Abstract
Background
Per current guidelines, patients with a first-degree relative (FDR) with adenomas should get screened at age 40. Data on the prevalence of adenomas and advanced adenomas (AAs) in these patients are lacking.
Objective
To examine the prevalence of adenomas and AAs in 40- to 49-year-old individuals undergoing screening colonoscopy because of a family history (FH) of polyps and to compare these data with those of a control population of similar age.
Design
Retrospective cross-sectional study.
Setting
Tertiary care academic medical center and Veterans Affairs medical center.
Patients
Study subjects included all 40- to 49-year-old asymptomatic individuals undergoing initial screening colonoscopy at our institution from January 1, 2006, to June 1, 2009, because of an FDR with polyps. The control population consisted of all 40- to 49-year-old individuals who underwent their first colonoscopy during the same period because of abdominal pain, diarrhea, or constipation without an FH of polyps or colorectal cancer.
Intervention
Colonoscopy.
Main Outcome Measurements
The prevalence of adenomas of any size, AAs, and risk factors associated with adenomas.
Results
The prevalence of adenomas was greater in the FH of polyps group (n = 176) compared with the control sample (n = 178) (26.7% vs 13.5%; P = .002) but was not statistically greater for AAs (5.7% vs 3.4%; P = .3). After adjusting for confounders, FH of a polyp was associated with an increased prevalence of adenomas (odds ratio 2.8 [95% CI, 1.4 –5.5]).
Limitations
Limited data on polyp histology in FDRs and limited sample size.
Conclusions
Among 40- to 49-year-old patients undergoing screening colonoscopy because of an FDR with polyps, the prevalence of adenomas was greater than in a control population. Prospective research is needed to quantify the prevalence of AAs in this group and to determine whether these individuals should undergo screening colonoscopy at age 40.
Colorectal cancer (CRC) is the third most common cancer in the United States and the second most common cause of cancer-related death, although the incidence and mortality are decreasing because of increased CRC screening.1–4 The current multisociety CRC screening guidelines recommend that patients with a first-degree relative (FDR) (parent, sibling, or child) with a diagnosis of an adenoma of any size may undergo screening colonoscopy starting at age 40.1 Specifically, these multisociety guidelines state that “[individuals with a] first-degree relative with…an adenomatous polyp at age >60 years … screening same as average risk, but starting at age 40.” The guideline also states that “[individuals with a] first-degree relative with … an adenomatous polyp at age <60 years … should have a colonoscopy every 5 years starting at age 40 or 10 years younger than the earliest diagnosis in the family, whichever comes first.” This recommendation is partly based on research that individuals with adenomas had a higher prevalence of a family history (FH) of CRC compared with a control population and that individuals with an FH of adenomas have an approximately 2 times increased risk of CRC over their lifetime.2–4 In a French case-control study, Cottet et al5 recruited 168 FDRs (40–75 years old) of patients with a large adenoma (>10 mm) to undergo a screening colonoscopy and reported a higher prevalence of large adenomas and CRC in these individuals compared with a matched control population. However, no information is reported about the prevalence of adenomas in the 40- to 49-year-old relatives who were a minority of the study population. Also, the study did not address adenoma prevalence in individuals with an FH of small (<10 mm) adenomas. No published study has specifically addressed the prevalence of 40- to 49-year-old individuals who undergo an initial screening colonoscopy for an FH of adenomas. Furthermore, no study has addressed the real-life situation faced by gastroenterologists: 40- to 49-year-old patients present with an FH of “polyp” but frequently have no data about whether their FDR had an adenoma or hyperplastic polyp or the number or size of the adenomas.
The lack of data on this topic is particularly concerning because CRC screening with colonoscopy in the average-risk population has greatly increased the number of individuals younger than 40 years old with an FH of adenomas. The prevalence of adenomas in 50- to 75-year-old average-risk individuals is as high as 37.5%.6 As more and more average-risk individuals older than 50 years old undergo screening colonoscopy, far more than 37.5% of individuals younger than 40 years old will have an FH of adenomas if both parents undergo screening colonoscopy. This concern has been raised by other authors,7 although data on these 40- to 49-year-old individuals are still lacking. In this retrospective cross-sectional study, we quantified the prevalence of adenomas and advanced adenomas (AAs) (≥10 mm) in 40- to 49-year-old patients undergoing screening colonoscopy because of an FH of “polyps” and compared these data with the prevalence of adenomas and AAs in a control sample of 40- to 49-year-old patients without an FH of CRC or polyps who were undergoing their first colonoscopy because of altered bowel habits or abdominal pain.
METHODS
Study design
This was a retrospective endoscopic database study conducted at the University of Michigan in-hospital endoscopy unit and satellite endoscopy centers. All colonoscopies were performed by board-certified gastroenterology staff or gastroenterology fellows under direct supervision of staff gastroenterologists. The study subjects’ electronic medical records (EMRs) were reviewed to assess eligibility and abstract demographic and clinical information. All data were kept on University of Michigan secure laptops; the patient’s medical record number was the only identifier. Institutional review board approval was obtained before the beginning of the data collection.
Study population
All colonoscopies performed between January 1, 2006, and June 1, 2009, were analyzed. Inclusion criteria for the FH of polyp group included the following: (1) age of 40 to 49 years old and (2) indication for screening colonoscopy documented as FH of polyps or FH of adenomas. The FH of polyp or adenoma was self-reported, and no attempt was made to determine or confirm the reported histology of the polyp in the family member. This approach was chosen because it represents the dilemma usually faced by endoscopists when they receive a referral to perform a colonoscopy in a 40- to 49-year-old patient because of an FH of adenoma or polyp. Subjects were excluded for the following reasons: (1) an FH of CRC; (2) a previous colonoscopy for any reason in the past 10 years; (3) a personal history of adenomas or CRC; (4) a history of familial adenomatous polyposis, hereditary nonpolyposis CRC, or inflammatory bowel disease; (5) a documented history of overt or occult GI bleeding, iron deficiency anemia, or unexplained weight loss in the 6 months before colonoscopy; and (6) colonoscopies with poor/inadequate bowel preparations or that were incomplete. (Note: If patients with poor/inadequate bowel preparations were rescheduled for colonoscopy and this procedure was subsequently completed, then the data from this colonoscopy were included.) For the control sample of average-risk 40- to 49-year-old individuals undergoing colonoscopy, the indication for colonoscopy was altered bowel habits (diarrhea or constipation) or abdominal pain/discomfort. All exclusion criteria were also applied to this group, and patients from the control sample were also excluded if they had an FH of polyps/adenomas.
Data extraction
A computerized database search was performed to identify all 40- to 49-year-old individuals who underwent colonoscopy for the indication of FH of polyp, FH of adenoma, abdominal pain/discomfort, diarrhea, constipation, or altered bowel habits between January 1, 2006, and June 1, 2009. After identifying these potential study subjects, an extensive review of the EMRs (eg, review of primary care notes, specialty consultation notes, laboratory values, and pathology reports) was conducted to apply inclusion and exclusion criteria. After identifying eligible patients, demographic and colonoscopy data and pathology reports were extracted from the EMRs. The University of Michigan has a fully operational EMR system that records data about medical care received at the University of Michigan from 1996 to the present.
For the FH of polyp group, data were collected on the specific family members (eg, mother, father, brother, sister) with a history of polyps, the number of relatives affected, the age of the relatives at diagnosis, and the histological diagnosis (if reported in the EMRs) of the polyp in the FDR. If multiple family members had a diagnosis of polyps, the earliest diagnosis of a polyp in an FDR was recorded, if available. However, if these data were unavailable, then patients were still included in the study as long as the indication for colonoscopy was an FH of polyp and no exclusion criteria were met based on a review of the EMRs.
Colonoscopy procedure data were abstracted on cecal intubation, the size and histology of the polyps, and the presence of a gastroenterology fellow. Recommendations for the interval between screening colonoscopies were recorded, and these recommendations were compared with the multisociety guideline recommendations. An AA was defined as an adenoma 10 mm or larger in size. Based on the policy of the GI pathologists at the University of Michigan, villous histology is not reported. For all study patients, demographic data on sex, race/ethnicity, and age at the time of colonoscopy, body mass index (BMI) (kg/m2), the presence or absence of diabetes, long-term use of aspirin (>2 tablets/week), hormone replacement therapy, or statins at the time of colonoscopy were also collected.
Statistical analysis
The subject’s age, BMI, the number of relatives with a diagnosis of polyps, and the number and diameter of adenomas were reported as continuous variables. Sex, previous diagnosis of diabetes, long-term use of aspirin (>2 tablets/week), hormone replacement therapy, or statins at the time of colonoscopy, the presence of a gastroenterology fellow, and the presence of polyps and adenomas were dichotomized. FDR age at the time of polyp/adenoma diagnosis was reported as a continuous and dichotomous (younger than 60 years vs 60 years and older) variable.
Statistical differences among prevalence rates of adenomas or AAs across various predictors were measured using the χ2 test. Multivariable logistic regression was used to measure the impact of FH of polyps on adenoma prevalence. The model was adjusted for the age of the patient, the age of the FDR with a diagnosis of polyps/adenomas, race/ethnicity, sex, BMI, and aspirin and statin use. Crude and adjusted odds ratios (ORs) are reported. All statistical analyses were conducted using STATA software, version 10.0 (StataCorp, College Station, Tex).
RESULTS
Demographic data
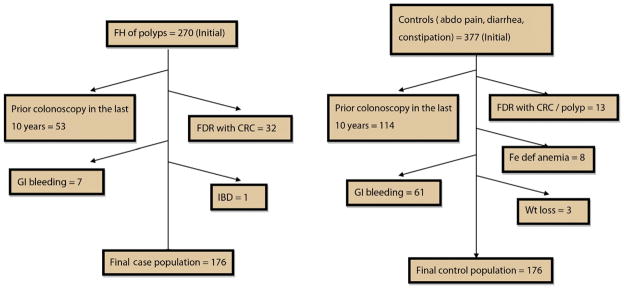
Between January 1, 2006, and June 1, 2009, a total of 647 individuals 40 to 49 years old underwent a screening colonoscopy because of an FH of polyps in an FDR (n = 270) or for evaluation of GI symptoms (n = 377). Ninety-four FH of polyp patients and 199 GI symptom patients fulfilled exclusion criteria (exclusion rates of 34.8% and 52.7%, respectively). As depicted in Figure 1, common exclusion criteria included FH of CRC in an FDR (n = 32) and colonoscopy in the past 10 years (n = 53). The final study sample included 176 FH of polyps subjects and 178 control subjects. Demographic data on study patients are provided in Table 1. No significant differences in age, BMI, sex, ethnicity, previous diagnosis of diabetes, long-term use of aspirin (>2 tablets/week), hormone replacement therapy, or statins were present in the 2 groups.
Figure 1.
Schematic representation of various reasons why patients were excluded from the study and how we arrived at the final sample size. Note: Patients with incomplete colonoscopies/poor bowel preparation were immediately rescheduled for colonoscopy. These procedures were automatically excluded based on the criteria for the computerized search of our endoscopic database and are not shown. When these patients underwent a repeat colonoscopy, then the results of this colonoscopy were included. abdo, abdominal; CRC, colorectal cancer; FDR, first-degree relative; Fe def, iron deficiency; FH, family history; IBD, inflammatory bowel disease; Wt, weight.
TABLE 1.
Demographic and clinical characteristics
| Age, y | Cases (n = 176) | Controls (n = 178) |
|---|---|---|
| Mean (SD) | 44.7 (0.2) | 44.7 (0.2) |
| 40–44, no. (%) | 82 (46.6) | 85 (47.8) |
| 45–49, no. (%) | 94 (53.4) | 93 (52.2) |
| Sex, no. (%) | Cases (n = 176) | Controls (n = 178) |
| Female | 113 (64.2) | 124 (69.7) |
| Male | 63 (35.8) | 54 (30.3) |
| BMI | Cases (n = 137) | Controls (n = 146) |
| Mean (SD) | 27.7 (6.1) | 28.2 (6.9) |
| ≥30, no. (%) | 40 (29.2) | 50 (34.2) |
| <30, no. (%) | 97 (70.8) | 96 (65.8) |
| Race, no. (%) | Cases (n = 176) | Controls (n = 178) |
| White | 158 (89.9) | 151 (84.8) |
| African American | 5 (2.8) | 11 (6.2) |
| Hispanic | 0 (0) | 3 (1.7) |
| Asian | 6 (3.4) | 6 (3.4) |
| Native American | — | — |
| Other/unknown | 7 (4) | 7 (3.9) |
| Clinical, no. (%) | Cases (n = 173) | Controls (n = 169) |
| Diabetes | 3 (1.7) | 5 (3) |
| Aspirin use | 10 (5.8) | 10 (5.9) |
| HRT | 3 (1.7) | 7 (4.1) |
| Statin use | 10 (5.8) | 10 (5.9) |
SD, Standard deviation; BMI, body mass index; HRT, hormone replacement therapy.
Adenoma prevalence, number, and size
The prevalence of adenomas (any size) was greater in the FH of polyps group (n = 176) compared with the control group (n = 178) of individuals with altered bowel habits or abdominal pain (26.7% vs 13.5%; P = .002), but was not greater for AAs (5.7% vs 3.4%; P = .3) (Table 2). No statistical differences in the mean size (6.5 ± 0.6 mm vs 5.8 ± 0.8 mm; P = .50) or number (1.4 ± 0.2 vs 1.3 ± 0.1; P = .45) of adenomas in the FH of polyp or control group were identified. FH of polyps patients had a significantly higher prevalence of adenomas in the 40- to 44-year-old subgroup compared with similarly aged patients in the control group (31.7% vs 10.6%; P = .001), although this comparison did not achieve statistical significance for the subgroup of 45- to 49-year-old patients with an FH of polyps compared with 45- to 49-year-old patients in the control group (22.3% vs 16.1%; P = not significant).
TABLE 2.
Prevalence of adenomas and advanced adenomas
| Cases
|
Controls
|
|||||
|---|---|---|---|---|---|---|
| No. | Adenoma (any size), no. (%) | Advanced adenoma, no. (%) | No. | Adenoma (any size), no. (%) | Advanced adenoma, no. (%) | |
| Overall prevalence | 176 | 47 (26.7)* | 10 (5.7) | 178 | 24 (13.5)* | 6 (3.4) |
|
| ||||||
| Prevalence by age of the patient | ||||||
|
| ||||||
| 40–44 y | 82 | 26 (31.7)† | 5 (6.1) | 85 | 9 (10.6)† | 3 (3.5) |
|
| ||||||
| 45–49 y | 94 | 21 (22.3) | 5 (5.3) | 93 | 15 (16.1) | 3 (3.2) |
|
| ||||||
| Prevalence by the age of FDR at the time of diagnosis of polyp | ||||||
|
| ||||||
| FDR <60 y | 37 | 11 (29.7) | 1 (2.7) | – | – | – |
|
| ||||||
| FDR ≥60 y | 9 | 3 (33.3) | 1 (11.1) | – | – | – |
|
| ||||||
| Prevalence by based on FH of adenoma or polyp (polyp histology unknown) | ||||||
|
| ||||||
| FH of adenoma | 105 | 27 (25.7) | 6 (5.7) | |||
|
| ||||||
| FH of polyp | 71 | 20 (28.1) | 4 (5.6) | |||
FDR, First-degree relative; FH, family history.
Prevalence of adenomas in cases significantly higher than controls (P = .002).
Prevalence of adenomas in cases significantly higher than controls (P = .001).
The precise age of the FDR when the polyp was diagnosed was documented in 46 of 176 cases (26.1%). No statistical differences in the adenoma or AA prevalence were identified among individuals whose FDR received a diagnosis of adenoma at younger than 60 years of age versus an FDR receiving a diagnosis of adenoma at 60 years of age or older. The histology of the polyp as adenoma in the FDR (ie, physician reported in the EMR that patient’s FDR had an adenoma) was documented in 105 cases (59.7%). There was no difference in the prevalence of adenomas or AAs based on whether the FDR’s polyp was documented as an adenoma or polyp. The size or number of adenomas did not differ based on the age of the FDR at the time of diagnosis of polyp or whether there was a documented history of an adenoma in the FDR.
Risk factors for adenomas
The multiple logistic regression analysis assessed the association between adenomas (any size) and an FH of polyp, adjusting for age, sex, race, BMI, the presence of a GI fellow during the colonoscopy, the presence of diabetes, and long-term ongoing aspirin use (Table 3). After adjusting for confounders, an FH of polyps is associated with an increased prevalence of adenomas (OR 2.8 [95% CI, 1.4–5.5]), and male sex is associated with an increased prevalence of AAs (OR 3.3 [95% CI, 1.1–10.0]). Among only the FH of polyp subjects (n = 176), multiple logistic regression analysis assessed whether the age of the FDR at the time of diagnosis, the number of FDRs with polyp, or having a documented FH of adenoma versus polyp had an impact on adenoma prevalence, and none of these factors were predictive of adenoma prevalence (Table 3).
TABLE 3.
Factors affecting the prevalence of adenomas and advanced adenomas
| Adenoma
|
Advanced adenomas
|
|||
|---|---|---|---|---|
| Crude | Adjusted | Crude | Adjusted | |
| All study subjects | ||||
|
| ||||
| FH of polyps | 2.3 (1.4–4.0) | 2.8 (1.4–5.5) | 1.7 (0.6–4.9) | 2.1 (0.6–7.0) |
|
| ||||
| Age | 1 (0.9–1.1) | 1 (0.9–1.2) | 1 (0.9–1.2) | 1.1 (0.9–1.3) |
|
| ||||
| Male sex | 1.5 (0.9–2.6) | 1.4 (0.7–2.7) | 2.7 (0.99–7.5) | 3.3 (1.1–10.0) |
|
| ||||
| Race (white vs other) | 1.0 (0.5–2.1) | 0.8 (0.3–2.0) | 2.2 (0.3–17.0) | 1.9 (0.2–15.0) |
|
| ||||
| Obesity | 1.0 (0.5–1.9) | 1 (0.6–2.1) | 2 (0.7–5.5) | 2 (0.7–5.9) |
|
| ||||
| Aspirin use | 1 (0.3–3.0) | 0.7 (0.2–1.6) | 1.1 (0.1–8.6) | 0.6 (0.1–5.0) |
|
| ||||
| Presence of a GI fellow | 0.8 (0.5–1.4) | 1.7 (0.7–2.8) | 1.1 (0.1–3.0) | 1 (0.3–3.3) |
|
| ||||
| Cases only | ||||
|
| ||||
| No. of FDRs with CRC | 1.8 (0.8–4.3) | 3.2 (0.6–18.0) | 1 (0.2–6.9) | — |
|
| ||||
| Age of the FDR at diagnosis >60 y | 1.2 (0.2–5.6) | 1.9 (0.2–15.0) | 4.5 (0.2–79.0) | — |
|
| ||||
| FH of adenoma (vs polyp) | 0.9 (0.4–1.7) | 0.3 (0.04–3.0) | 1 (0.3–3.7) | — |
FH, Family history; CRC, colorectal cancer; FDR, first-degree relative.
Recommended repeat interval after the screening colonoscopy
Per multisociety guidelines, if the FDR was younger than 60 years old when an adenoma was diagnosed, then the patient should undergo repeat screening colonoscopy at least every 5 years. However, if the FDR was 60 years of age or older at the time of adenoma diagnosis, then the patient should undergo repeat screening colonoscopy every 10 years if no adenomas are found. If an individual with an FH of polyp has a small adenoma, multiple (≥3) adenomas, or a large adenoma, then standard recommendations for surveillance colonoscopy apply. Therefore, in the FH of polyp group, it is necessary to know whether the FDR was younger or older than 60 years of age at the time of diagnosis of a polyp to recommend an appropriate interval for repeat colonoscopy if no adenomas were identified in the study subject.
The frequency of adherence to guideline recommendations for repeat screening could be ascertained for 90 colonoscopies in the FH of polyp group. The follow-up recommendation was consistent with guidelines in 56.7% (51/90) of cases (Table 4). Among recommendations for repeat colonoscopy that were not consistent with guidelines, a majority of subjects were not given any recommendations for a repeat colonoscopy (56.4% (22/39), whereas 38.5% (15/39) were called back at briefer intervals than recommended.
TABLE 4.
Frequency of adherence to guideline recommendation for repeat colonoscopy
| Recommended interval | No. (%) |
|---|---|
| Adherent | 51/90 (56.7) |
| Nonadherent | 39/90 (43.3) |
| If nonadherent | |
| Called back too soon | 15/39 (38.5) |
| Called back too late | 1/39 (2.6) |
| No follow-up recommendation given* | 22/39 (56.4) |
| Incomplete recommendations given† | 1/39 (2.6) |
Adherence to guideline recommendations is based on recommendations from the recent multisociety colorectal cancer screening guidelines.1
No recommendation for repeat colonoscopy in endoscopy report and no pathology letter sent to the patient with a recommendation for timing of next colonoscopy.
Endoscopy report recommends that the patient follow up with his or her primary care physician and discuss the timing of next colonoscopy.
DISCUSSION
The 2008 multisociety guidelines recommend that individuals with an FH of adenomas should get their first screening colonoscopy at age 40. As CRC screening with colonoscopy increases, many individuals younger than 40 years of age will have an FDR (mother, father, brother, or sister) who have received a diagnosis of an adenoma.7 However, we have virtually no direct data on the prevalence of adenomas in these 40- to 49-year-old subjects with an FH of adenoma. The relative lack of data may contribute to some variation in recommendations among different societies. For example, the 2008 American College of Gastroenterology CRC guideline now states that patients with a single FDR with a nonadvanced adenoma should undergo the same CRC screening as an average-risk individual.8
In this study, we quantified the prevalence of adenomas in 40- to 49-year-old individuals undergoing an initial screening colonoscopy because of an FH of polyp and compared these data with colonoscopy findings in a control sample of 40- to 49-year-old individuals undergoing an initial colonoscopy for altered bowel habits or abdominal pain. Based on our review of the literature, this is the only study that focuses solely on adenoma prevalence in 40- to 49-year-old individuals with an FH of polyps who undergo screening colonoscopy. We used stringent exclusion criteria and a detailed search of the EMRs to identify a sample of 176 asymptomatic 40- to 49-year-old patients with an FH of polyps who underwent screening colonoscopy and a control group of 178 average-risk, 40- to 49 –year-old individuals who underwent colonoscopy for altered bowel habits or abdominal pain. The prevalence of adenomas (any size) was greater in the FH of polyps group than in the control sample of individuals with altered bowel habits or abdominal pain (26.7% vs 13.5%; P = .002) and trended more greatly for AAs (5.7% vs 3.4%; P = .3) (Table 2). Furthermore, an FH of polyp was associated with adenomas after adjusting for multiple confounders (OR 2.8 [95% CI, 1.4–5.5]). Our study highlights important practical limitations in the implementation of these recommendations. First, it is frequently difficult for the patient to accurately report the histology of the polyp in their FDRs. In our study, the referring physician or endoscopist reported that the FDR’s polyp was an adenoma in approximately 60% of cases (105/176). However, it is difficult to estimate the accuracy of the patient’s self-report. Although studies demonstrate greater than 80% accuracy for self-report of an FH of CRC,9,10 there are no reported data on the accuracy of self-report of an FH of adenoma. Furthermore, it may be quite difficult to estimate the accuracy of a self-report of an FH of adenoma among U.S. individuals because of current Health Insurance Portability and Accountability Act privacy regulations and institutional review board restrictions on contacting family members of study patients. Currently, we are conducting a prospective study of 40- to 49 –year-old individuals who undergo initial screening colonoscopy because of an FH of polyp. As part of this study, we are seeking permission from the FDRs to review their endoscopy and histology reports. Because of Health Insurance Portability and Accountability Act privacy guidelines, our institutional review board only allows us to seek study participation of FDRs through an opt-in procedure. Thus, 40- to 49-year-old study patients are given a blank postcard to address and send to their FDR. The postcard briefly describes the study and provides a phone number for the FDR to call if he or she is willing to participate in the study. To date, only 1 FDR has opted in to participate in our prospective study among more than 90 study patients. With these opt-in procedures, it will be virtually impossible to review endoscopy reports and histology reports of FDRs, even in prospective studies. Nevertheless, our inability to obtain histology reports about polyps in FDRs may be a strength of our retrospective study. In the real world, endoscopists infrequently have histology reports on polyps in FDRs. Instead, endoscopists simply get the individual’s self-report of a polyp/adenoma in an FDR. Our study reflects this real-world practice. Another practical real-world limitation is that health professionals may incorrectly report whether a patient has an FH of CRC or adenoma because of limited chart documentation. As part of our study, we found that 11.8% of patients (32/270) in the original FH polyp group actually had an FH of CRC after detailed chart review (Fig. 1).
Our data also highlight a recurrent issue of the quality of CRC screening with colonoscopy. Endoscopists may not follow guideline recommendations for repeat colonoscopy and may favor recommending colonoscopy at shorter intervals. In our study, endoscopists were compliant with guideline recommendations in only 56.7% of cases. A large proportion of patients did not get any recommendations, which may reflect endoscopists’ uncertainty about guideline recommendations. Among patients who did get specific inappropriate recommendations, these subjects were virtually always instructed to return sooner than recommended. To the best of our knowledge, this is the first study that assessed compliance with guidelines for 40- to 49-year-old individuals who undergo screening colonoscopy for an FH of adenoma.
Our study had several limitations that need to be highlighted. It was a retrospective endoscopic database study that was supplemented with review of individual EMRs. It relied on the recall of study patients when they reported their FH information to their physicians. Most importantly, our study was relatively small: 176 individuals with an FH of polyp compared with 178 average-risk individuals who undergo colonoscopy for altered bowel habits or abdominal pain. With this relatively small sample size, our data cannot be used to change current guideline recommendations. Changes to current guideline recommendations will probably occur after data from prospective, multicenter studies of these patients are reported. Our intention is to start the discussion about the appropriateness of this guideline recommendation based on our retrospective database study.
In conclusion, the prevalence of adenomas (any size) was greater in the FH of polyps group than in the control group of individuals with altered bowel habits or abdominal pain (26.7% vs 13.5%; P = .002) and trended more greatly for AAs (5.7% vs 3.4%; P = .3) Furthermore, an FH of polyp was associated with adenomas after adjusting for multiple confounders in our multiple logistic regression analysis (OR 2.8 [95% CI, 1.4–5.5]). Therefore, our findings support the current 2008 multisociety guideline recommendation to perform screening colonoscopy at age 40 in individuals with an FH of polyp. This retrospective research is a first step to determine whether current guideline recommendations are appropriate, although prospective data from large multicenter studies will be needed to conclusively address this issue.
Take-home Message.
Adenomas are more frequent in 40- to 49-year-old individuals with a family history of polyps compared with average-risk 40- to 49-year-old individuals.
Results of this study support current guideline recommendations, although prospective studies should be performed to provide definitive data to support or refute this guideline recommendation.
Abbreviations
- AA
advanced adenoma
- BMI
body mass index
- CRC
colorectal cancer
- EMR
electronic medical record
- FDR
first-degree relative
- FH
family history
- OR
odds ratio
Footnotes
DISCLOSURE: Dr Schoenfeld was supported by grants NIDDK, NIH 1K24DK084208 and VA HSR&D NDA 06-205. No other financial relationships relevant to this publication were disclosed.
Presented at the American College of Gastroenterology annual meeting, 2009 meeting; Digestive Disease Week, 2009 (Am J Gastroenterol 2009; 104[Suppl3]:S42), and 2010 (Gastroenterology 2010;138[Suppl1]: S-477).
References
- 1.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 2.Winawer SJ, Zauber AG, Gerdes H, et al. Risk of colorectal cancer in the families of patients with adenomatous polyps. National Polyp Study Workgroup. N Engl J Med. 1996;334:82–7. doi: 10.1056/NEJM199601113340204. [DOI] [PubMed] [Google Scholar]
- 3.Lynch KL, Ahnen DJ, Byers T, et al. First-degree relatives of patients with advanced colorectal adenomas have an increased prevalence of colorectal cancer. Clin Gastroenterol Hepatol. 2003;1:96–102. doi: 10.1053/cgh.2003.50018. [DOI] [PubMed] [Google Scholar]
- 4.Ahsan H, Neugut AI, Garbowski GC, et al. Family history of colorectal adenomatous polyps and increased risk for colorectal cancer. Ann Intern Med. 1998;128:900–5. doi: 10.7326/0003-4819-128-11-199806010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Cottet V, Pariente A, Nalet B, et al. Colonoscopic screening of first-degree relatives of patients with large adenomas: increased risk of colorectal tumors. Gastroenterology. 2007;133:1086–92. doi: 10.1053/j.gastro.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman DA, Weiss DG, Bond JH, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–8. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 7.Austin GL, Goldstein JI, Peters SL, et al. Are colorectal cancer screening recommendations for first-degree relatives of patients with adenomas too aggressive? Clin Gastroenterol Hepatol. 2011;9:308–13. doi: 10.1016/j.cgh.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell RJ, Brewster D, Campbell H, et al. Accuracy of reporting of family history of colorectal cancer. Gut. 2004;53:291–5. doi: 10.1136/gut.2003.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aitken J, Bain C, Ward M, et al. How accurate is self-reported family history of colorectal cancer? Am J Epidemiol. 1995;141:863–71. doi: 10.1093/oxfordjournals.aje.a117522. [DOI] [PubMed] [Google Scholar]