Abstract
Objective
Bone morphogenetic proteins (Bmps) are important mediators of inflammation and atherosclerosis, though their mechanism of action is not fully understood. To better understand the contribution of the Bmp signaling pathway plays in vascular inflammation, we investigated the role of Bmper (Bmp-endothelial cell precursor-derived regulator), an extracellular Bmp modulator, in an induced in vivo model of inflammation and atherosclerosis.
Methods and Results
We crossed apolipoprotein E-deficient (ApoE−/−) mice with mice missing one allele of Bmper (Bmper+/−, used in place of Bmper−/− mice that die at birth) and measured the development of atherosclerosis in mice fed a high fat diet. Bmper haploinsufficiency in ApoE−/− mice (Bmper+/−;ApoE−/− mice) led to a more severe phenotype compared to Bmper+/+;ApoE−/− mice. Bmper+/−;ApoE−/− mice also exhibited increased Bmp activity in endothelial cells in both the greater and lesser curvatures of the aortic arch, suggesting a role for Bmper in regulating Bmp-mediated inflammation associated with laminar and oscillatory shear stress. siRNA knockdown of Bmper in human umbilical vein endothelial cells caused a dramatic increase in the inflammatory markers ICAM1 and VCAM1 at rest and following exposure to oscillatory and laminar shear stress.
Conclusion
We conclude that Bmper is a critical regulator of Bmp-mediated vascular inflammation and that the fine-tuning of Bmp and Bmper levels is essential in the maintenance of normal vascular homeostasis.
Keywords: Bone morphogenetic protein, Bmp endothelial cell precursor-derived regulator, atherosclerosis, inflammation, fluid shear stress
Atherosclerosis is a disease that results from plaque formation within arteries, resulting in arterial hardening and narrowing. It is mediated by a chronic inflammatory process characterized by the accumulation of lipids and inflammatory cells (plaques) along the inner walls of arteries1. Although plaque formation is a complex process, endothelial inflammation has been identified as one of the critical initiating factors1. Endothelial inflammation can be induced by decreases or disruptions in blood flow, making some regions of the vasculature more prone to plaque formation2, 3. For example, arterial regions that are exposed to uniform, unidirectional blood flow with high shear stress are protected from endothelial inflammation and have a lower incidence of atherosclerotic plaque formation3, 4. In comparison, atherosclerotic lesions develop predominantly at branches, bends and bifurcations in arteries5-7, where endothelial cells are exposed to low or disturbed fluid shear stress, resulting in low mean and oscillatory shear stress on the endothelial cells. In these lesion-prone regions, disturbed or oscillatory shear stress increases expression of bone morphogenetic proteins (Bmps) and their antagonists in the vascular endothelium8, 9. In turn, Bmps activate an inflammatory response characterized by the expression of adhesion molecules, like intracellular adhesion molecule 1 (ICAM1) and vascular cell adhesion molecule 1 (VCAM1) on the endothelial surface9. Despite this, animal studies examining the direct role of Bmp on vascular inflammation have been inconclusive, with some studies reporting a decrease in inflammatory responses and atherosclerotic lesion formation when Bmp activity is increased10, whereas others concluding that Bmp plays a pro-inflammatory role10, 11. Although each of these studies support a central role for Bmp-mediated endothelial inflammation in atherosclerosis, some uncertainty remains about the precise contribution of Bmp signaling to endothelial inflammation and atherosclerosis.
Bmps belong to the transforming growth factor (TGF-β) superfamily and play important roles in cellular processes such as bone formation, proliferation, differentiation, motility, vasculogenesis and angiogenesis (reviewed by Moreno-Miralles12). More specifically, Bmp4, together with Bmp2 and Bmp6, demonstrate important roles in endothelial differentiation, migration and angiogenesis13-16. Previously, we identified a novel extracellular modulator of Bmp, Bmp-endothelial cell precursor derived regulator (Bmper; also called crossveinless 2 in Drosophila melanogaster), which is required for hematopoietic and vascular development and hypoxia-induced retinal neovascularization13, 14, 17. We have also demonstrated that Bmper regulates Bmp4 activity in a dose-dependent manner13. Recent reports show that Bmper is induced by inflammatory-regulatory stimuli such as oscillatory shear stress and mevastatin, and inhibits tumor necrosis factor-α (TNFα)-induced endothelial inflammation18, 19, suggesting that Bmper acts in an anti-inflammatory capacity in endothelial cells by inhibiting Bmp activity. This led us to question whether Bmper may also inhibit the endothelial inflammation and subsequent pathology associated with atherosclerosis.
In this study, we used the apolipoprotein E (ApoE)−/− mouse atherosclerotic model to study the effects of Bmper haploinsufficiency on the development of atherosclerosis. We used Bmper+/− mice instead of Bmper−/− mice because Bmper−/− mice die at birth13. Bmper+/−;ApoE−/− mice fed a high fat diet displayed an exacerbated inflammatory vascular response compared to ApoE−/− mice with the wild-type Bmper gene (Bmper+/+;ApoE−/−). Mechanistically, we demonstrate that the protective effects afforded by Bmper are dependent on the inhibition of Bmp activity. These data demonstrate for the first time that Bmper is a novel player in the development of atherosclerosis and in fluid shear stress-modulated inflammatory responses in endothelial cells. Taken together, it suggests that Bmper is a novel protective regulator of vascular inflammation and vascular diseases such as atherosclerosis.
Methods
Animals and diets
Bmper+/− mice, previously generated in our laboratory on a C57BL/6J genetic background13, were crossed with ApoE−/− mice to generate Bmper+/−;ApoE−/− mice. We used the Bmper+/− mice instead of Bmper−/− mice because Bmper−/− mice die at birth13. All adult mice were fed with the standard chow or a high-fat/high-cholesterol diet (Western diet) (Harlan Laboratories, Indianapolis, IN) for 20 weeks. Body weight of mice was monitored before and after they were fed with different diets. Blood serum was obtained every four weeks. All animal experimental procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Committee for the Use of Animals in Research.
Lipid analysis
Mice were fasted for 18 hours before blood sampling. Less than 200 μl of blood was collected through sub-mandibular bleeding using a lancet. The total cholesterol level was measured enzymatically with a commercially available kit (Infinity kits, Thermo Scientific, Waltham, MA).
Lesion quantification
The mice were euthanized and perfusion fixed with 10% buffered formalin via the left ventricle for 5 minutes. The lesions located in the aorta and aortic sinuses were analyzed using Oil Red O staining. To measure lesions in the aorta, the whole aorta, including the ascending arch, thoracic and abdominal segments, was dissected, gently cleaned of adventitial tissue and stained with Oil Red O. The surface lesion area was quantified with ImageJ software and is presented as a percentage of the total surface area of the whole aorta. To measure the lesions in the aortic sinuses, the heart and proximal aorta were excised, and the apex and lower half of the ventricles were removed. The remaining sample was embedded in OCT (Tissue-Tek, Fisher Scientific, Pittsburgh, PA) and frozen on dry ice. Starting from the appearance of the aortic valve, serial frozen sections at 5-μm thickness were collected until the aortic valves were completely sectioned following the previously described protocol20. Sections were stained with eosin and Oil Red O. The slides were imaged by light microscopy, and the atherosclerotic lesion area located in aortic sinus area was quantified with ImageJ and averaged over a 280 μm region.
Calcification quantification
Deposited calcium in the aorta was detected by staining with von Kossa. The 5-μm cryosections of aortic sinus were prepared as described above and subjected to the von Kossa staining procedure. The calcification area from each section was quantified as a percentage of the total vessel cross-sectional area using ImageJ software.
ELISA measurements
Blood samples were drawn from mice after consuming the high fat diet or standard chow for 4 weeks. Soluble VCAM (sVCAM) and soluble ICAM (sICAM) were measured in plasma in triplicate using an enzyme-linked immunosorbent assay (ELISA) method (R&D Systems, Minneapolis, MN).
Reagents
Recombinant human Bmp4 and Bmper protein and antibodies recognizing Bmper and Bmp4 were obtained from R&D Systems. VCAM1 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) for Western blotting and Chemicon for immunofluorescence (Millipore, Billerica, MA). The ICAM1 antibody was purchased from Cell Signaling Technology (Danvers, MA) and used for western blotting experiments. An additional ICAM1 antibody (purchased from Chemicon) was used for immunofluorescence experiments. The pSmad1,5,8 antibody was purchased from Cell Signaling Technology and used for both western blotting and immunofluorescence experiments.
Cell culture and siRNA transfection
HUVECs (human umbilical vein endothelial cells) were purchased from Lonza and cultured in endothelial basal medium (EBM; Lonza, Allendale, NJ) supplemented with hydrocortisone, bovine brain extract, epidermal growth factor and 2% fetal calf serum. The cells from passages 4-8 were used for experiments. The stealth siRNA duplexes were obtained from Invitrogen (Grand Island, NY). The siRNAs against mouse Bmper are a mixture of the duplexes of 5′-gaauuucagccagaaggaagcaaau-3′ and 5′-ggagagaugugguccucuaucaauu-3′. The siRNA against mouse Bmp4 is a duplex of 5′-GCAUGUCAGGAUUAGCCGAUCGUUA-3′. The control siRNA is the Stealth™ RNAi negative control duplex (Cat. No. 12935-300) and was purchased from Invitrogen. The siRNAs were transfected into HUVECs according to the manufacturer's recommended protocol for Nucleofection (Amaxa; the HUVEC protocol). Briefly, for each sample, 2×105 HUVECs were transfected with 300 pmol siRNA. The experiments with Bmp4 or Bmper siRNA-transfected HUVECs were performed one day or four days later, respectively. The siRNAs resulted in more than 70% knockdown of the protein levels of Bmp4 and Bmper.
Shear stress assays
HUVECs were post-confluent for 48 hours before the performance of fluid shear stress experiment to decrease the background signals. Laminar shear stress assay was described previously21. Briefly, confluent cells in 10-cm dish were exposed to shear stress using the cone and plate flow chamber system for eight hours at 20 dyne cm-2 for laminar shear stress or ±5 dyn cm-2 for oscillatory shear stress experiments.
Immunoblotting
Cells were harvested in lysis buffer (1% Triton X-100, 50 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, 1 mmol/L Na3VO4 and 0.1% protease inhibitor mixture; Sigma) and clarified by centrifugation at 16,000 g. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes.
Immunofluorescence
The aortic arch segments were dissected out and gently cleaned of the adventitia. The aortic fragments located at the greater curvature (GC) and lesser curvature (LC) were separated and fixed in 3.7% formaldehyde for 10 minutes at room temperature. The aortic fragments were sequentially treated with 70% ethanol for 30 minutes and 5% hydrogen peroxide in methanol. Then, the segments were washed with water for 5 minutes. For the phospho-Smad 1, 5, 8 antibody, the samples were soaked in boiling citric acid buffer (10 mmol/L, pH 6.0) for 9 minutes to expose the antigens. Next, the aortic fragments or 5-μm cryosections of the aortic root were blocked with 5% heat-inactivated goat serum for 1 hour and then incubated overnight with primary antibodies against ICAM1, VCAM1, CD31 or CD68 diluted in the blocking solution. After three washes in TBS, cells were incubated in the dark with a second antibody conjugated with Alexa Fluor 488 or 568 (Molecular Probes, Eugene, OR) in blocking solution for 90 minutes at 37 °C. After 3 washes in TBS, the fragments were counterstained with DAPI for phospho-Smad1, 5 and 8 staining. The en face images of the endothelial layer and the cross-sectional images of the aortic root were visualized by confocal laser scanning microscopy.
Statistical analysis
Data are shown as the mean ± SE for 3 to 4 separate experiments. Differences were analyzed with two-way ANOVA and post-hoc analyses such as Student's t-test when needed. Values of P≤0.05 were considered statistically significant.
Results
Bmper expression protects against atherosclerotic lesion formation and calcification
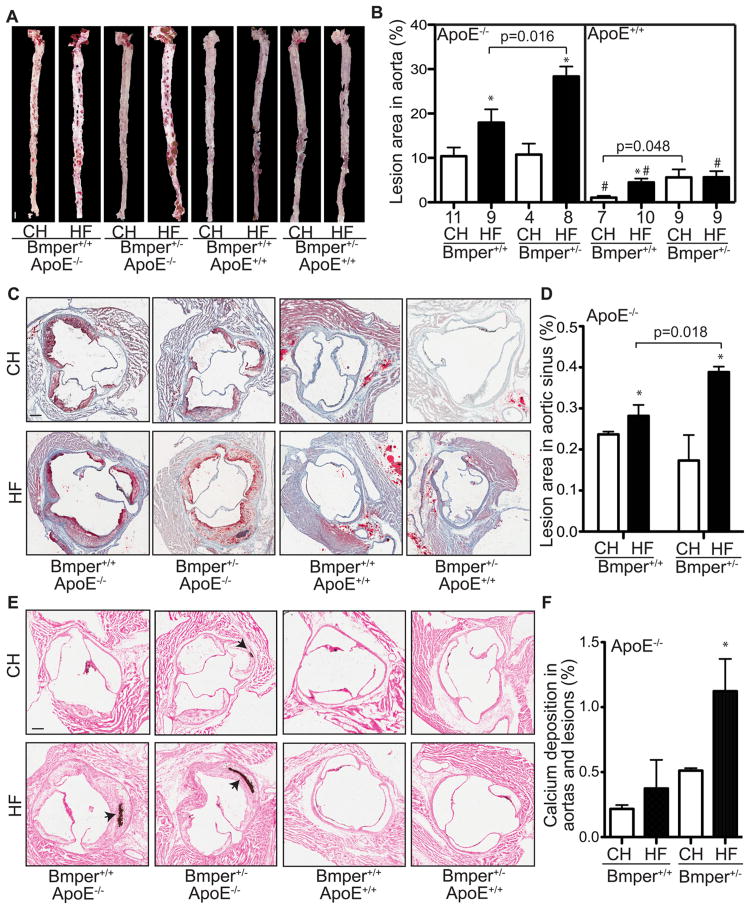
Accumulated evidence suggests that Bmper protects endothelial cells from inflammation by inhibiting Bmp activity18, 19. Therefore, we used the ApoE−/− mouse model, in which a high fat diet leads to accelerated atherosclerotic lesion formation and arterial calcification, to analyze the in vivo effect of reduced Bmper expression on vascular inflammation. We crossed wild-type or ApoE−/− mice with mice that had either one or two functional Bmper alleles, resulting in four genotypes of experimental mice: Bmper+/+;ApoE+/+, Bmper+/−;ApoE+/+, Bmper+/+;ApoE−/−, Bmper+/−;ApoE−/−. These mice were then fed either a standard chow (CH) or high fat (HF) diet for twenty weeks and the formation of atherosclerotic plaques in the aorta and aortic sinus regions was evaluated by Oil Red O staining. ApoE−/− mice that were haploinsufficient for Bmper expression (Bmper+/−;ApoE−/−) responded to the high fat diet with enhanced plaque formation compared to Bmper+/+;ApoE−/− mice (28.35±2.23% vs. 17.96±3.00%, P=0.016), as measured by en face staining of aortic lesions (including both thoracic and abdominal aorta, Figures 1A, 1B). In addition, cross-sectional analysis of aortic sinus lesions revealed that the plaques formed in the Bmper+/−;ApoE−/− mice were larger compared to lesions in Bmper+/+;ApoE−/− mice (0.38±0.02% vs. 0.29±0.03%, P=0.018), demonstrating a protective effect of Bmper expression on the degree of plaque growth (Figures 1C, 1D). When lesion calcification was compared between genotypes, Bmper+/−;ApoE−/− mice again showed an exacerbated response, with a 120% increase over baseline levels of calcification as determined by von Kossa staining, compared to a 68% increase in the Bmper+/+;ApoE−/− mice. This equated to an overall increase in total calcification of 1.12±0.25% in Bmper+/−;ApoE−/− mice vs. 0.37±0.22% in Bmper+/+;ApoE−/− mice. Bmper+/−;ApoE−/− mice fed the high fat diet showed no differences in body weight or serum cholesterol levels compared to Bmper+/+;ApoE−/− mice (Figures S1A, S1B), indicating that diet-induced increases in weight and lipid levels are not responsible for the more robust atherosclerotic phenotype in the Bmper+/;ApoE−/− mice. Together, these results indicate that Bmper plays a protective role in plaque formation and arterial calcification in an in vivo model of atherosclerosis.
Figure 1.
Bmper haploinsufficiency leads to aggravated atherosclerotic plaque formation and lesion calcification in ApoE−/− mice. Mice were fed a high fat diet (HF) or standard chow (CH) for twenty weeks. The aorta and heart were dissected out and stained with Oil Red O. A, Representative images of the Oil Red O staining of aortas. Scale bar, 1.5 mm. B. The lesions on the surface of each aorta were quantified as a percentage of the total area of the aorta. *, P<0.05, compared to mice with the same genotype but fed the control diet. #, P<0.05, compared to mice fed with the same diet but with the ApoE−/− genotype. The numbers below each column are the number of mice used in the experiments. C, Representative images of Oil Red O stained sections of aortic sinus regions from mice of the designated genotype and food groups. Scale bar, 0.2 mm. D, The lesions in the aortic sinus region were quantified as the percentage of the total lumenal area of aortas. *, P<0.05, compared to mice with the same genotype but fed the control diet, n≥4. There were no detectable lesions formed in the aortic sinus regions of ApoE+/+ mice. E, Representative images of calcification in the aortas and lesions as determined by Von Kossa staining (purple; indicated by black arrows). Scale bar, 0.2 mm. F, The area containing calcification deposition was measured as a percentage of the total lumenal cross-sectional area of aortas. *, P<0.05, compared to mice fed the same diet but with the ApoE−/− genotype, n≥5. No detectable calcification detected in aortas of ApoE+/+ mice.
Bmper expression inhibits aortic inflammation
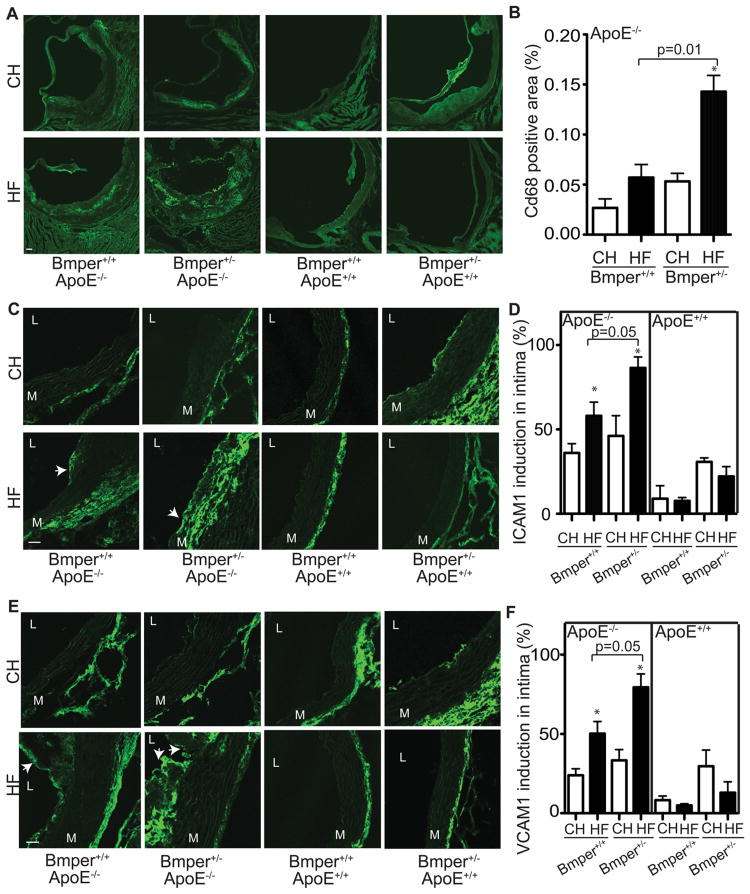
Several reports have demonstrated a central role for the Bmp signaling pathway in promoting endothelial inflammatory responses8, 9, 18, 19, 22. Therefore, we sought to determine if the protective influence of Bmper on the development of atherosclerotic lesions is due to changes in vascular inflammation. To test this, we measured the degree of macrophage infiltration (a common phenotype observed with the onset of atherosclerotic plaques1) and expression of inflammatory markers in atherosclerotic plaques in Bmper+/+;ApoE−/− and Bmper+/−;ApoE−/− mice. Quantitative analysis revealed a more robust degree of macrophage infiltration (determined by CD68 expression) in aortas of Bmper+/−;ApoE−/− mice compared to Bmper+/+;ApoE−/− mice (0.143±0.016% CD68 positive area per aortic cross-sectional area vs. 0.057±0.013%, respectively) following twenty weeks of the high fat diet (Figures 2A, 2B). Bmper+/−;ApoE−/− mice also exhibited a dramatic increase in expression of two inflammatory markers, ICAM1 and VCAM1, in the intima associated with aortic lesions, compared to Bmper+/+;ApoE−/− mice after twenty weeks of the high fat diet (Figures 2C and 2D), supporting the notion that Bmper functions as an anti-inflammatory mediator. Similar increases in ICAM1 and VCAM1 expression were also observed in serum from Bmper+/−;ApoE−/− mice compared to Bmper+/+;ApoE−/− mice after four weeks of the high fat diet (Figure S2). We also performed immunostaining with anti-Bmper and Bmp4 antibodies to determine whether there is a change in the level of expression of these proteins in response vascular inflammation induced by high fat diet. We observed a robust increase in Bmper (Figure S3A) and Bmp4 (Figure S3B) levels in mice fed a high fat diet compared to those fed a control diet. This increase in Bmper and Bmp4 correlated with the increased expression of ICAM1/VCAM1 and CD68 signals (Figure 2), further supporting the notion that the modulation of Bmp signaling by Bmper plays an important role in the inflammatory responses induced by high fat diet. Collectively, these data demonstrate that Bmper haploinsufficiency leads to phenotypic changes correlative with an increased chronic, vascular inflammatory response and likely contributes to the aggravated atherosclerotic lesion formation observed in the Bmper+/−;ApoE−/− mice.
Figure 2.
Bmper haploinsufficiency increases macrophage infiltration and intimal inflammation in ApoE−/− mice. A, Representative images showing the CD68-labeled macrophages infiltrate in atherosclerotic lesions of mice of the designated genotype and food group. Scale bars: 100 μm. B, Macrophage infiltration was quantified by measuring the area of CD68-positive cells and calculating this as a percentage of the total vessel area of aorta studied. *, P<0.05, compared to mice with the same genotype but fed the control diet. n=3. C-F, ICAM1 (green in C) and VCAM1 (green in E) immunofluorescence in aortic lesions of mice of the designated genotype and food group. Shown are representative images of 3-5 mice. Scale bars: 25 μm. L indicates lumen. M indicates media. Arrows indicate plaques. D, F, The intimal length containing ICAM1 (Figure C) and VCAM1 (Figure E) was measured as a percentage of the total intimal conference. *, P<0.05, compared to mice with the same genotype but fed the control diet, n≥3.
Bmper inhibits shear stress-dependent induction of inflammatory adhesion molecules in endothelium
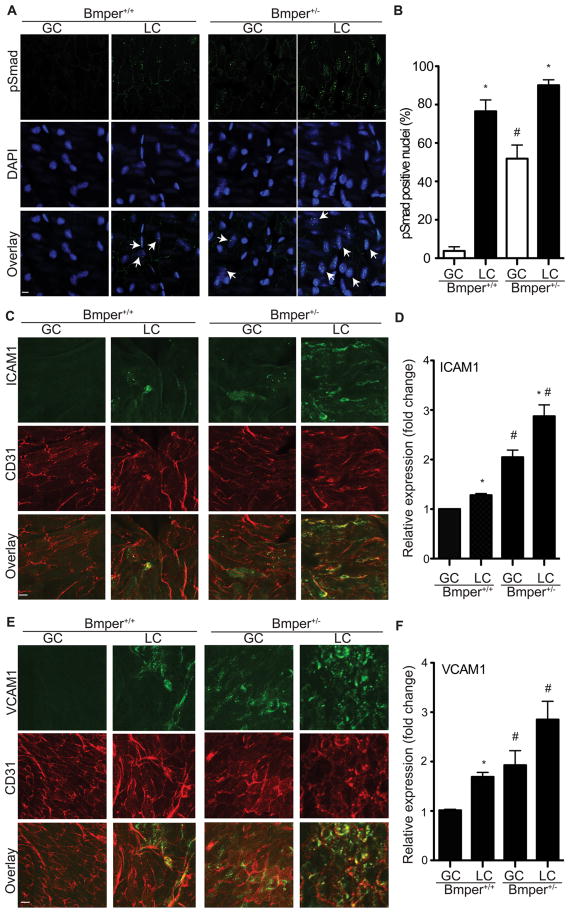
Aortic lesions develop predominantly in regions that are exposed to low or disturbed fluid shear stress, such as the lesser curvature of the aorta (LC). Even under normal hemodynamic conditions, previous studies demonstrate higher endothelial inflammation in the LC compared to other regions of the aorta, such as the greater curvature (GC)3, 4, possibly due to the increased expression of Bmps in the vascular endothelium of these regions8. This difference in lesion formation in the GC and LC was also observed in the Bmper atherosclerosis model. Specifically, we observed that there was a very significant increase in lesion area in the LC compared to GC of both Bmper+/+;ApoE−/− and Bmper+/−;ApoE−/− mice (Figure S4). Immunostaining of cross sections of the LC and GC with an antibody specific for Bmper demonstrated a dramatic increase of Bmper protein levels in intima and media of the LC compared to the GC in both Bmper+/+ and Bmper+/− mice (Figure S5A). To quantitatively determine the difference of Bmper protein level in the GC and LC, we also performed Western blotting with vessel lysates obtained from the GC and LC. We observed significantly more Bmper protein located in the LC than GC in both Bmper+/+ and Bmper+/− mice (S5B), suggesting that Bmper protein level is modulated by different fluid shear stress. Encouraged by these observations, we utilized this inherent difference in vascular inflammation between the LC and GC to analyze the effect of reduced Bmper expression on downstream mediators and effectors of BMP activation in these regions of mouse aortas. In order to simplify our experimental setup we only examined mice on the ApoE+/+ background forwarding subsequent experiments. Endothelial cells located in the LC region of aortas of Bmper+/+ mice displayed abundant Smad1, 5, 8 activation (as detected by phosphorylated Smad1, 5, 8 (pSmad) signals) compared to endothelial cells in the GC region (Figure 3A), consistent with previous reports11. In contrast, Bmper+/− mice exhibited an enhanced increase in Smad activation in the LC region in compared to Bmper+/+ mice, but in addition, Bmper+/− mice also had clearly detectable Smad activation in the GC region of aortas, consistent with our in vivo data described above indicating that Bmper functions as an anti-inflammatory mediator (Figure 3B). Expression patterns of both ICAM1 and VCAM1 paralleled that of Smad activation (Figures 3C-3F), demonstrating that Bmper inhibits the endothelial response to oscillatory shear stress-mediated induction of endothelial inflammatory adhesion molecules, and in the context of atherosclerosis, may directly inhibit the endothelial inflammatory response.
Figure 3.
Bmper haploinsufficiency results in increased Bmp activity and expression of inflammatory adhesion molecules in the ApoE+/+ endothelium of the greater curvature (GC) and lesser curvature (LC) of the aortic arch. A, En face staining of the GC and LC in the aortic arch of Bmper+/− and Bmper+/− mice was performed using an antibody specific for phospho-Smad 1, 5, 8 (pSmad, green). Nuclei were counterstained with DAPI (blue). Arrows indicate pSmad positive nuclei. Scale bar: 10 μm. B, pSmad-positive nuclei were quantified as a percentage of all nuclei per field. *, P<0.05, compared to pSmad-positive nuclei (%) in the GC region of the same mice; #, P<0.05, compared to pSmad-positive nuclei (%) in the same region of wild-type littermates, n=3. C-F, En face staining was performed for ICAM1 (green in C) and VCAM1 (green in E) and the endothelial marker CD31 (red in C and E). Scale bar: 10 μm. D and F, the average intensity of ICAM1 (D) and VCAM1 (F) was measured as a fold change over the level of intensity in the GC of wild-type mice. *, P<0.05, compared to the relative expression level in the GC of the same mice; #, P<0.05, compared to the relative expression level in the same region of wild-type littermates, n=3.
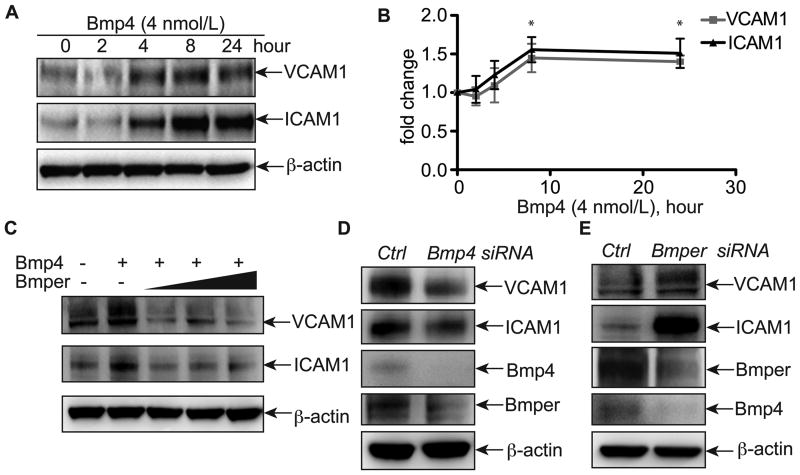
Bmper inhibits Bmp4-induced inflammatory gene expression in endothelial cells and prevents fluid shear stress-induced inflammatory responses
Our in vivo results clearly support a role for Bmper in suppressing shear stress-mediated inflammation. To determine the effects of Bmper on inflammation in the endothelial cell compartment, we established a cell-based model to determine if Bmper's ability to attenuate inflammation is Bmp4-depedent and in response to fluid shear stress. Treatment of primary human umbilical vein endothelial cells (HUVECs) with Bmp4 increased ICAM1 and VCAM1 expression at both the RNA and protein levels in a time-dependent manner, with peak expression occurring at 8 hours post-treatment (Figures 4A, 4B and data not shown), consistent with previous reports9, 18. This robust Bmp4-mediated increase in ICAM1 and VCAM1 expression was blocked in the presence of exogenous Bmper (Figure 4C), demonstrating that Bmper directly antagonizes Bmp4-mediated inflammatory signaling. Next we examined the ability of endogenous Bmp4 and Bmper to affect the expression of inflammatory markers. Given the robust inflammation seen in the normally quiescent GC region of the aorta in Bmper+/−;ApoE−/− mice, we hypothesized that reducing endogenous Bmp4 or Bmper expression in endothelial cells would affect the inflammatory signature of the cells even in the absence of exogenous mediators. As expected, Bmp4-targeted siRNA reduced expression of ICAM1 and VCAM1 in transfected cells, whereas siRNA-mediated reduction of endogenous Bmper expression resulted in a significant increase in these same inflammatory markers (Figures 4D, 4E). Collectively, our data demonstrate that the anti-inflammatory function of Bmper in endothelial cells is mediated, at least in part, through antagonizing Bmp activity.
Figure 4.
Bmper inhibits Bmp4-induced ICAM1 and VCAM1 expression. A, HUVECs were treated with 4 nmol/L of Bmp4 for different time periods as indicated, and then cell lysates were subjected to Western blotting with specific antibodies against ICAM1 and VCAM1. B, ICAM1 and VCAM1 band intensity was quantified with ImageJ and normalized against the actin level. *, P<0.05, compared to the sample without Bmp4 treatment, n=3. C, HUVECs were treated with 4 nmol/L Bmp4 and increasing concentrations of Bmper (5, 10, 20 nmol/L) for 8 hours. Cell lysates were then subjected to Western blotting with specific antibodies against ICAM1 and VCAM1. D, HUVECs were transfected with Bmp4 siRNA or control siRNA. Eighteen hours later, cells were harvested and subjected to Western blotting. E, HUVECs were transfected with Bmper siRNAs or control siRNA. Ninety-six hours later, cells were harvested and subjected to Western blotting.
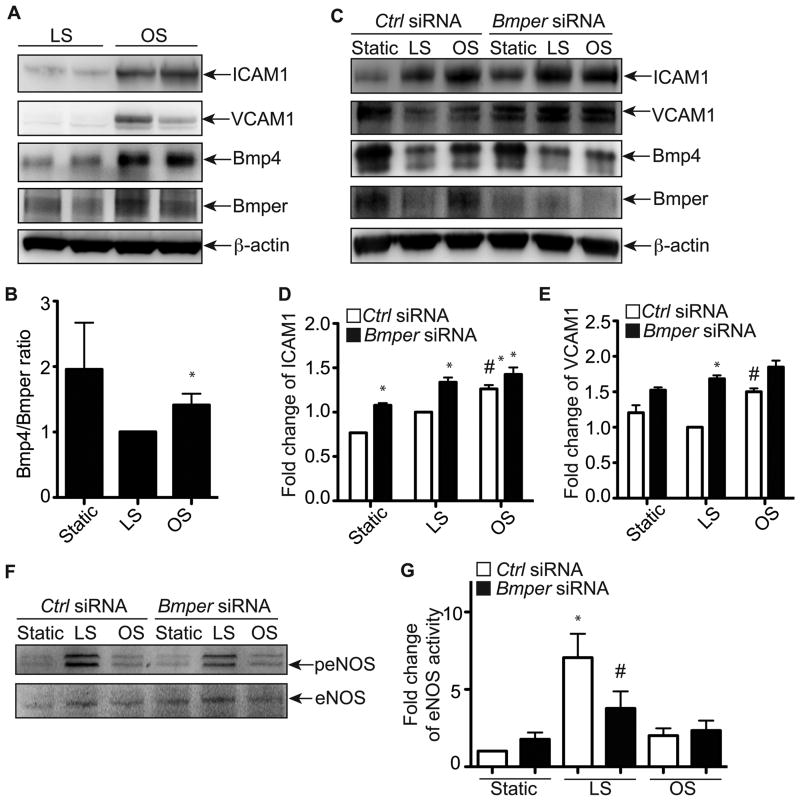
Given the effect of Bmper gene dosage on endothelial inflammation in vivo (Figure 3) and the in vitro effects of a reduction of Bmper expression on endothelial inflammation in the absence of any stimuli (Figure 4), we hypothesized that the inflammatory response to shear stress in the aorta may be mediated by changes in the levels of Bmper expression. To test this hypothesis directly, we subjected HUVEC to conditions that mimic the shear stress conditions in the LC and GC aortic regions using either oscillatory stress (±5 dyne cm-2) or laminar shear stress (20 dyne cm-2) for eight hours, respectively. Consistent with our in vivo data, endothelial cells subjected to oscillatory shear had a larger inflammatory response (a 2.33-fold and 4.56-fold increase in ICAM1 and VCAM1 expression, respectively) compared to cells subjected to laminar shear, respectively (Figure 5A). Interestingly, we also observed increases in Bmp4 and Bmper expression in oscillatory shear conditions compared to laminar shear conditions (Figure 5A). Moreover, the protein ratio of Bmp4 to Bmper increased more dramatically in oscillatory shear conditions compared to laminar shear conditions (Figure 5B). To determine if the inflammatory response observed in our shear stress model is mediated by changes in Bmper protein level, we used siRNA-mediated gene silencing of Bmper and compared the extent of inflammatory marker expression to control siRNA-treated cells. In control siRNA-treated cells, we detected higher ICAM1 and VCAM1 expression after oscillatory shear compared to laminar shear (Figures 5C-5E), with similar patterns of Bmp4 and Bmper expression as what was seen in untreated cells exposed to both modes of shear stress (Figure 5A). In Bmper siRNA-treated cells, we observed an increased inflammatory response in oscillatory shear conditions compared to control siRNA-treated cells (Figures 5C-5E), consistent with the increased inflammation we observe in LC regions in Bmper+/− aortas (Figure 3). Additionally, even in the low inflammatory environment (laminar shear stress), reducing Bmper expression resulted in 34% and 68% higher expression of ICAM1 and VCAM1, respectively, compared to control siRNA-treated cells (Figures 5C-5E), paralleling our observations seen in GC regions of Bmper+/− aortas (Figure 3). Given that laminar shear stress promotes endothelial survival and integrity by activating endothelial NOS (eNOS) signaling4, and that Bmper regulates eNOS protein expression and activity under static conditions19, we tested whether eNOS is regulated by Bmper under laminar or oscillatory shear conditions. Very excitingly, we observed that control siRNA-treated endothelial cells subjected to laminar shear stress for eight hours displayed a robust increase in eNOS phosphorylation compared to cells subjected to oscillatory shear stress (Figure 5F, G). However, in Bmper siRNA-treated cells, we observed a significant decrease in eNOS phosphorylation (Figure 5F, G) after laminar shear compared to the control-treated cells. This suggests that the protective effect of Bmper under laminar shear conditions might be mediated by increased eNOS activity. The detailed mechanism behind this eNOS regulation by Bmper under laminar shear conditions remains to be determined. Taken together, these data demonstrate a protective role for Bmper in laminar shear stress environments to reduce endothelial inflammation.
Figure 5.
Bmper is required for the regulation of ICAM1 and VCAM1 expression by fluid shear stress. A, HUVECs were subjected to oscillatory shear stress (OS, ±5 dyn cm-2) or laminar shear stress (LS, 20 dyn cm-2) or remained static in the incubator for 8 hours. Cell lysates were used for Western blotting to detect the expression of Bmper and Bmp4 and the induction of inflammatory adhesion molecules ICAM1 and VCAM1. B, The band intensity of Bmp4 and Bmper (A) was quantified with ImageJ and is presented as the ratio of Bmp4/Bmper protein level compared to the LS condition. *, P<0.05 compared to the cells exposed to laminar shear stress. n=4. C, HUVECs were transfected with Bmper or control siRNAs. Four days later, cells were exposed to oscillatory shear stress (±5 dyn cm-2), laminar shear stress (20 dyn cm-2) or static conditions for 8 hours. D~E, The band intensity of ICAM1 (D) and VCAM1 (E) was quantified with ImageJ and is presented as the relative fold change of protein level compared to the LS condition. *, P<0.05 compared to the cells transfected with control siRNAs, n=3; #, P<0.05 compared to the cells exposed to laminar shear stress, n=3. F, HUVECs were transfected with Bmper or control siRNAs. Four days later, cells were exposed to oscillatory shear stress (±5 dyn cm-2), laminar shear stress (20 dyn cm-2) or static conditions for 8 hours. Cell lysates were subjected for Western blotting with phospho-eNOS (Ser1177) and eNOS antibodies. G, The band intensity of phospho-eNOS (Ser1177)(E) was quantified with ImageJ and is presented as the relative fold change of protein level compared to the total eNOS protein level. *, P<0.02 compared to the cells at the static condition; #, P<0.03 compared to the cells transfected with control siRNA. n=3.
Discussion
In this study, we have identified a previously unrecognized, protective role for Bmper in the setting of atherosclerosis. ApoE−/− mice, who develop an atherosclerotic phenotype when fed a high fat diet, displayed significantly worsened symptoms when they carried only one phenocopy of the Bmper gene (Bmper+/−;ApoE−/− mice). Not only did the decrease in Bmper levels in these mice cause a dramatic increase in lesion size, it also resulted in increased arterial calcification and a heightened induction of endothelial inflammatory adhesion molecules in the aorta in regions subjected to oscillatory and laminar shear stress. Similar results were found in cultured cell studies, solidifying the notion that Bmper is a critical regulator of vascular inflammation and broadening our understanding of the role that Bmper plays in the myriad of events that result from the Bmp signaling pathway.
Previously, our studies revealed the essential roles of Bmp and Bmper signaling in endothelial cell differentiation, migration and angiogenesis13, 14, 16, 17, 23, 24. With the results from the present study, we can now add vascular inflammation to the growing list of Bmp signaling events that are regulated by Bmper. Bmper+/− mice demonstrated increased Smad activation and expression of the inflammatory markers ICAM1 and VCAM1 in the endothelium layer of the lesser curvature of the aorta, a region known to be predisposed to atherogenic activity due to Bmp-mediated vascular inflammation brought about by oscillatory shear stress effects on endothelial cells (Figure 3). In addition, decreased levels of Bmper in Bmper+/−;ApoE−/− mice led to an increase in the number of macrophages that were recruited and that migrated into the inflamed, atherogenic regions of the aortas (Figures 2A, 2B), supporting the notion that Bmper acts as a protective regulator of vascular inflammation. It is worth noting however, that Bmper may also play a role in maintaining general vascular health in addition to its role in inflammatory responses. Our analysis of aortas taken from ApoE+/+ mice that were haploinsufficient for Bmper (Bmper+/−;ApoE+/+ mice) revealed a significant increase in the area of atherosclerotic lesions in mouse fed a regular diet (Figures 1A, 1B). This suggests that Bmper may be important in maintaining vascular health even under basal conditions, a theory that will require further experiments to determine.
As compelling as these in vivo results are, however, it is important to remember that the decrease in Bmper expression in the Bmper+/− mouse is not limited to endothelial cells. Since atherosclerosis is a pathological condition that results from the dysfunction of multiple cell types and involves different cellular events, it is not possible, from these in vivo studies, to be able to localize the protective effect of Bmper to endothelial cells alone. Indeed, published reports demonstrate that Bmps enhance smooth muscle cell migration and induce proinflammatory factors such as inducible nitric oxide synthase (iNOS) and TNF in macrophages25, 26. The inhibition of Bmp activity by specific inhibitors and antagonist decreases vascular calcification, suggesting important roles of Bmp in vascular calcification as well as early vascular injury10, 11. Therefore, it is entirely possible that Bmper, a secreted extracellular Bmp modulator, may also be able to influence Bmp activity not only in endothelial cells but in additional cell types such as smooth muscle cells and macrophages. Therefore, the contribution of Bmper to protection against atherogenic processes and vascular inflammation will need further investigation.
As mentioned above, a number of published reports have detailed the role of Bmper/Bmp signaling in various aspects of endothelial cell function13, 14, 16, 17, 23, 24. However, the ability of Bmper to inhibit endothelial inflammatory responses has not been investigated directly. In order to examine this aspect of endothelial Bmp signaling, we cultured HUVECs and subjected them to fluid shear stress to induce an inflammatory response similar to what occurs within atherogenic areas of the aorta. We found that Bmper inhibits the inflammatory response usually elicited from endothelial cells in response to oscillatory shear stress (Figures 3 and 5). This result is consistent with other reports demonstrating that the expression of Bmper and other Bmp antagonists is increased by oscillatory shear stress compared to laminar shear stress8. Surprisingly, however, we also found that Bmper also exerts an anti-inflammatory effect on endothelial cells exposed to laminar shear stress. Laminar shear stress promotes endothelial survival and integrity by activating MAP kinases such as ERK/BMK1 and endothelial NOS (eNOS) signaling4. Our data have demonstrated that Bmper is also involved in protecting endothelial cells exposed to laminar shear stress, partly due to increases in activity of eNOS and decreases ICAM1 and VCAM1 expression (Figure 5C, F) that have recently been attributed to Bmper activity in endothelial cells19. The exact molecular mechanisms through which Bmper modulates eNOS activity and other signaling pathways of laminar shear stress need further investigation.
Bmper has been identified as a critical regulator of Bmp signaling activity, important in both vascular development and in hypoxia-induced retinal neovascularization13, 14. Previously we reported a gradient effect of Bmper's ability to influence Bmp signaling, whereby superstoichiometric concentrations of Bmper compared to Bmp inhibit Bmp signaling and substoichiometric concentrations of Bmper compared to Bmp activate Bmp signaling13. In this study, we observed 61% and 15% higher Bmp4 and Bmper expression in oscillatory shear stress conditions, resulting in a significantly lower Bmper to Bmp4 ratio compared to laminar shear stress conditions, an expression pattern consistent with an anti-inflammatory role for Bmper (Figure 5A, B). This observation further suggests that the fine-tuning of Bmp activity by Bmper is essential for modulating Bmp-mediated cellular functions. Collectively, the data presented in this report demonstrates that the regulation of Bmp activity by Bmper is essential for the maintenance of normal vascular homeostasis and its disruption increases the risk of inflammatory vascular diseases such as atherosclerosis.
Supplementary Material
Acknowledgments
We thank Kirk McNaughton and the UNC Histology Research Core Facility in the Department of Cell and Molecular Physiology for providing excellent histological services. We also thank Robert Bagnell, Victoria J. Madden and Steven J. Ray in the UNC Microscopy Services Laboratories for help with immunohistochemistry experiments.
Sources of funding: This work was supported in part by NIH grant R01-HL061656 (to C.P.).
Footnotes
Author disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 2.Surapisitchat J, Hoefen RJ, Pi X, Yoshizumi M, Yan C, Berk BC. Fluid shear stress inhibits TNF-alpha activation of JNK but not ERK1/2 or p38 in human umbilical vein endothelial cells: Inhibitory crosstalk among MAPK family members. Proc Natl Acad Sci U S A. 2001;98:6476–6481. doi: 10.1073/pnas.101134098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Traub O, Berk BC. Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 4.Berk BC, Min W, Yan C, Surapisitchat J, Liu Y, Hoefen R. Atheroprotective Mechanisms Activated by Fluid Shear Stress in Endothelial Cells. Drug News Perspect. 2002;15:133–139. doi: 10.1358/dnp.2002.15.3.704684. [DOI] [PubMed] [Google Scholar]
- 5.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5:293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 6.Asakura T, Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res. 1990;66:1045–1066. doi: 10.1161/01.res.66.4.1045. [DOI] [PubMed] [Google Scholar]
- 7.Moore JE, Jr, Xu C, Glagov S, Zarins CK, Ku DN. Fluid wall shear stress measurements in a model of the human abdominal aorta: oscillatory behavior and relationship to atherosclerosis. Atherosclerosis. 1994;110:225–240. doi: 10.1016/0021-9150(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 8.Chang K, Weiss D, Suo J, Vega JD, Giddens D, Taylor WR, Jo H. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation. 2007;116:1258–1266. doi: 10.1161/CIRCULATIONAHA.106.683227. [DOI] [PubMed] [Google Scholar]
- 9.Sucosky P, Balachandran K, Elhammali A, Jo H, Yoganathan AP. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-beta1-dependent pathway. Arterioscler Thromb Vasc Biol. 2009;29:254–260. doi: 10.1161/ATVBAHA.108.176347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, Bostrom KI. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 2010;107:485–494. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derwall M, Malhotra R, Lai CS, Beppu Y, Aikawa E, Seehra JS, Zapol WM, Bloch KD, Yu PB. Inhibition of Bone Morphogenetic Protein Signaling Reduces Vascular Calcification and Atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:613–622. doi: 10.1161/ATVBAHA.111.242594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno-Miralles I, Schisler JC, Patterson C. New insights into bone morphogenetic protein signaling: focus on angiogenesis. Curr Opin Hematol. 2009;16:195–201. doi: 10.1097/MOH.0b013e32832a07d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley R, Ren R, Pi X, Wu Y, Moreno I, Willis M, Moser M, Ross M, Podkowa M, Attisano L, Patterson C. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J Cell Biol. 2009;184:597–609. doi: 10.1083/jcb.200808064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno-Miralles I, Ren R, Moser M, Hartnett ME, Patterson C. Bone morphogenetic protein endothelial cell precursor-derived regulator regulates retinal angiogenesis in vivo in a mouse model of oxygen-induced retinopathy. Arterioscler Thromb Vasc Biol. 2011;31:2216–2222. doi: 10.1161/ATVBAHA.111.230235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moser M, Patterson C. Bone morphogenetic proteins and vascular differentiation: BMPing up vasculogenesis. Thromb Haemost. 2005;94:713–718. doi: 10.1160/TH05-05-0312. [DOI] [PubMed] [Google Scholar]
- 16.Pi X, Ren R, Kelley R, Zhang C, Moser M, Bohil AB, Divito M, Cheney RE, Patterson C. Sequential roles for myosin-X in BMP6-dependent filopodial extension, migration, and activation of BMP receptors. J Cell Biol. 2007;179:1569–1582. doi: 10.1083/jcb.200704010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moser M, Yu Q, Bode C, Xiong JW, Patterson C. BMPER is a conserved regulator of hematopoietic and vascular development in zebrafish. J Mol Cell Cardiol. 2007;43:243–253. doi: 10.1016/j.yjmcc.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helbing T, Rothweiler R, Heinke J, Goetz L, Diehl P, Zirlik A, Patterson C, Bode C, Moser M. BMPER is upregulated by statins and modulates endothelial inflammation by intercellular adhesion molecule-1. Arterioscler Thromb Vasc Biol. 2010;30:554–560. doi: 10.1161/ATVBAHA.109.201087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helbing T, Rothweiler R, Ketterer E, Goetz L, Heinke J, Grundmann S, Duerschmied D, Patterson C, Bode C, Moser M. BMP activity controlled by BMPER regulates the proinflammatory phenotype of endothelium. Blood. 2011;118:5040–5049. doi: 10.1182/blood-2011-03-339762. [DOI] [PubMed] [Google Scholar]
- 20.Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987;68:231–240. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z, Rubin J, Tzima E. Role of PECAM-1 in arteriogenesis and specification of preexisting collaterals. Circ Res. 2010;107:1355–1363. doi: 10.1161/CIRCRESAHA.110.229955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinke J, Kerber M, Rahner S, Mnich L, Lassmann S, Helbing T, Werner M, Patterson C, Bode C, Moser M. Bone morphogenetic protein modulator BMPER is highly expressed in malignant tumors and controls invasive cell behavior. Oncogene. 2011 doi: 10.1038/onc.2011.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moser M, Binder O, Wu Y, Aitsebaomo J, Ren R, Bode C, Bautch VL, Conlon FL, Patterson C. BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol Cell Biol. 2003;23:5664–5679. doi: 10.1128/MCB.23.16.5664-5679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren R, Charles PC, Zhang C, Wu Y, Wang H, Patterson C. Gene expression profiles identify a role for cyclooxygenase 2-dependent prostanoid generation in BMP6-induced angiogenic responses. Blood. 2007;109:2847–2853. doi: 10.1182/blood-2006-08-039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez VA, Ali Z, Alastalo TP, Ikeno F, Sawada H, Lai YJ, Kleisli T, Spiekerkoetter E, Qu X, Rubinos LH, Ashley E, Amieva M, Dedhar S, Rabinovitch M. BMP promotes motility and represses growth of smooth muscle cells by activation of tandem Wnt pathways. J Cell Biol. 192:171–188. doi: 10.1083/jcb.201008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong JH, Lee GT, Lee JH, Kwon SJ, Park SH, Kim SJ, Kim IY. Effect of bone morphogenetic protein-6 on macrophages. Immunology. 2009;128:e442–450. doi: 10.1111/j.1365-2567.2008.02998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.