Abstract
The prevalence of foodborne trematode (FBT) metacercariae was investigated in fish from 2 localities of northern Vietnam in 2004-2005. Freshwater fish (9 species) were collected from local markets in Hanoi City (n=76) and Nam Dinh Province (n=79), and were examined for FBT metacercariae using the artificial digestion technique. Adult flukes were obtained from hamsters experimentally infected with the metacercariae at day 8 post-infection. Three (Haplorchis pumilio, Centrocestus formosanus, and Procerovum varium) and 6 (Haplorchis taichui, H. pumilio, C. formosanus, P. varium, Stellantchasmus falcatus, and Heterophyopsis continua) species of FBT metacercariae were detected in the 2 regions, respectively. Overall, among the positive fish species, H. pumilio metacercariae were detected in 104 (80.0%) of 130 fish examined (metacercarial density per infected fish; 64.2). C. formosanus metacercariae were found in 37 (40.2%) of 92 fish (metacercarial density; 14.7). P. varium metacercariae were detected in 19 (63.3%) of 30 fish (Anabas testudineus and Mugil cephalus) (metacercarial density; 247.7). S. falcatus metacercariae were found in all 10 M. cephalus examined (metacercarial density; 84.4). H. continua metacercariae (2 in number) were detected in 1 fish of Coilia lindmani. Morphologic characteristics of the FBT metacercariae and their experimentally obtained adults were described. The results have demonstrated that various FBT species are prevalent in northen parts of Vietnam.
Keywords: Haplorchis taichui, Haplorchis pumilio, Centrocestus formosanus, Procerovum varium, Stellantchasmus falcatus, Heterophyopsis continua, foodborne trematode, metacercaria, fish, Vietnam
INTRODUCTION
Foodborne trematode (FBT) infections are an important public health concern in various Asian countries, including Lao PDR, Vietnam, Cambodia, Thailand, the Philippines, Taiwan, China, and the Republic of Korea. Human FBT infections are caused by habitual consumption of raw fish containing infective larvae (metacercariae) [1-3]. Studies on FBT metacercarial infections have revealed that some species of freshwater and brackish water fish play important roles as the source of human infections in endemic areas [4-7]. It is known that FBT are not only pathogenic to the human host provoking remarkable morbidities but also harmful to fish hosts causing a serious economic loss in the aquaculture fish industry [1].
Surveys on metacercarial infection in second intermediate hosts, in combination with surveys on adult fluke infections in humans, are essential to understand the epidemiology and host-parasite relationships of FBT infections in particular geographical areas. However, fecal examinations are not usually suitable to determine the exact infection status in humans, since the specific identification of eggs is very difficult in cases of mixed infections with small-sized trematode eggs, in particular, the liver fluke, heterophyids, gymnophallids, and lecithodendriid flukes [8-10]. Therefore, investigation of metacercarial infections in second intermediate hosts available in the area around can provide valuble information on the trematode epidemiology.
Hanoi City and Nam Dinh Province are located in the northern part of Vietnam. Through adult fluke recovery by Dung et al. [11], it has been known that many residents in Nam Dinh Province are infected with FBT, such as Clonorchis sinensis, heterophyids (Haplorchis pumilio, Haplorchis taichui, Haplorchis yokogawai, and Stellantchasmus falcatus), and Fasciolopsis buski [11]. After that survey, metacercatrial infections were investigated in fish intermediate hosts caught in several local areas in Vietnam [12-17]. In particular, Phan et al. [17] surveyed on FBT metacercarial infection in freshwater fish from small-scale farms (family-based household fish farm) in Nam Dinh Province. However, those studies did not provide quantitative details of FBT metacercarial infections in fish hosts, mainly focusing on qualitative aspects of commercially important cultured fish species. Therefore, in the present study, we intended to reveal the infection status (both qualitative and quantitative aspects) of FBT metacercariae in wild fish caught from 2 localities of northern Vietnam (Hanoi and Nam Dinh Province). In addition, we described the morphologic characteristics of FBT metacercariae and their adults obtained from experimentally infected hamsters.
MATERIALS AND METHODS
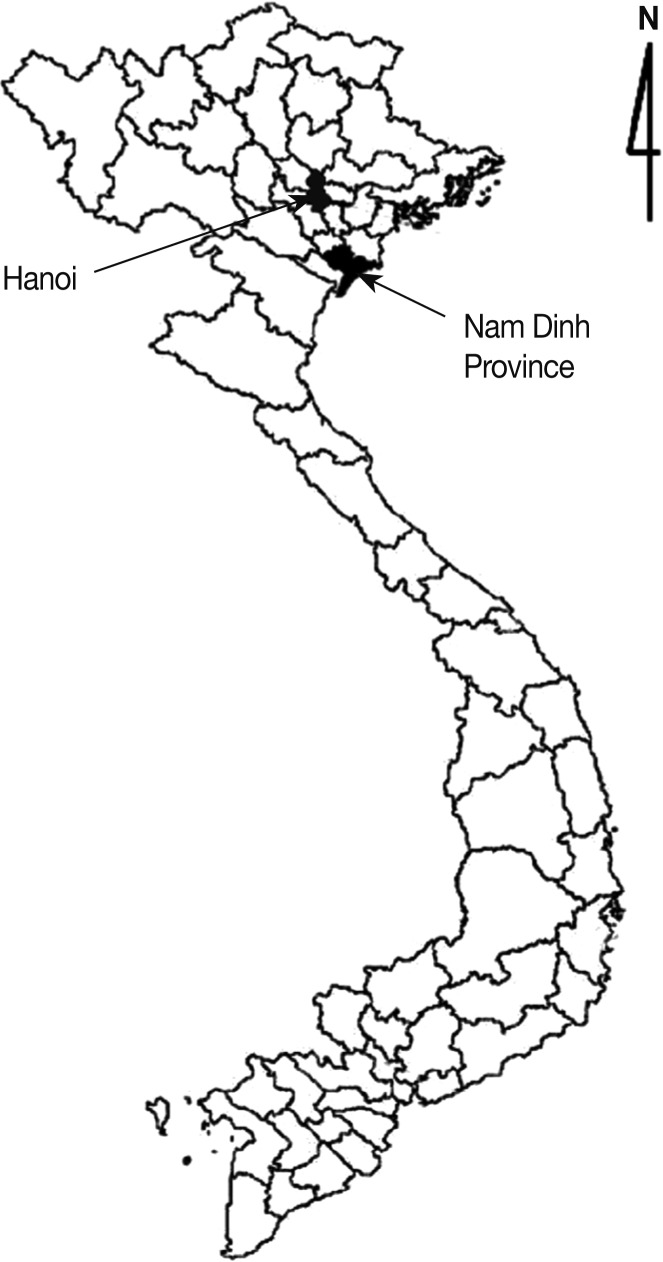
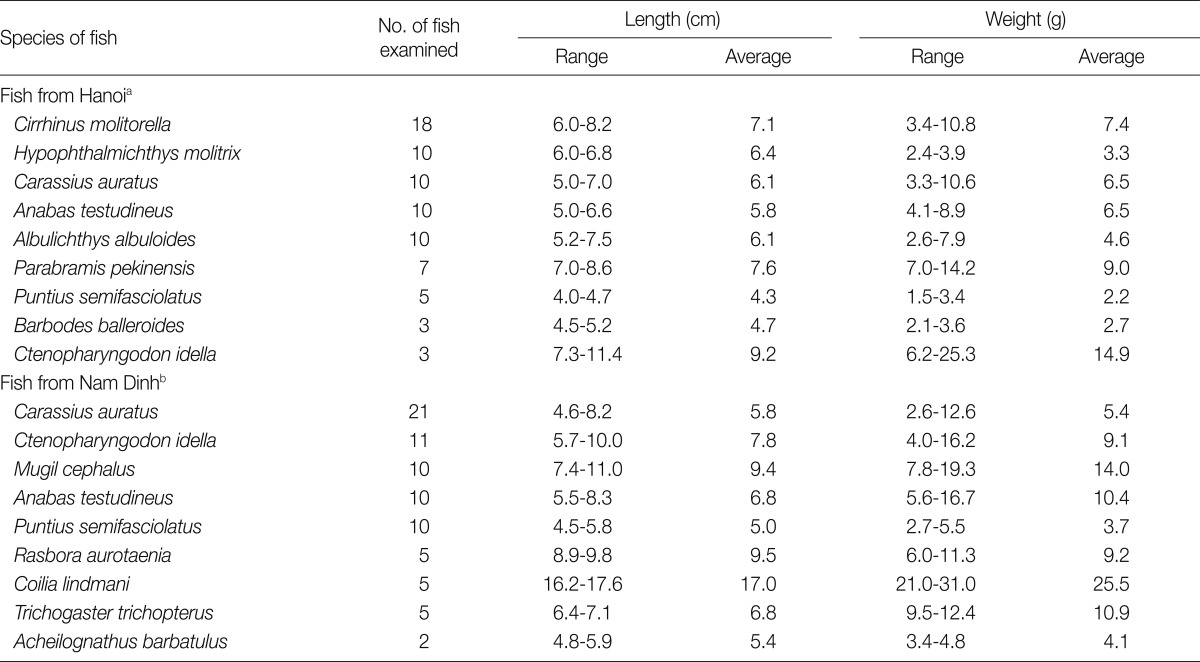
In Hanoi and Nam Dinh Province (Fig. 1), 76 and 79 fish (9 species each), respectively, were collected from local markets in November 2004 to April 2005. All collected fish were transferred with ice to the laboratory of Department of Parasitology, Gyeongsang National University School of Medicine, Jinju, Korea. Their length and weight were measured and the species were identified with the aid of FishBase site in internet (Table 1) [18]. Individual fish was ground with a mortar with pestle, or a grinder, and the ground fish meat was mixed with artificial gastric juice. The mixture was incubated at 36℃ for 2 hr. The digested material was filtered through a 1×1 mm mesh, and washed with 0.85% saline untill the supernatant became clear. The sediment was carefully examined using a stereomicroscope. The metacercariae were classified by their general features and were identified at the species level based on detailed morphologies and demensions using a light microscope. Identified metacercariae were experimentally infected to hamsters to obtain adult worms. At day 8 after infection, the hamsters were killed by cervical dislocation, and their small intestines were isolated and longitudinally opened with a scissors in a beaker containing 0.85% saline. Adult flukes were recovered from the sediment of the intestinal contents which were diluted and washed with 0.85% saline. Recovered worms were fixed with 10% formalin under a cover glass pressure, stained with Semichon's acetocarmine, and observed using a light microscope equipped with a micrometer.
Fig. 1.
Surveyed areas (▪) (Hanoi City and Nam Dinh Province), located in northern Vietnam.
Table 1.
Fish collected from local markets in Hanoi City and Nam Dinh Province, Vietnam
a76 fish and b79 fish in total (9 species each) were examined.
RESULTS
FBT metacercariae in fish from Hanoi
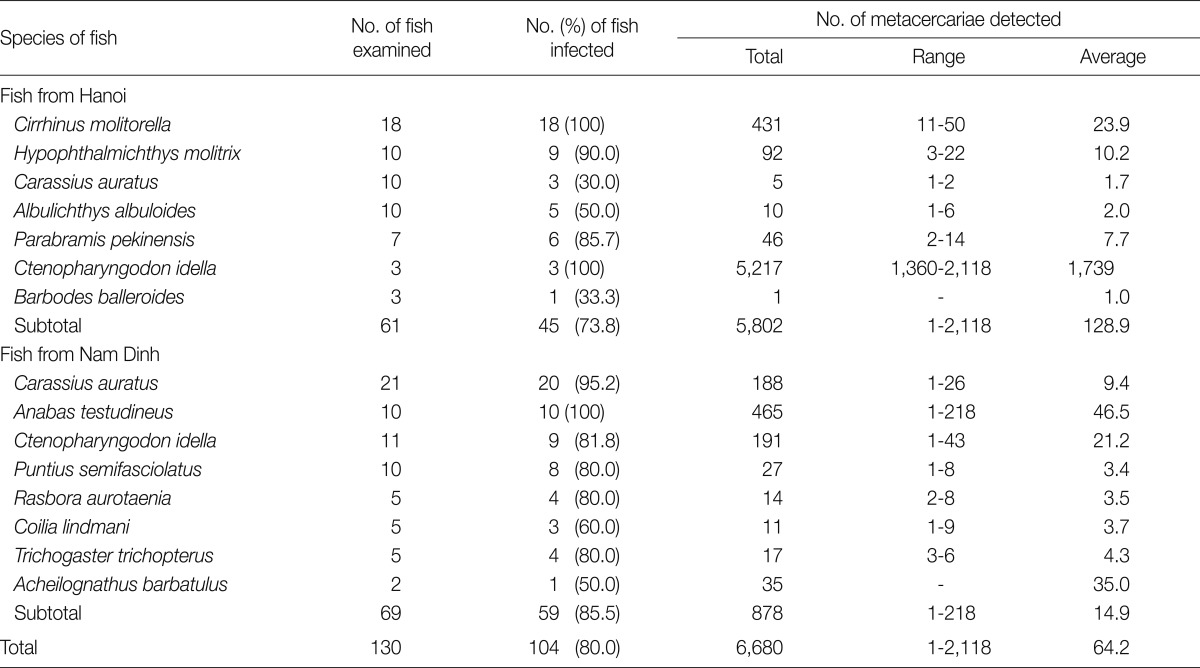
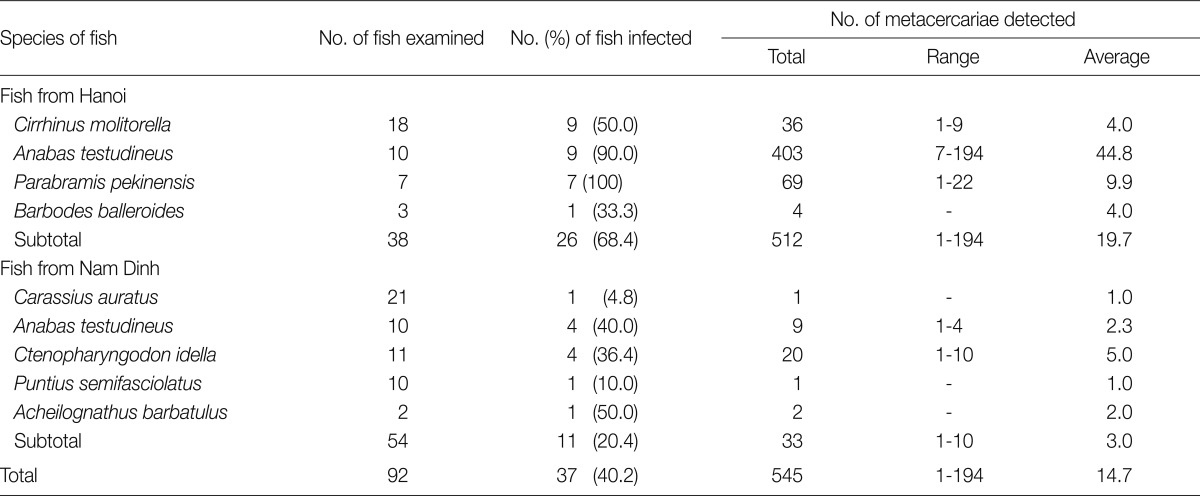
In fish from Hanoi, 3 species of metacercariae, H. pumilio, Centrocestus formosanus, and Procerovum varium were recovered. H. pumilio metacercariae were detected in 7 of 9 fish species examined. Among them, Ctenopharyngodon idella was most heavily infected (Table 2). C. formosanus metacercariae were found in 4 fish species, and Anabas testudineus revealed a relatively high infection rate and high metacercarial density (Table 3). P. varium metacercariae were detected in only 1 fish species, A. testudineus. The infection rate of this fish with P. varium metacercariae was 100%, and the average metacercarial density was 466 per fish (Table 4).
Table 2.
Haplorchis pumilio metacercarial infection in fish from local markets in Hanoi City and Nam Dinh Province, Vietnam
Table 3.
Centrocestus formosanus metacercarial infection in fish from local markets in Hanoi City and Nam Dinh Province, Vietnam
Table 4.
Procerovum varium metacercarial infection in fish from local markets in Hanoi City and Nam Dinh Province, Vietnam
FBT metacercariae in fish from Nam Dinh Province
In fish from Nam Dinh Province, 6 species of metacercariae, H. taichui, H. pumilio, C. formosanus, P. varium, S. falcatus, and Heterophyopsis continua, were recovered. H. taichui metacercariae (6 in total number) were detected in 3 (60%) of 5 Rasbora aurotaenia fish. H. pumilio metacercariae were detected in 8 of 9 fish species examined. Among them, A. testudineus revealed relatively higher prevalence (Table 2). C. formosanus metacercariae were recovered in 5 fish species, and their infection rate and densities were relatively low (Table 3). P. varium metacercariae were detected in 2 fish species (climbing perch A. testudineus and striped mullet Mugil cephalus). S. falcatus metacercariae were detected in all 10 M. cephalus, and the average metacercarial density was 84.4. Only 2 H. continua metacercariae were recovered in a Lindman's grenadier anchovy, Coilia lindmani.
Morphology of metacercariae (All measurement unit is µm)
H. taichui metacercariae (n=5) were elliptical, 190-220 (av. 205)×160-190 (175) in size, had a baseball glove-shaped ventrogenital sac with 11-18 rodlets and an O-shaped excretory bladder occupying the large portion of the posterior body. These metacercariae were identical with those from Laotian and Chinese fish [5,6].
H. pumilio metacercariae (n=20) were elliptical, 160-195 (179)×145-175 (159) in size, had 36-42 deer horn-like minute spines arranged in 1-2 rows around the ventrogenital complex, and an O-shaped excretory bladder occupying the large portion of the posterior body. They were identical with those from Chinese fish [6].
C. formosanus metacercariae (n=20) were elliptical, 155-190 (169)×123-150 (138) in size, had 32 circumoral spines around the oral sucker arranged in 2 rows, and a X-shaped excretory bladder occupying the greater portion of the posterior body. They were identical with those from Laotian fish [5].
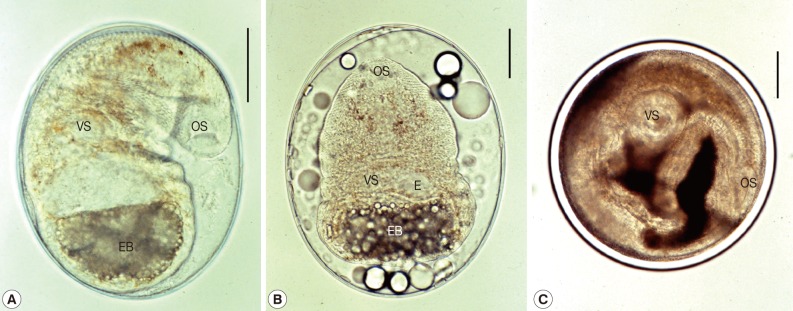
P. varium metacercariae (n=20) were elliptical, 165-208 (187)×115-163 (147) in size, had yellowish-brown pigment granules scattering in the area near the intestinal bifurcation, a pair of eyespots lateral to the pharynx, a submedian ventral sucker, a thick-walled bulb-like expulser, and a D-shaped (half moon-shaped) excretory bladder with grouped granules (Fig. 2A).
Fig. 2.
FBT metacercariae detected in fish hosts from Hanoi and Nam Dinh Province, Vietnam. A) P. varium metacercaria; elliptical and 187×147 µm in average size, having yellowish-brown pigment granules, a pair of eyespots, a muscular oral sucker (OS) and a submedian ventral sucker (VS), a thick-walled expulser, and a D-shaped excretory bladder (EB) with grouped granules. Scale bar=50 µm. B) S. falcatus metacercaria; elliptical and 297×232 µm in average size, having yellowish-brown pigment granules, a muscular oral sucker (OS), and a submedian ventral sucker (VS), a thick-walled expulser (E), and a V-shaped excretory bladder (EB). Scale bar=50 µm. C) H. continua metacercaria; round with relatively thick cyst wall and 458×453 µm in average size, having brownish pigments scattered all over the body, a muscular oral sucker (OS) and a ventral sucker (VS) located median, a genital sucker located just behind the ventral sucker, and a Y-shaped excretory bladder (EB). Scale bar=100 µm.
S. falcatus metacercariae (n=10) were elliptical, 255-330 (297)×225-250 (232) in size, had yellowish-brown pigment granules scattered all over the body, a submedian ventral sucker, a thick-walled bulb-like expulser, and a V-shaped excretory bladder (Fig. 2B).
H. continua metacercariae (n=2) were nearly round with relatively thick cyst wall, 440-475 (458)×435-470 (453) in size, had brownish pigment scattered all over the body, a ventral sucker located median, a genital sucker located just behind the ventral sucker, and a Y-shaped excretory bladder (Fig. 2C).
Morphology of adult flukes
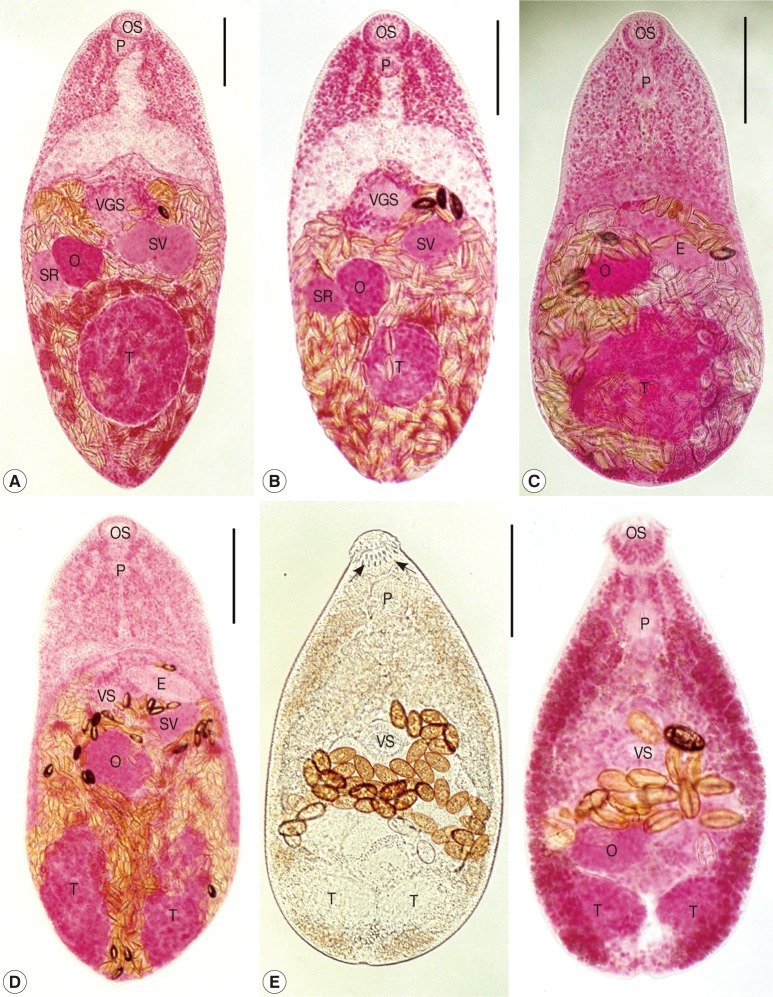
H. taichui (n=2; Fig. 3A): Body small, pear-shaped, 580-730 (av. 655) long and 300-310 (305) wide, with the greatest width at middle, the ovarian level. Oral sucker subterminal, 45 by 60 in size. Pharynx subglobular or elliptical, 35-38 (36) by 28-30 (29). Esophagus short, 75-95 (85) in length. Ventrogenital sac small with 11-18 rodlets, baseball glove-shaped, 88-100 (94) by 70-80 (75). Seminal vesicle saccular, bipartite, 113-125 (119) by 73-75 (74). Ovary spherical or subspherical, 85-88 (86) by 71-74 (73), dextral to midline. Seminal receptacle ellipsoidal, 80-88 (84) by 50-53 (51), lying at the right side of the ovary. Testis single, globular or subglobular, 160-193 (176) by 120-165 (143), lying in the posterior 1/4 of the body. Uterus with eggs occupying from the anterior 1/3 to the posterior end (most of the hind-body). Vitellaria follicular, distributing in post-ovarian fields. Eggs small, yellow, and 24-27 (26) by 12-14 (13).
Fig. 3.
Adult heterophyid flukes recovered from experimental hamsters at day 8 post-infection. All scale bars=100 µm. A) H. taichui adult. Body small, 655×305 µm in average size, having a muscular oral sucker (OS) and pharynx (P), a ventrogenital sac (VGS) armed with 11-18 rodlets, a saccular seminal vesicle (SV) and seminal receptacle (SR), a spherical ovary (O), single globular testis (T), and follicular vitellaria distributed in post-ovarian fields. B) H. pumilio adult. Body small, 496×217 µm in average size, having a muscular oral sucker (OS) and pharynx (P), a ventrogenital sac (VGS) equipped with 36-42 deer horn-like minute spines, a saccular seminal vesicle (SV) and seminal receptacle (SR), a spherical ovary (O), single globular testis (T), and follicular vitellaria distributed in post-ovarian fields. C) P. varium adult. Body small, 434×223 µm in average size, having a muscular oral sucker (OS) and a pharynx (P), a small ventral sucker and a long and thick-walled expulsor (E), a spherical ovary (O), single globular testis (T), and follicular vitellaria distributing in post-ovarian fields. D) S. falcatus adult. Body small, 481×239 µm in average size, having a muscular oral sucker (OS) and pharynx (P), a small ventral sucker (VS) and a long and thick-walled expulsor (E), a spherical ovary (O), 2 globular testes (T), and follicular vitellaria distributed in post-ovarian fields. E) C. formosanus adult (left, unstained; right, acetocarmine-stained). Body very small, 367×207 µm in average size, having an oral sucker (OS) armed with 32 circumoral spines (arrow marks), a muscular pharynx (P), a well-developed ventral sucker (VS), a spherical ovary (O), 2 globular testes (T), and follicular vitellaria distributed along extracecal margins from the pharyngeal level to the posterior end.
H. pumilio (n=15, Fig. 3B): Body small, pear-shaped, 415-550 (496) long and 195-245 (217) wide, with the greatest width at middle, the ovarian level. Oral sucker subterminal, 43-50 (46) by 50-58 (54). Pharynx subglobular or elliptical, 25-33 (29) by 20-30 (25). Esophagus short, 40-83 (57) in length. Ventrogenital sac small with 36-42 deer horn-like minute spines, 65-80 (74) by 50-75 (57). Seminal vesicle saccular, 28-100 (60) by 18-75 (45). Ovary spherical or subspherical, 55-70 (62) by 30-68 (52), slightly dextral to midline. Seminal receptacle elliptical, 38-85 (51) by 25-58 (38), lying at the right side of the ovary. Testis single, globular or subglobular, 65-103 (82) by 75-105 (85), lying at the posterior 1/4 of the body. Uterus with eggs occupying from the anterior 1/3 to the posterior end (most of the hind-body). Vitellaria follicular, distributing in post-ovarian fields. Eggs small, yellow, and 28-31 (30) by 15-18 (16).
P. varium (n=20, Fig. 3C): Body small, pear-shaped, 355-475 (434) long and 190-250 (223) wide, with the greatest width at posterior 1/3 of the body. Oral sucker subterminal, 35-43 (38) by 38-48 (42). Pharynx subglobular or elliptical, 23-30 (27) by 18-23 (20). Esophagus short, 33-75 (55) in length. Ventral sucker very small, 15-30 (25) by 18-33 (27), embedded in the ventrogenital sac. Expulsor long and thick-walled, 88-138 (115) by 25-35 (29). Ovary spherical or subspherical, 45-75 (59) by 38-70 (48), slightly dextral to midline. Seminal receptacle saccular, 45-83 (63) by 33-55 (44), lying at the right side of the testis. Testis single, globular or subglobular, 105-150 (127) by 100-180 (127), situated at the middle of the hind-body. Uterus with eggs occupying from the anterior 1/3 to the posterior end (most of the hind-body). Vitellaria follicular, distributing from the posterior border of the ovary to the posterior extremity. Eggs small, yellow, and 25-28 (26) by 11-15 (13).
S. falcatus (n=15, Fig. 3D): Body small, 400-650 (481) long and 200-300 (239) wide. Oral sucker subterminal, 30-43 (38) by 45-55 (49). Pharynx subglobular, 23-30 (27) by 20-38 (24). Esophagus slender, 45-118 (72) in length. Ventral sucker small, 25-30 (27) by 28-30 (29). Expulsor long and thick-walled, 75-100 (87) by 30-38 (33). Seminal vesicle saccate, 63-70 (66) by 33-40 (37). Ovary spherical, 63-100 (79) by 43-75 (62). Testes paired, ovoid or globular, slightly oblique and widely separated; right 83-180 (111) by 45-93 (62); left 83-188 (108) by 30-88 (55). Uterus with eggs occupying from the anterior 1/3 to the posterior end (most of the hind-body). Vitellaria follicular, distributing in the post-ovarian fields. Eggs small, yellow, and 20-23 (21) by 11-13 (12).
C. formosanus (n=15, Fig. 3E): Body very small, 320-470 (367) long and 170-235 (207) wide. Oral sucker subterminal, 35-43 (40) by 38-50 (42), armed with about 32 circumoral spines. Prepharynx very short, 10-25 (17) long. Pharynx globular, 30-43 (35) by 20-28 (23). Esophagus very short. Ventral sucker round or elliptical, 33-43 (37) by 45-53 (51). Ovary elliptical, 50-75 (59) by 30-53 (39), dextral to midline. Seminal receptacle large and saccular, 50-75 (63) by 40-55 (49). Two testes ellipsoidal, side by side near the posterior end; right 60-88 (72) by 33-50 (41), left 55-88 (66) by 30-43 (36). Vitellaria follicular, distributing along the extracecal margins from the pharyngeal level to the posterior end. Eggs small, yellow, and 29-33 (30) by 15-18 (17).
DISCUSSION
The 6 species of FBT metacercariae (H. taichui, H. pumilio, C. formosanus, P. varium, S. falcatus, and H. continua) detected in this study were all minute intestinal flukes and members of the Heterophyidae. Among them, H. pumilio was the dominant species. It is of note that Dung et al. [11] reported a high prevalence (64.9%) of small trematode eggs and also high prevalence for soil-transmitted helminths among residents of 2 communes in Nam Dinh Province, northern Vietnam, in April 2005. They recovered adult flukes of 6 trematode species (Clonorchis sinensis, H. pumilio, H. taichui, H. yokogawai, S. falcatus, and Fasciolopsis buski) from 33 peoples who revealed over 1,000 eggs per gram of feces (EPG) for small trematode eggs after praziquantel treatment and purgation. Among the 6 trematode species, H. pumilio was recovered in all 33 (100%) residents and the worm load averaged 416 per person [11]. Accordingly, it is presumed that more than 8 species of FBT, including H. pumilio, may be distributed in the northern part of Vietnam, although we could not find C. sinensis and H. yokogawai metacercariae.
Recently, studies on FBT metacercarial infections in Vietnam have been performed popularly. Thu et al. [12] surveyed on metacercarial infections in cultured catfish and snakeheads, and also in several wild fish species from An Giang Province, a major fish production area in the Mekong Delta of Vietnam in 2005-2006. Thien et al. [13] examined 13 major cultured fish species from Tien Giang Province and Can Tho City, located in central areas of the Mekong Delta of Vietnam, in 2005-2006. Hop et al. [14] examined FBT metacercarial infections in wastewater-fed aquaculture fish in northern Vietnam [14]. Chi et al. [15] performed a cross-sectional survey of FBT metacercariae in farmed fish in Nghe An Province, about 300 km south of Hanoi, in 2005 [15]. Vo et al. [16] surveyed 2 species of groupers (Epinephelus coioides and Epinepheles bleekeri), and mullet (M. cephalus) in a central coastal area of Vietnam (Khanh Hoa Province) in 2008 [16]. Phan et al. [17] surveyed on the infection status of FBT metacercariae in freshwater fish from small-scale farms (family-based household fish farm) in Nam Dinh Province in 2010 [17]. These studies focused mainly on metacercarial infections in commercially important fish species and in qualitative aspects. Studies on quantitative aspects of FBT metacercarial infections in each species of fish and thereby the suitability and susceptability of fish hosts for each FBT species have not been available.
In the present study, H. pumilio metacercariae were detected in 104 (80.0%) of 130 fish examined, and the metacercarial density was 64.2 per fish infected. The metacercarial density was higher in fish from Hanoi (av. 129 metacercariae) than those from Nam Dinh Province (av. 15 metacercariae). The most heavily infected fish with H. pumilio metacercariae was the grass carp (Ctenopharyngodon idella) caught from Hanoi. The high susceptibility of this fish species was also shown in the study of Phan et al. [17] which was performed in Nam Dinh Province [17]. A similar finding was shown in Guangxi Zhuang Autonomous Region located in the southern part of China, slightly north of our surveyed area [6]. In this area of China, 18 fish species (Carassius auratus, Acheilognathus tonkinensis, Hemibarbus maculatus, Hypophthalmichthys molitrix, Hemiculter leucisculus, C. idella, Toxabramis houdemeri, Microphysogobio fukiensis, Pseudohemiculter dispar, Opsariichthys bidens, Squalidus argentatus, Metzia lineata, Cyprinus carpio, Puntius semifasciolatus, Saurogobio dabryi, Culter recurviceps, Chanodichthys dabryi, and Pseudorasbora parva) were listed as hosts for H. pumilio [6]. On the other hand, in Nam Dinh Province of Vietnam, Phan et al. [17] listed 17 fish species (Labeo rohita, H. molitrix, Cirrhinus mrigala, C. idella, C. auratus, Squaliobarbus curriculus, Piaractus brachypomum, C. carpio, Cirrhinus molitorella, Hypophthalmichthys nobilis, Barbonymus gonionotus, H. leucisculus, Anabas testudineus, Oreochromis niloticus, Clarias batrachus, Channa orientalis, and Notopterus notopterus) for the host for H. pumilio. In the present study, 7 more fish species (Acheilognathus barbatulus, Albulichthys albuloides, Barbodes balleroides, Coilia lindmani, Parabramis pekinensis, Rasbora aurotaenia, and Trichogaster trichopterus) have been newly added as the host for H. pumilio.
C. formosanus metacercariae were detected in 8 fish species (A. barbatulus, A. testudineus, B. balleroides, C. auratus, C. molitorella, C. idella, P. pekinensis, and P. semifasciolatus) in the present study. Phan et al. [17] detected the same species of metacercariae in 10 fish species (L. rohita, H. molitrix, C. mrigala, C. idella, S. curriculus, P. brachypomum, C. carpio, C. batrachus, C. orientalis, and A. testudineus) in Nam Dinh Province [17]. In China, Sohn et al. [6] reported 10 fish species (M. fukiensis, A. tonkinensis, S. argentatus, C. carpio, H. molitrix, A. rivularis, H. leucisculus, M. lineata, S. dabryi and P. parva) as the fish hosts for C. formosanus [6]. Therefore, it appears that more than 24 fish species play the role of second intermediate hosts for C. formosanus in Vietnam and China.
It has been known that 2 species of liver flukes (Clonorchis sinensis and Opisthorchis viverrini) distribute in Vietnam. C. sinensis is distributed in the northern part, whereas O. viverrini is found in the southern part. However, the metacercariae of these liver flukes were only rarely detected in fish from Vietnam. In An Giang Province, a southern part, only 19 metacercariae of O. viverrini were detected in 1.9% of 108 fish examined [12]. Thien et al. [13] could not find any liver fluke metacercariae in all 13 important cultured fish species from Tien Giang Province and Can Tho City, in the Mekong Delta of Vietnam [13]. No liver fluke metacercariae were detected in a cross-sectional survey of FBT metacercariae in farmed fish from Nghe An Province [15]. Meanwhile, only a small number of C. sinensis metacercariae were found in 1 of 1,185 silver carps (H. molitrix) examined in small-scale farms in Nam Dinh Province. In the present study, we could not find any C. sinensis metacercariae in fish from the same study area. By contrast, Dung et al. [11] reported a high positive rate (51.5%) of C. sinensis adult worms (their worm load was not so high; 1-18 per individual) from 33 residents with over 1,000 EPG for small trematode eggs in Nam Dinh Province [11]. This discrepancy between the metacercarial infection in fish hosts and the adult fluke infections in humans should be clarified in the near future.
In the present study, P. varium metacercariae were detected in 2 fish species (A. testudineus and M. cephalus), although these metacercariae were previously recorded in 9 fish species (L. rohita, H. molitrix, C. mrigala, C. idella, S. curriculus, P. brachypomum, C. batrachus, B. gonionotus, and A. testudineus) in Nam Dinh Province [17]. Vo et al. [16] also detected P. varium metacercariae together with 2 other heterophyid metacercariae (H. continua and P. summa) in 2 species of groupers and mullet from Khanh Hoa Province in Vietnam. On the other hand, S. falcatus and H. continua metacercariae were found in M. cephalus and C. lindmani, respectively, in the present study.
Although morphologic characteristics of FBT metacercariae had previously been reported, we redescribed some of their characteristic features to provide a useful aid for epidemiologic studies in Southeast Asian countries. Among the FBT metacercariae detected in our study, 3 species (H. taichui, H. pumilio, and C. formosanus) were morphologically identical with those from China and Lao PDR [5,6]. On the other hand, the metacercariae of P. varium (187×147 µm) were smaller than those (210×180) reported in a freshwater fish (Oryzias melastigma) from Visakhapatnam, India [19]. However, our P. varium metacercariae were almost identical with those found in groupers from Nha Trang district in Khanh Hoa Province, Vietnam [16]. With regard to S. falcatus metacercariae, they were previously detected in cultured giant gouramy (Osphronemus gourami) from Tien Giang Province and Can Tho City [13], and also in the common carp (C. carpio) and grass carp (C. idella) from Nghe An Province, Vietnam [15]. However, no morphologic descriptions were available on S. falcatus metacercariae in Vietnam. H. continua metacercariae (458×453 µm) detected in a C. lindmani fish in the present study were similar in shape with but larger than those (380 in diameter) recovered in mullets from Khanh Hoa Province [16]. In the Republic of Korea, it is agreed that H. continua metacercariae detected in various fish species (Laterolabrax japonicus, Acanthogobius flavimanus, Clupanodon punctatus, Plecoglossus altivelis, Conger myriaster, Boleophthalmus pectinirostris, and Scartelaos sp.) show wide size ranges by fish hosts examined [20].
Among the FBT species distributed in Vietnam, 2 species of liver flukes (C. sinensis and O. viverrini) are highly important in clinical and pathological aspects, although their metacercariae were not detected in this study. Being similar in general shape, the liver fluke metacercariae can be distinguished from those of heterophyid flukes by the presence of a large well-developed ventral sucker which is nearly equal in size with the oral sucker. The ventral suckers of H. taichui, H. pumilio, C. formosanus, P. varium, and S. falcatus are characteristically smaller than their oral suckers. Nevertheless, when these metacercariae were mixed together, it is not easy to distinguish them unless each metacercaria is subjected to a detailed observation under a light microscope.
The experimentally obtained adults of the 5 species of FBT were morphologically compatible with those previously reported. Dung et al. [11] only briefly mentioned on the adult worm morphologies of H. pumilio, H. taichui, H. yokogawai, and S. falcatus after recovery in residents of Nam Dinh Province, Vietnam. The size of H. taichui (756×421) and H. pumilio (632×291) adults obtained from residents were slightly larger than those from our study, whereas the adult of S. falcatus (468×298) from residents was smaller in size than our specimens [11].
ACKNOWLEDGMENTS
We thank the staff of the Fishborne Zoonotic Parasites in Vietnam (FIBOZOPA project), in particular, Dr. K. Darwin Murrell, Mr. Jesper Clausen, and Mr. Bui Thanh, for their kind cooperation. We also thank Jung-A Kim, Department of Parasitology, Gyeongsang National University School of Medicine, Jinju, Korea, for her help in fish examinations.
References
- 1.WHO. Food-borne trematode infections in Asia. Report of Joint WHO/FAO Workshop. Hanoi. Vietnam: 2002. [Google Scholar]
- 2.Chai JY, Murrell KD, Lymbry A. Fishborne parasitic zoonoses: Status and issues. Int J Parasitol. 2005;35:1233–1254. doi: 10.1016/j.ijpara.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Chai JY. Intestinal flukes. In: Murrell KD, Fried B, editors. Food-Borne Parasitic Zoonoses. New York, NY, USA: Springer; 2007. pp. 53–115. [Google Scholar]
- 4.Scholz T, Ditrich O, Giboda M. Larval stages of medically important flukes (Trematoda) from Vientiane Province, Laos. Part I. Metacercariae. Ann Parasitol Hum Comp. 1990;65:238–243. doi: 10.1051/parasite/199267375. [DOI] [PubMed] [Google Scholar]
- 5.Rim HJ, Sohn WM, Yong TS, Eom KS, Chai JY, Min DY, Lee SH, Hoang EH, Phommasack B, Insisengmay S. Fishborne trematode metacercariae detected in freshwater fish from Vientiane Municipality and Savannakhet Province, Lao PDR. Korean J Parasitol. 2008;46:253–260. doi: 10.3347/kjp.2008.46.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sohn WM, Eom KS, Min DY, Rim HJ, Hoang EH, Yang Y, Li X. Fishborne trematode metacercariae in freshwater fish from Guangxi Zhuang Autonomous Region, China. Korean J Parasitol. 2009;47:249–257. doi: 10.3347/kjp.2009.47.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rim HJ, Sohn WM, Yong TS, Eom KS, Chai JY, Min DY, Lee SH, Hoang EH, Phommasack B, Insisengmay S. Fishborne trematode metacercariae in fish from three Provinces, Luang Prabang, Khammuane, and Salavan, in Lao PDR. Korean J Parasitol. 2013;51 doi: 10.3347/kjp.2013.51.1.107. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rim HJ, Chai JY, Min DY, Cho SY, Eom KS, Hong SJ, Sohn WM, Yong TS, Deodato G, Standgaard H, Phommasack B, Yun CY, Hoang EH. Prevalence of intestinal parasite infections on a national scale among primary schoolchildren in Laos. Parasitol Res. 2003;91:267–272. doi: 10.1007/s00436-003-0963-x. [DOI] [PubMed] [Google Scholar]
- 9.Chai JY, Park JH, Han ET, Guk SM, Lin A, Kim JL, Sohn WM, Yong TS, Eom KS, Min DY, Hwang EH, Phommasack B, Insisiengmay B, Rim HJ. Mixed infections with Opisthorchis viverrini and intestinal flukes in residents of Vientiane Municipality and Saravane Province in Laos. J Helminthol. 2005;79:283–289. doi: 10.1079/joh2005302. [DOI] [PubMed] [Google Scholar]
- 10.Chai JY, Han EK, Guk SM, Shin EH, Sohn WM, Yong TS, Eom KS, Lee KH, Jeong HG, Ryong YS, Hoang EH, Phommasack B, Insisiengmay B, Lee SH, Rim HJ. High prevalence of liver and intestinal fluke infection among residents of Savannakhet Province in Laos. Korean J Parasitol. 2007;45:213–218. doi: 10.3347/kjp.2007.45.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dung DT, De NV, Waikagul J, Dalsgaard A, Chai JY, Sohn WM, Murrell KD. Fishborne intestinal zoonotic trematodiasis, Vietnam. Emerg Infect Dis. 2007;13:1828–1833. doi: 10.3201/eid1312.070554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thu ND, Loan TT, Dalsgaard A, Murrell KD. Survey for zoonotic liver and intestinal trematode metacercariae in cultured and wild fish in An Giang Province, Vietnam. Korean J Parasitol. 2007;45:45–54. doi: 10.3347/kjp.2007.45.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thien PC, Dalsgaard A, Bui NT, Olsen A, Murrell KD. Prevalence of fishborne zoonotic parasites in important cultured fish species in the Mekong Delta, Vietnam. Parasitol Res. 2007;101:1277–1284. doi: 10.1007/s00436-007-0633-5. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen TH, De NV, Murrell KD, Dalsgaard A. Occurrence and species distribution of fishborne zoonotic trematodes in wastewater-fed aquaculture in northern Vietnam. Trop Med Int Health. 2007;12:66–72. doi: 10.1111/j.1365-3156.2007.01943.x. [DOI] [PubMed] [Google Scholar]
- 15.Chi TTK, Dalsgaard A, Turnbull JF, Tuan PA, Murrell KD. Prevalence of zoonotic trematodes in fish from a Vietnamese fish farming community. J Parasitol. 2008;94:423–428. doi: 10.1645/GE-1389.1. [DOI] [PubMed] [Google Scholar]
- 16.Vo DT, Murrell D, Dalsgaard A, Bristow G, Nguyen DH, Bui TN, Vo DT. Prevalence of zoonotic metacercariae in two species of grouper, Epinephelus coioides and Epinephelus bleekeri, and flathead mullet, Mugil cephalus, in Vietnam. Korean J Parasitol. 2008;46:77–82. doi: 10.3347/kjp.2008.46.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phan VT, Ersbøll AK, Nguyen KV, Madsen H, Dalsgaard A. Farm-level risk factors for fishborne zoonotic trematode infection in integrated small-scale fish farms in northern Vietnam. PLoS Negl Trop Dis. 2010;4:e742–e750. doi: 10.1371/journal.pntd.0000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Search FishBase. http://www.fishbase.org/search.php.
- 19.Umadevi K, Madhavi R. Observations on the morphology and life-cycle of Procerovum varium (Onji & Nishio, 1916) (Trematoda: Heterophyidae) Syst Parasitol. 2000;46:215–225. doi: 10.1023/a:1006398205390. [DOI] [PubMed] [Google Scholar]
- 20.Sohn WM. Fish-borne zoonotic trematode metacercariae in the Republic of Korea. Korean J Parasitol. 2009;47(suppl):S103–S113. doi: 10.3347/kjp.2009.47.S.S103. [DOI] [PMC free article] [PubMed] [Google Scholar]