Abstract
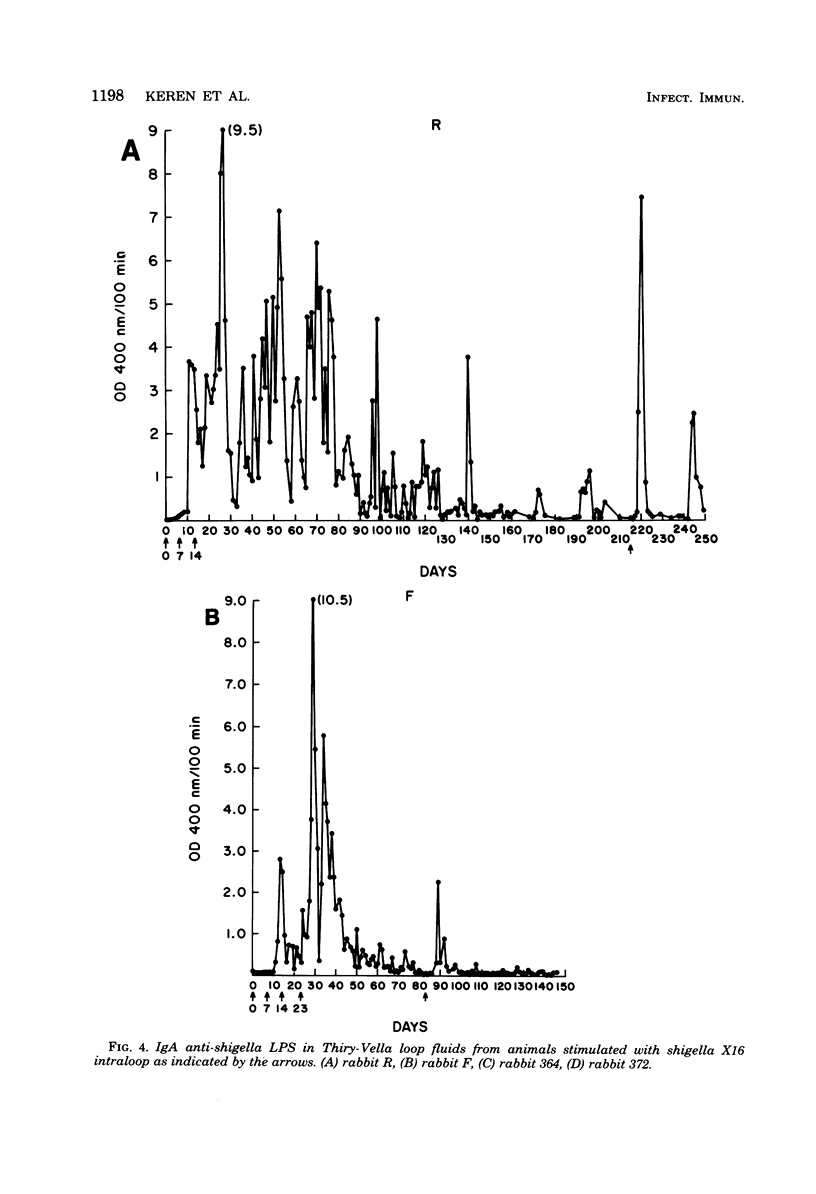
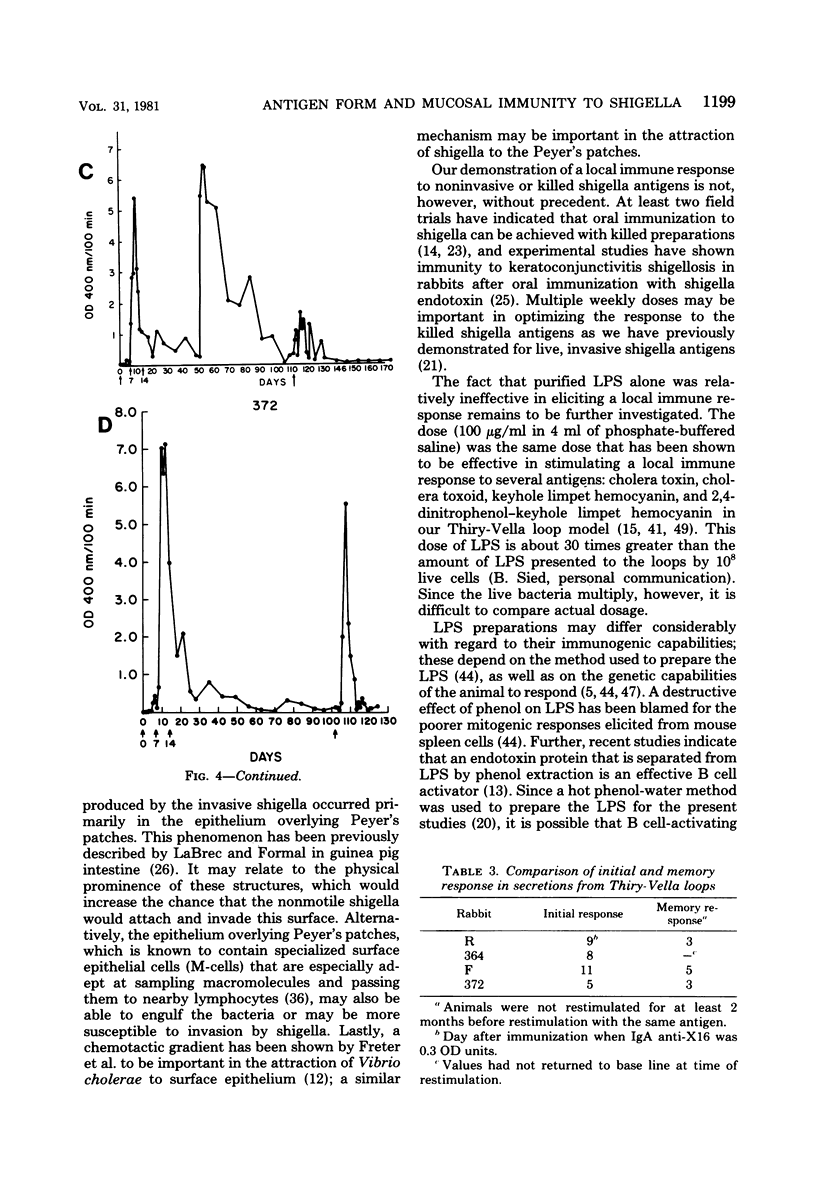
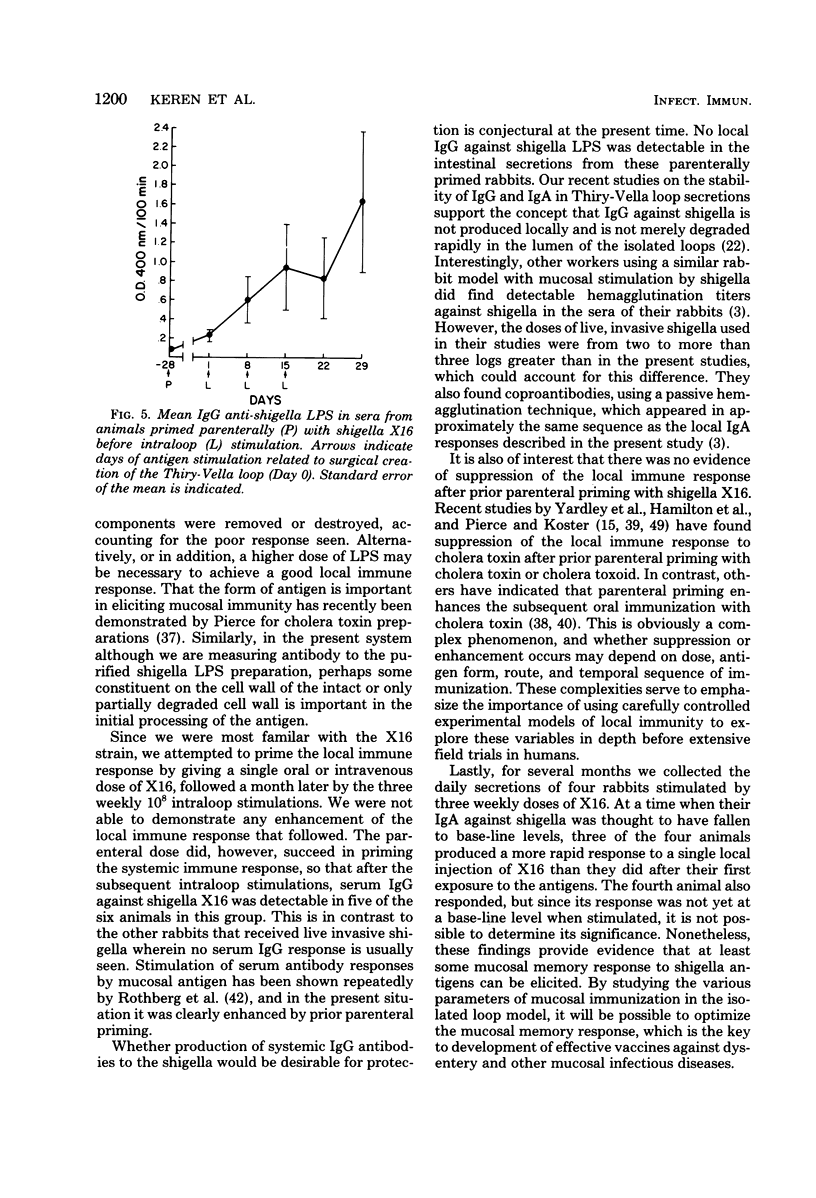
One major stumbling block in the development of an effective means to immunize against shigellosis and other enteric diseases has been the lack of a means to assess sequential mucosal immune responses to different potential immunogens. In the present study, we compared the abilities of live invasive organisms, noninvasive organisms, and nonviable antigen preparations of shigella to elicit mucosal immune responses. Whereas previous studies have found that effective immunity was produced best by vaccination with live invasive strains of shigella, in the present study, live noninvasive strains that did not produce any histopathological damage were consistently able to produce local (immunoglobulin A) immune responses as vigorous as those of the invasive strains. Further, acetone-killed shigella antigen was also an effective mucosal immunogen, whereas hot phenol-water-extracted shigella lipopolysaccharide was ineffective, possibly due to the method of preparation. A single oral or parenteral priming was ineffective in enhancing the mucosal immune response when restimulated 1 month later with the same antigen. However, a mucosal memory response was found to be present several months after a triple mucosal stimulation with a locally invasive vaccine strain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARON L. S., FORMAL S. B., SPILMAN W. The virulence and immunogenicity of Salmonella typhosa grown in continuous culture. J Bacteriol. 1956 Aug;72(2):168–175. doi: 10.1128/jb.72.2.168-175.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denchev V., Trifonova A., Jeleva M., Duncheva S., Bratanova V., Dimitrova Z., Kovachev P. Immunity against dysentery after enteral immunization with live vaccine. Acta Microbiol Acad Sci Hung. 1974;21(1-2):71–74. [PubMed] [Google Scholar]
- DuPont H. L., Hornick R. B., Snyder M. J., Libonati J. P., Formal S. B., Gangarosa E. J. Immunity in shigellosis. II. Protection induced by oral live vaccine or primary infection. J Infect Dis. 1972 Jan;125(1):12–16. doi: 10.1093/infdis/125.1.12. [DOI] [PubMed] [Google Scholar]
- Eisenstein T. K., Angerman C. R. Immunity to experimental Salmonella infection: studies on the protective capacity and immunogenicity of lipopolysaccharide, acetone-killed cells, and ribosome-rich extracts of Salmonella typhimurium in C3H/HeJ and CD-1 mice. J Immunol. 1978 Sep;121(3):1010–1014. [PubMed] [Google Scholar]
- FORMAL S. B., LABREC E. H., KENT T. H., FALKOW S. ABORTIVE INTESTINAL INFECTION WITH AN ESCHERICHIA COLI-SHIGELLA FLEXNERI HYBRID STRAIN. J Bacteriol. 1965 May;89:1374–1382. doi: 10.1128/jb.89.5.1374-1382.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Kent T. H., Austin S., Labrec E. H. Fluorescent-antibody and histological study of vaccinated and control monkeys challenged with Shigella flexneri. J Bacteriol. 1966 Jun;91(6):2368–2376. doi: 10.1128/jb.91.6.2368-2376.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Kent T. H., May H. C., Palmer A., Falkow S., LaBrec E. H. Protection of monkeys against experimental shigellosis with a living attenuated oral polyvalent dysentery vaccine. J Bacteriol. 1966 Jul;92(1):17–22. doi: 10.1128/jb.92.1.17-22.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Labrec E. H., Palmer A., Falkow S. Protection of Monkeys Against Experimental Shigellosis with Attenuated Vaccines. J Bacteriol. 1965 Jul;90(1):63–68. doi: 10.1128/jb.90.1.63-68.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Maenza R. M., Austin S., LaBrec E. H. Failure of parenteral vaccines to protect monkeys against experimental shigellosis. Proc Soc Exp Biol Med. 1967 Jun;125(2):347–349. doi: 10.3181/00379727-125-32087. [DOI] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C., Macsai M. S. Effect of chemotaxis on the interaction of cholera vibrios with intestinal mucosa. Am J Clin Nutr. 1979 Jan;32(1):128–132. doi: 10.1093/ajcn/32.1.128. [DOI] [PubMed] [Google Scholar]
- Goodman G. W., Sultzer B. M. Characterization of the chemical and physical properties of a novel B-lymphocyte activator, endotoxin protein. Infect Immun. 1979 Jun;24(3):685–696. doi: 10.1128/iai.24.3.685-696.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahneis H. Successful field trials with oral killed Shigella sonnei vaccine. Acta Microbiol Acad Sci Hung. 1974;21(1-2):75–80. [PubMed] [Google Scholar]
- HIGGINS A. R., FLOYD T. M., KADER M. A. Studies in shigellosis. III. A controlled evaluation of a monovalent Shigella vaccine in a highly endemic environment. Am J Trop Med Hyg. 1955 Mar;4(2):281–288. [PubMed] [Google Scholar]
- Hamilton S. R., Yardley J. H., Brown G. D. Suppression of local intestinal immunoglobulin A immune response to cholera toxin by subcutaneous administration of cholera toxoids. Infect Immun. 1979 May;24(2):422–426. doi: 10.1128/iai.24.2.422-426.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren D. F., Elliott H. L., Brown G. D., Yardley J. H. Atrophy of villi with hypertrophy and hyperplasia of Paneth cells in isolated (thiry-Vella) ileal loops in rabbits. Light-microscopic studies. Gastroenterology. 1975 Jan;68(1):83–93. [PubMed] [Google Scholar]
- Keren D. F. Enzyme-linked immunosorbent assay for immunoglobulin G and immunoglobulin A antibodies to Shigella flexneri antigens. Infect Immun. 1979 May;24(2):441–448. doi: 10.1128/iai.24.2.441-448.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren D. F., Holt P. S., Collins H. H., Gemski P., Formal S. B. The role of Peyer's patches in the local immune response of rabbit ileum to live bacteria. J Immunol. 1978 Jun;120(6):1892–1896. [PubMed] [Google Scholar]
- Keren D. F., Holt P. S., Collins H. H., Gemski P., Formal S. B. Variables affecting local immune response in ileal loops: role of immunization schedule, bacterial flora, and postsurgical inflammation. Infect Immun. 1980 Jun;28(3):950–956. doi: 10.1128/iai.28.3.950-956.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren D. F., Scott P. J., Bauer D. Variables affecting the local immune response in Thiry-Vella loops. II. Stability of antigen-specific IgG and secretory IgA in acute and chronic Thiry-Vella loops. J Immunol. 1980 Jun;124(6):2620–2624. [PubMed] [Google Scholar]
- Keusch G. T., Grady G. F., Mata L. J., McIver J. The pathogenesis of Shigella diarrhea. I. Enterotoxin production by Shigella dysenteriae I. J Clin Invest. 1972 May;51(5):1212–1218. doi: 10.1172/JCI106915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalewska D., Mulczyk M. Immunity to keratoconjunctivitis shigellosa in rabbits after oral immunization with Shigella sonnei free endotoxin. Acta Microbiol Acad Sci Hung. 1974;21(1-2):99–102. [PubMed] [Google Scholar]
- Kétyi I., Rauss K., Vertényi A. Oral immunization against dysentery. Acta Microbiol Acad Sci Hung. 1974;21(1-2):81–85. [PubMed] [Google Scholar]
- LABREC E. H., FORMAL S. B. Experimental Shigella infections. IV. Fluorescent antibody studies of an infection in guinea pigs. J Immunol. 1961 Nov;87:562–572. [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., DuPont H. L., Formal S. B., Hornick R. B., Takeuchi A., Gangarosa E. J., Snyder M. J., Libonati J. P. Pathogenesis of Shigella dysenteriae 1 (Shiga) dysentery. J Infect Dis. 1973 Mar;127(3):261–270. doi: 10.1093/infdis/127.3.261. [DOI] [PubMed] [Google Scholar]
- MACKEL D. C., LANGLEY L. F., VENICE L. A. The use of the guinea-pig conjunctivae as an experimental model for the study of virulence of Shigella organisms. Am J Hyg. 1961 Mar;73:219–223. doi: 10.1093/oxfordjournals.aje.a120179. [DOI] [PubMed] [Google Scholar]
- Meitert T., Sulea I. T., Gogulescu L., Baron E., Tempea C., Istrati G. Immunogenic value of live, inactivated and cell-free dysentery vaccines. Acta Microbiol Acad Sci Hung. 1974;21(1-2):103–107. [PubMed] [Google Scholar]
- Mel D. M., Papo R. G., Terzin A. L., Vuksić L. Studies on vaccination against bacillary dysentery. 2. Safety tests and reactogenicity studies on a live dysentery vaccine intended for use in field trials. Bull World Health Organ. 1965;32(5):637–645. [PMC free article] [PubMed] [Google Scholar]
- Mel D. M., Terzin A. L., Vuksić L. Studies on vaccination against bacillary dysentery. 1. Immunization of mice against experimental Shigella infection. Bull World Health Organ. 1965;32(5):633–636. [PMC free article] [PubMed] [Google Scholar]
- Mel D., Gangarosa E. J., Radovanovic M. L., Arsic B. L., Litvinjenko S. Studies on vaccination against bacillary dysentery. 6. Protection of children by oral immunization with streptomycin-dependent Shigella strains. Bull World Health Organ. 1971;45(4):457–464. [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Gentry M. K., Thompson M. R., Doctor B. P., Gemski P., Formal S. B. Shigellosis and Escherichia coli diarrhea: relative importance of invasive and toxigenic mechanisms. Am J Clin Nutr. 1979 Jan;32(1):229–233. doi: 10.1093/ajcn/32.1.229. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Koster F. T. Priming and suppression of the intestinal immune response to cholera toxoid/toxin by parenteral toxoid in rats. J Immunol. 1980 Jan;124(1):307–311. [PubMed] [Google Scholar]
- Pierce N. F., Sack R. B., Sircar B. K. Immunity to experimental cholera. III. Enhanced duration of protection after sequential parenteral-oral administration of toxoid to dogs. J Infect Dis. 1977 Jun;135(6):888–896. doi: 10.1093/infdis/135.6.888. [DOI] [PubMed] [Google Scholar]
- Pierce N. F. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J Exp Med. 1978 Jul 1;148(1):195–206. doi: 10.1084/jem.148.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg R. M., Kraft S. C., Michalek S. M. Systemic immunity after local antigenic stimulation of the lymphoid tissue of the gastrointestinal tract. J Immunol. 1973 Dec;111(6):1906–1913. [PubMed] [Google Scholar]
- Rout W. R., Formal S. B., Giannella R. A., Dammin G. J. Pathophysiology of Shigella diarrhea in the rhesus monkey: intestinal transport, morphological, and bacteriological studies. Gastroenterology. 1975 Feb;68(2):270–278. [PubMed] [Google Scholar]
- SWIFT H., HRUBAN Z. FOCAL DEGRADATION AS A BIOLOGICAL PROCESS. Fed Proc. 1964 Sep-Oct;23:1026–1037. [PubMed] [Google Scholar]
- Skidmore B. J., Morrison D. C., Chiller J. M., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS). II. The unresponsiveness of C3H/HeJ Mouse spleen cells to LPS-induced mitogenesis is dependent on the method used to extract LPS. J Exp Med. 1975 Dec 1;142(6):1488–1508. doi: 10.1084/jem.142.6.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Sprinz H., LaBrec E. H., Formal S. B. Experimental bacillary dysentery. An electron microscopic study of the response of the intestinal mucosa to bacterial invasion. Am J Pathol. 1965 Dec;47(6):1011–1044. [PMC free article] [PubMed] [Google Scholar]
- Watson J., Largen M., McAdam K. P. Genetic control of endotoxic responses in mice. J Exp Med. 1978 Jan 1;147(1):39–49. doi: 10.1084/jem.147.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley J. H., Keren D. F., Hamilton S. R., Brown G. D. Local (immunoglobulin A) immune response by the intestine to cholera toxin and its partial suppression with combined systemic and intra-intestinal immunization. Infect Immun. 1978 Feb;19(2):589–597. doi: 10.1128/iai.19.2.589-597.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]