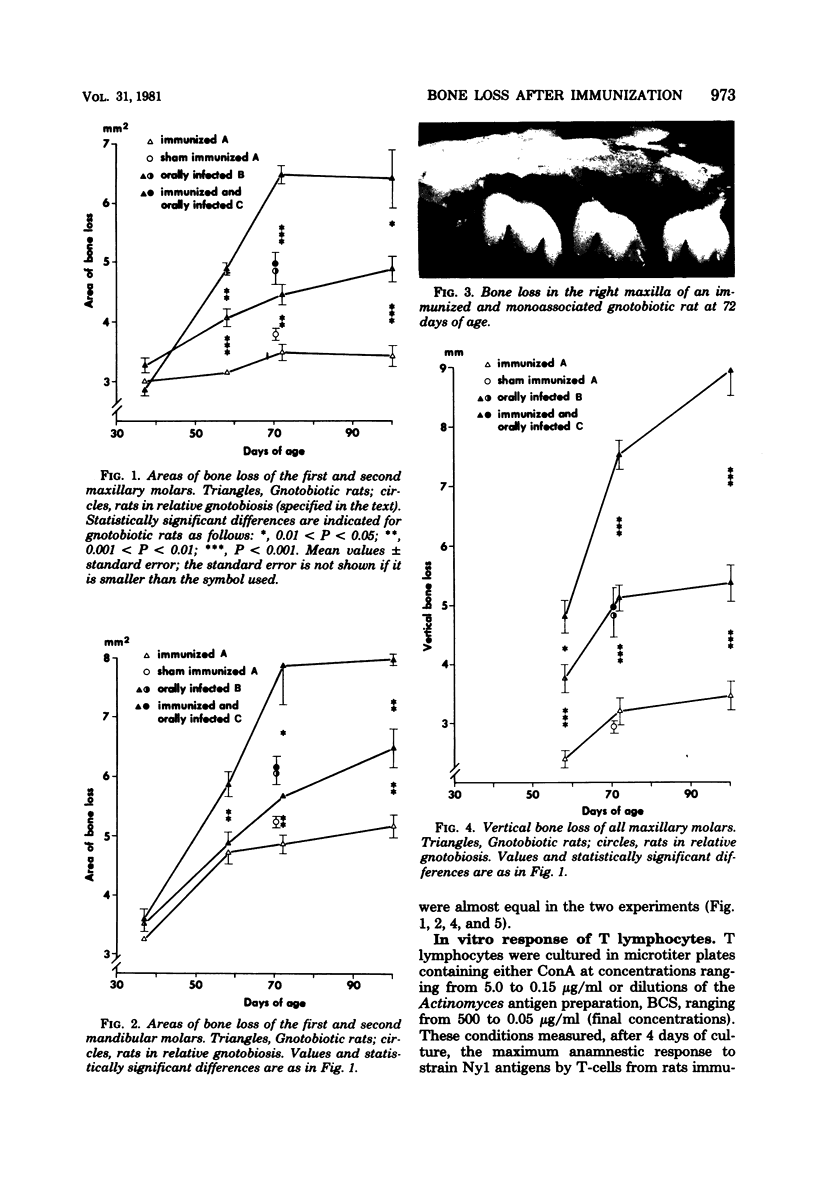
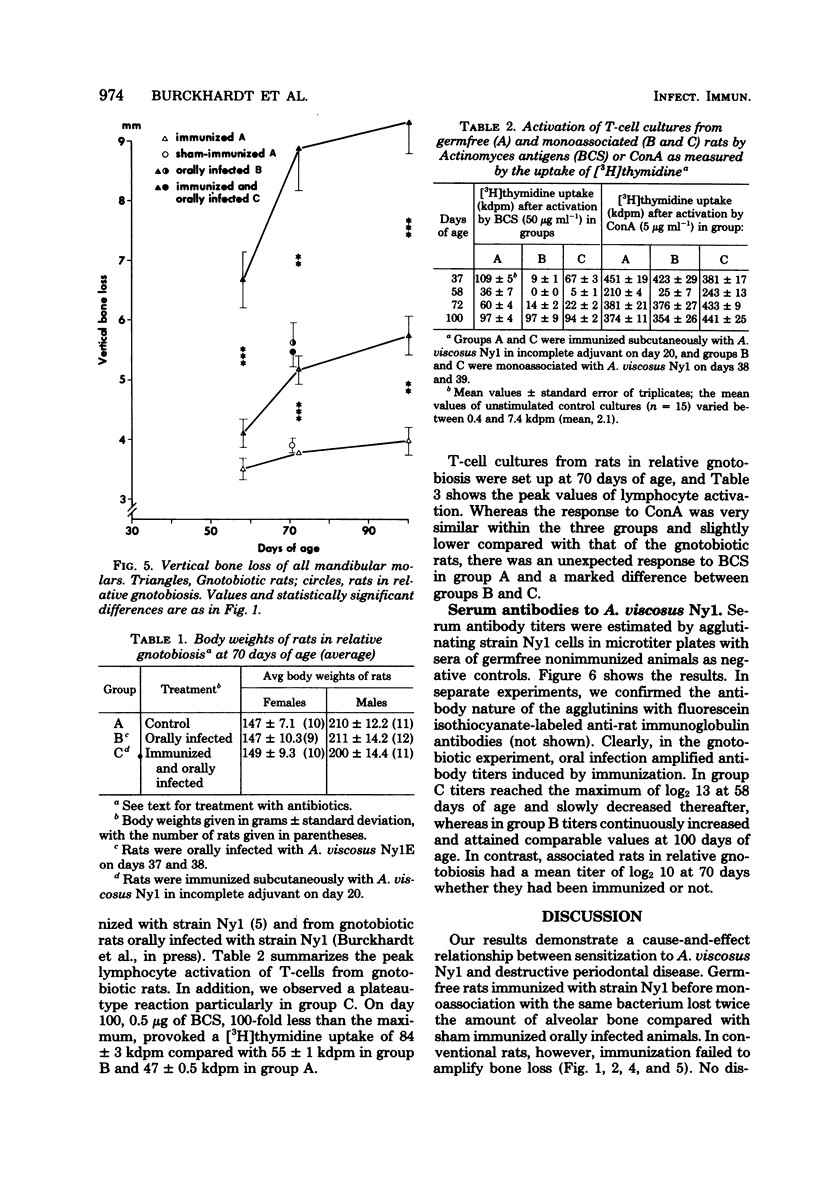
We investigated a possible cause-and-effect relationship between sensitization against Actinomyces viscosus Nyl and destructive periodontal disease in RIC-Sprague-Dawley rats. Germfree rats (66) were either immunized with A. viscosus Nyl (day 20) or orally infected with A. viscosus Nyl (days 38 and 39) or both. We measured alveolar bone loss in maxillary and mandibular molars, in vitro T-lymphocyte responsiveness, and serum antibody titers. In immunized and monoassociated rats bone loss in both jaws progressed rapidly between days 37 and 72, whereas the rate of further resorption decreased until day 100. In monoassociated rats, development of bone loss was much slower, and the maximum resorption measured was, at best, half of the bone loss compared with the former group. However, no amplification of bone loss by immunization was observed in a second experiment using 63 conventional rats kept in relative gnotobiosis. Antibody titers to A. viscosus Nyl in gnotobiotic monoassociated rats were higher in immunized animals, whereas no difference was found in the respective groups of the relative gnotobiotic experiment. The fact that immunization more than doubled alveolar bone loss in gnotobiotic monoassociated rats confirms the allergic nature of the disease. The lack of such an effect under conventional conditions points to suppressor mechanisms whose decrease might convert stable periodontal lesions into progressive ones.
Full text
PDF

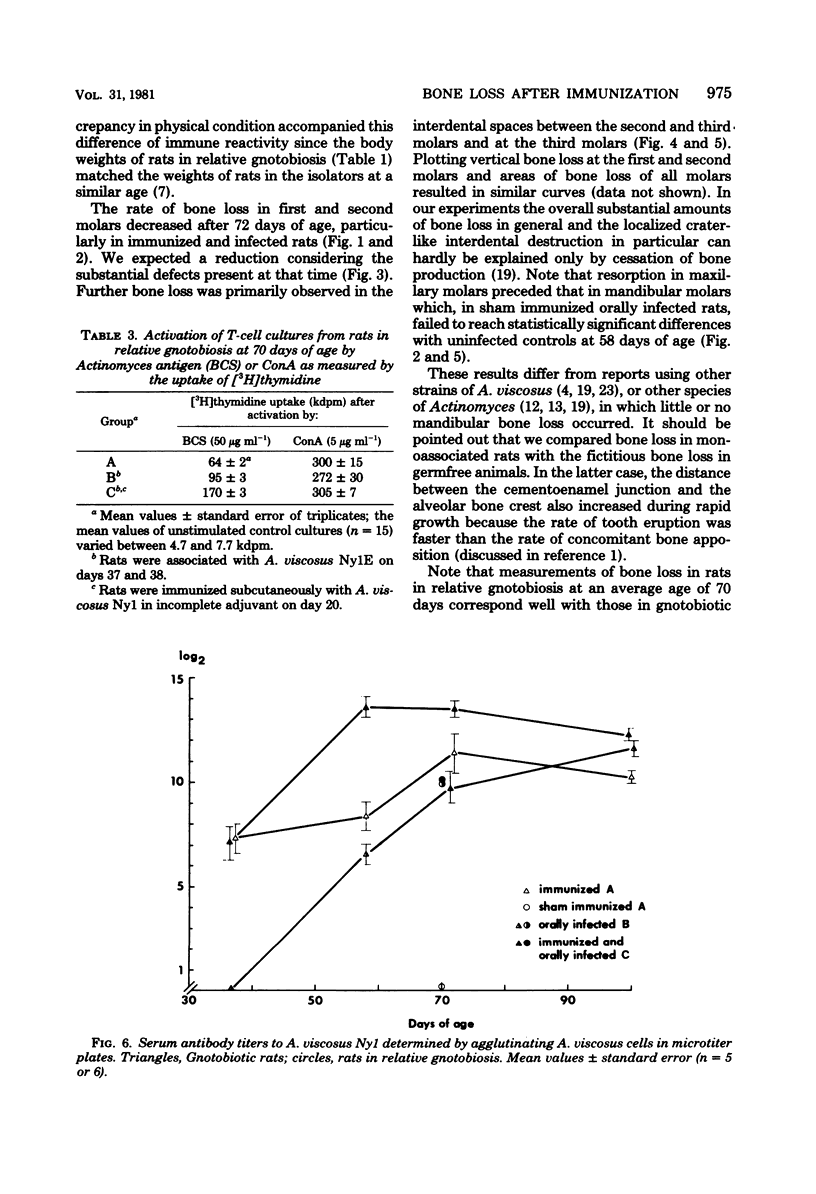


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amstad-Jossi M., Schroeder H. E. Age-related alterations of periodontal structures around the cemento-enamel junction and of the gingival connective tissue composition in germ-free rats. J Periodontal Res. 1978 Jan;13(1):76–90. doi: 10.1111/j.1600-0765.1978.tb00156.x. [DOI] [PubMed] [Google Scholar]
- Baker J. J., Chan S. P., Socransky S. S., Oppenheim J. J., Mergenhagen S. E. Importance of Actinomyces and certain gram-negative anaerobic organisms in the transformation of lymphocytes from patients with periodontal disease. Infect Immun. 1976 May;13(5):1363–1368. doi: 10.1128/iai.13.5.1363-1368.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden G. H., Hardie J. M., Fillery E. D. Antigens from Actinomyces species and their value in identification. J Dent Res. 1976 Jan;55:A192–A204. doi: 10.1177/002203457605500112011. [DOI] [PubMed] [Google Scholar]
- Brecher S. M., van Houte J., Hammond B. F. Role of colonization in the virulence of Actinomyces viscosus strains T14-Vi and T14-Av. Infect Immun. 1978 Nov;22(2):603–614. doi: 10.1128/iai.22.2.603-614.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt J. J., Guggenheim B., Hefti A. Are Actinomyces viscosus antigens B cell mitogens? J Immunol. 1977 Apr;118(4):1460–1465. [PubMed] [Google Scholar]
- Burckhardt J. J., Guggenheim B. Increased smooth-surface caries incidence in gnotobiotic rats immunized with Actinomyces viscosus. Caries Res. 1980;14(1):56–59. doi: 10.1159/000260435. [DOI] [PubMed] [Google Scholar]
- Burckhardt J. J. Rat memory T lymphocytes. II. Differences in macrophage-dependent activation shown by Actinomyces viscosus antigens and by mitogens, using silica in vitro. Scand J Immunol. 1979;10(3):229–235. doi: 10.1111/j.1365-3083.1979.tb01344.x. [DOI] [PubMed] [Google Scholar]
- Burckhardt J. J. Rat memory T lymphocytes: in vitro proliferation induced by antigens of Actinomyces viscosus. Scand J Immunol. 1978;7(2):167–172. doi: 10.1111/j.1365-3083.1978.tb00440.x. [DOI] [PubMed] [Google Scholar]
- Chen C., Hirsch J. G. The effects of mercaptoethanol and of peritoneal macrophages on the antibody-forming capacity of nonadherent mouse spleen cells in vitro. J Exp Med. 1972 Sep 1;136(3):604–617. doi: 10.1084/jem.136.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Click R. E., Benck L., Alter B. J. Immune responses in vitro. I. Culture conditions for antibody synthesis. Cell Immunol. 1972 Feb;3(2):264–276. doi: 10.1016/0008-8749(72)90165-7. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Koffler D. Immune complex disease in experimental animals and man. Adv Immunol. 1973;16(0):185–264. doi: 10.1016/s0065-2776(08)60298-9. [DOI] [PubMed] [Google Scholar]
- Crawford J. M., Taubman M. A., Smith D. J. The effects of local immunization with periodontopathic microorganisms on periodontal bone loss in gnotobiotic rats. J Periodontal Res. 1978 Sep;13(5):445–459. doi: 10.1111/j.1600-0765.1978.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Crawford J. M., Taubman M. A., Smith D. J. The natural history of periodontal bone loss in germfree and gnotobiotic rats infected with periodontopathic microorganisms. J Periodontal Res. 1978 Jul;13(4):316–325. doi: 10.1111/j.1600-0765.1978.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Garant P. R. An electron microscopic study of the periodontal tissues of germfree rats and rats monoinfected with Actinomyces naeslundii. J Periodontal Res. 1976;(15):3–79. [PubMed] [Google Scholar]
- Genco R. J., Mashimo P. A., Krygier G., Ellison S. A. Antibody-mediated effects on the periodontium. J Periodontol. 1974 May;45(5):330–337. doi: 10.1902/jop.1974.45.5.330. [DOI] [PubMed] [Google Scholar]
- Gershon R. K. T cell control of antibody production. Contemp Top Immunobiol. 1974;3:1–40. doi: 10.1007/978-1-4684-3045-5_1. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Reactions in the periodontium to continuous antigenic stimulation in sensitized gnotobiotic rats. Infect Immun. 1974 Sep;10(3):565–577. doi: 10.1128/iai.10.3.565-577.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving J. T., Socransky S. S., Heeley J. D. Histological changes in experimental periodontal disease in gnotobiotic rats and conventional hamsters. J Periodontal Res. 1974;9(2):73–80. doi: 10.1111/j.1600-0765.1974.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Ivanyi L., Lehner T. Lymphocyte transformation by sonicates of dental plaque in human periodontal disease. Arch Oral Biol. 1971 Sep;16(9):1117–1121. doi: 10.1016/0003-9969(71)90216-0. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Johnson D. A., Behling U. H., Lai C. H., Listgarten M., Socransky S., Nowotny A. Role of bacterial products in periodontitis: immune response in gnotobiotic rats monoinfected with Eikenella corrodens. Infect Immun. 1978 Jan;19(1):246–253. doi: 10.1128/iai.19.1.246-253.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan H. V., Fitzgerald R. J., Stanley H. R. Plaque formation and periodontal pathology in gnotobiotic rats infected with an oral actinomycete. Am J Pathol. 1965 Dec;47(6):1157–1167. [PMC free article] [PubMed] [Google Scholar]
- Keller R. Major changes in lymphocyte proliferation evoked by activated macrophages. Cell Immunol. 1975 Jun;17(2):542–551. doi: 10.1016/s0008-8749(75)80058-x. [DOI] [PubMed] [Google Scholar]
- Kysela S., Steinberg A. D. Increased survival of NZB-W mice given multiple syngeneic young thymus grafts. Clin Immunol Immunopathol. 1973 Nov;2(1):133–136. doi: 10.1016/0090-1229(73)90043-3. [DOI] [PubMed] [Google Scholar]
- König K. G., Guggenheim B. Implantation of antibiotic-resistant bacteria and the production of dental caries in rats. Adv Oral Biol. 1968;3:217–252. doi: 10.1016/b978-1-4832-3119-8.50014-7. [DOI] [PubMed] [Google Scholar]
- Lindenmann J. Speculations on idiotypes and homobodies. Ann Immunol (Paris) 1973 May;124(2):171–184. [PubMed] [Google Scholar]
- Mackler B. F., Altman L. C., Wahl S., Rosenstreich D. L., Oppenheim J. J., Mergenhagen S. E. Blastogenesis and lymphokine synthesis by T and B lymphocytes from patients with periodontal disease. Infect Immun. 1974 Oct;10(4):844–850. doi: 10.1128/iai.10.4.844-850.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackler B. F., Frostad K. B., Robertson P. B., Levy B. M. Immunoglobulin bearing lymphocytes and plasma cells in human periodontal disease. J Periodontal Res. 1977 Jan;12(1):37–45. doi: 10.1111/j.1600-0765.1977.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Mattingly J. A., Eardley D. D., Kemp J. D., Gershon R. K. Induction of suppressor cells in rat spleen: influence of microbial stimulation. J Immunol. 1979 Mar;122(3):787–790. [PubMed] [Google Scholar]
- Metzger Z., Hoffeld J. T., Oppenheim J. J. Macrophage-mediated suppression. I. Evidence for participation of both hdyrogen peroxide and prostaglandins in suppression of murine lymphocyte proliferation. J Immunol. 1980 Feb;124(2):983–988. [PubMed] [Google Scholar]
- Page R. C., Schroeder H. E. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976 Mar;34(3):235–249. [PubMed] [Google Scholar]
- Seymour G. J., Greenspan J. S. The phenotypic characterization of lymphocyte subpopulations in established human periodontal disease. J Periodontal Res. 1979 Jan;14(1):39–46. doi: 10.1111/j.1600-0765.1979.tb00216.x. [DOI] [PubMed] [Google Scholar]
- Seymour G. J., Powell R. N., Davies W. I. Conversion of a stable T-cell lesion to a progressive B-cell lesion in the pathogenesis of chronic inflammatory periodontal disease: an hypothesis. J Clin Periodontol. 1979 Oct;6(5):267–277. doi: 10.1111/j.1600-051x.1979.tb01930.x. [DOI] [PubMed] [Google Scholar]
- Smith F. N., Lang N. P. Lymphocyte blastogenesis to plaque antigens in human periodontal disease. ii. The relationship to clinical parameters. J Periodontal Res. 1977 Jul;12(4):310–317. doi: 10.1111/j.1600-0765.1977.tb00135.x. [DOI] [PubMed] [Google Scholar]