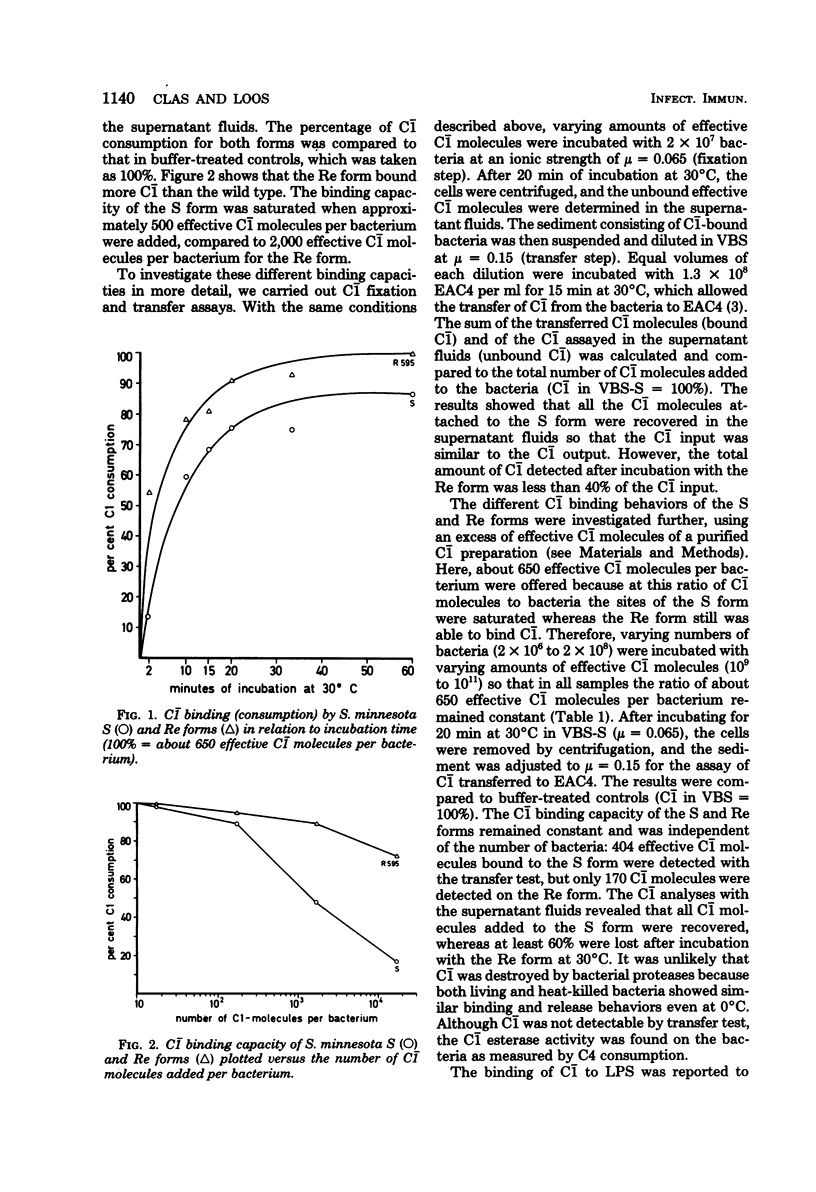
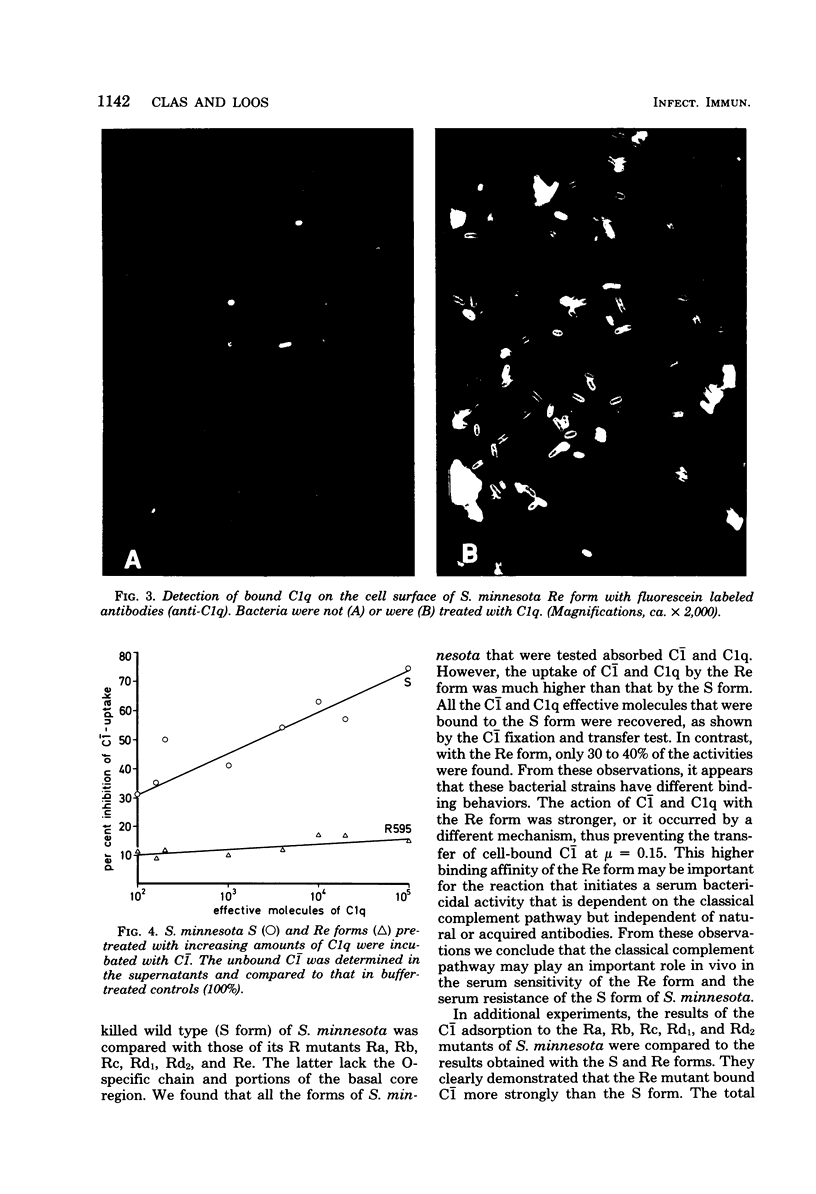
Abstract
Strong bactericidal effects of normal guinea pig and human sera against the Salmonella minnesota S form and an R form (Re) depend on Ca2+, complement component C4, and subcomponent C1q of complement component C1. Therefore, the interaction of C1 and C1q with these forms was investigated. The bacteria directly bound subcomponent C1q, as demonstrated by fixation and transfer tests and by fluorescent methods. Binding of macromolecular C1 was shown by fixation and transfer tests and by C4 consumption. C1 fixation and transfer tests provide evidence that C1 and C1q were bound more tightly to the Re form than to the S form. At physiological ionic strength, all cell-bound molecules were released from the S form, whereas at least 60% remained on the cell surface of the Re form. The Re form showed another binding behavior for C1: preincubation of bacteria with purified C1q totally prevented C1 uptake by the S form, compared to only 10% inhibition of the uptake by the Re form. Therefore, we conclude that macromolecular C1 is bound differently by the S form than by the Re form. The analysis of five other core-deficient mutants of S. minnesota (Ra, Rb, Rc, Rd1, and Rd2) revealed that the difference could be explained by a deficiency of the O-specific polysaccharide. In contrast, all the C1q bound to Ra, Rb, and Rc mutants was detectable by the transfer test. Therefore, we postulate that binding of macromolecular C1 to these mutants must be due to an additional C1 subcomponent besides C1q.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomew R. M., Esser A. F. Differences in activation of human and guinea pig complement by retroviruses. J Immunol. 1978 Nov;121(5):1748–1751. [PubMed] [Google Scholar]
- Borsos T., Rapp H. J. Hemolysin titration based on fixation of the activated first component of complement: evidence that one molecule of hemolysin suffices to sensitize an erythrocyte. J Immunol. 1965 Sep;95(3):559–566. [PubMed] [Google Scholar]
- Clas F., Loos M. Killing of the S and Re forms of Salmonella minnesota via the classical pathway of complement activation in guinea-pig and human sera. Immunology. 1980 Aug;40(4):547–556. [PMC free article] [PubMed] [Google Scholar]
- Colten H. R., Bond H. E., Borsos T., Rapp H. J. Purification of the first component of complement by zonal ultracentrifugation. J Immunol. 1969 Oct;103(4):862–865. [PubMed] [Google Scholar]
- Dierich M. P., Bitter-Suermann D., König W., Hadding U., Galanos C., Rietschel E. T. Analysis of bypass activation of C3 by endotoxic LPS and loss of this potency. Immunology. 1973 Apr;24(4):721–733. [PMC free article] [PubMed] [Google Scholar]
- Gewurz H., Shin H. S., Mergenhagen S. E. Interactions of the complement system with endotoxic lipopolysaccharide: consumption of each of the six terminal complement components. J Exp Med. 1968 Nov 1;128(5):1049–1057. doi: 10.1084/jem.128.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos M., Bitter-Suermann D., Dierich M. Interaction of the first (C1), the second (C2) and the fourth (C4) component of complement with different preparations of bacterial lipopolysaccharides and with lipid A. J Immunol. 1974 Mar;112(3):935–940. [PubMed] [Google Scholar]
- Loos M., Wellek B., Thesen R., Opferkuch W. Antibody-independent interaction of the first component of complement with Gram-negative bacteria. Infect Immun. 1978 Oct;22(1):5–9. doi: 10.1128/iai.22.1.5-9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHAEL J. G., WHITBY J. L., LANDY M. Studies on natural antibodies to gram-negative bacteria. J Exp Med. 1962 Jan 1;115:131–146. doi: 10.1084/jem.115.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus R. L., Shin H. S., Mayer M. M. An alternate complement pathway: C-3 cleaving activity, not due to C4,2a, on endotoxic lipopolysaccharide after treatment with guinea pig serum; relation to properdin. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1351–1354. doi: 10.1073/pnas.68.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Rowley D. Sensitivity of rough gram-negative bacteria to the bactericidal action of serum. J Bacteriol. 1968 May;95(5):1647–1650. doi: 10.1128/jb.95.5.1647-1650.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERZL J., PESAK V., KOSTKA J., JILEK M. THE RELATION BETWEEN THE BACTERICIDAL ACTIVITY OF COMPLEMENT AND THE CHARACTER OF THE BACTERIAL SURFACES. Folia Microbiol (Praha) 1964 Sep;89:284–298. doi: 10.1007/BF02873307. [DOI] [PubMed] [Google Scholar]
- Schultz D. R., Loos M., Bub F., Arnold P. I. Differentiation of hemolytically active fluid-phase and cell-bound human C1q by an ant venom-derived polysaccharide. J Immunol. 1980 Mar;124(3):1251–1257. [PubMed] [Google Scholar]
- Volanakis J. E., Stroud R. M. Rabbit C1q: purification, functional and structural studies. J Immunol Methods. 1972 Nov;2(1):25–34. doi: 10.1016/0022-1759(72)90014-2. [DOI] [PubMed] [Google Scholar]




