Abstract
The present study was performed to determine the infection status of anisakid larvae in marine fish collected from 3 sea areas of the Republic of Korea. Total 86 marine fish (8 species) collected from the East Sea (Goseong-gun, Gangwon-do), 171 fish (10 species) from the South Sea (Sacheon-si, Gyeongsangnam-do), and 92 fish (7 species) from the Yellow Sea (Incheon Metropolitan City) were examined by both naked eyes and artificial digestion method. Among the total of 349 fish examined, 213 (61.0%) were infected with 8 species of anisakid larvae, i.e., Anisakis simplex, 6 types of Contracaecum spp., and Raphidascaris sp., and the mean larval density was 13.8 per infected fish. Anisakid larvae were detected in 45 fish (52.3%) from the East Sea, 131 fish (76.6%) from the South Sea, and 37 fish (40.2%) from the Yellow Sea. The average numbers of larvae detected were 4.0, 16.6, and 15.9, respectively. Anisakis simplex larvae were detected in 149 fish (42.7%), and the mean larval density was 9.0 per infected fish. They were found in 26 fish (30.2%) collected from the East Sea, 96 fish (56.1%) from the South Sea, and 27 fish (29.3%) from the Yellow Sea. The average numbers of larvae detected were 2.9, 10.3, and 10.5, respectively. Conclusively, the present study suggests that the infection rate and density of anisakid larvae are more or less higher in the fish from the South Sea than those from the East Sea or the Yellow Sea.
Keywords: Anisakis simplex, anisakid larva, marine fish, the East Sea, the South Sea, the Yellow Sea
INTRODUCTION
Anisakidosis (anisakid larvae infection) is one of the foodborne parasitic zoonoses, especially in localities that have the custom of eating raw marine fish and squids. With regard to the etiologic agents of this disease, it has been known that the third-stage larvae (L3) of Anisakis simplex, Anisakis physeteris, and Pseudoterranova decipiens are mainly involved in prevalent countries. In particular, A. simplex larvae are most frequently found in human cases [1-3]. Some marine fish, i.e., the spotted chub mackerel (Scomber japonicum), olive flounder (Paralichthys olivaceus), spotted sardine (Sardinops melanostictus), whitespotted conger (Conger myriaster), and Japanese amberjack (Seriola quinqueradiata), and Japanese common squid (Todarodes pacificus) have been known as the main infection sources of this disease in Japan and Korea [4-8].
Several studies on the infection status of anisakid larvae in marine fish and cephalopods have been performed in Korea. However, most of the studies were performed on only 1 fish species, such as the yellow croaker (Pseudosciaena polyactis), white-spotted conger, salmon (Onchorhynchus keta), sea trout (Oncorhynchus masou), or anchovy (Engraulis japonicus) [9-14], and on the fish species could be collected in limited areas of Korea [15,16]. Recently, Choi et al. [17] extensively surveyed on the infection status of A. simplex larvae in fish and cephalopods purchased from the Cooperative Fish Market in Busan, Korea. However, there have been no comparative studies on anisakid larvae in fish from 3 different sea areas, the East Sea, South Sea, and Yellow Sea, of the Republic of Korea. Therefore, we surveyed on the infection status of anisakid larvae in marine fish, which are popularly eaten raw, collected from 3 different sea areas of Korea.
MATERIALS AND METHODS
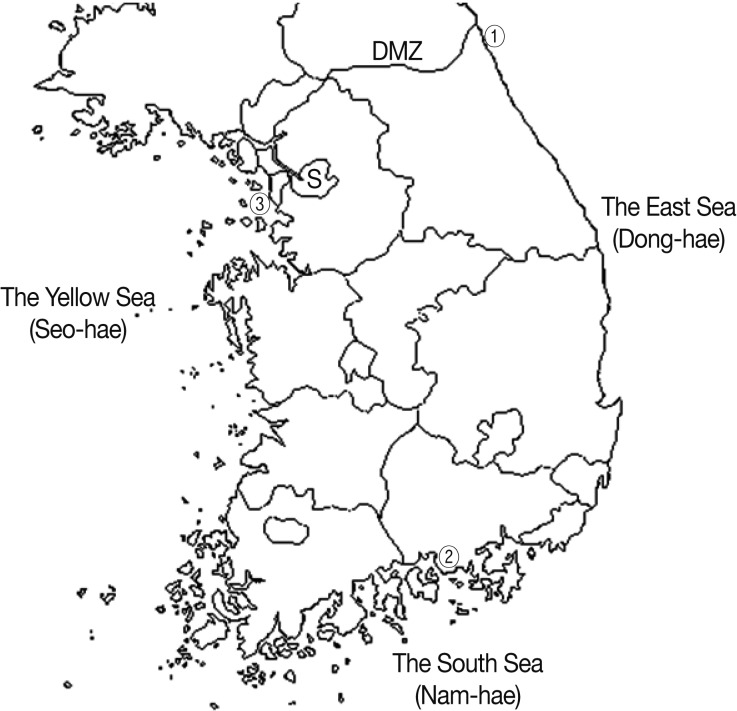
Total 86 marine fish (8 species) were collected from a fish market in Goseong-gun, Gangwon-do (the East Sea), 171 fish (10 species) were from Sacheon-si, Gyeongsangnam-do (the South Sea), and 92 fish (7 species) were from Incheon Metropolitan City (the Yellow Sea), in March to May, 2008 (Fig. 1). Kinds of fish and numbers by collection sites were as follows:
Fig. 1.
Surveyed areas. ① Goseong-gun in Gangwon-do (the East Sea), ② Sacheon-si in Gyeongsangnam-do (the South Sea) and ③ Incheon Metropolitan City (the Yellow Sea).
In the East Sea, 15 Clupea pallasii (Pacific herring), 11 Oncorhynchus masou masou (trout), 10 Pleurogrammus azonus (arabesque greenling), 10 Hexagrammos otakii (greenling), 10 Thamnaconus modestus (black scraper), 10 Sebastes schlegeli (jacopever), 10 Paralichthys olivaceus (olive flounder), and 10 Hyppoglosoides pinetorum (pointhead flounder) were examined.
In the South Sea, 30 S. schlegeli, 30 Pleuronichthys cornutus (frog flounder), 20 Conger myriaster (whitespotted conger), 20 H. otakii, 20 Sebastes thompson (goldeye rockfish), 11 Sebastes inermis (dark-banded rockfish), 10 Acanthopagrus schlegeli (black sea bream), 10 Evynnis japonica (Crimson sea bream), 10 Scorpaenodes littoralis (shore rockfish), and 10 Platycephalus indicus (bartail flathead) were subjected for study.
In the Yellow Sea, 22 Mugil cephalus (gray mullet), 20 H. otakii, 15 Konosirus punctatus (dotted gizzard shad), 10 S. schlegeli, 10 C. myriaster, 10 Seriola quinqueradiata (Japanese amberjack), and 10 Lateolabrax japonicus (sea perch) were examined.
All collected fish were transported with ice to the Parasitology Laboratory, Gyeongsang National University School of Medicine, Jinju, Korea. The visceral organs of each fish were isolated from the abdominal cavities. After examination with naked eyes, the organs were incubated with pepsin-HCl solution in a 36℃ incubator for 2-3 hr to isolate deeply embedded and entangled fine larvae. After washing the mixture several times with 0.85% saline, all nematodes liberated were collected from the digested materials using a stereomicroscope. The collected nematodes, excluding A. simplex larvae (these were easily diagnosed), were fixed with 10% hot formalin, cleared in alcohol-glycerin solution, mounted in glycerin-jelly, and observed using a light microscope with a micrometer. Species and type of larvae were determined by their morphological characteristics, measurement data, and other identification indices.
RESULTS
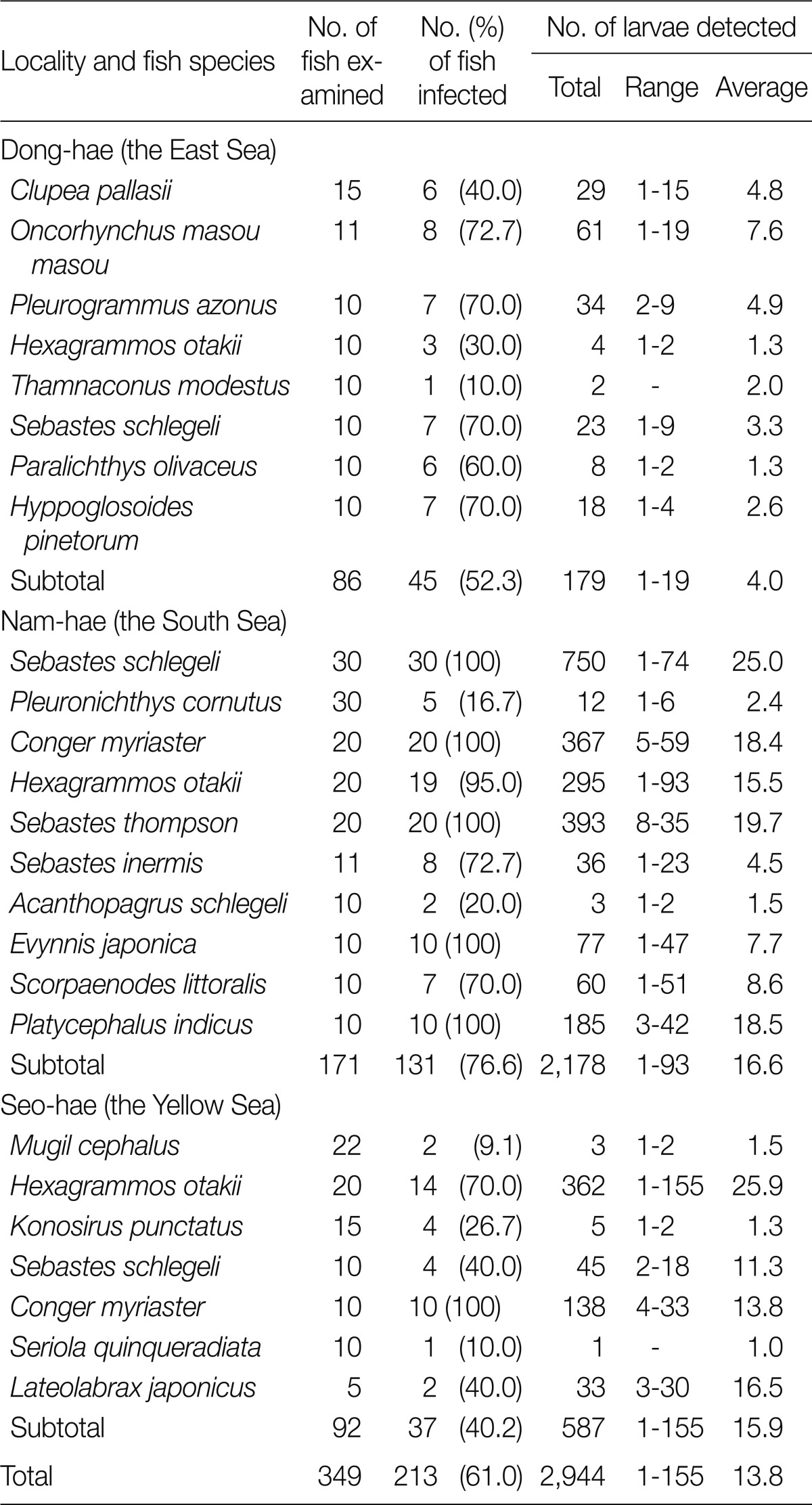
A total of 179 anisakid larvae (4.0 per infected fish), including those of A. simplex, were detected in 45 (52.3%) of 86 fish collected from the East Sea. In the South Sea, 131 (76.6%) of 171 fish were infected with a total of 2,178 anisakid larvae (16.6 per infected fish), which were comprised of larvae of A. simplex, 6 types of Contracaecum spp., and Raphidascaris sp. In the Yellow Sea, total 587 anisakid larvae (15.9 per infected fish) were detected in 37 (40.2%) out of 92 fish examined; the larvae comprised of A. simplex, 6 types of Contracaecum spp., and Raphidascaris sp. The infection status by the fish species and collection sites is shown in detail in Table 1.
Table 1.
Infection status of anisakid larvae in fish from 3 sea areas of the Republic of Korea
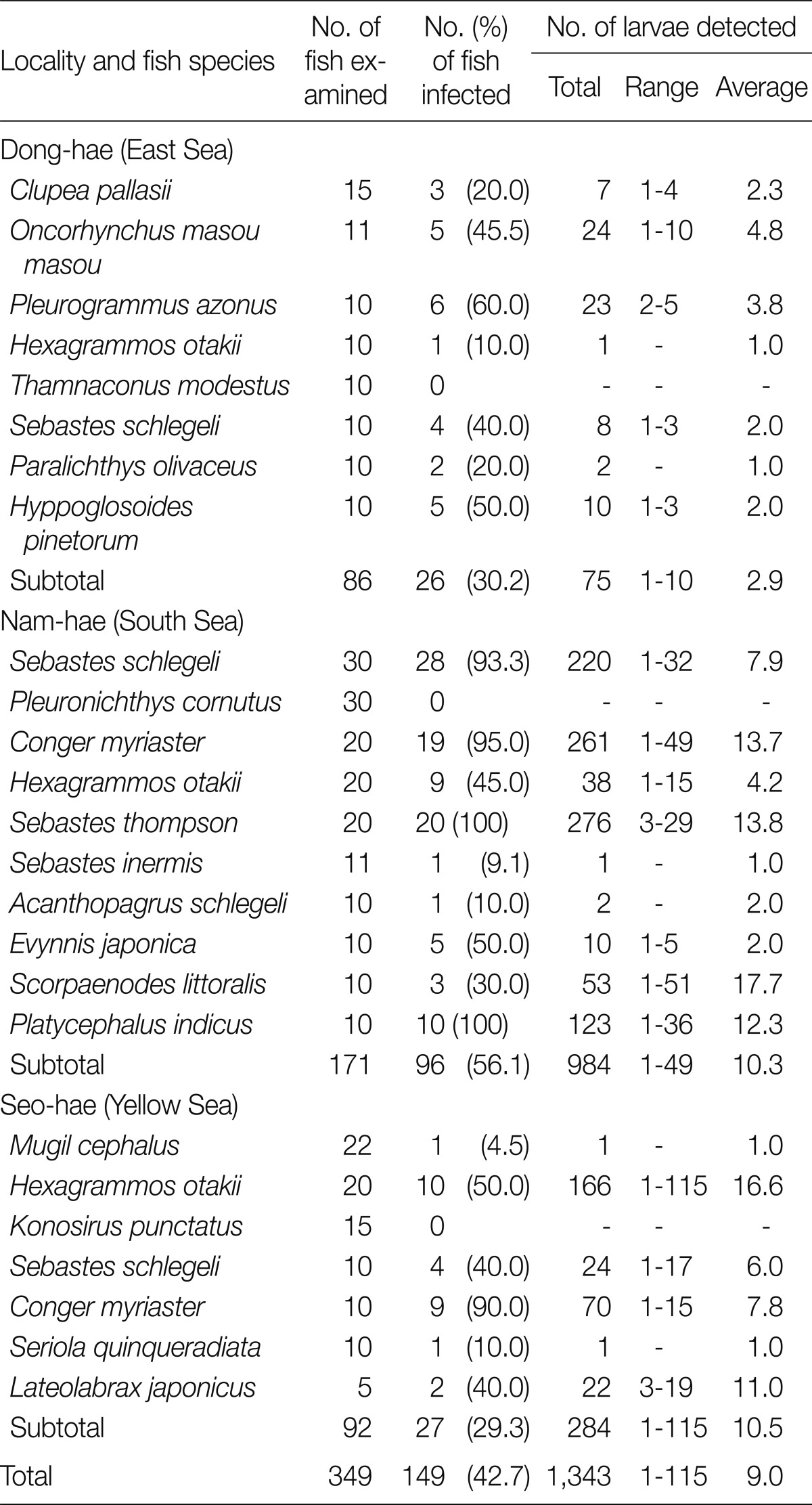
A. simplex larvae were detected in 26 (30.2%) fish collected from the East Sea, 96 fish (56.1%) from the South Sea, and 27 fish (29.3%) from the Yellow Sea. The average numbers per infected fish were 2.9, 10.3, and 10.5 larvae, respectively. No larvae were found in 10 T. modestus from the East Sea, 30 P. cornutus from the South Sea, and 15 K. punctatus from the Yellow Sea. The infection status of A. simplex larvae by fish species and collection sites is shown in Table 2.
Table 2.
Infection status of Anisakis simplex larvae in fish from 3 sea areas of the Republic of Korea
In the East Sea, 104 (58.1%) anisakid larvae, excluding A. simplex (41.9%), were detected. Contracaecum type A larvae were detected in 18 (20.9%) of 86 fish examind, Contracaecum type C in 3 fish (3.5%), Contracaecum type C' in 6 fish (7.0%), Contracaecum type D in 7 fish (8.1%), Contracaecum type D' in 1 fish (1.2%), Contracaecum type V in 1 fish (1.2%), and Raphidascaris sp. in 5 fish (5.8%).
In the South Sea, a total of 1,194 (54.8%) larvae, excluding A. simplex (45.2%), were collected. Contracaecum type A larvae were detected in 60 (35.1%) of 171 fish examined, Contracaecum type C in 4 fish (2.3%), Contracaecum type C' in 70 fish (40.9%), Contracaecum type D in 23 fish (13.5%), Contracaecum type D' in 18 fish (10.5%), Contracaecum type V in 4 fish (2.3%), and Raphidascaris sp. in 6 fish (3.5%).
In the Yellow Sea, total 281 (48.8%) anisakid larvae, excluding A. simplex (51.2%), were detected. Contracaecum type A larvae were detected in 17 (18.5%) of 92 fish examined, Contracaecum type C in 2 fish (2.2%), Contracaecum type C' in 11 fish (12.0%), Contracaecum type D in 5 fish (5.4%), Contracaecum type D' in 5 fish (5.4%), Contracaecum type V in 3 fish (3.3%), and Raphidascaris sp. in 6 fish (6.5%).
DISCUSSION
The number of fish and the species of fish examined were more or less different in 3 surveyed sites; partly for this reason, the general trends of larval infection appeared to be comparable by the survey sites. The infection rates and densities of anisakid larvae were higher in fish from the South Sea than in those from the East Sea and the Yellow Sea. Comparing the East Sea and the Yellow Sea, the infection rate was higher in the East Sea (52.3%) than in the Yellow Sea (40.2%). However, the average infection density was higher in fish from the Yellow Sea (15.9 larvae) than in those from the East Sea (4.0 larvae). Chun et al. [15] also reported that the infection density of anisakid larvae was much higher in fish from the South Sea (43.3 per fish) than in those from the Yellow Sea (8.7 per fish) [15].
Among the fish species examined in the present study, only 2 species, H. otakii and S. schlegeli, were commonly collected in 3 sea areas. The infection rates of anisakid larvae were 30%, 95%, and 70% in H. otakii from the East Sea, South Sea, and Yellow Sea respectively, and the average infection densities were 1.3, 15.5, and 25.9 larvae per infected fish. In case of S. schlegeli, the infection rates were 70%, 100%, and 40%, and the average infection densities were 3.3, 25.0, and 11.3 larvae per infected fish from the East Sea, South Sea, and Yellow Sea, respectively. In the 2 fish species commonly collected in 3 sea areas, the infection status with anisakid larvae was also higher in those from the South Sea.
A species of the sea eel, the whitespotted conger (Conger myriaster), has been suspected as one of the most important fish hosts for human anisakidosis in Korea [6-8], and frequently examined for anisakid larvae infections by Korean researchers [10,11,14,17]. Chai et al. [10] collected a total of 1,351 anisakid larvae (90 larvae per infected fish) in 15 (57.7%) of 26 whitespotted congers collected from the Noryangjin fish market in Seoul, Korea [10]. Song and Hwang [11] reported 67.8% (positive rate) and 4.6 larvae (mean intensities of infection) in 382 whitespotted congers collected in Busan. A total of 804 anisakid larvae (9.6 larvae per infected fish) were collected from 84 (90.3%) of 93 whitespotted congers collected in a local fish market of Chungmu-si (presently Tongyoung-si), Gyeongsangnam-do, Korea [14]. The present study showed that the infection rates were all 100% in whitespotted congers from the South Sea (n=20) and the Yellow Sea (n=10), and the mean intensities of infection were 18.4 and 13.8 larvae per infected fish, respectively. On the other hand, Choi et al. [17] reported 76.5% (positive rate) and 14.3 A. simplex larvae (mean intensities of infection) in 68 whitespotted congers collected in the Cooperative Fish Market in Busan, Korea [17]. In the present study, the infection rates of whitespotted congers with A. simplex larvae were 95.0% and 90.0%, and mean intensities of infection were 13.7 and 7.8 larvae, in the South Sea and the Yellow Sea, respectively. These data suggest that the sea eel (whitespotted conger) is still an important fish host for anisakidosis in Korea.
It has been known that A. simplex larvae (Anisakis type I larvae) are most frequently found in human cases and also in the second intermediate hosts (fish and cephalopods) in Korea. A total of 2,944 anisakid larvae comprising of A. simplex (1,343 larvae: 45.6%), 6 types of Contracaecum spp., and Raphidascaris sp. were detected in 213 of 349 fish examined in the present study. However, other etiologic agents of anisakidosis, A. physeteris (Anisakis type II) and P. decipiens (Terranova type A) larvae, were not detected at all. On the other hand, the infection rate with A. simplex larvae was higher in fish from the South Sea (56.1%) than in those from the East Sea (30.2%) and the Yellow Sea (29.3%). The mean intensity of infection was higher in fish from the Yellow Sea (10.5 larvae) than in those from the South Sea (10.3 larvae) and the East Sea (2.9 larvae). Choi et al. [17] reported that the overall infection rate with A. simplex larvae was 34.3%, and the mean intensity was 17.1 larvae in fish and cephalopods from the Cooperative Fish Market in Busan, Korea.
No A. simplex larvae were detected in 10 T. modestus from the East Sea, 30 P. cornutus from the South Sea, and 15 K. punctatus from the Yellow Sea, respectively. Choi et al. [17] also could not find any larvae in 5 fish species, Pseudosciaena crocea, Cynoglossus semilaevis, K. punctatus, Oplegnathus fascitus, and Chromis notata, and 1 cephalopod species, Loligo bleekeri. Especially, they could not detect A. simplex larvae in 174 dotted gizzard shad, K. punctatus, examined, which is in accordance with the finding in the present study. In case of 2 fish species, H. otakii and S. schlegeli, commonly collected in 3 sea areas, the infection rate was higher in fish from the South Sea (74.0%) than in fish from the Yellow Sea (46.7%) and the East Sea (25.0%). However, the mean intensity of infection was higher in fish from the Yellow Sea (13.6 larvae) than in those from the South Sea (7.0 larvae) and the East Sea (1.8 larvae). From the findings of the present study, it is difficult to explain what kinds of ecological factors would be responsible for the difference of infection status with anisakid larvae in fish hosts from 3 different sea sites of the Korean peninsula.
With regard to the genus Anisakis, it has been reported that Anisakis type I larvae (morphological type) actually comprise several different species genetically, including A. simplex sensu stricto, Anisakis pegreffii, and A. simplex C [18,19]. As we did not perform molecular studies on the larval worms in this study, the presence of A. pegreffii, A. simplex C, or other genetic species among our specimens cannot be ruled out.
ACKNOWLEDGMENTS
This study was supported by a grant from the National Institute of Health (20071114961-00), Centers for Disease Control and Prevention, the Republic of Korea. We thank A-Ra Cho and Hae-In Ryu (Department of Parasitology, Gyeongsang National University School of Medicine, Jinju, Korea), for their help in the examination of fish.
References
- 1.Sakanari JA, Mckerrow JH. Anisakiasis. Clin Microbiol Rev. 1989;2:278–284. doi: 10.1128/cmr.2.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chai JY, Murrell KD, Lymbery AJ. Fish-borne parasitic zoonoses: Status and issues. Int J Parasitol. 2005;35:1233–1254. doi: 10.1016/j.ijpara.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Sohn WM, Chai JY. Anisakiosis (Anisakidosis) In: Palmer SR, et al., editors. Oxford Textbook of Zoonoses-Biology, Clinical Practice, and Public Health Control. 2nd ed. Oxford, UK: Oxford University Press; 2011. pp. 774–786. [Google Scholar]
- 4.Nagasawa K, Moravec F. Larval anisakid nematodes of Japanese common squid (Todarodes pacificus) from the Sea of Japan. J Parasitol. 1995;81:69–75. [PubMed] [Google Scholar]
- 5.Ishikura H, Takahashi S, Ishikura H. Anisakidae and anisakidosis in Japan; Proceedings of the 2nd Japan-Korea Parasitologists' Seminar (Forum Cheju-2); 1996. pp. 50–63. [Google Scholar]
- 6.Seol SY, Ok SC, Pyo JS, Kim IH, Lee SH, Chung JM, Choi HJ, Jung SJ, Sohn WM. Twenty cases of gastric anisakiasis caused by Anisakis type I larva. Korean J Gastroenterol. 1994;26:17–24. (in Korean) [Google Scholar]
- 7.Im KI, Shin HJ, Kim BH, Moon SI. Gastric anisakiasis cases in Cheju-do, Korea. Korean J Parasitol. 1995;33:179–186. doi: 10.3347/kjp.1995.33.3.179. [DOI] [PubMed] [Google Scholar]
- 8.Song TJ, Cho SW, Joo KH. Endoscopic findings of acute gastric anisakiasis. Thirty-nine cases in Inchon City. Korean J Gastrointest Endosc. 1999;19:878–884. (in Korean) [Google Scholar]
- 9.Chai JY, Cho YM, Sohn WM, Lee SH. Larval anisakids collected from the yellow corvine in Korea. Korean J Parasitol. 1986;24:1–11. doi: 10.3347/kjp.1986.24.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Chai JY, Cho SR, Kook J, Lee SH. Infection status of the sea eel (Astroconger myriaster) purchased from the Noryangjin fish market with anisakid larvae. Korean J Parasitol. 1992;30:157–162. doi: 10.3347/kjp.1992.30.3.157. [DOI] [PubMed] [Google Scholar]
- 11.Song SB, Hwang EG. Infection status of larval anisakids in Astroconger myriaster collected from the Southern Sea near Pusan. Korean J Parasitol. 1992;30:263–267. doi: 10.3347/kjp.1992.30.4.263. [DOI] [PubMed] [Google Scholar]
- 12.Kim KH, Joo KH, Rim HJ. A study about infection state of anisakis larvae and parasitic helminths in salmon (Onchorhynchus keta) and sea trout (Oncorhynchus masou) which were caught from Taepo Port, Kangwon Do. Korean J Rural Med. 1990;15:27–32. [Google Scholar]
- 13.Song SB, Lee SR, Chung HH, Han NS. Infection status of anisakid larvae in anchovies purchased from local fishery market near southern and eastern sea in Korea. Korean J Parasitol. 1995;33:95–99. doi: 10.3347/kjp.1995.33.2.95. (in Korean) [DOI] [PubMed] [Google Scholar]
- 14.Chun KS. Infection status of the sea eel (Astroconger myriaster) with anisakid larvae in the markets from Chungmu. Korean J Environ Health Soc. 1997;23:14–17. (in Korean) [Google Scholar]
- 15.Chun SK, Chung BK, Ryu BS. Studies on Anisakis sp. (1) On the infection state of anisakis-like larvae isolated from various marine fishes. Korean J Fish Aquat Sci. 1968;1:1–6. (in Korean) [Google Scholar]
- 16.Woo HC, Kim JA. The infection status and identification of anisakid larvae in marine fish caught from the sea near Cheju island. Korean J Vet Public Health. 2000;24:307–317. (in Korean) [Google Scholar]
- 17.Choi SH, Kim J, Jo JO, Cho MK, Yu HS, Cha HJ, Ock MS. Anisakis simplex larvae: infection status in marine fish and cephalopods purchased from the Cooperative Fish Market in Busan, Korea. Korean J Parasitol. 2011;49:39–44. doi: 10.3347/kjp.2011.49.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umehara A, Kawakami Y, Matsui T, Araki J, Uchida A. Molecular identification of Anisakis simplex sensu stricto and Anisakis pegreffii (Nematoda: Anisakidae) from fish and cetacean in Japanese waters. Parasitol Int. 2006;55:267–271. doi: 10.1016/j.parint.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Arizono N, Yamada M, Tegoshi T, Yoshikawa M. Anisakis simplex sensu stricto and Anisakis pegreffii: biological characteristics and pathogenic potential in human anisakiasis. Foodborne Pathog Dis. 2012;9:517–521. doi: 10.1089/fpd.2011.1076. [DOI] [PubMed] [Google Scholar]