Abstract
Background
FGF signaling plays numerous roles during organogenesis of the embryonic gut tube. Mouse explant studies suggest that different thresholds of FGF signaling from the cardiogenic mesoderm induce lung, liver, and pancreas lineages from the ventral foregut progenitor cells. The mechanisms that regulate FGF dose in vivo are unknown. Here we use Xenopus embryos to examine the hypothesis that a prolonged duration of FGF signaling from the mesoderm is required to induce foregut organs.
Results
We show that both mesoderm and FGF signaling are required for liver and lung development in Xenopus; formally demonstrating that this important step in organ induction is conserved with other vertebrate species. Prolonged contact with the mesoderm and persistent FGF signaling through both MEK and PI3K over an extended period of time are required for liver and lung specification. Inhibition of FGF signaling results in reduced liver and lung development, with a modest expansion of the pancreas/duodenum progenitor domain. Hyper-activation of FGF signaling has the opposite effect expanding liver and lung gene expression and repressing pancreatic markers. We show that FGF signaling is cell autonomously required in the endoderm and that a dominant negative FGF receptor decreases the ability of ventral foregut progenitor cells to contribute to the lung and liver buds.
Conclusions
These results suggest that the liver and lungs are specified at progressively later times in development requiring mesoderm contact for different lengths of time. Our data suggest that this is achieved at least in part through prolonged FGF signaling. In addition to providing a foundation for further mechanistic studies on foregut organogenesis using the experimental advantages of the Xenopus system, these data have implications for the directed differentiation of stem cells into foregut lineages.
Keywords: FGF, ERK, AKT, Lung, Liver, Foregut, Endoderm, Organ induction, Xenopus
Background
In the Fibroblast Growth Factor (FGF) signaling pathway, secreted ligands bind to transmembrane tyrosine kinase FGF receptors causing dimerization and activation of a number of intracellular signal transduction cascades including the mitogen-activated protein kinase (MEK) and phosphoinositide 3-kinase (PI3K), which phosphorylate Erk and Akt, respectively [1]. FGF signals regulate cellular differentiation, proliferation, and survival in many contexts and studies in mice, chick, and zebrafish have shown that FGF mediated mesenchymal-epithelial interactions play numerous roles in the developing gut tube [2-4]. During gastrulation, FGF signaling patterns the primitive gut tube by promoting posterior over anterior cell fate in the endoderm [5]. Then only hours later, FGF signals from the anterior lateral plate and cardiac mesoderm segregate the pancreas, liver, and lung lineages from a pool of ventral foregut progenitor cells [6-12]. Recent studies in zebrafish suggest that FGF signaling acts in part by restricting hepatic competence of the endoderm along the anterior-posterior (A-P) axis [13,14]. Additionally, FGFs are important for the outgrowth and morphogenesis of many organ buds during fetal development; for instance mesenchymal FGF10 controls lung branching [15,16], pancreas proliferation and growth [17,18], stomach morphogenesis [19], and hepatopancreatic fate [20]. Considering these multiple context-dependent activities, it is likely that a better understanding of the precise temporal roles of FGF signaling during endoderm organogenesis will inform approaches to direct the differentiation of human stem cells in vitro[2,21].
In this study we investigated the role that FGF signaling plays in the specification of foregut organs in Xenopus embryos. In zebrafish and chick, FGF signals (along with BMP and Wnt) have been shown to be essential for hepatic specification [6,7]. Additionally, in vitro studies using mouse embryo foregut explant cultures from 0–7 somite-stages (ss) of development have suggested that FGF signals from the cardiac and lateral plate mesoderm regulate the induction of the pancreas, liver, and lungs in a dose-dependent manner [8,10]. Little or no FGF signaling is required for ventral endodermal explants from early somite-stage mouse embryos to turn on the pancreas marker Pdx1, whereas explants cultured with cardiac mesoderm or recombinant FGF2 express the liver marker Albumin[12] and higher FGF doses stimulate expression of the thyroid/lung marker Nkx2.1[8]. Similar dose-responsive FGF effects have been observed during the differentiation of human ES cells to foregut lineages [22,23]. Downstream of FGF receptor signaling, it has been shown that in mouse embryos the MEK branch of the FGF pathway is necessary for liver Albumin and Alpha-fetoprotein expression, while the PI3K branch promotes hepatoblast proliferation [9]. These data have led to a model of foregut organ development where different doses of FGF specify the different foregut lineages: very low or absent FGF levels are required for pancreas, intermediate FGF levels promote liver, and high FGF levels are required for lung. The mechanisms by which different thresholds of FGF are achieved in vivo are unknown, in part because mouse embryos are difficult to manipulate at these early stages in development.
Xenopus embryos have increasingly proven to be a valuable model to study the mechanisms of organogenesis [24,25]. Horb and Slack have shown that signals from the mesoderm between stages NF15 (0 ss) to NF42 (organ bud stage) are required for Xenopus endodermal explants to become regionally specified into anterior and posterior organ lineages [26]. Consistent with a conserved role for FGF signaling in Xenopus foregut organ induction, multiple FGF ligands and receptors are expressed in the Xenopus foregut mesenchyme and endoderm between stages NF15-42 [27] . Moreover, FGF signaling is necessary for the induction of liver gene expression in cultured ectodermal explants in vitro[28] and Akt signaling is required for later pancreas and stomach progenitor cell survival [29].
Despite these suggestive data, the role of FGF signaling in Xenopus foregut organ specification in vivo has not formally been determined. In this study, we show that:
1. The pancreas, liver and lung are specified at progressively later times in Xenopus development, and that the liver and lungs require progressively longer mesoderm contact;
2. FGF signaling is required for lung and liver specification in vivo and this is, at least in part, a cell autonomous requirement in the endoderm;
3. Foregut endoderm in which FGF signaling is experimentally blocked appears to remain in a progenitor-like state;
4. Ectopic activation of the FGF pathway expands liver and lung development, and represses pancreatic fate;
5. The high levels of FGF necessary for lung and liver induction appear to be achieved through FGF signaling over an extended period of time via both the MEK and PI3K pathways.
This temporal requirement for FGF signaling in foregut lineage segregation provides the foundation for future mechanistic studies in Xenopus, and may impact studies aimed at inducing different foregut lineages from pluripotent stem cells.
Methods
Embryo manipulations
Xenopus laevis embryos were cultured as previously described [30]. All animal experiments complied with the “Animal Research: Reporting in vivo Experiments” (ARRIVE) guidelines and were approved by the Cincinnati Children’s Research Foundation IACUC committee (protocol #0B12097). For microdissection, ventral explants were dissected from embryos using eyelash blades and hair loops. At the indicated developmental time half of the explants had the mesoderm separated using hair loops in 5 units/ml Dispase (BD Biosciences) for 10–15 min (Figure 1A). Endoderm explants (+meso and –meso) were then cultured in 0.5xMBS to Stage 37 for analysis. Synthetic RNA for microinjection was transcribed using the mMessage Kits (Ambion) and purified on Microspin-6 columns (BioRad). The following plasmids were used for mRNA synthesis (enzymes used to linearize DNA templates, RNA polymerase): pRN3 GFP (SfiI, T3); pCS2 + β-gal (NotI, Sp6); dnFGFR (pxFD/XSS) (EcoRI, SP6); caFGFR (iFGFR1) (Not1, SP6). Embryos with clear dorsal-ventral pigment were selected for injections into the bottom of dorsal-vegetal blastomeres at the 4-8-cell stage to target large regions of the foregut mesendoderm and into the D2.1 cells at the 16-cell stage to target foregut endoderm avoiding the mesoderm. Lineage labels were used to confirm the correct targeting. Cell soluble inhibitors were dissolved in DMSO and added to the media at the following working concentrations: PD173074 (300 μM; TOCRIS), U0126 (Cell Signaling, 300 μM), LY294003 (Cell Signaling, 40 μM), and SU5402 (10 μM with 0.1 M ATP). B/B Homodimerizer (Clontech) was dissolved in 100% ethanol and used at 1.25 μM working concentration. Explants were cultured in 100 ng/ml of human recombinant FGF2 (Invitrogen) in 0.5XMBS + 0.1%BSA.
Figure 1.
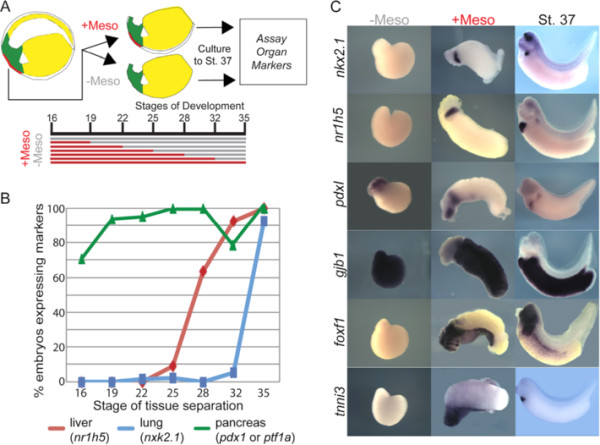
Foregut organ specification requires prolonged contact with the cardiac-lateral plate mesoderm. (A) Diagram of the experimental design. Ventral explants were cultured with (+Meso) or without mesoderm (−Meso) during the indicated time points and assayed by in situ hybridization at stage NF37. (B) Summary of experimental results showing the percentage of explants with mesoderm removed at different times expressing liver (nr1h5), lung/thyroid (nkx2.1) and pancreas (pdx1 or ptf1a) markers at stage NF37, n > 20 explants for each condition and each probe. (C) Representative explants cultured from stage NF16 to NF37 with or without mesoderm and corresponding whole embryo controls assayed by in situ hybridization with the indicated markers. Endodermal (gjb1) or mesodermal (foxf1 and tnni3) specific markers demonstrated clean separation of the tissues.
In situ hybridization
In situ hybridizations were performed as previously described [31] using the following probes: nr1h5 (formally for1, Xenbase.org) [32], pdx1[33], ptf1α[34], nkx2.1[35], tnni3 (formally cardiac-troponin, Xenbase.org) [36], foxf1[37], gjb1[38], hhex[39], nkx2.5[40], hnf4α[41]. Image-J software was used to measure the average size of the hhex and pdx1 expression domains +/− S.D. in injected and control sibling embryos.
Immunostaining and Western blot analysis
For Western blots, five embryos per sample were lysed in a TLB buffer (1% Triton X 100, 25 mM Tris pH 7.4, 150 mM NaCl, 2 mM Na3VO4, 2.5 mM NaF and 25 mM B-glycerophosphate) with protease and phosphatase inhibitors each diluted 1:100: phosphatase inhibitor cocktail II (Sigma), PhosSTOP (Roche), protease inhibitor cocktail (Sigma). Samples were run on a 10% polyacrylamide gel and transferred to an Immobilon membrane (Millipore). The membrane was incubated with the following antibodies: mouse anti-dpErk1/2 (1:250, Sigma), rabbit anti-Erk2 (1:1000, Cell Signaling), rabbit anti-pAkt (1:1000, Cell Signaling), and rabbit anti-AKT (1:1000, Cell Signaling) and analyzed using the ECL Plus system (GE Healthcare) and a FUJIFILM LAS-4000 luminescent analyzer.
For immunofluorescence, Xenopus embryos were fixed in MEMFA for 2 h at RT, bisected with a razor blade, and stored in Dent’s fixative (80%MetOH + 20%DMSO). For confocal analysis, the embryo pieces were rehydrated though a methanol series, blocked with BBT (PBS, 1%bovine albumin, 0.1%Triton) for 2 h and BBT with 5% serum for 1 h, incubated with primary antibody overnight at 4°, washed in PBS 0.2%Triton, incubated with secondary antibody overnight at 4°, washed again, dehydrated in methanol, then cleared with a Benzyl Benzoate and Benzyl Alcohol mix (2:1). For immunofluorescence, the following antibodies were used, primary: rabbit anti-dpErk1/2 (1:300, Cell Signaling), mouse anti-GFP (1:300, Clonetech), rabbit anti-phospho-Histone H3 (1:300, Cell Signaling), and rabbit anti-active-Caspase 3 (1:300, Cell Signaling); and secondary antibodies: anti-rabbit-CY5 (1:500, Jackson), anti-mouse-CY2 (1:500, Jackson) or goat anti-rabbit-AP (1:5000, Jackson).
Results
Pancreas, liver, and lung are specified at progressively later times in development through prolonged interactions with cardiac-lateral plate mesoderm
As a first step in characterizing the potential role of FGFs in Xenopus foregut organ induction we carefully examined when during development different foregut lineages were specified. Horb and Slack have previously demonstrated in Xenopus that mesodermal signals between stages NF15 to NF42 are required in order for the endoderm to become regionally specified and express pancreas, liver, and intestinal markers at stage NF42 [26]. In order to determine whether distinct lineages are specified at different times, we performed a series of microdissection experiments isolating ventral explants and removing the ventral cardiac/lateral plate mesoderm from the endoderm at different times in development (Figure 1A). Explants were cultured until stage NF37 (~40 ss) and analyzed by in situ hybridization for expression of early lineage markers of the pancreas (pdx1 and pft1a; [33,42]), liver (nr1h5; [32]) and lung/thyroid (nkx2.1; [35]) (Figure 1B, C). As controls to verify effective separation of the endoderm from mesoderm tissue, we examined the expression of the pan-endodermal marker gjb1[38], the lateral plate mesoderm marker foxf1[37], and the cardiac mesoderm marker tnni3[36] (Figure 1C). These controls demonstrated that our method of removing the mesoderm by dispase treatment and manual pealing off the tissue with hairloops effectively produced endoderm explants without foxf1+ and tnni3+ mesoderm.
These experiments showed that the expression of early pancreas, liver, or lung markers in the endoderm required mesoderm contact for different periods of time. Interestingly, we observed the pancreas-duodenum marker pdx1 was expressed in explants >75% of the time regardless of when the mesoderm was removed between stages NF16-35 (Figure 1B,C). We obtained similar results with another pancreas marker ptf1a, suggesting that as early as stage NF16 the endoderm has received sufficient signals to activate expression of pancreatic progenitor markers by stage NF35. In contrast, expression of the lung and liver markers required longer durations of mesodermal contact. Expression of the liver marker nr1h5 required mesoderm contact until stages NF25-28, after which point the mesoderm was no longer required (Figure 1B,C). In contrast, nkx2.1 expression was not induced in endoderm explants unless mesoderm was kept in contact throughout stage NF35 (36 ss; Figure 1B,C). In explants cultured with mesoderm through stage NF35, the nkx2.1+ tissue was observed in two discreet domains immediately dorsal-posterior to the heart, indicative of lung tissue. We conclude that the pancreas, liver, and lungs are specified at progressively later times in development in a caudal-to-rostral progression along the A-P axis. The most caudal tissue the pancreas is specified first, followed by liver, which requires mesoderm contact until NF31 and then the most rostral organ the lung is specified last requiring mesoderm contact up to NF35.
FGF signaling is active in the Xenopus foregut endoderm during organ induction
Our tissue separation experiments show that complete organ induction requires mesodermal contact between stages NF16-35. A survey of the literature indicates that many FGF ligands (FGF1-4,6,8,9,14,19,22,23) and receptors are expressed in the Xenopus foregut region during this time in development [27,43]. To investigate if and when the Xenopus ventral foregut endoderm is responding to FGF signaling we examined di-phosphorylated Erk1/2 (pErk) immunostaining as a read-out of active FGF/MEK signaling. In the gastrula embryo, pErk was not detected in the endoderm and was restricted to the involuting mesoderm, as previously described [44]. We first detected a low level of pErk in the anterior mesendoderm at stage NF15 as it migrates to its final position in the ventral foregut (Figure 2A). Between stages NF19-28 robust pErk was present in the ventral foregut progenitors and in the adjacent cardiac and lateral plate mesoderm (Figure 2B-D). By stage NF35 when lung and liver specification markers begin to be expressed pErk is detected in the thickening hepatic epithelium and the nascent lung buds, as well as in the heart and lateral plate mesoderm (Figure 2E, F). In stage NF42 gut tubes, we detected pErk in the liver and pancreatic buds, stomach, and the distal tips of the lung buds consistent with a role for FGF signaling during organ bud outgrowth (Figure 2G, H). This data reveals that between stages NF15-35, the period when mesoderm is required for liver and lung specification, the endoderm is experiencing active pErk signaling.
Figure 2.
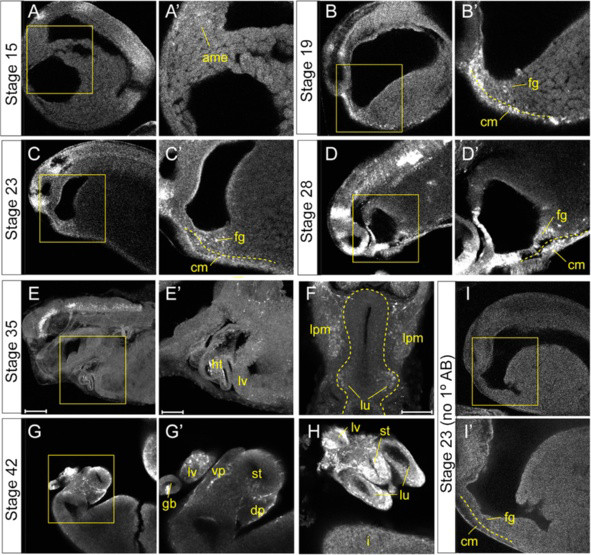
FGF signaling is active in the foregut endoderm during organ induction. Confocal immunostaining of bisected Xenopus embryos show active FGF-MEK signaling with di-phospho ERK1/2 (pErk) (white) in the developing foregut tissue at the indicated stages (anterior left, ventral down). Images are 10X magnification with independent scans of the boxed regions at 20X magnification. (A) Stage NF15 embryos show a low level of FGF signaling in the migrating anterior mesendoderm (ame). (B) Stage NF19, (C) NF23 and (D) NF28 show pErk staining in the ventral foregut endoderm (fg) as well as the underlying cardiac mesoderm (cm). The dashed yellow line indicates the boundary between the endoderm and mesoderm. (E) Mid-sagittal and (F) transverse optical sections of stage NF35 embryos show pErk staining in the liver epithelium (lv), heart (ht) and nascent lung buds (lu) and lateral plate mesoderm (lpm). (G and H) Stage NF42 gut tubes show pErk in liver bud (lv), gal bladder (gb), dorsal (dp) and ventral pancreatic buds (vp), the stomach (st) and the distal tips of the lung buds (lu). (I) Stage NF23 control embryos with no primary antibody.
FGF signaling induces lung and liver, and represses early pancreas fate
Multiple FGF ligands and receptors are expressed in the Xenopus foregut during organ induction [27,43]. In order to inhibit all FGF signaling in the foregut endoderm, we cultured embryos from stage NF18 to NF35 with a small molecule inhibitor PD173074 (FGFRi), which blocks FGF receptor activity [45] and analyzed them for mature liver (nr1h5), pancreas (ptf1α), lung (nkx2.1), and heart (tnni3) markers (Figure 3A). Western blot analysis confirmed that FGFRi treatment dramatically reduced FGF/pErk and FGF/pAkt activity (Figure 3B) and blocked expression of the FGF target gene spry2 (Figure 3A). FGFRi treatment resulted in a dramatic reduction or complete loss of liver (97% of embryos, n = 104) and lung (71%, n = 34) marker expression (Figure 3 A,C). We did not observe any obvious impact on nkx2.1 expression in the thyroid region. This FGF requirement in lung development is similar to that recently described by Wang et al.[46]. In contrast, expression of the early pancreas marker ptf1α was only reduced in 37% (n = 56) of the FGF-inhibited embryos, suggesting that pancreas specification requires little if any FGF signaling during these stages (Figure 3 A,C). Treatment with a second independent FGF receptor inhibitor SU5402, or injection of RNA encoding a dominant negative FGF receptor (dnFGFR) into the presumptive foregut mesendoderm at the 4–8 cell stage [47] caused a similar loss of liver and lung gene expression at stage NF35, with little if any impact on the pancreas gene expression relative to controls (Figure 3C, data not shown).
Figure 3.
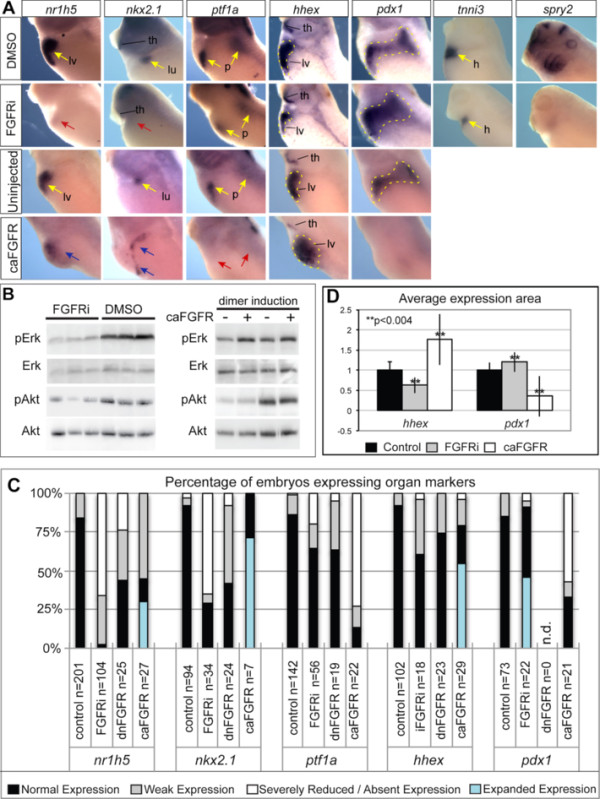
FGF signaling is required for lung and liver specification at the expense of pancreas. (A) In situ hybridization with the indicated probes in control, FGF-inhibited or FGF-activated embryos. Embryos were cultured in DMSO or PD173074 (FGFRi) from NF18 to NF35 to inhibit FGF signaling. Control uninjected or embryos injected with RNA encoding an inducible FGF receptor (caFGFR; 20 pg) into vegetal blastomeres at the 4/8-cell stage, were treated with the homodimerizing drug from NF18 to NF35 to activate FGF signaling. Yellow arrows indicate normal expression, red arrows absent expression, blue arrows ectopic expression; yellow dash outlines expression boundaries. p; pancreas, lv; liver, lu; lung, th; thyroid, h; heart. (B) Western blot analysis of pErk, total Erk, pAkt and total Akt in embryos cultured in either PD173074 (FGFRi) or DMSO from stage NF18 to NF35 (in triplicate). Western blot analysis of embryos injected with caFGFR(+) and treated from NF18 to NF35 with B/B Homodimerizer show increased FGF/pErk signaling compared to uninjected controls at Stage NF 23 (lanes1 + 2) and Stage NF 35 (lanes3 + 4). (C) Summary of gene expression in FGF-inhibited (FGFRi or dnFGFR; 3 ng) and FGF-activated (caFGFR; 20 pg) embryos. Controls include DMSO treated, β-gal injected and uninjected B/B dimerizing drug treated, none of which had an obvious impact on gene expression. (D) Quantification of average hhex and pdx1 expression areas in control and FGF-manipulated embryos. ImageJ software was used to measure the expression area with the average expression area of controls set to 1. Averages are based on n > 13 embryos for each condition and marker from at least two independent experiments, standard deviation and significance based on t-test (**p < 0.004).
To test whether FGF signaling was sufficient to induce lung and liver fate we cultured foregut endoderm explants lacking mesoderm with recombinant FGF2, but this was not sufficient to induce nr1h5 or nkx2.1 expression (Additional file 1: Figure S1). This suggests that other mesodermal signals in additional to FGFs are also required, the most likely candidate being BMPs, which we have recently shown is also required to maintain foregut progenitors [48]. To overcome this complication, we injected the presumptive foregut mesendoderm with a drug inducible FGFR1 construct (caFGFR) [49]. When injected embryos were treated from stage NF18 to NF35 with the drug “B/B-Homodimerizer” (Clontech) this causes receptor clustering and ligand-independent FGFR signaling as measured by increased pErk levels (Figure 3B). Activation of the caFGFR caused an expansion of liver (nr1h5) and lung (nkx2.1) expression domains in 30% and 75% of the embryos respectively (Figure 3A,C). In contrast, the pancreas marker ptf1α was reduced or absent in 86% of the activated caFGFR embryos (Figure 3A,C).
We next wanted to determine what became of the presumptive liver and lung foregut endoderm when FGF signaling was blocked. One possibility was that it adopted a more posterior fate, but analysis of FGFRi embryos with intestinal markers indicated that this was not the case (data not shown). We next tested whether FGF signaling was required to maintain early foregut progenitor identity prior to lineage segregation. We therefore examined the expression of hhex, which is initially expressed throughout the foregut endoderm, but then becomes restricted to the liver and thyroid as organ induction proceeds [39]. However, in embryos treated with the FGFRi from stage NF15 to NF23, or embryos injected with dnFGFR RNA we found that hhex expression was largely unaffected at stage NF23 (Additional file 2: Figure S2A), indicating that FGF signaling is not required to maintain the foregut progenitors at this stage. In addition when we examined hhex expression in embryos treated with FGFRi from NF18-35; hhex was still present in most embryos, although the size of the expression domain was significantly reduced (Figure 3A,C,D). This result was in contrast to the liver specification marker nr1h5, which was absent in most FGFRi embryos, suggesting that when FGF signaling is inhibited the foregut cells are blocked in a progenitor-like state.
We also closely examined pdx1 expression because mouse explant experiments suggest that in the absence of cardiac mesoderm or absence of FGF, the foregut adopts a pdx1+ pancreatic/duodenal fate rather than liver fate [12]. In Xenopus pdx1 is expressed in the pancreatic/duodenal progenitors prior to the expression of ptf1a. FGFRi treatment caused a modest, but significant expansion in the size of the pdx1+ expression domain (Figure 3A,C,D). Examination of hhex and pdx1 in embryos where FGF signaling was ectopically activated by the caFGFR revealed the opposite phenotype with a significant expansion of hhex and a reduced or absent pdx1 expression domain (Figure 3A,C,D).
Together these results indicate that FGF signaling is required for hepatic specification from hhex + progenitors. The data further suggests that similar to what has been reported in mice, FGF regulates the choice of Xenopus endoderm progenitors cells to adopt liver versus pancreas fates.
FGF signaling is required autonomously in the endoderm
The Xenopus lateral plate and pre-cardiac mesoderm is also patterned by FGF signaling [50-52]. Since the cardiac mesoderm, in particular, is implicated in foregut organ induction it was possible that the effects on endoderm gene expression we observed in FGF-inhibited embryos were secondary to mesodermal changes. We therefore examined the expression of the cardiac progenitor marker nkx2.5 at stage NF23 and the myocardial differentiation marker tnni3 at stage NF35. Although nkx2.5 and tnni3 expression were reduced in approximately 50% of FGF-inhibited embryos, both heart markers were almost always present (Figure 3A; Additional file 2: Figures S2A and Additional file 3: Figure S3A). This suggested that impaired cardiac development was unlikely to completely explain the loss of liver and lungs.
To directly test the cell-autonomous need for FGF signaling in foregut endoderm progenitors, we injected RNA encoding GFP along with dnFGFR or β-galactosidase (β-gal) RNA into the D2.1 blastomeres at the 16-cell stage, which targets the RNA to the foregut endoderm, and avoids the mesoderm. Injection of the dnFGFR into the mesoderm frequently causes gastrulation defects [47] and we observed this in about 50% of our 4-cell stage injections in Figure 3. In contrast the foregut-targeted embryos gastrulated normally and the lineage label showed that the anterior mesendoderm migrated correctly to the ventral foregut position (Figure 4A). Immunostaining of injected embryos confirmed that dnFGFR/GFP expressing cells lack robust pErk activity at stage NF23 (Figure 4A). We then scored isolated gut tubes at stage NF42 to determine which tissues the lineage labeled cells had contributed.
Figure 4.
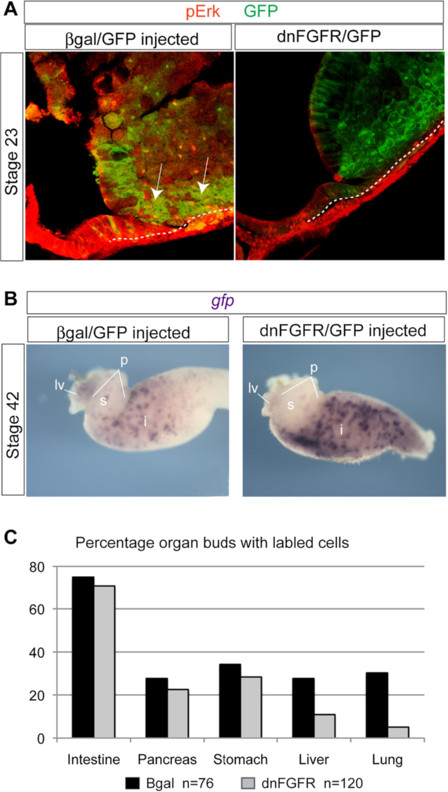
Inhibition of FGF signaling in Xenopus embryos. (A) Confocal immunostaining of pErk (red) and a GFP lineage tracer (green) in the foregut region of bisected stage NF23 Xenopus embryos that were injected into the presumptive foregut endoderm cells at the 16-cell stage with RNA encoding either β-gal (3 ng) and GFP or dnFGFR (3 ng) and GFP. The β-gal/GFP injected embryos show pErk in GPF + cells whereas in dnFGFR/GFP injected embryos pErk is undetectable in GFP + foregut cells. (B) A representative stage NF42 gut tube from βgal/GFP and dnFGFR/GFP injected embryos assayed by in situ hybridization for gfp RNA to show mosaic contribution of labeled cells to different organ buds. p; pancreas, lv; liver, s; stomach, i; intestine. (C) Summary showing percentage of organ buds containing labeled cells indicates a decreased contribution to the lung and liver buds in dnFGFR injected embryos (n = 120) compared to βgal controls (n = 70).
The most severe phenotype in these experiments was a complete loss of all discernable foregut organ buds, which never occurred in controls (data not shown); consistent with foregut endoderm requiring FGF signaling to induce organ lineages. In gut tubes with mosaic expression of labeled cells, we found that control β-gal/GFP cells contributed to the liver in 28% and the lung in approximately 30% of embryos, whereas dnFGFR/GFP-expressing cells only contributed to the liver in 11% and the lung in 5% of embryos (Figure 4B, C). Importantly, gfp positive cells were not detected in the cardiac mesoderm, verifying that the RNA was specifically targeted to the endoderm. This excludes the possibility that the defects in liver and lung contribution were only secondary to cardiac defects and demonstrates a cell-autonomous requirement for FGF signaling in the endoderm. There was little difference in contribution of control βgal or dnFGFR expressing cells to the intestine, pancreatic buds, or stomach (Figure 4B, C), suggesting that robust FGF signaling is not required for cells to populate these tissues. We conclude that ventral foregut endoderm progenitors that cannot receive an FGF signal are less likely to contribute to lung and liver buds.
Prolonged FGF signaling is required for lung and liver induction
In vitro mouse explant experiments using high doses of recombinant FGF signaling induce Nkx2.1 expressing lung tissue, whereas moderate doses of FGF induce liver-specific gene expression [8]. To test whether Xenopus liver and lung fate exhibit a similar dose-dependent need for FGF signaling in vivo we treated embryos with different concentrations of the FGFRi from stages NF18 to NF35. This resulted in the expected dose-dependent reduction in endogenous pErk (data not shown). However, both liver (nr1h5) and lung (nkx2.1) genes exhibited a similar dose responsive reduction in expression (Additional file 2: Figure S2B), suggesting that the level of FGFR activity per se does not regulate lung versus liver fate in Xenopus. We therefore considered the alternative hypothesis that FGF signaling might induce the liver and lungs at different times in development. To test these temporal requirements for FGF signaling, we treated embryos for different time periods with the FGF receptor inhibitor (Figure 5A) and scored liver and lung specification at stage NF35 (Figure 5B).
Figure 5.
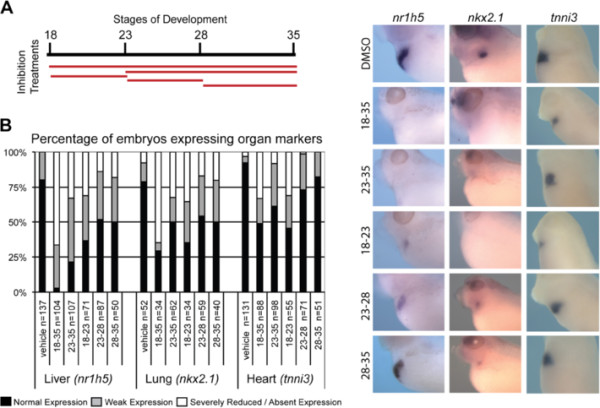
Prolonged FGF signaling is required for lung and liver induction. (A) Experimental design showing the time-course of FGFRi treatment. (B) Embryos treated with DMSO or FGFRi at the indicated stages were assayed at stage NF35 by in situ hybridization for markers of liver (nr1h5), lung (nkx2.1), and heart (tnni3). Representative examples are shown and the graph summarizes the % of embryos with normal, weak, or severely reduced/absent expression.
We found that inhibition of FGF signaling for shorter periods of time (NF18-23, NF23-28, and NF23-25) all resulted in a reduction in liver and lung marker expression, which was less dramatic than when FGF signaling was inhibited for the entire NF18-35 period (Figure 5B). We noticed that the level of reduction in nkx2.1-expressing lung tissue was similar in the various intermediate treatments, whereas the reduction in liver gene expression was more dramatic at earlier time points. These results are consistent with the tissue separation experiments (Figure 1), which indicate that the liver is specified earlier than the lung. Cardiac tnni3 expression was mildly reduced across FGF inhibition treatments, with the long and early periods showing the most effects (Figure 5B, Additional file 3: Figure S3A) [50]. These data suggest that prolonged FGF signaling throughout the organ induction period is necessary to specify both liver and lung, and that lung fate requires the longest duration of active FGF signaling.
PI3K and MEK branches both contribute to lung and liver development
There is evidence from mouse foregut explants that different branches of FGF signaling play distinct roles in foregut development, with MEK being required for liver gene expression whist the PI3K branch regulates proliferation [9]. To determine if a distinct FGF signaling pathways were required for foregut lineages in Xenopus, we cultured the embryos in either LY294002 (a PI3K inhibitor) or U0126 (a MEK1/2 inhibitor) [53,54] from stage NF18 to NF35 and analyzed the foregut organ lineage markers. Western blot analysis confirmed that PI3K inhibition (PI3Ki) resulted in a significant decrease in phospho-Akt (pAkt), whereas MEK1/2 inhibition (MEKi) resulted in a significant decrease in pErk compared to vehicle treated controls (Figure 6A). PI3Ki and MEKi treatments caused reduced or absent liver expression in 62% or 34% of the embryos respectively, which was not as dramatic as the reductions caused by FGFRi treatment (Figure 6B,C), suggesting that both the PI3K and MEK branches are both involved in Xenopus hepatic induction. Nkx2.1 expression was reduced or absent in 83% of PI3Ki embryos, similar to FGFRi, but in only 45% of MEKi embryos, suggesting prolonged PI3K activity is particularly important in lung specification (Figure 6B,C). If we removed the FGFRi at NF35 and isolated embryonic gut tubes at stage NF42, 46% of the lung and 75% of the liver buds were dramatically reduced in size, while the pancreas, stomach, and intestine were largely unaffected (Figure 6B). In comparison, the PI3Ki or MEKi treatments only caused modest reductions in foregut organ bud size at NF42, which were not as dramatic as those seen with the FGFRi (Figure 6B). We also tested whether PI3K or MEK were required at different times in development by treating embryos for shorter time periods. Similar to the results with the FGFRi, treatment with either MEKi or PI3Ki for a variety of shorter durations between stage NF18-35 all resulted in a modest reduction in lung and liver gene expression which was less severe than the FGFRi treatment over the similar period; and caused little change in pancreas specification consistent with the FGFRi data (Additional file 3: Figure S3A). This data suggests there is not a specific time when the PI3K or MEK branches act, but support a duration model where both branches are necessary over a prolonged period (NF18-35).
Figure 6.
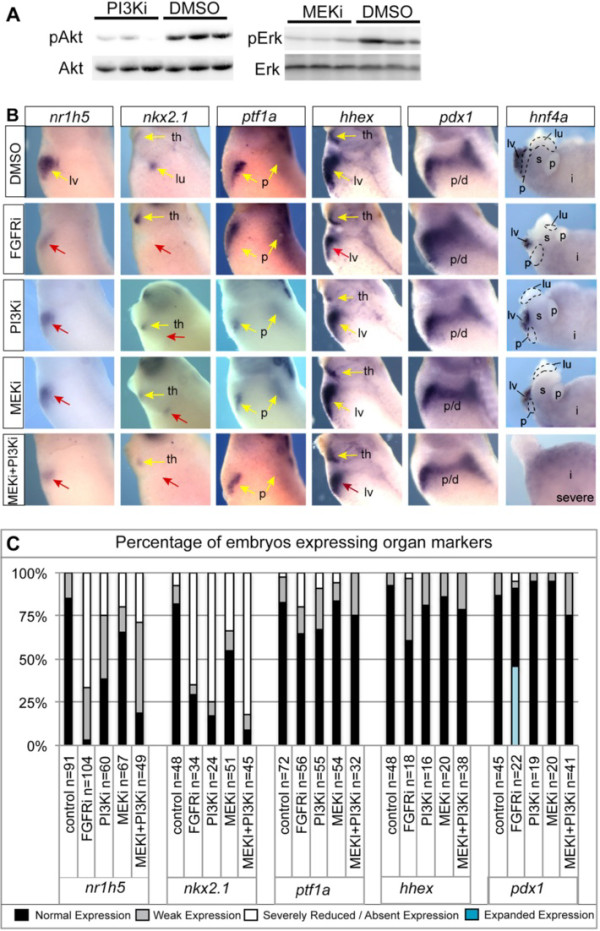
Both PI3K and MEK signaling contribute to lung and liver development. (A) Western blot analysis of embryos cultured in DMSO, PI3Ki (LY294002), or MEKi (U0126) from stages NF18 to NF35 in triplicate shows a dramatic decrease in pAkt and pErk levels with PI3Ki and MEKi treatment respectively. (B) Embryos cultured in DMSO or the indicated inhibitors from NF18 to NF35 were analyzed at stage NF35 for liver (nr1h5), lung (nkx2.1), pancreas (ptf1α), liver/thyroid (hhex), and pancreatic/duodenal (pdx1) expression. Embryos cultured in DMSO or the indicated inhibitors from NF18 to NF35 were analyzed at stage NF42 for organ bud appearance with hnf4α-stained gut tubes. Yellow arrows indicate normal expression, red arrows indicate reduced or absent expression. Foregut organ buds are outlined in dashed lines (lu; lung, p; pancreas, lv; liver, s; stomach, i; intestine, th; thyroid, p/d; pancreas/duodenum). (C) Summary of the percentage of inhibited embryos with expanded, normal, weak, or severely reduced/absent foregut marker expression compared to controls with the number of embryos analyzed listed for each condition. FGFRi data is repeated from Figure 3 for comparison.
The fact that PI3Ki and MEKi both resulted in moderate phenotypes less severe than FGFRi treatment suggests that PI3K and MEK branches act in parallel. To directly test the dual need for PI3K and MEK signaling, we treated embryos with both inhibitors and observed a dramatic loss of liver and lung markers similar to FGFRi treatment (Figure 6B,C). Additionally, although many MEKi + PI3Ki embryos died by stage NF42, some remaining embryos exhibited severe foregut organ agenesis (Figure 6B). Treatment with PI3Ki, MEKi, or MEKi + PI3Ki did not disrupt expression of hhex or pdx1 in the embryos at stage NF35 (Figure 6B,C), with the exception of a modest decrease in the hhex expression area in PI3Ki treated embryos similar to FGFRi (Additional file 3: Figure S3B). The observation that combined MEK + PI3Ki treatment did not expand pdx1 expression like the FGFRi embryos suggests either that another pathway downstream of FGFR represses pancreatic fate, and/or that PI3K and MEK have additional FGF-independent pro-pancreatic roles in the foregut (Figure 6B,C, Additional file 3: Figure S3B).
We therefore examined the impact that FGF, PI3K, and MEK inhibition had on proliferation and cell death. Embryos treated with FGFRi, PI3Ki, or MEKi did not exhibit significantly changed foregut cell proliferation at stages NF23 or NF35 relative to vehicle treated controls as measured by phospho-histone H3 (pHH3) (Additional file 4: Figure S4A, B). However, at stage NF42, when organ buds undergo extensive outgrowth [55], FGFRi and PI3Ki embryos exhibited a significant decrease in foregut cell proliferation (Additional file 4: Figure S4A,B). Analysis of apoptosis by activated caspase-3 immunostaining demonstrated that PI3Ki (but not FGFRi or MEKi) treatment increased foregut endoderm cell death at stage NF23 and NF35 (Additional file 4: Figure S4C,D). The fact that PI3Ki causes increased cell death, whereas the FGFRi did not supports the idea that additional FGF-independent PI3K-mediated signaling pathway(s) promote foregut cell survival.
We conclude that both the PI3K and MEK branches of FGF signaling contribute to lung and liver specification in Xenopus with the PI3K branch being particularly important for lung. In addition FGFR and PI3K regulate organ bud proliferation with FGF-independent PI3K activity also promoting Xenopus foregut cell survival.
Discussion
The role of FGF signaling in Xenopus foregut organ development has not been systematically examined to date. In this study we show that:
1. The pancreas, liver and lung are specified at progressively later times in development and that the liver and lung require progressively longer mesodermal contact between stages NF16-35;
2. Activated pErk, indicative of FGF signaling, is detected in the developing foregut endoderm throughout this period of development;
3. Prolonged FGF signaling via both the MEK and PI3K pathways is required for Xenopus lung and liver specification in vivo and this is at least in part a cell autonomous requirement in the endoderm;
4. FGF signaling regulates the allocation of hhex + liver progenitors versus pdx1+ pancreatic/duodenum progenitors.
Horb and Slack have previously shown that contact with the mesoderm between stages NF15-42 was essential for the endoderm to express liver and pancreas genes at stage NF42 [26]. Our work extends this study by examining if different lineages are specified at different time points during development and tests the role of FGF signaling in this process. Our data show that the liver and lung lineages are specified at progressively later times in development, requiring mesodermal contact for increasingly longer times. The critical period of mesodermal contact between stages NF16-35 correlates with the presence of pErk in the foregut endoderm and the necessity for prolonged FGF signaling for the expression of liver and lung, but not pancreas, specification markers. We demonstrated that specification of liver and lung lineages requires mesoderm contact from stages NF16-31 and NF16-35 respectively. Interestingly, we found that as early as stage NF16-19 endoderm explants without mesoderm were able to express pancreatic markers pdx1 and ptf1a at stage 35. This result contrasts with those of Horb and Slack who found that the mesoderm was required between stages NF25-42 for anterior endoderm to express pdx1 at stage NF42 when organ buds begin to form. This suggests that although we detect pdx1 expression in endoderm explants cultured without mesoderm at stage NF35, pancreatic fate might not yet be stably committed; thus mesodermal contact could be required through stage NF42 to maintain later pancreatic gene expression. This is consistent with the role of FGF/PI3K-mediated signaling promoting proliferation of a pdx1+ cell population.
Our data suggest that the prolonged requirement for the mesoderm in liver and lung specification can, at least in part, be accounted for by prolonged FGF signaling. Together with the recent report by Drysdale and colleagues who similarly found that FGF signaling was required for Xenopus lung development [46], our data demonstrate that the critical role for FGF signaling in liver and lung is highly conserved in vertebrates [3,6-10,56]. Our results are consistent with findings from chick [7,57] and mouse [8,10,12] explant cultures showing that mesodermal contact and FGF signaling are required for the specification of liver and lung. In addition, although the lateral plate mesoderm is clearly patterned by FGFs [50], our results targeting the dnFGFR to the foregut endoderm demonstrates a cell autonomous requirement for active FGF signaling in liver and lung development similar to endoderm-specific transgenic analysis in mice [9]. It is worth noting that while the molecular pathways are conserved between different species the relative timing of lineage specification is somewhat different. We show that liver and lung fate is specified in Xenopus between the 30–37 somite stages (ss), which is similar to zebrafish at 26–30 ss [58]; however in mouse and chick these fates are specified between the 7–10 ss [8,59]. We speculate that these differences could be influenced in part by the relatively early heart development in amniotes.
In vitro mouse foregut explants studies suggest that different doses of FGF segregate the foregut endoderm into pancreas, liver and lung lineages with little or no FGF being required for pancreas, intermediate FGF doses promoting liver and high FGF concentration promoting lung fate in the foregut explants [8]. Similarly hhex expression in hepatoblasts is FGF-dependent in zebrafish embryos [6] and chick explants [7]. Our results are in general agreement with these studies; we also found that FGF signaling promoted liver and lung and repressed pancreas. However we did not find any evidence supporting a dose requirement for liver versus lung induction in Xenopus, although we cannot totally rule out this mechanism. We also did not find discrete short periods in development when liver versus lung fate was induced. Rather our data support a duration model where prolonged FGF signaling is required to specify both liver and lung lineages, with the lung seeming to require a longer duration of FGF activity. Different durations of FGF signaling are important in the development of other systems as well, for instance, in lens epithelial cells it has been shown that low doses of FGF signaling (associated with a short duration of active pErk signaling) promote proliferation while high doses of FGF (associated with prolonged pErk signaling) promote fiber differentiation [60].
We also postulate that the spatial requirement for FGF signaling in vivo is also likely to be important factor in regulating the duration of exposure to a FGF signal. We observed that in some FGF inhibited embryos the residual liver gene (nr1h5) expression was immediately next to the cardiac mesoderm, suggesting liver gene expression was only induced in the cells closest to a source of FGF. It is important to point out that our experiments do not formally demonstrate that the mesoderm alone is the source of the FGF ligands; evidence from chick and Xenopus embryos shows the endoderm also expresses FGF ligands [7,52] and thus autocrine signaling within the endoderm could be involved.
Our data indicate that both the MEK and PI3K branches of the FGF response contribute to lung and liver induction. Inhibition of either branch resulted in intermediate phenotypes compared to FGFRi, while combining MEKi and PI3Ki caused a dramatic loss of mature liver and lung markers recapitulating the FGFRi treatment. Our data also indicate that the PI3K activity is important for cell survival and proliferation, and some of this activity appears to be FGF-independent, consistent with a report that pAkt signaling has an anti-apoptotic role in Xenopus stomach/pancreas development [29]. Our in vivo findings that both MEK and PI3K are involved in liver and lung specification differ somewhat from explant studies of the mouse liver, where the MEK branch is important for hepatic gene expression while the PI3K branch is important for explant growth [9].
We demonstrated that specification of liver and lung lineages requires mesoderm contact from stages NF16-31 and NF16-35 respectively and while our data suggest that prolonged FGF signaling accounts for this in part, it is likely that additional signals also differentially promote these lineages at later stages. Indeed BMP, Wnt and Retinoic acid are also required for foregut organogenesis [6,7,11,25,48,61,62] and these probably interact with the FGF pathway. Recent examples of such signaling cross talk have come from studies in zebrafish suggest that FGF signaling acts along the anterior-posterior axis to restrict the competence of the endoderm to respond to hepatic-inducing Wnt signals [13,14]. Consistent with other signaling factors acting in concert with FGFs, we found that exogenous FGF2 was not sufficient to support liver or lung fate in foregut explants lacking mesoderm, whereas in vivo, and presumably in the presence of other signals, activated caFGFR was sufficient to expand liver and lung at the expense of pancreas. This suggests either that different FGF ligands are specifically required for liver and lung induction and/or that other mesodermal signals are required to potentiate the inducing activity of FGF2. Candidates for additional signals include BMPs. It is also possible that the mesoderm provides important cell-cell or cell-ECM contacts that might be necessary to support foregut organ cell fate. Indeed, we have recently shown that fibronectin-dependent BMP signaling is required to maintain foregut progenitors [48]. In the future it will be important to better understand the mechanisms by which the FGF pathway interacts with other pathways during foregut organogenesis.
Conclusions
The Xenopus embryo is increasingly being used to study the development of endoderm derived organs, but the molecular basis foregut lineage specification is poorly understood. We demonstrate that the liver and lung lineages are specified at progressively later times in development requiring progressively longer mesoderm contact between stages NF15-35. We show that FGF signaling is active in the foregut endoderm at this time and that lung and liver induction requires prolonged FGF signaling through both the MEK and PI3K transduction pathways. We conclude that FGF-mediated foregut organ development in Xenopus is highly conserved with that described in mammals. Moreover our results highlight a previously unappreciated role for the temporal regulation of signaling during organ induction, which may impact strategies to direct the differentiation of stem cells.
Abbreviations
FGF: Fibroblast growth factor; FGFR: Fibroblast growth factor receptor; ERK: Extracellular-signal regulated kinase; pErk: Phosphorylated extracellular-signal regulated kinase; AKT: Protein kinase B; pAkt: Phosphorylated protein kinase B; MEK: Mitogen-activated protein kinase; PI3K: Phosphoinositide 3-kinase; BMP: Bone morphogenetic protein; NF: Nieuwkoop and faber stage; FGFRi: Fibroblast growth factor receptor inhibitor (PD173074); PI3Ki: Phosphoinositide 3-kinase inhibitor (LY294002); MEKi: Mitogen-activated protein kinase inhibitor (U0126); dnFGFR: Dominant negative FGF receptor; caFGFR: Inducible constitutively active FGF receptor; ss: Somite stage.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ETS performed most of the experiments and co-wrote the paper. APK performed the explant experiments and co-wrote the paper. SAR participated in the experimental design, performed some of the in situ analysis and co-wrote the paper. AMZ guided the project, helped design the experiments and co-wrote the paper. All authors read and approved the final manuscript.
Supplementary Material
Figure S1. Exogenous FGF is not sufficient to induce lung or liver lineages in explants. (A) Foregut explants with or without mesoderm were cultured from stage NF18 to NF35 in BSA or FGF2 and analyzed for expression of liver (nr1h5), lung (nkx2.1) and pancreas (pdx1) markers. (B) Western blot analysis of explants shows an increase in pErk levels upon FGF2 treatment.
Figure S2. FGF signaling is not required for maintaining foregut progenitors, but is required for lung and liver induction in a dose-dependent manner. (A) Embryos cultured in DMSO and FGFRi from stages NF15 to NF23 or injected with RNA encoding β-gal (3 ng) or dnFGFR (3 ng) were analyzed for expression of the foregut progenitor marker hhex and the cardiac progenitor marker nkx2.5. The graph summarizes the percentage of embryos with normal, weak or severely reduced/absent expression. (B) Embryos cultured from stages NF18 to NF35 in DMSO or FGFRi at the indicated concentrations and the percent of embryos with normal, weak, or severely reduced/absent nr1h5 and nkx2.1 expression was scored at stage NF35.
Figure S3. Prolonged MEK and PI3K signaling are required for a full lung and liver induction. (A) Percentage of embryos treated with either; DMSO, FGFRi, PI3Ki or MEKi for the indicated stages that exhibit with normal expression of nr1h5, nkx2.1, ptf1α, or tnni3 (n > 14 embryos for each condition and probe). Inhibition of MEK or PI3K over various intermediate durations results in a reduction in specification markers, suggesting that prolonged signaling is required for full foregut organ gene expression. (B) Quantification of average hhex and pdx1 expression areas in control and inhibited embryos. ImageJ software was used to measure the expression area with the average expression area of controls set to 1. Averages are based on n > 13 embryos for each condition and marker from at least two independent experiments, standard deviation and significance based on t-test (**p < .004) as indicated. FGFRi data is repeated from Figure 3 for comparison.
Figure S4. Cell Proliferation and apoptosis in FGF-inhibited embryos. (A) Analysis of cell proliferation by phospho-histone H3 (pHH3) immunostaining in embryos treated with DMSO, FGFRi, PI3Ki or MEKi. At stages NF23 (mid-sagittal) and NF35 (transverse section) embryos were assayed by 80 μM confocal Z-stack of pHH3 immunostaining (white dots), whereas stage NF42 isolated gut tubes were assayed by pHH3 immunohistochemistry (blue dots). Yellow dashed lines outline the foregut region quantified. (B) Summary of mean number of pHH3+ cells in the foregut region +/− SD (n > 5 embryos for each condition and stage). (C) Analysis of apoptosis by activated Caspase-3 immunostaining in embryos treated with DMSO, FGFRi, PI3Ki or MEKi. At stages NF23 (mid-sagittal) and NF35 (transverse section) embryos were assayed by 80 μM confocal Z-stack immunostaining (white dots). Yellow dashed lines outline the foregut region quantified. (D) Summary of mean number of active caspase-3+ cells in the foregut region +/− SD (n > 5 embryos for each condition and stage).
Contributor Information
Emily T Shifley, Email: emily.shifley@cchmc.org.
Alan P Kenny, Email: alan.kenny@cchmc.org.
Scott A Rankin, Email: scott.rankin@cchmc.org.
Aaron M Zorn, Email: aaron.zorn@cchmc.org.
Acknowledgments
We are grateful to Harv Issacs for the inducible FGFR construct. ETS was supported by an NIH Training Program in Perinatal Endocrinology T32 HD07463. APK was supported by a Procter Scholarship from Cincinnati Children’s Hospital Medical Center and by NIH grant K08 HL105661. This work was supported by NIH grant R01 DK078392 to AMZ.
References
- Dorey K, Amaya E. FGF signalling: diverse roles during early vertebrate embryogenesis. Development. 2010;137:3731–3742. doi: 10.1242/dev.037689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessimoz J, Opoka R, Kordich JJ, Grapin-Botton A, Wells JM. FGF signaling is necessary for establishing gut tube domains along the anterior-posterior axis in vivo. Mech Dev. 2006;123:42–55. doi: 10.1016/j.mod.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Shin D, Shin CH, Tucker J, Ober EA, Rentzsch F, Poss KD, Hammerschmidt M, Mullins MC, Stainier DY. Bmp and Fgf signaling are essential for liver specification in zebrafish. Development. 2007;134:2041–2050. doi: 10.1242/dev.000281. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yatskievych TA, Baker RK, Antin PB. Regulation of Hex gene expression and initial stages of avian hepatogenesis by Bmp and Fgf signaling. Dev Biol. 2004;268:312–326. doi: 10.1016/j.ydbio.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132:35–47. doi: 10.1242/dev.01570. [DOI] [PubMed] [Google Scholar]
- Calmont A, Wandzioch E, Tremblay KD, Minowada G, Kaestner KH, Martin GR, Zaret KS. An FGF response pathway that mediates hepatic gene induction in embryonic endoderm cells. Dev Cell. 2006;11:339–348. doi: 10.1016/j.devcel.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- Wandzioch E, Zaret KS. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science. 2009;324:1707–1710. doi: 10.1126/science.1174497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch G, Jung J, Zheng M, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- Shin D, Lee Y, Poss KD, Stainier DY. Restriction of hepatic competence by Fgf signaling. Development. 2011;138:1339–1348. doi: 10.1242/dev.054395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, Weidinger G, Moon RT, Stainier DY. Intrinsic and extrinsic modifiers of the regulative capacity of the developing liver. Mech Dev. 2012;128:525–535. doi: 10.1016/j.mod.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver M, Dunn NR, Hogan BL. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development. 2000;127:2695–2704. doi: 10.1242/dev.127.12.2695. [DOI] [PubMed] [Google Scholar]
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–4878. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S, Scharfmann R. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001;128:5109–5117. doi: 10.1242/dev.128.24.5109. [DOI] [PubMed] [Google Scholar]
- Jacquemin P, Yoshitomi H, Kashima Y, Rousseau GG, Lemaigre FP, Zaret KS. An endothelial-mesenchymal relay pathway regulates early phases of pancreas development. Dev Biol. 2006;290:189–199. doi: 10.1016/j.ydbio.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Nyeng P, Norgaard GA, Kobberup S, Jensen J. FGF10 signaling controls stomach morphogenesis. Dev Biol. 2007;303:295–310. doi: 10.1016/j.ydbio.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong PD, Munson CA, Norton W, Crosnier C, Pan X, Gong Z, Neumann CJ, Stainier DY. Fgf10 regulates hepatopancreatic ductal system patterning and differentiation. Nat Genet. 2007;39:397–402. doi: 10.1038/ng1961. [DOI] [PubMed] [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM. et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameri J, Stahlberg A, Pedersen J, Johansson JK, Johannesson MM, Artner I, Semb H. FGF2 specifies hESC-derived definitive endoderm into foregut/midgut cell lineages in a concentration-dependent manner. Stem Cells. 2010;28:45–56. doi: 10.1002/stem.249. [DOI] [PubMed] [Google Scholar]
- Johannesson M, Stahlberg A, Ameri J, Sand FW, Norrman K, Semb H. FGF4 and retinoic acid direct differentiation of hESCs into PDX1-expressing foregut endoderm in a time- and concentration-dependent manner. PLoS One. 2009;4:e4794. doi: 10.1371/journal.pone.0004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl EJ, Bilogan CK, Mukhi S, Brown DD, Horb ME. Xenopus pancreas development. Dev Dyn Off Pub Am Assoc Anat. 2009;238:1271–1286. doi: 10.1002/dvdy.21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Rankin SA, Sinner D, Kenny AP, Krieg PA, Zorn AM. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008;22:3050–3063. doi: 10.1101/gad.1687308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horb ME, Slack JM. Endoderm specification and differentiation in xenopus embryos. Dev Biol. 2001;236:330–343. doi: 10.1006/dbio.2001.0347. [DOI] [PubMed] [Google Scholar]
- Lea R, Papalopulu N, Amaya E, Dorey K. Temporal and spatial expression of FGF ligands and receptors during xenopus development. Developmental dynamics: an official publication of the American Association of Anatomists. 2009;238:1467–1479. doi: 10.1002/dvdy.21913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Jurgens K, Hollemann T, Claussen M, Ramadori G, Pieler T. Cell-autonomous and signal-dependent expression of liver and intestine marker genes in pluripotent precursor cells from xenopus embryos. Mech Dev. 2003;120:277–288. doi: 10.1016/s0925-4773(02)00460-4. [DOI] [PubMed] [Google Scholar]
- Wen L, Yang Y, Wang Y, Xu A, Wu D, Chen Y. App l1 is essential for the survival of xenopus pancreas, duodenum, and stomach progenitor cells. Developmental dynamics: an official publication of the American Association of Anatomists. 2010;239:2198–2207. doi: 10.1002/dvdy.22356. [DOI] [PubMed] [Google Scholar]
- Zorn AM, Butler K, Gurdon JB. Anterior endomesoderm specification in xenopus by Wnt/beta-catenin and TGF-beta signalling pathways. Dev Biol. 1999;209:282–297. doi: 10.1006/dbio.1999.9257. [DOI] [PubMed] [Google Scholar]
- Costa RM, Mason J, Lee M, Amaya E, Zorn AM. Novel gene expression domains reveal early patterning of the xenopus endoderm. Gene Expr Patterns. 2003;3:509–519. doi: 10.1016/s1567-133x(03)00086-3. [DOI] [PubMed] [Google Scholar]
- Seo YW, Sanyal S, Kim HJ, Won DH, An JY, Amano T, Zavacki AM, Kwon HB, Shi YB, Kim WS. et al. FOR, a novel orphan nuclear receptor related to farnesoid X receptor. J Biol Chem. 2002;277:17836–17844. doi: 10.1074/jbc.M111795200. [DOI] [PubMed] [Google Scholar]
- Wright CV, Schnegelsberg P, De Robertis EM. XlHbox 8: a novel xenopus homeo protein restricted to a narrow band of endoderm. Development. 1989;105:787–794. doi: 10.1242/dev.105.4.787. [DOI] [PubMed] [Google Scholar]
- Jarikji ZH, Vanamala S, Beck CW, Wright CV, Leach SD, Horb ME. Differential ability of Ptf1a and Ptf1a-VP16 to convert stomach, duodenum and liver to pancreas. Dev Biol. 2007;304(2):786–799. doi: 10.1016/j.ydbio.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small EM, Vokes SA, Garriock RJ, Li D, Krieg PA. Developmental expression of the xenopus Nkx2-1 and Nkx2-4 genes. Mech Dev. 2000;96:259–262. doi: 10.1016/s0925-4773(00)00400-7. [DOI] [PubMed] [Google Scholar]
- Drysdale TA, Tonissen KF, Patterson KD, Crawford MJ, Krieg PA. Cardiac troponin I is a heart-specific marker in the xenopus embryo: expression during abnormal heart morphogenesis. Dev Biol. 1994;165:432–441. doi: 10.1006/dbio.1994.1265. [DOI] [PubMed] [Google Scholar]
- Tseng HT, Shah R, Jamrich M. Function and regulation of FoxF1 during xenopus gut development. Development. 2004;131:3637–3647. doi: 10.1242/dev.01234. [DOI] [PubMed] [Google Scholar]
- Sinner D, Kirilenko P, Rankin S, Wei E, Howard L, Kofron M, Heasman J, Woodland HR, Zorn AM. Global analysis of the transcriptional network controlling xenopus endoderm formation. Development. 2006;133:1955–1966. doi: 10.1242/dev.02358. [DOI] [PubMed] [Google Scholar]
- Newman CS, Chia F, Krieg PA. The XHex homeobox gene is expressed during development of the vascular endothelium: overexpression leads to an increase in vascular endothelial cell number. Mech Dev. 1997;66:83–93. doi: 10.1016/s0925-4773(97)00092-0. [DOI] [PubMed] [Google Scholar]
- Tonissen KF, Drysdale TA, Lints TJ, Harvey RP, Krieg PA. XNkx-2.5, a xenopus gene related to Nkx-2.5 and tinman: evidence for a conserved role in cardiac development. Dev Biol. 1994;162:325–328. doi: 10.1006/dbio.1994.1089. [DOI] [PubMed] [Google Scholar]
- Holewa B, Strandmann EP, Zapp D, Lorenz P, Ryffel GU. Transcriptional hierarchy in xenopus embryogenesis: HNF4 a maternal factor involved in the developmental activation of the gene encoding the tissue specific transcription factor HNF1 alpha (LFB1) Mech Dev. 1996;54:45–57. doi: 10.1016/0925-4773(95)00460-2. [DOI] [PubMed] [Google Scholar]
- Afelik S, Chen Y, Pieler T. Combined ectopic expression of Pdx1 and Ptf1a/p48 results in the stable conversion of posterior endoderm into endocrine and exocrine pancreatic tissue. Genes Dev. 2006;20:1441–1446. doi: 10.1101/gad.378706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub R, Adelman Z, Clementi J, Weiss R, Bonasera J, Servetnick M. Evolutionarily conserved and divergent expression of members of the FGF receptor family among vertebrate embryos, as revealed by FGFR expression patterns in xenopus. Dev Genes Evol. 2000;210:345–357. doi: 10.1007/s004270000076. [DOI] [PubMed] [Google Scholar]
- Christen B, Slack JM. Spatial response to fibroblast growth factor signalling in xenopus embryos. Development. 1999;126:119–125. doi: 10.1242/dev.126.1.119. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Froum S, Hamby JM, Schroeder MC, Panek RL, Lu GH, Eliseenkova AV, Green D, Schlessinger J, Hubbard SR. Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. EMBO J. 1998;17:5896–5904. doi: 10.1093/emboj/17.20.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Deimling SJ, D'Alessandro NE, Zhao L, Possmayer F, Drysdale TA. Retinoic acid is a key regulatory switch determining the difference between lung and thyroid fates in xenopus laevis. BMC Dev Biol. 2011;11:75. doi: 10.1186/1471-213X-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Kenny AP, Rankin SA, Allbee AW, Prewitt AR, Zhang Z, Tabangin ME, Shifley ET, Louza MP, Zorn AM. Sizzled-Tolloid Interactions Maintain Foregut Progenitors by Regulating Fibronectin-Dependent BMP Signaling. Dev Cell. 2012;23(2):292–304. doi: 10.1016/j.devcel.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pownall ME, Welm BE, Freeman KW, Spencer DM, Rosen JM, Isaacs HV. An inducible system for the study of FGF signalling in early amphibian development. Dev Biol. 2003;256:89–99. doi: 10.1016/s0012-1606(02)00120-3. [DOI] [PubMed] [Google Scholar]
- Deimling SJ, Drysdale TA. Fgf is required to regulate anterior-posterior patterning in the xenopus lateral plate mesoderm. Mech Dev. 2011;128:327–341. doi: 10.1016/j.mod.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Keren-Politansky A, Keren A, Bengal E. Neural ectoderm-secreted FGF initiates the expression of Nkx2.5 in cardiac progenitors via a p38 MAPK/CREB pathway. Dev Biol. 2009;335:374–384. doi: 10.1016/j.ydbio.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Samuel LJ, Latinkic BV. Early activation of FGF and nodal pathways mediates cardiac specification independently of Wnt/beta-catenin signaling. PLoS One. 2009;4:e7650. doi: 10.1371/journal.pone.0007650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F. et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Horb LD, Horb ME. BrunoL1 regulates endoderm proliferation through translational enhancement of cyclin A2 mRNA. Dev Biol. 2010;345:156–169. doi: 10.1016/j.ydbio.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naye F, Voz ML, Detry N, Hammerschmidt M, Peers B, Manfroid I. Essential roles of zebrafish bmp2a, fgf10, and fgf24 in the specification of the ventral pancreas. Mol Biol Cell. 2012;23:945–954. doi: 10.1091/mbc.E11-08-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N, Houssaint E. Role of the mesoderm in the induction of the synthesis of glycogen during differentiation of the hepatic endoderm. C R Acad Sci Hebd Seances Acad Sci D. 1967;264:1872–1874. [PubMed] [Google Scholar]
- Field HA, Ober EA, Roeser T, Stainier DY. Formation of the digestive system in zebrafish. I. Liver morphogenesis. Dev Biol. 2003;253:279–290. doi: 10.1016/s0012-1606(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Gualdi R, Bossard P, Zheng M, Hamada Y, Coleman JR, Zaret KS. Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev. 1996;10:1670–1682. doi: 10.1101/gad.10.13.1670. [DOI] [PubMed] [Google Scholar]
- Iyengar L, Wang Q, Rasko JE, McAvoy JW, Lovicu FJ. Duration of ERK1/2 phosphorylation induced by FGF or ocular media determines lens cell fate. Differ Res in Biol Divers. 2007;75:662–668. doi: 10.1111/j.1432-0436.2007.00167.x. [DOI] [PubMed] [Google Scholar]
- Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Exogenous FGF is not sufficient to induce lung or liver lineages in explants. (A) Foregut explants with or without mesoderm were cultured from stage NF18 to NF35 in BSA or FGF2 and analyzed for expression of liver (nr1h5), lung (nkx2.1) and pancreas (pdx1) markers. (B) Western blot analysis of explants shows an increase in pErk levels upon FGF2 treatment.
Figure S2. FGF signaling is not required for maintaining foregut progenitors, but is required for lung and liver induction in a dose-dependent manner. (A) Embryos cultured in DMSO and FGFRi from stages NF15 to NF23 or injected with RNA encoding β-gal (3 ng) or dnFGFR (3 ng) were analyzed for expression of the foregut progenitor marker hhex and the cardiac progenitor marker nkx2.5. The graph summarizes the percentage of embryos with normal, weak or severely reduced/absent expression. (B) Embryos cultured from stages NF18 to NF35 in DMSO or FGFRi at the indicated concentrations and the percent of embryos with normal, weak, or severely reduced/absent nr1h5 and nkx2.1 expression was scored at stage NF35.
Figure S3. Prolonged MEK and PI3K signaling are required for a full lung and liver induction. (A) Percentage of embryos treated with either; DMSO, FGFRi, PI3Ki or MEKi for the indicated stages that exhibit with normal expression of nr1h5, nkx2.1, ptf1α, or tnni3 (n > 14 embryos for each condition and probe). Inhibition of MEK or PI3K over various intermediate durations results in a reduction in specification markers, suggesting that prolonged signaling is required for full foregut organ gene expression. (B) Quantification of average hhex and pdx1 expression areas in control and inhibited embryos. ImageJ software was used to measure the expression area with the average expression area of controls set to 1. Averages are based on n > 13 embryos for each condition and marker from at least two independent experiments, standard deviation and significance based on t-test (**p < .004) as indicated. FGFRi data is repeated from Figure 3 for comparison.
Figure S4. Cell Proliferation and apoptosis in FGF-inhibited embryos. (A) Analysis of cell proliferation by phospho-histone H3 (pHH3) immunostaining in embryos treated with DMSO, FGFRi, PI3Ki or MEKi. At stages NF23 (mid-sagittal) and NF35 (transverse section) embryos were assayed by 80 μM confocal Z-stack of pHH3 immunostaining (white dots), whereas stage NF42 isolated gut tubes were assayed by pHH3 immunohistochemistry (blue dots). Yellow dashed lines outline the foregut region quantified. (B) Summary of mean number of pHH3+ cells in the foregut region +/− SD (n > 5 embryos for each condition and stage). (C) Analysis of apoptosis by activated Caspase-3 immunostaining in embryos treated with DMSO, FGFRi, PI3Ki or MEKi. At stages NF23 (mid-sagittal) and NF35 (transverse section) embryos were assayed by 80 μM confocal Z-stack immunostaining (white dots). Yellow dashed lines outline the foregut region quantified. (D) Summary of mean number of active caspase-3+ cells in the foregut region +/− SD (n > 5 embryos for each condition and stage).


