Abstract
Background
In 2009, the American Society of Clinical Oncology recommended that patients with metastatic colorectal cancer (mCRC) who are candidates for anti-epidermal growth factor receptor (EGFR) therapy have their tumors tested for KRAS mutations because tumors with such mutations do not respond to anti-EGFR therapy. Limiting anti-EGFR therapy to those without KRAS mutations will reserve treatment for those likely to benefit while avoiding unnecessary costs and harm to those who would not. Similarly, tumors with BRAF genetic mutations may not respond to anti-EGFR therapy, though this is less clear. Economic analyses of mutation testing have not fully explored the roles of alternative therapies and resection of metastases.
Methods
This paper is based on a decision analytic framework that forms the basis of a cost-effectiveness analysis of screening for KRAS and BRAF mutations in mCRC in the context of treatment with cetuximab. A cohort of 50 000 patients with mCRC is simulated 10 000 times, with attributes randomly assigned on the basis of distributions from randomized controlled trials.
Results
Screening for both KRAS and BRAF mutations compared with the base strategy (of no anti-EGFR therapy) increases expected overall survival by 0.034 years at a cost of $22 033, yielding an incremental cost-effectiveness ratio of approximately $650 000 per additional year of life. Compared with anti-EGFR therapy without screening, adding KRAS testing saves approximately $7500 per patient; adding BRAF testing saves another $1023, with little reduction in expected survival.
Conclusions
Screening for KRAS and BFAF mutation improves the cost-effectiveness of anti-EGFR therapy, but the incremental cost effectiveness ratio remains above the generally accepted threshold for acceptable cost effectiveness ratio of $100 000/quality adjusted life year.
Overall survival of patients with metastatic colorectal cancer (mCRC) has considerably improved in the last decade (1). Improvements in the treatment of mCRC have reportedly led to six to eight months of survival with best supportive care alone, and more than twenty months with different lines of treatments (2). About 20% of patients with CRC present with synchronous colorectal liver metastases at diagnosis, and 80% to 90% of them are unresectable (3–6). Roughly, 14% to 16% of unresectable colorectal liver metastasis could become resectable with chemotherapy (7,8). Of those whose cancer metastasizes later, nearly 60% have metastases of the liver (3,9,10). Developments in surgery and chemobiologic therapies have allowed more patients to undergo hepatic resection, the potential cure for mCRC (11–13). Patients who have hepatic resections have a nearly 20% probability that they will be alive after 10 years (3,14).
Cetuximab, a monoclonal antibody acting against epidermal growth factor receptor (EGFR), was approved by the US Food and Drug Administration (FDA) in 2004 for use with irinotecan for the treatment for EGFR-expressing mCRC in patients refractory to irinotecan-based chemotherapy. Cetuximab is also approved for use as a single agent in patients with recurrent EGFR-expressing mCRC who do not tolerate irinotecan-based therapy. Approval was based on objective response rates. No data demonstrated an improvement in survival (15). In 2007, cetuximab received regular approval after findings from a multinational open-label trial by NCI-Canada in patients with EGFR-expressing mCRC following both oxaliplatin- and irinotecan-containing treatments. The trial found statistically significant differences in overall survival (6.1 months vs. 4.6 months) between patients randomly assigned to receive best supportive care only and those receiving both best supportive care and cetuximab (15). In 2009, retrospective subset analyses of trials with patients with mCRC whose tumors have codon 12 and 13 V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations noted a lack of benefit from cetuximab (and panitumumab, which is also a monoclonal antibody acting against EGFR that was approved in 2006) (16). Bevacizumab, a monoclonal antibody that inhibits the function of vascular endothelial growth factor (VEGF), was approved by the FDA in 2004, for first-line treatment in patients with mCRC. In 2006, following a trial (E3200) demonstrating a statistically significant difference in overall survival (13.0 months vs. 10.8 months) between patients on 5-FU + folinic acid + oxaliplatin (FOLFOX4) and bevacizumab and those on FOLFOX4 only, the FDA extended approval for its use as second-line therapy with 5-fluoroucil-based chemotherapy (17). On the other hand, the effect on overall survival of genetic mutations in the serine/threonine-protein kinase B-Raf (BRAF-V600, or BRAF) is unclear (4,18,19). Patients with BRAF mutations appear to have a poorer prognosis. Retrospective subset analyses suggest that, regardless of BRAF status, patients might benefit from anti-EGFR therapies in the first-line setting. There appears to be no benefit after the patient has progressed after first-line therapy (4). KRAS and BRAF mutations are mutually exclusive in individuals. Both carry additional potential harms (16,20).
In 2009, the American Society of Clinical Oncology issued a provisional clinical opinion recommending that all patients with mCRC who are candidates for anti-EGFR treatments have their tumors tested for KRAS mutations (21). Screening for and then limiting anti-EGFR therapy to those without KRAS mutations is aimed at providing the treatment to those who are likely to benefit from it while avoiding unnecessary costs and harm to those who are not likely to benefit. Anti-EGFR treatments are provided to patients with both curative and palliative intent. In the case of treatments administered with a curative intent, the only potential cure is resection of the metastases. Such a successful cure carries additional costs. The monoclonal antibody treatments, especially anti-EGFR therapy, are considerably more expensive than other existing treatments (22,23). In addition, for many patients without a KRAS mutation who do receive anti-EGFR treatment, there may be no meaningful gain in terms of length of survival and only harmful side effects and expenses. In the case of BRAF screening, an economic analysis is warranted to understand the effect of poorer prognosis and lack of effect following progression after first-line treatment.
Blank et al. (24) reports cost-effectiveness analyses of screening for KRAS and BRAF mutations in mCRC in the Swiss health systems. They estimated anti-EGFR therapy combined with screening costs at $83 147 (€62 653) per quality-adjusted life year (QALY) saved. For the United States, it has been estimated that KRAS testing would save more than $700 million compared with providing anti-EGFR screening to all mCRC patients, excluding the costs of additional resections (25). Another study has also advocated the use of screening for KRAS mutations, with very accommodating breakeven pricing of $3500 per screening (26). These studies lack transparency regarding how they analyze the treatments, resection of metastases, and survival for the different types of metastases. These aspects have major effects on the costs and effects in the analysis. This paper aims to evaluate the costs and effects of screening for KRAS and BRAF mutations in mCRC in the context of targeting the use of cetuximab to those without these mutations. The analysis uses the available evidence base, including the use of treatments, resection probabilities, and length of survival to create and calibrate a comprehensive analytical framework. Reported overall survival rates from different trials are used to develop best estimates to analyze the base case and conduct subsequent sensitivity analyses. The paper provides insight into the major costs in the treatment of mCRC, including resection costs, and clarifies the economic case for anti-EGFR therapy and associated screening in the United States.
Methods
This paper is based on a decision analytic framework. The framework forms the basis of a cost effectiveness analysis of undertaking screening for KRAS and BRAF mutations in mCRC in the context of treatment with cetuximab. The framework operates within a rich probability structure that “creates” individual patient profiles from probability distributions and passes them through treatment regimens to analyze costs and effects. The parameters in the analytical framework are based on the available evidence from clinical trials and other literature in clinical oncology. The framework is designed to provide answers to questions about the suitability of screening mCRC patients for KRAS and BRAF genetic mutations under varying circumstances.
Outcomes Evaluated
Our primary outcome of interest is overall survival, which, in a setting comparing a base strategy to alternatives, is measured in terms of life years saved (LYS) through the implementation of the alternatives. Average LYS and costs are calculated in 10 000 simulations, each with 50 000 mCRC patients. Separately, the two averages provide comparison of health impact and costs among strategies, and combined they provide the incremental cost-effectiveness ratio (ICER) in terms of US Dollars per LYS: ICER = (Cost of Strategy1 – Cost of Strategy2) / (Effect of Strategy1 – Effect of Strategy2).
Model Structure
We compared four strategies (Figure 1): No anti-EGFR therapy (best supportive care); anti-EGFR therapy without screening; screening for KRAS mutations only (before providing anti-EGFR therapy); and screening for KRAS and BRAF mutations (before providing anti-EGFR therapy). These strategies are evaluated in the context of two broad strategies of treatments: anti-EGFR and best supportive care, which can include all treatments other than anti-EGFR, such as chemotherapy, radiotherapy, anti-VEGF, and surgery. Anti-EGFR and anti-VEGF strategies or paths of treatment will include lines of treatment that might belong to the other types of strategies of treatments. None of these treatments by themselves can “cure” mCRC. Only complete resection may cure it. These treatments are aimed at either increasing the possibility of resecting the metastasis or providing palliative care. Although we include BRAF testing in the last strategy, evidence suggests that tumor BRAF mutation status does not predict response to treatment (like KRAS) but is simply a predictor of poor survival, independent of anti-EGFR therapy (4, 27). This strategy is included to test the potential contribution of BRAF testing if BRAF mutation proves to predict anti-EGFR therapy effectiveness.
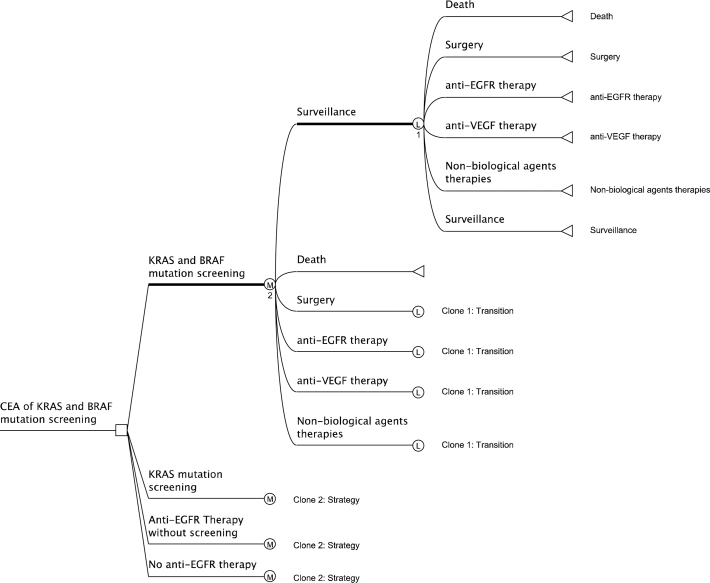
Figure 1.
Markov model of disease progression and treatment in metastatic colorectal cancer. Clone 1, Clone 2 are clone copies of the master clone subtrees 1 and 2, indicated by the respectively numbered heavy lines. The clone copies have the same structure as but can have different calculations from the master clone subtrees. Legend:  : Terminal node (the outcome because of following a path);
: Terminal node (the outcome because of following a path);  : Logical node (logical decisions are made based on logical structure in the node);
: Logical node (logical decisions are made based on logical structure in the node);  : Markov node (indicates the presence of a hidden or shown Markov subtree at the node);
: Markov node (indicates the presence of a hidden or shown Markov subtree at the node);  : Decision node (indicates the point of decision, which in our case is choosing between the four strategies); KRAS = V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog, BRAF = serine/threonine-protein kinase B-Raf, EGFR = epidermal growth factor receptor, VEGF = vascular endothelial growth factor.
: Decision node (indicates the point of decision, which in our case is choosing between the four strategies); KRAS = V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog, BRAF = serine/threonine-protein kinase B-Raf, EGFR = epidermal growth factor receptor, VEGF = vascular endothelial growth factor.
A cohort of patients with mCRC is simulated with attributes randomly assigned based on distributions from available randomized controlled trials. The initial randomly assigned attributes include the nature of metastases (synchronous lung and liver, metachronous lung and liver, synchronous abdominal and peritoneal metastases, and initial resectability), weekly transition probabilities for survival and treatment, and weekly costs. Along the treatment paths, a patient can enter the following states: anti-EGFR treatment, anti-VEGF treatment, oxaliplatin- or irinotecan-based treatments, surgery, surveillance, or death. As in a typical decision analysis, death is an “absorbing” state that forms the end of tracking a simulated patient. Patients can enter, leave, and reenter the other states based on conditional transition probabilities. Patients can transition on a weekly basis (ie, model cycle length = 1 week).
The values of parameters needed for the various lines of treatment are estimated using observations from randomized controlled trials. The model is calibrated such that the overall survival for different strategies built on the observations and probabilities for treatments and resections should not be statistically significantly different from findings in randomized controlled trials. Chemorefractory patients are eligible for anti-EGFR therapy. We do not differentiate between the cumulative toxicities of non-cetuximab treatments, which can inhibit their repeated usage in different combinations. The model’s parameters and the values used in the analysis are provided in Table 1.
Table 1.
Model parameters*
| Parameters | Estimates base case | Values (in 2010 US$) | Estimates range (%) | Sources |
| Proportion of patients with KRAS mutations | 28% | – | 25–60 | (49, 50) |
| Proportion of patients with BRAF mutations | 4% | – | 3–10 | (49, 50) |
| Proportion of patients with initially resectable metastases | 20% | – | 10–25 | (4, 9) |
| Proportions of patients with metachronous/ synchronous/synchronous abdominal-peritoneal metastases | 30%/20%/50% | – | † | (4, 10, 33, 34, 51-54) |
| Median number of cycles of conversion therapy | 22 cycles | – | † | (13) |
| Gap between treatment with bevacizumab and resection of metastases (because bevacizumab increases the risk of bleeding and hinders wound healing) | 6 weeks | – | † | (4) |
| Interaction of KRAS mutation on chemotherapiesʹ effectiveness | No effect‡ | – | 4–21 decrease | (22, 55) |
| Effect of cetuximab when KRAS mutation is present | No effect | – | † | (41) |
| Probability of conversion (to resectable) with:ChemotherapyCetuximab and chemotherapy | 14%25% | – | 14-3020-30 | (13, 45, 56, 57) |
| Probability of conversion (to resectable) with bevacizumab | 14% | – | 20 | Interpolation; (4) |
| Maximum overall survival under palliative care | 28 months | – | † | (30) |
| Probability of 1-, 3-, 5-, 10-year survival following hepatic resection | 85%, 39%, 25%, and 11% | – | † | (32-35) |
| Recurrence after hepatic resection | 46% within 12 months | – | † | (36) |
| Repeat hepatic resection rates | 13%–53% | – | † | (38) |
| Third hepatic resections | 16% of the patients who experienced recurrence had third hepatectomy 3–32 mo between second and third hepatectomy with a median of 13–15 mo | – | † | (32, 37, 38) |
| Costs | ||||
| Chemotherapy Rx costs per week(Costs proportioned according to the usage of FOLFOX, FOLFIRI and CapeOX) | – | 451 | +50 | (46) |
| Cetuximab Rx costs per week (regular) | – | 4180‡ | +50 | (46) |
| Cetuximab Rx costs per week (initial) | – | 6653‡ | +50 | (46) |
| Bevacizumab + 5-FU Rx average costs per week | – | 1278 | +50 | (46) |
| Physician costs of administering treatment per cycle | – | 189 | +50 | (58) |
| Monitoring costsCarcinoembryonic antigen (every 3 mo or 2 y; 6 mo for 3-5 y)Chest, abdominal, and/or pelvic CT scan (every 3–6 mo for 2 y; 6–12 mo up to a total of 5 y)Colonoscopy at 1, 3, and then every 5 y | – | 771160706 | +50 | (58, 59) |
| KRAS screening costs | – | 224 | ±50 | (58) |
| KRAS + BRAF screening costs | – | 303 | ±50 | (58) |
| Hepatic surgery costsHospital and professional costsMortality costs (in 5% cases)Morbidity costs (in 30% cases) | – | 40 30020 65712 394 | +50 | (48) |
* FOLFOX = Leucovorin Calcium, Fluorouracil and Oxaliplatin; FOLFIRI = Leucovorin Calcium, Fluorouracil and Irinotecan Hydrochloride; CapeOX = Capecitabine and Oxaliplatin; Rx = Treatment; CT = Computed Tomography; – = not applicable.
† A blank cell in the “estimates range” column indicates that we used only the base case estimate of the variable in the analysis.
‡ Cetuximab is given in combination with chemotherapies, so there is at least the “standard” treatment effect of the chemotherapies given in combination. In this case, we used the cost of a cetuximab regimen, which includes irinotecan, every 2 weeks (4).
Clinical Inputs
Treatment sequence and curative vs palliative pathways. The model does not differentiate between the effectiveness of the sequence in which the different treatments are administered, as exposure to chemotherapy itself appears to be more important than the sequence (28,29). Nor does the model differentiate between curative and palliative care. In all intervention strategies, all patients are eligible for anti-VEGF therapy. We identified no data indicating whether testing for KRAS or BRAF mutations influences the choice between curative and palliative paths, which are not well-differentiated in guidelines or practice. The model allows patients to undergo both neo-adjuvant and adjuvant treatments. In the case of neo-adjuvant treatments, we assume that the primary role of the treatment is to convert unresectable metastases of the liver to resectable ones.
Metastases. We incorporate three types of metastases: synchronous lung and liver, metachronous lung and liver, and synchronous abdominal and peritoneal metastases. Approximately 15% to 25% of patients with CRC present with synchronous liver cancer (4,5). Among those with mCRC, evidence supports the association of synchronous liver metastases with a worse prognosis than metachronous metastatic liver cancer (4). Nearly 50% of patients with metastatic CRC have either synchronous abdominal or peritoneal metastases, for which there is only palliative treatment.
Chemotherapies. In the model, we also combine oxaliplatin-, irinotecan-, and capecitabine-based treatments into a single type of treatment. Combination treatments that use cetuximab with either oxaliplatin or irinotecan are considered anti-EGFR treatments. We include only cetuximab as an anti-EGFR treatment in the analysis because it is, by a wide margin, the most common anti-EGFR treatment used in the United States.
Resection and Survival. For patients following palliative treatment paths, the maximum survival is 28 months (30). For patients on curative treatment paths, the major factor driving survival rates is curative resection of the liver metastases. Studies providing an understanding of survival rates for patients under different types of treatments, independent of surgeries, are uncommon (9). Even with major advancements in treatment therapies for mCRC, the survival of patients in nonresected cases does not exceed 5 years. For patients who do not undergo any resection, nearly 2% survived for 3 years, but none were alive at 5 years (9,31). Of the patients who have undergone hepatic resection, 85%, 39%, 25%, and 11% are alive 1, 3, 5, or 10 years after the surgery, respectively (9,32–35). In addition, these patients have nearly always experienced recurrence (13).
From 10% to 25% of patients presenting with lung or liver cancer are initially resectable (4,9). For the remainder of the patients, depending on their suitability for potential conversion to becoming resectable, they undergo conversion therapies that can include the chemotherapies bevacizumab and cetuximab. A median of 22 cycles of treatments are needed for conversion to resectability. Conversion and resection are followed by recurrence in 46% of the patients within a year of the resection (36). In most cases, the first curative hepatic resection leaves the remainder of the liver unviable for another resection. Still, a second resection and third resection are performed in 6% to 11% and 16% of the patients, respectively (32,37,38). Survival rates after the second hepatectomy are similar to the ones after the first hepatectomy (32). The estimates available for parameterizing a patient’s overall survival after having undergone treatments and monitoring vary considerably. Generally, randomized controlled trials report outcomes in two ways: the first is the proportion having that outcome (ie, alive or progression-free) at certain milestones (after 3, 5, or 10 years) or the median value of that outcome (ie, median survival time in months or years). We took advantage of the availability of two forms of survival data by parameterizing our model to the first set of expressions (proportion reaching a 3-, 5-, or 10-year milestone). We used the standard conversion technique of converting the percentage for a fixed period (say, 30% being alive at 3 years) to an annual rate, followed by a second conversion of the annual rate to a percentage for the desired period which, in our case, is a week (39). We used the second expression, median survival in months or years, as a calibration measure.
The overall survival rate varies considerably with trials. In non-cetuximab therapies, mean overall survival has been reported between 4.8 months and 20 months. In cetuximab-based therapies, mean overall survival varies between 8.1 months and 23.5 months (1,40-45). Such substantial variation is expected because patient profiles can vary considerably between trials. The primary source of variation, which appears to go unrecognized in such discussions, is likely to be the time of diagnosis of the metastases.
Cost Inputs
To approximate the market price of the chemotherapies, we used wholesale pricing estimated by the average selling price (46). To these we added the median Medicare average payment for physician services for administration of the chemotherapies (47). We test the sensitivity of the results to higher costs by increasing them by 50%. The costs are based on doses per 80kg, 5 ft 11 in, body surface area 2.0 m2. Among chemotherapies, costs vary widely between therapies with and without capecitabine. Among HMO Research Network sites participating in the CERGEN study, we observed that nearly 15% of the patients with mCRC were given capecitabine-based therapies. Using this information and the treatment regimens recommended by the National Comprehensive Cancer Network, we created a weighted average of the cost of the treatments (4). Hepatic resection costs are prominent for being nearly a magnitude greater (48). Costs of postoperative morbidity, observed in 30% of the cases, and postoperative mortality, observed in less than 5% of the cases, were also included (48). All costs were inflation-adjusted to 2010 US dollars, and future costs and life years discounted at 3%.
Cost-Effectiveness Analysis
The analysis was conducted as a two-level simulation using TreeAge Pro 2009 Suite, TreeAge Software, Inc, Williamstown, MA (Figure 1). A cohort of 50 000 patients is analyzed 10 000 times. Each time the cohort is analyzed, the system records the mean values of several variables, including cost and overall survival. The 10 000 mean values of each variable are sufficient to derive statistical inference for the population (49). The model follows each patient for a maximum of 10 years. In addition to showing the broad Markov structure, Figure 1 also shows that the basic algorithm is the same for the screening strategies compared in the model. Sensitivity analyses are described in Supplementary Material (available online).
Results
Table 2 shows the details of costs and effects of three anti-EGFR interventions and no anti-EGFR. The interventions are listed in order of increasing effect, as is standard in cost-effectiveness league tables. Compared with testing only for KRAS, providing anti-EGFR therapy to all mCRC patients without testing increases survival by 0.0026 years and costs $7493 more, with an ICER of approximately $2.8 million/LYS. Screening for both KRAS and BRAF mutations prior to administering anti-EGFR therapy compared with the base strategy (of no anti-EGFR therapy), increases OS by 0.034 years (12.4 days) at a cost of $22 033, yielding an ICER of approximately $650 000 per LYS. Looking at the results from a slightly different perspective, compared with providing anti-EGFR therapy without screening, adding KRAS testing alone saves $7493 per patient, and adding BRAF testing saves an additional $1023 with little reduction in expected survival.
Table 2.
Incremental Cost-Effectiveness Ratios (ICER)*
| Strategy | Cost over 10 years ($) | Incremental cost ($) | Effect (overall survival in years) | Incremental effect | ICER: $ per life year saved | |||||
| No anti-EGFR therapy | 34 291 | NA | 0.6686 | NA | NA | |||||
| KRAS and BRAF screening with anti-EGFR therapy | 56 324 | 22 033 | 0.7025 | 0.0340 | 648 396 | |||||
| KRAS screening with anti-EGFR therapy | 57 348 | 1023 | 0.7029 | 0.0004 | 2 814 338 | |||||
| Anti-EGFR therapy without screening | 64 841 | 7493 | 0.7055 | 0.0026 | 2 932 767 |
* NA = not applicable; EGFR = epidermal growth factor receptor.
Underlying the results in Table 2 are several processes and, therefore, costs associated with undertaking any strategy. To better understand these costs, we also analyzed how the different processes contribute to the costs when implemented across populations of patients in the model. When we do not provide any anti-EGFR treatment, the average resection cost is $8600, whereas the total average costs are $34 291. When we screen for both KRAS and BRAF mutations, average resection costs are $11 300 out of total average costs of $56 234. When anti-EGFR therapy is provided without screening, average resection costs are $11 500 out of total average costs of $64 841, accounting for 2% of the increase in average costs (compared with the screening scenario). The vast majority of additional treatment costs associated with anti-EGFR therapy is attributable to chemotherapy, with comparatively little increase in resection costs and subsequent improvement in overall survival.
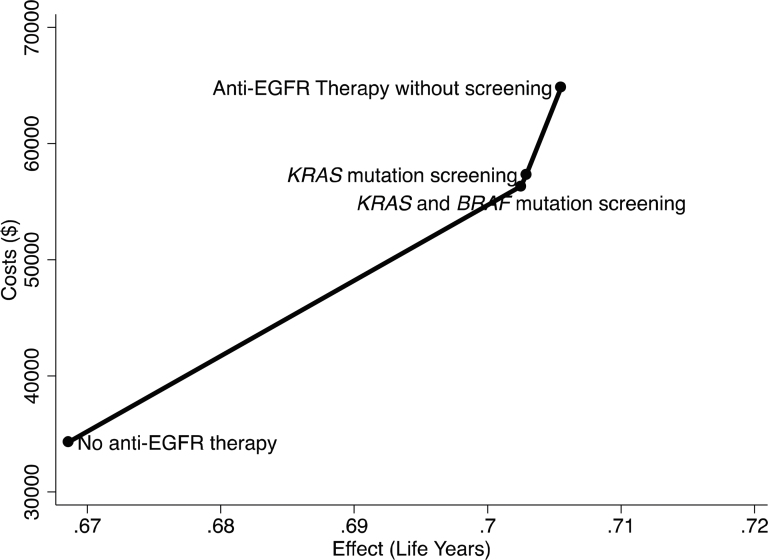
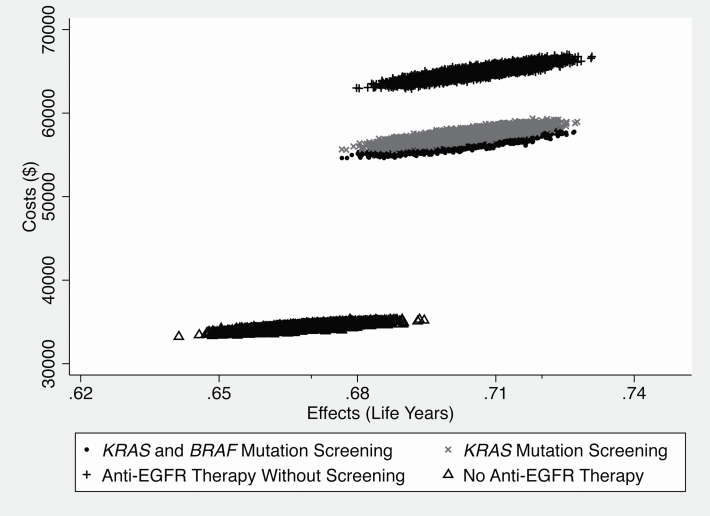
A comparison of all four strategies’ costs and effects is pictured in Figure 2. The largest gain in survival and the largest increase in costs occurs from introducing anti-EGFR with screening for KRAS and BRAF mutations; however, the ICER for forgoing screening is extremely high (as shown by the steep slope of the line), because the increment in survival is less than a day. Figure 2 gives the impression that the estimates are deterministic in nature. Figure 3 provides a visual representation of the spread in costs and effects of the different strategies. In Figure 3, the scatter of average costs and effects under different strategies highlights that the major differences are in costs of the strategies with major overlap in effectiveness. Estimated standard errors for the treatment effects are: no anti-EGFR therapy: 0.0476; KRAS and BRAF screening with anti-EGFR therapy: 0.0495; KRAS screening with anti-EGFR therapy: 0.0496; anti-EGFR therapy without screening: 0.0500.
Figure 2.
Cost-effectiveness analysis of four strategies with no anti-EGFR therapy as the base strategy.
Figure 3.
Costs and effectiveness scatter plot.
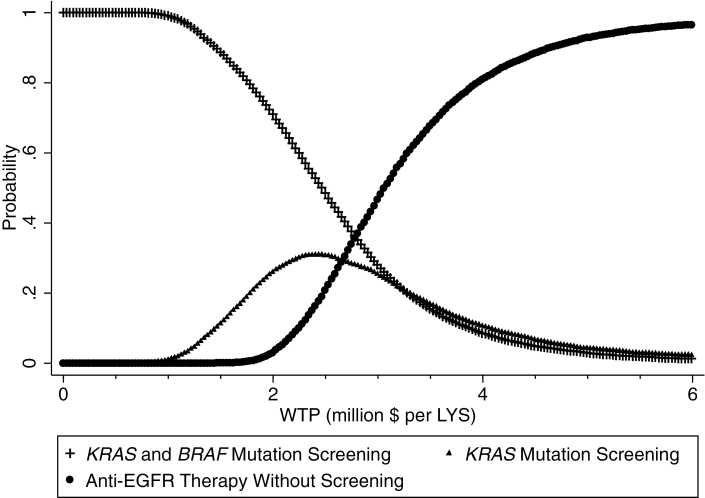
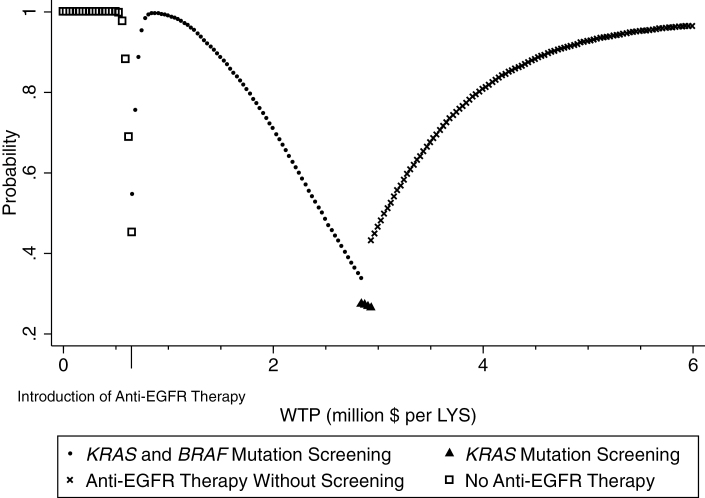
The difference in costs of the strategy with no anti-EGFR therapy and therapies with anti-EGFR therapy, with much overlap in effectiveness, stands out. In most cases, we expend considerably more by adopting an anti-EGFR therapy strategy, with a highly uncertain small gain in overall survival. To understand the simultaneous uncertainty in both costs and effects, we generated cost-effectiveness acceptability curves (CEACs). Net benefit CEACs in Figure 4 show the probability that an intervention is cost-effective compared with all other interventions for a range of threshold values of willingness to pay, which is an elicited maximum amount a potential payer would pay for an increment in health improvement (50,51). At willingness to pay thresholds at less than $1 million per life-year saved, providing anti-EGFR therapy with screening is definitely preferable. The probability that either screening strategy (only KRAS or both KRAS and BRAF) will be cost-effective decreases considerably at higher willingness-to-pay thresholds. Anti-EGFR therapy without screening has the highest probability of being considered cost-effective only for willing to pay thresholds of $3 million or more per additional year of survival. This aligns with the baseline ICERs in Table 2.
Figure 4.
Cost-effectiveness acceptability curves of anti-EGFR therapies. WTP: Willingness to pay, LYS = Life year saved, KRAS = V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog, BRAF = serine/threonine-protein kinase B-Raf, EGFR = epidermal growth factor receptor.
Figure 5 shows an acceptability frontier formed by the envelope of strategies that would be favored at specific willingness-to-pay thresholds. The acceptability frontier is derived from Figure 4, which excludes no anti-EGFR therapy for clarity but is based on the same information. Figure 5 simplifies the depiction of the selection from among the different strategies at specific willingness-to-pay thresholds by displaying only the optimal strategy at each threshold. At the lowest levels of willingness to pay, not using anti-EGFR therapy is preferred with certainty. With an increase in willingness to pay, the screening strategies are preferred. After about $3 million per year willingness to pay, providing anti-EGFR treatment to everyone is most preferred.
Figure 5.
Acceptability frontier. WTP = Willingness to pay, LYS = Life year saved, KRAS = V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog, BRAF = serine/threonine-protein kinase B-Raf, EGFR = epidermal growth factor receptor.
As we move right toward anti-EGFR therapy without screening, we can see that starting at a willingness to pay equal to the ICER of strategy of screening for KRAS and BRAF, the willingness-to-pay thresholds that would support more widespread use of anti-EGFRs are magnitudes higher than the oft-cited $50 000/QALY figure, which is considered a reasonable threshold (52).
We conducted the following sensitivity analyses, which are described and presented in the Supplementary Material (available online): 1) Conversion probability for chemotherapy is 30% [bevacizumab=30%, cetuximab=50%]; 2) conversion probability for bevacizumab is +10%; cetuximab is +20%; 3) cost of surgery is +50%; 4) cost of screening (a) +50% and (b) -50%; and 5) prognostic decrease in overall survival with BRAF (regardless of treatment).
The sensitivity analyses provide insight into the responsiveness of the results to changes in the parameters. From among all sensitivity analyses, increasing the probabilities of conversion in sensitivity analysis 1 results in the highest response (increase) in overall survival. Nevertheless, the increment in survival is not proportionate to the change in the parameter values. In sensitivity analysis 1, increasing the probability of conversion using cetuximab regimens by 100% increases the mean overall survival only by 17%. This can be attributed to the successive Markov decision paths through which a patient must pass, which tend to reduce the net overall effect.
Discussion
Anti-EGFR treatment is costly. In mCRC, when the treatment is provided without screening for genetic mutations, it is the most costly strategy and carries a very small improvement in mean survival. We estimate that screening for KRAS and/or BRAF mutations can reduce the cost of anti-EGFR treatment but with a very small reduction in overall survival. The screening interventions are cost saving compared with providing anti-EGFR therapy to all mCRC patients.
At the lowest WTP levels, the preference is to forgo anti-EGFR therapy altogether. This is because, even with the cost savings resulting from the use of screening, the higher net cost of treatment, which allows anti-EGFR treatments for patients without genetic mutations, is unacceptably high if WTP is less than $350 000/LYS. These “lower” levels of willingness to pay for increased survival are quite high relative to commonly cited thresholds, such as $50 000/LYS or less for reasonable value and $100 000/LYS or more for questionable value.
Our results are considerably different from Blank et al. (24). The cost of base case (€3983) in their cost-effectiveness table is very low, which could be because of the exclusion of resection costs. Similarly, costs for other strategies are about half of ours. They appear to have excluded repeated surgeries, associated costs in all cases, and repeated application of treatments, including anti-EGFR (53). However, the major difference between their results and those presented here appears to be in the effect on overall survival of anti-EGFR treatments. Blank et al. estimated nearly double the increase in lifetime overall survival with use of anti-EGFR therapy compared with no anti-EGFR therapy. Blank et al.’s estimates of overall survival match the randomized controlled trial findings for a subset of patients with wild-type KRAS being evaluated for cetuximab and basic supportive care treatment versus BSC treatment only (22). Our analysis is based on a general population of mCRC patients, of whom roughly 50% have synchronous abdominal and peritoneal metastases with a maximum overall survival of 6.5 months (54). It is difficult to compare findings from such a patient base to those from randomized controlled trials and other literature because of the variation in inclusion criteria for patients. Excluding those with abdominal/peritoneal metastases from anti-EGFR therapy might bring the cost effectiveness ratio closer to those in other studies. Including this exclusion in the model is not possible because of insufficient data. Ideally, to compare our estimates with the available evidence, we should compare our findings to the overall randomized patient base being treated and not differentiate overall survival based on mutation status.
In order to compare our findings and those of other researchers, we can see that only NCIC CO.17 reported a statistically significant difference in the effect of cetuximab on overall survival. Statistically insignificant gains in overall survival resulting from treatment with cetuximab align with our estimates. Vijayaraghavan et al. (55) provide a cost-effectiveness analysis of using anti-EGFR treatments in combination therapies compared with anti-EGFR treatments alone. Their cost structures also reflect a lack of retreatment with earlier treatments and inclusion of costs of resections (55). Compared with Vijayaraghavan et al. and Blank et al., our analytical structure assumes the adoption of multiple chemotherapies in basic supportive care to optimally improve overall survival (28,29).
Our analysis points to two types of potential cost savings that a policymaker or payer could obtain depending on their willingness to pay. At the lowest willingness to pay, the payer who decides against the use of anti-EGFR therapy can save about $20 000 per patient. A willingness to adopt anti-EGFR testing for both KRAS and BRAF could together save roughly $8000 compared with providing anti-EGFR treatment without any screening.
In a conference abstract, Shankaran et al. (25) estimated that $740 million in annual savings would be realized in the United States by providing KRAS testing to all 29 762 mCRC patients. Using the same number of cases and their rate of prevalence of KRAS mutation, we estimate that savings would be closer to $103 million annually. The amount of detail available in their abstract prevents assessment of the reasons for the difference between the estimates.
This study has several limitations. First, we did not provide progression-free survival outcomes for the different strategies. In the case of mCRC patients, progression-free survival does not play a major role in deciding the course of treatment. Second, we did not incorporate differences among the treatment options in quality of life. There is an insufficient evidence base to undertake such an analysis. Mittmann et al. (22) and other papers based on the CO.17 trial have provided direct information regarding the quality of life for the patients, but it is for a much shorter duration: 24 weeks. Mittmann et al. report QALYs for two cases: patients without KRAS mutation who are subjected to cetuximab and those on non–anti-EGFR therapy. Without separate utility values for those administered cetuximab and having KRAS mutation, there is no way to incorporate the quality-of-life impact of side effects from cetuximab and basic supportive care. Our results show a small incremental gain in overall survival when forgoing KRAS testing before treatment. These gains may not have been seen if the health impact of treatment were expressed in quality-adjusted survival. Differences in quality of life among patients receiving alternate treatments have not been quantified in a way that allows quality-adjusted survival to be modeled.
This analysis did not consider patient preferences regarding discontinuation of cetuximab treatment because of the reduced quality of life from adverse reactions, which has been observed in 3% to 10% of patients (16). Dermatologic toxicities with treatment include an acneiform rash in 76% to 88% of the patients, with severe rash in 1% to 17% of the patients (16). Other common adverse reactions include headache, diarrhea, and infection (16).
Our analysis for BRAF testing assumes that 100% of the difference in survival between cetuximab users with BRAF mutations, similar to those with KRAS mutations, is because of a lack of response to cetuximab. However, evidence from randomized controlled trials suggests that BRAF mutations may independently predict prognosis (56–58). Our estimated savings from BRAF testing are overstated if any part of the survival difference is because of BRAF predicting of survival independent of therapy choice.
In general, our results are less supportive of the use of anti-EGFR therapy than previous analyses, and they indicate lower cost savings from KRAS testing than previously reported. Although we cannot confirm that anti-EGFR therapy is a cost-effective use of health care resources, we can affirm that KRAS testing is cost-saving. BRAF testing may offer additional savings.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (RC2CA148471). Co-PIs were Katrina A. Goddard, Lawrence H. Kushi, and Evelyn P. Whitlock. Affiliation and Awardee Organization: Kaiser Foundation Research Organization.
Supplementary Material
This research was conducted at multiple sites of the HMO Cancer Research Network (CRN). The CRN consists of the research programs, enrollee populations, and databases of 14 HMO members of the HMO Research Network. The overall goal of the CRN is to conduct collaborative research to determine the effectiveness of preventive, curative, and supportive interventions for major cancers that span the natural history of those cancers among diverse populations and health systems. The program sponsor did not have a role in the design of the study, analysis, and interpretation of the data, the writing of the article, or the decision to submit the article for publication. DLV served as a consultant to Genetech, Novartis, and Medco.
References
- 1. Winder T Lenz HJ. Vascular endothelial growth factor and epidermal growth factor signaling pathways as therapeutic targets for colorectal cancer. Gastroenterology. 2010; 138(6):2163–2176 [DOI] [PubMed] [Google Scholar]
- 2. Goldberg RM Rothenberg ML Van Cutsem E et al. The continuum of care: a paradigm for the management of metastatic colorectal cancer. Oncologist. 2007; 12(1):38–50 [DOI] [PubMed] [Google Scholar]
- 3. Power DG Kemeny NE. Role of adjuvant therapy after resection of colorectal cancer liver metastases. J Clin Oncol. 2010; 28(13):23002309 [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer NetworkColon Cancer.. In: NCCN Clinical Practice Guidelines in Oncology.. v.3.2010 ed. Fort Washington, PA: National Comprehensive Cancer Network, Inc; 2011; [Google Scholar]
- 5. Van Cutsem E Nordlinger B Adam R et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006; 42(14):2212–2221 [DOI] [PubMed] [Google Scholar]
- 6. Kemeny N. Management of liver metastases from colorectal cancer. Oncology. 2006; 20(10):1161–1186 [PubMed] [Google Scholar]
- 7. Bismuth H Adam R Levi F et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996; 224(4):509–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute for Health and Clinical ExcellenceBevacizumab and cetuximab for the treatment of metastatic colorectal cancer. London: National Institute for Health and Clinical Excellence; [Google Scholar]
- 9. Kemeny N. Presurgical chemotherapy in patients being considered for liver resection. Oncologist. 2007; 12(7):825–839 [DOI] [PubMed] [Google Scholar]
- 10. Leonard GD Brenner B Kemeny NE. Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2005; 23(9):2038–2048 [DOI] [PubMed] [Google Scholar]
- 11. Kopetz S Chang GJ Overman MJ et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. . J Clin Oncol.. 2009; 27(22):3677–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parks R Gonen M Kemeny N et al. Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. . J Am Coll Surg. 2007; 204(5):753–763 [DOI] [PubMed] [Google Scholar]
- 13. Adam R Aloia T Levi F et al. Hepatic resection after rescue cetuximab treatment for colorectal liver metastases previously refractory to conventional systemic therapy. J Clin Oncol. 2007; 25(29):4593–4602 [DOI] [PubMed] [Google Scholar]
- 14. Tomlinson JS Jarnagin WR DeMatteo RP et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007; 25(29):4575–4580 [DOI] [PubMed] [Google Scholar]
- 15. Pazdur R. FDA Approval for Cetuximab. http://www.cancer.gov/cancertopics/druginfo/fda-cetuximab - Anchor-Colorecta-60318 Accessed December 10, 2011. [Google Scholar]
- 16. ImClone LLC. Prescribing information for Erbitux.. In: Company ELa., ed. Indianapolis, IN: ImClone LLC; 2012; [Google Scholar]
- 17. Pazdur R. FDA Approval for Bevacizumab. http://www.cancer.gov/cancertopics/druginfo/fda-bevacizumab - Anchor-Approva-51277 Accessed December 11, 2011. [Google Scholar]
- 18. De Roock W De Vriendt V Normanno N et al. KRAS, BRAF, PIK3CA, and PTEN mutations: implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol. 2011; 12(6):594–603 [DOI] [PubMed] [Google Scholar]
- 19. De Roock W Claes B Bernasconi D et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010; 11(8):753–762 [DOI] [PubMed] [Google Scholar]
- 20.Genentech IncPrescribing information for Avastin. South San Francisco, CA: Bevacizumab-Genentech, Inc; 2011; [Google Scholar]
- 21.American Society of Clinical OncologyASCO Releases Provisional Clinical Opinion Recommending Routine KRAS Gene Testing to Guide Treatment for Metastatic Colorectal Cancer. http://www.asco.org/ascov2/Press+Center/Latest+News+Releases/Meetings+News/ASCO+Releases+Provisional+Clinical+Opinion+Recommending+Routine+KRAS+Gene+Testing+to+Guide+Treatment+for+Metastatic+Colorectal+Cancer Accessed August 20, 2011. [Google Scholar]
- 22. Mittmann N Au HJ Tu D et al. Prospective cost-effectiveness analysis of cetuximab in metastatic colorectal cancer: evaluation of National Cancer Institute of Canada Clinical Trials Group CO.17 trial. J Natl Cancer Inst. 2009; 101(17):1182–1192 [DOI] [PubMed] [Google Scholar]
- 23. Starling N Tilden D White J et al. Cost-effectiveness analysis of cetuximab/irinotecan vs active/best supportive care for the treatment of metastatic colorectal cancer patients who have failed previous chemotherapy treatment. Brit J Cancer.. 2007; 96(2):206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blank PR Moch H Szucs TD et al. KRAS and BRAF mutation analysis in metastatic colorectal cancer: a cost-effectiveness analysis from a Swiss perspective. Clin Cancer Res. 2011; 17(19):6338–6346 [DOI] [PubMed] [Google Scholar]
- 25. Shankaran V Bentrem DJ Mulcahy MF et al. Economic implications of Kras testing in metastatic colorectal cancer (mCRC). 2009 Gastrointestinal Cancers Symposium. Chicago, IL: American Society of Clinical Ontology; 2009; [Google Scholar]
- 26. Mancl EE Kolesar JM Vermeulen LC. Clinical and economic value of screening for Kras mutations as predictors of response to epidermal growth factor receptor inhibitors. Am J Health Syst Pharm. 2009; 66(23): 2105–2112 [DOI] [PubMed] [Google Scholar]
- 27. Lin JS Webber EM Senger CA et al. Systematic review of pharmacogenetic testing for predicting clinical benefit to anti-EGFR therapy in metastatic colorectal cancer. Am J Cancer Res. 2011; 1(5):650–662 [PMC free article] [PubMed] [Google Scholar]
- 28. Grothey A Sargent D. Overall survival of patients with advanced colorectal cancer correlates with availability of fluorouracil, irinotecan, and oxaliplatin regardless of whether doublet or single-agent therapy is used first line. J Clin Oncol.. 2005; 23(36):9441–9442 [DOI] [PubMed] [Google Scholar]
- 29. Van Cutsem E. Challenges in the use of epidermal growth factor receptor inhibitors in colorectal cancer. Oncologist. 2006; 11(9):1010–1017 [DOI] [PubMed] [Google Scholar]
- 30. Mitry E Lièvre A Bachet J-B et al. Irinotecan as palliative chemotherapy for metastatic colorectal cancer: evolving tactics following initial treatment. Int J of Colorectal Disease. 2009; 24(6):605–612 [DOI] [PubMed] [Google Scholar]
- 31. Scheele J Stangl R Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990; 77(11):1241–1246 [DOI] [PubMed] [Google Scholar]
- 32. Nordlinger B Jaeck D Guiget M et al. Surgical resection of hepatic metastases: multicentric retrospective study by the French Association of Surgery.. In: Cancer Research UK. 1992; 129–161 [Google Scholar]
- 33. Pool AEvd Lalmahomed ZS Özbay Y et al. “Staged” liver resection in synchronous and metachronous colorectal hepatic metastases: differences in clinicopathological features and outcome. Colorectal Dis. 2009; 12(10 Online):e229–235 [DOI] [PubMed] [Google Scholar]
- 34. Fong Y Cohen A Fortner J et al. Liver resection for colorectal metastases. J Clin Oncol. 1997; 15(3):938–946 [DOI] [PubMed] [Google Scholar]
- 35. Pawlik TM Scoggins CR Zorzi D et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005; 241(5):715–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fernandez-Trigo V Shamsa F Sugarbaker PH. Repeat liver resections from colorectal metastasis. Repeat Hepatic Metastases Registry. Surgery. 1995; 117(3):296–304 [DOI] [PubMed] [Google Scholar]
- 37. Nordlinger B Vaillant J Guiguet M et al. Survival benefit of repeat liver resections for recurrent colorectal metastases: 143 cases. Association Francaise de Chirurgie. J Clin Oncol. 1994; 12(7):1491–1496 [DOI] [PubMed] [Google Scholar]
- 38. Wanebo HJ Chu QD Avradopoulos KA et al. Current perspectives on repeat hepatic resection for colorectal carcinoma: a review. Surgery. 1996; 119(4):361–371 [DOI] [PubMed] [Google Scholar]
- 39. Prabhu VS Farnham PG Hutchinson AB et al. Cost-effectiveness of HIV screening in STD clinics, emergency departments, and inpatient units: a model-based analysis. PLOS ONE. 2011; 6(5):e19936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Van Cutsem E Rougier A Kohne CH et al. Meta-analysis of the CRYSTAL and OPUS studies combining cetuximab with chemotherapy (CT) as 1st-line treatment for patients (pts) with metastatic colorectal cancer (mCRC): results according to KRAS and BRAF mutation status. . Eur J Cancer. 2009; 7: 6077 [Google Scholar]
- 41. Karapetis CS Khambata-Ford S Jonker DJ. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008; 359: 1757–1765 [DOI] [PubMed] [Google Scholar]
- 42. Youssoufian H. Role of K-ras mutation status in optimizing selection of colorectal cancer patients for treatment with Erbitux (Cetuximab). http://www.fda.gov/ohrms/dockets/ac/08/slides/2008-4409s1-04-ImClone.pdf Accessed June 11, 2011. [Google Scholar]
- 43. Van Cutsem E Lang I Folprecht G et al. Cetuximab plus FOLFIRI in the treatment of metastatic colorectal cancer (mCRC): the influence of KRAS and BRAF biomarkers on outcome: updated data from the CRYSTAL trial.. In 2010 Gastrointestinal Cancers Symposium. Orlando, FL: American Society of Clinical Oncology; 2010; [Google Scholar]
- 44. Maughan T Adams RA Smith CG et al. Addition of cetuximab to oxaliplatin-based combination chemotherapy in patients with KRAS wild-type advanced colorectal cancer: a randomised superiority trial (MRC COIN). Eur J Cancer.. 2009; 7(Suppl):6LBA [Google Scholar]
- 45. Cunningham D Atkin W Lenz H-J et al. Colorectal cancer. Lancet. 2010; 375: 1030–1047 [DOI] [PubMed] [Google Scholar]
- 46.Centers for Medicare and Medicaid Services2010 ASP Drug Pricing Files. https://http://www.cms.gov/McrPartBDrugAvgSalesPrice/01_overview.asp - TopOfPage Accessed November 13, 2011. [Google Scholar]
- 47.IngenixNational Fee Analyzer Charge Data for Evaluating Fees Nationally. Salt Lake City, UT: Ingenix, Inc; 2004; [Google Scholar]
- 48. Gazelle GS Hunink MGM Kuntz KM et al. Cost-effectiveness of hepatic metastasectomy in patients with metastatic colorectal carcinoma: a state-transition Monte Carlo decision analysis. Ann Surg. 2003; 237(4):544–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. O'Hagan A Stevenson M Madan J. Monte Carlo probabilistic sensitivity analysis for patient level simulation models: efficient estimation of mean and variance using ANOVA. Health Econ. 2007; 16(10):1009–1023 [DOI] [PubMed] [Google Scholar]
- 50. Fenwick E Byford S. A guide to cost-effectiveness acceptability curves. Brit J Psychiatry. 2005; 187: 106–108 [DOI] [PubMed] [Google Scholar]
- 51. Fenwick E Marshall D Levy A et al. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv Res. 2006; 6(1):52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mekenkamp LJ Koopman M Teerenstra S et al. Clinicopathological features and outcome in advanced colorectal cancer patients with synchronous vs metachronous metastases. Brit J Cancer. 2010; 103(2):159-–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Santini D Vincenzi B Addeo R et al. Cetuximab rechallenge in metastatic colorectal cancer patients: how to come away from acquired resistance?. Annals Oncol. 2012;;23(9):2313–2318. [DOI] [PubMed] [Google Scholar]
- 54. Mitry E Lievre A Bachet JB et al. Irinotecan as palliative chemotherapy for metastatic colorectal cancer: evolving tactics following initial treatment. Int J Colorectal Dis. 2009; 24(6):605–612 [DOI] [PubMed] [Google Scholar]
- 55. Vijayaraghavan A Efrusy MB Goke B et al. Cost-effectiveness of KRAS testing in metastatic colorectal cancer patients in the United States and Germany. Int J Cancer. 2011; 131(2):438–445 [DOI] [PubMed] [Google Scholar]
- 56. Bokemeyer C Bondarenko I Hartmann JT et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011; 22(7):1535–1546 [DOI] [PubMed] [Google Scholar]
- 57. Van Cutsem E Kohne C-H Lang I et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011; 29(15):2011–2019 [DOI] [PubMed] [Google Scholar]
- 58. Tol J Dijkstra JR Klomp M et al. Markers for EGFR pathway activation as predictor of outcome in metastatic colorectal cancer patients treated with or without cetuximab. Eur J Cancer. 2010; 46(11):1997–2009 [DOI] [PubMed] [Google Scholar]
- 59. Andreyev H Norman A Cunningham D et al. Kirsten ras mutations in patients with colorectal cancer: the multicenter “RASCAL” study. J Natl Cancer Inst. 1998; 90(9):675–684 [DOI] [PubMed] [Google Scholar]
- 60. Yuen ST Davies H Chan TL et al. Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia. Cancer Res. 2002; 62(22):6451–6455 [PubMed] [Google Scholar]
- 61. Hurley RW. Treatment and progression of metastatic colorectal cancer.. In: Behl AS, ed. Expert Opinion: In-Person Meeting. St Paul, MN; 2010; [Google Scholar]
- 62. Van Dessel ELS Fierens K Pattyn P et al. Defining the optimal therapy sequence in synchronous resectable liver metastases from colorectal cancer: a decision analysis approach. Acta Chirurgica Belgica. 2009; 109(3):317–20 [DOI] [PubMed] [Google Scholar]
- 63. Bramhall SR Gur U Coldham C et al. Liver resection for colorectal metastases. Ann Royal Coll Surg Engl. 2003; 85(5):334–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tsai M-S Su Y-H Ho M-C et al. Clinicopathological features and prognosis in resectable synchronous and metachronous colorectal liver metastasis. Ann Surg Oncol. 2007; 14(2):786–794 [DOI] [PubMed] [Google Scholar]
- 65. Bokemeyer C Bondarenko I Makhson A et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009; 27(5):663–671 [DOI] [PubMed] [Google Scholar]
- 66. Adam R Delvart V Pascal G et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004; 240(4):644–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gunnar F Thomas G Wolf OB et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010; 11(1):38–47 [DOI] [PubMed] [Google Scholar]
- 68.IngenixNational Fee Analyzer 2010. Eden Prairie, MN: Ingenix; 2009; [Google Scholar]
- 69. Lansdorp-Vogelaar I van Ballegooijen M Zauber AG et al. At what costs will screening with CT colonography be competitive? A cost-effectiveness approach. Intl J Cancer. 2009; 124(5):1161–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.