Abstract
Retroviral genome recognition is mediated by interactions between the nucleocapsid (NC) domain of the virally encoded Gag polyprotein and cognate RNA packaging elements that, for most retroviruses, appear to reside primarily within the 5′-untranslated region (5′-UTR) of the genome. Recent studies suggest that a major packaging determinant of Bovine Leukemia Virus (BLV), a member of the human T-cell leukemia virus (HTLV)/BLV family and a non-primate animal model for HTLV-induced leukemogenesis, resides within the gag open reading frame. We have prepared and purified the recombinant BLV NC protein and conducted electrophoretic mobility shift and isothermal titration calorimetry studies with RNA fragments corresponding to these proposed packaging elements. The gag-derived RNAs did not exhibit significant affinity for NC, suggesting an alternate role in packaging. However, an 83-nucleotide fragment of the 5′-UTR that resides just upstream of the gag start codon binds NC stoichiometrically and with high affinity (Kd = 136 ± 21 nM). These nucleotides were predicted to form tandem hairpin structures, and studies with smaller fragments indicate that the NC binding site resides exclusively within the distal hairpin (residues G369- U399, Kd = 67 ± 8 nM at physiological ionic strength). Unlike all other structurally characterized retroviral NC binding RNAs, this fragment is not expected to contain exposed guanosines, suggesting that RNA binding may be mediated by a previously uncharacterized mechanism.
Keywords: Bovine Leukemia Virus (BLV), ribonucleic acid (RNA), packaging signal, nucleocapsid protein (NC), isothermal titration calorimetry (ITC)
1. Introduction
During the Late (post-integration) phase of the retroviral replication cycle, two copies of the unspliced, positive-sense RNA genome are selected for packaging from a cellular pool that contains a substantial excess of non-viral and spliced viral mRNAs (Goff, 1990). Extensive genetic and biochemical studies of several retroviruses indicate that genome selection is mediated by interactions between the nucleocapsid (NC) domains of the virally encoded Gag polyproteins and packaging elements near the 5′ end of the viral genome (ψ-site) (Berkowitz et al., 1996; D’Souza and Summers, 2005; Darlix et al., 2011; Greatorex, 2004; Greatorex and Lever, 1998; Jewell and Mansky, 2000; Lu et al., 2011b; Paillart et al., 1996; Paillart et al., 2004; Rein, 1994; Russell et al., 2004). The ψ-sites appear to consist of discrete, sometimes discontinuous elements that overlap with the segments that promote RNA dimerization and splicing, providing potential mechanisms for the discriminate packaging of an unspliced, diploid genome. The packaging signal of the murine leukemia virus (MLV) appears to reside exclusively within the upstream 350-nucleotides of the 5′-Untranslated Region (5′-UTR) of the viral RNA, and a “core encapsidation signal” comprising fewer than 100-nucleotides has been identified that is capable of directing heterologous RNAs into virus-like particles (Adam and Miller, 1988; Mann and Baltimore, 1985; Mann et al., 1983; Miyazaki et al., 2010a; Miyazaki et al., 2010b; Mougel and Barklis, 1997). Similarly, packaging of the human immunodeficiency type-1 virus (HIV-1) RNA appears to be directed mainly by residues within the 5′-UTR and the immediately adjacent region of gag (Heng et al., 2012; Lu et al., 2011a; Lu et al., 2011b; McBride and Panganiban, 1996; McBride and Panganiban, 1997; McBride et al., 1997).
Recently, efforts have been made to identify the packaging signals and mechanism of Bovine Leukemia Virus (BLV), a member of the delta retrovirus genus that is closely related to the human T-cell lymphoma viruses (HTLV-I/IV) -- the etiologic agent implicated in human adult T-cell leukemia and T-cell lymphoma (Gillet et al., 2007). HTLV-1 has infected about 20 million people worldwide, of which 2 to 3% have developed acute T-cell leukemia (ATL). An additional 2 to 3% have developed chronic inflammatory syndromes, including HTLV-Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP, an inflammatory disease of the central nervous system) (Kabeya et al., 2001). No satisfactory treatments are currently available for HTLV-related diseases. Given its close genetic relationship with HTLV, BLV appears to be a suitable non-primate model for studying disease processes and viral mechanisms associated with this genus of retroviruses (Gillet et al., 2007).
Computational and mutagenesis studies identified segments of the BLV genome that are important for packaging (Fig. 1A). Rice and co-workers identified a nucleotide sequence located immediately downstream of the Primer binding site that is conserved among divergent retroviruses (U340-C356 for BLV) (Rice et al., 1985) and residues U321-C400 were predicted to form a tandem stem loop structure (Fig 1B) (Kurg et al., 1995). Mutations designed to disrupt stem-loop V resulted in modest but significant (~3-fold) reductions in genomic RNA packaging, supporting the hypothesis that these residues are important for efficient packaging. Mansky and co-workers showed that deletion of a segment that extends from SLV to residue 71 of the gag open reading frame causes major reductions (~50-fold) in genome packaging and virus production (Mansky et al., 1995) (Fig. 1B,C). Computational and mutagenesis studies indicated that residues A417-U472 form tandem stem loops (SL1-2gag, Fig. 1C), which were proposed to function as the “primary packaging signal” (Mansky and Wisniewski, 1998). Deletions of nucleotides in the gag open reading frame that encode for residues of the capsid (CA) domain also modestly reduced packaging levels (~ 7-fold) (Mansky et al., 1995; Mansky and Wisniewski, 1998), but other deletions near the 3′ end of gag and in the pol and env genes did not significantly affect genome packaging (Mansky et al., 1995). These findings collectively suggested that the packaging signal of BLV consists of discrete elements that dispersed within the 5′-UTR and gag open reading frame.
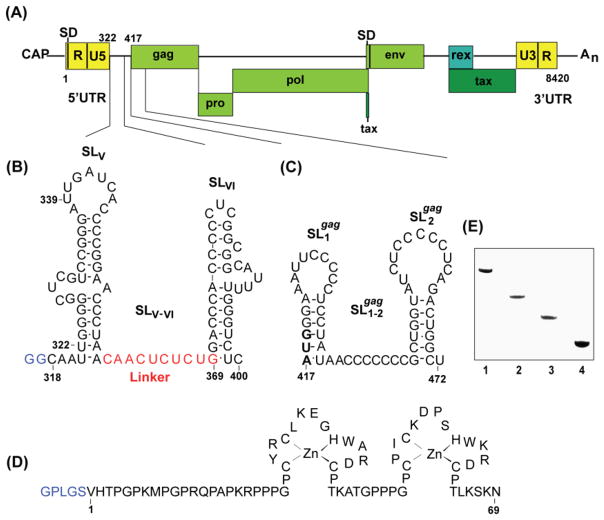
Figure 1.
(A) Representation of the Bovine Leukemia Virus (BLV) RNA genome showing relative locations of the splice donor site (SD) and RNA packaging signal. Nucleotides are numbered using the first residue after the 5′-cap as position number 1. The 18 nucleotide Primer Binding Site was indicated by numbers nt322 and nt339. Secondary structure of a segment of the BLV (B) 5′UTR (shown in red is the 10-nucleotide linker connecting the SLV and SLVI stem loops) (C) 5′gag. (D) Denaturing PAGE results (12 %(w/v) polyacrylamide) showing relative electrophoretic migration and sample purity for the RNA constructs used in these studies. Lane numbers 1, 2, 3, 4, 5 correspond to RNA constructs SLV-VI, SL1-2gag, SLV, SLVI respectively. To increase the transcription yields, non-native GG residues shown in blue were added at the 5′ termini of SLV-VI. 5′ termini GG of SLVI are native (E) Amino acid sequence and the zinc-binding mode of the BLV NC protein. Non-native residues derived from the PreScission protease cleavage site (used to cleave GST during purification) are shown in blue.
In an effort to more clearly identify the protein-RNA interactions that may be involved in BLV genome recognition, and to specifically probe for sites that interact with NC, we have prepared the recombinant BLV NC protein (Fig. 1D) and conducted RNA binding studies by gel shift and ITC methods using oligoribonucleotides corresponding to proposed RNA packaging elements.
2. Materials and Methods
2.1 Cloning of recombinant BLV NC protein
The native BLV NC cDNA sequence was optimized by substituting all the less favorable codons with those having the highest usage frequencies by E. coli (2001 Novagen catalog). Eight single stranded DNAs, altogether covering the full-length optimized cDNA sequence were purchased from Oligos Etc. Inc. (Wilsonville, OR) in 50 nm scales. Double stranded DNAs, due to sequence complementarity, were obtained by equimolar mixing of two single-stranded DNAs (10 μM Tris-HCl, pH 7.5; three minutes incubation at 90 °C followed by slow cooling to room temperature). The full-length double stranded DNA was derived from these four pairs of double stranded DNAs by ligation using T4 DNA ligase (New England Biolabs). Two oligonucleotides BAM (5′-GGATCGCGGATCCGCTGTTG-TTAACCGTGA-3′) and XHO (5′-GTGGCCGCTCGAGTT-AGGAAACAGCCGGCG-3′) (underlined are BamHI and XhoI recognition site, respectively) were used as the primers in the polymerase chain reaction (PCR) to amplify the full-length cDNA and to introduce the BamHI and XhoI recognition sites. The PCR was performed with Ready to Go PCR beads (Amersham Pharmacia Biotech) and consisted of 35 cycles at 94 °C for one minute, 55 °C for one minute, and 72 °C for 1.5 minutes followed by a final extension at 72 °C for ten minutes. The PCR product was gel-purified with the Gel-extraction Kit (Qiagen), digested with the BamHI and XhoI (New England Biolabs), and ligated with likewise digested expression vector Pgex-6p-1 (Amersham Pharmacia Bio-tech) using T4 DNA ligase. The ligation product was then transformed into DH5α E. coli using heat shock. Transformants were screened for the presence of insert by plasmid mini-preps (Quick-Spin DNA extraction kit; Qiagen) and restriction enzyme digestions. Clones containing the NC cDNA insert were verified by sequencing the complete insert by the dideoxynucleotide method (DNA Sequencing Resource Center at the Rockefeller University). The primer used for sequencing was the pGEX 5′ sequencing primer (5′-GGGCTGGCAAGCCACGTTTGGTG-3′). The clone used for over expression of the fusion GST-NC was named pBLVNC.
2.2 Expression and Purification of the BLV NC protein
The fusion protein was expressed in E. coli BL21 (DE) GOLD (Invitrogen) and purified by affinity chromatography on glutathione-Sepharose (GE Healthcare). GST-NC protein expression was induced with 1 mM isopropyl β-D-thiogalactoside (IPTG) at 37 °C (post induction growth for four hours at 30 °C), bacteria were collected by centrifugation (8,000 rpm for 15 minutes) and were washed once in phosphate-saline buffer (PBS). All protein purification steps were performed at 4 °C. The bacterial pellet was re-suspended in PBS and lysed by Micro fluidizer (Microfluidics, Newton, MA). The nucleic acids were precipitated using polyethyleneimine with a final concentration of 0.45 % (w/v). Debris was pelleted by centrifugation (17,000 rpm for 30 minutes) and the resulting supernatant was filtered through 0.4 μ filter and then allowed for an hour binding to the sepharose beads. The beads were washed with 2 L of PBS and 500 ml cleavage buffer (50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.5 mM DTT). Cleavage of N-terminal GST protein from the GST-NC fusion was done) for 16 hours with the enzyme PreScission protease (GE Healthcare). Completion of digestion was monitored by SDS-PAGE. The purified NC protein was exchanged into buffer (10mM Tris-HCl (pH 7.0), 5–10 mM NaCl, 0.1 mM BME) using Amicon-3 (Millipore) and the concentration was determined by optical absorbance at 280 nM.
2.3 Preparation of RNA samples
The template for SLV-VI was prepared and sequenced by Gene Gateway (Hayward, CA) as an insert in the PUC 18 plasmid with a 3′ SmaI restriction site for lineralization and the T7 promoter sequence directly upstream of the template sequence followed by non-native GG to increase the transcription yield. Template plasmids for SLV-VI were amplified in E. coli DH5α. The plasmids were extracted from the cells using Plasmid Mega Kit (Qiagen) and lineralized with SmaI enzyme (New England Biolabs) at 1 μg/unit overnight at room temperature. The cut plasmid was then extracted twice with phenol/chloroform and precipitated with ethanol. The pellet was washed with 70 % ethanol (v/v), and dissolved in sterile distilled water. The DNA template for making SLVI was ordered from Dharmacon Research Inc. and purified by denaturing gel electrophoresis (PAGE). The template was designed with having the T7 RNA polymerase promoter at the 3′ end of the complementary sequence of the transcript required. The template was mixed with 1.5 times the top strand of the T7 promoter (5′-TAATACGACTCACTATA-3′), heated and then cooled to anneal the two strands of the double stranded promoter.
SLV-VI and SLVI oligonucleotides were synthesized using standard in vitro transcription methods previously described (Milligan et al., 1987) with T7 polymerase and purified to a single nucleotide resolution by gel electrophoresis (Milligan and Uhlenbeck, 1989; Wyatt et al., 1991). The DNA transcripts were transcribed in vitro by T7 RNA polymerase in 30 ml reactions containing the optimized amount of template, 20 mM MgCl2, 2 mM spermidine, 80 mM Tris-HCl (pH 8.1), 2 mM DTT, 10 % DMSO, 0.3 mg T7 RNA polymerase and optimized amount of each NTP (4 mM ATP, 12 mM GTP, 16 mM CTP, 9.4 mM UTP for SLVI and 4 mM ATP, 6.5 mM GTP, 8.5 mM CTP, 5.1 mM UTP for SLV-VI). Reactions were incubated at 37 °C for three hours and quenched with 25 mM EDTA. The RNA was ethanol precipitated, air dried and re-suspended in water containing 4 M urea. After denaturation at 96 °C for five minutes, the RNA was purified by electrophoresis on urea-containing polyacrylamide denaturing gels. The concentration of each sample was determined by measuring the optical absorbance at 260 nM.
Oligoribonucleotides SLV, SL1-2gag, SL1gag, SL2gag and 10nt linker were purchased from Dharmacon Research Inc (Lafayette, CO, USA). The 2′-protecting groups for minimizing the chance of RNA degradation of these RNAs were removed before use as recommended. Each tube was briefly centrifuged and 400 μl of 2′ deprotection buffer was added into the RNA. The RNA was completely dissolved, vortexed for 10 seconds and centrifuged for 10 seconds. Then the RNA was incubated at 60 °C for 3 hours-to-overnight. This was followed by precipitation of RNA with 100 % ethanol at −20 °C over night. The pelleted RNA was further purified with urea-containing denaturing PAGE (% 16–20) to a single nucleotide resolution and RNA was recovered using an electro elution system.
2.4 Native polyacrylamide gel electrophoresis
All RNA and NC samples were prepared at 0.3 mM stock solution in buffer (10 mM Tris-HCl (pH 7.0), 5–150 mM NaCl, 0–1 mM MgCl2, 0.1 mM BME). RNAs were heated at 95 °C for two minutes and cooled on ice. Then 1 μl of a 0.3 mM NC solution in buffer was added to 1μl of RNA to give a ratio of 1:1 NC:RNA. Increasing amounts of 0.3 mM NC were added to each of 1 μl of RNA giving rise to NC:RNA ratios ranging from 0:1 to 1.25:1. Buffer was added (10 μl final volume), and samples were incubated at room temperature for 30 minutes. Sucrose (40 w/v; 2 μl) was then added and samples (~1.2 μg RNA) were loaded onto 12 % (for SL1-2gag and SLV-VI) native polyacrylamide gels at 160 V, 4 °C in 1XTris-Borate buffer (89 mM Tris-Base, 89 mM boric acid). After electrophoresis, the gel was stained with Stains-all (Sigma) which stains negatively charged molecules, and photographed with a Kodak digital camera.
2.5 Isothermal titration calorimetry
Dissociation constants for BLV NC binding to SL1-2gag, SLV-VI, SLV, SLVI and 10 nt linker were determined by standard ITC methods using a VP-isothermal titration calorimeter (MicroCal Corp., Northamton, MA) (Wiseman et al., 1989). The RNA and NC samples containing buffer (10 mM Tris-Base (pH 7.0), 0.1 mM BME and varying NaCl (5–300 mM NaCl) and MgCl2 (0–1 mM) concentrations were degassed prior to titration. Protein and RNA concentrations were established by UV-Vis absorption measurements. Heats of reaction (μcal/sec) were measured at 30 °C for 28 injections of NC protein (75 μM) into 1.41 ml of RNA (5μM). The heats of dilution were obtained by titrating the identical protein sample into a cell containing sample buffer and subtracted from the raw data prior to analysis. Heats of dilution were typically 0.03 μcal/s and the maximum heats of binding were 0.3 μcal/s, giving an apparent signal-to-noise ratio of 10. The syringe mixing speed was 310 rpm as recommended by the vendor. Binding curves were analyzed by non-linear least-square fitting of the baseline-corrected ITC data to a single binding site or two-site binding model (ITC Origin program, V2.8: Microcal, Northampton, MA). The dissociation constant for each RNA is reported as the mean ± the standard deviation obtained from three independent titration experiments.
3. Results
3.1 BLV NC protein expression and purification
The native BLV NC gene could not be readily expressed due to the presence of several Arg and Pro codons that are used rarely in E. coli. A sequence-optimized insert obtained by site-directed mutagenesis was cloned and inserted into the pGEX expression vector as a GST fusion gene, and the sequence of the clone confirmed by nucleotide sequencing (Rockefeller Protein/DNA Technology Center). This optimized clone over-expressed efficiently in E. coli. Treatment of the resulting protein with PreScission protease to remove the GST tag afforded a 74- residue protein (referred throughout this paper as NC) that contains five non-native residues at the N-terminus due to cleavage specificity of the PreScission protease enzyme: NH3+- GPLSG-V1HTPGPKMPG10PRQPAPKRPP20PGPCYRCLKE30GHWARDCPTK40ATGPPPGPCP50I CKDPSHWKR60DCPTLKSKN69 (the zinc fingers are underlined). About 10 mg-purified protein per liter of culture media was obtained. Coomasie stained SDS-PAGE of the purified protein showed a single band at about 7.9 kDa. The molecular weight of the purified 15N-labeled apoprotein (7965.07 ± 0.1 Da, determined by ion-spray mass spectrometry) was as expected (MWcalc = 7964.97 Da).
3.2 Preparation of RNA samples
Oligoribonucleotides SL1-2gag, SLV and the 10mer linker connecting the SLV and SLVI were purchased from Dharmacon Research Inc. After deprotection of the 2′-O-bis (acetoxyethoxy)-methyl (ACE) group, these RNAs were purified by ethanol precipitation followed by denaturing PAGE. Oligoribonucleotides SLV-VI and SLVI were prepared using standard in vitro transcription methods (Milligan et al., 1987). Template for SLV-VI was prepared from cDNA clones, whereas the template for the SLVI constructs was obtained synthetically (KECK Foundation Biotechnology Research Center) and purified by denaturing polyacrylamide gel electrophoresis (PAGE). Reactions were optimized to give maximum yields by incorporating non-native guanosines for SLV-VI at the 5′terminus (For SLVI, the guanosines were native) (Fig. 1B). RNA samples were purified by preparative scale denaturing PAGE (Milligan and Uhlenbeck, 1989; Wyatt et al., 1991), with yields typically in the range of 1.0–3.0 μmoles of purified RNA per 90 ml reaction solution (see Methods section for details). Denaturing polyacrylamide gels showing relative electrophoretic mobility and sample purity are shown in Fig. 1E.
3.3 BLV NC exhibits weak affinity for SL1-2gag
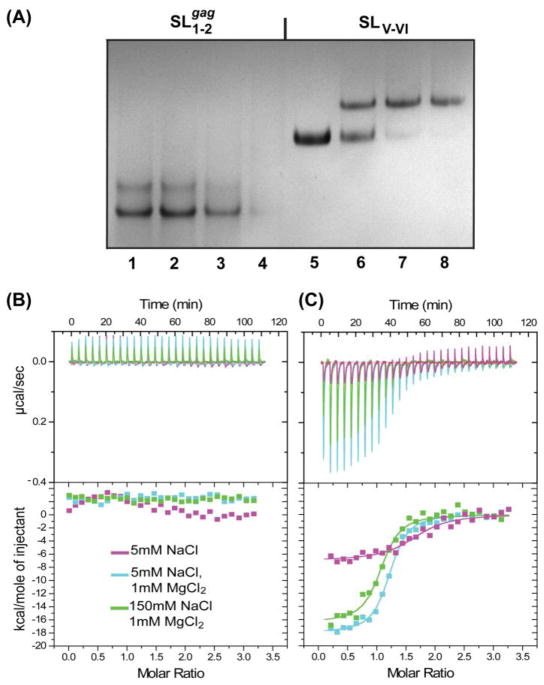
SL1-2gag forms both monomer (major) and oligomer (minor) species under conditions of 150 mM NaCl and 1 mM MgCl2, based on native polyacrylamide gel electrophoresis (PAGE) mobilities, Fig. 2A. Titration with NC led to smearing of the RNA bands at higher NC concentrations, but not to discrete band shifts observed previously for high-affinity NC binding by other retroviral NC proteins (Amarasinghe et al., 2001; Zhou et al., 2005). Similar results were obtained for smaller RNA constructs corresponding to the isolated SL1gag and SL2gag stem loops (data not shown). RNAse contamination tests ruled out the possibility of RNA degradation; thus the smearing of RNA bands upon addition of NC, along with the lack of any distinct band shift, was attributed to the formation of weakly interacting RNA-NC species. NC proteins are generally capable of binding single stranded nucleotides non-specifically and destabilizing nucleic acid helices(Guo et al., 1997; Johnson et al., 2000; Khan and Giedroc, 1992; Tsuchihashi and Brown, 1994; Wu et al., 1996), which may explain the smearing observed upon addition of NC to gag-derived RNAs. Isothermal Titration Calorimetry (ITC) experiments confirmed that, under physiological-like conditions ([NaCl] = 150mM, [MgCl2] = 1mM, pH=7.0), NC interacts weakly with SL1-2gag, Fig. 2B.
Figure 2.
NATIVE-PAGE (12%) data showing titration of BLV NC with RNA segments (A) from 5′gag, SL1-2gag (lanes 1–4) and from 5′UTR, SLV-VI (lanes 5–8) ([RNA]=30μM; lanes 1–4 and 5–8 correspond to addition of 0.0, 0.5, 1.0, 1.25 equivalents of NC to SL1-2gag and SLV-VI, respectively). The presence of smearing RNA bands with NC increments for NC- SL1-2gag titration indicates that NC binds SL1-2gag via nonspecific-weak interactions, whereas formation of a well defined, electrophoretic band shift upon addition of NC into SLV-VI indicates that high affinity NC binding site resides within the 5′UTR. Representative ITC data obtained for NC binding to (B) SL1-2gag and (C) SLV-VI under different ionic conditions (shown in the inset). Top panel: raw data with each peak corresponding to the heat produced upon addition of NC ([NC] = 75 μM, 10μl per injection) to RNA, ([RNA]=5 μM, 1.41 ml). Bottom panel: binding isotherms obtained after peak integration.
3.4 BLV NC binds SLV-VI with high affinity
The 85 nucleotide SLV-VI RNA migrates as a single band on native PAGE gels with mobility of a monomer. In contrast to results obtained for SL1-2gag, titration of SLV-VI with NC under conditions of physiological ionic strength led to discrete, stoichiometric band shifts consistent with high affinity binding and formation of a 1:1 NC- SLV-VI adduct, Fig. 2A. Binding was also monitored by ITC (Fig. 2C). Under conditions of near-physiological ionic strength (150 mM NaCl, 1 mM MgCl2), an exothermic binding isotherm was obtained (Kd = 136 ± 21 nM), consistent with results obtained previously for other NC-RNA complexes (D’Souza et al., 2001; Dey et al., 2005; Spriggs et al., 2008; Zhou et al., 2005). Unlike previous findings, binding appeared to be much weaker at low ionic strength (5 mM NaCl, no added MgCl2), and gave rise to a binding isotherm consistent with weaker binding by multiple NC molecules. This was surprising, since BLV NC is highly basic, and electrostatic interactions were expected to contribute favorably to NC-RNA interactions. Tight, stoichiometric binding appears to require Mg2+ and the highest affinity was observed for samples containing low concentrations of NaCl (5.0 mM) and physiological concentrations of MgCl2 (1.0 mM) (Kd = 93 ± 12 nM). Mg2+ is known to promote the proper folding and structural stabilization of a number of RNAs, including tRNAs (Draper et al., 2005; Romer and Hach, 1975; Serra et al., 2002; Summers et al., 1992), and our findings suggest that the proper folding of SLV-VI may also be Mg2+ dependent.
3.5 BLV NC binds specifically to SLVI
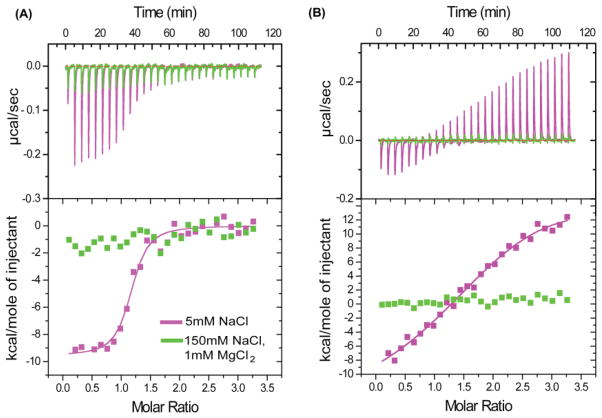
SLV-VI is predicted to consist of two stem loops connected by a short linker (SLV+linker+SLVI). The 10nt linker connecting the SLV and the SLVI stem loop contains a UCUG motif, which was shown to be the primary recognition sequence of the MMLV packaging signal, and is exposed upon dimer induced conformational changes in the genome (D’Souza and Summers, 2004). MMLV NC binds to this motif with a dissociation constant of ~95nM when the surrounding sequences are included (Dey et al., 2005). For this reason we investigated whether BLV NC interaction with the UCUG containing BLV linker resembles it in BLV genome recognition. Unlike SLV, an investigation of the linker, 5′-CAACUCUCUG-3′, NC interaction by ITC afforded favorable, negative enthalpic heats that can be fit to a 1:1 binding isotherm with a dissociation constant of Kd = 92 ± 16 nM under low ionic strength (5 mM NaCl) (Fig. 3A). These findings initially suggested to us that BLV and MLV NC proteins behave similarly in that they target unstructured RNA sequences. However, unlike observations for MLV NC, no binding was detected by ITC under conditions of physiological-like ionic strength (150 mM NaCl + 1 mM MgCl2) (Fig. 3A). Thus, the binding of BLV NC to the SLV-VI linker at low ionic strength appears to be mediated exclusively by electrostatic interactions, and it is unlikely that the SLV-VI linker participates directly in NC binding under physiological conditions.
Figure 3.
Representative ITC data obtained upon titration of BLV NC with (A) 10nt SLV-VI Linker RNA (5′-CAACUCUCUG-3′) (B) SLV hairpin RNA. Different colors denote the different conditions employed (shown in the inset). Under low ionic strength (magenta panels) NC binds to SLV via very weak interactions whereas to Linker via tight interactions (Equilibrium dissociation constant for NC-Linker complex, Kd = 92 ± 16 nM). However, under conditions of physiological-like ionic strength (green panels), NC did not bind to either the Linker or SLV RNAs.
Similar results were obtained for the isolated SLV RNA. Under conditions of low ionic strength, SLV exhibited weak affinity for NC characterized by positive binding enthalpies at NC:RNA ratios greater than ~1.2:1 (Fig. 3B). Positive NC-RNA binding enthalpies are attributed to NC-induced destabilization and unfolding of RNA structure resulting from the chaperone activity of the protein (Feng et al., 1999; Urbaneja et al., 2002). Under conditions of physiological-like ionic strength (150mM NaCl, 1mM MgCl2), which should stabilize the SLV RNA structure and inhibit weak electrostatic NC: RNA interactions, no binding could be detected by ITC (Fig. 3B).
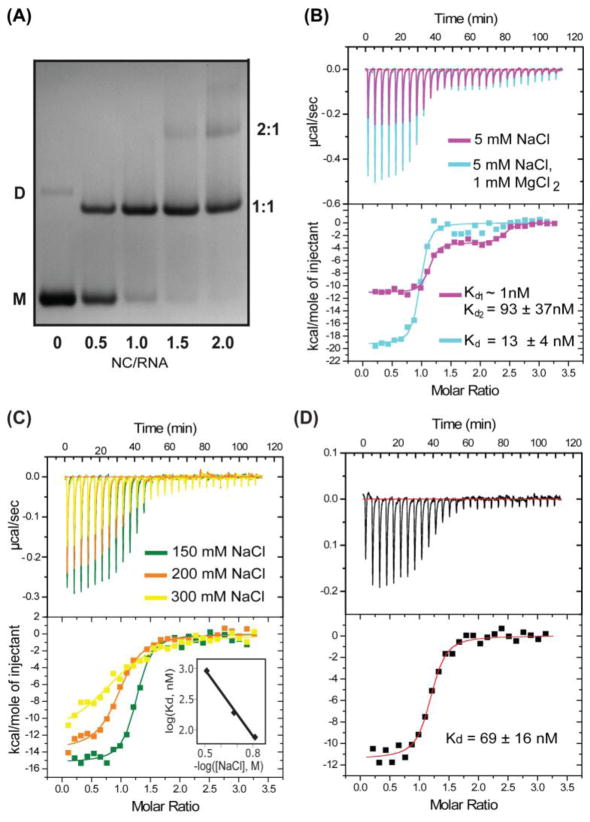
In contrast, a 31 nt RNA corresponding to the SLVI stem loop exhibited high affinity for BLV NC. Under conditions of low ionic strength (5 mM NaCl) a well-defined, stoichiometric band shift was observed by native PAGE for SLVI upon titration with NC, Fig. 4A. Beyond NC: SLVI ratios of 1:1, an additional band was observed indicative of the formation of a NC2:SLVI complex. The fact that the second band shift did not appear at NC:RNA ratio below 1:1 indicates that SLVI binds the second NC molecule with affinity that is significantly (> 10 fold) reduced compared to the initial NC binding. Similar sequential binding behavior was observed by ITC (Fig. 4B). The fitted equilibrium dissociation constant for the first site (Kd =0.15 nM) was too low to be considered reliable under the experimental conditions employed (low ionic strength). The fitted dissociation constant for the second NC binding site (93 ± 37 nM) is at least ~100 fold weaker than that of the first site. Binding to the second site was almost completely inhibited in the presence of 1 mM MgCl2 (Fig. 4B), even at low overall ionic strength (5 mM NaCl), and the best fits for the data obtained under these conditions were achieved with a 1:1 NC:RNA binding mode (Kd = 13 ± 4 nM, Fig. 4B). However, as observed in experiments performed without added salts, the ITC data exhibited steep transitions and saturation behavior near NC:RNA stoichiometries of 1:1, indicating that NC binding to SLVI is still too tight for accurate measurement under these conditions. At physiological ionic strength (150 mM NaCl, 1 mM MgCl2), SLVI consisted primarily of a monomer species, based on native PAGE behavior, and addition of NC afforded a well-defined, stoichiometric band shift at and above 1:1 NC: RNA ratios. ITC experiments performed under these conditions confirmed that NC interacts with SLVI stoichiometrically and with affinity (Kd = 67 ± 8 nM; Fig. 4C) that is approximately 2.5 times greater than that observed for HIV-1 NC binding to it’s cognate SL3 stem loop packaging element under similar conditions. Replacement of Na+ with the physiological cation, K+, in the binding buffer resulted in a similar NC-RNA binding affinity (Kd = 69 ± 16nM, 150mM KCl, 1mM MgCl2) (Fig. 4D).
Figure 4.
(A) Native-PAGE (14%) data showing results of titrating SLVI with NC (5mM NaCl, 1 mM MgCl2). Under the conditions employed, the SLVI RNA migrates as monomeric (major, M) and dimeric (minor, D) species. Addition of NC leads to formation of a specific 1:1 NC-RNA complex that migrates faster than the dimeric RNA and 2:1 NC: SLVI complex that migrates slower than the dimeric RNA. (B) Representative ITC data obtained for titration of SLVI (5 μM, 1.41ml) with NC (75 μM, 10 μl per injection) under conditions of 5mM NaCl (magenta panel) and 5mM NaCl+1mM MgCl2 (cyan panel). Under low ionic strength (5mM NaCl), SLVI exhibits two NC binding sites (Equilibrium dissociation constants for the first site and the second site are Kd1 < 1nM and Kd2 = 93 ± 37nM respectively). Weaker binding site is inhibited upon addition of 1mM MgCl2. (C) Representative ITC data obtained for titration of SLVI in the presence of varying concentrations of NaCl. Different colors denote the different NaCl concentrations employed (shown in the inset). The equilibrium dissociation constants were measured as 67 ± 8 nM ([NaCl] = 150 mM), 181 ± 49 nM ([NaCl] = 200 mM) and 932 ± 245 nM ([NaCl] = 300 mM). The Plot of the log (Kd, nM) versus −log ([NaCl], M) (correlation coefficient=0.9982) (see the inset), which afforded an extrapolated dissociation constant of 2 ± 0.6 pM at [NaCl] =10mM. (D) ITC data obtained upon titration of SLVI with NC under physiological-like ionic conditions (140 mM KCl, 1 mM MgCl2) showing that replacement of Na+ with K+ does not affect the binding affinity (Kd= 69 ± 16nM).
Because several previous NC-RNA binding studies were conducted using low ionic strength sample conditions (5–10 mM NaCl), we conducted ITC experiments over a range of NaCl concentrations (with [MgCl2] = 1 mM) (Fig. 4C), which allowed us to estimate the affinity of NC for SLVI at lower ionic strength where binding was too tight for accurate measurement. Extrapolation of a log [Kd] versus −log [NaCl] plot (Fig. 4C) afforded an estimated NC dissociation constant at low salt ([NaCl] = 10 mM; [MgCl2] = 1.0 mM) of (extrapolated dissociation constant Kd = 2 ± 0.6 pM), which is five orders of magnitude tighter than the value measured for HIV-1 NC binding to the ψ-RNA stem loop SL3 (Amarasinghe et al., 2000), and three orders of magnitude tighter than the affinity measured for the Rous sarcoma virus NC protein binding to its minimal, 75 nt packaging element (Zhou et al., 2005).
4. Discussion
Retroviral genome packaging is initiated by the formation of a complex between a small number of Gag proteins (perhaps a dozen or fewer (Jouvenet et al., 2008; Jouvenet et al., 2009; Jouvenet et al., 2011; Kutluay and Bieniasz, 2010)) and RNA elements located near the 5′ end of the unspliced genome (Berkhout, 1996; Berkowitz et al., 1996; Jewell and Mansky, 2000; Rein, 1994). Non-viral vectors that contain the 5′-most 734 nucleotides of the BLV genome can be efficiently packaged into BLV virus-like particles, indicating that the packaging signal residues exclusively reside within this region (Jewell and Mansky, 2005). Deletion experiments identified discrete elements within the 5′-UTR (Kurg et al., 1995) and gag ORF (Mansky et al., 1995) that are critical for efficient packaging. For example, deletion of 5′-UTR residues (U340-A417) led to a 5-fold reduction in packaging, and mutation of six bases (C346-G351) in the conserved consensus sequence immediately adjacent to the primer binding-site (U340-C356) (Rice et al., 1985) resulted in a 3-fold decrease in packaging (Kurg et al., 1995). These studies indicate that residues U340-A417 within the 5′UTR are critical for packaging. Deletion analysis indicate that BLV packaging signal was contained within a 147nt-region spanning the residues, U340-G487 (Mansky et al., 1995). Secondary structure predictions, coupled with site directed mutagenesis experiments, have indicated that tandem stem loops in the gag ORF, SL1-2gag (A417-U472) (Mansky and Gajary, 2002; Mansky et al., 1995; Mansky and Wisniewski, 1998) is the critical element. The finding that critical elements of the packaging signal reside within the gag open reading frame was unusual (although the most efficient packaging has been found to depend on residues within gag (Bender et al., 1987; Berkowitz et al., 1995)), and suggested that the mechanism of BLV genome packaging may differ, to some extent, from that employed by other retroviruses.
We have now shown that BLV NC does not exhibit significant affinity for SL1-2gag. This does not rule out the possibility that this element serves some other role in packaging, such as stabilizing a critical RNA structure or interacting with cellular or other viral constituents. Interestingly, gel-shift mobility assays revealed that the BLV matrix protein (MA) also binds with specificity to gag-derived RNAs that contain SL1-2gag (Katoh et al., 1991; Katoh et al., 1993), which led to suggestions that MA, in addition to NC, may function in packaging by binding to specific regions of the viral genome (Wang et al., 2003). It is thus conceivable that the SL1-2gag element promotes packaging by binding to the BLV MA domain, rather than the NC domain, and further studies along these lines are warranted.
Our studies indicate that BLV NC binds with high affinity to SLV-VI, consistent with the in vivo packaging experiments of Kurg and colleagues (Kurg et al., 1995). Additionally, under physiological-like sample conditions, the binding site residues reside exclusively within the SLVI fragment. Binding under these conditions (Kd = 67 ± 8 nM) is significantly tighter than that observed previously for HIV-1 NC binding to RNA tetra loops in low-salt buffers (Kd ~ 150 nM; [NaCl] = 10 mM; [MgCl2] = 0 mM) (Amarasinghe et al., 2000) or MLV NC binding to single stranded RNAs containing Py-Py-Py-G (Py = pyrimidine) sequences in similar low-salt buffers (~100–300 nM) (D’Souza et al., 2001; Dey et al., 2005). The affinities of HIV-1 and MLV NC for their cognate RNAs decrease with increasing ionic strength, and it is likely that, under similar, native-like conditions, BLV NC-SLVI interactions may be more than an order of magnitude tighter than those of HIV-1 and MLV. Surprisingly, the extrapolated BLV NC:SLVI dissociation constant at low ionic strength (10 mM NaCl, 1 mM MgCl2) is more than four orders of magnitude tighter than the values measured for HIV-1 NC:SL3 and NC:SL2 binding under similar conditions (Amarasinghe et al., 2000). It is not immediately obvious why different retroviral NC proteins exhibit such different affinities for their RNA packaging signals. One possibility is that the affinity of the packaging signals for NC is context-dependent, and highest affinity (and perhaps specificity) requires the intact 5′-UTR. It is also conceivable that genome recognition by different retroviruses is mediated by different NC: RNA stoichiometries. For example, BLV genome packaging might be mediated by as few as one or two NC proteins that bind with very high affinity, whereas HIV-1 genome packaging might require several NC domains that bind with reduced affinities.
In all previously characterized retroviral NC-RNA structures, binding is mediated, at least partly, by insertion of one or more exposed guanosines to hydrophobic pockets on the surfaces of the CCHC-type zinc fingers. Binding is promoted by the formation of hydrogen bonds between the guanosine O6, N1H and N2H21 groups and backbone NH and O atoms of peptide residues located at the bottom of the pocket (Amarasinghe et al., 2000; De Guzman et al., 1998; South and Summers, 1993). The N-and C-terminal HIV-1 NC zinc fingers bind in this manner to the second and last exposed guanosines of the SL2 and SL3 GG (A/U) G tetra loops (Amarasinghe et al., 2000; De Guzman et al., 1998). The single MLV NC zinc finger binds in an analogous manner to exposed guanosines in unstructured UAUCUG elements (D’Souza and Summers, 2004; Dey et al., 2005), and the N-terminal zinc finger of the RSV NC protein binds to the single exposed guanosine of a U(217)GCG tetraloop (Zhou et al., 2007). It is noteworthy that the C-terminal RSV NC zinc finger does not bind to exposed guanosines, but instead interacts with exposed adenosine bases in unstructured strands that link adjacent stem loops (Zhou et al., 2007). Interestingly, the predicted secondary structure of SLVI does not contain a single exposed guanosine, but instead contains an apical CUC loop, a bulged C, and a larger CAUUU bulge. Although NC molecules can have potent chaperone activity (Darlix et al., 2011), preliminary NMR studies indicate that NC does not induce unwinding of the SLVI RNA (Yildiz and Summers, unpublished). The RNA is also unusual relative to other NC targets in that it only binds tightly to NC in the presence of Mg+2, which can sometimes inhibit NC binding and chaperone activity (Summers et al., 1992). Together, these observations suggest that the NC-RNA structures responsible for directing BLV genome recognition and packaging may be significantly different from those of other retroviruses. High-resolution structural studies of the BLV NC:SLVI designed to test this hypothesis are underway.
Highlights.
Previously proposed packaging signals tested.
Only one of several proposed packaging elements binds NC with high-affinity.
NC binding element unexpectedly lacks features common to other retroviral NC binding sites.
Acknowledgments
Support from the NIH (GM42561 to MFS) is gratefully acknowledged. We thank Dr. Mansky (Ohio State) for providing the BLV plasmid construct (pBLV-SVNEO), David King (University of California, Berkeley) for mass spectrometry measurements, Rasheeda Johnson (UMBC) for her help in the wet lab, and Dr. Holly Summers (UMBC) for technical help. K.B. was supported by an NIGMS initiative for minority student development grant (R25-GM55036).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam MA, Miller AD. Identification of a signal in a murine retrovirus that is sufficient for packaging of nonretroviral RNA into virions. J Virol. 1988;62(10):3802–3806. doi: 10.1128/jvi.62.10.3802-3806.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarasinghe GK, De Guzman RN, Turner RB, Chancellor K, Wu ZR, Summers MF. NMR structure of the HIV-1 nucleocapsid protein bound to stem-loop SL2 of the ψ-RNA packaging signal. J Mol Biol. 2000;301:491–511. doi: 10.1006/jmbi.2000.3979. [DOI] [PubMed] [Google Scholar]
- Amarasinghe GK, Zhou J, Miskimon M, Chancellor KJ, McDonald JA, Matthews AG, Miller RA, Rouse MD, Summers MF. Stem-loop SL4 of the HIV-1 ψ-RNA packaging signal exhibits weak affinity for the nucleocapsid protein. Structural studies and implications for genome recognition. J Mol Biol. 2001;314:961–969. doi: 10.1006/jmbi.2000.5182. [DOI] [PubMed] [Google Scholar]
- Bender MA, Palmer TD, Gelinas RE, Miller AD. Evidence that the packaging signal of Moloney murine leukemia virus extends into the gag region. J Virol. 1987;61(5):1639–1646. doi: 10.1128/jvi.61.5.1639-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhout B. Prog Nucl Acid Res and Mol Biol. Vol. 54. Academic Press, Inc; 1996. Structure and function of the human immunodeficiency virus leader RNA; pp. 1–34. [DOI] [PubMed] [Google Scholar]
- Berkowitz R, Fisher J, Goff SP. RNA packaging. Curr Top Microbiol Immun. 1996;214:177–218. doi: 10.1007/978-3-642-80145-7_6. [DOI] [PubMed] [Google Scholar]
- Berkowitz RD, Hammarskjold ML, Helga-Maria C, Rekosh D, Goff SP. 5′ Regions of HIV-1 RNAs are not sufficient for encapsidation: Implications for the HIV-1 packaging signal. Virology. 1995;212:718–723. doi: 10.1006/viro.1995.1530. [DOI] [PubMed] [Google Scholar]
- D’Souza V, Melamed J, Habib D, Pullen K, Wallace K, Summers MF. Identification of a high-affinity nucleocapsid protein binding site within the Moloney Murine Leukemia Virus Ψ-RNA packaging signal. Implications for genome recognition. J Mol Biol. 2001;314:217–232. doi: 10.1006/jmbi.2001.5139. [DOI] [PubMed] [Google Scholar]
- D’Souza V, Summers MF. Structural basis for packaging the dimeric genome of Moloney Murine Leukaemia Virus. Nature. 2004;431:586–590. doi: 10.1038/nature02944. [DOI] [PubMed] [Google Scholar]
- D’Souza V, Summers MF. How retroviruses select their genomes. Nature Revs Microbiol. 2005;3:643–655. doi: 10.1038/nrmicro1210. [DOI] [PubMed] [Google Scholar]
- Darlix JL, Godet J, Ivanyi-Nagy R, Fosse P, Mauffret O, Mely Y. Flexible nature and specific functions of the HIV-1 nucleocapsid protein. J Mol Biol. 2011;410:565–581. doi: 10.1016/j.jmb.2011.03.037. [DOI] [PubMed] [Google Scholar]
- De Guzman RN, Wu ZR, Stalling CC, Pappalardo L, Borer PN, Summers MF. Structure of the HIV-1 nucleocapsid protein bound to the SL3 ψ-RNA recognition element. Science. 1998;279:384–388. doi: 10.1126/science.279.5349.384. [DOI] [PubMed] [Google Scholar]
- Dey A, York D, Smalls-Mantey A, Summers MF. Composition and sequence dependent binding of RNA to the nucleocapsid protein of Moloney Murine Leukemia Virus. Biochemistry. 2005;44:3735–3744. doi: 10.1021/bi047639q. [DOI] [PubMed] [Google Scholar]
- Draper DE, Grilley D, Soto AM. Ions and RNA folding. Annu Rev Biophys Biomol Struct. 2005;34:221–243. doi: 10.1146/annurev.biophys.34.040204.144511. [DOI] [PubMed] [Google Scholar]
- Feng YX, Campbell S, Harvin D, Ehresmann B, Ehresmann C, Rein A. The human immunodeficiency virus type 1 Gag polyprotein has nucleic acid chaperone activity: Possible role in dimerization of genomic RNA and placement of tRNA on the primer binding site. J Virol. 1999;73:4251–4256. doi: 10.1128/jvi.73.5.4251-4256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillet N, Florins A, Boxus M, Burteau C, Nigro A, Vandermeers F, Balon H, Bouzar AB, Defoiche J, Burny A, Reichert M, Kettmann R, Willems L. Mechanisms of leukemogenesis induced by bovine leukemia virus: prospects for novel anti-retroviral therapies in human. Retrovirology. 2007;4:18. doi: 10.1186/1742-4690-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff SP. Retroviral reverse transcriptase: Synthesis, structure and function. J Aquired Immune Defic Syndr. 1990;3:817–831. [PubMed] [Google Scholar]
- Greatorex J. The retroviral RNA dimer linkage: different structures may reflect different roles. Retrovirology. 2004;1(22) doi: 10.1186/1742-4690-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greatorex J, Lever A. Retroviral RNA dimer linkage. J Gen Virol. 1998;79:2877–2882. doi: 10.1099/0022-1317-79-12-2877. [DOI] [PubMed] [Google Scholar]
- Guo J, Henderson LE, Bess J, Kane B, Levin JG. Human immunodeficiency virus type 1 nucleocapsid protein promotes efficient strand transfer and specific viral DNA synthesis by inhibiting TAR-dependent self-priming from minus-strand strong-stop DNA. J Virol. 1997;71:5178–5188. doi: 10.1128/jvi.71.7.5178-5188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng X, Kharytonchyk S, Garcia EL, Lu K, Sachin Divakaruni S, LaCotti C, Edme K, Telesnitsky A, Summers MF. Identification of a minimal HIV-1 RNA packaging signal. J Mol Biol. 2012;417:224–239. doi: 10.1016/j.jmb.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell NA, Mansky LM. In the beginning: genome recognition, RNA encapsidation and the initiation of complex retrovirus assembly. J Gen Virol. 2000;81:1889–1899. doi: 10.1099/0022-1317-81-8-1889. [DOI] [PubMed] [Google Scholar]
- Jewell NA, Mansky LM. Packaging of heterologous RNAs by a minimal bovine leukemia virus RNA packaging signal into virus particles. Arch Virol. 2005;150(6):1161–1173. doi: 10.1007/s00705-004-0476-7. [DOI] [PubMed] [Google Scholar]
- Johnson PE, Turner RB, Wu ZR, Hairston L, Guo J, Levin JG, Summers MF. A mechanism for (+) strand transfer enhancement by the HIV-1 nucleocapsid protein during reverse transcription. Biochemistry. 2000;39:9084–9091. doi: 10.1021/bi000841i. [DOI] [PubMed] [Google Scholar]
- Jouvenet N, Bieniasz PD, Simon SM. Imaging the biogenesis of individual HIV-1 virions in live cells. Nature. 2008;454:236–240. doi: 10.1038/nature06998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvenet N, Simon SM, Bieniasz PD. Imaging the interaction of HIV-1 genomes and Gag during assembly of individual viral particles. Proc Natl Acad Sci USA. 2009;106(45):19114–19119. doi: 10.1073/pnas.0907364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvenet N, Simon SM, Bieniasz PD. Visualizing HIV-1 assembly. J Mol Biol. 2011;410(4):501–511. doi: 10.1016/j.jmb.2011.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya H, Ohashi K, Onuma M. Host immune responses in the course of bovine leukemia virus infection. J Vet Med Sci. 2001;63(7):703–708. doi: 10.1292/jvms.63.703. [DOI] [PubMed] [Google Scholar]
- Katoh I, Kyushiki H, Sakamoto Y, Ikawa Y, Yoshinaka Y. Bovine Leukemia Virus Matrix-Associated Protein MA(p15): Further Processing and Formation of a Specific Complex with the Dimer of the 5′-Terminal Genomic RNA Fragment. J Virol. 1991;65:6845–6855. doi: 10.1128/jvi.65.12.6845-6855.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh I, Yasunaga T, Yoshinaka Y. Bovine Leukemia Virus RNA Sequences Involved in Dimerization and Specific gag Protein Binding: Close Relation to the Packaging Sites of Avian, Murine, and Human Retroviruses. J Virol. 1993;67:1830–1839. doi: 10.1128/jvi.67.4.1830-1839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R, Giedroc DP. Recombinant human immunodeficiency virus type 1 nucleocapsid NCp7 protein unwinds tRNA. J Biol Chem. 1992;267(10):6689–6695. [PubMed] [Google Scholar]
- Kurg A, Sommer G, Metspalu A. An RNA stem-loop structure involved in the packaging of bovine leukemia virus genomic RNA in vivo. Virology. 1995;211:434–442. doi: 10.1006/viro.1995.1425. [DOI] [PubMed] [Google Scholar]
- Kutluay SB, Bieniasz PD. Analysis of the initiating events in HIV-1 particle assembly and genome packaging. PLoS pathogens. 2010;6(11):e1001200. doi: 10.1371/journal.ppat.1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Heng X, Garyu L, Monti S, Garcia E, Kharytonchyk S, Dorjsuren B, Kulandaivel G, Jones S, Hiremath A, Sachin Divakaruni S, LaCotti C, Barton S, Tummillo D, Hosic A, Edme K, Albrecht S, Telesnitsky A, Summers MF. NMR detection of structures in the HIV-1 5′-leader RNA that regulate genome packaging. Science. 2011a;344:242–245. doi: 10.1126/science.1210460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Heng X, Summers MF. Structural determinants and mechanism of HIV-1 genome packaging. J Mol Biol. 2011b;410:609–633. doi: 10.1016/j.jmb.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R, Baltimore D. Varying the position of a retrovirus packaging sequence results in the encapsidation of both unspliced and spliced RNA. J Virol. 1985;54:401–407. doi: 10.1128/jvi.54.2.401-407.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R, Mulligan RC, Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983;33:153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Mansky LM, Gajary LC. The primary nucleotide sequence of the bovine leukemia virus RNA packaging signal can influence efficient RNA packaging and virus replication. J Virol. 2002;301:272–280. doi: 10.1006/viro.2002.1578. [DOI] [PubMed] [Google Scholar]
- Mansky LM, Krueger AE, Temin HM. The bovine leukemia virus encapsidation signal is discontinuous and extends into the 5′ end of the gag gene. J Virol. 1995;69:3282–3289. doi: 10.1128/jvi.69.6.3282-3289.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansky LM, Wisniewski RM. The bovine leukemia virus encapsidation signal is composed of RNA secondary structures. J Virol. 1998;72:3196–3204. doi: 10.1128/jvi.72.4.3196-3204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride MS, Panganiban AT. The human immunodeficiency virus type 1 encapsidation site is a multipartite RNA element composed of functional hairpin structures. J Virol. 1996;70:2963–2973. doi: 10.1128/jvi.70.5.2963-2973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride MS, Panganiban AT. Position dependence of functional hairpins important for human immunodeficiency virus type 1 RNA encapsidation in vivo. J Virol. 1997;71:2050–2058. doi: 10.1128/jvi.71.3.2050-2058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride MS, Schwartz MD, Panganiban AT. Efficient encapsidation of human immunodeficiency virus type 1 vectors and further characterization of cis elements required for encapsidation. J Virol. 1997;71:4544–4554. doi: 10.1128/jvi.71.6.4544-4554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan JF, Groebe DR, Witherell GW, Uhlenbeck OC. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Research. 1987;15(21):8783–9798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan JF, Uhlenbeck OC. Synthesis of small RNAs using T7 RNA polymerase. Meth Enzymol. 1989;180:51–62. doi: 10.1016/0076-6879(89)80091-6. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Garcia E, King SR, Iyalla K, Loeliger K, Starck P, Syed S, Telesnitsky A, Summers MF. An RNA structural switch regulates diploid genome packaging by Moloney Murine Leukemia Virus. J Mol Biol. 2010a;396:141–152. doi: 10.1016/j.jmb.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y, Irobalieva RN, Tolbert BS, Smalls-Manty A, Iyalla K, Loeliger K, D’Souza V, Khant H, Schmid MF, Garcia E, Telesnitsky A, Chiu W, Summers MF. Structure of a conserved retroviral RNA packaging element by NMR spectroscopy and cryo-electron tomography. J Mol Biol. 2010b;404:751–772. doi: 10.1016/j.jmb.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougel M, Barklis E. A role for two hairpin structures as a core RNA encapsidation signal in murine leukemia virus virions. J Virol. 1997;71:8061–8065. doi: 10.1128/jvi.71.10.8061-8065.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillart JC, Marquet R, Skripkin E, Ehresmann C, Ehresmann B. Dimerization of retroviral genomic RNAs: Structural and functional implications. Biochimie. 1996;78:639–653. doi: 10.1016/s0300-9084(96)80010-1. [DOI] [PubMed] [Google Scholar]
- Paillart JC, Shehu-Xhilaga M, Marquet R, Mak J. Dimerization of retroviral RNA genomes: An inseparable pair. Nature Revs Microbiol. 2004;2:461–472. doi: 10.1038/nrmicro903. [DOI] [PubMed] [Google Scholar]
- Rein A. Retroviral RNA packaging: A review. Arch Virology. 1994;9:513–522. doi: 10.1007/978-3-7091-9326-6_49. [DOI] [PubMed] [Google Scholar]
- Rice NR, Stephens RM, Burny A, Gilden RV. The gag and pol genes of bovine leukemia virus: nucleotide sequence and analysis. Virology. 1985;142(2):357–377. doi: 10.1016/0042-6822(85)90344-7. [DOI] [PubMed] [Google Scholar]
- Romer R, Hach R. tRNA conformation and magnesium binding. A study of a yeast phenylalanine-specific tRNA by a fluorescent indicator and differential melting curves. Eur J Biochem. 1975;55(1):271–284. doi: 10.1111/j.1432-1033.1975.tb02160.x. [DOI] [PubMed] [Google Scholar]
- Russell RS, Liang C, Wainberg MA. Is HIV-1 RNA dimerization a prerequisite for packaging? Yes, no, probably? Retrovirology. 2004;1(23) doi: 10.1186/1742-4690-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra MJ, Baird JD, Dale T, Fey BL, Retatagos K, Westhof E. Effects of magnesium ions on the stabilization of RNA oligomers of defined structures. RNA. 2002;8(3):307–323. doi: 10.1017/s1355838202024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South TL, Summers MF. Zinc- and sequence-dependent binding to nucleic acids by the N-terminal zinc finger of the HIV-1 nucleocapsid protein: NMR structure of the complex with the Psi-site analog, dACGCC. Protein Sci. 1993;2(1):3–19. doi: 10.1002/pro.5560020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs S, Garyu L, Connor R, Summers MF. Potential intra- and intermolecular interactions involving the Unique-5′ Region of the HIV-1 5′-UTR. Biochemistry. 2008;46:13064–13073. doi: 10.1021/bi8014373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers MF, Henderson LE, Chance MR, Bess JWJ, South TL, Blake PR, Sagi I, Perez-Alvarado G, Sowder RCI, Hare DR, Arthur LO. Nucleocapsid zinc fingers detected in retroviruses: EXAFS studies of intact viruses and the solution-state structure of the nucleocapsid protein from HIV-1. Protein Sci. 1992;1:563–574. doi: 10.1002/pro.5560010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchihashi Z, Brown PO. DNA strand exchange and selective DNA annealing promoted by the human immunodeficiency virus type 1 nucleocapsid protein. J Virol. 1994:5863–5870. doi: 10.1128/jvi.68.9.5863-5870.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbaneja MA, Wu M, Casas-Finet J, Karpel RL. HIV-1 nucleocapsid protein as a nucleic acid chaperone-spectroscopic study of it’s helix-destablilizing properties, structural binding specificity, and annealing activity. J Mol Biol. 2002;318:749–769. doi: 10.1016/S0022-2836(02)00043-8. [DOI] [PubMed] [Google Scholar]
- Wang H, Norris KM, Mansky LM. Involvement of the matrix and nucleocapsid domains of the bovine leukemia virus Gag polyprotein precursor in viral RNA packaging. J Virol. 2003;77:9431–9438. doi: 10.1128/JVI.77.17.9431-9438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman T, Williston S, Brandts JF, Lin LN. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal Biochem. 1989;179:131–137. doi: 10.1016/0003-2697(89)90213-3. [DOI] [PubMed] [Google Scholar]
- Wu W, Henderson LE, Copeland TD, Gorelick RJ, Bosche WJ, Rein A, Levin JG. Human immunodeficiency virus type 1 nucleocapsid protein reduces reverse transcriptase pausing at a secondary structure near the murine leukemia virus polypurine tract. J Virol. 1996;70:7132–7142. doi: 10.1128/jvi.70.10.7132-7142.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt JR, Chastain M, Puglisi JD. Synthesis and purification of large amounts of RNA oligonucleotides. BioTechniques. 1991;11:764–769. [PubMed] [Google Scholar]
- Zhou J, McAllen K, Tailor Y, Summers MF. High affinity nucleocapsid protein binding to the μΨ RNA packaging signal of Rous sarcoma virus. J Mol Biol. 2005;349:976–988. doi: 10.1016/j.jmb.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Zhou T, Xu L, Dey B, Hessel AJ, Ryk DV, Xiang SH, Yang X, Zhang MY, Zwick MB, Arthos J, Burton DR, Dimitrov DS, Sodroski J, Wyatt R, Nabel GJ, Kwong PD. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature. 2007;445:732–737. doi: 10.1038/nature05580. [DOI] [PMC free article] [PubMed] [Google Scholar]