Abstract
Monthly surveys were conducted to investigate the occurrence of chigger mites and seroprevalence of scrub typhus among small mammals in Jeollanam-do, the southwestern part of Korea, from November 2006 through October 2007. Fifty-eight small mammals, including 57 Apodemus agrarius (98.3%) and 1 Crocidura lasiura (1.7%), were captured, and a total of 4,675 chigger mites representing 4 genera and 8 species were collected from them. The chigger infestation rate among small mammals was 69.0%. The most predominant species in A. agrarius was Leptotrombidium scutellare (54.0%), followed by Leptotrombidium pallidum (39.4%), Leptotrombidium orientale (4.4%), Leptotrombidium palpale (1.1%), Neotrombicula tamiyai (0.6%), Eushoengastia koreaensis (0.3%), Neotrombicula gardellai (0.3%), and Cheladonta ikaoensis (<0.1%). The chigger index of A. agrarius was the highest in October (740.0), followed by November (242.0), September (134.6), March (98.3), February (38.2), January (35.3), December (34.5), April (30.8), and May (1.7). The average antibody positive rate of scrub typhus in wild rodents was 50.0%. The seropositive rates were high in October (100.0%) and November (83.3%), whereas those in other months were relatively low (28.6-57.1%). The chigger index of L. scutellare rapidly increased in September to form an acuminate peak in October, followed by a gradual decline. These results suggest that the outbreak of scrub typhus in the southwestern part of Korean peninsula is mostly due to L. scutellare.
Keywords: Leptotrombidium scutellare, Leptotrombidium pallidum, Apodemus agrarius, scrub typhus, monthly occurrence
INTRODUCTION
Scrub typhus (tsutsugamushi disease) caused by the obligate intracellular bacterium Orientia tsutsugamushi is an acute febrile infectious disease transmitted through larval bites of trombiculid mites, also known as chigger mites [1,2]. O. tsutsugamushi is naturally maintained with the cycles of chigger mites and wild rodents. It can be transovarially transmitted in chigger mites [3,4]. Scrub typhus is endemic in geographic regions, including Korea, China, Japan, Thailand, and other Asian countries [1,2]. Since the first Korean case of scrub typhus was reported in 1985 [5], the annual number of cases has increased to more than 5,000 in 2005-2008 [6]. The southwestern and middle-western parts of Korea, such as Jeollanam-do, Jeollabuk-do, and Chungcheongnam-do, showed higher prevalence than other areas [7]. Although several epidemiological data showing relative abundance of vector mites, their hosts, and geographic distribution were published, there are no reports demonstrating monthly occurrence of chigger mites and wild rodents during a whole year in the Republic of Korea. The present study was conducted to identify monthly population dynamics of chigger mites and rodents, and seroprevalence rates of scrub typhus in wild rodents in an endemic area of Jeollanam-do which is located in the southwestern part of the Korean peninsula.
MATERIALS AND METHODS
Collection localities
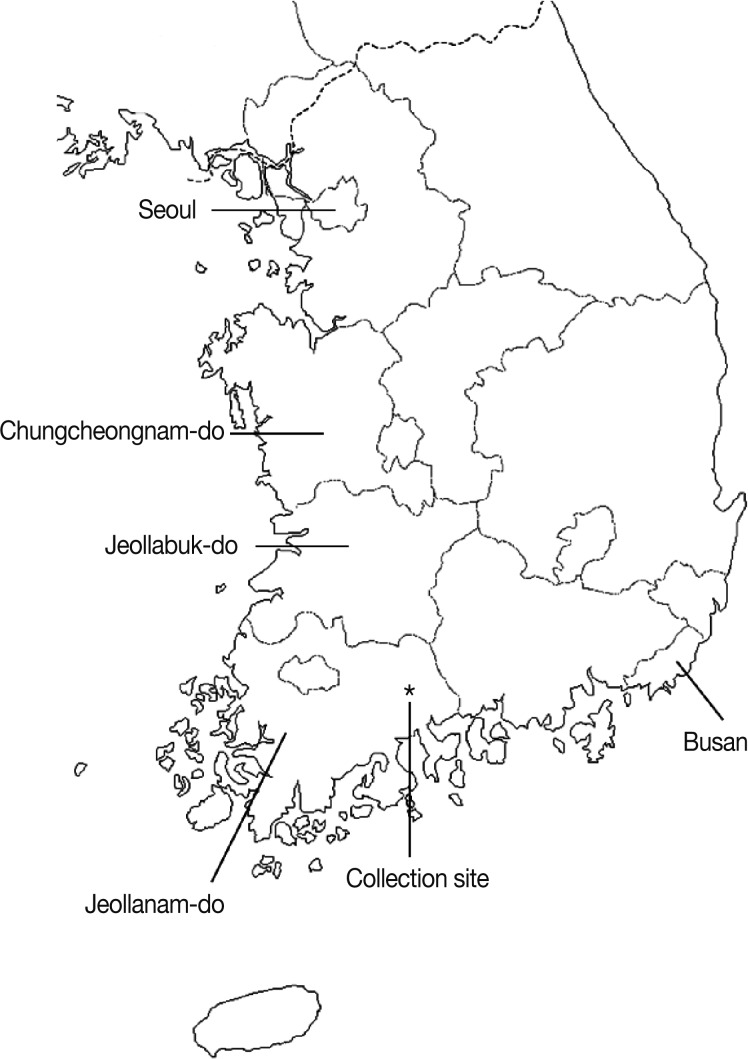
Monthly surveillance of field rodents was conducted from November 2006 through October 2007. The collection site was located in an endemic region of scrub typhus in Hoeryong-ri, Hwangjeon-myeon, Suncheon-si, Jeollanam-do, Korea (127° 30'12.8''E, 35°36'11.5''N) (Fig. 1). The monthly average temperature and precipitation in Jeollanam-do was based on the data of Korea Meteorological Administration.
Fig. 1.
Map of the collection site.
Collection of field rodents and chigger mites
Field rodents were captured by Sherman live traps (7.6×8.9×22.9 cm; H.B. Sherman, Tallahassee, Florida, USA) baited with peanut butter placed between 2 saltine crackers. The traps were set up before sunset and collected early in the next morning. Live-captured rodents were transported to the laboratory, where they were anesthetized. After identifying the species, their blood was taken for detection of antibodies. The bodies of the rodents were hung individually over a 1,000 ml beaker filled to a depth of 1 cm with tap water for harvesting the larval mites. The mites which fell into the water were removed with a fine brush and placed in 75% ethanol until mounted on slides with polyvinyl alcohol media. The larval mites were identified under a light microscope using morphological keys prepared by Ree [8].
Detection of O. tsutsugamushi-specific antibodies
Blood samples were centrifuged at 1,000 g for 10 min, and sera were maintained at -70℃ until assayed for the presence of antibodies against O. tsutsugamushi. The sera were diluted 1:32 in PBS and examined for IgG antibodies against O. tsutsugamushi Karp and Gilliam stains by the indirect immunofluorescence assay (IFA). The IFA antigen slide was placed in a moist chamber to maintain the humidity throughout the procedure. Diluted sera to be tested were deposited on a spot slide and incubated at 37℃ for 30 min. After washing 3 times with PBS for 5 min, fluorescein isothiocyanate-conjugated goat anti-mouse antibody (MP Biomedicals, Aurora, Ohio, USA) was pipetted onto each spot, and the slide was incubated in a humidified chamber at 37℃ for 30 min. The slide was washed 3 times each for 5 min with PBS and then air-dried. The slide spots were mounted with glycine-buffered glycerol under cover slips and examined for characteristic cytoplasmic fluorescent patterns with a fluorescence microscope (50W, Zeiss Co, Mainz, Germany).
RESULTS
Monthly occurrence of field rodents and chigger mites
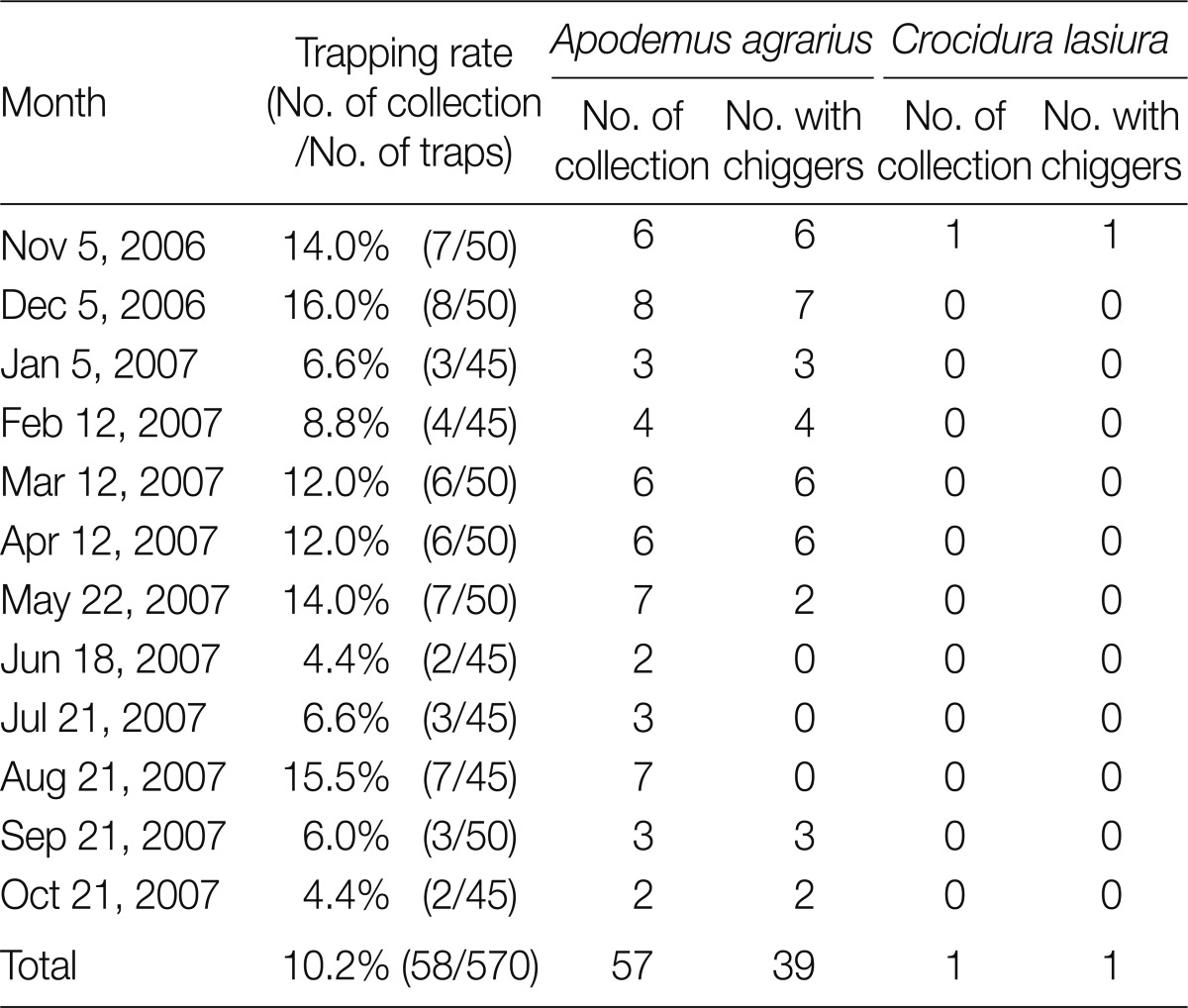
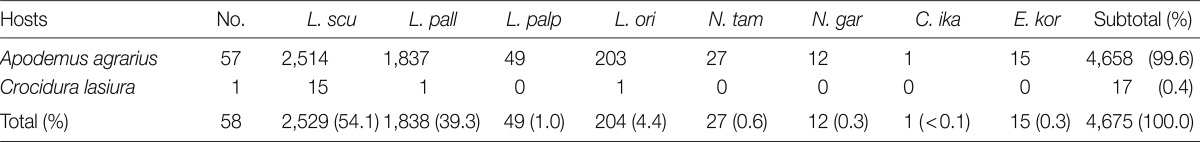
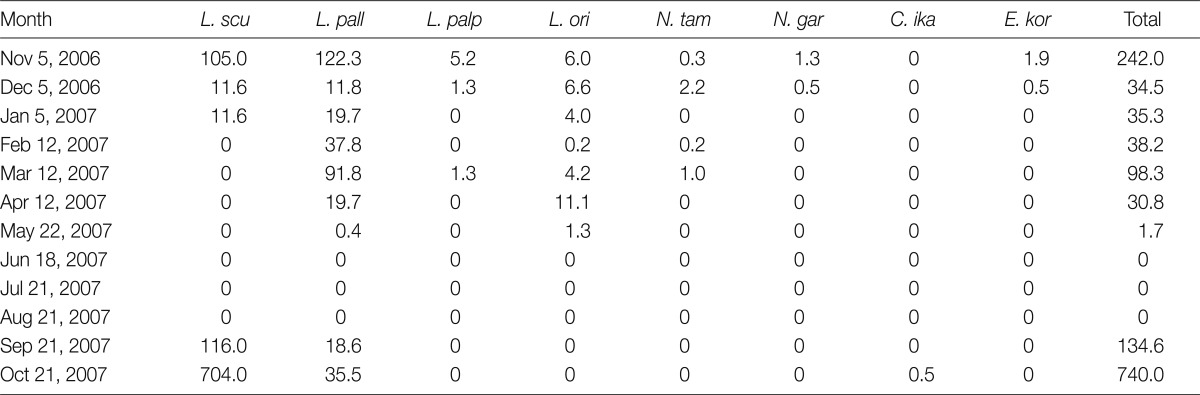
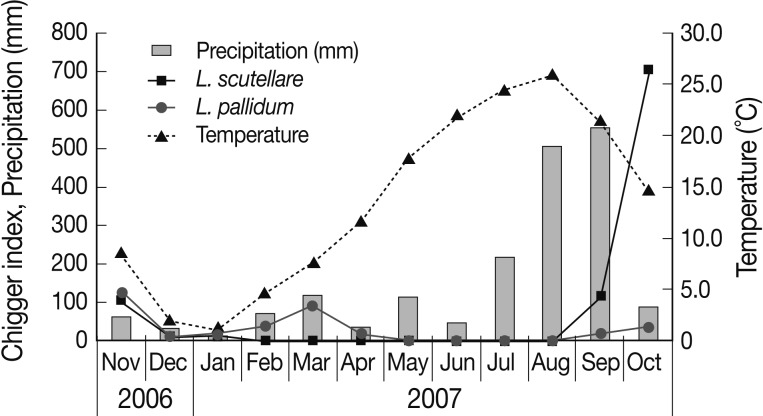
A total of 58 small mammals representing 2 genera and 2 species were collected. The common striped field mouse, Apodemus agrarius (98.3%) was predominantly collected, while only 1 insectivore Crocidura lasiura (1.7%) was collected. Thirty-nine of 57 (68.4%) A. agrarius and 1 C. lasiura were infested with larval mites. The monthly trapping rates ranged from 4.4% to 16.0% (Table 1). In total, 4,675 chiggers, representing 4 genera and 8 species, were collected from small mammals. Of 4,658 chiggers (99.6%) collected from A. agrarius, Leptotrombidium scutellare was the most predominant species (54.0%), followed by Leptotrombidium pallidum (39.4%), Leptotrombidium orientale (4.4%), Leptotrombidium palpale (1.1%), Neotrombicula tamiyai (0.6%), Eushoengastia koreaensis (0.3%), Neotrombicula gardellai (0.3%), and Cheladonta ikaoensis (<0.1%) (Table 2). The monthly occurrence and chigger indices of each chigger mite from A. agrarius are shown in Table 3 and Fig. 2. The chigger index was the highest in October (740.0), followed by November (242.0), September (134.6), March (98.3), February (38.2), January (35.3), December (34.5), April (30.8), and May (1.7). There was no chigger during the summer season (June-August). The chigger index of L. scutellare increased rapidly in September to form an acuminate peak in October, followed by a gradual decline, whereas that of L. pallidum reached a peak in November.
Table 1.
Monthly trapping rates and chigger infestation status of field small mammals in Jeollanam-do from November 2006 through October 2007
Table 2.
Number of chigger mites by species collected from small mammals in Jeollanam-do from November 2006 through October 2007
L. scu: Leptotrombidium scutellare; L. pall: L. pallidum; L. palp: L. palpale; L. ori: L. orientale; N. tam: Neotrombicula tamiyai; N. gar: N. gardellai; C. ika: Cheladonta ikaoensis; E. kor: Eushoengastia koreaensis.
Table 3.
Monthly chigger indices by species collected from A. agrarius in Jeollanam-do from November 2006 through October 2007
L. scu: Leptotrombidium scutellare; L. pall: L. pallidum; L. palp: L. palpale; L. ori: L. orientale; N. tam: Neotrombicula tamiyai; N. gar: N. gardellai; C. ika: Cheladonta ikaoensis; E. kor: Eushoengastia koreaensis.
Fig. 2.
Monthly average temperature, precipitation, and chigger indices of A. agrarius in Jeollanam-do from November 2006 through October 2007.
O. tsutsugamushi-specific antibodies in field rodents
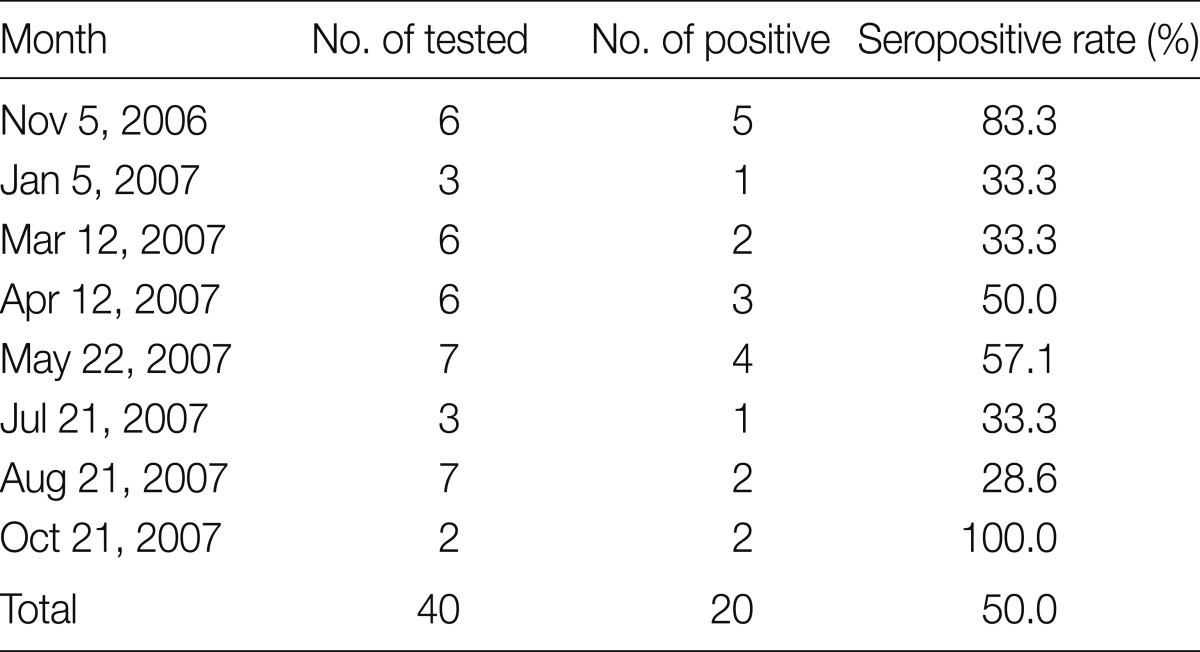
Of 40 A. agrarius, 20 (50.0%) were positive for antibodies to O. tsutsugamushi. Seropositive rates of scrub typhus were higher in October (100%) and November (83.3%), whereas those in other months were relatively lower (28.6-57.1%) (Table 4).
Table 4.
Seropositive rates of scrub typhus in A. agrarius in Jeollanam-do from November 2006 through October 2007
DISCUSSION
Scrub typhus is regarded as one of the most widespread diseases in Korea which occur mainly in autumn. In this study, we have shown that A. agrarius (98.3%) accounted for nearly all of the live-captured small mammals in the field. This agrees to previous results that A. agrarius was predominant in rural areas of the Korean peninsula [5,9-11]. Leptotrombidium species, including L. pallidum, L. scutellare, L. palpale, and L. orientale, were known as vectors of scrub typhus in Korea [5,12,13], of which L. pallidum was predominant in the middle and northern parts of Korea, whereas L. scutellare was predominant in the southern parts of Korea [12,13]. Our data showed that L. scutellare was the dominant species in Jeollanam-do, followed by L. pallidum.
The monthly prevalence of scrub typhus in an endemic region, Jeollanam-do, from November 2006 through October 2007 was shown in Table 5 [6]. In Jeollanam-do, the outbreaks increased drastically in October, peaked in November, and started to decrease in December, when the coldest season begins. Of 617 cases, 598 (96.9%) were reported during these 3 months, while several cases were reported in other months. In the present study, 50% of A. agrarius were positive for O. tsutsugamushi-specific antibodies, and the seropositive rate in A. agrarius was higher in autumn (November and October) than in other seasons (Table 4). L. scutellare and L. pallidum are active and take meals in cool dry autumn conditions. These findings indicate that A. agrarius is the main reservoir animal of scrub typhus. The chigger index was also the highest in October (740.0), followed by November (242.0) (Table 3). The discrepancy of the peak period between the outbreaks of scrub typhus and the population density of chiggers might be due to the incubation period of the disease. Scrub typhus has an incubation period of 5-20 days [1].
Table 5.
Monthly prevalence of scrub typhus in Jeollanam-do from November 2006 through October 2007
The chigger index of L. scutellare in the autumn season (September and October) was higher than that of other species, while none were collected from February through August (Table 3). The chigger index of L. palpale, L. orientale, and E. koreaensis, which are also known as vectors of scrub typhus, was quite low in the autumn season. In October, the chigger index of L. scutellare in Jeollanam-do was 704.0, whereas those in Jeollabuk-do which is located in the middle-western part of the Korean peninsula ranged from 19.4 to 77.6 [14].
The population density of L. scutellare in Jeollanam-do was 10 times higher than that in Jeollabuk-do. The annual mean air temperature of the collection site was 13.5℃ (Fig. 2). These results support the hypothesis that the most important limited factor for L. scutellare distribution is annual mean air temperature above 10℃ [13]. Previous reports indicated that the infection rate of O. tsutsugamushi among chiggers in Korea was the highest for L. palpale (5.3%), followed by Neotrombicula japonica (4.3%), L. scutellare (3.7%), L. orientale (3.6%), E. koreaensis (1.9%), and L. pallidum (1.5%) [15]. Based on our results and also previous studies, we suggest that the outbreak of scrub typhus in autumn is mainly by L. scutellare, whereas a few cases in other seasons are possibly by other species, including L. pallidum, L. palpale, L. orientale, and E. koreaensis. The incidence of scrub typhus was positively correlated with temperature and humidity during summer [7]. The arthropod vectors of scrub typhus are ectothermic. Temperature and precipitation can strongly influence their development, reproduction, and population dynamics [16]. As shown in Fig. 2, the chigger index was increased after the hot and humid summer. Because of global warming, L. scutellare could expand its distribution to middle or northern parts of the Korean peninsula, and its abundance and population peak could be changed.
The epidemiological study on monthly distributions and population densities of the vectors and reservoir hosts of scrub typhus will be helpful to establish strategies for monitoring the disease transmission, management, and preventive plans.
ACKNOWLEDGMENTS
We are grateful to Dr. Heung Chul Kim, the 5th Medical Detachment, 168th Multifunctional Medical Battalion, US army MDDAC-Korea, for his assistance in data analysis. This work was supported by Konkuk University in 2012.
References
- 1.Seong SY, Choi MS, Kim IS. Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect. 2001;3:11–21. doi: 10.1016/s1286-4579(00)01352-6. [DOI] [PubMed] [Google Scholar]
- 2.Bang HA, Lee MJ, Lee WC. Comparative research on epidemiological aspects of tsutsugamushi disease (scrub typhus) between Korea and Japan. Jpn J Infect Dis. 2008;61:148–150. [PubMed] [Google Scholar]
- 3.Duong V, Blassdell K, May TT, Sreyrath L, Gavotte L, Morand S, Frutos R, Buchy P. Diversity of Orientia tsutsugamushi clinical isolates in Cambodia reveals active selection and recombination process. Infect Genet Evol. 2010 doi: 10.1016/j.meegid.2010.08.015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Chang WH. Current status of tsutsugamushi disease in Korea. J Korean Med Sci. 1995;10:227–238. doi: 10.3346/jkms.1995.10.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ree HI, Lee IY, Cho MK. Determination of the vector species of tsutsugamushi disease in Korea. Korean J Parasitol. 1991;29:87–92. doi: 10.3347/kjp.1991.29.1.87. [DOI] [PubMed] [Google Scholar]
- 6.Korea Centers for Disease Control and Prevention. Diseases Web Statistics System 2009. http://stat.cdc.go.kr/
- 7.Kim SH, Jang JY. Correlations between climate change-related infectious diseases and meteorological factors in Korea. J Prev Med Public Health. 2010;43:436–444. doi: 10.3961/jpmph.2010.43.5.436. [DOI] [PubMed] [Google Scholar]
- 8.Ree HI. Fauna and key to the chigger mites of Korea (Acarina: Trombiculidae and Leeuwenhoekiidae) Korean J Syst Zool. 1990;6:57–70. [Google Scholar]
- 9.Ree HI, Kim TE, Lee IY, Jeon SH, Hwang UW, Chang WH. Determination and geographical distribution of Orientia tsutsugamushi serotypes in Korea by nested polymerase chain reaction. Am J Trop Med Hyg. 2001;65:528–534. doi: 10.4269/ajtmh.2001.65.528. [DOI] [PubMed] [Google Scholar]
- 10.Kim HC, Lee IY, Chong ST, Richards AL, Gu SH, Song JW, Lee JS, Klein TA. Serosurveillance of scrub typhus in small mammals collected from military training sites near the DMZ, Northern Gyeonggi-do, Korea, and analysis of the relative abundance of chiggers from mammals examined. Korean J Parasitol. 2010;48:237–243. doi: 10.3347/kjp.2010.48.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HC, Yang YC, Chong ST, Ko SJ, Lee SE, Klein TA, Chae JS. Detection of Rickettsia typhi and seasonal prevalence of fleas collected from small mammals in the Republic of Korea. J Wildl Dis. 2010;46:165–172. doi: 10.7589/0090-3558-46.1.165. [DOI] [PubMed] [Google Scholar]
- 12.Ree HI, Lee IY, Cho MK. Study on vector mites of tsutsugamushi disease in Cheju Island, Korea. Korean J Parasitol. 1992;30:341–348. doi: 10.3347/kjp.1992.30.4.341. [DOI] [PubMed] [Google Scholar]
- 13.Ree HI, Lee IY, Jeon SH, Yoshida Y. Geographical distribution of vectors and sero-strains of tsutsugamushi disease at mid-south inland of Korea. Korean J Parasitol. 1997;35:171–179. doi: 10.3347/kjp.1997.35.3.171. [DOI] [PubMed] [Google Scholar]
- 14.Lee IY, Ree HI, Hong HK. Seosonal prevalence and geographical distribution of Trombiculid mites (Acarina: Trombiculidae) in Korea. Korean J Zool. 1993;36:408–415. [Google Scholar]
- 15.Lee HI, Shim SK, Song BG, Choi EN, Hwang KJ, Park MY, Park C, Shin EH. Detection of Orientia tsutsugamushi, the causative agent of scrub typhus, in a novel mite species, Eushoengastia koreaensis, in Korea. Vector Borne Zoonotic Dis. 2011;11:209–214. doi: 10.1089/vbz.2009.0180. [DOI] [PubMed] [Google Scholar]
- 16.Gage KL, Burkot TR, Eisen RJ, Hayes EB. Climate and vectorborne diseases. Am J Prev Med. 2008;35:436–450. doi: 10.1016/j.amepre.2008.08.030. [DOI] [PubMed] [Google Scholar]