Abstract
Background
Most countries carrying out campaigns of bovine tuberculosis (TB) eradication impose a ban on the use of mycobacterial vaccines in cattle. However, vaccination against paratuberculosis (PTB) in goats is often allowed even when its effect on TB diagnosis has not been fully evaluated. To address this issue, goat kids previously vaccinated against PTB were experimentally infected with TB.
Results
Evaluation of interferon-γ (IFN-γ) secretion induced by avian and bovine tuberculins (PPD) showed a predominant avian PPD-biased response in the vaccinated group from week 4 post-vaccination onward. Although 60% of the animals were bovine reactors at week 14, avian PPD-biased responses returned at week 16. After challenge with M. caprae, the IFN-γ responses radically changed to show predominant bovine PPD-biased responses from week 18 onward. In addition, cross-reactions with bovine PPD that had been observed in the vaccinated group at week 14 were reduced when using the M. tuberculosis complex-specific antigens ESAT-6/CFP-10 and Rv3615c as new DIVA (differentiation of infected and vaccinated animals) reagents, which further maintained sensitivity post-challenge. Ninety percent of the animals reacted positively to the tuberculin cervical comparative intradermal test performed at 12 weeks post-infection. Furthermore, post-mortem analysis showed reductions in tuberculous lesions and bacterial burden in some vaccinated animals, particularly expressed in terms of the degree of extrapulmonary dissemination of TB infection.
Conclusions
Our results suggest a degree of interference of PTB vaccination with current TB diagnostics that can be fully mitigated when using new DIVA reagents. A partial protective effect associated with vaccination was also observed in some vaccinated animals.
Keywords: Tuberculosis, Paratuberculosis, Goat, Vaccine, Diagnostic, Interferon gamma
Background
Caprine tuberculosis (TB), caused either by Mycobacterium bovis or M. caprae, and paratuberculosis (PTB), caused by M. avium subsp. paratuberculosis (Map), are endemic diseases in goat herds of the Iberian Peninsula [1-3]. Both infections may have an impact in terms of economic loss. Moreover, M. caprae and Map can be transmitted between domestic hosts and wildlife species [2,4]. In addition, M. caprae is a zoonotic agent [5-7] and Map has been associated with Crohn’s disease [8,9].
Control of PTB in small ruminants can be facilitated by vaccination because the bacterial burden is greatly reduced, containing the spread of the disease and preventing clinical expression [10]. Furthermore, vaccination against PTB with Map-killed formulations has been demonstrated to be an economically efficient strategy to achieve control of the disease in small ruminants [11-13] and cattle [14].
On the other hand, it has been shown that exposure to M. avium can interfere with the diagnosis of M. tuberculosis complex organisms such as M. bovis or M. caprae[15-17]. Similarly, there are some reports showing that natural infection with Map compromises TB diagnosis in cattle [18,19] and goats [20]. Therefore, PTB could also affect the specificity of diagnostic tests used in TB control programs. The Spanish eradication program of bovine TB expressly forbids the use of Map-based vaccines in cattle because of this potential interference with TB diagnostics. In contrast, vaccination against PTB in goats is permitted with the exception of a minority of herds that are subjected to TB control in some regions. To date, the potential effect of Map-based vaccines on the diagnosis of TB has only been investigated post hoc under field conditions [21,22].
It has been reported that TB diagnostic tests currently used in cattle, such as the skin test or the interferon-gamma (IFN-γ) assay, can be used for diagnosis of TB in goats [23-25]. Moreover, in recent years, new IFN-γ assays based on antigens secreted by active growing bacilli, such as the peptide cocktail ESAT-6/CFP-10 (E/C) or Rv3615c [26,27], have been developed as alternatives to bovine and avian tuberculins. These antigens are not present in either M. bovis BCG or Map and can be viewed as novel DIVA (differentiation of infected and vaccinated animals) regents able to distinguish TB-infected from TB- or PTB-vaccinated animals.
The aim of the present work was to assess how vaccination against Map affects standard and novel diagnostic tests in goats. Thus, we evaluated the interference of a commercial Map-killed vaccine on TB diagnosis in goats vaccinated and subsequently challenged with M. caprae. The objectives of the present work were: (1) to investigate the possible interference of vaccination before and after M. caprae infection on TB diagnostic tests (single and comparative skin tests and IFN-γ assay); (2) to assess the usefulness of DIVA-peptide candidates E/C and Rv3615c; and (3) to evaluate immunological and post-mortem indicators of the effects of PTB vaccination on M. caprae infection.
Results
Assessment of diagnostic tests
The effect of Map vaccination and subsequent infection with M. caprae on TB diagnostic assays based on cell-mediated immunity was assessed during this study. Four vaccinated goats were subjected to the tuberculin cervical comparative intradermal test (CIT) at week 14, and all were negative for TB and correctly classified as avian reactors. However, the four goats were classified as positive for TB if only the bovine tuberculin (PPD-B) result (as a single intradermal tuberculin test [SIT]) was considered (data not shown), which indicated that the specificity of the SIT was severely compromised by PTB vaccination. The skin test was repeated in all goats at week 26 (12 weeks post-infection [wpi] with M. caprae). In the vaccinated group, 9 of 10 goats were bovine reactors (positive for TB) using the CIT (1 goat was an avian reactor), but all goats were classified as TB reactors by applying the SIT interpretation. All unvaccinated goats were positive for TB with both tests (Table 1). Differences between groups in the mean specific thickness increase for each tuberculin were statistically significant only for ΔPPD-A (avian tuberculin), with a mean increase of 14.4 mm (11.9–17, 95% CI) in the vaccinated group versus a mean increase of 10.8 mm (8.8–12.9, 95% CI) in the control group (p < 0.05) (Table 1). Differences were also found in the mean value of ΔPPD-B minus ΔPPD-A. This value was significantly lower in the vaccinated group (5.2 mm, 2.9–7.5, 95% CI) than in the unvaccinated group (10.3 mm, 8.8–11.9, 95% CI) (p < 0.005) (Table 1).
Table 1.
Increases in skin-fold thickness and corresponding results of tuberculin skin tests
| Group | Goat | ΔAva | ΔBovb | ΔBov-ΔAv | CIT | SIT |
|---|---|---|---|---|---|---|
| Vaccinated |
1 |
14.2 |
22.6 |
8.4 |
+ |
+ |
| 2 |
15.2 |
26.0 |
10.8 |
+ |
+ |
|
| 3 |
17.6 |
20.9 |
3.3 |
+ |
+ |
|
| 4 |
10.6 |
19.5 |
8.9 |
+ |
+ |
|
| 5 |
16.2 |
18.1 |
1.9 |
+ |
+ |
|
| 6 |
14.5 |
16.5 |
2.0 |
+ |
+ |
|
| 7 |
6.2 |
14.2 |
8.0 |
+ |
+ |
|
| 8 |
14.6 |
17.4 |
2.8 |
+ |
+ |
|
| 9 |
13.2 |
19.4 |
6.2 |
+ |
+ |
|
| 10 |
22.0 |
21.7 |
−0.3 |
- e |
+ |
|
| Mean (95% CI) |
14.4 (11.9-17)*f |
19.6 (17.5-21.7) |
5.2 (2.9-7.5)** |
|
|
|
| Unvaccinated |
11 |
11.3 |
19.7 |
8.4 |
+ |
+ |
| |
12 |
15.0 |
24.1 |
9.1 |
+ |
+ |
| |
13 |
3.3 |
14.5 |
11.2 |
+ |
+ |
| |
14 |
9.8 |
18.3 |
8.5 |
+ |
+ |
| |
15 |
11.3 |
21.2 |
9.9 |
+ |
+ |
| |
16 |
8.7 |
24.6 |
16.0 |
+ |
+ |
| |
17 |
11.4 |
19.1 |
7.7 |
+ |
+ |
| |
18 |
10.1 |
22.5 |
12.4 |
+ |
+ |
| |
19 |
14.6 |
25.8 |
11.2 |
+ |
+ |
| |
20 |
12.7 |
21.6 |
8.9 |
+ |
+ |
| Mean (95% CI) | 10.8 (8.8-12.9) | 21.1 (19–23.2) | 10.3 (8.8-11.9) |
Test results are scored as (−) negative or (+) positive.
a ΔAv, increase in skin-fold thickness (mm) 72 h after PPD-A application.
b ΔBov, increase in skin-fold thickness (mm) 72 h after PPD-B application.
c CIT, Cervical comparative intradermal test.
d SIT, Single intradermal test.
e Avian reactor.
f *p < 0.05; **p < 0.005 (unpaired two-sample Student’s t-test).
The tuberculin-based IFN-γ test was performed every 2 weeks throughout the experiment. The kinetics of IFN-γ responses of the vaccinated group showed a PPD-A-biased response, which switched to a PPD-B-biased mean response 4 weeks after M. caprae infection (at week 18 of the experiment) (Figure 1). In the unvaccinated control group, a PPD-B-biased response was obtained 4 weeks after M. caprae infection, whereas no response was observed before this time point in any of the goats (data not shown). When the results were analyzed individually (Table 2), Map-vaccinated goats began showing responses to PPD-A (“avian reactors”) at week 2 after vaccination (2 of 10 goats), and all of them subsequently became avian reactors between weeks 4 and 10. However, 1 of 10 goats became a “bovine reactor” (positive to PPD-B) at week 12 of the experiment (12 weeks post-vaccination [wpv]), and this figure rose to 6 of 10 at week 14, in the blood samples taken just before infection with M. caprae. Interestingly, in the next blood sampling at 2 wpi (week 16), again 8 of 10 goats showed a stronger reaction to PPD-A than to PPD-B, and the 2 other vaccinated goats were negative to both tuberculins. By contrast, at week 18 (4 weeks after M. caprae challenge), 9 of 10 (90%) of the vaccinated goats were bovine reactors. At week 20, all goats were positive to PPD-B (bovine reactors), and these results were maintained during the rest of the trial with the exception of one goat that tested bovine-negative at week 24 and another that was an avian reactor at week 28 (see Table 2). In the unvaccinated group, all goats were bovine reactors at week 18 and remained so until the end of the experiment, with the exception of two goats that were negative at week 24 but returned to positivity at weeks 26 and 28 (data not shown).
Figure 1.
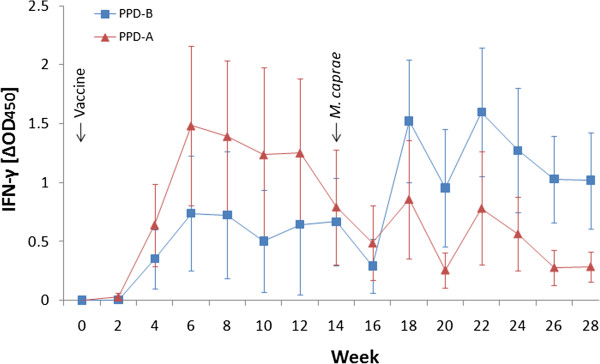
Kinetics of IFN-γ released after stimulation of whole blood with tuberculins in the vaccinated group. Avian tuberculin (PPD-A, ▲), bovine tuberculin (PPD-B, ■). Results are expressed as mean ΔOD450 (mean of OD450 of antigen-stimulated values with unstimulated values subtracted) ± 95% confidence interval.
Table 2.
Results of the standard IFN-γ assay (Bovigam™)
|
Goat |
Week |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | 24 | 26 | 28 | |
| 1 |
N |
N |
Av |
Av |
Av |
Av |
Av |
Av |
Av |
Bov |
Bov |
Bov |
Bov |
Bov |
Bov |
| 2 |
N |
Av |
Av |
Av |
Av |
Av |
Av |
Av |
Av |
Bov |
Bov |
Bov |
Bov |
Bov |
Bov |
| 3 |
N |
N |
Av |
Av |
Av |
Av |
Av |
Bov |
Av |
Bov |
Bov |
Bov |
Bov |
Bov |
Bov |
| 4 |
N |
N |
Av |
Av |
Av |
Av |
Bov |
Bov |
Av |
Bov |
Bov |
Bov |
N |
Bov |
Bov |
| 5 |
N |
N |
Av |
Av |
Av |
Av |
Av |
Bov |
N |
Bov |
Bov |
Bov |
Bov |
Bov |
Bov |
| 6 |
N |
N |
Av |
Av |
Av |
Av |
Av |
Av |
Av |
Bov |
Bov |
Bov |
Bov |
Bov |
Bov |
| 7 |
N |
N |
Av |
Av |
Av |
Av |
Av |
Bov |
N |
Bov |
Bov |
Bov |
Bov |
Bov |
Av |
| 8 |
N |
N |
Av |
Av |
Av |
Av |
Av |
Av |
Av |
Av |
Bov |
Bov |
Bov |
Bov |
Bov |
| 9 |
N |
Av |
Av |
Av |
Av |
Av |
Av |
Bov |
Av |
Bov |
Bov |
Bov |
Bov |
Bov |
Bov |
| 10 | N | N | Av | Av | Av | Av | Av | Bov | Av | Bov | Bov | Bov | Bov | Bov | Bov |
Test results are classified as: Av, positive response to PPD-A; Bov, positive response to PPD-B; or N, negative.
Next, we assessed the interference of Map vaccination with the IFN-γ assay by employing the defined antigen reagents E/C and Rv3615c. Responses were assessed at the same five time points described in the Materials and Methods section. At weeks 14 (0 wpi) and 16, all goats were defined as TB-negative (ΔOD450 ≤ 0.05) according to the test results. At weeks 20, 24, and 28, 90%, 100%, and 70% of vaccinated goats were positive with the E/C cocktail and 100%, 90%, and 80% were positive with Rv3615c, respectively (Table 3). However, these proportions increased to 100%, 100%, and 90% at weeks 20, 24, and 28, respectively, when the E/C and Rv3615c results were considered together. Very similar results were obtained in the unvaccinated group, in which 100%, 80%, and 90% of goats were positive to E/C at weeks 20, 24, and 28, respectively; 100%, 80%, and 80% of goats were positive to Rv3615c; and 100%, 80%, and 90% of goats were positive again when combining the results for both antigens at the same time points (data not shown).
Table 3.
Results of the IFN-γ assay using two DIVA reagents: ESAT-6/CFP10 (E/C) and Rv3615c
|
Goat |
Week |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
14 |
16 |
20 |
24 |
28 |
|||||
| E/C | Rv3615c | E/C | Rv3615c | E/C | Rv3615c | E/C | Rv3615c | E/C | Rv3615c | |
| 1 |
- |
- |
- |
- |
+ |
+ |
+ |
- |
+ |
- |
| 2 |
- |
- |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
| 3 |
- |
- |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
| 4 |
- |
- |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
| 5 |
- |
- |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
| 6 |
- |
- |
- |
- |
- |
+ |
+ |
+ |
- |
+ |
| 7 |
- |
- |
- |
- |
+ |
+ |
+ |
+ |
- |
+ |
| 8 |
- |
- |
- |
- |
+ |
+ |
+ |
+ |
- |
- |
| 9 |
- |
- |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
| 10 | - | - | - | - | + | + | + | + | + | + |
Test results are classified as (−) negative or (+) positive.
Seropositivity to Map was assessed using the Paratub.Serum-S ELISA kit (Institut Pourquier, Montpellier, France) applying the cut-off defined above. All goats (n = 20) were negative at week 0. Unvaccinated goats also remained negative during the experiment (data not shown). In contrast, 1 of 10 (10%) and 5 of 10 (50%) vaccinated goats were positive or doubtful at weeks 14 (0 wpi) and 28 (14 wpi), respectively, with different degrees of intensity (Table 4). The mean intensity of antibody response (%S/P) increased moderately, but was statistically significant (p < 0.05), from 20% (4%–36%, 95% CI) at week 14 to 55% (19%–91%, 95% CI) at week 28 (2 weeks after CIT).
Table 4.
Seroreactivity to Map (Paratub.Serum-S™ ELISA)
|
Goat |
Week |
|||||
|---|---|---|---|---|---|---|
| |
0 |
14 |
28a |
|||
| Result | S/Pb | Result | S/P | Result | S/P | |
| 1 |
- |
0% |
- |
30% |
+ |
130% |
| 2 |
- |
0% |
- |
0% |
- |
0% |
| 3 |
- |
1% |
- |
8% |
d |
51% |
| 4 |
- |
0% |
- |
3% |
+ |
113% |
| 5 |
- |
0% |
- |
36% |
+ |
56% |
| 6 |
- |
0% |
- |
16% |
- |
19% |
| 7 |
- |
0% |
- |
6% |
- |
4% |
| 8 |
- |
0% |
+ |
86% |
+ |
157% |
| 9 |
- |
0% |
- |
4% |
- |
9% |
| 10 | - | 0% | - | 11% | - | 11% |
Test results are classified as (−) negative, (+) positive, or (d) doubtful.
a Two weeks after CIT.
b S/P, Seroreactivity rates.
Pathology and bacteriology
At necropsy on week 28 (14 wpi), visible pathology typical of TB was observed in the lungs and pulmonary lymph nodes (LN) of all animals irrespective of their vaccination status. Of the seven lung lobes evaluated, the vaccinated group presented an average of three lobes with gross lesions (2–4, 95% CI), slightly lower than the mean of four lobes (3–5, 95% CI) found in the unvaccinated group, but this difference was not statistically significant (p = 0.324).
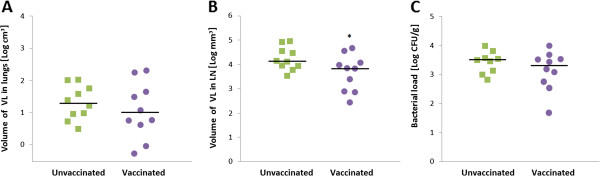
The mean log10-transformed volume of gross lesions in lungs was similar in the unvaccinated (1.3 log10 cm3; 1–1.7, 95% CI) and vaccinated (1.1 log10 cm3; 0.5–1.6, 95% CI) groups (p = 0.470), but values were spread over a wider range in the vaccinated group. By contrast, statistically significant differences between vaccinated and control goats were found after assessing the gross pathology in LN: The unvaccinated animals presented with a mean log10-transformed volume of visibly affected tissue of 3.9 log10 mm3 (3.6–4.3, 95% CI) compared with 3.3 log10 mm3 (2.7–3.8, 95% CI) obtained in the vaccinated group (p < 0.05). The individual pathological parameters are represented in Figure 2A and B.
Figure 2.
Post-mortem analysis as measured by gross pathology and bacterial burden. Results are plotted for individual goats. (A) Total volume of visible lesions (VL) in lungs as log10 cm3. (B) Total volume of VL in respiratory lymph nodes (LN) as log10 mm3. (C) Bacterial load as log10 cfu/g. (●) vaccinated goats, (■) unvaccinated goats. Horizontal lines indicate median values. Significance determined by unpaired t-test: *p < 0.05.
In addition, none of the vaccinated goats showed extrapulmonary gross lesions, whereas dissemination was observed in 4 of 10 unvaccinated goats that presented with gross lesions beyond the thoracic area (2 goats with lesions in retropharyngeal LN, 2 goats with lesions in mesenteric LN, and 1 goat with lesions in the spleen). These lesions were histopathologically confirmed to be tuberculous.
In three animals, focal lesions were also found in the LN draining the Map vaccine inoculation point (axillary, prescapular, or subcutaneous LN). These lesions were attributed to the vaccine inoculation because of their localization and histological characteristics: lesions predominantly necrotizing rather than granulomatous, without Langerhans cells, but surrounded by dispersed polymorphonuclear cells and abundant fibrosis.
Finally, the mycobacterial load in LN was calculated as log10 cfu/g. The bacterial burden in the pulmonary and retropharyngeal LN of all animals (n = 20) ranged from 2 to 4 log10 cfu/g (Figure 2C). The total bacterial load in the unvaccinated group was 3.5 log10 cfu/g (3.2–3.7, 95% CI), slightly higher than that in the vaccinated group (3.1 log10 cfu/g, 2.7–3.6, 95% CI). However, this difference was not statistically significant (p = 0.203).
Cross-sectional analysis
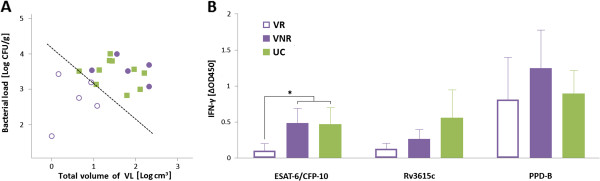
To evaluate the vaccine effect in terms of heterologous protection, post-mortem parameters were also assessed in combination. A mild positive correlation between bacteriological and pathological findings was found by correlating individual logarithmic values of bacterial load and total volume of visible lesions (r = 0.386, p < 0.05). However, a higher dispersion of individual values was observed in the vaccinated group compared with the unvaccinated group (Figure 3A), and a stronger positive correlation was found when only vaccinated goats were assessed (r = 0.523, p < 0.05), whereas results obtained from unvaccinated goats did not show significant correlations (r = -0.274, p > 0.05).
Figure 3.
Protective efficacy measured by post-mortem results, and cross-sectional comparison of IFN-γ results with vaccination outcome. (A) Correlation between pathological and bacteriological parameters of all goats (n = 20). Dashed line (y = −x + 4.16) indicates the threshold defined to divide vaccine responders (VR) and vaccine non-responders (VNR). Symbols represent vaccine cross-protection outcome: (■) unvaccinated controls (UC), (○) VR goats, (●) VNR goats. (B) Specific IFN-γ responses at week 28 of the experiment as determined by ELISA after stimulation of whole blood with PPD-B, ESAT-6/CFP-10, or Rv3615c. Results are represented in relation to vaccine cross-protective outcome and are expressed as mean ΔOD450 ± 95% confidence interval. Significances determined by non-parametric Kruskal-Wallis test: *p < 0.05.
To further analyze the reductions in post-mortem parameters associated with vaccination, a cross-sectional study was performed on the basis of the criteria described in a previous TB vaccine efficacy trial [28]. The vaccinated animals were divided into two subgroups according to the severity of the pathology and the bacterial burden. This division was established by combining the data of the total volume of gross lesions and the bacterial load in LN. The mean between the volume of lesions (log10 cm3) and the bacterial load (log10 cfu) was thereby calculated for each animal, and the cut-off point to classify the animals into categories of distinct vaccination outcomes was defined as the minimum value obtained among the unvaccinated control animals (Figure 3A). Subsequently, a “vaccine responders” (VR) subgroup of five goats and another “vaccine non-responders” (VNR) subgroup of five goats were defined (Figure 3A, open and closed circles).
Once the subgroup division was applied as described in the previous paragraph, the assumed pathological and bacteriological differences between the new subgroups were checked. The total volume of visible lesions and the bacterial load were significantly lower in VR compared with VNR and unvaccinated control (UC) animals (p < 0.05).
The release of IFN-γ specific to E/C, Rv3615c, and PPD-B at week 28 (performed just before sacrifice of the animals) was also compared between the VR, VNR, and UC groups (Figure 3B). A significantly lower IFN-γ to E/C response was found in VR animals than in VNR and UC animals (p < 0.05). The relationship between E/C-specific IFN-γ response and disease severity was confirmed by finding a positive correlation of IFN-γ-specific secretion to E/C at week 28 with the total volume of gross lesions (Spearman rank = 0.546, p < 0.01) and the bacterial load (but at the statistical limit of significance [Spearman rank = 0.379, p = 0.050], data not shown).
Discussion and conclusions
The present experimental trial in goats was designed with the aim to determine the effects of Map vaccination on current TB diagnostic tests and assess the effects of PTB vaccination on TB infection.
All experimental goats became infected irrespective of vaccination status. However, a reduction in pulmonary pathology was observed in some vaccinated individuals compared with the unvaccinated group. Another remarkable finding was that all vaccinated goats showed only TB lesions at the site of infection (i.e., lungs and associated LN) in contrast to the increased dissemination frequency in non-vaccinated animals. This finding is analogous to the results of subcutaneous vaccination against PTB with the Map-killed vaccine in cattle subsequently challenged with Map. The vaccine in those studies induced systemic immunity, preventing bacteremia and, consequently, dissemination of mycobacteria from the primary infection site. However, they did not prevent the establishment of the initial infection [10]. Similar results were obtained in another experimental infection with M. caprae in which three of six (50%) infected goats showed gross TB lesions in mesenteric LN [29]. This outcome is also consistent with findings observed in field cases of TB in goats [30-32].
In this sense, the present results indicate a certain degree of containment of dissemination of the infection from the primary complex, even when a significant reduction in the bacterial load in pulmonary drainage LN has not been observed. Nevertheless, this containment may not be sufficient to effectively prevent excretion of mycobacteria and horizontal transmission within a herd.
The role of the immunological status of vaccinated animals, especially the T-cell response, can be critical in terms of control or spread of the infection from the primary respiratory focus. Similarly, Hope et al. found few lesions at necropsy and smaller bacterial loads in LN in calves inoculated with M. avium and subsequently challenged with M. bovis[17]. The authors suggested that T-cell responses resulting from M. avium infection enhanced a protective secondary response after challenge with M. bovis.
After conducting cross-sectional analysis, we found positive correlations of the IFN-γ response to E/C with pathology severity, and more weakly with bacterial load. A similar result was obtained with the single ESAT-6-specific IFN-γ response compared with pathology scores in goats [30] and calves [33]. Furthermore, a positive correlation between the E/C-specific IFN-γ response and bacterial load was also previously described in goats [24] and cattle [34]. Moreover, after analyzing post-mortem data, clear differences in the pathological and bacteriological results were found within the vaccinated group. The IFN-γ responses of vaccinated goats with low and high post-mortem scores (VR and VNR, respectively) were compared. The significantly lower E/C-specific IFN-γ responses obtained in VR animals demonstrated the capacity of this immunological biomarker to predict the severity of the disease and, by default, to predict the vaccine outcome.
In terms of assessment of TB diagnostic tests, we have adapted the diagnostic tests routinely used in cattle in bovine TB eradication programs. We have also introduced new DIVA reagents to perform the IFN-γ assay. Sensitivities obtained in all tests were higher than those obtained in previous studies in naturally infected goats [20,23]. Notwithstanding this, the enhanced sensitivity described in these works when combining skin tests and IFN-γ tests was also confirmed in the present experimental trial.
The sensitivities of skin tests performed in vaccinated animals (100% and 90% for SIT and CIT, respectively), were higher than those obtained in another study carried out with goat herds naturally co-infected with TB and PTB (71% and 42.7%, respectively) [20]. In this work, the authors reported many animals with false-negative CIT results that showed higher skin-fold thickness to PPD-A than to PPD-B. It is important to note that the sensitivity of CIT in the present study was achieved using strict interpretation of the test. With the standard interpretation (ΔBov-ΔAv ≥ 3 mm), the sensitivity would be reduced to 60%.
By contrast, in our trial, only a slightly higher sensitivity was found when applying the SIT in comparison with the CIT. However, the results showed serious compromise of the specificity of the SIT in vaccinated animals prior to challenge (4 of 4 false-positive reactors), which completely disappeared when using the CIT. These results are in accordance with those found in another study performed in a PTB-vaccinated dairy goat herd free of TB [21]. Similarly, compromise of the specificity of the SIT in PTB-vaccinated deer has also been reported [35,36].
Compared with the SIT, the standard IFN-γ assay seemed to be more robust in terms of specificity. False-positive results were concentrated at the interval between weeks 12 and 14, and then disappeared at week 16 when there was not yet a response to M. caprae infection. After 12 wpv, the IFN-γ responses to PPD-A decreased faster than did those to PPD-B (see Figure 1). As a consequence, 60% of the animals were false-positive “bovine reactors” at week 14. More long-term trials must be performed to study the kinetics of the IFN-γ response to Map vaccination.
Encouragingly, the sensitivity obtained for the standard IFN-γ test after M. caprae challenge was very high considering that elevated whole-blood IFN-γ responses to PPD-A were previously observed in Map-vaccinated cattle [37]. In this sense, from weeks 18 to 28 (4–14 wpi), we only detected a masking due to PPD-A in two vaccinated animals. However, the high IFN-γ responses observed in our study (measured shortly after experimental infection) may decrease with the progression of natural TB infection under field conditions.
In the last decade, much effort has been focused on the development of novel antigens for bovine TB diagnosis that are more sensitive and specific than the avian and bovine tuberculins. This research has already resulted in the identification of several antigens, such as ESAT-6, CFP-10, and Rv3615c, that reduce cross-reactive immune responses to different mycobacterial infections or vaccinations in cattle [26,27,38] and goats [39,40]. In the present study, we showed the capacity of both E/C and Rv3615c to distinguish PTB-vaccinated and TB-infected goats. Furthermore, no differences in sensitivity were observed between the experimental groups. Importantly, when combining the positive results of E/C and Rv3615c IFN-γ assays, the sensitivity was identical to that obtained by the tuberculin-based IFN-γ assay, which is currently being employed as an ancillary test to the skin test in some eradication campaigns. A similar pattern was previously described in cattle experimentally infected with M. bovis, in which the sensitivity increased from 77.9% to 91% when considering the results of Rv3615c and E/C IFN-γ assay together [27].
By contrast, serological responses to the Map vaccine were moderate; only one and four vaccinated goats were seropositive to PTB at weeks 14 and 28, respectively. These data are not in agreement with the 50% positivity previously obtained in PTB-infected goats [41]. Interestingly, a boost effect on the Map ELISA due to CIT might be found at week 28 (2 weeks after tuberculin testing) when comparing the results with the test performed at week 14. The boost effect on the IgG response due to the skin test was previously described in M. bovis-infected cattle [34] and M. caprae-infected goats [24].
Vaccination against Map represents an important advance in controlling PTB and improving the economic balance of affected farms. Therefore, the pros and cons of its application must be exhaustively evaluated. An attractive speculation is that the partial protection to TB infection observed in some PTB-vaccinated animals could indirectly facilitate the control of TB, although long-term field studies are required to confirm this perspective. Moreover, we have demonstrated that to some degree, PTB vaccination interfered (after M. caprae infection) with the sensitivity of tuberculin-based TB diagnostic tests. However, beyond the first 2 weeks post-infection, only 5% false-negative results were obtained in the IFN-γ assay. In addition, these animals reacted positively in more than 80% of the remaining post-challenge tests. Thus, considering the collective basis on which TB tests are usually applied, it is unlikely that an infected herd could be undiagnosed upon extrapolation to a larger population. On the other hand, our results confirm that the interference with the specificity can be fully overcome by using defined DIVA reagents. Thus, developing and subsequently introducing these reagents into routine diagnostics could represent an improvement in both strategies: control of PTB by vaccination and control of TB by rapid detection of infected animals.
Methods
Experimental animals
The experiment was carried out in 20 Murciano-Granadina female goats between 2 and 3 months of age from a herd free of TB and PTB in the Region of Murcia (southwest Spain). Before the experiment, goats were submitted to standard tests for diagnosis of TB: the CIT and IFN-γ assay (Bovigam™; Prionics AG, Schlieren, Switzerland). Experimental animals were confirmed to be negative for both tests. In addition, all goats had negative results for PTB (Paratub.Serum-S; Institut Pourquier, Montpellier, France).
Vaccination and infection
A group of 10 goats was subcutaneously inoculated with a single dose of 1 ml (2.5 mg/ml) of Silirum® (CZ Veterinaria, Porriño, Pontevedra, Spain), a commercial heat-inactivated and oil-adjuvanted Map vaccine. Another group of 10 goats was maintained as unvaccinated controls. After 14 weeks, all animals were housed and acclimatized in two experimental boxes (level 3 biocontainment) for 1 week prior to experimental infection.
The field strain of M. caprae SB0416 (http://www.mbovis.org) was used as the inoculum and was prepared as previously described [24]. Goats were anesthetized by intravenous administration of 4 to 6 mg/kg of propofol (Propofol Lipuro®) and 0.2 mg/kg of midazolam (Dormicum®), and subsequently inoculated with approximately 1.5 × 103 cfu of M. caprae suspended in 0.5 ml of phosphate-buffered saline (PBS) by the endobronchial route [24].
All experimental procedures were approved by the Animal Welfare Committee of the Universitat Autònoma de Barcelona in agreement with the European Union Laws for protection of experimental animals.
Sampling and clinical observations
Animals were observed twice daily at feeding time. Prior to vaccination and infection and every 2 weeks throughout the experiment, animals were weighed and their rectal temperature was taken and blood samples were collected from the jugular vein into heparinized blood tubes for immunological studies.
Diagnostic tests
Skin tests
Skin tests were carried out in all goats at 26 wpv and 12 wpi. Four goats were also tested at 14 wpv (the remaining goats were not tested to minimize the potential effect of the intradermal tuberculin inoculation on the IFN-γ assay). Tests were performed by inoculating 0.1 ml (2500 IU) of bovine (PPD-B) and avian (PPD-A) tuberculins (Porriño, Pontevedra, Spain) on the left and the right side of the neck, respectively. The skin-fold thickness was recorded just before inoculation and after 72 h. Results were interpreted either by considering only the increase in thickness for PPD-B (as in a single intradermal test, SIT) or for both PPD-B and PPD-A (as in a comparative intradermal test, CIT). The results were read with the strict interpretation used in bovine TB eradication programs. Goats were considered positive to SIT if the increase in skin-fold thickness at the PPD-B site was ≥2 mm. For the CIT interpretation, goats were considered positive if the increase in skin-fold thickness at the PPD-B site was ≥2 mm and higher than the increase at the PPD-A site.
IFN-γ assay
Whole-blood cultures were performed every 2 weeks in 96-well cell culture plates. Heparinized blood (1 ml per well) was incubated for 24 h at 37°C and 5% CO2 with either PPD-B or PPD-A at a final concentration of 10 μg/ml. Phytohemagglutinin (PHA) (Sigma-Aldrich, Steinheim, Germany) was used as positive control at 10 μg/ml; PBS was used as a negative control. In addition, at 0, 2, 6, 10, and 14 wpi, the peptide cocktail ESAT-6/CFP-10 (E/C) and Rv3615c (Animal Health and Veterinary Laboratories Agency, Weybridge, UK) were used at a final concentration of 5 μg/ml. Plasma supernatants were collected after centrifugation and transferred to a 96-well plate. The IFN-γ enzyme-linked immunosorbent assay (ELISA) was performed according to the manufacturer’s instructions (Bovigam™). Optical density was measured at 450 nm (OD450) using a microplate reader (PowerWave XS; BioTek, Winooski, VT). Results were expressed as ΔOD450 (OD450 of antigen-stimulated sample minus OD450 of non-stimulated sample). A result was positive when ΔOD450 > 0.05, whereas in the case of the standard test (using PPD-A and PPD-B as stimuli), a result was positive when ΔOD450 of PPD-B > 0.05 and OD450 of PPD-B > OD450 of PPD-A.
Map IgG ELISA
Plasma samples were analyzed in duplicate for antibodies to Map with Paratub.Serum-S™ ELISA before vaccination (week 0), before infection (week 14), and before the end of the experiment (week 28). The assay was performed according to the manufacturer’s instructions. The ELISA reaction was also measured at OD450. The results were expressed as S/P (%), calculated as (mean of sample OD450 - mean of negative control OD450)/(mean of positive control OD450 - mean of negative control OD450) × 100. According to the criterion described by the manufacturer, a sample with an S/P of <45% was considered to be negative, of <55% and ≥45% to be doubtful, and of ≥55% to be positive.
Post-mortem examination
All goats were euthanized at 14 wpi (28 wpv) with an overdose of sodium pentobarbital administered intravenously. They were immediately necropsied to assess the presence and volume of tuberculous lesions in the lungs and pulmonary LN.
Lungs
Ten percent-buffered formalin was poured into the trachea, which then was tied, and the lungs were subsequently immersed in a container with formalin. After fixation, the lungs were sliced at 4- to 5-mm intervals, and each slice was photographed. Gross lesions were analyzed using image analyzer software (ImageJ 1.43u; National Institutes of Health, USA). The volume of gross lesions in each slice was calculated by multiplying the affected area and slice thickness. The total volume of gross lesions was calculated by adding the partial volumes of gross lesions obtained for each slice.
Lymph nodes
The number of gross lesions and their diameter were recorded for each LN at the time of necropsy. Data were recorded by the same pathologist to ensure measurement consistency. LN pathology scoring was calculated by the approximated total volume of granulomas per sample, calculated using the sphere formula (4/3 × π × r3). After pathological evaluation, the whole LN was processed for bacterial culture to calculate the bacterial load.
Bacterial count
Cranial and caudal mediastinal, tracheobronchial, and retropharyngeal LN were individually weighed and homogenized with 10 ml of sterile distilled water using a tissue homogenizer (Masticator; IUL Instruments, Barcelona, Catalonia, Spain). Homogenates were decontaminated with a final concentration of 0.35% w/v hexadecylpyridinium chloride [42] for 15 min with orbital shaking. Decontaminated homogenates were centrifuged at 2471 × g for 30 min, and pellets were resuspended in 10 ml of PBS containing 0.05% Tween 80. Aliquots of 0.1 ml of a 10-fold serial dilution of each homogenate were plated on Middlebrook 7H11 agar (BD Diagnostics, Sparks, MD). Plates were incubated at 37°C for 28 days, and colonies were then counted and the bacterial load (cfu/g) for each sample was calculated.
Statistical analysis
Student’s unpaired two-sample t-test was used for comparisons between the groups in terms of differences in thickness increases in the CIT, S/P values of Map IgG ELISA, number of affected lung lobes, logarithm-transformed data of volumes of gross lesions (log10 mm3), and bacterial loads in the LN (log10 cfu/g). Correlations between pathology and bacterial burden were assessed using linear regression analysis, whereas the non-parametric Spearman rank test was used to analyze correlations between IFN-γ responses (ΔOD450) and post-mortem data. After cross-sectional analysis, IFN-γ responses and post-mortem parameters in the resulting groups were compared by applying the non-parametric Kruskal-Wallis test and Dunn’s post hoc multiple comparison test. The normality of the data and the homogeneity of variances between treatment groups were assessed using the Shapiro-Wilk and Levene tests, respectively. Statistical analysis of the data was performed using SPSS Statistical Package version 17.0 (IBM Inc., Chicago, IL).
Competing interests
The co-author Eugenia Puentes belongs to CZ Veterinaria S.A., which produces Silirum® as a prophylactic vaccine against PTB. This does not alter the author’s adherence to the journal’s policy on sharing data. The rest of the authors declare that they have no competing interests.
Authors’ contributions
BPV, RJ, and MD conceived and designed the experiments, analyzed the data, and drafted the manuscript. Regarding conduction of experiments, MD, SL-S, and MN performed the necropsy and evaluated the pathological records, and BPV and MM performed the immunological and bacteriological assays. JG, HMV, BVR, and EP contributed substantively to the scientific discussion of the results. All authors have read and approved the final manuscript.
Contributor Information
Bernat Pérez de Val, Email: bernat.perez@cresa.uab.cat.
Miquel Nofrarías, Email: miquel.nofrarias@cresa.uab.cat.
Sergio López-Soria, Email: sergio.lopez@cresa.uab.cat.
Joseba M Garrido, Email: jgarrido@neiker.net.
H Martin Vordermeier, Email: Martin.Vordermeier@ahvla.gsi.gov.uk.
Bernardo Villarreal-Ramos, Email: Bernardo.Villarreal@ahvla.gsi.gov.uk.
Maite Martín, Email: maite.martin@cresa.uab.cat.
Eugenia Puentes, Email: e.puentes@czveterinaria.com.
Ramón A Juste, Email: rjuste@neiker.net.
Mariano Domingo, Email: mariano.domingo@cresa.uab.cat.
Acknowledgements
This study was founded by European Union Project TB-STEP (FP7-KBBE-2007-1-3-04, no. 212414).
We would like to thank Félix García, Ana Andaluz, and Xavier Moll from the Department of Animal Medicine and Surgery of the Universitat Autònoma de Barcelona for their assistance in performing the experimental infection.
We also thank the staff members of the Level 3 Biocontainment Unit of CReSA for their technical and clinical assistance.
We are grateful to the Animal Health and Veterinary Laboratories Agency for supplying the mycobacterial peptide cocktails used as DIVA reagents.
References
- de Juan L, Alvarez J, Aranaz A, Rodriguez A, Romero B, Bezos J, Mateos A, Dominguez L. Molecular epidemiology of Types I/III strains of Mycobacterium avium subspecies paratuberculosis isolated from goats and cattle. Vet Microbiol. 2006;115(1–3):102–110. doi: 10.1016/j.vetmic.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Duarte EL, Domingos M, Amado A, Botelho A. Spoligotype diversity of Mycobacterium bovis and Mycobacterium caprae animal isolates. Vet Microbiol. 2008;130(3–4):415–421. doi: 10.1016/j.vetmic.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Rodriguez S, Bezos J, Romero B, de Juan L, Alvarez J, Castellanos E, Moya N, Lozano F, Javed MT, Saez-Llorente JL, Liebana E, Mateos A, Dominguez L, Aranaz A. The Spanish Network on Surveillance and Monitoring of Animal Tuberculosis. Mycobacterium caprae Infection in Livestock and Wildlife, Spain. Emerg Infect Dis. 2011;17(3):532–535. doi: 10.3201/eid1703.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson K, Alvarez J, Bakker D, Biet F, de Juan L, Denham S, Dimareli Z, Dohmann K, Gerlach GF, Heron I, Kopecna M, May L, Pavlik I, Sharp JM, Thibault VC, Willemsen P, Zadoks RN, Greig A. Occurrence of Mycobacterium avium subspecies paratuberculosis across host species and European countries with evidence for transmission between wildlife and domestic ruminants. BMC Microbiol. 2009;9:212. doi: 10.1186/1471-2180-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubica T, Rusch-Gerdes S, Niemann S. Mycobacterium bovis subsp. caprae caused one-third of human M. bovis-associated tuberculosis cases reported in Germany between 1999 and 2001. J Clin Microbiol. 2003;41(7):3070–3077. doi: 10.1128/JCM.41.7.3070-3077.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetnic Z, Katalinic-Jankovic V, Sostaric B, Katalinic-Jankovic V, Obrovac M, Marjanovic S, Benic M, Kirin BK, Katalinic-Jankovic V. Mycobacterium caprae in cattle and humans in Croatia. Int J Tuberc Lung Dis. 2007;11:658. [PubMed] [Google Scholar]
- Prodinger WM, Brandstatter A, Naumann L, Pacciarini M, Kubica T, Boschiroli ML, Aranaz A, Nagy G, Cvetnic Z, Ocepek M, Skrypnyk A, Erler W, Niemann S, Pavlik I, Moser I. Characterization of mycobacterium caprae isolates from Europe by mycobacterial interspersed repetitive unit genotyping. J Clin Microbiol. 2005;43(10):4984–4992. doi: 10.1128/JCM.43.10.4984-4992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant IR. Zoonotic potential of Mycobacterium avium ssp. paratuberculosis: the current position. J Appl Microbiol. 2005;98(6):1282–1293. doi: 10.1111/j.1365-2672.2005.02598.x. [DOI] [PubMed] [Google Scholar]
- Juste RA, Elguezabal N, Pavón A, Garrido JM, Geijo M, Sevilla I, Cabriada JL, Tejada A, García-Campos F, Casado R, Ochotorena I, Izeta A. Association between Mycobacterium avium subsp. paratuberculosis DNA in blood and cellular and humoral immune response in inflammatory bowel disease patients and controls. Int J Infect Dis. 2009;13(2):247–254. doi: 10.1016/j.ijid.2008.06.034. [DOI] [PubMed] [Google Scholar]
- Uzonna JE, Chilton P, Whitlock RH, Habecker PL, Scott P, Sweeney RW. Efficacy of commercial and field-strain Mycobacterium paratuberculosis vaccinations with recombinant IL-12 in a bovine experimental infection model. Vaccine. 2003;21(23):3101–3109. doi: 10.1016/S0264-410X(03)00261-5. [DOI] [PubMed] [Google Scholar]
- Reddacliff L, Eppleston J, Windsor P, Whittington R, Jones S. Efficacy of a killed vaccine for the control of paratuberculosis in Australian sheep flocks. Vet Microbiol. 2006;115(1–3):77–90. doi: 10.1016/j.vetmic.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Singh SV, Singh PK, Singh AV, Sohal JS, Gupta VK, Vihan VS. Comparative efficacy of an indigenous ‘inactivated vaccine’ using highly pathogenic field strain of Mycobacterium avium subspecies paratuberculosis ‘Bison type’ with a commercial vaccine for the control of Capri-paratuberculosis in India. Vaccine. 2007;25(41):7102–7110. doi: 10.1016/j.vaccine.2007.07.054. [DOI] [PubMed] [Google Scholar]
- Juste RA, Casal J. An economic and epidemiologic simulation of different control strategies for ovine paratuberculosis. Prev Vet Med. 1993;15(2–3):101–115. [Google Scholar]
- Juste R, Alonso-Hearn M, Molina E, Geijo M, Vazquez P, Sevilla I, Garrido J. Significant reduction in bacterial shedding and improvement in milk production in dairy farms after the use of a new inactivated paratuberculosis vaccine in a field trial. BMC Res Notes. 2009;2(1):233. doi: 10.1186/1756-0500-2-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadori M, Tagliabue S, Lauzi S, Finazzi G, Lombardi G, Telo P, Pacciarini L, Bonizzi L. Diagnosis of Mycobacterium bovis infection in calves sensitized by mycobacteria of the avium/intracellulare group. J Vet Med B Infect Dis Vet Public Health. 2002;49(2):89–96. doi: 10.1046/j.1439-0450.2002.00513.x. [DOI] [PubMed] [Google Scholar]
- Howard CJ, Kwong LS, Villarreal-Ramos B, Sopp P, Hope JC. Exposure to Mycobacterium avium primes the immune system of calves for vaccination with Mycobacterium bovis BCG. Clin Exp Immunol. 2002;130(2):190–195. doi: 10.1046/j.1365-2249.2002.01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope JC, Thom ML, Villarreal-Ramos B, Vordermeier HM, Hewinson RG, Howard CJ. Exposure to Mycobacterium avium induces low-level protection from Mycobacterium bovis infection but compromises diagnosis of disease in cattle. Clin Exp Immunol. 2005;141(3):432–439. doi: 10.1111/j.1365-2249.2005.02882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez J, de Juan L, Bezos J, Romero B, Sáez JL, Marqués S, Domínguez C, Mínguez O, Fernández-Mardomingo B, Mateos A, Domínguez L, Aranaz A. Effect of paratuberculosis on the diagnosis of bovine tuberculosis in a cattle herd with a mixed infection using interferon-gamma detection assay. Vet Microbiol. 2009;135(3–4):389–393. doi: 10.1016/j.vetmic.2008.09.060. [DOI] [PubMed] [Google Scholar]
- Muskens J, van Zijderveld F, Eger A, Bakker D. Evaluation of the long-term immune response in cattle after vaccination against paratuberculosis in two Dutch dairy herds. Vet Microbiol. 2002;86(3):269–278. doi: 10.1016/S0378-1135(02)00006-8. [DOI] [PubMed] [Google Scholar]
- Álvarez J, de Juan L, Bezos J, Romero B, Sáez JL, Gordejo FJR, Briones V, Moreno MÁ, Mateos A, Domínguez L, Aranaz A. Interference of paratuberculosis with the diagnosis of tuberculosis in a goat flock with a natural mixed infection. Vet Microbiol. 2008;128(1–2):72–80. doi: 10.1016/j.vetmic.2007.08.034. [DOI] [PubMed] [Google Scholar]
- Chartier C, Mercier P, Pellet M, Vialard J. Effect of an inactivated paratuberculosis vaccine on the intradermal testing of goats for tuberculosis. Vet J. 2012;191(3):360–363. doi: 10.1016/j.tvjl.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Bezos J, Álvarez J, Mínguez O, Marqués S, Martín O, Vigo V, Pieltain C, Romero B, Rodríguez S, Casal C, Mateos A, Domínguez L, de Juan L. Evaluation of specificity of tuberculosis diagnostic assays in caprine flocks under different epidemiological situations. Res Vet Sci. 2012;93(2):636–640. doi: 10.1016/j.rvsc.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Gutiérrez M, Tellechea J, García Marín JF. Evaluation of cellular and serological diagnostic tests for the detection of Mycobacterium bovis-infected goats. Vet Microbiol. 1998;62(4):281–290. doi: 10.1016/S0378-1135(98)00217-X. [DOI] [PubMed] [Google Scholar]
- Pérez de Val B, López-Soria S, Nofrarías M, Martín M, Vordermeier HM, Villarreal-Ramos B, Romera N, Escobar M, Solanes D, Cardona PJ, Domingo M. Experimental Model of Tuberculosis in the Domestic Goat after Endobronchial Infection with Mycobacterium caprae. Clin Vaccine Immunol. 2011;18(11):1872–1881. doi: 10.1128/CVI.05323-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebana E, Aranaz A, Urquía JJ, Mateos A, Dominguez L. Evaluation of the gamma-interferon assay for eradication of tuberculosis in a goat herd. Aust Vet J. 1998;76(1):50–53. doi: 10.1111/j.1751-0813.1998.tb15686.x. [DOI] [PubMed] [Google Scholar]
- Vordermeier HM, Whelan A, Cockle PJ, Farrant L, Palmer N, Hewinson RG. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in Cattle. Clin Diagn Lab Immunol. 2001;8(3):571–578. doi: 10.1128/CDLI.8.3.571-578.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidders B, Pirson C, Hogarth PJ, Hewinson RG, Stoker NG, Vordermeier HM, Ewer K. Screening of highly expressed Mycobacterial genes identifies Rv3615c as a useful differential diagnostic antigen for the Mycobacterium tuberculosis complex. Infect Immun. 2008;76(9):3932–3939. doi: 10.1128/IAI.00150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vordermeier HM, Villarreal-Ramos B, Cockle PJ, McAulay M, Rhodes SG, Thacker T, Gilbert SC, McShane H, Hill AVS, Xing Z, Hewinson RG. Viral booster vaccines improve Mycobacterium bovis BCG-Induced protection against bovine tuberculosis. Infect Immun. 2009;77(8):3364–3373. doi: 10.1128/IAI.00287-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezos J, de Juan L, Romero B, Álvarez J, Mazzucchelli F, Mateos A, Domínguez L, Aranaz A. Experimental infection with Mycobacterium caprae in goats and evaluation of immunological status in tuberculosis and paratuberculosis co-infected animals. Vet Immunol Immunopathol. 2010;133(269):275. doi: 10.1016/j.vetimm.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Domingo M, Gil O, Serrano E, Guirado E, Nofrarias M, Grassa M, Cáceres N, Pérez B, Vilaplana C, Cardona PJ. Effectiveness and safety of a treatment regimen based on isoniazid plus vaccination with Mycobacterium tuberculosis cells? Fragments: field-study with naturally Mycobacterium caprae-infected Goats. Scand J Immunol. 2009;69(6):500–507. doi: 10.1111/j.1365-3083.2009.02251.x. [DOI] [PubMed] [Google Scholar]
- Daniel R, Evans H, Rolfe S, de la Rua-Domenech R, Crawshaw T, Higgins RJ, Schock A, Clifton-Hadley R. Outbreak of tuberculosis caused by Mycobacterium bovis in golden Guernsey goats in Great Britain. Vet Rec. 2009;165(12):335–342. doi: 10.1136/vr.165.12.335. [DOI] [PubMed] [Google Scholar]
- Quintas H, Reis J, Pires I, Alegria N. Tuberculosis in goats. Vet Rec. 2010;166(14):437–438. doi: 10.1136/vr.c1678. [DOI] [PubMed] [Google Scholar]
- Vordermeier HM, Chambers MA, Cockle PJ, Whelan AO, Simmons J, Hewinson RG. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect Immun. 2002;70(6):3026–3032. doi: 10.1128/IAI.70.6.3026-3032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyashchenko K, Whelan AO, Greenwald R, Pollock JM, Andersen P, Hewinson RG, Vordermeier HM. Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with Mycobacterium bovis BCG and infected with M-bovis. Infect Immun. 2004;72(5):2462–2467. doi: 10.1128/IAI.72.5.2462-2467.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh CG, Labes RE, Griffin JFT. The effect of Johne’s vaccination on tuberculin testing in farmed red deer (Cervus elaphus) N Z Vet J. 2005;53(4):216–222. doi: 10.1080/00480169.2005.36549. [DOI] [PubMed] [Google Scholar]
- Stringer LA, Wilson PR, Heuer C, Hunnam JC, Mackintosh CG. Effect of vaccination and natural infection with Mycobacterium avium subsp. paratuberculosis on specificity of diagnostic tests for bovine tuberculosis in farmed red deer (Cervus elaphus) N Z Vet J. 2011;59(5):218–224. doi: 10.1080/00480169.2011.596182. [DOI] [PubMed] [Google Scholar]
- Platt R, Thoen CO, Stalberger RJ, Chiang Y, Roth JA. Evaluation of the cell-mediated immune response to reduced doses of Mycobacterium avium ssp. paratuberculosis vaccine in cattle. Vet Immunol Immunopathol. 2010;136(1–2):122–126. doi: 10.1016/j.vetimm.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Cockle PJ, Gordon SV, Hewinson RG, Vordermeier HA. Field evaluation of a novel differential diagnostic reagent for detection of Mycobacterium bovis in cattle. Clin Vaccine Immunol. 2006;13(10):1119–1124. doi: 10.1128/CVI.00209-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezos J, Álvarez J, de Juan L, Romero B, Rodríguez S, Fernández-De-Mera IG, Hewinson RG, Vordermeier M, Mateos A, Domínguez L, Aranaz A. Assessment of in vivo and in vitro tuberculosis diagnostic tests in Mycobacterium caprae naturally infected caprine flocks. Prev Vet Med. 2011;100(3–4):187–192. doi: 10.1016/j.prevetmed.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Pérez de Val B, Villarreal-Ramos B, Nofrarias M, Lopez-Soria S, Romera N, Singh M, Abad FX, Xing Z, Vordermeier HM, Domingo M. Goats primed with BCG and boosted with a recombinant adenovirus expressing Ag85A show enhanced protection agaist tuberculosis. Clin Vaccine Immunol. 2012;19(9):1339–1347. doi: 10.1128/CVI.00275-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumber S, Eamens G, Whittington RJ. Evaluation of a Pourquier ELISA kit in relation to agar gel immunodiffusion (AGID) test for assessment of the humoral immune response in sheep and goats with and without Mycobacterium paratuberculosis infection. Vet Microbiol. 2006;115(1–3):91–101. doi: 10.1016/j.vetmic.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Corner LAL, Trajstman AC. An evaluation of 1-hexadecylpyridinium chloride as a decontaminant in the primary isolation of Mycobacterium bovis from bovine lesions. Vet Microbiol. 1988;18(2):127–134. doi: 10.1016/0378-1135(88)90058-2. [DOI] [PubMed] [Google Scholar]