Abstract
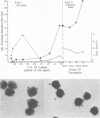
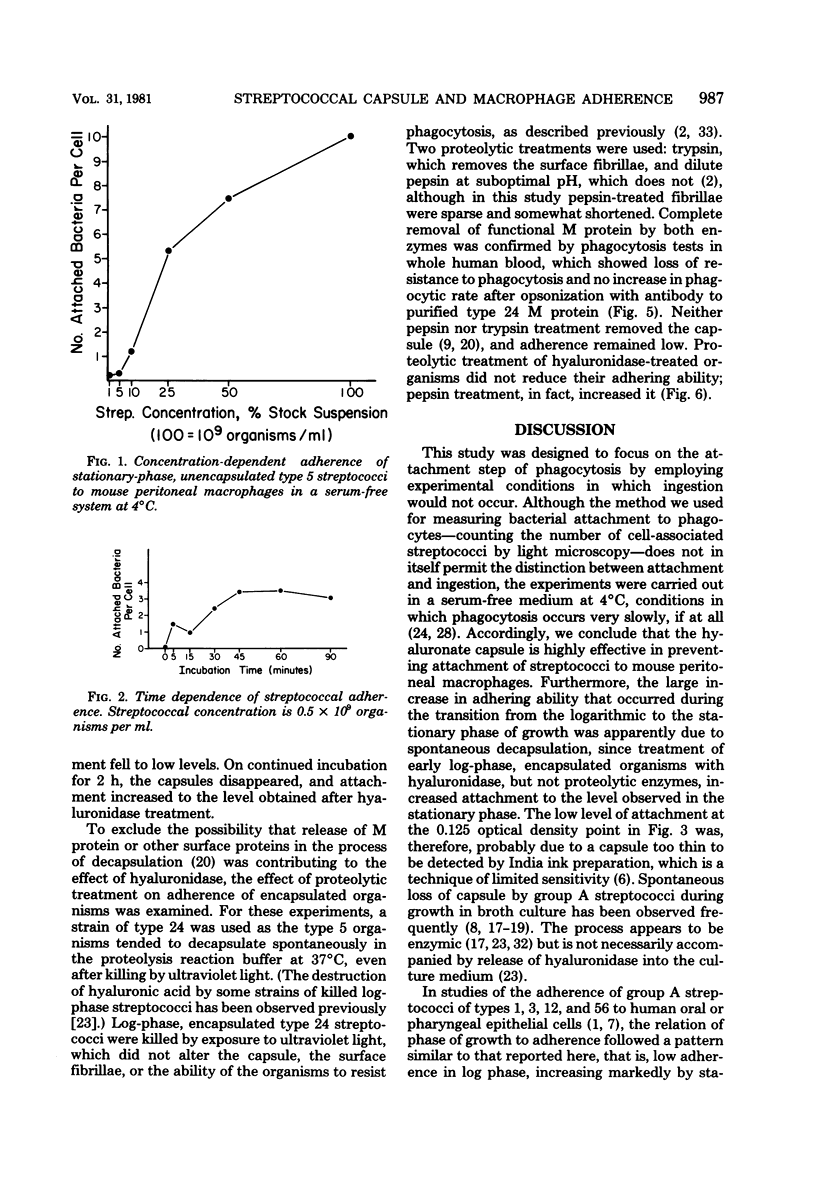
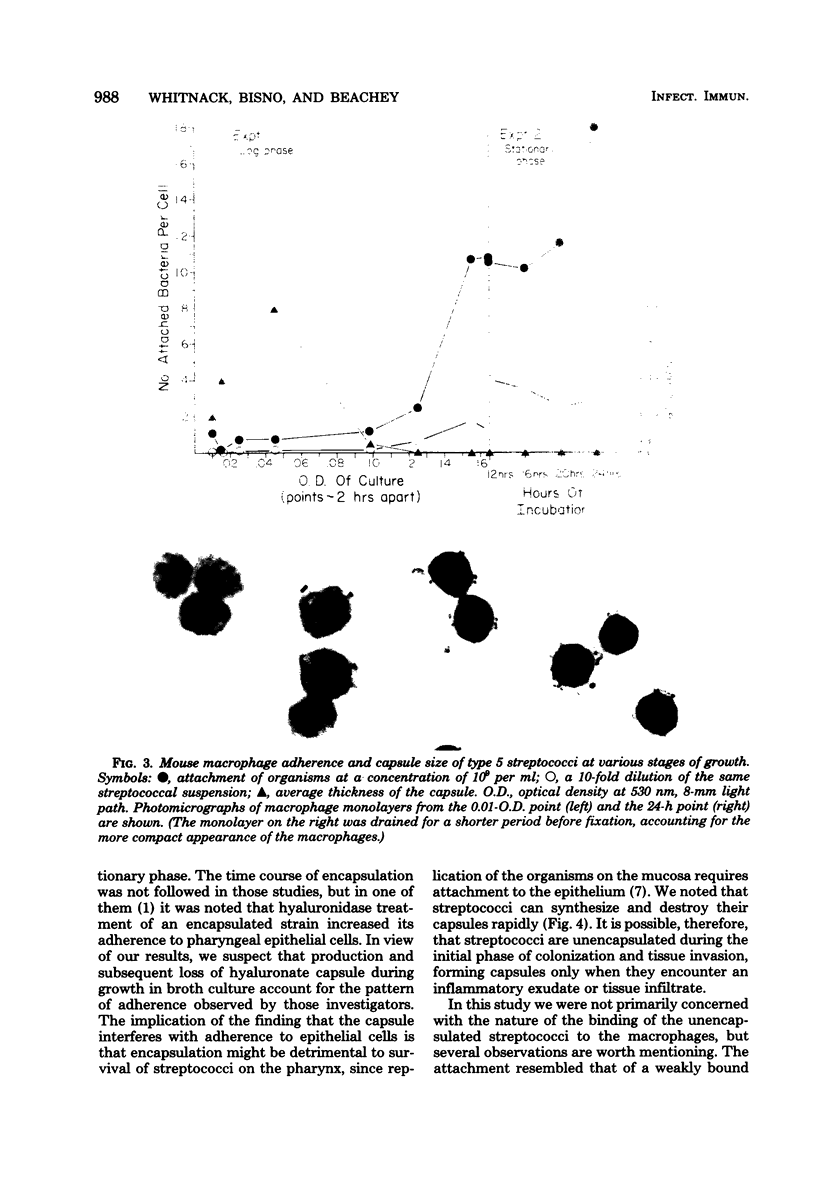
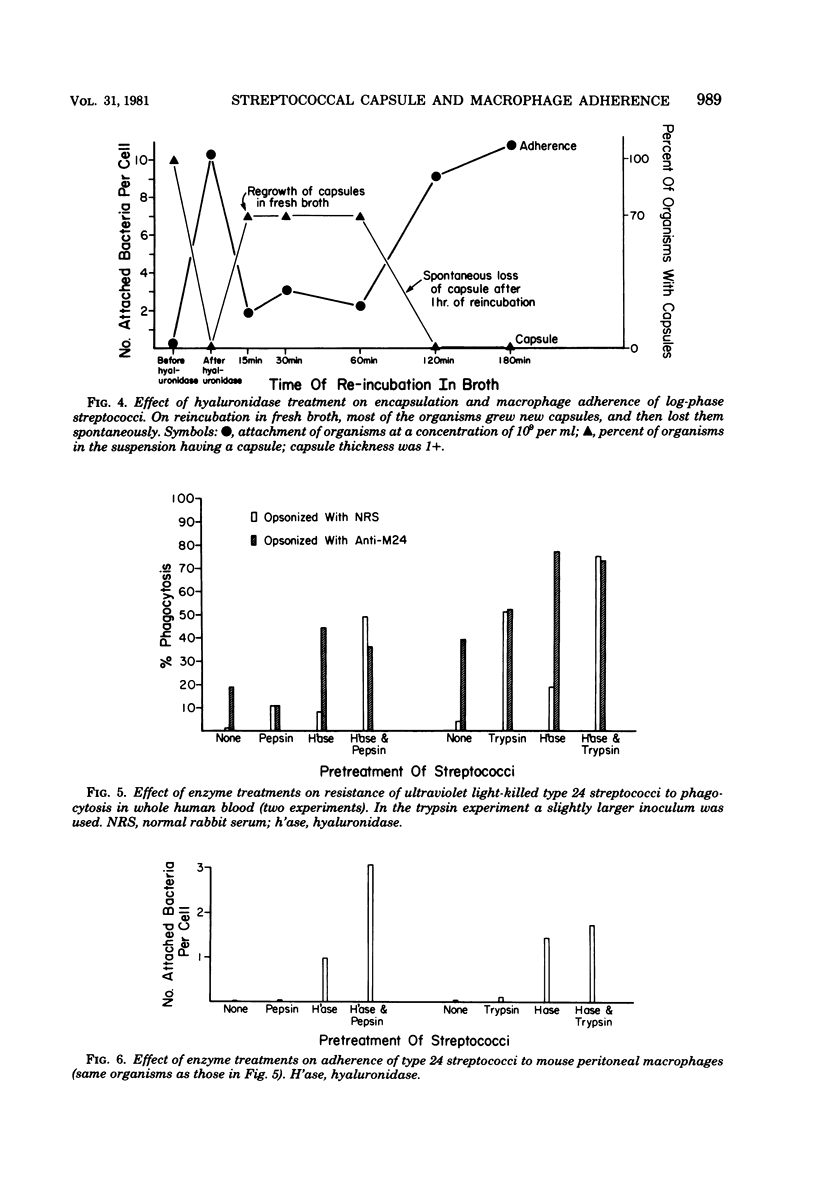
The antiphagocytic properties of the streptococcal hyaluronic acid capsule were explored in a system in which binding of the organism to the phagocyte, but not ingestion, could occur. The capsule was found to be highly effective in preventing attachment of two strains of group A streptococci to mouse peritoneal macrophages. Variation in attachment with phase of growth in broth culture (low in early log phase, high in late-log phase and thereafter) could be accounted for by production and subsequent loss of capsule. Hyaluronidase treatment removed the capsule and increased adherence; treatment with proteolytic enzymes removed M protein and decreased resistance to phagocytosis in whole human blood but did not remove the capsule or increase adherence to the mouse peritoneal macrophages.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartelt M. A., Duncan J. L. Adherence of group A streptococci to human epithelial cells. Infect Immun. 1978 Apr;20(1):200–208. doi: 10.1128/iai.20.1.200-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976 Apr 1;143(4):759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Stollerman G. H., Chiang E. Y., Chiang T. M., Seyer J. M., Kang A. H. Purification and properties of M protein extracted from group A streptococci with pepsin: covalent structure of the amino terminal region of type 24 M antigen. J Exp Med. 1977 Jun 1;145(6):1469–1483. doi: 10.1084/jem.145.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisno A. L. Alternate complement pathway activation by group A streptococci: role of M-protein. Infect Immun. 1979 Dec;26(3):1172–1176. doi: 10.1128/iai.26.3.1172-1176.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGUID J. P. The demonstration of bacterial capsules and slime. J Pathol Bacteriol. 1951 Oct;63(4):673–685. doi: 10.1002/path.1700630413. [DOI] [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Gonococcal interactions with polymorphonuclear neutrophils: importance of the phagosome for bactericidal activity. J Clin Invest. 1978 Dec;62(6):1161–1171. doi: 10.1172/JCI109235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. Parameters affecting the adherence and tissue tropisms of Streptococcus pyogenes. Infect Immun. 1974 Jan;9(1):85–91. doi: 10.1128/iai.9.1.85-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FABER V., ROSENDAL K. Streptococcal hyaluronidase. II. Studies on the production of hyaluronidase and hyaluronic acid by representatives of all types of hemolytic streptococci belonging to group A. Acta Pathol Microbiol Scand. 1954;35(2):159–164. [PubMed] [Google Scholar]
- FOLEY M. J., WOOD W. B., Jr Studies on the pathogenicity of group A streptococci. II. The antiphagocytic effects of the M protein and the capsular gel. J Exp Med. 1959 Oct 1;110:617–628. doi: 10.1084/jem.110.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. N. M proteins of group A streptococci. Bacteriol Rev. 1974 Mar;38(1):57–86. doi: 10.1128/br.38.1.57-86.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL M. J., JAMES A. M., MAXTED W. R. Some physical investigations of the behaviour of bacterial surfaces. VIII. Studies on the capsular material of Streptococcus pyogenes. Biochim Biophys Acta. 1963 Mar 19;66:264–274. doi: 10.1016/0006-3002(63)91193-4. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G., CHURCH A. B. Studies of phagocytosis of group A streptococci by polymorphonuclear leucocytes in vitro. J Exp Med. 1960 Mar 1;111:309–322. doi: 10.1084/jem.111.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980 Jan;65(1):82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- MACLENNAN A. P. The production of capsules, hyaluronic acid and hyaluronidase by group A and group C streptococci. J Gen Microbiol. 1956 Feb;14(1):134–142. doi: 10.1099/00221287-14-1-134. [DOI] [PubMed] [Google Scholar]
- MORRIS M., SEASTONE C. V. The relationship of M protein and resistance to phagocytosis in the beta hemolytic streptococci. J Bacteriol. 1955 Feb;69(2):195–203. doi: 10.1128/jb.69.2.195-203.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M. The dissociation of the attachment and ingestion phases of phagocytosis by macrophages. Exp Cell Res. 1967 Apr;46(1):19–28. doi: 10.1016/0014-4827(67)90405-3. [DOI] [PubMed] [Google Scholar]
- STOLLERMAN G. H., RYTEL M., ORTIZ J. Accessory plasma factors involved in the bactericidal test for type-specific antibody to group A Streptococci. II. Human plasma cofactor (s) enhancing opsonization of encapsulated organisms. J Exp Med. 1963 Jan 1;117:1–17. doi: 10.1084/jem.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger D. S., Reed W. P. Pneumococcal adherence to human epithelial cells. Infect Immun. 1979 Feb;23(2):545–548. doi: 10.1128/iai.23.2.545-548.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis: recognition and ingestion. Semin Hematol. 1975 Jan;12(1):83–116. [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. II. Contact angles and phagocytosis of encapsulated bacteria before and after opsonization by specific antiserum and complement. J Reticuloendothel Soc. 1972 Nov;12(5):497–502. [PubMed] [Google Scholar]
- WEINER L. M., SEASTONE C. V. Chemical inhibition of decapsulation in the beta hemolytic streptococcus. Proc Soc Exp Biol Med. 1951 Nov;78(2):466–469. doi: 10.3181/00379727-78-19105. [DOI] [PubMed] [Google Scholar]
- WILEY G. G., WILSON A. T. The ability of group A streptococci killed by heat or mercury arc irradiation to resist ingestion by phagocytes. J Exp Med. 1956 Jan 1;103(1):15–36. doi: 10.1084/jem.103.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]