Abstract
Organic cation transporters have previously been implicated in cisplatin nephrotoxicity. In this study, we found that renal tubular secretion of cisplatin is abolished in mice lacking the Oct1 and Oct2 transporters [Oct1/2(−/−) mice], and these mice are protected from experiencing severe cisplatin-induced renal damage. Compared to wildtype mice, Oct1/2(−/−) mice also experienced a significantly decreased change in urinary activity of N-acetyl-β-D-glucosaminidase (NAG) following cisplatin administration (~4-fold, P=0.0016). A cutoff for cumulative urinary NAG activity of >0.4 AU was associated with a 21-fold increased odds for severe nephrotoxicity (P=0.0017), which in turn was linked with overall survival [hazard ratio (95%CI), 8.1 (2.1–31), P=0.0078]. Next, we screened 16 agents at varying concentrations for inhibitory potential against the human homolog transporter, OCT2, using transfected 293Flp-In cells. We focused further on the possible utility of cimetidine as an OCT2 inhibitor because of its strong potency (>95% inhibition), and because this agent is not routinely co-administered with cisplatin. In mice, we found that cimetidine inhibited cisplatin-induced urinary NAG activity changes to a degree significantly different from vehicle-control treated mice (P=0.016), but similar to that seen in Oct1/2(−/−) mice (P=0.91). Interestingly, cimetidine did not affect the uptake of cisplatin into SKOV-3 cells, the NCI60 cell line with the highest OCT2 expression. Collectively, this study suggests that OCT2 inhibitors can completely inhibit transporter-mediated uptake of cisplatin in renal proximal tubular cells, and subsequently ameliorate cisplatin nephrotoxicity without affecting the accumulation in tumor cells.
Keywords: Cisplatin, nephrotoxicity, organic cation transporters, OCT2, N-acetyl-β-D-glucosaminidase
Introduction
Cisplatin is a DNA-crosslinking agent that is among the most widely used anticancer drugs worldwide in both adult and pediatric populations. The dose-limiting side effects associated with cisplatin-based chemotherapy include renal tubular dysfunction (nephrotoxicity), and to a lesser extent, hearing loss (ototoxicity) (1). Severe and irreversible damage to the kidney is a tremendous health problem and remains the single most important complication of cisplatin treatment as it may limit further treatment or even threaten life. This side effect primarily affects the S3 segment of the renal proximal tubules and occurs in up to 40% of patients despite intensive prophylactic measures (2), including extensive pre- and posthydration regimens with hypertonic saline.
Over the last 3 decades, various approaches have been reported to afford reno-protection during cisplatin treatment, although most of these have not been evaluated in animal models or humans (3). The clinical application of many of these strategies has been hampered by the recognition that (a) cisplatin has multiple intracellular targets and hence blocking a single injurious event will only have partial protective effects in the kidney, and (b) the renoprotective approach may diminish the antitumor effects of cisplatin given the overlap in cell death signaling pathways between normal kidney cells and tumor cells (3). Therefore, an ideal approach of renoprotection is to protect the kidneys without affecting the therapeutic effects in tumors.
Using transfected mammalian cells, we previously reported that cisplatin is a substrate for the organic cation transporter OCT2 (4), which is highly expressed at the basolateral membrane of renal tubular epithelial cells and is considered a major transporter in the active secretion of xenobiotics from the circulation into the kidney. More recently, we found that mice lacking the ortholog transporters Oct1 and Oct2 [Oct1/2(−/−) mice] have impaired urinary excretion of cisplatin and that these animals are protected from experiencing severe cisplatin nephrotoxicity (5). This suggests that these transporters are key proteins involved in the initial step toward renal elimination of cisplatin. Based on these observations, we hypothesized that the extent to which cisplatin interacts with Oct 1 and Oct2 (or OCT2 in humans) and subsequently affects nephrotoxicity is dependent on intracellular drug concentrations in the renal tubule and subsequent activation of pathways resulting in tubular apoptosis. The objectives of the current study were to (a) assess the extent of renal secretion of cisplatin in wildtype mice and Oct1/2(−/−) mice, (b) determine the influence of cisplatin treatment on the time course of biomarkers of nephrotoxicity and overall survival, and (c) evaluate the effects of OCT2 inhibitors on cisplatin transport and nephrotoxicity.
Materials and Methods
Chemical and reagents
The Flp-In transfection system, Dulbecco’s Modified Eagle’s Medium (DMEM), phosphate-buffered saline (PBS), Lipofectamine 2000, hygromycin, zeocin, Opti-MEM reduced serum medium, TRIzol, Superscript III first strand synthesis system, and fetal bovine serum (FBS) were all obtained from Invitrogen. The human full length cDNA clone of OCT2 was purchased from Origene. Cisplatin and [14C]tetraethylammonium bromide (TEA) were obtained from Sigma Chemical Co. and American Radiolabeled Chemicals, respectively. All other compounds used in this study were of reagent grade or better.
Animals
Adult (8–12 week old) male FVB wild-type and Oct1/2(−/−) mice were purchased from Taconic. Mice deficient in Oct1 [Oct1(−/−) mice ] or Oct2 [Oct2(−/−) mice] were provided by Dr. Alfred H. Schinkel (6). All animals were housed and handled in accordance with the Institutional Animal Care and Use Committee of St. Jude Children’s Research Hospital. Animals were housed in a temperature controlled environment with a 12-h light cycle and were given a standard diet and water ad libitum.
Plasma pharmacokinetic studies
Blood samples were obtained at 0.25, 0.75, 1, 2, 4, and 8 h after an i.p. dose of cisplatin at 10 mg/kg, centrifuged to obtain plasma, and flameless atomic absorption spectrometry (AAS) was used to determine total platinum concentrations (5). The unbound platinum concentration was determined following ethanolic plasma-protein precipitation (7). Pharmacokinetic parameters were calculated using the software package PK Solutions 2.0 (Summit Research Services).
Urinary platinum excretion
For experiments involving collection of urine, animals were housed in metabolic cages in a temperature controlled environment with a 12-h reverse light cycle. After the animals had acclimated to metabolic cages, a 24-h baseline urine sample was collected 16 h after the onset of light. The next day, mice were given a single i.p. injection of cisplatin at a dose of 10 mg/kg. Urine was collected before drug administration and at 4, 24, 48, and 72 h after administration. Urine samples were diluted with nitric acid (0.2%) and analyzed for total platinum using AAS (5). An estimate of the renal clearance (CLR) of total platinum was determined as follows: CLR = Aurine (0–24)/AUC(0–24), where Aurine (0–24) and AUC(0–24) are the cumulative urinary excretion of platinum up to 24 h and the area under the plasma concentration-time curve for protein-unbound platinum up to 24 h, calculated using the linear trapezoidal rule, respectively.
Assessment of renal biomarkers
Glomerular fltration rate (GFR) at baseline was estimated in male FVB mice and Oct1/2(−/−) mice from body weight (BW) from intraspecies allometry using the following equation: GFR = 0.036 × BW0.74±0.15 (8). The validity of this approach is supported by the previous finding that GFR determined from [14C]inulin clearance was not significantly different between wildtype mice and Oct1/2(−/−) mice and corresponded well to the GFR estimated from BW alone (9). Levels of blood urea nitrogen (BUN), serum creatinine and urinary creatinine were determined using a Vetscan autoanalyzer (Abaxis). Urinary N-acetyl-β-D-glucosaminidase (NAG) activity before and after cisplatin treatment with or without an i.v. dose of cimetidine (30 mg/kg) or vehicle control was measured in samples obtained from wildtype, Oct1(−/−), Oct2(−/−) and Oct1/2(−/−) mice using a kit from Diazyme Laboratories (Poway) using detection at 505 nm on a µQuant microplate spectrophotometer (BioTek Instruments).
Survival analysis
Overall survival was assessed in male wild-type mice and age- and weight-matched male Oct1/2 (−/−) mice after administration of cisplatin by i.p. injection at a single dose of 10 or 20 mg/kg. Overall survival was assessed by the Kaplan-Meier method, using an exact log-rank test and a Cox-Mantel proportional hazard model to determine the impact of mouse genotype and nephrotoxicity.
Histopathological analysis
For microscopic examination, tissues were fixed in 4% phosphate-buffered formalin, embedded in paraffin, sectioned at 4 µm, and stained with hematoxylin and eosin in accordance with standard procedures.
Cellular transport studies
The Flp-In transfection system was used as described to produce stably transfected 293Flp-In cells expressing OCT2 or an empty vector (4). These cell lines were collected and reseeded in complete DMEM containing 10% FBS and hygromycin B (100 µg/mL). All uptake experiments were performed on monolayer cultures in 6-well plates at 37°C, as described (4). For the transport inhibition assays, monolayers were washed once with warm PBS then pre-incubated with DMEM containing potential OCT2 inhibitors at initial concentrations of 1 mM. This medium was removed and replaced with the same concentration of inhibitor in DMEM solution with TEA (2 µM) and allowed to incubate for an additional 30 min at 37°C. After incubation, cells were washed twice with cold PBS, collected and solubilized in 1N sodium hydroxide. Radioactivity was assessed by liquid scintillation counting, platinum levels were measured using AAS (5), and protein concentrations were measured using a bicinchoninic acid (BCA) protein-assay kit (Pierce Biotechnology). Compounds that were found to be significant inhibitors of TEA uptake were subsequently tested at a concentration of 1 µM.
The potential functional change in transport associated with a common OCT2 variant (p.270Ala>Ser) was assessed using cisplatin transport in the presence and absence of cimetidine. Briefly, HEK293 cells expressing either an empty vector, wildtype OCT2 (containing reference sequence), or a protein variant of OCT2 containing the p.270Ala>Ser amino acid substitution (10) were tested under similar conditions mentioned above.
Gene expression in NCI60 cancer cell lines
RNA from the NCI60 cancer cell lines were provided by the National Cancer Institute tumor repository (Bethesda). SKOV-3 cells were from ATCC (catalog #HTB-77). RNA was reverse transcribed using SuperScript III first strand synthesis supermix for qRT-PCR (Invitrogen) according to manufacturer’s recommendations. Gene transcripts were quantified using SYBR Green PCR mastermix (Qiagen) and primers previously described (11). Reactions were carried out in triplicate unless otherwise stated as previously reported (4). Briefly, 25 µL volumes were used with the following PCR variables: 95 °C for 15 min then 40 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s, followed by dissociation cycles. Transcripts of each sample were normalized to the housekeeping gene, GAPDH.
The genomic sequence of the OCT2 gene, SLC22A2, was obtained by Genbank (accession no. NM 003058) and primers were designed as previously described (4). Samples were cleaned with ExoSAP-IT reagent (USB Corporation) following PCR and sequenced in both forward and reverse directions using Big Dye Terminator version 3.1 on an Applied Biosystems 3730XL DNA analyzer. Sequencher software version 4.7 (Gene Codes Corporation) was used for sequencing analysis.
Statistical analysis
All data are presented as mean and standard error (SE), unless otherwise stated. Statistical analyses were done using a two-tailed t test, and P values < 0.05 were considered to be statistically significant. All statistical calculations were performed using the software package NCSS version 2004 (Number Cruncher Statistical System).
Results
Renal secretion of cisplatin is abolished in Oct1/2(−/−) mice
We recently reported that in the mouse, cisplatin is recognized as a substrate by both Oct1 and Oct2 (5). To further investigate the roles of Oct1 and Oct2 in renal secretion of cisplatin, we compared the plasma and urinary pharmacokinetics of this drug in Oct1/2(−/−) mice. At 24 h after cisplatin administration to Oct1/2(−/−) mice, a cumulative urinary excretion of 57% of the administered dose was observed compared to 91% in wildtype animals (P<0.001), while the plasma AUC of unbound platinum was not dependent on mouse genotype (5.65±1.95 vs 5.60±1.42 mg·h/L). The renal clearance of platinum was greatly reduced in the Oct1/2(−/−) mice, although there were no differences in the estimated GFR at baseline (16.4±0.21 vs 16.8±0.52 mL/h) (Supplementary Table ST1). The ratio of renal clearance to GFR was about 1.5 for wild-type mice, which is comparable to what has been found by others (15). In Oct1/2(−/−) mice, however, this ratio was reduced to about 1, indicating that the net tubular secretion of platinum was completely abolished in these animals.
Renal biomarker changes in response to cisplatin
We found that in wildtype mice receiving cisplatin, the widely used biomarkers for assessing cisplatin nephrotoxicity, BUN and serum creatinine, are less than ideal because increases only occur after substantial kidney damage, and then with a time delay (Fig. 1A and 1B), consistent with previous findings (16). Indeed, BUN and serum creatinine did not show significant elevation in the mice until 72 h after administration of cisplatin. This is despite the notion that histopathological analysis indicated proximal tubular damage as early as 24 h following drug administration (Fig. 1C and 1D). Furthermore, we found that the ratio of renal creatinine clearance to estimated GFR is about 1 in Oct1/2(−/−) mice but significantly increased in wildtype mice (P=0.025), suggesting that creatinine also undergoes Oct1/Oct2-mediated renal tubular secretion (Supplementary Fig. S1).
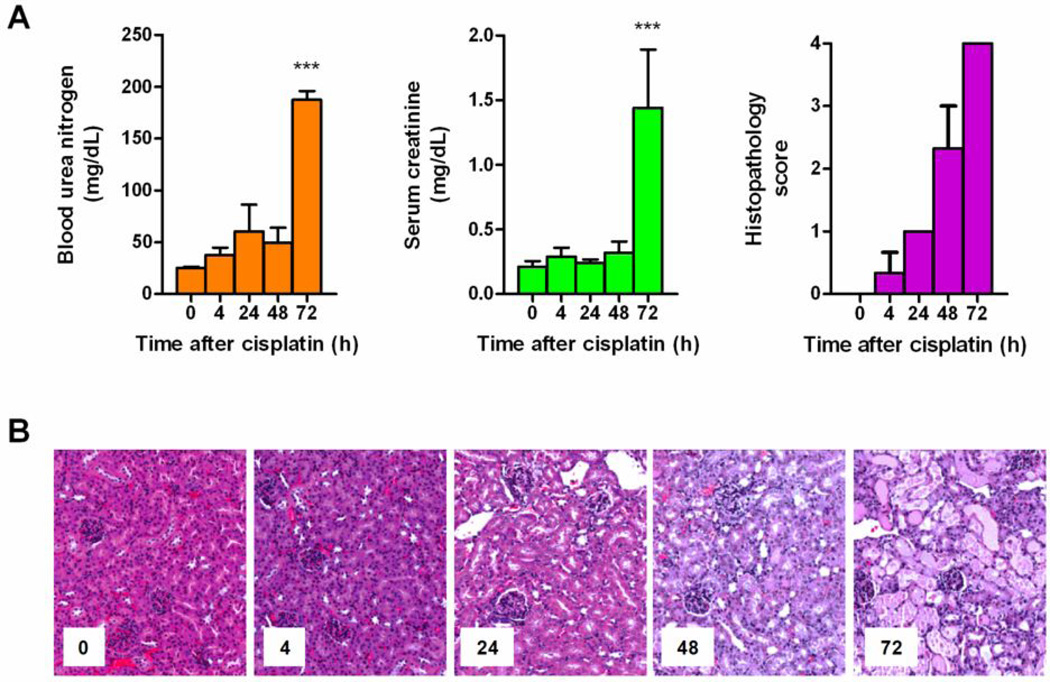
Figure 1.
Measures of nephrotoxicity in adult male FVB mice after administration of cisplatin (17.5 mg/kg, i.p.; n=3 per time point). (A) Left panel, mean of blood urea nitrogen (BUN); Middle panel, mean of serum creatinine; Right panel, mean histopathology scores determined from the extent of acute renal tubular necrosis based on the percentage of observed damaged tubules: 0, absent; 1, <25% (rare); 2, 25–50% (mild); 3, 50–75% (moderate); 4, >75% (severe). Data are shown as mean (bars) and SE (error bars); (B) Representative kidney histopathology before and after administration of cisplatin. ***, P<0.001.
NAG as a biomarker of cisplatin nephrotoxicity
As an alternative to BUN and creatinine, we explored the utility of measuring urinary NAG for evaluating the role of renal transporters in cisplatin nephrotoxicity. In contrast to BUN and creatinine, we found that NAG is a sensitive quantitative biomarker for detection of cisplatin nephrotoxicity with significant changes in activity occurring as early as 4 h (P<0.0001) (Fig. 2A). Cisplatin-induced changes in urinary NAG activity were much less pronounced in Oct1/2(−/−) mice compared with wildtype mice over the studied time interval (Fig. 2A), consistent with the reduced nephrotoxicity observed in the transporter-deficient animals (5). Additional analysis on mice following a single dose of cisplatin indicated that a cut-off point of >0.4 AU for the cumulative urinary NAG activity over 48 h is highly predictive of severe nephrotoxicity, with a ‘severe/not-severe’ odds ratio (95% confidence limits) of 21.0 (2.91 – 151) (P=0.0017).
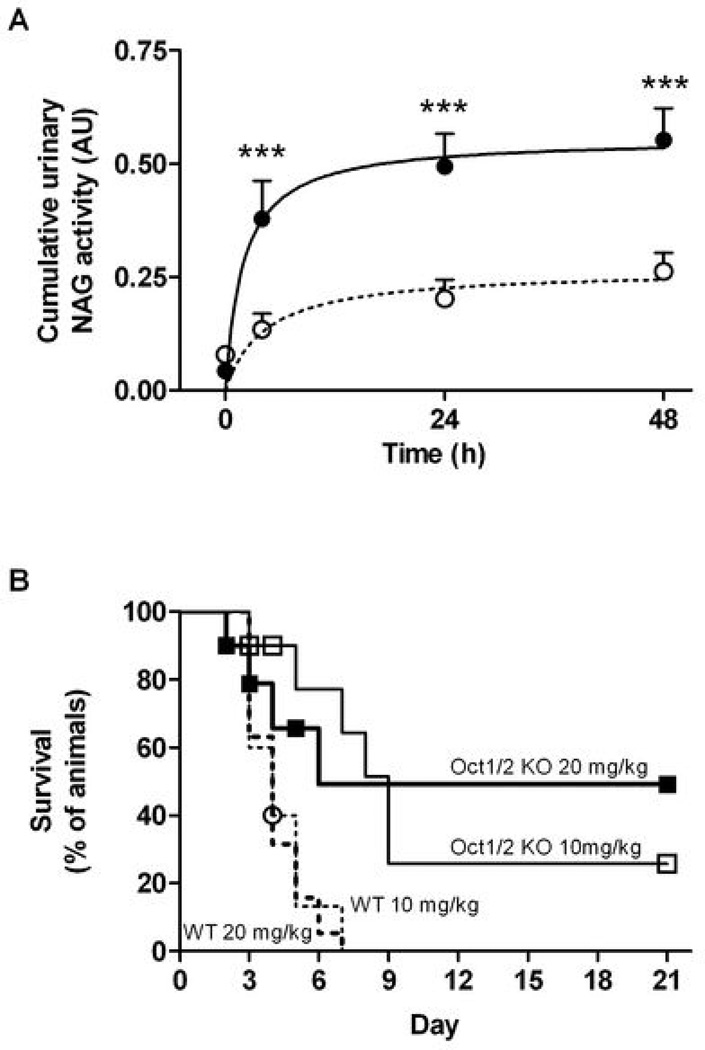
Figure 2.
Influence of Oct1/Oct2-deficiency on cisplatin-induced changes in N-acetyl-β-D-glucosaminidase (NAG) and overall survival (n=9–19 per dose per genotype). (A) Cumulative urinary NAG activity in adult male FVB mice (closed symbols, solid line; n=12) and Oct1/2(−/−) mice (open symbols, dotted line; n=12) after administration of cisplatin (10 mg/kg, i.p.). Data are shown as mean (bars) and SE (error bars); (B) Overall survival curves in adult male FVB wildtype (dotted lines) and Oct1/2(−/−) mice (solid lines) after administration of cisplatin at a dose of 10 mg/kg (thin lines) or 20 mg/kg (thick lines). Symbols are shown for censored observations. ***, P<0.001.
The absence of severe cisplatin nephrotoxicity (i.e., when >75% of tubules damaged) in Oct1/2(−/−) mice is a critical difference from the observed side effects in wildtype mice, since the severity of renal damage is linked with mortality following a single dose of cisplatin. Indeed, the decreased toxicity in the Oct1/2(−/−) mice is associated with a significantly prolonged overall survival (Fig. 2B). For example, at a cisplatin dose of 10 mg/kg, the ‘wildtype/Oct1/2(−/−)’ Cox-Mantel hazard ratio (95% confidence limits) was found to be 8.1 (2.1–31) (P=0.0078). Interestingly, while all wildtype mice died by day 7, a subset of Oct1/2(−/−) mice appeared to be completely protected from cisplatin-induced death.
Cimetidine inhibits cisplatin transport by OCT2
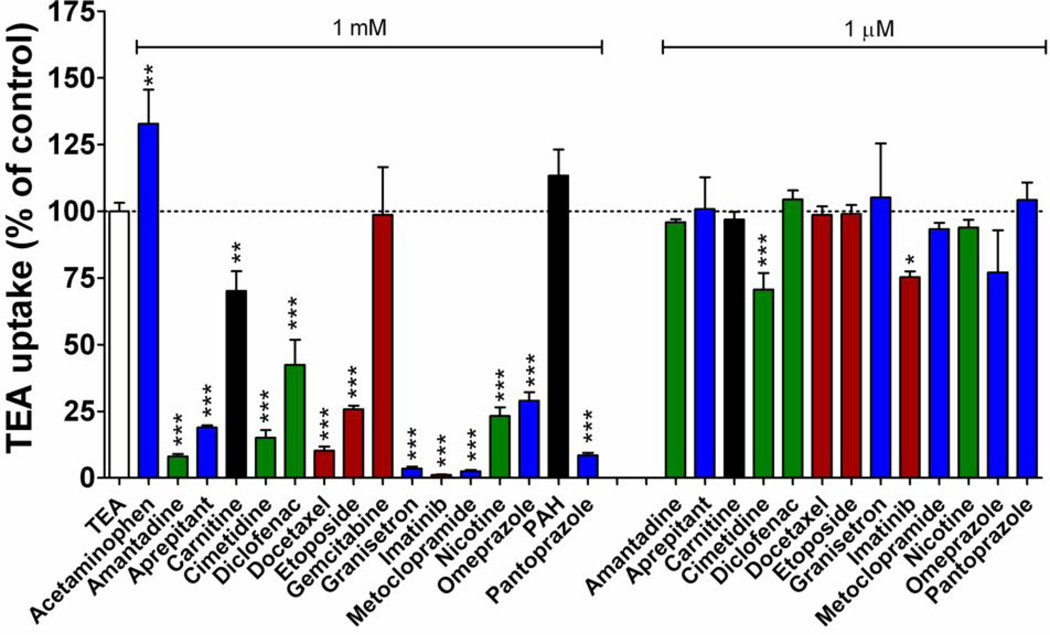
To explore the possible utility of chemical inhibitors of OCT2 as a strategy to prevent severe cisplatin nephrotoxicity, we screened 16 agents at varying concentrations for OCT2 inhibitory potential in transfected 293Flp-In cells using TEA as a test substrate (Fig. 3). At a substrate-to-inhibitor concentration ratio of 1:500, all of the known OCT2 inhibitors (amantadine, cimetidine, diclofenac, imatinib, and nicotine) used in this screen demonstrated significant inhibition (8–42% of control values). A number of supportive care drugs (aprepitant, granisetron, metoclopramide, omeprazole, and pantoprazole) and chemotherapeutic agents (docetaxel and etoposide) also showed significant inhibition of OCT2 function (by 1–29% of control). When tested at clinically relevant concentrations (1 µM), however, only imatinib (75% of control, P=0.012) and cimetidine (70% of control, P=0.0006) were found to be inhibitors of OCT2 function.
Figure 3.
Inhibition of OCT2-mediated transport of tetraethylammonium bromide (TEA) by various prescription drugs. Transport of [14C]TEA (2 µM) during a 30-min incubation was assessed in 293Flip-In cells transfected with an empty vector (VC) or with OCT2 in the presence and absence of the inhibitors at a substrate-to-inhibitor concentration ratio of 1:500 (1 mM of inhibitor) or 1:0.5 (1 µM of inhibitor). Data represent the extent of TEA uptake in OCT2-overexpressing cells corrected for nonspecific uptake in VC cells, and were expressed as a percentage of uptake in the absence of inhibitors, which was set at 100%. Data are shown as mean (bars) and SE (error bars) of at least three experiments performed in triplicate. Red, cancer drugs; Blue, supportive-care drugs; Green, known OCT2 inhibitors; Black, other. *, P<0.05 vs control; **, P<0.01 vs control; ***, P<0.001 vs control.
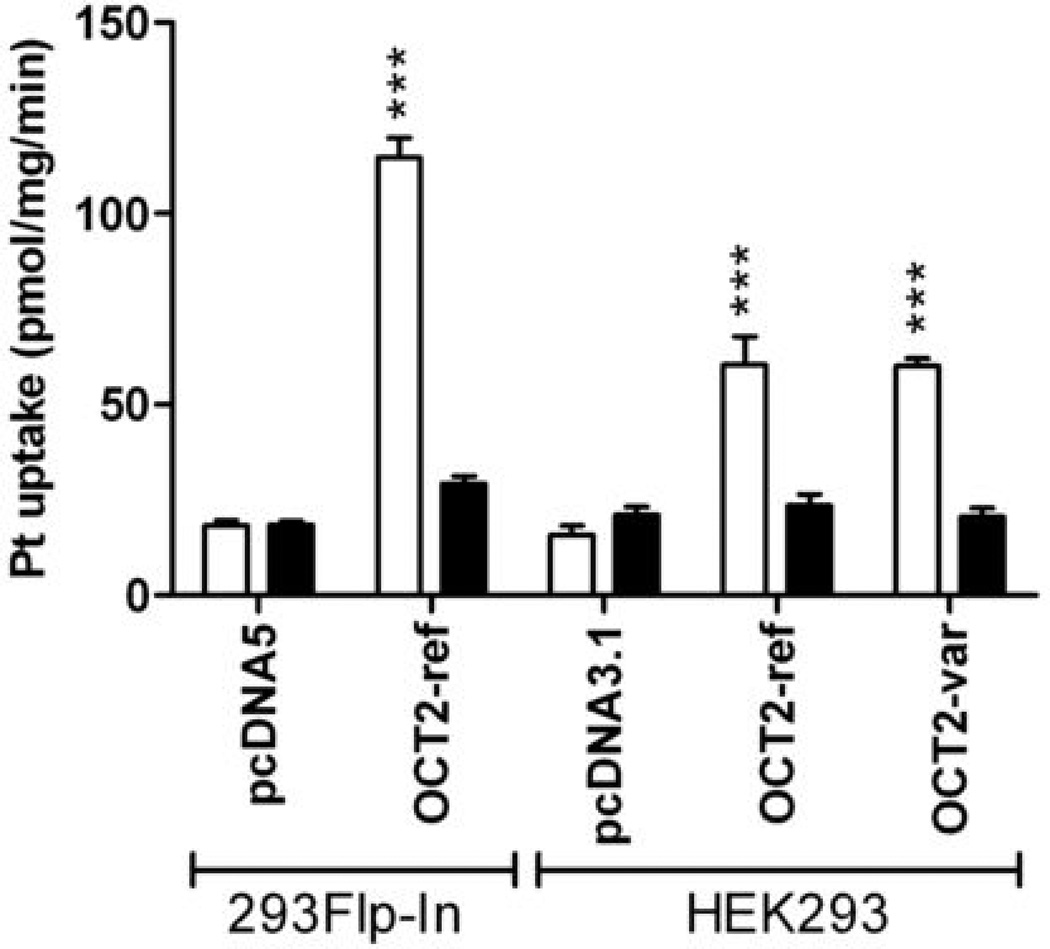
The ability of cimetidine to inhibit OCT2 function was further confirmed using cisplatin as a test substrate in three different OCT2-overexpressing cell lines (Fig. 4). These results indicated that cimetidine, at a substrate-to-inhibitor concentration ratio of 1:2, completely abolished the uptake of cisplatin (P<0.0001). Interestingly, cisplatin transport in the absence or presence of cimetidine was not altered in HEK293 cells transfected with a common OCT2 protein variant (p.270Ala>Ser) associated with the rs316019 (808G>T) single-nucleotide polymorphism (Fig. 4). This suggests that this particular OCT2 variant is not associated with altered substrate recognition for cisplatin.
Figure 4.
Effect of cimetidine (1 mM) on the accumulation of cisplatin (500 µM, for 30 minutes) in 293Flp-In cells transfected with empty vector (VC) or OCT2, or HEK293 cells transfected with empty vector (VC), wildtype OCT2, or the OCT2 p.270Ala>Ser variant (OCT2-Var). Open bars represent cisplatin alone, black bars represent cisplatin in the presence of cimetidine. Data are shown as mean (bars) and SE (error bars) of three experiments performed in triplicate. ***, P<0.001.
Cimetidine prevents cisplatin nephrotoxicity
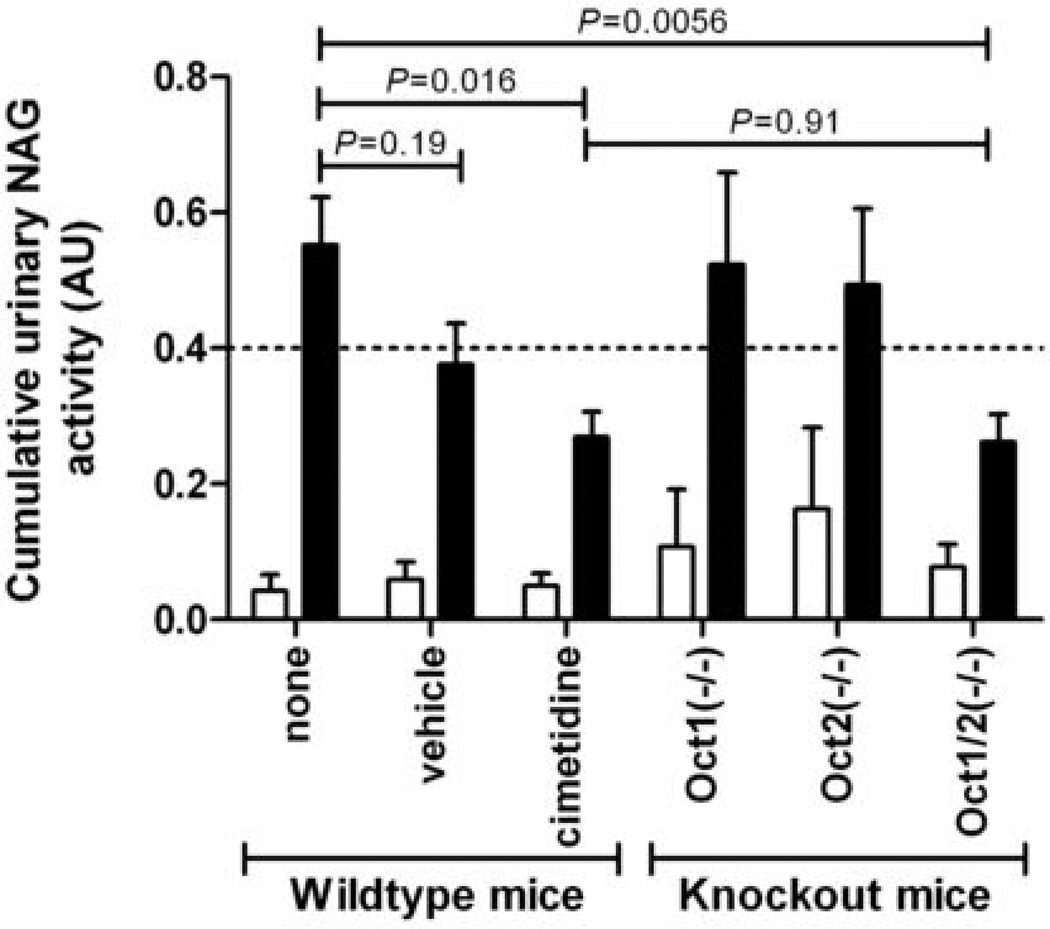
To obtain preliminary evidence for the usefulness of adding cimetidine to cisplatin-based therapy to ameliorate nephrotoxicity, urinary NAG activity was determined in mice receiving cisplatin (10 mg/kg i.p.) in the presence and absence of cimetidine (30 mg/kg i.v.). As expected based on urinary excretion data for cisplatin (5), no difference in urinary NAG activity was observed between wildtype mice and Oct1(−/−) or Oct2(−/−) single knockout mice, whereas activity was significantly reduced in the Oct1/2(−/−) double knockout animals (P=0.0056) (Fig. 5). NAG activity was slightly reduced in wildtype mice in which cisplatin administration was preceded by an i.v. injection of the aqueous vehicle used for cimetidine. Although the difference in NAG activity in this group compared with mice receiving cisplatin alone was not statistically significant (P=0.19), the observation is consistent with prior clinical data suggesting that i.v. hydration can reduce cisplatin nephrotoxicity to some extent (17). Most importantly, wildtype mice receiving concurrent cimetidine with cisplatin had urinary NAG values that were significantly reduced compared with mice receiving cisplatin only (P=0.016) and similar to levels in Oct1/2(−/−) treated with cisplatin alone (P=0.91). This suggests that cimetidine is able to completely inhibit Oct1/Oct2-mediated uptake of cisplatin in renal proximal tubular cells, and subsequently ameliorate cisplatin nephrotoxicity.
Figure 5.
Cisplatin-induced (10 mg/kg, i.p.) changes in urinary NAG activity in Oct1(−/−) (n=6), Oct2(−/−) (n=6), Oct1/2(−/−) (n=12), or wildtype mice receiving no pretreatment (n=12), vehicle injection (saline, i.v.) (n=5), or cimetidine (30 mg/kg, i.v.) (n=6). Open bars represent pretreatment levels of urinary NAG activity, black bars are cumulative urinary NAG activity over 48 h. Data are shown as mean (bars) and SE (error bars).
Cimetidine does not inhibit cisplatin uptake into cancer cells
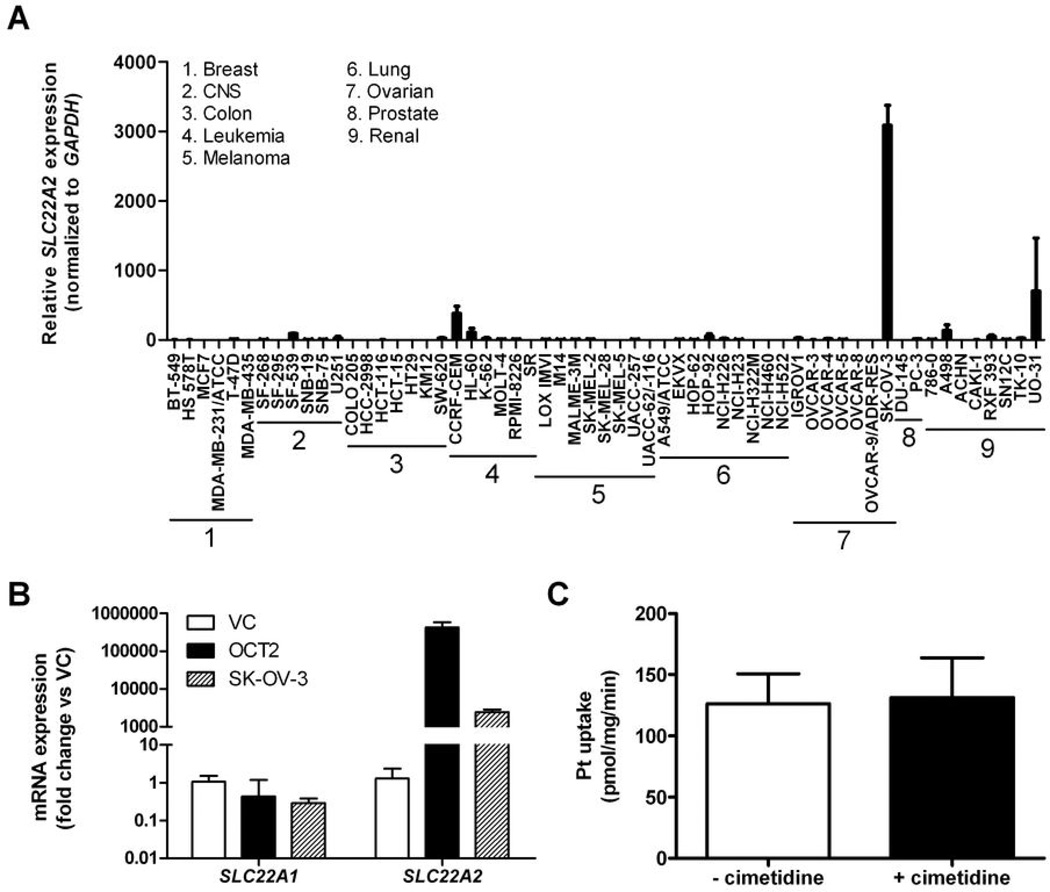
The contribution of OCT2 to the uptake of cisplatin in human tumor cells is currently unknown. We previously reported that the OCT2 gene, SLC22A2, was either absent or detectable at very low levels in multiple human tumors, including those for which cisplatin is clinically used, such as lung cancer and ovarian cancer (5). Amongst the NCI60 cancer cell line panel, we identified the ovarian cancer cell line SKOV-3 as having the highest expression of SLC22A2 (Fig. 6A). However, we found that cellular sensitivity to cisplatin in the NCI60 cancer cell line panel was not significantly associated with the expression of SLC22A2 (R2=0.009, P=0.47; data not shown). We also found that expression of SLC22A2 was approximately 175-fold lower in SKOV-3 cells compared with our OCT2-transfected 293Flp-In cells, and that the expression of other genes of putative relevance to cisplatin transport, such as SLC22A1 (encoding OCT1), were very low in all of the celI models tested (Fig. 6B). Although the absolute uptake of cisplatin in SKOV-3 cells was quite substantial, the presence of an excess amount of cimetidine had no influence on the cellular uptake and retention of cisplatin in this model (Fig. 6C). This finding is consistent with the possibility that substantial overexpression of OCT2, such as that observed in our transfected 293Flp-In cells -or under normal physiological conditions in human kidney-, is required before its quantitative contribution to cisplatin transport can be discerned.
Figure 6.
Expression of the OCT2 gene, SLC22A2, in the NCI60 cancer cell lines and its influence on cisplatin transport. (A) Real-time PCR expression levels of SLC22A2 (normalized to GAPDH) in the NCI60 panel. Abbreviation: CNS, central nervous system; (B) Real-time PCR expression of the OCT1 gene, SLC22A1, and SLC22A2 in 293Flip-In cells transfected with an empty vector (VC), 293Flip-In cells transfected with OCT2, and SKOV-3 cells; (C) Influence of cimetidine (1 mM) on the uptake of cisplatin (500 µM) in SKOV-3 cells. Data are shown as mean (bars) and SE (error bars) of three experiments performed in triplicate.
Discussion
This study provides direct in vivo demonstration that organic cation transporters (OCT2 in humans, Oct1 and Oct2 in mice) are essential for the active secretion of cisplatin into renal proximal tubular cells, and that these proteins play a crucial role in the development of cisplatin nephrotoxicity. Our collective in vitro and in vivo data have potentially important clinical implications for the optimization of cisplatin usage, and strongly support the hypothesis that pharmacological inhibitors of OCT2 can be used to prevent cisplatin-induced kidney damage.
A role of organic cation transporters in the renal uptake of cisplatin has been suggested previously by several studies. This was initially deduced from in vitro studies that showed that cisplatin could inhibit the cellular uptake of the prototypical OCT2 substrate, TEA (18, 19). Furthermore, in vivo administration of cisplatin inhibits the renal clearance of other organic cations (20), and conversely, several organic cations can affect the renal clearance of cisplatin (21) without affecting GFR (22). Recently, in vitro methods using OCT2-overexpressing cells have been used to confirm that cisplatin is itself a substrate for this transporter (4). Moreover, our previous study indicates that functional loss of the mouse ortholog transporters Oct1 and Oct2 is associated with a marked reduction in urinary excretion of platinum and nephrotoxicity, as determined by histopathological staining (5). Our current findings that Oct1/2(−/−) mice completely lack renal secretion of platinum and also have an increase in the median survival time compared to wildtype mice receiving the same cisplatin dose further highlight the importance of Oct1 and Oct2 in the pharmacology and toxicology of cisplatin.
It has been long recognized that better biomarkers for cisplatin nephrotoxicity are needed both for animal studies and for use in humans where early detection of kidney injury will influence therapy and potentially morbidity and mortality. Here, we found that routinely used measures of kidney injury, such as BUN and serum creatinine, increase significantly only after substantial damage occurs and then with a substantial time delay, which renders BUN and creatinine useless as a biomarker in the context of our studies. Furthermore, our current data indicate that creatinine itself is undergoing Oct1/Oct2-mediated renal secretion, which is further independently supported by the notion (a) that creatinine is a known transported substrate of rat Oct2 and human OCT2 (23), and (b) our previous observation that serum creatinine levels at baseline are about 40% higher in Oct1/2(−/−) mice compared with wildtype mice (5). These newly identified features of creatinine not only add to the problem of using creatinine clearance as a surrogate for measuring glomerular filtration, but pose specific concerns in the context of its use as an indicator of renal function following cisplatin treatment.
It should be pointed out that our in vivo findings on creatinine are at odds with a recent study by Eisner et al. indicating that creatinine secretion was, in fact, identical in wildtype mice and Oct1/2(−/−) mice (24), and hypothesized to be mediated by the organic anion transporter, Oat1. Further study is required to understand the mechanism underlying the differential impact of Oct1/Oct2-deficiency on the renal secretion of creatinine, including studies involving determining creatinine clearance in Oct1/2(−/−) mice following the administration of an Oat1 inhibitor, such as para-aminohippuric acid.
Beyond serum markers, many urinary proteins have been previously evaluated as putative noninvasive indicators of general renal injury, including α- and π-glutathione-Stransferases, neutrophil gelatinase-associated lipocalin, cysteine-rich protein 61, interleukin-18, clusterin, and F-actin (25). However, problems with the reliability of these proteins to identify and monitor kidney injury includes instability in the urine, modification due to physicochemical properties of the urine, absence of sustained elevation and lack of a high-throughput detection method. Based on these considerations, we evaluated cisplatin-induced changes in urinary NAG, a high molecular weight lysosomal enzyme that is abundant in the proximal tubular epithelia (26, 27). Due to its large size this molecule is not known to undergo filtration, but is rather released into the urine when renal proximal tubular cells are damaged (26). Urinary NAG activity has been previously used in evaluating lead-mediated damage (27) and cisplatin nephrotoxicity in rats (28). These prior studies and our current work in wildtype, Oct1(−/−), Oct2(−/−), and Oct1/2(−/−) mice strongly support the hypothesis that urinary NAG activity is a highly sensitive, stable, and early biomarker for cisplatin nephrotoxicity, especially compared to BUN and creatinine. The ability of cimetidine to completely block Oct1/Oct2-mediated uptake of cisplatin in renal proximal tubular cells, and subsequently ameliorate cisplatin-induced changes in NAG activity, support further efforts to explore the use of this biomarker in rodents and patients receiving cisplatin.
Most of the approaches reported in the literature to afford renoprotection during cisplatin treatment have not separately considered the possible implications on the anticancer actions of cisplatin in tumors. It is therefore imperative to conclusively demonstrate that intentional inhibition of OCT2 function does not negatively influence cisplatin effects on the tumor. The mechanism of cellular uptake of cisplatin in tumor cells is not entirely clear and may vary from one cell type to another (3). Preclinical studies have demonstrated that a high-affinity transporter known as copper transporter 1 (CTR1, SLC31A1) plays a critical role in the uptake of cisplatin in yeast and several tumor cell lines. However, the accumulation of cisplatin in human cancer cells expressing this transporter is limited by the fact that cisplatin triggers the down-regulation and proteasomal degradation of CTR1, thereby limiting its own uptake (29). Our current studies demonstrate that even the presence of an excess amount of cimetidine had no influence on the cellular uptake and retention of cisplatin in SKOV-3 cancer cells, which carry the highest SLC22A2 expression amongst the NCI60 cell lines. In addition to this in vitro observation, it is noteworthy that a quantitative real-time PCR analysis of 80 ovarian tumor specimens revealed recently that the vast majority of tumors had low (n=12) or undetectable (n=68) levels of SLC22A2 (30). Of the 80 specimens, 51 were obtained from patients treated with cisplatin-containing chemotherapy, 42 of whom responded to the treatment (30). No difference, either in expression level or frequency of SLC22A2-positive tumors, was apparent between the non-responders and responders. These in vitro and ex vivo results suggest that OCT2 function is not a critical determinant of the antitumor effects of cisplatin, and thus that the use of OCT2 inhibitors should not affect the intracellular accumulation of cisplatin into tumors.
Collectively, our study suggests that the use of a pharmacological inhibitor of OCT2 is a feasible mechanism to exploit for preventing cisplatin nephrotoxicity without influencing effects on the tumor. Furthermore, this study indicates that urinary NAG activity serves a sensitive early biomarker for nephrotoxicity. If this dose-limiting toxicity can be alleviated in patients, the therapeutic window of cisplatin in cancer treatment can be dramatically improved. We are currently conducting a prospective clinical trial to evaluate the effects of cimetidine as a conjunctive therapy to reduce or eliminate cisplatin nephrotoxicity. While cimetidine will be used here as a pharmacological tool for establishing proof-of-concept, our future studies will be aimed at developing and evaluating more selective agents that could provide better protection against the renal damage associated with cisplatin use.
Supplementary Material
Supplementary Figure 1.
Ratio of renal clearance (CL) of creatinine and estimated glomerular filtration rate (GFR) in wildtype mice and Oct1/2(−/−) mice at baseline (n=12 per group).
Acknowledgment
Grant Support: Supported by the American Lebanese Syrian Associated Charities (ALSAC) and United States Public Health Service Cancer Center Support Grant 3P30CA021765.
We thank John Killmar, Peter Vogel, and Laura Janke for assistance with data analysis; the National Cancer Institute Tumor Repository for providing RNA and DNA from the NCI60 cell lines; and Alfred Schinkel for providing Oct1(−/−) and Oct2(−/−) mice.
Footnotes
Conflict of interest statement
The authors have no conflict of interest.
References
- 1.Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003;23:460–464. doi: 10.1016/s0270-9295(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 2.de Jongh FE, van Veen RN, Veltman SJ, et al. Weekly high-dose cisplatin is a feasible treatment option: analysis on prognostic factors for toxicity in 400 patients. Br J Cancer. 2003;88:1199–1206. doi: 10.1038/sj.bjc.6600884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 4.Filipski KK, Loos WJ, Verweij J, Sparreboom A. Interaction of cisplatin with the human organic cation transporter 2. Clin Cancer Res. 2008;14:3875–3880. doi: 10.1158/1078-0432.CCR-07-4793. [DOI] [PubMed] [Google Scholar]
- 5.Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009;86:396–402. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonker JW, Wagenaar E, Mol CA, et al. Reduced hepatic uptake and intestinal excretion of organic cations in mice with a targeted disruption of the organic cation transporter 1 (Oct1 [Slc22a1]) gene. Mol Cell Biol. 2001;21:5471–5477. doi: 10.1128/MCB.21.16.5471-5477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma J, Stoter G, Verweij J, Schellens JH. Comparison of ethanol plasma-protein precipitation with plasma ultrafiltration and trichloroacetic acid protein precipitation for the measurement of unbound platinum concentrations. Cancer Chemother Pharmacol. 1996;38:391–394. doi: 10.1007/s002800050501. [DOI] [PubMed] [Google Scholar]
- 8.Hackbarth H, Buttner D, Gartner K. Intraspecies allometry: correlation between kidney weight and glomerular filtration rate vs. body weight. Am J Physiol. 1982;242:R303–R305. doi: 10.1152/ajpregu.1982.242.3.R303. [DOI] [PubMed] [Google Scholar]
- 9.Jonker JW, Wagenaar E, Van Eijl S, Schinkel AH. Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol Cell Biol. 2003;23:7902–7908. doi: 10.1128/MCB.23.21.7902-7908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zolk O, Solbach TF, Konig J, Fromm MF. Functional characterization of the human organic cation transporter 2 variant p.270Ala>Ser. Drug Metab Dispos. 2009;37:1312–1318. doi: 10.1124/dmd.108.023762. [DOI] [PubMed] [Google Scholar]
- 11.Shu Y, Bello CL, Mangravite LM, Feng B, Giacomini KM. Functional characteristics and steroid hormone-mediated regulation of an organic cation transporter in Madin-Darby canine kidney cells. J Pharmacol Exp Ther. 2001;299:392–398. [PubMed] [Google Scholar]
- 12.Ludwig T, Riethmuller C, Gekle M, Schwerdt G, Oberleithner H. Nephrotoxicity of platinum complexes is related to basolateral organic cation transport. Kidney Int. 2004;66:196–202. doi: 10.1111/j.1523-1755.2004.00720.x. [DOI] [PubMed] [Google Scholar]
- 13.Ciarimboli G, Ludwig T, Lang D, et al. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol. 2005;167:1477–1484. doi: 10.1016/S0002-9440(10)61234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Lovejoy KS, Shima JE, et al. Organic cation transporters are determinants of oxaliplatin cytotoxicity. Cancer Res. 2006;66:8847–8857. doi: 10.1158/0008-5472.CAN-06-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddik ZH, Newell DR, Boxall FE, Harrap KR. The comparative pharmacokinetics of carboplatin and cisplatin in mice and rats. Biochem Pharmacol. 1987;36:1925–1932. doi: 10.1016/0006-2952(87)90490-4. [DOI] [PubMed] [Google Scholar]
- 16.Hewitt SM, Dear J, Star RA. Discovery of protein biomarkers for renal diseases. J Am Soc Nephrol. 2004;15:1677–1689. doi: 10.1097/01.asn.0000129114.92265.32. [DOI] [PubMed] [Google Scholar]
- 17.Launay-Vacher V, Rey JB, Isnard-Bagnis C, Deray G, Daouphars M. Prevention of cisplatin nephrotoxicity: state of the art and recommendations from the European Society of Clinical Pharmacy Special Interest Group on Cancer Care. Cancer Chemother Pharmacol. 2008;61:903–909. doi: 10.1007/s00280-008-0711-0. [DOI] [PubMed] [Google Scholar]
- 18.Nelson JA, Santos G, Herbert BH. Mechanisms for the renal secretion of cisplatin. Cancer Treat Rep. 1984;68:849–853. [PubMed] [Google Scholar]
- 19.Williams PD, Hottendorf GH. Effect of cisplatin on organic ion transport in membrane vesicles from rat kidney cortex. Cancer Treat Rep. 1985;69:875–880. [PubMed] [Google Scholar]
- 20.Miura K, Goldstein RS, Pasino DA, Hook JB. Cisplatin nephrotoxicity: role of filtration and tubular transport of cisplatin in isolated perfused kidneys. Toxicology. 1987;44:147–158. doi: 10.1016/0300-483x(87)90145-4. [DOI] [PubMed] [Google Scholar]
- 21.Endo T, Kimura O, Sakata M. Carrier-mediated uptake of cisplatin by the OK renal epithelial cell line. Toxicology. 2000;146:187–195. doi: 10.1016/s0300-483x(00)00176-1. [DOI] [PubMed] [Google Scholar]
- 22.Klein J, Bentur Y, Cheung D, Moselhy G, Koren G. Renal handling of cisplatin: interactions with organic anions and cations in the dog. Clin Invest Med. 1991;14:388–394. [PubMed] [Google Scholar]
- 23.Urakami Y, Kimura N, Okuda M, Inui K. Creatinine transport by basolateral organic cation transporter hOCT2 in the human kidney. Pharm Res. 2004;21:976–981. doi: 10.1023/b:pham.0000029286.45788.ad. [DOI] [PubMed] [Google Scholar]
- 24.Eisner C, Faulhaber-Walter R, Wangi Y, et al. Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int. 2010 doi: 10.1038/ki.2009.501. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 26.Kavukcu S, Soylu A, Turkmen M. The clinical value of urinary N-acetyl-beta-Dglucosaminidase levels in childhood age group. Acta Med Okayama. 2002;56:7–11. doi: 10.18926/AMO/31721. [DOI] [PubMed] [Google Scholar]
- 27.Chia KS, Mutti A, Tani C, et al. Urinary N-acetyl-beta-D-glucosaminidase activity in workers exposed to inorganic lead. Occup Environ Med. 1994;51:125–129. doi: 10.1136/oem.51.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yonezawa A, Masuda S, Nishihara K, Yano I, Katsura T, Inui K. Association between tubular toxicity of cisplatin and expression of organic cation transporter rOCT2 (Slc22a2) in the rat. Biochem Pharmacol. 2005;70:1823–1831. doi: 10.1016/j.bcp.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 29.Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol Pharmacol. 2006;70:1390–1394. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- 30.Burger H, Zoumaro-Djayoon A, Boersma AW, et al. Differential transport of platinum compounds by the human organic cation transporter hOCT2 (hSLC22A2) Br J Pharmacol. 2010 doi: 10.1111/j.1476-5381.2009.00569.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1.
Ratio of renal clearance (CL) of creatinine and estimated glomerular filtration rate (GFR) in wildtype mice and Oct1/2(−/−) mice at baseline (n=12 per group).