Abstract
Noroviruses (NoVs) are important pathogens causing epidemic acute gastroenteritis that affects millions of people worldwide. The protruding (P) domain of the NoV capsid protein, the surface antigen of NoV, forms a 24-mer subviral particle called the P particle that is an excellent candidate vaccine against NoVs. The P particles are easily produced in Escherichia coli, highly stable and highly immunogenic. Each P domain has three surface loops that can be used for foreign antigen presentation, making the P particles a useful platform for vaccine development against other infectious diseases. This article summarizes the discovery, structure, development and applications of the P particles as a vaccine against NoVs, as well as a vaccine platform against rotavirus, influenza virus and possibly other pathogens in the future.
Keywords: antigen presentation, bivalent vaccine, calicivirus, influenza virus, M2e epitope, norovirus, P domain, P particle, rotavirus VP8, vaccine platform
The application of recombinant technology to produce subunit vaccines based on virus-like particles (VLPs) and other subviral particles has led to a new direction in modern medicine. Such laboratory-made nanoparticles assemble spontaneously when the viral capsid proteins or portions of the viral capsid proteins are expressed through an in vitro expression system. These particles are usually multivalent complexes retaining the antigenic structures of the native virions, and are therefore an attractive candidate subunit vaccine. Because of the lack of a viral genome, these subviral particles are noninfectious and therefore safe as vaccines. Successful examples of subunit vaccines include four US FDA-approved VLP vaccines: Recombivax HB® (Merck, NJ, USA) and Energix-B® (GlaxoSmithKline, London, UK) against hepatitis B virus and Gardasil® (Merck) and Cervarix® (GlaxoSmithKline) against human papilloma virus. In addition, many other subunit vaccines, including the norovirus (NoV) VLP vaccine [1,2], are under extensive development. Thus, subunit vaccines, through recombinant technology, represents an innovative vaccine strategy complementary to the conventional vaccine approaches.
NoV subviral particles
NoVs are single-stranded, positive-sense RNA viruses in the calicivirus family. Structurally, the NoV genome is encapsulated by a protein capsid that is formed by a single major structural protein (VP1). Human NoVs cannot be effectively cultivated in cell cultures or infect small animals and therefore a subunit vaccine is a choice for NoVs. NoV VLPs (Figure 1A) assemble spontaneously when the capsid protein is produced in a eukaryotic expression system including insect cells through recombinant baculovi-ruses [3,4], mammalian cells via a Venezuelan equine encephalitis replicon [5] or plasmid [6], yeast [7] and several transgenic plants [8,9]. However, VLP production was unsuccessful in a prokaryotic expression system [10].
Figure 1. Structures of the five norovirus complexes that are formed by full-length or truncated norovirus VP1.
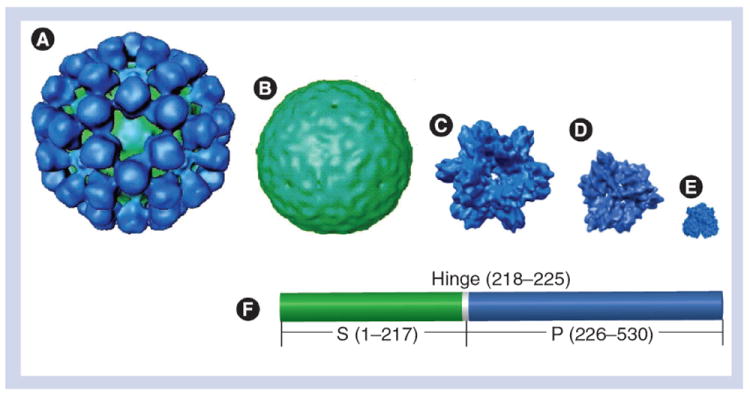
(A) Virus-like particle (180-mer: ~37 nm). (B) S particle (180-mer: ~27 nm). (C) P particle (24-mer: ~20 nm). (D)Small P particle (12-mer: ~14 nm). (E) P dimer (~6 nm). (F) Linear structure of norovirus VP1 with indications of the S (green) and the P (blue) domains that are linked by a short hinge. Numbers are based on Norwalk virus VP1.
P: Protruding; S: Shell.
Adapted with permission from [28].
While the VLP-based NoV vaccine has been extensively investigated, including successful Phase I and II clinical trials on baculovirus-derived VLPs [1,11,12], another type of NoV subviral particles, the protruding (P) particles (Figure 1C), has been developed that could be an alternative or second generation vaccine against NoVs. The NoV capsid protein contains two major domains, the shell (S) and the P domains, linked by a short hinge (Figure 1F) [13]. The P particles are formed by the P domain and revealed the same antigenic types as VLPs [14,15]. They are highly immunogenic and very stable and most importantly, they can be efficiently produced in Escherichia coli. In fact, the laboratory-made P particle has been developed through several artificial modifications for higher yield and stability in the E. coli expression system (see below). A further feature of the P particle is the usefulness of its three surface loops for presentation of foreign antigens for immune enhancement [16,17]. Thus, P particles can be applied not only as a vaccine for NoVs, but also as a platform for novel vaccine development against other viral and bacterial pathogens.
This article summarizes the discovery and subsequent research on the potential applications of the P particles as a candidate vaccine against NoVs as well as a vaccine platform for multiple presentations of different foreign antigens as vaccines against different infectious pathogens. Examples on the presentation of the rotavirus spike protein VP8* and the influenza viral M2e epitope are given. Principles of P particle formation and stability via intermolecular interactions, and factors to enhance these interactions are also discussed. The knowledge gained from the NoV P domain and P particles will help our understanding of analog proteins of other virus and bacterial pathogens for potentially similar applications.
Discovery of NoV P particles
The NoV P particle was first discovered in a study to characterize the interaction between the human NoV capsid and the host histo-blood group antigens (HBGAs), the attachment factors of NoVs [18-23]. The S and P domains of NoV capsid were found to be structurally and functionally independent. Expression of the S domain alone in insect cells through a recombinant baculovirus formed smaller thin-layer S particles with a smooth surface (~27 nm) (Figure 1B) [24,25]. The S particle structurally corresponds to the interior shell of the NoV capsid and does not bind to HBGAs [24,25].
When the P domain is expressed in E. coli, it forms three different P domain complexes: the 24-mer P particle (~20 nm) (Figure 1C) [14,15,26,27]; the 12-mer small P particle (~14 nm) (Figure 1D) [28]; and the P dimer (~6 nm) (Figure 1E) [25,29-34]. Native mass spectrometry (MS) showed that the 24-mer P particles can be disassociated in reducing conditions into P dimers that can reassemble into the 12-mer small P particles and two further P domain complexes, the 18-mer and the 36-mer P particles [35]. The structures of the latter two complexes, however, remain unknown. Thus, these P domain complexes are interchangeable, with the P dimer as the building block. In contrast to the S particle, all three P domain complexes revealed binding function to HBGAs, indicating that the P domain is the HBGA-binding domain. These P complexes have been extensively used as tools for the study of NoV–host interaction [14-16,25-28,36-38].
Among the three P complexes, the 24-mer P particles have been extensively studied and several applications of the P particles have been proposed based on its high stability, easy production in the E. coli system and its capability to accommodate foreign antigens [15-17,26,27]. Most importantly, its large size and multivalent nature make it highly immunogenic, and thus, an excellent vaccine candidate and a versatile vaccine platform for foreign antigen presentation for enhanced immune response [16,17,39].
The structure of NoV P particles
Stable P particles can be produced through expression of the P domain with an end-linked cysteine-containing tag (Figure 2B) [15]. 3D structure reconstruction of the P particle by electron cryomicroscopy revealed an octahedral symmetry with the P dimer as the basic unit [14]. The P particle appears spherical, having a center cavity and 12 P dimer spikes protruding outward (Figure 2B). The orientation of the arch-like P dimer in the P particle is similar to that in the NoV VLP, in which the P2 subdomain constitutes the top or the head, while the P1 subdomain builds the base or the leg of the P dimer spike [14]. As a result, the P particles and their parental VLPs share similar surface antigenic structure (Figure 2E) and HBGA-binding properties, which have been confirmed by ELISA and HBGA-binding assays [14,36].
Figure 2. Formation and structural properties of norovirus P particles.
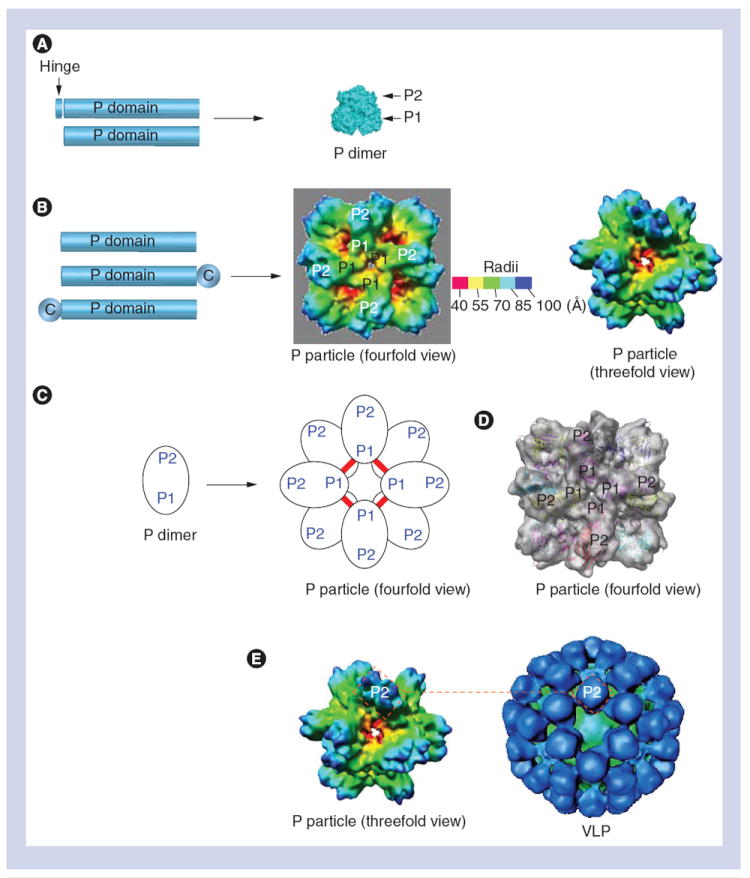
(A) The expression of the P domain with or without the hinge (left) forms a P dimer (right). (B) While the expression of the P domain (left) forms P particles (right), an end-linked C stabilizes the P particle formation. (C) P particle formation by P dimer. Red lines indicate the inter-P dimer disulfide bonds. (D)Fitting of the crystal structure of the norovirus (NoV) P domain into the density map of the P particle. P1 and P2 subdomains are indicated. (E) The NoV P particle shares similar surface antigenic structures to NoV. The VLP (right) is constituted by 180 VP1s with the P dimers on its outermost surfaces, while the P particle (left) is composed of 12 P dimers with similar orientation to those of the VLP. The two rectangles (red dashed lines) indicate the P2 region of a P dimer, representing the surface antigenic structures of both particles.
C: Cysteine-containing tag; P: Protruding; VLP: Virus-like particle.
The structure of the P particles also explains the role of the end-linked cysteine-containing tag at the P1 subdomain for stabilizing the P particle formation through the inter-P dimer disulfide bonds (Figure 2C). The presence and role of these disulfide bonds have been proven by an instability of the P particles in a strong reducing condition [35]. In addition, fitting of the crystal structure of the P domain into the 3D structure of the P particle allows three surface loops on the outermost surface of each protrusion of the P particles to be defined (Figure 2D & 3) [14,16,39]. These exposed loops have been shown to be capable of accommodating foreign small-to-large antigens without affecting the formation of the chimeric P particles [16,17,39]. Thus, this detailed structure information laid a solid foundation for research design and application of the P particles.
Figure 3. The principle of antigen presentation by norovirus P particles.
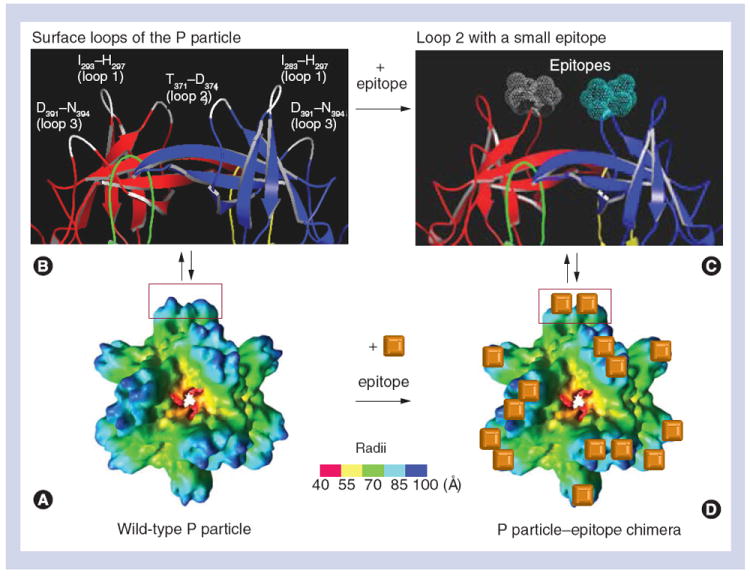
(A) The structure of a wild-type P particle reconstructed by electron cryomicroscopy. (B) Crystal structure (cartoon model) of a protrusion of the P particle with indications of three surface loops (white color) that were labeled with the starting and the ending amino acids. (C) A small epitope (dot model in gray and cyan colors) is inserted into loop 2 of the P dimer. The two P domains of the P dimer in (B) and (C) that form the protrusion of the P particle were colored in red (P2)/green (P1) and yellow (P1)/blue (P2), respectively. (D) Twenty four copies of the epitope (orange cube) are indicated on the outermost surface of the chimeric P particle.
P: Protruding.
Adapted with permission from [39].
Factors affecting P particle formation & stability
There are multiple intermolecular interactions occurring in the P1 regions of P dimers that may be the driving forces of the P particle formation (Figure 2B & C) [13]. Therefore, modifications at the ends of the P protein, the P1 subdomain, strongly affected the efficiency and stability of the P particle formation. While the full-length P domains were able to form both P dimer and P particle in vitro, which were dynamically interchangeable [15], expression of the P domain with an eight amino-acid hinge formed P dimers only (Figure 2A & B) [25]. However, when a cysteine-containing tag (CCT) was added to the N- or C-terminus of the P domain, it efficiently formed stable P particles [14,15,26]. The most commonly used CCTs are CNGRC and CDCRGDCFC (RGD4C), although a number of other CCTs have also been demonstrated to be effective [15]. In fact, an addition of a CCT to the end of the P domain can compensate the negative effect from the hinge, resulting in stable P particles. A further factor that positively affects the P particle formation is the C-terminal arginine-cluster (R-cluster) of the P protein. A deletion of the R-cluster completely prevented the P particle formation [38], even with the presence of a CCT. These data suggest that additional inter-P dimer interactions at the P1 regions are critical for P particle formation and stability. The role of the CCTs in P particle formation may be to increase the interaction among P domain subunits through intermolecular disulfide bonds within a P particle [14,15]. The addition of a non-cysteine-containing sequence, such as the hinge, apparently weakens such interactions. However, the role of the R-cluster in the P particle formation remains unclear. These factors should be considered for high efficient production of the P particles.
NoV P particle as a vaccine candidate
The P particle is composed of 12 P dimers that represent the surface antigenic structures of NoVs and contain all elements for viral receptor interaction and immune responses of the virus (Figure 2D) [14-16]. With the optimal conditions described above, a yield of 20 mg soluble P protein per liter of bacteria culture can be easily obtained, in which >90% of the P proteins form stable P particles [15]. A yield of 7.5 mg P particle per liter of yeast Pichia was also gained [14]. Highly purified P particles can be obtained by fast performance liquid chromatography using a size exclusion and/or an anion-exchange column [14,16,21] that can be easily scaled up for a large yield. Recent expression of the P particle in E. coli without using any purification tags has been successful through hydrophobic and anion-exchange chromatography [Richardson C, Jiang X, Unpublished data], providing an even simpler approach for larger production of the P particle vaccine. In addition, P particles are stable at a wide range of storage temperatures from cryogenic freezing to ambient temperatures and can be lyophilized [Tan M, Jiang X, Unpublished data]. Native MS with solution phase perturbation indicated that the P particles were stable in different ionic strengths and pHs [35]. Furthermore, native MS with gas phase perturbations, including collision induced dissociation and tandem MS (MS/MS), confirmed the high stability of the P particles. These features allow the P particles to be easily stored and transported, which are particularly important for distribution of the future P particle vaccine to low-income developing countries and remote areas where the NoV vaccine is in high demand.
The P particle is highly immunogenic. Immunization of mice with the P particles in a dose range of 15–50 μg per mouse for three-to-four times, intranasally without an adjuvant or subcutaneously with an adjuvant, resulted in high antibody responses against NoV VLPs [14,16,17,36]. The NoV-specific antibody titers induced by the P particles were comparable with that induced by NoV VLPs, which were significantly higher than that induced by the P dimers [14,16]. After immunization with the P particle, the mouse sera were able to block the interaction of NoV VLP with HBGA receptors, a blocking assay that correlated with the antibody protection against NoV infection and illness [40]. Therefore, the NoV P particle is a promising subunit vaccine against human NoVs. However, in a recent study, Tamminen et al. reported a weaker immune response induced by the P particles than that by VLPs [41]. In this study, the P protein was produced using an end-fused His tag without cysteines, which is unlikely to have a high efficiency of P particle formation [31]. Thus, when a new approach is employed to produce the NoV P particle, a simple gel-filtration analysis would be necessary to verify the P particle formation before further immunogenicity experiments are performed [42].
NoV P particle as a vaccine platform for antigen presentation
There are three surface loops on the distal end of each P domain, corresponding to the outermost surface of the P particle (Figure 3). These loops have been shown to be good sites for foreign antigen insertion for improved immune responses of the inserted antigens [16,17,39]. While all three surface loops have been shown to be capable for foreign antigen insertion and presentation [39], loop 2 has been extensively studied (Figure 3). Following these studies, several basic findings have been made: a foreign antigen, ranging from small peptides with a few amino acids to a large protein with up to 238 amino acids (green fluorescence proteins), can be inserted into the P particle without disruption of the P particle formation [16,17,39]; the success of an antigen insertion and presentation by the P particle appears to be protein-sequence dependent, but not dependent on the size of the antigen. Although a general rule remains lacking, an insertion of a hydrophilic antigen appeared to have a higher success rate than the insertion of a hydrophobic antigen; simultaneous insertion of different epitopes to more than one loop was feasible. Using VLPs as a vaccine platform has also been reported in several viruses, including flock house virus [43], human hepatitis B virus [44-46] and cowpea mosaic virus [47,48]. The NoV P particle represents another vaccine platform for diverse foreign antigen presentation for novel vaccine development.
P particle-based bivalent vaccine development
Two P particle-based bivalent vaccine candidates against NoVs and rotaviruses, as well as NoVs and influenza viruses, have been developed and examined by preclinical animal trials. Both vaccines demonstrated strong immune responses and protection against infection of corresponding pathogens, providing a new concept of a dual vaccine against two infectious diseases.
A bivalent vaccine against NoV & rotavirus
Rotavirus is another important cause of acute gastroenteritis in children. The proposed bivalent vaccine against NoV and rotavirus was a chimeric P particle containing one of the major neutralization antigens (VP8*) of rotavirus. The VP8* protein (159 amino acids) was inserted into loop 2 of the P protein by a molecular cloning approach [16,39]. The resulting chimeric P proteins formed a new type of P particle with the VP8* anchored on the outermost surfaces of the chimeric P particle, which is larger than wild-type P particles (Figure 4). Evidence of surface presentation of the VP8* by the P particle was obtained through structural reconstruction of electron cryomicroscopy followed by insertion of the crystal structure of rotavirus VP8* into the extended density map of the chimeric P particle (Figure 4) [16].
Figure 4. Structure of the protruding particle–VP8* chimera reconstructed by electron cryomicroscopy.
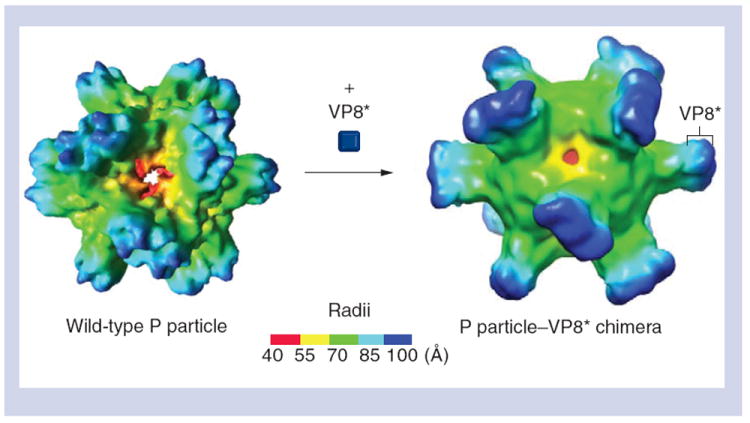
(A) The wild-type P particle before the VP8* antigen is inserted. (B) The P particle–VP8* chimera with the VP8* antigen on its outermost surface. Compared with the wild-type P particle, the chimera shows extended protrusions with nicks in the middle, suggesting the boundary between the P2 subdomain and the inserted VP8* antigen. The radii of the particles are indicated by the color schemes.
P: Protruding.
Adapted with permission from [16].
Immunization of the P particle–VP8* chimeric vaccine to mice resulted in significantly higher VP8*-specific antibody responses compared with that induced by the free VP8* [16]. The resulting mouse sera also showed significantly higher neutralization titers against homologous rotavirus replication in cell cultures than that of the sera induced by the free VP8*. A challenge study further demonstrated that the chimeric vaccine provided mice approximately 90% protection against infection of a murine rotavirus (EDIM strain), which was measured by the reduction of viral shedding in stools of the animals [16]. Heterotypic immune responses to rotavirus VP8* presented by the P particle were also studied, which showed a modest increase of cross-neutralization antibodies against rotavirus infection in cell cultures by plaque reduction assays between DS1 (P[4]) and Wa (P[8]) compared with those induced by the VP8* alone [16]. Since P[4] and P[8] rotaviruses cause the vast majority of epidemics, a combined chimeric P particle vaccine containing VP8* derived from both virus types would probably have broader protection. Importantly, the chimeric P particles also induced high titers of the NoV-specific antibody which was able to block the binding of NoV VLP to HBGA receptors; this is believed to be associated with the protection against NoV infection and illness [40]. Therefore, the P particle–VP8* chimera appeared to be a promising dual vaccine against both NoV and rotavirus.
A bivalent vaccine against NoV & influenza virus
The proposed bivalent vaccine against the NoV and influenza virus was a chimeric P particle that contains the short peptide epitope M2e (23 amino acids) of the influenza virus [17]. Influenza remains a globally important disease with significant morbidity and mortality, causing more than 20,000 hospitalizations in the USA and 500,000 deaths worldwide each year [49,50]. M2e is the ectodomain of the M2 protein of the influenza virus that functions as an ion channel and is abundantly expressed in the infected cells [51,52]. The sequence of M2e is highly conserved among most influenza A strains [53,54], making it an attractive target for a universal vaccine.
The M2e epitope was also inserted into loop 2 of the P particle and the resulting P particle–M2e chimera induced significantly higher titers of the M2e-specific antibody in mice than that induced by the free M2e peptide, even when five-times the amount of peptide was used [17]. Animal challenge studies showed that the P particle–M2e chimera provided 100% protection to mice against lethal challenge of a mouse-adapted influenza virus (PR8 strain; H1N1), which was significantly higher than that provided by the free M2e epitope (12.5%). The responses of antibody subclasses to the chimeric vaccine were also studied. IgG1 titer was high, similar to that of the total IgG, suggesting that the chimeric vaccine induced a strong Th2-type response and that IgG1 may play an important role in the protective immunity. As expected, the chimeric vaccine also induced high antibody response to the P particle platform and the antibody strongly inhibited binding of NoV VLP to viral receptors. These data supported the notion that the P particle–M2e chimera is a promising dual vaccine against both the NoV and influenza virus.
Conclusion & future perspective
Using recombinant technology, a novel subviral particle of the NoV, the P particle, has been constructed. This P particle has shown to be promising as a candidate vaccine against NoVs and as an innovative platform for vaccine development against other viral and bacterial pathogens. Two P particle-based bivalent vaccines have been proved to be promising vaccines against NoV/rotavirus and NoV/influenza virus. The fact that both chimeric P particle vaccines induced specific antibody and immune protection indicates that the P particle is able to present both linear and conformational epitopes of the antigens. In fact, the well-fitting of the crystal structure of rotavirus VP8* in the chimeric P particle provided direct evidence of structural preservation of the antigen on the chimeric P particle (Figure 4) [16]. For future studies, further evaluation of these dual vaccines in humans would be necessary. In addition, it would be significant to systematically evaluate the capability and capacity of the three surface loops of the P particle. Based on these outcomes, P particle-based multivalent vaccines with multiple insertions of different antigens should be explored, including complex insertions of antigen and other immune stimulus factors, such as a universal T-cell epitope, for further improved immune responses. Furthermore, the nature of the immune response induced by the P particle and the effect of adjuvants in immunization with the P particle in comparison with VLPs need to be studied. Finally, while not discussed in this article, other possible applications of the P particle platform should also be evaluated. For example, the P particle platform can be used to produce antibodies specific to disease biomarkers for diagnosis, and/or as carriers for target-directed drug delivery.
Executive summary.
Norovirus protruding particle
-
▪
Norovirus (NoV) protruding (P) particle is composed of 24 P monomers forming 12 P dimers in an octahedral symmetry. The P particle contains the major antigenic structures and host receptor interacting elements of NoV.
-
▪
The P particle assembles spontaneously when the P protein is expressed in Escherichia coli and a fusion of a cysteine-containing tag to the ends of the P domain leads to the highly efficient production of the P particle.
P particle as a vaccine candidate
-
▪
The P particle is stable, highly immunogenic, and able to induce a neutralizing antibody that blocks NoV virus-like particle binding to the histo-blood group antigen receptors, and therefore it is a promising candidate vaccine against NoV.
P particle as a vaccine platform for antigen presentation
-
▪
Insertion of foreign antigens into one of the three surface loops of each P domain resulted in new chimeric P particles with enhanced immunogenicity of the inserted antigens, providing an excellent platform for vaccine development.
-
▪
The resulting chimeric P particles also maintained the major antigenic epitopes of the P particles, allowing the production of dual vaccine against NoV and the inserted antigens to be developed.
P particle-based bivalent vaccine development
-
▪
Preclinical animal trials have demonstrated the P particle–VP8* chimera as a promising dual vaccine against rotavirus and NoV and the P particle–M2e chimera against influenza virus and NoV.
Acknowledgments
The research described in this article was supported by the NIH, the National Institute of Allergy and Infectious Diseases (R21 AI092434-01A1 to M Tan, R01AI089634 to X Jiang) and by an Institutional Clinical and Translational Science Award (NIH/NCRR grant number 1UL1RR026314-01 to M Tan). M Tan and X Jiang receive a royalty from LigoCyte for a license on the P particle vaccine technology.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Atmar RL, Bernstein DI, Harro CD, et al. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med. 2011;365(23):2178–2187. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbst-Kralovetz M, Mason HS, Chen Q. Norwalk virus-like particles as vaccines. Expert Rev Vaccines. 2010;9(3):299–307. doi: 10.1586/erv.09.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang X, Matson DO, Ruiz-Palacios GM, Hu J, Treanor J, Pickering LK. Expression, self-assembly, and antigenicity of a snow mountain agent-like calicivirus capsid protein. J Clin Microbiol. 1995;33(6):1452–1455. doi: 10.1128/jcm.33.6.1452-1455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang X, Wang M, Graham DY, Estes MK. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol. 1992;66(11):6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrington PR, Yount B, Johnston RE, Davis N, Moe C, Baric RS. Systemic, mucosal, and heterotypic immune induction in mice inoculated with Venezuelan equine encephalitis replicons expressing Norwalk virus-like particles. J Virol. 2002;76(2):730–742. doi: 10.1128/JVI.76.2.730-742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taube S, Kurth A, Schreier E. Generation of recombinant norovirus-like particles (VLP) in the human endothelial kidney cell line 293T. Arch Virol. 2005;150(7):1425–1431. doi: 10.1007/s00705-005-0517-x. [DOI] [PubMed] [Google Scholar]
- 7.Xia M, Farkas T, Jiang X. Norovirus capsid protein expressed in yeast forms virus-like particles and stimulates systemic and mucosal immunity in mice following an oral administration of raw yeast extracts. J Med Virol. 2007;79(1):74–83. doi: 10.1002/jmv.20762. [DOI] [PubMed] [Google Scholar]
- 8.Mason HS, Ball JM, Shi JJ, Jiang X, Estes MK, Arntzen CJ. Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc Natl Acad Sci USA. 1996;93(11):5335–5340. doi: 10.1073/pnas.93.11.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tacket CO, Mason HS, Losonsky G, Estes MK, Levine MM, Arntzen CJ. Human immune responses to a novel norwalk virus vaccine delivered in transgenic potatoes. J Infect Dis. 2000;182(1):302–305. doi: 10.1086/315653. [DOI] [PubMed] [Google Scholar]
- 10.Tan M, Zhong W, Song D, Thornton S, Jiang X. E. coli-expressed recombinant norovirus capsid proteins maintain authentic antigenicity and receptor binding capability. J Med Virol. 2004;74(4):641–649. doi: 10.1002/jmv.20228. [DOI] [PubMed] [Google Scholar]
- 11.El-Kamary SS, Pasetti MF, Mendelman PM, et al. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis. 2010;202(11):1649–1658. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ball JM, Graham DY, Opekun AR, Gilger MA, Guerrero RA, Estes MK. Recombinant Norwalk virus-like particles given orally to volunteers: Phase I study. Gastroenterology. 1999;117(1):40–48. doi: 10.1016/s0016-5085(99)70548-2. [DOI] [PubMed] [Google Scholar]
- 13.Prasad BV, Hardy ME, Dokland T, Bella J, Rossmann MG, Estes MK. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999;286(5438):287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- 14.Tan M, Fang P, Chachiyo T, et al. Noroviral P particle: structure, function and applications in virus-host interaction. Virology. 2008;382:115–123. doi: 10.1016/j.virol.2008.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan M, Jiang X. The P domain of norovirus capsid protein forms a subviral particle that binds to histo-blood group antigen receptors. J Virol. 2005;79(22):14017–14030. doi: 10.1128/JVI.79.22.14017-14030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan M, Huang P, Xia M, et al. Norovirus P particle, a novel platform for vaccine development and antibody production. J Virol. 2011;85(2):753–764. doi: 10.1128/JVI.01835-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia M, Tan M, Wei C, et al. A candidate dual vaccine against influenza and noroviruses. Vaccine. 2011;29(44):7670–7677. doi: 10.1016/j.vaccine.2011.07.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindesmith L, Moe C, Marionneau S, et al. Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9:548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- 19.Hutson AM, Atmar RL, Graham DY, Estes MK. Norwalk virus infection and disease is associated with ABO histo-blood group type. J Infect Dis. 2002;185(9):1335–1337. doi: 10.1086/339883. [DOI] [PubMed] [Google Scholar]
- 20.Huang P, Farkas T, Marionneau S, et al. Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: identification of 4 distinct strain-specific patterns. J Infect Dis. 2003;188(1):19–31. doi: 10.1086/375742. [DOI] [PubMed] [Google Scholar]
- 21.Tan M, Jiang X. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol. 2005;13(6):285–293. doi: 10.1016/j.tim.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Tan M, Jiang X. Norovirus-host interaction: implications for disease control and prevention. Expert Rev Mol Med. 2007;9(19):1–22. doi: 10.1017/S1462399407000348. [DOI] [PubMed] [Google Scholar]
- 23.Tan M, Jiang X. Norovirus-host interaction: multi-selections by human histo-blood group antigens. Trends Microbiol. 2011;19(8):382–388. doi: 10.1016/j.tim.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertolotti-Ciarlet A, White LJ, Chen R, Prasad BV, Estes MK. Structural requirements for the assembly of Norwalk virus-like particles. J Virol. 2002;76(8):4044–4055. doi: 10.1128/JVI.76.8.4044-4055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan M, Hegde RS, Jiang X. The P domain of norovirus capsid protein forms dimer and binds to histo-blood group antigen receptors. J Virol. 2004;78(12):6233–6242. doi: 10.1128/JVI.78.12.6233-6242.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan M, Xia M, Cao S, et al. Elucidation of strain-specific interaction of a GII-4 norovirus with HBGA receptors by site-directed mutagenesis study. Virology. 2008;379(2):324–334. doi: 10.1016/j.virol.2008.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan M, Xia M, Chen Y, et al. Conservation of carbohydrate binding interfaces: evidence of human HBGA selection in norovirus evolution. PLoS One. 2009;4(4):E5058. doi: 10.1371/journal.pone.0005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan M, Fang PA, Xia M, Chachiyo T, Jiang W, Jiang X. Terminal modifications of norovirus P domain resulted in a new type of subviral particles, the small P particles. Virology. 2011;410(2):345–352. doi: 10.1016/j.virol.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao S, Lou Z, Tan M, et al. Structural basis for the recognition of blood group trisaccharides by norovirus. J Virol. 2007;81(11):5949–5957. doi: 10.1128/JVI.00219-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Tan M, Xia M, et al. Crystallography of a lewis-binding norovirus, elucidation of strain-specificity to the polymorphic human histo-blood group antigens. PLoS Pathog. 2011;7(7):E1002152. doi: 10.1371/journal.ppat.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansman GS, Biertumpfel C, Georgiev I, et al. Crystal structures of GII.10 and GII.12 norovirus protruding domains in complex with histo-blood group antigens reveal details for a potential site of vulnerability. J Virol. 2011;85(13):6687–6701. doi: 10.1128/JVI.00246-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bu W, Mamedova A, Tan M, Xia M, Jiang X, Hegde RS. Structural basis for the receptor binding specificity of Norwalk virus. J Virol. 2008;82(11):5340–5347. doi: 10.1128/JVI.00135-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi JM, Hutson AM, Estes MK, Prasad BV. Atomic resolution structural characterization of recognition of histo-blood group antigens by Norwalk virus. Proc Natl Acad Sci USA. 2008;105(27):9175–9180. doi: 10.1073/pnas.0803275105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shanker S, Choi JM, Sankaran B, Atmar RL, Estes MK, Prasad BV. Structural analysis of histo-blood group antigen binding specificity in a norovirus GII.4 epidemic variant: implications for epochal evolution. J Virol. 2011;85(17):8635–8645. doi: 10.1128/JVI.00848-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bereszczak JZ, Barbu IM, Tan M, et al. Structure, stability and dynamics of norovirus P domain derived protein complexes studied by native mass spectrometry. J Struct Biol. 2012;177(2):273–282. doi: 10.1016/j.jsb.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Xia M, Tan M, et al. Genetic and phenotypic characterization of GII-4 noroviruses that circulated during 1987 to 2008. J Virol. 2010;84(18):9595–9607. doi: 10.1128/JVI.02614-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan M, Jin M, Xie H, Duan Z, Jiang X, Fang Z. Outbreak studies of a GII-3 and a GII-4 norovirus revealed an association between HBGA phenotypes and viral infection. J Med Virol. 2008;80(7):1296–1301. doi: 10.1002/jmv.21200. [DOI] [PubMed] [Google Scholar]
- 38.Tan M, Meller J, Jiang X. C-terminal arginine cluster is essential for receptor binding of norovirus capsid protein. J Virol. 2006;80(15):7322–7331. doi: 10.1128/JVI.00233-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan M, Xia M, Huang P, et al. Norovirus P particle as a platform for antigen presentation. Procedia Vaccinol. 2011;4:19–26. [Google Scholar]
- 40.Reeck A, Kavanagh O, Estes MK, et al. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis. 2010;202(8):1212–1218. doi: 10.1086/656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamminen K, Huhti L, Koho T, et al. A comparison of immunogenicity of norovirus GII-4 virus-like particles and P-particles. Immunology. 2012;135(1):89–99. doi: 10.1111/j.1365-2567.2011.03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan M, Jiang X. The formation of P particle increased immunogenicity norovirus P protein. Immunology. 2012;136(1):28–29. doi: 10.1111/j.1365-2567.2012.03555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manayani DJ, Thomas D, Dryden KA, et al. A viral nanoparticle with dual function as an anthrax antitoxin and vaccine. PLoS Pathog. 2007;3(10):E142. doi: 10.1371/journal.ppat.0030142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kratz PA, Bottcher B, Nassal M. Native display of complete foreign protein domains on the surface of hepatitis B virus capsids. Proc Natl Acad Sci USA. 1999;96(5):1915–1920. doi: 10.1073/pnas.96.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nassal M, Skamel C, Kratz PA, Wallich R, Stehle T, Simon MM. A fusion product of the complete Borrelia burgdorferi outer surface protein A (OspA) and the hepatitis B virus capsid protein is highly immunogenic and induces protective immunity similar to that seen with an effective lipidated OspA vaccine formula. Eur J Immunol. 2005;35(2):655–665. doi: 10.1002/eji.200425449. [DOI] [PubMed] [Google Scholar]
- 46.Nassal M, Skamel C, Vogel M, et al. Development of hepatitis B virus capsids into a whole-chain protein antigen display platform: new particulate Lyme disease vaccines. Int J Med Microbiol. 2008;298(1–2):135–142. doi: 10.1016/j.ijmm.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Chatterji A, Burns LL, Taylor SS, et al. Cowpea mosaic virus: from the presentation of antigenic peptides to the display of active biomaterials. Intervirology. 2002;45(4–6):362–370. doi: 10.1159/000067929. [DOI] [PubMed] [Google Scholar]
- 48.Chatterji A, Ochoa W, Shamieh L, et al. Chemical conjugation of heterologous proteins on the surface of Cowpea mosaic virus. Bioconjug Chem. 2004;15(4):807–813. doi: 10.1021/bc0402888. [DOI] [PubMed] [Google Scholar]
- 49.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 50.Grandea AG, 3rd, Olsen OA, Cox TC, et al. Human antibodies reveal a protective epitope that is highly conserved among human and nonhuman influenza A viruses. Proc Natl Acad Sci USA. 2010;107(28):12658–12663. doi: 10.1073/pnas.0911806107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zebedee SL, Richardson CD, Lamb RA. Characterization of the influenza virus M2 integral membrane protein and expression at the infected-cell surface from cloned cDNA. J Virol. 1985;56(2):502–511. doi: 10.1128/jvi.56.2.502-511.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lamb RA, Zebedee SL, Richardson CD. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985;40(3):627–633. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- 53.Ito T, Gorman OT, Kawaoka Y, Bean WJ, Webster RG. Evolutionary analysis of the influenza A virus M gene with comparison of the M1 and M2 proteins. J Virol. 1991;65(10):5491–5498. doi: 10.1128/jvi.65.10.5491-5498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu W, Zou P, Ding J, Lu Y, Chen YH. Sequence comparison between the extracellular domain of M2 protein human and avian influenza A virus provides new information for bivalent influenza vaccine design. Microbes Infect. 2005;7(2):171–177. doi: 10.1016/j.micinf.2004.10.006. [DOI] [PubMed] [Google Scholar]


