Abstract
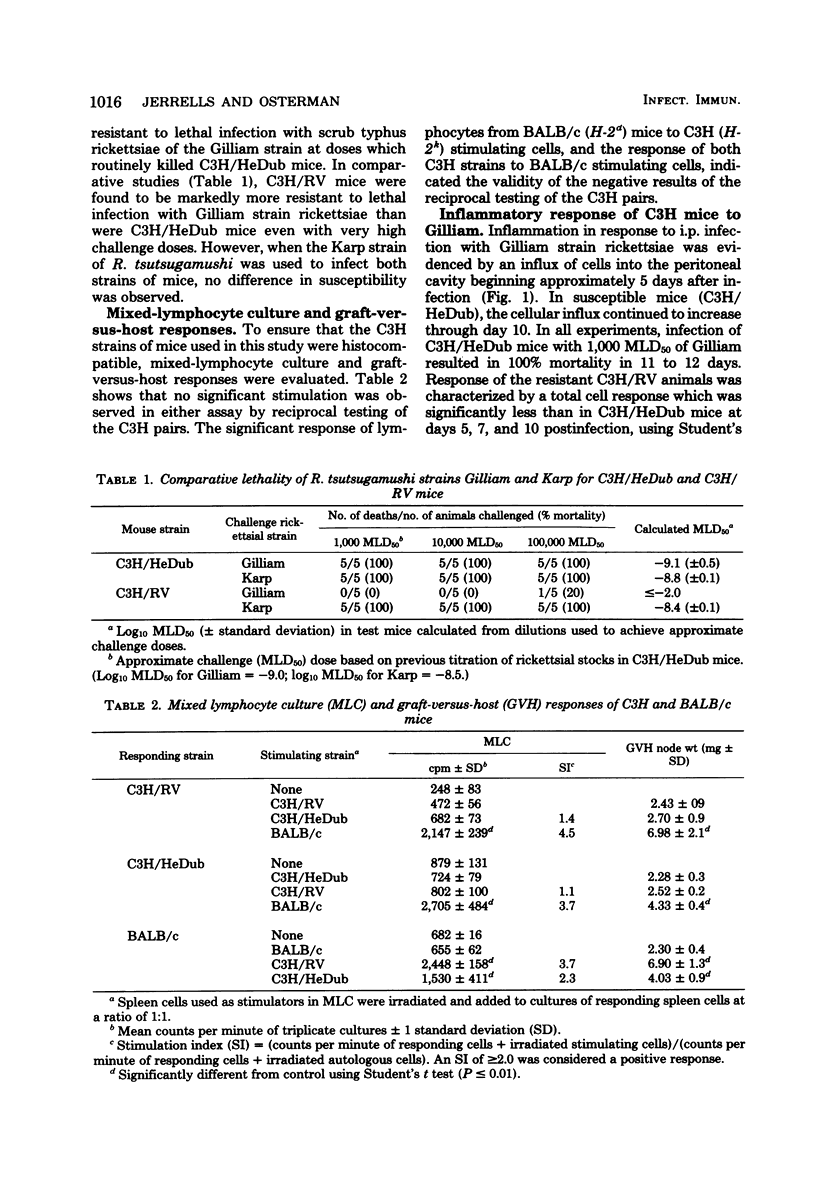
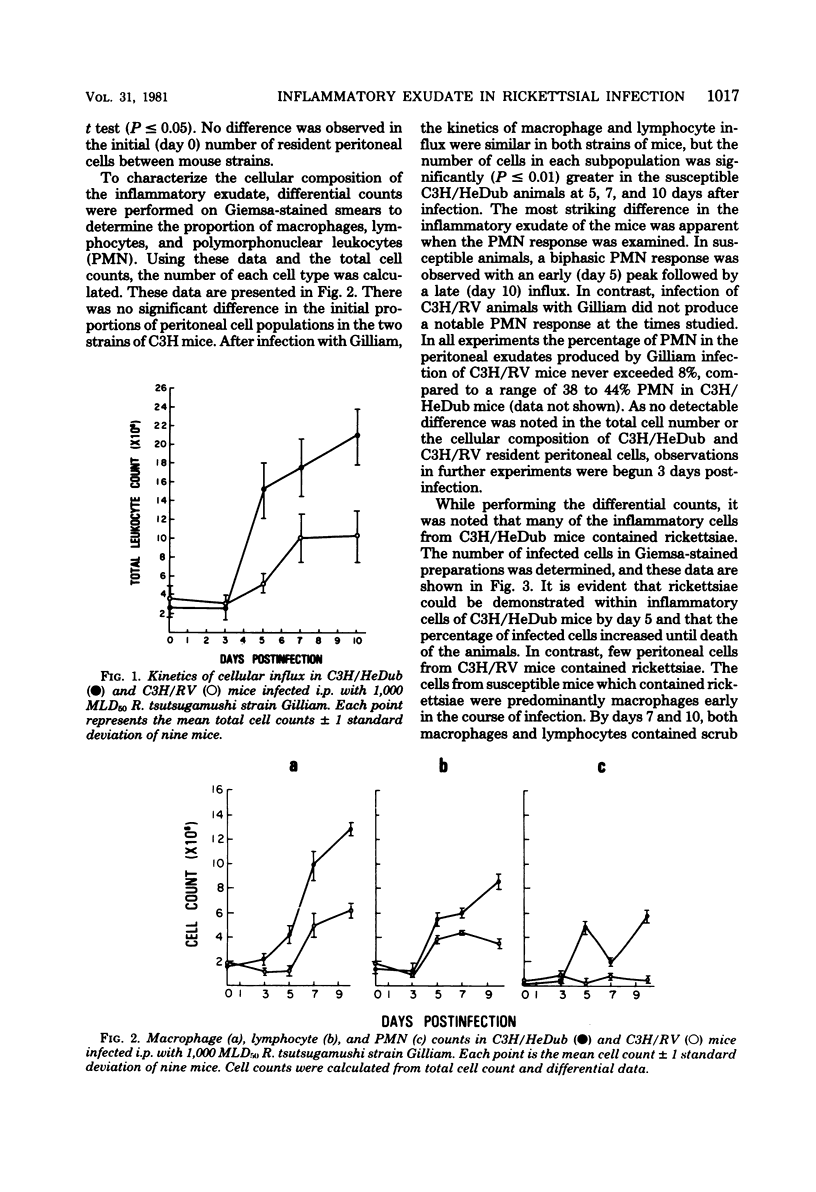
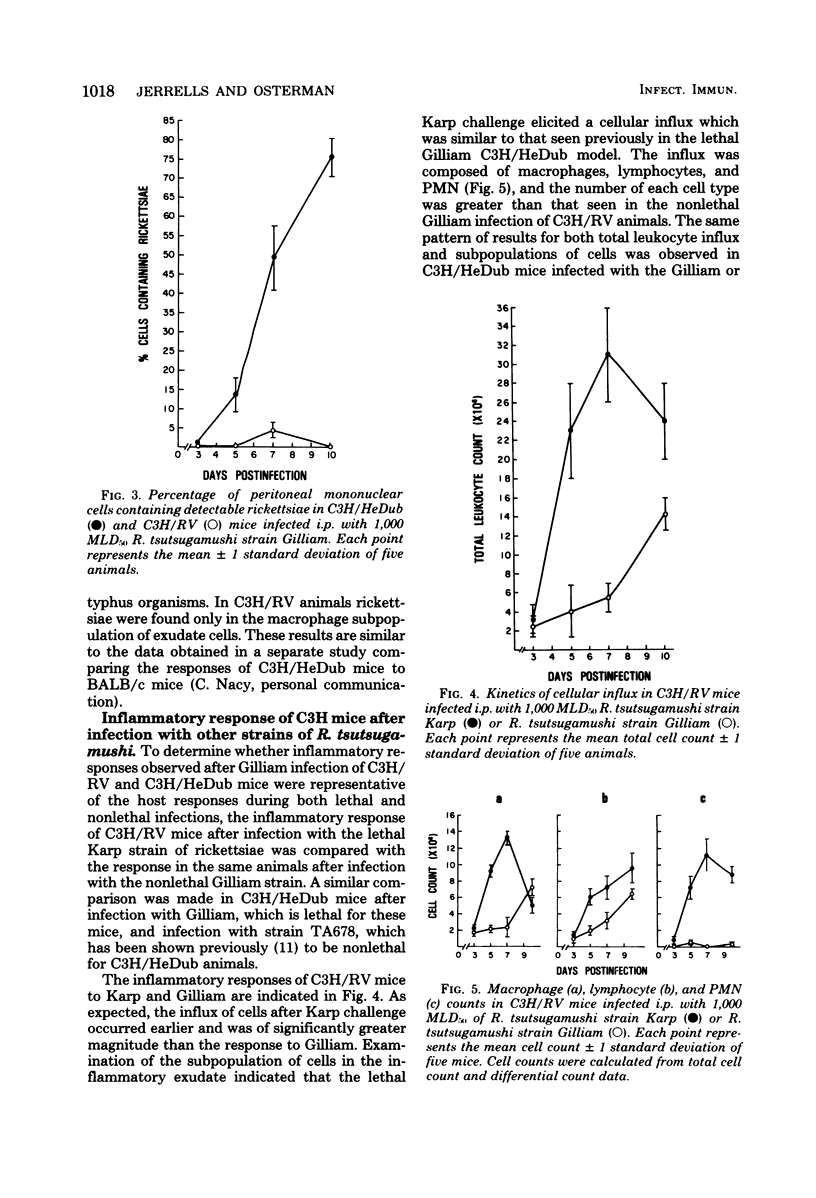
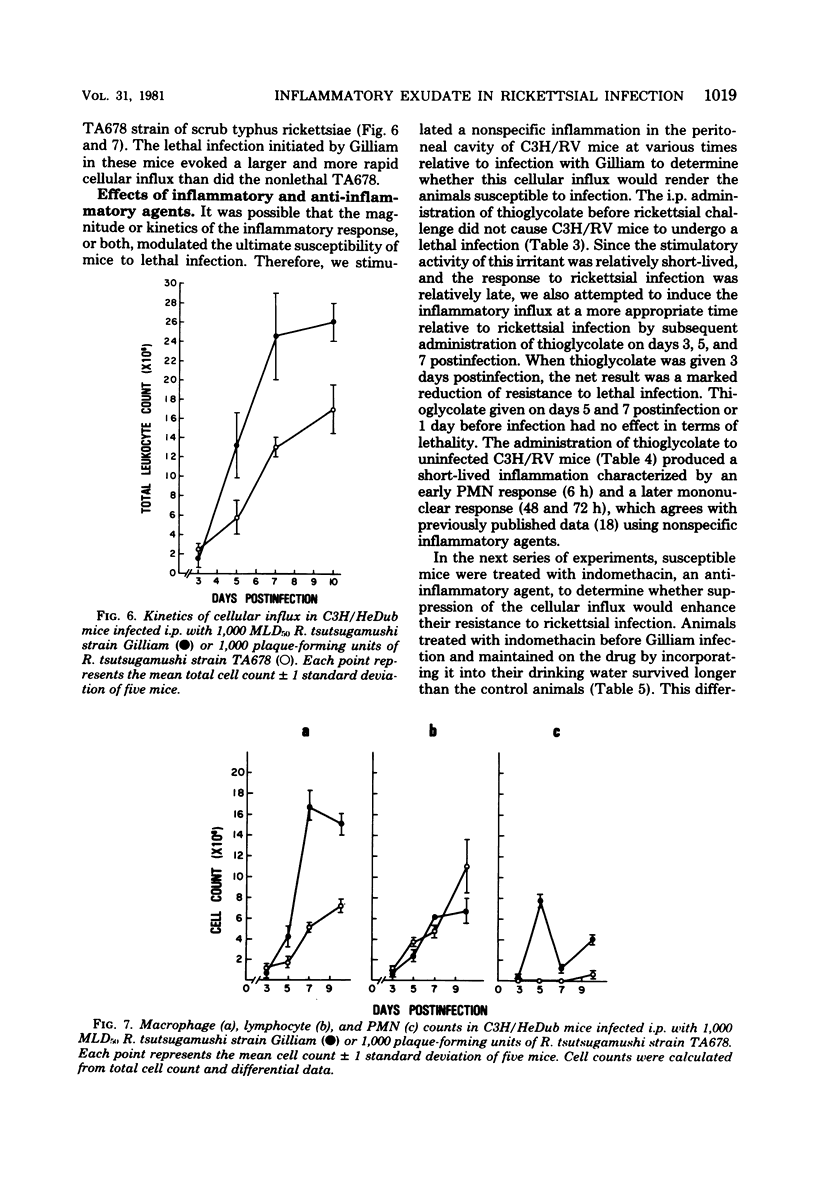
Two strains of C3H mice differed in their susceptibility to lethal infection with Rickettsia tsutsugamushi strain Gilliam. Adult C3H/RV mice were markedly more resistant to lethal infection than C3H/HeDub mice, and both were histocompatible as assessed by mixed-lymphocyte cultures and graft-versus-host responses. The inflammatory response of susceptible C3H/HeDub mice to intraperitoneal infection was evident approximately 5 days postinfection, and the magnitude of the cellular influx increased until death of the animal. The inflammation consisted of an early polymorphonuclear leukocyte response, followed by a mononuclear cell influx which persisted until death of the animal. The C3H/RV mice evidenced similar kinetics of cell influx, but the inflammatory response was significantly reduced in magnitude, and the response of C3H/RV animals to Gilliam was predominantly mononuclear in nature, with little influx of polymorphonuclear leukocytes into the peritoneal cavity. C3H/RV mice were rendered susceptible to Gilliam infection by induction of a nonspecific inflammation with thioglycolate if given 3 days after infection. Conversely, treatment of C3H/HeDub mice with indomethacin, an anti-inflammatory agent, prolonged survival after infection with Gilliam. The results of this study indicate that genetic resistance to Gilliam is not due simply to a greater host response to infection or, conversely, that susceptibility is due to a host response quantitatively lacking in a cellular component necessary for antirickettsial immunity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. W., Jr, Osterman J. V. Host defenses in experimental rickettsialpox: genetics of natural resistance to infection. Infect Immun. 1980 Apr;28(1):132–136. doi: 10.1128/iai.28.1.132-136.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker L. A., Campbell P. A. Thioglycolate medium decreases resistance to bacterial infection in mice. Infect Immun. 1980 Feb;27(2):455–460. doi: 10.1128/iai.27.2.455-460.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt P. N., Jacoby R. O. Genetic resistance to lethal flavivrus encephalitis. II. Effect of immunosuppression. J Infect Dis. 1976 Aug;134(2):166–173. doi: 10.1093/infdis/134.2.166. [DOI] [PubMed] [Google Scholar]
- Catanzaro P. J., Shirai A., Hilderbrandt P. K., Osterman J. V. Host defenses in experimental scrub typhus: histopathological correlates. Infect Immun. 1976 Mar;13(3):861–875. doi: 10.1128/iai.13.3.861-875.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect Immun. 1978 Mar;19(3):755–762. doi: 10.1128/iai.19.3.755-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell M. B., Koprowski H., Lagerspetz K. Genetically determined resistance to infection with group B arboviruses. I. Distribution of the resistance gene among various mouse populations and characteristics of gene expression in vivo. J Infect Dis. 1974 Mar;129(3):240–247. doi: 10.1093/infdis/129.3.240. [DOI] [PubMed] [Google Scholar]
- Ewing E. P., Jr, Takeuchi A., Shirai A., Osterman J. V. Experimental infection of mouse peritoneal mesothelium with scrub typhus rickettsiae: an ultrastructural study. Infect Immun. 1978 Mar;19(3):1068–1075. doi: 10.1128/iai.19.3.1068-1075.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford W. L., Burr W., Simonsen M. A lymph node weight assay for the graft-versus-host activity of rat lymphoid cells. Transplantation. 1970 Sep;10(3):258–266. doi: 10.1097/00007890-197009000-00007. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Webb D. R. Regulation of the immune response by prostaglandins. Clin Immunol Immunopathol. 1980 Jan;15(1):106–122. doi: 10.1016/0090-1229(80)90024-0. [DOI] [PubMed] [Google Scholar]
- Groves M. G., Osterman J. V. Host defenses in experimental scrub typhus: genetics of natural resistance to infection. Infect Immun. 1978 Feb;19(2):583–588. doi: 10.1128/iai.19.2.583-588.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves M. G., Rosenstreich D. L., Taylor B. A., Osterman J. V. Host defenses in experimental scrub typhus: mapping the gene that controls natural resistance in mice. J Immunol. 1980 Sep;125(3):1395–1399. [PubMed] [Google Scholar]
- Ito M., Ralph P., Moore M. A. In vitro stimulation of phagocytosis in a macrophage cell line measured by a convenient radiolabeled latex bead assay. Cell Immunol. 1979 Aug;46(1):48–56. doi: 10.1016/0008-8749(79)90244-2. [DOI] [PubMed] [Google Scholar]
- Jacoby R. O., Bhatt P. N. Genetic resistance to lethal flavivirus encephalitis. I. Infection of congenic mice with Banzi virus. J Infect Dis. 1976 Aug;134(2):158–165. doi: 10.1093/infdis/134.2.158. [DOI] [PubMed] [Google Scholar]
- Jerrells T. R., Dean J. H., Richardson G. L., McCoy J. L., Herberman R. B. Role of suppressor cells in depression of in vitro lymphoproliferative responses of lung cancer and breast cancer patients. J Natl Cancer Inst. 1978 Oct;61(4):1001–1009. [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978 Mar 1;147(3):952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch N. R., Salomon J. C. Tumor growth inhibition and potentiation of immunotherapy by indomethacin in mice. J Natl Cancer Inst. 1979 Jan;62(1):117–121. [PubMed] [Google Scholar]
- Moeller G. R., Terry L., Snyderman R. The inflammatory response and resistance to endotoxin in mice. J Immunol. 1978 Jan;120(1):116–123. [PubMed] [Google Scholar]
- Nacy C. A., Osterman J. V. Host defenses in experimental scrub typhus: role of normal and activated macrophages. Infect Immun. 1979 Nov;26(2):744–750. doi: 10.1128/iai.26.2.744-750.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks S. C., Jr, Osterman J. V., Hetrick F. M. Plaque assay and cloning of scrub typhus rickettsiae in irradiated L-929 cells. J Clin Microbiol. 1977 Jul;6(1):76–80. doi: 10.1128/jcm.6.1.76-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Rikihisa Y., Ito S. Intracellular localization of Rickettsia tsutsugamushi in polymorphonuclear leukocytes. J Exp Med. 1979 Sep 19;150(3):703–708. doi: 10.1084/jem.150.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. J., Phares H. F., Graessle O. E. Effects of indomethacin on acute, subacute, and latent infections in mice and rats. J Bacteriol. 1968 Jul;96(1):6–13. doi: 10.1128/jb.96.1.6-13.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Phillips S. M., Osterman J. V. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun. 1976 Jul;14(1):39–46. doi: 10.1128/iai.14.1.39-46.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


