Abstract
Successful hematopoietic stem cell transplant (HSCT) requires the infusion of a sufficient number of hematopoietic stem/progenitor cells (HSPCs) that are capable of homing to the bone marrow cavity and regenerating durable trilineage hematopoiesis in a timely fashion. Stem cells harvested from peripheral blood are the most commonly used graft source in HSCT. While granulocyte colony-stimulating factor (G-CSF) is the most frequently used agent for stem cell mobilization, the use of G-CSF alone results in suboptimal stem cell yields in a significant proportion of patients. Both the chemokine receptor CXCR4 and the integrin α4β1 (VLA-4) play important roles in the homing and retention of HSPCs within the bone marrow microenvironment. Preclinical and/or clinical studies have shown that targeted disruption of the interaction of CXCR4 or VLA-4 with their ligands results in the rapid and reversible mobilization of hematopoietic stem cells into the peripheral circulation and is synergistic when combined with G-CSF. In this review we discuss the development of small molecule CXCR4 and VLA-4 inhibitors and how they may improve the utility and convenience of peripheral blood stem cell transplantation.
Keywords: Hematopoietic stem cell transplantation, hematopoietic stem cell mobilization, CXCR4, VLA-4, plerixafor
Introduction
The majority of hematopoietic stem and progenitor cells (HSPCs) reside in the bone marrow in a highly organized microenvironment consisting of marrow stromal cells, osteoblasts, osteoclasts and other extracellular matrix proteins (e.g., collagens, fibronectins, proteoglycans).1–5 HSPCs express a number of cell surface molecules such as very late antigen 4 (VLA-4), CXCR4, CXCR2, CD44, CD62L, lymphocyte function-associated antigen-1 (LFA-1), CD117 (c-kit), and Robo4 that mediate their adherence in the BM microenvironment.3, 4, 6, 7 These interactions play important roles in regulating HSPC trafficking, as well as self-renewal, proliferation and differentiation processes.4, 8
Mobilized HSPCs collected from peripheral blood have essentially replaced bone marrow as a source of stem cells for autologous and allogeneic transplantation. There was initial concern regarding the use of mobilized peripheral blood stem cells (PBSCs) as a source of graft for allogeneic stem cell transplantation. This concern was based on the presence of a 10- to 50-fold increase in the T cell content of the mobilized peripheral blood products, which could potentially lead to higher rates of acute and chronic GVHD. However, transplantation with mobilized PBSCs was associated with faster engraftment, reduced infectious complications, enhanced immune reconstitution, shorter hospitalization, and reduced costs.9–12
Currently, granulocyte colony-stimulating factor (G-CSF) alone or G-CSF plus chemotherapy are the most commonly used methods for stem cell mobilization. Unfortunately, 5–30% of patients do not respond to these agents.13 New strategies are needed to manage patients who fail initial mobilization, decrease the number of leukaphereses required to collect adequate number of HSPCs, improve immune reconstitution and decrease total cost. This article will first briefly review the standard approaches to HSPC mobilization using G-CSF. We will then discuss new strategies to mobilize stem cells through the use of CXCR4 and VLA-4 small molecule antagonists.
Standard approaches to hematopoietic stem and progenitor cell mobilization
Cytokines, especially G-CSF, can be used alone or in combination with chemotherapy (disease-specific chemotherapy or cyclophosphamide) to increase the number of circulating CD34+ cells, the surrogate marker of HSPCs in humans.13–15 Studies have shown that although mobilization with G-CSF plus chemotherapy generates higher HSPC yield when compared to G-CSF alone, failure rates are not different between the two mobilization groups (5–30%).16–18 Factors that may limit the collection of an adequate amount of CD34+ HSPC include age, sex, extent and type of chemotherapy, previous use of immunomodulatory drugs, previous exposure to radiation, previous attempts at mobilization, depressed peripheral blood CD34+ HSPC counts before mobilization, disease status, and involvement of bone marrow in the disease process.19,20 The optimal time for collection of stem cells after mobilization with chemotherapy plus G-CSF is 10–14 days as opposed to 4–5 days when G-CSF is used as single agent.13 Although mobilization with chemotherapy plus G-CSF generally requires fewer leukaphereses to collect an adequate number of stem cells, individual differences in response to chemotherapy, and the potential for complications result in more unpredictable collections that can delay the transplant and result in increased morbidity and cost. The use of chemotherapy as a part of mobilization is associated with significantly higher toxicities including increased risk for secondary malignances, impairment of fertility, cardiac toxicity, hemorrhagic cystitis, anaphylactic reactions, and higher cost.
G-CSF is well tolerated, with skeletal pain, fatigue and nausea being most frequent side effects. Rare episodes of spontaneous splenic rupture have been reported.21–24 Strategies to manage patients who fail their initial mobilization include dose escalation of G-CSF (12.5–50 μg/kg/day), addition of another cytokine such as GM-CSF, addition of chemotherapy or harvesting the bone marrow; however, no standard approach exists. In general, patients who fail initial mobilization are more likely to fail remobilization regardless of the remobilization regimen.17, 25 Furthermore, additional mobilization efforts often result in poorer patient outcomes and increased resource utilization. For these reasons, novel mobilization agents that are less toxic, more rapid and increase the yield of collected CD34+ cells for transplantation are needed. Plerixafor (AMD3100, Mozobil; Genzyme, Massachusetts, USA), a novel small molecule antagonist of CXCR4, was approved by the United States Food and Drug Administration (FDA) for use with G-CSF in December of 2008 to mobilize HSPC to the peripheral blood for collection and subsequent autologous transplantation in patients with non-Hodgkin’s lymphoma (NHL) and multiple myeloma (MM). Below we will briefly discuss CXCR4/CXCL12 biology and clinical results obtained using plerixafor as a mobilizing agent in autologous and allogeneic transplantation. We will then discuss some of the limitations with the use of plerixafor for HSPC mobilization and new agents in clinical development that target the CXCR4/CXCL12 axis.
HSPC mobilizing agents that target the CXCL12/CXCR4 axis
CXCL12/CXCR4 axis
CXCL12 or stromal cell derived factor 1α (SDF-1α) is a chemokine that is constitutively produced at high levels in the bone marrow by stromal cells such as osteoblasts, endothelial cells and a subset of reticular cells.26–30 It is a potent chemoattractant for HSPCs and has been shown to regulate cell adhesion, survival, and cell-cycle status.31–33 CXCL12 requires the amino terminus of CXCR4 for binding and activates downstream signaling pathways via the second extracellular loop.34 Further structure-function studies indicate that the third intracellular loop (IL3) of CXCR4 is necessary for Gi-dependent signaling, and intracellular loops 2 and 3 as well as the C-terminus part of CXCR4 are required for chemotaxis.35 Interestingly, CXCL12 gene polymorphism has been proposed as a conditional factor for human CD34+ stem cell mobilization, with the presence of the SDF1-3′A allele as a predictive factor of good CD34+ cell mobilization.36, 37 More recently, a second receptor, CXCR7, was identified that binds CXCL12 with an affinity that is approximately tenfold higher than the affinity for CXCR4.38, 39 Though the role of CXCR7 in CXCL12-dependent chemotaxis is not fully understood, there is evidence that CXCR7 lacks intrinsic chemotactic activity towards CXCL12 and rather functions by sequestering CXCL12 and modifying CXCR4 signaling.40–43
CXCR4 is a member of the large family of seven transmembrane domain receptors coupled to heterotrimeric Gi proteins and functions as a coreceptor for human immunodeficiency virus type 1 (HIV-1) cell entry.44–48 CXCR4 exists in different isoforms as the result of differential splicing, which affect the length of its N-terminus.49 Post-translational modifications of CXCR4 include N-glycosylation, tyrosine sulfation, and modification of tyrosine-21 by chondroitin sulfate.49 The binding of CXCR4 to CXCL12 results in activation of multiple signal transduction pathways ultimately triggering chemotaxis.49, 50 More recently, both trefoil factor family 2 (TFF2)51 and macrophage migrating inhibiting factor (MIF)52 were described as additional ligands of CXCR4. Finally, the expression of CXCR4 on human CD34+ stem cells is dynamic and Flt3-ligand,53 SCF,54 IL-3,55 IL-8,56 hepatocyte growth factor,57 andG-CSF58 are all known to modulate the SDF/CXCR4 pathway.
Genetic alteration of CXCR4
Mice deficient for CXCL12 or CXCR4 die perinatally and exhibit similar defects in neuron migration, organ vascularization, and hematopoiesis. CXCR4−/− and CXCL12−/−embryos have severely reduced B cell lymphopoiesis, reduced myelopoiesis in fetal liver, and nearly complete loss of myelopoiesis in the bone marrow.59–62 This data, along with the observation of increased numbers of circulating myeloid cells in the fetal blood of CXCR4-deficient embryos,63 demonstrated the pivotal role for the CXCR4/CXCL12 axis in bone marrow colonization.
Since the lethality caused by deficiency in CXCR4 prevents analysis of its role in adult hematopoiesis, investigators generated hematopoietic chimeras by transferring CXCR4-deficient cells into lethally irradiated wild type recipients. Unexpectedly, CXCR4−/− fetal liver cells engrafted in the marrow of the irradiated recipients when high cell numbers were transplanted.63–65 These results imply that early HSPC homing to the BM can occur in the absence of CXCR4. However, mice reconstituted with CXCR4-deficient fetal liver cells exhibited significantly reduced numbers of donor derived B220+ B cells, Mac-1+ granulocytic-monocytic cells, Gr-1+ granulocytic cells, and CD61+ megakaryocytic cells in the bone marrow.63–65 Furthermore, there was a marked increase in the number of circulating immature granulocytes, CFU-Cs (30-fold increase), and Lin-Sca-1+c-kit+ (LSK) cells in mice stably engrafted with CXCR4-deficient fetal liver cells.63–65 In separate studies, CXCR4−/− and wild-type BM cells were competitively transplanted into lethally irradiated congenic recipients, and HSPC engraftment was evaluated by their multi-lineage contribution to the peripheral blood at 8 weeks post-injection.66 These experiments also revealed that CXCR4−/− BM cells exhibited impaired engrafting capacity, with 5-fold more CXCR4−/− BM cells being required to achieve chimerism levels similar to the wild-type BM cells. Taken together, these data suggest that the impaired hematopoietic reconstitution potential of adoptively transferred CXCR4−/− cells is related to their altered homing and retention within the bone marrow microenvironment.
To further characterize the role of CXCR4 in hematopoietic stem cells, two groups have independently used the Cre-loxP recombination system to selectively delete CXCR4 in the hematopoietic system. In these studies, CXCR4-floxed mice were crossed to either MxCre33 or tamoxifen-inducible Cre66 transgenic mice and Cre was activated by injecting poly(I)-poly(C) or tamoxifen, respectively. Consistent with the phenotype of mice reconstituted with CXCR4-deficient fetal liver cells, conditional ablation of CXCR4 in mice caused a severe defect in B cell lymphopoiesis and a significant increase in the number of circulating LSK stem cells. In both studies, primitive hematopoietic stem cells (LSK cells) were retained in the bone marrow and became hyperproliferative following CXCR4 inactivation.33, 66 In the study by Nie et al.66, elevated numbers of bone marrow and circulating LSK cell numbers as well as sustained hematopoiesis were observed for at least 8 months after tamoxifen induction of Cre and consequent excision of CXCR4. In contrast, Sugiyama et al.33 reported a drastically reduced number of LSK cells in the bone marrow and impaired hematopoiesis 4 months after poly(I)-poly(C)-mediated induction of Cre and deletion of CXCR4. Nie et al.66 suggested that these discrepancies in the maintenance of LSK cells and hematopoiesis following CXCR4 ablation might be related to the toxicity of poly(I)-poly(C) on LSK cells. Whatever the explanation, the data indicate that HSPCs can be retained in the BM microenvironment through a CXCR4-independent mechanism. Furthermore, the hyperproliferative state of LSK cells following CXCR4 ablation indicates that CXCR4 acts intrinsically in primitive HSPCs to enforce quiescence.66
Additional evidence for the critical role that CXCR4 plays in leukocyte trafficking has been obtained from patients with the genetic immunodeficiency syndrome WHIM (warts, hypogammaglobulinemia, infections, myelokathexis). Most cases of WHIM syndrome have been linked to autosomal dominant mutations in CXCR4, all of which truncate the C-terminal tail of CXCR4.67–69 Multiple studies have demonstrated that loss of the intracellular tail of CXCR4 prevents its internalization and desensitization in response to CXCL12.67, 68, 70 This loss of homologous desensitization leads to long-lasting activation of G-proteins and sustained functional activity of the chemokine receptor as evidenced by increased chemotaxis to CXCL12, F-actin polymerization, intracellular calcium release, and endothelial adhesion.67, 68, 71 Some functional consequences of dysregulated CXCR4 signaling in WHIM syndrome is the failure of mature neutrophils to exit the bone marrow (myelokathexis) resulting in peripheral neutropenia, defects in B-cell development, reduced immune function, and other similarities to murine CXCL12 and CXCR4 deficient embryos.72, 73
The data obtained from WHIM patients and studies using genetically modified mice supporta model in which CXCL12 signaling through CXCR4 provides a keyretention signal for HSPCs in the bone marrow. Furthermore, multiple preclinical and clinical studies have shown that pharmacologic disruption of the CXCL12/CXCR4 axis using various CXCR4 modulators, including small molecule antagonists, peptide agonists, and anti-CXCR4 antibodies stimulate HSPC mobilization in a target-dependent manner.74, 75 Below we will discuss some of these different types of CXCR inhibitors in greater detail.
Plerixafor
Plerixafor is a bicyclam derivative that reversibly competes with and inhibits CXCL12 binding to CXCR4. This compound was originally tested clinically as an agent for treatment of human immunodeficiency virus (HIV) infection by blocking the HIV entry into CD4+ T cells.76, 77 Initial studies in healthy volunteers showed good tolerance with minimal and reversible adverse effects.78, 79 During those clinical trials, leukocytosis was noted after a single intravenous dose of plerixafor. Further investigation demonstrated that CD34+ cells were one component of this generalized leukocytosis. Bioavailability after subcutaneous (sc) injection was 87% with a dose dependent increase in CD34+ cells in the peripheral blood. No drug was detected after oral administration.
A single subcutaneous dose of plerixafor at 160–240 μg/kg resulted in a 6- to 10-fold increase in CD34+ cell count starting 1 hour, peaking at 9 hours after injection and declining to baseline within 24 hours.80 Pharmacokinetic studies of plerixafor have identified no differences between older and younger patients. Thus, no dose adjustment beyond that based on renal function is recommended in elderly patients. The use of plerixafor has not been studied in individuals aged <18 years. Most frequently noted adverse effects were transient pain and injection site erythema, headache, paresthesias, diarrhea, bloating and nausea. An additional study showed plerixafor could be combined with G-CSF to further increase the yield of CD34+ cells. On the basis of these results plerixafor was further pursued for HSPC mobilization in the clinical setting.81 Testing of plerixafor as a HIV drug was abandoned due to a lack of antiviral effect and the occurrence of asymptomatic premature ventricular contractions in two patients.
Phase I studies with plerixafor
Devine et al.82 assessed safety and clinical effects of plerixafor in patients with NHL and multiple myeloma. Plerixafor caused a rapid and statistically significant increase in the total WBC and peripheral blood CD34+ cell counts at both 4 and 6 hours following a single injection. The absolute number of circulating CD34+ cells at 4 and 6 hours after plerixafor administration were higher in the 240 μg/kg group compared with the 160 μg/kg group with a maximum 6-fold increase in circulating CD34+ cells.
Phase II studies with plerixafor
The initial clinical trial of plerixafor in human HSPC mobilization by Flomenberg et al.83 were based on the hypothesis that the combination of plerixafor plus G-CSF would be superior to G-CSF alone and that the plerixafor plus G-CSF-mobilized cells would engraft at least as well as their G-CSF-mobilized counterparts. Patients with MM or NHL in first or second complete or partial remission were eligible for enrollment. Initially, patients were randomly assigned to receive plerixafor plus G-CSF or G-CSF alone as their mobilizing regimen, followed by a 2-week washout period and remobilization with the alternate regimen. Subsequently, randomization was discontinued and all patients received G-CSF alone as the initial mobilizing regimen. G-CSF mobilization consisted of the daily subcutaneous administration of the drug at a dose of 10 μg/kg and pheresis was begun on day 4 or 5 of administration. During plerixafor plus G-CSF mobilization, plerixafor was administered subcutaneously at a dose of 160–240 μg/kg beginning on day 4 or 5 followed 6 hours later by apheresis. The apheresis procedure was limited to 3 blood volumes per day. Patients treated with plerixafor plus G-CSF mobilized more CD34+ cells per leukapheresis, required fewer leukaphereses to reach the target CD34+ cell count, and mobilized higher total CD34+ cell yield. Fifty-six percent of patients mobilized with plerixafor plus G-CSF yielded at least 2 × 106 CD34+ cells/kg recipient body weight after one leukapheresis and 100% after two leukaphereses (compared to 64% with G-CSF alone). In addition, all 9 of 25 patients who failed to yield the minimum CD34+ cells/kg after four leukaphereses with G-CSF alone were successfully remobilized with plerixafor plus G-CSF.
Plerixafor given with G-CSF has been shown to mobilize CD34+ cells in NHL, MM, and Hodgkin’s disease (HD) patients who could not collect sufficient cells for autologous transplant following other mobilization regimens. These poor mobilizers were excluded from company-sponsored trials, but have been included in a plerixafor Single Patient Use protocol, referred to as a Compassionate Use Protocol (CUP). A cohort of 115 data-audited poor mobilizers in CUP were assessed, with the objective being to collect 2 × 106 CD34+ cells per kg following plerixafor plus G-CSF mobilization. The rates of successful CD34+ cell collection were similar for patients who previously failed chemotherapy mobilization or cytokine-only mobilization: NHL—60.3%, MM—71.4% and HD—76.5%. Following transplant, median times to neutrophil and PLT engraftment were 11 days and 18 days, respectively. Engraftment was durable.84
Several additional studies in heavily pretreated patients with NHL, Hodgkin’s lymphoma and multiple myeloma had similar results, confirming that plerixafor is a well-tolerated and effective mobilizing agent.85–87 In addition, these studies support the use of plerixafor plus G-CSF in those patients who have failed initial attempts at mobilization with G-CSF or chemotherapy plus G-CSF. Pharmacokinetic and pharmacodynamic studies of plerixafor in patients with lymphoma and multiple myeloma were comparable to those in healthy volunteers, supporting the current recommended dosing of plerixafor (240 mg/kg subcutaneous).88 Plerixafor was also tested in combination with chemotherapy and G-CSF for HSPC mobilization in patients with multiple myeloma and NHL. Addition of plerixafor to chemo-mobilization accelerated the rate of increase in CD34+ cells.89 However, the use of chemotherapy in plerixafor-based mobilization regimens is unlikely to be justified since adequate yield of HSPCs can be collected without subjecting the patient to the toxicities of chemotherapy. A retrospective study comparing plerixafor plus G-CSF to a historical cohort mobilized with chemotherapy plus G-CSF showed similar cost but more predictable days of leukapheresis and less hospitalization in the plerixafor plus G-CSF group.90
Phase III studies with plerixafor
Two phase III, multicenter, randomized, double-blind, placebo-controlled studies compared plerixafor plus G-CSF to G-CSF alone for mobilization of stem cells in patients with MM and NHL.91, 92 Patients received G-CSF at 10 μg/kg/day for 4 days and on the evening of the fourth day they received either plerixafor at 240 μg/kg sc or placebo. The leukaphereses were started on day 5, after the morning dose of G-CSF, and continued until CD34+ cell yield was ≥5 × 106/kg (NHL) or ≥6 × 106/kg (MM) or a total of 4 leukaphereses. Patients continued receiving their morning dose of G-CSF and evening dose of study drug until collection was completed. Patients who failed to collect ≥2 × 106 CD34+ cells/kg with G-CSF alone were eligible for rescue with plerixafor plus G-CSF.
In the NHL trial (N=298), 59% of patients in the plerixafor arm reached the primary endpoint of 5 × 106 CD34+ cells/kg compared to 20% in the placebo arm (P < 0.001). Importantly, 130/150 (87%) of patients in the plerixafor arm and only 70/148 (47%) in the placebo arm reached the secondary endpoint of at least 2 × 106 CD34+ cells/kg (P < 0.001). Patients failing to yield at least 2 × 106 CD34+ cells/kg were eligible for ‘rescue’ mobilization with plerixafor plus G-CSF. After rescue therapy, 33/52 patients from the placebo arm, and 4/10 patients from the plerixafor arm had successful remobilization.93 A total of 35% of patients in the placebo arm failed the mobilization process versus 7% of patients in the plerixafor arm. In the multiple myeloma trial (N=302), the primary endpoint of 6 × 106 CD34+ cells/kg was met in 72% of patients from the plerixafor group and only 34% from the placebo group (P < 0.001).
In both the MM and NHL studies plerixafor was well tolerated with minimal side-effects. Patients receiving transplants had rapid hematopoietic recovery and durable grafts across all treatment groups.91, 92 On the basis of the results of these two phase III randomized placebo controlled trials, plerixafor was FDA-approved, in combination with G-CSF, for HSPC mobilization in patients with NHL and multiple myeloma in December 2008.
Use of plerixafor in allogeneic transplantation
Plerixafor was tested for HSPC mobilization in allogeneic transplantation.94 Normal sibling donors were mobilized with plerixafor 240 μg/kg subcutaneously and underwent leukapheresis 4 hours later. The FDA mandated for the first 8 patients that we also collect, after a 10-day washout period, a G-CSF mobilized backup product. Two-thirds of the donors mobilized with plerixafor alone yielded the minimum goal of 2 × 106 CD34+ cells/kg recipient body weight after a single leukapheresis (100% after two collections; 20L/apheresis). Allografts mobilized with plerixafor contained less CD34+ cells and higher numbers of T, B and NK cells compared to G-CSF mobilized allografts (Table 1). With a median follow-up of 277 days after allo-transplantation, engraftment was prompt, acute GVHD (grades 2–4) occurred in 35% of patients, and no unexpected adverse events were observed. It is possible that the allografts would have contained higher yields of CD34+ cells if leukapheresis were started 6–10 hours after plerixafor, which is considered the peak of mobilization in both patients and normal allogeneic donors. Several ongoing studies are testing different routes of administration (intravenous vs. subcutaneous), doses, and schedules of plerixafor alone or in combination with G-CSF for HSPC mobilization (Table 2).
Table 1.
Comparison of HSPC mobilization by plerixafor and/or G-CSF
| Characteristic | Species | Comparison of Plerixafor to G-CSF | Reference |
|---|---|---|---|
| Time of peak CD34+ cell mobilization | Human | Plerixafor induces peak mobilization of CD34+ cells within 4–9 hours while G-CSF requires 4–5 days | 80, 94, 226, 254–256 |
| Magnitude of CD34+ cell mobilization | Human | Plerixafor alone mobilizes fewer CD34+ cells/μL blood than G-CSF and plerixafor-mobilized grafts contain fewer CD34+ cells/kg recipient body weight compared with G-CSF. | 94 |
| Human | Plerixafor significantly increases both G-CSF-stimulated mobilization of CD34+ cells and leukapheresis yields of CD34+ cells. | 83, 84, 86, 87, 91–93, 228 | |
| CD34+ stem cell phenotype | Human | Plerixafor preferentially mobilizes a CD34dimCD45RA+CD123hiCXCR4hi plasmacytoid DC precursor | 97 |
| Primitive CD34+CD38− HSC mobilization | Human | Plerixafor and G-CSF-mobilized grafts have a higher proportion of primitive 34+38− cells than grafts mobilized with G-CSF alone | 228 |
| CFU content | Human | Plerixafor mobilizes decreased numbers of BFU-E & CFU-GEMM compared to G-CSF | 226 |
| LTC-IC content | Human | Plerixafor mobilizes a higher frequency of LTC-IC compared to G-CSF | 228 |
| DC content | Human | Plerixafor and G-CSF-mobilized grafts have 2-fold more DCs (DC1 & DC2) than grafts mobilized with G-CSF alone | 257 |
| Frequency of SRCs | Human | Plerixafor mobilizes increased numbers of SRCs compared to G-CSF | 226, 258 |
| Cell cycle status | Rhesus macaque | Plerixafor-mobilized grafts have a higher proportion of CD34+ cells in G1 phase of cell cycle | 229 |
| CXCR4 expression | Rhesus macaque | Plerixafor-mobilized grafts have a higher percentage of CD34+ cells expressing CXCR4 and VLA-4 | 229 |
| Human | Plerixafor and G-CSF-mobilized CD34+ cells express more CXCR4 than CD34+ cells mobilized with G-CSF alone | 227, 228 | |
| VLA-4 expression | Rhesus macaque | Plerixafor-mobilized grafts have a higher percentage of CD34+ cells expressing VLA-4 | 229 |
| microRNA and cDNA expression | Rhesus macaque and Human | Plerixafor-mobilized HSPCs have unique microRNA and cDNA expression profiles compared to CD34+ cells mobilized with G-CSF alone | 227, 229, 259, 260 |
| Lymphocyte Content | Human | Grafts mobilized with plerixafor contain more T, B and NK cells | 94 |
Abbreviations: HSPC, hematopoietic stem and progenitor cell; G-CSF, granulocyte colony-stimulating factor; BFU-E, burst-forming unit-erythroid; CFU, colony forming unit; GEMM, granulocyte erythroid macrophage, megakaryocyte; LTC-IC, long-term culture-initiating cell; DC, dendritic cell; DC1, type 1 DC; DC2, type 2 DC; SRC, SCID repopulating cell
Table 2.
Ongoing clinical trials using CXCR4 inhibitors for HSPC mobilization
| Indication | Institution | Study Title | ClinicalTrials. gov Identifier |
|---|---|---|---|
| Autologous transplantation | Washington University School of Medicine | A phase I/II study of intravenous AMD3100 added to a mobilization regimen of G-CSF to increase the number of autologous peripheral blood stem cells collected from patients with lymphoma | NCT00733824 |
| Autologous transplantation | Cincinnati Children’s Hospital | AMD3100 in combination with G-CSF to mobilize peripheral blood stem cells in patients with Fanconi anemia: a phase I/II study | NCT00479115 |
| Autologous transplantation | Duke University | An observational study of plerixafor mobilization rescue for autologous stem cell transplant patients with inadequate response to G-CSF | NCT00901225 |
| Autologous transplantation | Mayo Clinic | Phase II trial of intravenously administered AMD3100 for stem cell mobilization in patients with multiple myeloma undergoing autologous stem cell transplantation following a lenalidomide-based initial therapy | NCT00998049 |
| Autologous transplantation | CancerCare Manitoba | Open Label Phase II Trial of an Augmented Mobilization Strategy With Plerixafor in a Population at Risk for Poor Stem Cell Mobilization | NCT01037517 |
| Autologous transplantation | City of Hope Medical Center | A Phase I/Pilot Study of Intravenous Plerixafor Following Cyclophosphamide Mobilization in Patients With Multiple Myeloma | NCT01074060 |
| Autologous transplantation | Emory University | Evaluation of Plerixafor in Combination With Chemotherapy and G-CSF for CD34+ Cell Mobilization | NCT01095757 |
| Autologous transplantation | Emory University | WCI1680-09: Evaluation of Alterations in Time of Administration of Plerixafor in Combination With G-CSF on Safety and CD34+ Cell Mobilization | NCT01149863 |
| Autologous transplantation | Fred Hutchinson Cancer Research Center | Mobilization of Autologous Peripheral Blood Stem Cells in CD20+ Lymphoma Patients Using RICE, G-CSF, and Plerixafor | NCT01097057 |
| Autologous transplantation | Sheba Medical Center | Plerixafor + Recombinant Human G-CSF for Autologous Peripheral Blood Stem Cell Transplantation in Hard to Mobilize Patients: a Phase IIB Study | NCT01164345 |
| Autologous transplantation | University of Liverpool | A Comparison of Plerixafor/G-CSF With Chemotherapy/G-CSF for Stem Cell Mobilisation | NCT01186224 |
| Autologous transplantation | University of Heidelberg | A Phase IIa, Proof of Concept Study is to Determine the Degree of Mobilisation of CD34+ Cells Following Administration of POL6326 in Patients With Multiple Myeloma | NCT01105403 |
| Autologous transplantation | Chaim Sheba Medical Center | A Phase I/IIA, Non-Randomized, Open Label, Single Dose, Dose-Escalation, Safety Study of BKT140, a CXCR4 Antagonist in Patients With Multiple Myeloma | NCT01010880 |
| Autologous transplantation | Multiple | A Phase II, Randomized, Open-Label, Multi-Center Study to Evaluate the Safety, Pharmacokinetics, and Hematopoietic Stem Cell Mobilization of TG-0054 in Patients With Multiple Myeloma, Non-Hodgkin Lymphoma or Hodgkin Disease | NCT01018979 |
| Allogeneic transplantation | Washington University School of Medicine | A phase II study evaluating the safety and efficacy of intravenous AMD3100 for the mobilization and transplantation of HLA-matched sibling donor hematopoietic stem cells in patients with advanced hematological malignancies | NCT00914849 |
| Healthy donors | Charite Research Organisation GmbH | A Single Center, Open-label, Repeated Dose, Phase I Study in Healthy Subjects to Assess the Safety, Tolerability, Pharmacokinetics and the Effect on Mobilization of Hematopoietic Stem Cells of NOX-A12 Alone and in Combination With Filgrastim | NCT01194934 |
| Allogeneic transplantation | MD Anderson Cancer Center | G-CSF and plerixafor with busulfan and fludarabine for allogeneic stem cell transplantation for myeloid leukemias | NCT00822770 |
| Allogeneic transplantation | Fred Hutchinson Cancer Research Center | A Pilot Study of Combined Plerixafor + Filgrastim for Mobilization of Peripheral Blood Stem Cells From Normal Donors | NCT01076270 |
| Allogeneic transplantation | St. Jude Children’s Research Hospital | A Pediatric Study of a Plerixafor Containing Regimen In Second Allogeneic Stem Cell Transplantation | NCT01068301 |
Abbreviations: G-CSF, granulocyte colony stimulating factor.
The pharmacokinetics of subcutaneous plerixafor requires that it be administered the night before leukapheresis so that the morning collection would correspond to the peak of the circulating HSPCs. Such administration is associated with inconvenience and additional cost. To improve the kinetics of mobilization, intravenous plerixafor is being tested in both autologous and allogeneic HSPC transplant clinical trials (Table 1). In our Phase I allogeneic transplant trial, twenty-one healthy donors were initially mobilized with increasing doses of intravenous plerixafor (80, 160, 240, 320, 400 or 480 μg/kg).95 After 4 days of drug clearance, the same donors were then mobilized with a single subcutaneous dose of 240 μg/kg plerixafor followed 4 hours later by leukapheresis.
Peak numbers of circulating CD34+ cells were observed 4–6 hours after intravenous dosing (vs. 6–9 hours after subcutaneous dosing) and donors given 240 μg/kg intravenous plerixafor, had higher peak levels of CD34+ cells/μL compared to the same donors who received 240 μg/kg subcutaneous plerixafor. There was a clear dose-response relationship of intravenous plerixafor on mobilization of CD34+ HSPCs, with the 320 μg/kg dose yielding a maximum 8-fold increase in circulating CD34+ cells at 4 hours after injection. We also noted that intravenous dosing (especially doses > 240 μg/kg) resulted in prolonged mobilization of CD34+ cells such that levels approached 20 CD34+ cells/μL at 24 hours after intravenous dosing.
In our Phase II study, twenty-eight sibling donors were treated with plerixafor at a dose of 320 μg/kg by intravenous injection, followed 4 hours later by leukapheresis. Successful mobilization was defined as a minimum leukapheresis yield of ≥ 2.0 × 106/kg CD34+ cell/kg actual recipient body weight. Six of twenty-three evaluable donors (26%) did not achieve the minimum cell dose of 2 × 106 CD34+ cells/kg in a single 20 liter leukapheresis procedure.96 This mobilization failure rate of 26% (6/23) with 320 μg/kg intravenous plerixafor is similar to the failure rate of 33% (15/45) we observe following administration of 240 μg/kg subcutaneous plerixafor. Four of the six patients who failed to collect ≥ 2.0 × 106 CD34+ cells/kg recipient body weight after their first leukapheresis procedure met goal after a second mobilization and collection procedure. In both subcutaneous and intravenous plerixafor studies only 3/68 normal allogeneic donors (4.4%) failed to mobilize > 2 × 106 CD34+ cells/kg after two 20 liter apheresis procedures.
Plerixafor and G-CSF mobilize phenotypically different CD34+ cell subsets
While evaluating the CD34+ cells obtained from the 8 healthy donors mobilized sequentially with plerixafor and G-CSF in our allogeneic transplant trial, we found that plerixafor mobilized a population of CD34dim cells which were present on average in nearly 5-fold higher numbers compared to the G-CSF-mobilized CD34+ cells.97 Previous studies by others have demonstrated that human CD34+ cells can be divided into at least three distinct subsets based on their cell surface expression of CD45RA and CD123 (IL-3Rα): (i.) CD34+CD45RA−CD123+/− cells containing common myeloid progenitors (CMPs), megakaryocyte/erythrocyte progenitors (MEPs) and more primitive HSPCs, (ii.) CD34+CD45RA+CD123+/− cells containing more committed granulocyte/macrophage (GMP) and lymphoid progenitors, and (iii.) CD34dimCD45RA+CD123hi cells.98, 99 Strikingly, we observed that plerixafor preferentially mobilized CD45RA+CD123hi cells, with the relative frequency of this CD34dim population increasing from 2% before treatment to 18% at 4 hours after the administration of the drug. In contrast, G-CSF mobilized grafts were enriched for CD34+CD45RA−CD123+/− cells (86% of the CD34+ cells) and contained less than 1% of the CD34dimCD45RA+CD123hi cells. Further analyses by flow cytometry determined that the CD34dim population represents a plasmacytoid pro-DC2 (for progenitor of pre-dendritic cell type 2) progenitor compartment as indicated by their CD45RA+CD123hiBDCA-2+BDCA-4+CD36+CD4dimCD25−CD13− phenotype. Of interest, two of the key molecules responsible for stem cell homing, retention and trafficking, CXCR4 and VLA-4, were significantly over-expressed in the CD34dimCD45RA+ subset compared to the CD34+CD45RA− and CD34+CD45RA+ cells.100
A summary of the major differences between G-CSF and plerixafor-mobilized stem cell grafts is shown in in Table 1. These differences were recently reviewed by Fruehauf et al.101 and clearly indicate that the composition of G-CSF and plerixafor-mobilized grafts are significantly different. What impact these different types of grafts have on the engraftment and function of HSPCs after hematopoietic stem cell transplantation remains largely unknown.
Alternative drugs in development targeting the CXCL12/CXCR4 axis
The success of plerixafor significantly addresses a number of the limitations involved with the use of G-CSF in hematopoietic stem cell mobilization. However, nearly one-third of healthy donors mobilized with plerixafor alone require more than one apheresis to obtain the minimal number of CD34+ cells (≥2.0 × 106 CD34+ cells/kg) necessary for transplantation. Furthermore, plerixafor was withdrawn from trials involving HIV patients due to its lack of oral bioavailability and cardiotoxicity. Importantly, the cardiac-related side effect of plerixafor was not a result of the compound’s ability to block CXCR4 function, but rather due to its presumed structural capacity for encapsulating metals. Because of these limitations, efforts to discover and develop potent and orally available inhibitors of the CXCR4/CXCL12 axis continue. Below we will discuss the status of some lead drug candidates that target the CXCR4/CXCL12 axis.
POL6326 (Polyphor) is a selective and reversible CXCR4 inhibitor belonging to a novel drug class based on Polyphor’s proprietary β-hairpin Protein Epitope Mimetics (PEM) Technology.102 The parent compound to POL6326, POL3026, was designed and optimized starting from the naturally occurring CXCR4 inhibitor polyphemusin-II.103, 104 The design involved incorporating residues from a truncated polyphemusin-II analogue (TC14011) into a macrocyclic template-bound β-hairpin mimetic. POL3026 binds at least 50–100-fold better to CXCR4 than does plerixafor. POL6326 was synthesized from POL3026 to improve its pharmacological properties while maintaining or improving potency and selectivity for CXCR4. A single injection of POL6326 to mice produces an 11–12-fold increase in circulating progenitor cells with a peak at 2–4 hours post-dosing.102 A Phase I clinical trial involving administration of POL6326 to 74 healthy volunteers was successfully completed in the UK in July 2008. A Phase IIa, proof of concept study to determine the degree of mobilization of CD34+ cells following intravenous administration of POL6326 in patients with multiple myeloma is ongoing (ClinicalTrials.gov Identifier NCT01105403).105
BKT-140 (4F-benzoyl-TN14003; Biokine Therapeutics) is a 14-residue polypeptide antagonist of CXCR4 that was also downsized from polyphemusin-II.106 BKT-140 binds CXCR4 with an affinity of approximately 1 nM and a single injection of the drug alone results in mobilization of murine CFU-Cs (> 6-fold increase over baseline) that exhibit long-term hematopoietic reconstituting activity.107, 108 Peripheral blood CFU-C levels peaked 2 hours after BKT-140 administration and returned to baseline by 24 hours. BKT-140 and G-CSF act synergistically to mobilize WBC and HSPCs. Comparative studies between BKT-140 and plerixafor suggest that the two compounds bind and inhibit CXCR4 via different mechanisms.108–110 BKT-140 functions as an inverse agonist and binds CXCR4 residues in extracellular domains and regions of the hydrophobic core proximal to the cell surface. In contrast, plerixafor functions as an antagonist111 or weak partial agonist109, 110 and binds amino acids in the central hydrophobic core of CXCR4. Interestingly BKT140, but not plerixafor, selectively, specifically and rapidly stimulates human leukemia and myeloma cell death in vitro and in vivo.112 A Phase I/IIA, non-randomized, open label, single dose, dose-escalation, and safety study of BKT140 in patients with MM is now open (ClinicalTrials.gov Identifier NCT01010880).113
NOX-A12 (NOXXON Pharma) is a L-enantiomeric RNA oligonucleotide (Spiegelmer) that binds and neutralizes CXCL12.114 Spiegelmers are mirror-image nuclease-resistant ribonucleotides that bind and inhibit target molecules in a manner conceptually similar to antibodies.115, 116 The 45 nucleotide long NOX-A12 binds CXCL12 with an affinity of less than 1 nM and has an IC50 value of 300 pM in a Jurkat cell migration assay.114 Preclinical studies in the mouse and monkey indicate that NOX-A12 induces a dose-dependent mobilization of murine CFU-Cs and a >3-fold reversible mobilization of monkey WBCs with peak numbers of circulating cells being maintained between 3 and 8 hours post-injection. A first-in-man clinical trial with NOX-A12 was successfully completed in May 2010 (ClinicalTrials.gov Identifier NCT00976378) and a multiple-dose Phase I trial started in August 2010 (ClinicalTrials.gov Identifier NCT01194934). This second clinical study aims to determine the safety, tolerability, pharmacokinetics, and pharmacodynamics of repeated intravenous doses of NOX-A12 alone and in combination with G-CSF in healthy subjects.
TG-0054 (Taigen Biotechnology) is a CXCR4 antagonist of undisclosed structure. The compound blocks CXCL12 binding to CXCR4 with an IC50 value of 10 nM.117 In Phase I study, the drug exhibited a favorable safety and pharmacokinetic profile in healthy subjects.118 A single dose of TG-0054 at 1.12–4.40 mg/kg resulted in a 3- to 14-fold increase in CD34+ cell count starting at 2 hours, peaking at 4–6 hours after injection and declining to baseline within 24 hours. The mean number of circulating CD34+ cells averaged approximately 30 cells/μL at peak mobilization.118 A phase II study to evaluate the safety, pharmacokinetics, and hematopoietic stem cell mobilization of TG-0054 in patients with multiple myeloma, non-Hodgkin lymphoma or Hodgkin disease is currently open (ClinicalTrials.gov Identifier NCT01018979).
MDX-1338 (BMS-936564; Medarex/Bristol-Myers Squibb) is a fully human antibody that targets CXCR4.119, 120 Since MDX-1338 is an IgG4 antibody, it lacks complement dependent cytotoxicity (CDC) activity and antibody dependent cell mediated cytotoxicity (ADCC) activity. Preclinical studies have shown that MDX-1338 binds to CXCR4-expressing cells with low nanomolar affinity, blocks CXCL12 ligand binding to CXCR4 expressing cells and inhibits CXCL12 induced migration and calcium flux with low nanomolar EC50 values.120 In addition, MDX-1338 also reduced tumor growth in acute myelogenous leukemia and lymphoma xenograft models.119 A Phase I, dose escalation study of MDX-1338 as a monotherapy with chemotherapy is expected to enroll up to 34 patients with relapsed/refractory AML (ClinicalTrials.gov Identifier NCT01120457). Moreover, this trial is designed to establish and evaluate the safety, tolerability and maximum tolerated dose, as well as preliminary pharmacodynamics and efficacy of MDX-1338.
CXCR4 pepducins (Anchor Therapeutics) are highly stable synthetic lipopeptide pharmacophores that modulate CXCR4 activity from inside the cell membrane.121 The CXCR4 receptor compounds are derived from the intracellular loops of CXCR4 and act as antagonists of CXCR4 G-protein signaling.121 The pepducins PZ-218 and PZ-305 are based on the first intracellular loop of CXCR4, while PZ-210 targets the third intracellular loop.122 Preclinical studies have shown that all three of these CXCR4 pepducins inhibit CXCL12-mediated calcium flux and chemotaxis of human neutrophils.122 Furthermore, CXCR4 pepducins mobilized murine HSPCs that exhibit long-term hematopoietic reconstituting activity with efficacy similar to plerixafor.
ALX-0651 (Ablynx) is an anti-CXCR4 Nanobody® which is being developed for hematopoietic stem cell mobilization.123 Nanobodies® are a novel class of therapeutic single-domain proteins derived from the smallest antibody-binding fragment of camelid heavy chain only antibodies.124 Their discovery dates back to the early 1990s when Hamers-Casterman et al.125 found that sera of camels, dromedaries and llamas contain fully functional antibodies that lack light chains. These heavy-chain antibodies contain a single variable domain (VHH) and two constant domains (CH2 and CH3). The VHH domain is a perfectly stable polypeptide harboring the full antigen-binding capacity of the original heavy-chain antibody and forms the basis of the Nanobody technology. Ablynx reported the successful generation of two types of Nanobodies against CXCR4: highly potent antagonistic Nanobodies, as well as anti-CXCR4 Nanobodies with inverse agonist function.123 Furthermore, they report that a single, intravenous administration of anti-CXCR4 Nanobody resulted in rapid mobilization of stem cells in a pre-clinical animal model. ALX-0651 is reportedly a biparatopic Nanobody directed against two different epitopes of CXCR4.
AMD070 (Genzyme Corp.) is an orally bioavailable non-cyclam CXCR4 antagonist derived from plerixafor.126 Administration of AMD070 to HIV-1 infected patients suppressed the replication of X4 and dual/mixed strains of viruses.127, 128 Leukocytosis followed AMD070 administration in all subjects, ranging from 1.3-fold (50-mg single dose subject) to 2.9-fold (400-mg single dose subject) above baseline, with a peak between 2 and 4 hours after injection.128 Similar kinetics and magnitudes of mobilization where observed across al leukocyte subsets tested, which included neutrophils, lymphocytes, monocytes, basophils, eosinophils, CD4+ T cells, and CD34+ stem cells. AMD070 was slowly eliminated, with a terminal elimination half-life of 11 to 16 hours.128 Unfortunately, the FDA had to place AMD070 on clinical hold due to liver histology changes in long-term preclinical toxicity studies.
GSK812397 (GlaxoSmithKline) is a novel, orally bioavailable noncompetitive CXCR4 antagonist.129 The compound was developed from a structure-avidity study aimed at improving the antiviral properties of AMD070 through iterative structural modifications. Preclinical studies have shown that GSK812397 binds to CXCR4-expressing cells with low nanomolar affinity, blocks infection of host cells by X4-utilizing HIV-1 viruses and inhibits CXCL12 induced migration and calcium flux with low nanomolar EC50 values.129 The bioavailability of GSK812397 was similar across several species, including rat (21%), dog (21%), and monkey (17%). Furthermore, the half-life was greater than 12 h in both dog and monkey. No data has been published on the effects of GSK812397 on WBC and HSPC mobilization.
KRH-3955 (Kureha Chemical Industries) is an orally bioavailable, non-cyclam, non-peptide small molecule CXCR4 antagonist designed from KRH-1636.130 KRH-1636 was discovered through screening more than 1000 compounds from the Kureha Corp chemical library.131 Preclinical studies have shown that KRH-3955 binds to CXCR4-expressing cells with low nanomolar affinity, blocks infection of host cells by X4-utilizing HIV-1 viruses and inhibits CXCL12 induced calcium flux.130 The compound shows an oral bioavailability of 26% in rats and an intravenous half-life of 99 hours. The authors suggest that the long half-life of KRH-3955 is likely caused by its accumulation in tissues which may be disadvantageous in terms of toxicity.130 No data has been published on the effects of KRH-3955 on WBC and HSPC mobilization.
FC131 is a cyclopentapeptide [c(Gly1-D-Tyr2-Arg3-Arg4-Nal5); Nal is 2-naphthylalanine] developed by molecular size reduction of the 14-residue T140 CXCR4 antagonist.132 The size reduction (from T140 to FC131) was done based on the identification of the four bioactive amino acid residues Arg2, Nal3, Tyr5, and Arg14 of the T140 molecule. Fuji et al132 reported that FC131 exerts anti-HIV activity as well as CXCR4 antagonist activity equipotent to T140. Several analogues of FC131 have been reported, but all show lower potency. No data has been published on the effects of KRH-3955 on WBC and HSPC mobilization.
WZ811 (N1,N4-di-2-pyridinyl-1,4-benzenedimethanamine) is the lead candidate drug in a new class of CXCR4 antagonists that contain two aromatic amine moieties connected by a para-xylylene group.133 WZ811 is a potent inhibitor of binding of an SDF-1 peptide mimic to CXCR4 (EC50 = 0.3 nM), prevents CXCL12-mediated modulation of cAMP (EC50 = 1.2 nM) in cells and blocks CXCL12-induced Matrigel invasion by MDA-MB-231 human breast adenocarcinoma cells (EC50 = 5.2 nM).133 Studies to improve the pharmacokinetic profile of WZ811 led to the discovery of MSX-122 (WZ40). MSX-122 is an orally bioavailable small molecule that was produced by Metastatix, Inc. There is a report that MSX-122 induces leukocytosis in monkeys beginning 4–6 hours after administration with a peak total white blood cell count at 12–18 hours that was 1.5–2 fold higher than baseline.134 A phase I trial to determine the safety and pharmacokinetics of oral MSX-122 in patients with refractory metastatic or locally advanced solid tumors was suspended in June 2008 (ClinicalTrials.gov Identifier NCT00591682). No data has been published on the effects of WZ811 or MSX-122 on HSPC mobilization.
HSPC mobilizing agents that target VLA-4
Integrins
Integrins are a large family of transmembrane glycoproteins that mediate cell-cell and cell-matrix interaction and communication.135–137 All integrins are non-covalently linked, heterodimeric molecules containing a α and a β subunit. Each subunit contains a small cytoplasmic domain, a single transmembrane spanning region, and a large extracellular domain.138, 139 In vertebrates, 18 α and 8 β subunit genes have been identified and can combine to form 24 different integrin receptors, which vary in their ligand specificity, expression and signaling.140 Integrin β1 (CD29) associates with at least 12 different α subunits (α1- α11 and αV) and forms the largest integrin subfamily. The integrin chains α4, α5, α6 and α9 are expressed with β1 on HSPCs and play important roles in hematopoietic stem cell migration, homing and engraftment.141, 142
Integrins mediate a wide variety of physiological processes including adhesion, migration, survival, and differentiation of cells. These functions are largely controlled by the strength of the ligand-binding affinity of the integrin. Integrin affinity for extracellular ligands is regulated through receptor clustering by multivalent ligands and/or conformational changes in the integrins.143, 144 Extensive research has shown that integrins can adopt at least three major conformational states: (i.) inactive (low affinity), (ii.) primed or activated (high affinity) and (iii.) ligand occupied.144 Integrin activity is regulated through both the interactions of intracellular proteins with integrin cytoplasmic domains (inside-out activation) or by interactions with extracellular ligands (outside-in activation).144–146
The α4 integrin family
The α4 integrin family consists of two integrins, α4β1 (VLA-4)147 and α4β7148. Like most integrins, α4β1 normally exists in the inactive (low affinity) state. Although the precise mechanism of activation in vivo is not clear, VLA-4 can be activated in vitro in a variety of ways, including by ligand149, divalent cations149–151, monoclonal antibodies149–151, CXCL12152–155, IL-3156, c-kit ligand156, 157, GM-CSF156, stem cell factor158 and phorbol esters. Once activated, the primary counterligands for VLA-4 are vascular cell adhesion molecule-1 (VCAM-1; CD106)159, 160 and the alternatively spliced connecting segment 1 (CS-1) domain of fibronectin161, 162 found in the extracellular matrix.163 VLA-4 also binds to osteopontin, Mucosal Addressin Cell Adhesion Molecule 1 (MadCAM-1), thrombospondin, ICAM-4, and ADAM family members; however, the biological significance of these interactions is less clear.140 The primary ligand for α4β7 integrin is the immunoglobulin adhesion ligand MAdCAM-1.164, 165 Both VCAM-1 and MadCAM are normally expressed in the gastrointestinal tract, however VCAM expression extends into peripheral organs166, 167, while MadCAM expression is confined to organs of the gut.166, 168, 169
VLA-4 is constitutively expressed on most leukocytes, including monocytes, lymphocytes, eosinophils, basophils, and CD34+ hematopoietic precursorcells. The expression of VLA-4 on CD34+ HSPCs is known to be upregulated by IL-3 and SCF170 and down-regulated by G-CSF170–175. Studies by Lichterfeld et al.175 showed that the affinity and avidity of VLA-4 for a soluble VCAM1-Ig fusion protein was significantly reduced on circulating CD34+ after treatment with G-CSF in comparison to CD34+ cells from steady-state BM. Furthermore, the authors found that the number of circulating CD34+ cells following G-CSF administration was inversely related to the number of CD34+/VCAM1-Ig+ cells in the periphery, indicating that a low activation state of VLA-4 on CD34+ cells during G-CSF mobilization is associated with a higher number of circulating HSPCs.175 These observations suggest that both reduced VLA-4-expression and reduced VLA-4 avidity are associated with mobilization of CD34+ cells by G-CSF.
Genetic ablation of VLA-4 in mice
Both the α4 and β1 subunits of VLA-4 have been inactivated in mice. Table 3 summarizes the phenotypes of mice following genetic ablation of the α4 or β1 integrin subunits, with a particular emphasis on the impact each deletion has on HSPC formation, maintenance, distribution and migration. Since lack of α4 or β1 integrin is embryonic lethal due to nonhematological defects176–178, somatic chimeric mice were generated by injecting α4 or β1 integrin-deficient embryonic stem (ES) cells into wild-type (wt) or recombination activating gene (RAG)-deficient blastocysts. Although β1−/− HSPCs formed and were capable of differentiating into multiple cell lineages in vitro, they failed to migrate to the fetal liver (as well as adult sites of hematopoiesis) and establish hematopoiesis in the β1−/−/wt chimeras.178–180 In contrast, α4 integrins were not essential for HSPC colonization of the fetal liver in the somatic chimeric mice.181, 182 These data suggest that α subunits other than α4 integrin mediate the migration of integrin β1+ HSPCs to sites of hematopoiesis during fetal development. However, α4 integrin was absolutely required for hematopoietic maintenance and development, as the α4−/−/wt chimeras were incapable of completing erythroid, myeloid and lymphoid (B and T cell) differentiation in adult mice.181, 182
Table 3.
Phenotype of mice following genetic ablation of α4 or β1 integrin subunits
| Integrin | Model | Phenotype | Reference |
|---|---|---|---|
| β1 | β1−/− ES cells injected into wt or Rag-2−/− blastocysts | β1−/− HSPCs develop and can differentiate into different lineages in vitro, but fail to migrate to fetal liver | 178–180 |
| α4 | α4−/− ES cells injected into wt or Rag-2−/− blastocysts | α4−/− HSPCs develop and migrate to fetal liver, spleen and BM, but exhibit decreased erythroid, myeloid and lymphoid hematopoiesis | 181, 182 |
| α4 | MxCre-mediated ablation of floxed α4 in hematopoietic and nonhematopoietic cells | Eight-fold increase in circulating α4−/− CFU-Cs and increased sequestration of CFU-Cs in spleen. | 183 |
| α4 | Tie2Cre-mediated ablation of floxed α4 in hematopoietic and endothelial cells | Increased numbers of circulating CFU-Cs | 184 |
| α4 | Transplantation of wt and/or α4−/− BM cells into adult irradiated recipients | α4−/− BM cells exhibit impaired homing to the BM, increased numbers of circulating CFU-Cs and decreased competitive repopulating activity | 183–185 |
| α4 | E12.5 cells from wt or α4−/− mice cotransplanted with wt BM cells into adult irradiated recipients | α4−/− HSPCs displayedsimilar engraftment, multilineage differentiation, and competitive repopulation capacity compared to wt cells | 186 |
| β1 | β1−/− cells generated ex vivo were injected into lethally irradiated recipients | β1−/− HSPCs were not radioprotective and failed to migrate to the spleen and BM | 179 |
| β1 | MxCre-mediated ablation of floxed β1 in hematopoietic cells | No significant alterations in hematopoiesis or HSPC biodistribution | 187 |
| β1 & β7 | MxCre-mediated ablation of floxed β1 in β7−/− hematopoietic cells | Transient increase of CFU-Cs in the BM and peripheral blood at 2 mos after β1 deletion with return to baseline at 10 mos. | 188 |
| β1 & β7 | MxCre-mediated ablation of floxed β1 in β7−/− hematopoietic and nonhematopoietic cells | Eight-fold increase in circulating HSPCs (CFU-C). | 188 |
Abbreviations: ES, embryonic stem; Rag, recombination activating gene; E12.5; HSPC, hematopoietic stem and progenitor cell; BM, bone marrow; CFU-C, colony-forming unit-cells; wt, wild type; Embryonic day 12.5.
The Cre/loxP system of conditional gene ablation, where one or more critical exons is flanked by loxP (floxed) sites to allow cell-specific knockout, has been used to examine the role of α4 and β1 integrins on adult BM-derived HSPCs. In contrast to the striking inability of α4 or β1 integrin-deficient HSPCs to be maintained and differentiate in somatic chimeric mice, conditional deletion of α4 or β1 integrin in adult mice caused much less severe perturbations in hematopoiesis. Genetic ablation of floxed α4 integrin in both hematopoietic and nonhematopoietic compartments using Mx-Cre induced a 8-fold increase in the number of circulating HSPCs (CFU-Cs) compared with α4+/+ controls at 2 weeks after deletion.183 Interestingly, the circulating levels of CFU-Cs remained significantly elevated for at least 50 weeks after α4 integrin ablation and were accompanied by sequestration of CFU-Cs in the spleen. Similar long-term release of CFU-Cs into the peripheral blood was reported following deletion of floxed α4 integrin in hematopoietic and endothelial cells using Tie2Cre.184
To more clearly determine whether the alterations in progenitor biodistrubution were solely caused by the loss of α4 integrin on hematopoietic cells, BM cells from wild type (α4+/+) and α4-deficient animals were injected into lethally irradiated recipients and their homing, distribution and hematopoietic reconstitution potential were evaluated. Cells lacking α4 integrins displayed impaired homing to the BM, increased numbers of circulating CFU-Cs and a competitive disadvantage in both short-term and long-term hematopoietic reconstitution compared to normal competitors.183–185 These data suggest that expression of α4 integrin on HSPCs plays an important role in their retention within the BM microenvironment. Of note, different results were obtained following transplantation of wild-type or α4-deficient cells isolated from the tissues of embryonic day 12.5 (E12.5) embryos into lethally irradiated adult recipients.186 Here, α4 integrin-deficient cells displayedsimilar hematopoietic engraftment, multilineage differentiation, and competitive repopulation capacity compared to α4+/+ cells. The reason for this discrepancy between the two transplant models remains unclear.
Somewhat conflicting results have also been reported following genetic ablation of β1 integrin in adult BM-derived HSPCs. Initial experiments generated β1 integrin deficient HSPCs in vitro by transducing BM cells of mice homozygous for a floxed β1 integrin gene with a Cre recombinase retrovirus. Transplantation of these ex-vivo-generated β1−/− HSPCs into lethally irradiated recipients failed to provide radioprotection and BM engraftment.179 In contrast, no significant alterations were reported in the number, distribution, or function of β1 integrin-deficient HSPCs when they were generated in vivo using polyIC and the MxCre/loxP system.187 A similar lack of overt phenotype was reported when both β1 and β7, which are the only known partners of α4 integrin, were conditionally ablated in the hematopoietic system.188 However, a 8-fold increase in the number of circulating HSPCs was reported following genetic ablation of β1 in both the hematopoietic and non-hematopoietic systems of β7-deficient mice.188 This increase in circulating HSPCs is similar to the 8-fold increase in circulating CFU-Cs observed following genetic ablation of α4,183 and suggests that loss of β1 integrin function on nonhematopoietic cells might contribute to the release of HSPCs from the bone marrow to the peripheral blood.
Mobilization of HSPCs by anti-α4 integrin antibodies
Studies with antibodies have also suggested that the VLA-4 receptor plays an important role during the migration of HSPCs to the BM and their retention in the BM. Treatment of donor BM cells with an α4 integrin antibody before injection into lethally irradiated recipients inhibits their homing to the femurs of recipient mice.189, 190 This inhibition of BM homing was accompanied by an increase in the number of HSPCs in the peripheral blood and spleen. Since similar results were obtained by pre-treatment of recipients with an antibody to VCAM-1,189 a primary ligand for VLA-4, the data indicate that the VLA-4/VCAM-1 axis plays an important role in the initial stages of HSPC homing to the BM.
Additional studies have shown that in vivo administration of antibodies to VLA-4 increases the number of circulating HSPCs in mice, primates, and humans. The blockade of α4 integrin in mice189–194 and nonhuman primates191, 193, 195 results in rapid (<8 hours) and prolonged (> 10 days) mobilization of HSPCs. Both CFU-C assays and transplantation experiments definitively demonstrated that the anti-α4 integrin antibody mobilized committed progenitors and long-term repopulating cells. A functional kit receptor is required for HSPC mobilization by anti-α4 integrin antibody194 and either additive or synergistic mobilization of HSPCs was observed when the antibody was combined with G-CSF193, 195, plerixafor191, kit ligand193 and/or Flt3-ligand193. This enhanced mobilization of HSPCs following addition of G-CSF, plerixafor and/or Flt3-ligand to the anti-α4 integrin antibody therapy is likely mediated via their disruption of CXCR4/CXCL12 signaling in the bone marrow.196 Finally, although no significant mobilization is observed following blockade of β2 integrin alone, synergistic mobilization of murine and primate HSPCs occurs following concurrent inhibition of β2 integrin and blockade of VLA-4 by administration of an anti-α4 integrin antibody.192
Elevated circulating levels of HSPCs are also observed following treatment of multiple sclerosis(MS) patients with an anti-VLA4 antibody. Natalizumab (Tysabri;Biogen/Idec, Cambridge, MA) is a recombinant humanized neutralizing IgG4 monoclonal antibody that binds to the α4 subunit of the α4 β1 (VLA-4) and α4 β7 integrins. Natalizumab is approved by the FDA for the treatment of Crohn’s disease and relapsing MS and is postulated to function in these conditions by inhibiting the transmigration of leukocytes through the blood-brain barrier (MS) and intestines (Crohn’s). Similar to mice and nonhuman primates following anti-α4 integrin antibody administration, natalizumab-treated MS patients display a rapid and sustained increase in circulating HSPCs.197–199 Both CD34+ cell counts and CFU-Cs were significantly increased within 24 hours after a single injection of natalizumab to previously untreated MS patients. Overall, the number of circulating CD34+ cells/μL blood increased 3- to 5-fold during the first 72 hours after treatment and remained elevated at these levels (8–10 CD34+ cells/μL) for at least a month after natalizumab injection.197–199 Somewhat surprisingly, repeated administration of the antibody failed to mobilize additional CD34+ cells. To put the magnitude of CD34+ cell mobilization by natalizumab in context with other mobilizing regimens, the number of circulating CD34+ cells in natalizumab-treated MS patients are approximately one-sixth and one-third of those observed in G-CSF- and plerixafor-treated healthy donors, respectively.13, 94, 197–199 Finally, limited phenotyping studies on the CD34+ cells mobilized by natalizumab indicated that they were primarily quiescent (>90% in G0), failed to transmigrate towards SDF-1, and belonged to the subset of more committed progenitors co-expressing CD38 and enriched for erythroid BFU-E.197–199
Although natalizumab has shown clinical efficacy and provided validation for the involvement of the α4 integrin pathway in autoimmune and inflammatory conditions, its use as a clinical stem cell mobilizing agent in normal healthy donors is not justifiable because of its prolonged immune-modulating effects. More specifically, in addition to the prolonged mobilization of CD34+ HSPCs, natalizumab also produces a lymphocytosis that can be sustained for at least a month after antibody administration.200–204 This prolonged disruption of normal lymphocyte trafficking poses unacceptable risks to a healthy individual. Indeed, MS patients undergoing natalizumab monotherapy are at risk of developing progressive multifocal leukoencephalopathy (PML).205, 206 PML is a rare and often fatal demyelinating disease of the central nervous system caused by infection of oligodendrocytes and astrocytes by the John Cunningham polyomavirus. As of May 2010, a total of 49 cases of PML were reported among approximately 67,700 natalizumab-treated patients worldwide.205
Clinical trials with small molecule α4 integrin antagonists
Because natalizumab has shown clinical efficacy in the treatment of MS and Crohn’s disease, the development of small molecule VLA-4 antagonists is seen as a promising pharmaceutical approach to a novel class of therapeutic agents.207–210 Small molecule antagonist research in the α4 integrin area has been ongoing for nearly 20 years and over 250 patents describing α4 integrin antagonists as potential anti-inflammatory agents have been published.207 The small molecule antagonists of α4 integrin that have progressed into clinical trials are summarized in Table 4 and can be classified into two major structures: (1) the tripeptide motif of leucine–aspartic acid–valine (LDV) mimics, whose sequence is responsible for VLA-4 interaction with fibronectin or (2) N-acylphenylalanine-based compounds.208–210 In general, the LDV-based antagonists show high potency and selectivity for α4β1 (VLA-4) whereas the N-acylphenylalanine derivatives exhibit dual inhibitory activity for both α4β1 and α4β7. Early studies with the α4 integrin antagonists suffered from poor bioavailability and a short half-life. More recent trials with orally active new generation antagonists like SB-683699/Firategrast208, 211, AJM-300212, 213, and Compound 14e214 suggest that these compounds exhibit more favorable pharmacological properties. Finally, although many of the clinical trials listed in Table 4 have reported a transient lymphocytosis following antagonist administration, there is no published information on the effects these different α4 integrin antagonists have on CD34+ HSPC mobilization.
Table 4.
Summary of α4 integrin antagonists tested in human clinical trials
| Drug | Company | Class | Disease | Clinical trial status | Reference |
|---|---|---|---|---|---|
| Bio-1211 | Biogen/Merck | LDV | Asthma | Phase II | 261 |
| IVL-745 | Aventis | LDV | Asthma | Phase II | 262 |
| TBC-4746 | Encysive/Schering Plough | LDV | Asthma/MS | Phase I | 208 |
| DW-908e | Daiichi-Sankyp/Pharmacopeia | LDV | Asthma | Phase I | 263 |
| R-411/Valetegrast | Roche | N-acyl phenylalanine | Asthma | Phase II | 264 |
| AJM-300 | Ajinomoto | N-acyl phenylalanine | IBD | Phase II | 212, 213 |
| SB-683699/Firategrast | GlaxoSmithKliine | N-acyl phenylalanine | MS | Phase II | 208, 211 |
| CDP323 | UCB/Biogen | N-acyl phenylalanine | MS | Phase II | 207 |
| Compound 14e | Daiichi Sankyo | N-acyl phenylalanine | NA | Phase I | 214 |
Abbreviations: LDV, leucine–aspartic acid–valine; MS, multiple sclerosis; IBD, inflammatory bowel disease.
Mobilization of murine HSPCs by small molecule antagonists of VLA-4
The mobilization of murine, primate and human HSPCs by α4 integrin antibodies led to the hypothesis that small molecule antagonists of α4 integrins would represent a novel approach to mobilizing HSPCs. BIO5192 [2(S)-{[1-3,5-dichloro-benzenesulfonyl)-pyrrolidine-2(S)-carbonyl]-amino}-4-[4-methyl-2(S)-(methyl-{2-[4-(3-o-tolyl-ureido)-phenyl]acetyl}-amino)-pentanoylamino]-butyricacid] is a potent (Kd of < 10 pM) and highlyselective small molecule inhibitor of both the unactivated and activated forms of human, mouse, and rat α4β1 integrin.215 BIO5192 is an LDV-based α4 integrin antagonist and exhibits high affinity for α4β1 integrin because of an extremely slow dissociation rate (dissociation half-life >12h) of the inhibitor from both the unactivated and activated states of α4β1 integrin. The affinity of BIO5192 for α4β1 integrin is at least 250-fold higher than for α4β7 integrin.215
Similar to the clinical trials involving α4 integrin antagonists discussed above (Table 4), initial preclinical studies using murine experimental models of autoimmune encephalomyelitis and other inflammatory diseases showed induction of leukocytosis and lymphocytosis following BIO5192 administration.215 To determine whether HSPCs can be mobilized into the peripheral circulation by BIO5192, we treated mice with BIO5192 alone or in combination with plerixafor and/or G-CSF. We reported that blockade of α4β1 integrin with a single injection of BIO5192 alone results in a 30-fold increase in mobilization of murine CFU-Cs over basal levels.216 Peripheral blood CFU-GM levels peaked 0.5 to 1 hour after BIO5192 administration and returned to baseline by 6 hours. A similar magnitude of CFU-C mobilization was observed following treatment with plerixafor alone. Interestingly, an additive affect on HSPC mobilization was observed when BIO5192 was combined with plerixafor (3-fold compared to plerixafor alone), G-CSF (5-fold compared to G-CSF alone) or their combination (17-fold compared to G-CSF alone).216 Importantly, BIO5192 mobilized long-term repopulating cells that successfully engraft and expand in a multi-lineage fashion in secondary transplant experiments. Since BIO5192 will not be clinically developed, we have performed identical studies with similar results using the small molecule VLA-4 antagonist firategrast, which is currently in clinical development for the treatment of multiple sclerosis by GlaxoSmithKline (data not shown). These data provided the first evidence for the utility of small molecule inhibitors of VLA-4 either alone or in combination with G-CSF or plerixafor for mobilization of HSPCs.
To better understand the relationship between CXCR4 and VLA-4 in HSPC mobilization, Christopher et al.196 generated CXCR4−/− bone marrow chimeras by transplanting CXCR4−/− fetal liver cells into lethally irradiated wild type recipient mice. Following hematopoietic reconstitution, the CXCR4−/− chimeras were used to investigate the function of different mobilization agents in the absence of CXCR4/CXCL12 signaling. Surprisingly, mobilization by G-CSF or Groβ was completely abrogated in CXCR4−/− bone marrow chimeras.196 In contrast, HSPC mobilization by BIO5192 was robust; exhibiting a nearly 3000-fold increase in the number of circulating CFU-Cs in the CXCR4−/− chimeras compared to untreated control mice. These observations suggest that α4 integrin antagonist-induced mobilization of HSPCs occurs independently of CXCR4/CXCL12 signaling and further support the notion that CXCR4 and VLA-4 are the dominant receptors regulating HSPC migration from and retention within the bone marrow.
Summary
Plerixafor represents a significant advance in stem cell mobilization. Mobilization with G-CSF plus plerixafor in autologous stem cell mobilization decreases the number of patients who fail to collect the minimum number of CD34+ stem cells necessary for transplantation.91, 92 Even in patients who are unlikely to fail mobilization with G-CSF alone, plerixafor provides the benefits of a higher stem cell yield and fewer apheresis procedures. Consequently, fewer patients will be transplanted with suboptimal numbers of HSPCs, which can lead to delayed hematopoietic recovery and the associated increases in blood transfusions, rates of infection and length of hospitalization.
Plerixafor is also effective as a single agent in the allogeneic transplant setting. We have demonstrated that hematopoietic stem cells mobilized by plerixafor alone are functional and provide prompt and durable hematopoietic engraftment following transplantation into HLA-identical siblings with advanced hematological malignancies.94 These preliminary data demonstrate that the length of time required to mobilize and procure a functional hematopoietic allograft can be reduced from a 5-day to a 1-day process by directly targeting the CXCR4/CXCL12 axis. Unfortunately, nearly one-third of healthy donors mobilized with plerixafor alone fail to collect the minimal number of CD34+ cells (≥2.0 × 106 CD34+ cells/kg recipient body weight) necessary for transplantation in a single leukapheresis. One strategy being explored to reduce this mobilization failure rate is to design and develop a more potent CXCR4 inhibitor. Several novel CXCR4 inhibitors in various stages of clinical development were discussed in this review. Alternatively, since an additive affect on murine HSPC mobilization is observed when plerixafor is combined with the VLA-4 antagonist BIO5192216, a more successful mobilization regimen might involve the co-administration of small molecule inhibitors to CXCR4 and VLA-4. In general, the development of a G-CSF-free mobilization regimen is attractive both to avoid potential toxicities of G-CSF and to save time and resources during a 4–5 day G-CSF-based mobilization.
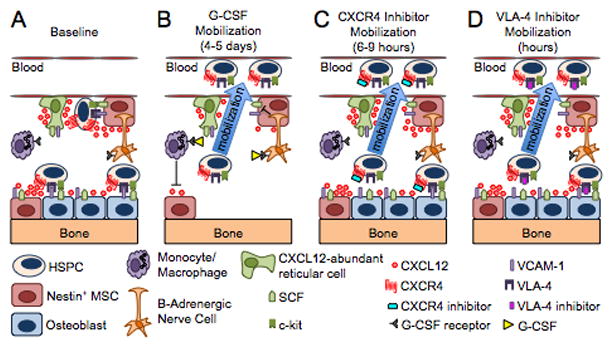
Recent research has shown that G-CSF induces HSPC mobilization by phagocyte depletion and modulation of the sympathetic nervous system (Figure 1).217–224 Cxcl12 down-regulation is critical in both of these processes217–224, and no G-CSF-mediated mobilization is observed following neutralization of CXCR4 with monoclonal antibodies225 or in CXCR4−/− BM chimeras196. These data indicate that disruption of the CXCR4/CXCL12 axis plays a dominant role in HSPC mobilization by G-CSF. However, the observation that a single injection of plerixafor can synergize with multiple injections of G-CSF indicate that the mechanisms involved in G-CSF and plerixafor HSPC mobilization are not completely overlapping.91, 92, 226 Combining the effects of G-CSF (phagocyte signaling and depletion, loss of osteoblasts, down-regulation of HSPC retention genes like Cxcl12 in Nestin+ niche cells), with pharmacologic inhibition of CXCR4 by plerixafor more effectively inhibits the CXCR4/CXCL12 axis and results in increased HSPC mobilization in vivo. Indeed, Chow et al.217 recently showed that mimicking one effect of G-CSF administration, in vivo phagocyte depletion, prior to plerixafor treatment, induced a two-fold increase in the magnitude of HSPC mobilization by the drug. Further, others and we have shown that plerixafor mobilizes HSPCs expressing higher levels of CXCR4 both before and during G-CSF administration.7, 100, 227–229 Although not tested experimentally, it is tempting to speculate that these high CXCR4 expressing HSPCs are tethered to the BM microenvironment in a predominantly CXCR4-dependent manner and are less reliant on other adhesive interactions for the their retention in the bone marrow niche. Alternatively, G-CSF and plerixafor may mobilize HSPCs from different niches within the bone marrow environment. In this regard, putative HSPCs have been found near the endosteum lined by osteoblasts (endosteal niche) and in association with sinusoidal endothelium (perivascular niche).4, 8
Figure 1. Models of HSPC mobilization by G-CSF and inhibitors of CXCR4 and VLA-4.
(A) Bone marrow environment at baseline. HSPCs in the endosteal niche are likely in close contact with osteoblasts and nestin+ mesenchymal stem cells (MSCs), both of which express numerous HSPC retention factors, including CXCL12, VCAM-1 and SCF. The perivascular niche is more distant from the endosteum and includes both nestin+ MSCs and CXCL12 abundant reticular cells. Cells of the monocytic lineage support the maintenance of osteoblasts and MSCs. β-adrenergic nerve cells of the sympathetic nervous system regulate MSC proliferation and induce circadian oscillations of CXCL12 expression. (B) Model of G-CSF-induced HSPC mobilization. Cells of the monocytic lineage express the receptor for G-CSF and provide factors that support the survival of MSCs and osteoblasts. Upon 4 to 5 days of stimulation with G-CSF, the monocytes/macrophages disappear, leading to the loss of osteoblast lineage cells and reduced expression of CXCL12, VCAM-1, and SCF on MSCs. Reduced expression of these key HSPC retention factors is also observed following G-CSF signaling through β-adrenergic nerve cells. The net effect of these signaling cascades is the disruption of HSPC retention interactions and mobilization of HSPCs into the peripheral blood. (C–D) Model of HSPC mobilization by inhibitors of CXCR4 or VLA-4. Targeted disruption of the interaction of CXCR4 or VLA-4 with their ligands results in the rapid (within hours) and reversible mobilization of HSPCs into the peripheral circulation. An additive or synergistic affect on HSPC mobilization is observed when a CXCR4 inhibitor is combined with a VLA-4 antagonist, G-CSF or their combination. When used alone, inhibitors of CXCR4 and VLA-4 mobilize fewer HSPCs than G-CSF.
Numerous reports have documented the importance of the VCAM-1/VLA-4 axis in modulating the homing and retention of HSPCs within the BM microenvironment. The observation that HSPCs are rapidly released into the peripheral circulation following targeted disruption of the VCAM-1/VLA-4 axis indicate that this interaction provides an alternative pathway for HSPC mobilization that is independent of the CXCR4/CXCL12 axis.189–194, 196–199, 216 This concept is further supported by the additive or synergistic mobilization of HSPCs that is observed following the addition of a VLA-4 inhibitor to a plerixafor and/or G-CSF-based mobilization regimen.191, 193, 216 Interestingly, although disruption of the CXCR4/CXCL12 axis via Cxcl12 down-regulation appears to play a dominant role in HSPC mobilization by G-CSF196, it is important to note that the growth factor also down-regulates the expression of transcripts encoding other HSPC retention genes, including SCF and VCAM-1, in cells that comprise the BM niche.222 Downregulation of these alternative genes involved in HSPC retention within the BM microenvironment may be an additional mechanism whereby G-CSF induces greater mobilization of HSPCs relative to a specific inhibitor of CXCR4 like plerixafor. It is also important to note that the binding of CXCL12 to CXCR4 enhances the adhesive properties of HSCs by inside-out signaling leading to activation of the integrins VLA-4, VLA-5, and LFA-1.153, 230–233 Since the CXCR4/CXCL12 and VCAM-1/VLA-4 axes interact in regulating HSPC trafficking and adhesion to the BM, others and we have sought to increase HSPC mobilization by co-administering inhibitors to both CXCR4 and VLA-4.191, 216 This dual inhibitor approach may ultimately provide a more efficient method to collect a functional hematopoietic graft in a single day.
Following disruption of the adhesive interactions mediating stem cell retention in the bone marrow niche, HSPCs must transit from the bone marrow parenchyma through the endothelium and into the sinusoids, where they are temporarily retained until their release into the general circulation. Relatively little is known about the physiologic mechanisms that govern the egress of HSPCs into the blood. Recent investigations have unveiled a role for the sphingosine-1-phosphate receptor 1 (SIP1) in mobilizing HSPCs from the BM under steady state conditions and following CXCR4 antagonist mediated mobilization.234–236 Receptors for sphingosine-1-phosphate (S1P) are present on human CD34+ HSPCs and mediate their chemotactic response towards S1P as well as S1P-induced upregulation of integrins.236–240 Although controversial, a shifted balance between intra- and extra-BM S1P might therefore play a role on HSPC mobilization into the peripheral blood. Interestingly, administration of a S1P1 agonist, SEW2871, enhanced plerixafor-mediated mobilization.235 These data imply that S1P receptors, particularly S1P1, regulate the egress of HSPCs from the bone marrow.
Finally, CXCR4 and VLA-4 inhibitors are likely to find important uses beyond hematopoietic stem cell mobilization. CXCR4 inhibitors may function as therapeutic agents in cancer therapy as anti-metastatic or chemosensitization agents241–245, as well as in inflammation and tissue repair.246–253 Furthermore, several studies have indicated an important role of VLA-4 in cell adhesion-mediated inflammatory pathologies including asthma, multiple sclerosis (MS), rheumatoid arthritis (RA), atherosclerosis, and inflammatory bowel disease (IBD).208–210 Specific orally available small molecule inhibitors of the VLA-4 interaction with its ligands would thus be expected to be of therapeutic benefit.
Acknowledgments
We apologize to colleagues whose work we are not able to discuss because of space limitations. This work was supported in part by research funding from The Barnes Jewish Foundation (St Louis, MO), Genzyme Corporation (Cambridge, MA) and National Institutes of Health grant R21 CA141523-01 to J.F.D. The project described was also supported by Grant Numbers 1 UL1 RR024992-01, 1 TL1 RR024995-01 and 1 KL2 RR 024994-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Conflict-of-interest disclosure
M.P.R. and J.F.D have received honoraria from Genzyme Corp.
References
- 1.Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 2.Kiger AA, White-Cooper H, Fuller MT. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature. 2000;407:750–754. doi: 10.1038/35037606. [DOI] [PubMed] [Google Scholar]
- 3.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 4.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 5.Kollet O, Dar A, Lapidot T. Themultiple roles of osteoclasts in host defense: bone remodeling and hematopoietic stem cell mobilization. Annu Rev Immunol. 2007;25:51–69. doi: 10.1146/annurev.immunol.25.022106.141631. [DOI] [PubMed] [Google Scholar]
- 6.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 7.Smith-Berdan S, Nguyen A, Hassanein D, Zimmer M, Ugarte F, Ciriza J, et al. Robo4 cooperates with CXCR4 to specify hematopoietic stem cell localization to bone marrow niches. Cell Stem Cell. 2011;8:72–83. doi: 10.1016/j.stem.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- 9.Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients withhematologic cancers. N Engl J Med. 2001;344:175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 10.Beyer J, Schwella N, Zingsem J, Strohscheer I, Schwaner I, Oettle H, et al. Hematopoietic rescue after high-dose chemotherapy using autologous peripheral-blood progenitor cells or bone marrow: a randomized comparison. J Clin Oncol. 1995;13:1328–1335. doi: 10.1200/JCO.1995.13.6.1328. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann O, Le Corroller AG, Blaise D, Michon J, Philip I, Norol F, et al. Peripheral blood stem cell and bone marrow transplantation for solid tumors and lymphomas: hematologic recovery and costs. A randomized, controlled trial. Ann Intern Med. 1997;126:600–607. doi: 10.7326/0003-4819-126-8-199704150-00002. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz N, Linch DC, Dreger P, Goldstone AH, Boogaerts MA, Ferrant A, et al. Randomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet. 1996;347:353–357. doi: 10.1016/s0140-6736(96)90536-x. [DOI] [PubMed] [Google Scholar]
- 13.Bensinger W, Dipersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009 doi: 10.1038/bmt.2008.410. [DOI] [PubMed] [Google Scholar]
- 14.Kessinger A, Armitage JO, Landmark JD, Weisenburger DD. Reconstitution of human hematopoietic function with autologous cryopreserved circulating stem cells. Exp Hematol. 1986;14:192–196. [PubMed] [Google Scholar]
- 15.Korbling M, Dorken B, Ho AD, Pezzutto A, Hunstein W, Fliedner TM. Autologous transplantation of blood-derived hemopoietic stem cells after myeloablative therapy in a patient with Burkitt’s lymphoma. Blood. 1986;67:529–532. [PubMed] [Google Scholar]
- 16.Dreger P, Kloss M, Petersen B, Haferlach T, Loffler H, Loeffler M, et al. Autologous progenitor cell transplantation: prior exposure to stem cell-toxic drugs determines yield and engraftment of peripheral blood progenitor cell but not of bone marrow grafts. Blood. 1995;86:3970–3978. [PubMed] [Google Scholar]
- 17.Pusic I, Jiang SY, Landua S, Uy GL, Rettig MP, Cashen AF, et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 2008;14:1045–1056. doi: 10.1016/j.bbmt.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Weaver CH, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86:3961–3969. [PubMed] [Google Scholar]
- 19.Haas R, Mohle R, Fruhauf S, Goldschmidt H, Witt B, Flentje M, et al. Patient characteristics associated with successful mobilizing and autografting of peripheral blood progenitor cells in malignant lymphoma. Blood. 1994;83:3787–3794. [PubMed] [Google Scholar]
- 20.Sugrue MW, Williams K, Pollock BH, Khan S, Peracha S, Wingard JR, et al. Characterization and outcome of “hard to mobilize”’ lymphoma patients undergoing autologous stem cell transplantation. Leuk Lymphoma. 2000;39:509–519. doi: 10.3109/10428190009113381. [DOI] [PubMed] [Google Scholar]
- 21.Anderlini P, Przepiorka D, Seong D, Miller P, Sundberg J, Lichtiger B, et al. Clinical toxicity and laboratory effects of granulocyte-colony-stimulating factor (filgrastim) mobilization and blood stem cell apheresis from normal donors, and analysis of charges for the procedures. Transfusion. 1996;36:590–595. doi: 10.1046/j.1537-2995.1996.36796323057.x. [DOI] [PubMed] [Google Scholar]
- 22.Becker PS, Wagle M, Matous S, Swanson RS, Pihan G, Lowry PA, et al. Spontaneous splenic rupture following administration of granulocyte colony-stimulating factor (G-CSF): occurrence in an allogeneic donor of peripheral blood stem cells. Biol Blood Marrow Transplant. 1997;3:45–49. [PubMed] [Google Scholar]
- 23.Falzetti F, Aversa F, Minelli O, Tabilio A. Spontaneous rupture of spleen during peripheral blood stem-cell mobilisation in a healthy donor. Lancet. 1999;353:555. doi: 10.1016/S0140-6736(99)00268-8. [DOI] [PubMed] [Google Scholar]
- 24.Fortanier C, Kuentz M, Sutton L, Milpied N, Michalet M, Macquart-Moulin G, et al. Healthy sibling donor anxiety and pain during bone marrow or peripheral blood stem cell harvesting for allogeneic transplantation: results of a randomised study. Bone Marrow Transplant. 2002;29:145–149. doi: 10.1038/sj.bmt.1703338. [DOI] [PubMed] [Google Scholar]
- 25.Goterris R, Hernandez-Boluda JC, Teruel A, Gomez C, Lis MJ, Terol MJ, et al. Impact of different strategies of second-line stem cell harvest on the outcome of autologous transplantation in poor peripheral blood stem cell mobilizers. Bone Marrow Transplant. 2005;36:847–853. doi: 10.1038/sj.bmt.1705147. [DOI] [PubMed] [Google Scholar]
- 26.Dar A, Goichberg P, Shinder V, Kalinkovich A, Kollet O, Netzer N, et al. Chemokine receptor CXCR4-dependent internalization and resecretion of functional chemokine SDF-1 by bone marrow endothelial and stromal cells. Nat Immunol. 2005;6:1038–1046. doi: 10.1038/ni1251. [DOI] [PubMed] [Google Scholar]
- 27.Imai K, Kobayashi M, Wang J, Shinobu N, Yoshida H, Hamada J, et al. Selective secretion of chemoattractants for haemopoietic progenitor cells by bone marrow endothelial cells: a possible role in homing of haemopoietic progenitor cells to bone marrow. Br J Haematol. 1999;106:905–911. doi: 10.1046/j.1365-2141.1999.01644.x. [DOI] [PubMed] [Google Scholar]
- 28.Jung Y, Wang J, Schneider A, Sun YX, Koh-Paige AJ, Osman NI, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38:497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 31.Watt SM, Forde SP. The central role of the chemokine receptor, CXCR4, in haemopoietic stem cell transplantation: will CXCR4 antagonists contribute to the treatment of blood disorders? Vox Sang. 2008;94:18–32. doi: 10.1111/j.1423-0410.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- 32.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 33.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Doranz BJ, Orsini MJ, Turner JD, Hoffman TL, Berson JF, Hoxie JA, et al. Identification of CXCR4 domains that support coreceptor and chemokine receptor functions. J Virol. 1999;73:2752–2761. doi: 10.1128/jvi.73.4.2752-2761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roland J, Murphy BJ, Ahr B, Robert-Hebmann V, Delauzun V, Nye KE, et al. Role of the intracellular domains of CXCR4 in SDF-1-mediated signaling. Blood. 2003;101:399–406. doi: 10.1182/blood-2002-03-0978. [DOI] [PubMed] [Google Scholar]
- 36.Benboubker L, Watier H, Carion A, Georget MT, Desbois I, Colombat P, et al. Association between the SDF1-3′A allele and high levels of CD34(+) progenitor cells mobilized into peripheral blood in humans. Br J Haematol. 2001;113:247–250. doi: 10.1046/j.1365-2141.2001.02717.x. [DOI] [PubMed] [Google Scholar]
- 37.Bogunia-Kubik K, Gieryng A, Dlubek D, Lange A. The CXCL12-3′A allele is associated with a higher mobilization yield of CD34 progenitors to the peripheral blood of healthy donors for allogeneic transplantation. Bone Marrow Transplant. 2009;44:273–278. doi: 10.1038/bmt.2009.30. [DOI] [PubMed] [Google Scholar]
- 38.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 40.Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, Li M, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A. 2007;104:14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, et al. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 42.Hartmann TN, Grabovsky V, Pasvolsky R, Shulman Z, Buss EC, Spiegel A, et al. A crosstalk between intracellular CXCR7 and CXCR4 involved in rapid CXCL12-triggered integrin activation but not in chemokine-triggered motility of human T lymphocytes and CD34+ cells. J Leukoc Biol. 2008;84:1130–1140. doi: 10.1189/jlb.0208088. [DOI] [PubMed] [Google Scholar]
- 43.Naumann U, Cameroni E, Pruenster M, Mahabaleshwar H, Raz E, Zerwes HG, et al. CXCR7 functions as a scavenger for CXCL12 and CXCL11. PLoS One. 2010;5:e9175. doi: 10.1371/journal.pone.0009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loetscher M, Geiser T, O’Reilly T, Zwahlen R, Baggiolini M, Moser B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem. 1994;269:232–237. [PubMed] [Google Scholar]
- 45.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 46.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 47.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 48.Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 49.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. 2007;1768:952–963. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, et al. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 51.Dubeykovskaya Z, Dubeykovskiy A, Solal-Cohen J, Wang TC. Secreted trefoil factor 2 activates the CXCR4 receptor in epithelial and lymphocytic cancer cell lines. J Biol Chem. 2009;284:3650–3662. doi: 10.1074/jbc.M804935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 53.Fukuda S, Broxmeyer HE, Pelus LM. Flt3 ligand and the Flt3 receptor regulate hematopoietic cell migration by modulating the SDF-1alpha(CXCL12)/CXCR4 axis. Blood. 2005;105:3117–3126. doi: 10.1182/blood-2004-04-1440. [DOI] [PubMed] [Google Scholar]
- 54.Dutt P, Wang JF, Groopman JE. Stromal cell-derived factor-1 alpha and stem cell factor/kit ligand share signaling pathways in hemopoietic progenitors: a potential mechanism for cooperative induction of chemotaxis. J Immunol. 1998;161:3652–3658. [PubMed] [Google Scholar]
- 55.Aiuti A, Tavian M, Cipponi A, Ficara F, Zappone E, Hoxie J, et al. Expression of CXCR4, the receptor for stromal cell-derived factor-1 on fetal and adult human lympho-hematopoietic progenitors. Eur J Immunol. 1999;29:1823–1831. doi: 10.1002/(SICI)1521-4141(199906)29:06<1823::AID-IMMU1823>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 56.Pruijt JF, Fibbe WE, Laterveer L, Pieters RA, Lindley IJ, Paemen L, et al. Prevention of interleukin-8-induced mobilization of hematopoietic progenitor cells in rhesus monkeys by inhibitory antibodies against the metalloproteinase gelatinase B (MMP-9) Proc Natl Acad Sci U S A. 1999;96:10863–10868. doi: 10.1073/pnas.96.19.10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 60.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 61.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 62.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4-and SDF-1-deficient mice. Proc Natl Acad Sci US A. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 64.Foudi A, Jarrier P, Zhang Y, Wittner M, Geay JF, Lecluse Y, et al. Reduced retention of radioprotective hematopoietic cells within the bone marrow microenvironment in CXCR4−/− chimeric mice. Blood. 2006;107:2243–2251. doi: 10.1182/blood-2005-02-0581. [DOI] [PubMed] [Google Scholar]
- 65.Kawabata K, Ujikawa M, Egawa T, Kawamoto H, Tachibana K, Iizasa H, et al. A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proc Natl Acad Sci U S A. 1999;96:5663–5667. doi: 10.1073/pnas.96.10.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205:777–783. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Balabanian K, Lagane B, Pablos JL, Laurent L, Planchenault T, Verola O, et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105:2449–2457. doi: 10.1182/blood-2004-06-2289. [DOI] [PubMed] [Google Scholar]
- 68.Gulino AV, Moratto D, Sozzani S, Cavadini P, Otero K, Tassone L, et al. Altered leukocyte response to CXCL12 in patients with warts hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome. Blood. 2004;104:444–452. doi: 10.1182/blood-2003-10-3532. [DOI] [PubMed] [Google Scholar]
- 69.Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34:70–74. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- 70.Kawai T, Choi U, Whiting-Theobald NL, Linton GF, Brenner S, Sechler JM, et al. Enhanced function with decreased internalization of carboxy-terminus truncated CXCR4 responsible for WHIM syndrome. Exp Hematol. 2005;33:460–468. doi: 10.1016/j.exphem.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Balabanian K, Levoye A, Klemm L, Lagane B, Hermine O, Harriague J, et al. Leukocyte analysis from WHIM syndrome patients reveals a pivotal role for GRK3 in CXCR4 signaling. J Clin Invest. 2008;118:1074–1084. doi: 10.1172/JCI33187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gorlin RJ, Gelb B, Diaz GA, Lofsness KG, Pittelkow MR, Fenyk JR., Jr WHIM syndrome, an autosomal dominant disorder: clinical, hematological, and molecular studies. Am J Med Genet. 2000;91:368–376. [PubMed] [Google Scholar]
- 73.Gulino AV. WHIM syndrome: a genetic disorder of leukocyte trafficking. Curr Opin Allergy Clin Immunol. 2003;3:443–450. doi: 10.1097/00130832-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 74.Pelus LM. Peripheral blood stem cell mobilization: new regimens, new cells, where do we stand. Curr Opin Hematol. 2008;15:285–292. doi: 10.1097/MOH.0b013e328302f43a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- 76.De Clercq E. The bicyclam AMD3100 story. Nat Rev Drug Discov. 2003;2:581–587. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- 77.Donzella GA, Schols D, Lin SW, Este JA, Nagashima KA, Maddon PJ, et al. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 78.Hendrix CW, Flexner C, MacFarland RT, Giandomenico C, Fuchs EJ, Redpath E, et al. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44:1667–1673. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lack NA, Green B, Dale DC, Calandra GB, Lee H, MacFarland RT, et al. A pharmacokinetic-pharmacodynamic model for the mobilization of CD34+ hematopoietic progenitor cells by AMD3100. Clin Pharmacol Ther. 2005;77:427–436. doi: 10.1016/j.clpt.2004.12.268. [DOI] [PubMed] [Google Scholar]
- 80.Liles WC, Broxmeyer HE, Rodger E, Wood B, Hubel K, Cooper S, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 81.Liles WC, Rodger E, Broxmeyer HE, Dehner C, Badel K, Calandra G, et al. Augmented mobilization and collection of CD34+ hematopoietic cells from normal human volunteers stimulated with granulocyte-colony-stimulating factor by single-dose administration of AMD3100, a CXCR4 antagonist. Transfusion. 2005;45:295–300. doi: 10.1111/j.1537-2995.2005.04222.x. [DOI] [PubMed] [Google Scholar]
- 82.Devine SM, Flomenberg N, Vesole DH, Liesveld J, Weisdorf D, Badel K, et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin’s lymphoma. J Clin Oncol. 2004;22:1095–1102. doi: 10.1200/JCO.2004.07.131. [DOI] [PubMed] [Google Scholar]
- 83.Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, Rowley SD, et al. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106:1867–1874. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]
- 84.Calandra G, McCarty J, McGuirk J, Tricot G, Crocker SA, Badel K, et al. AMD3100 plus G-CSF can successfully mobilize CD34+ cells from non-Hodgkin’s lymphoma, Hodgkin’s disease and multiple myeloma patients previously failing mobilization with chemotherapy and/or cytokine treatment: compassionate use data. Bone Marrow Transplant. 2008;41:331–338. doi: 10.1038/sj.bmt.1705908. [DOI] [PubMed] [Google Scholar]
- 85.Cashen A, Lopez S, Gao F, Calandra G, MacFarland R, Badel K, et al. A phase II study of plerixafor (AMD3100) plus G-CSF for autologous hematopoietic progenitor cell mobilization in patients with Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14:1253–1261. doi: 10.1016/j.bbmt.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 86.Fowler CJ, Dunn A, Hayes-Lattin B, Hansen K, Hansen L, Lanier K, et al. Rescue from failed growth factor and/or chemotherapy HSC mobilization with G-CSF and plerixafor(AMD3100): an institutional experience. Bone Marrow Transplant. 2009;43:909–917. doi: 10.1038/bmt.2008.409. [DOI] [PubMed] [Google Scholar]
- 87.Stiff P, Micallef I, McCarthy P, Magalhaes-Silverman M, Weisdorf D, Territo M, et al. Treatment with plerixafor in non-Hodgkin’s lymphoma and multiple myeloma patients to increase the number of peripheral blood stem cells when given a mobilizing regimen of G-CSF: implications for the heavily pretreated patient. Biol Blood Marrow Transplant. 2009;15:249–256. doi: 10.1016/j.bbmt.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 88.Stewart DA, Smith C, MacFarland R, Calandra G. Pharmacokinetics and pharmacodynamics of plerixafor in patients with non-Hodgkin lymphoma and multiple myeloma. Biol Blood Marrow Transplant. 2009;15:39–46. doi: 10.1016/j.bbmt.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 89.Dugan MJ, Maziarz RT, Bensinger WI, Nademanee A, Liesveld J, Badel K, et al. Safety and preliminary efficacy of plerixafor (Mozobil) in combination with chemotherapy and G-CSF: an open-label, multicenter, exploratory trial in patients with multiple myeloma and non-Hodgkin’s lymphoma undergoing stem cell mobilization. Bone Marrow Transplant. 2010;45:39–47. doi: 10.1038/bmt.2009.119. [DOI] [PubMed] [Google Scholar]
- 90.Shaughnessy P, Islas-Ohlmayer M, Murphy J, Hougham M, MacPherson J, Winkler K, et al. Plerixafor Plus G-CSF Compared to Chemotherapy Plus G-CSF for Mobilization of Autologous CD34+ Cells Resulted in Similar Cost but More Predictable Days of Apheresis and Less Hospitalization. Blood (ASH Annual Meeting Abstracts) 2009;114:abstract [2277]. [Google Scholar]
- 91.DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:4767–4773. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 92.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 93.Micallef IN, Stiff PJ, DiPersio JF, Maziarz RT, McCarty JM, Bridger G, et al. Successful stem cell remobilization using plerixafor (mozobil) plus granulocyte colony-stimulating factor in patients with non-hodgkin lymphoma: results from the plerixafor NHL phase 3 study rescue protocol. Biol Blood Marrow Transplant. 2009;15:1578–1586. doi: 10.1016/j.bbmt.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 94.Devine SM, Vij R, Rettig M, Todt L, McGlauchlen K, Fisher N, et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood. 2008;112:990–998. doi: 10.1182/blood-2007-12-130179. [DOI] [PubMed] [Google Scholar]
- 95.Rettig MP, Shannon WD, Ritchey J, Holt M, McFarland K, Lopez S, et al. Characterization of human CD34+ hematopoietic stem cells following administration of G-CSF or plerixafor. Blood (ASH Annual Meeting Abstracts) 2008;112:3476. [Google Scholar]
- 96.Rettig M, Lopez S, McFarland K, DiPersio JF. Rapid and Prolonged Mobilization of Human CD34+ Hematopoietic Stem Cells Following Intravenous (IV) Administration of Plerixafor. Blood (ASH Annual Meeting Abstracts) 2010;116:2261. [Google Scholar]
- 97.Rettig M, McFarland K, Ritchey J, Holt M, Deych E, Lopez S, et al. Preferential Mobilization of CD34+ Plasmacytoid Dendritic Cell Precursors by Plerixafor. Blood (ASH Annual Meeting Abstracts) 2009;114:32. [Google Scholar]
- 98.Manz MG, Miyamoto T, Akashi K, Weissman IL. Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci U SA. 2002;99:11872–11877. doi: 10.1073/pnas.172384399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112:3543–3553. doi: 10.1182/blood-2008-08-078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rettig MP, Ramirez P, Nervi B, DiPersio JF. CXCR4 and mobilization of hematopoietic precursors. Methods Enzymol. 2009;460:57–90. doi: 10.1016/S0076-6879(09)05203-3. [DOI] [PubMed] [Google Scholar]
- 101.Fruehauf S, Tricot G. Comparison of unmobilized and mobilized graft characteristics and the implications of cell subsets on autologous and allogeneic transplantation outcomes. Biol Blood Marrow Transplant. 2010;16:1629–1648. doi: 10.1016/j.bbmt.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 102.Robinson JA, Demarco S, Gombert F, Moehle K, Obrecht D. The design, structures and therapeutic potential of protein epitope mimetics. Drug Discov Today. 2008;13:944–951. doi: 10.1016/j.drudis.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 103.DeMarco SJ, Henze H, Lederer A, Moehle K, Mukherjee R, Romagnoli B, et al. Discovery of novel, highly potent and selective beta-hairpin mimetic CXCR4 inhibitors with excellent anti-HIV activity and pharmacokinetic profiles. Bioorg Med Chem. 2006;14:8396–8404. doi: 10.1016/j.bmc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 104.Moncunill G, Armand-Ugon M, Clotet-Codina I, Pauls E, Ballana E, Llano A, et al. Anti-HIV activity and resistance profile of the CXC chemokine receptor 4 antagonist POL3026. Mol Pharmacol. 2008;73:1264–1273. doi: 10.1124/mol.107.042911. [DOI] [PubMed] [Google Scholar]
- 105.Schmitt S, Weinhold N, Dembowsky K, Neben K, Witzens-Harig M, Braun M, et al. First Results of a Phase-II Study with the New CXCR4 Antagonist POL6326 to Mobilize Hematopoietic Stem Cells (HSC) In Multiple Myeloma (MM) Blood (ASH Annual Meeting Abstracts) 2010;116:824. [Google Scholar]
- 106.Juarez J, Bradstock KF, Gottlieb DJ, Bendall LJ. Effects of inhibitors of the chemokine receptor CXCR4 on acute lymphoblastic leukemia cells in vitro. Leukemia. 2003;17:1294–1300. doi: 10.1038/sj.leu.2402998. [DOI] [PubMed] [Google Scholar]
- 107.Abraham M, Beider K, Wald H, Weiss ID, Zipori D, Galun E, et al. The CXCR4 antagonist 4F-benzoyl-TN14003 stimulates the recovery of the bone marrow after transplantation. Leukemia. 2009;23:1378–1388. doi: 10.1038/leu.2009.56. [DOI] [PubMed] [Google Scholar]
- 108.Abraham M, Biyder K, Begin M, Wald H, Weiss ID, Galun E, et al. Enhanced unique pattern of hematopoietic cell mobilization induced by the CXCR4 antagonist 4F-benzoyl-TN14003. Stem Cells. 2007;25:2158–2166. doi: 10.1634/stemcells.2007-0161. [DOI] [PubMed] [Google Scholar]
- 109.Trent JO, Wang ZX, Murray JL, Shao W, Tamamura H, Fujii N, et al. Lipid bilayer simulations of CXCR4 with inverse agonists and weak partial agonists. J Biol Chem. 2003;278:47136–47144. doi: 10.1074/jbc.M307850200. [DOI] [PubMed] [Google Scholar]
- 110.Zhang WB, Navenot JM, Haribabu B, Tamamura H, Hiramatu K, Omagari A, et al. A point mutation that confers constitutive activity to CXCR4 reveals that T140 is an inverse agonist and that AMD3100 and ALX40-4C are weak partial agonists. J Biol Chem. 2002;277:24515–24521. doi: 10.1074/jbc.M200889200. [DOI] [PubMed] [Google Scholar]
- 111.Schols D, Struyf S, Van Damme J, Este JA, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beider K, Begin M, Abraham M, Wald H, Weiss I, Wald O, et al. Anti-Leukemia and Multiple Myeloma Selective Activity of CXCR4 Antagonist 4F-Benzoyl-TN14003 Involves Apoptotic Death Pathway. Blood (ASH Annual Meeting Abstracts) 2009;114:3857. [Google Scholar]
- 113.Nagler A, Shimoni A, Avivi I, Rowe JM, Beider K, Hardan I, et al. BKT140 Is a Novel CXCR4 Antagonist with Stem Cell Mobilization and Antimyeloma Effects: An Open-Label First Human Trial In Patients with Multiple Myeloma Undergoing Stem Cell Mobilization for Autologous Transplantation. Blood (ASH Annual Meeting Abstracts) 2010;116:2260. [Google Scholar]
- 114.Sayyed SG, Hagele H, Kulkarni OP, Endlich K, Segerer S, Eulberg D, et al. Podocytes produce homeostatic chemokine stromal cell-derived factor-1/CXCL12, which contributes to glomerulosclerosis, podocyte loss and albuminuria in a mouse model of type 2 diabetes. Diabetologia. 2009;52:2445–2454. doi: 10.1007/s00125-009-1493-6. [DOI] [PubMed] [Google Scholar]
- 115.Eulberg D, Klussmann S. Spiegelmers: biostable aptamers. Chembiochem. 2003;4:979–983. doi: 10.1002/cbic.200300663. [DOI] [PubMed] [Google Scholar]
- 116.Klussmann S, Nolte A, Bald R, Erdmann VA, Furste JP. Mirror-image RNA that binds D-adenosine. Nat Biotechnol. 1996;14:1112–1115. doi: 10.1038/nbt0996-1112. [DOI] [PubMed] [Google Scholar]
- 117.Huang Y, Liu Y, Yen C, Chen H, Chen S, King CR, et al. Rapid Mobilization of Murine Hematopoietic Stem and Progenitor Cells with TG-0054, a Novel CXCR4 Antagonist. Blood (ASH Annual Meeting Abstracts) 2009;114:3542. [Google Scholar]
- 118.Chung DT, Chang L, Huang Y, Tsai C, Hsu C, King CR, et al. TG-0054, a Novel and Potent Stem Cell Mobilizer, Displays Excellent PK/PD and Safety Profile in Phase I Trial. Blood (ASH Annual Meeting Abstracts) 2009;114:866. [Google Scholar]
- 119.Garber K. First results for agents targeting cancer-related inflammation. J Natl Cancer Inst. 2009;101:1110–1112. doi: 10.1093/jnci/djp266. [DOI] [PubMed] [Google Scholar]
- 120.Kuhne MR, Mulvey T, Belanger B, Chen S, Pan C, Chong C, et al. A fully human anti-CXCR4 antibody induces apoptosis in vitro and shows anti tumor activity iin vivo. Proceedings of the 100th Annual Meeting of the American Association for Cancer Research; 2009. p. abstract[LB-150]. [DOI] [PubMed] [Google Scholar]
- 121.Kaneider NC, Agarwal A, Leger AJ, Kuliopulos A. Reversing systemic inflammatory response syndrome with chemokine receptor pepducins. Nat Med. 2005;11:661–665. doi: 10.1038/nm1245. [DOI] [PubMed] [Google Scholar]
- 122.O’Callaghan KM, Hsieh M, VanEtten RA, Covic L, Kuliopulos A. CXCR4 Pepducins in Stem Cell Mobilization. Blood (ASH Annual Meeting Abstracts) 2009;114:2440. [Google Scholar]
- 123.Moses E, Law D. Ablynx’s R&D Investor Day. 2010 http://wwwablynxcom/investorrelations/english/documents/RDDayUSA_12Feb_2010_FINALVERSIONpdf.
- 124.Kolkman JA, Law DA. Nanobodies–from llamas to therapeutic proteins. dRUG Discov Today: tECHNOL. 2010 doi: 10.1016/j.ddtec.2010.03.002. doi: 10.1016/ [DOI] [PubMed] [Google Scholar]
- 125.Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 126.Skerlj RT, Bridger GJ, Kaller A, McEachern EJ, Crawford JB, Zhou Y, et al. Discovery of novel small molecule orally bioavailable C-X-C chemokine receptor 4 antagonists that are potent inhibitors of T-tropic (X4) HIV-1 replication. J Med Chem. 2010;53:3376–3388. doi: 10.1021/jm100073m. [DOI] [PubMed] [Google Scholar]
- 127.Moyle G, DeJesus E, Boffito M, Wong RS, Gibney C, Badel K, et al. Proof of activity with AMD11070, an orally bioavailable inhibitor of CXCR4-tropic HIV type 1. Clin Infect Dis. 2009;48:798–805. doi: 10.1086/597097. [DOI] [PubMed] [Google Scholar]
- 128.Stone ND, Dunaway SB, Flexner C, Tierney C, Calandra GB, Becker S, et al. Multiple-dose escalation study of the safety, pharmacokinetics, and biologic activity of oral AMD070, a selectiveCXCR4 receptor inhibitor, in human subjects. Antimicrob Agents Chemother. 2007;51:2351–2358. doi: 10.1128/AAC.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jenkinson S, Thomson M, McCoy D, Edelstein M, Danehower S, Lawrence W, et al. Blockade of X4-tropic HIV-1 cellular entry by GSK812397, a potent noncompetitive CXCR4 receptor antagonist. Antimicrob Agents Chemother. 2010;54:817–824. doi: 10.1128/AAC.01293-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Murakami T, Kumakura S, Yamazaki T, Tanaka R, Hamatake M, Okuma K, et al. The novel CXCR4 antagonist KRH-3955 is an orally bioavailable and extremely potent inhibitor of human immunodeficiency virus type 1 infection: comparative studies with AMD3100. Antimicrob Agents Chemother. 2009;53:2940–2948. doi: 10.1128/AAC.01727-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ichiyama K, Yokoyama-Kumakura S, Tanaka Y, Tanaka R, Hirose K, Bannai K, et al. A duodenally absorbable CXC chemokine receptor 4 antagonist, KRH-1636, exhibits a potent and selective anti-HIV-1 activity. Proc Natl Acad Sci U S A. 2003;100:4185–4190. doi: 10.1073/pnas.0630420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fujii N, Oishi S, Hiramatsu K, Araki T, Ueda S, Tamamura H, et al. Molecular-size reduction of a potent CXCR4-chemokine antagonist using orthogonal combination of conformation-and sequence-based libraries. Angew Chem Int Ed Engl. 2003;42:3251–3253. doi: 10.1002/anie.200351024. [DOI] [PubMed] [Google Scholar]
- 133.Zhan W, Liang Z, Zhu A, Kurtkaya S, Shim H, Snyder JP, et al. Discovery of small molecule CXCR4 antagonists. J Med Chem. 2007;50:5655–5664. doi: 10.1021/jm070679i. [DOI] [PubMed] [Google Scholar]
- 134.Natchus M, Arrendale R, Donald L. MSX-122, an orally available small molecule CXCR4 antagonist, promotes leukocytosis in monkeys at doses that were well tolerated in a 28 day toxicology study. AACR Annual Meeting 2009 Proceedings; 2008. Apr, p. 49.p. abstract [1189]. [Google Scholar]
- 135.Humphries MJ. Integrin structure. Biochem Soc Trans. 2000;28:311–339. [PubMed] [Google Scholar]
- 136.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 137.Johnson MS, Lu N, Denessiouk K, Heino J, Gullberg D. Integrins during evolution: evolutionary trees and model organisms. Biochim Biophys Acta. 2009;1788:779–789. doi: 10.1016/j.bbamem.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 138.Springer TA, Wang JH. The three-dimensional structure of integrins and their ligands, and conformational regulation of cell adhesion. Adv Protein Chem. 2004;68:29–63. doi: 10.1016/S0065-3233(04)68002-8. [DOI] [PubMed] [Google Scholar]
- 139.Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- 140.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Imai Y, Shimaoka M, Kurokawa M. Essential roles of VLA-4 in the hematopoietic system. Int J Hematol. 2010;91:569–575. doi: 10.1007/s12185-010-0555-3. [DOI] [PubMed] [Google Scholar]
- 143.Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15:547–556. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 144.Askari JA, Buckley PA, Mould AP, Humphries MJ. Linking integrin conformation to function. J Cell Sci. 2009;122:165–170. doi: 10.1242/jcs.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liddington RC, Ginsberg MH. Integrin activation takes shape. J Cell Biol. 2002;158:833–839. doi: 10.1083/jcb.200206011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chigaev A, Zwartz G, Graves SW, Dwyer DC, Tsuji H, Foutz TD, et al. Alpha4beta1 integrin affinity changes govern cell adhesion. J Biol Chem. 2003;278:38174–38182. doi: 10.1074/jbc.M210472200. [DOI] [PubMed] [Google Scholar]
- 147.Hemler ME, Elices MJ, Parker C, Takada Y. Structure of the integrin VLA-4 and its cell-cell and cell-matrix adhesion functions. Immunol Rev. 1990;114:45–65. doi: 10.1111/j.1600-065x.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 148.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 149.Masumoto A, Hemler ME. Multiple activation states of VLA-4. Mechanistic differences between adhesion to CS1/fibronectin and to vascular cell adhesion molecule-1. J Biol Chem. 1993;268:228–234. [PubMed] [Google Scholar]
- 150.Lobb RR, Antognetti G, Pepinsky RB, Burkly LC, Leone DR, Whitty A. A direct binding assay for the vascular cell adhesion molecule-1 (VCAM1) interaction with alpha 4 integrins. Cell Adhes Commun. 1995;3:385–397. doi: 10.3109/15419069509081293. [DOI] [PubMed] [Google Scholar]
- 151.Jakubowski A, Rosa MD, Bixler S, Lobb R, Burkly LC. Vascular cell adhesion molecule (VCAM)-Ig fusion protein defines distinct affinity states of the very late antigen-4 (VLA-4) receptor. Cell Adhes Commun. 1995;3:131–142. doi: 10.3109/15419069509081282. [DOI] [PubMed] [Google Scholar]
- 152.Petty JM, Lenox CC, Weiss DJ, Poynter ME, Suratt BT. Crosstalk between CXCR4/stromal derived factor-1 and VLA-4/VCAM-1 pathways regulates neutrophil retention in the bone marrow. J Immunol. 2009;182:604–612. doi: 10.4049/jimmunol.182.1.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.DiVietro JA, Brown DC, Sklar LA, Larson RS, Lawrence MB. Immobilized stromal cell-derived factor-1alpha triggers rapid VLA-4 affinity increases to stabilize lymphocyte tethers on VCAM-1 and subsequently initiate firm adhesion. J Immunol. 2007;178:3903–3911. doi: 10.4049/jimmunol.178.6.3903. [DOI] [PubMed] [Google Scholar]
- 155.Sanz-Rodriguez F, Hidalgo A, Teixido J. Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-mediated multiple myeloma cell adhesion to CS-1/fibronectin and VCAM-1. Blood. 2001;97:346–351. doi: 10.1182/blood.v97.2.346. [DOI] [PubMed] [Google Scholar]
- 156.Levesque JP, Leavesley DI, Niutta S, Vadas M, Simmons PJ. Cytokines increase human hemopoietic cell adhesiveness by activation of very late antigen (VLA)-4 and VLA-5 integrins. J Exp Med. 1995;181:1805–1815. doi: 10.1084/jem.181.5.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kodama H, Nose M, Niida S, Nishikawa S. Involvement of the c-kit receptor in the adhesion of hematopoietic stem cells to stromal cells. Exp Hematol. 1994;22:979–984. [PubMed] [Google Scholar]
- 158.Kovach NL, Lin N, Yednock T, Harlan JM, Broudy VC. Stem cell factor modulates avidity of alpha 4 beta 1 and alpha 5 beta 1 integrins expressed on hematopoietic cell lines. Blood. 1995;85:159–167. [PubMed] [Google Scholar]
- 159.Makarem R, Newham P, Askari JA, Green LJ, Clements J, Edwards M, et al. Competitive binding of vascular cell adhesion molecule-1 and the HepII/IIICS domain of fibronectin to the integrin alpha 4 beta 1. J Biol Chem. 1994;269:4005–4011. [PubMed] [Google Scholar]
- 160.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 161.Guan JL, Hynes RO. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990;60:53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- 162.Wayner EA, Garcia-Pardo A, Humphries MJ, McDonald JA, Carter WG. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989;109:1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Williams DA, Rios M, Stephens C, Patel VP. Fibronectin and VLA-4 in haematopoietic stem cell-microenvironment interactions. Nature. 1991;352:438–441. doi: 10.1038/352438a0. [DOI] [PubMed] [Google Scholar]
- 164.Fong S, Jones S, Renz ME, Chiu HH, Ryan AM, Presta LG, et al. Mucosal addressin cell adhesion molecule-1(MAdCAM-1). Its binding motif for alpha 4 beta 7 and role in experimental colitis. Immunol Res. 1997;16:299–311. doi: 10.1007/BF02786396. [DOI] [PubMed] [Google Scholar]
- 165.Viney JL, Jones S, Chiu HH, Lagrimas B, Renz ME, Presta LG, et al. Mucosal addressin cell adhesion molecule-1: a structural and functional analysis demarcates the integrin binding motif. J Immunol. 1996;157:2488–2497. [PubMed] [Google Scholar]
- 166.Connor EM, Eppihimer MJ, Morise Z, Granger DN, Grisham MB. Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in acute and chronic inflammation. J Leukoc Biol. 1999;65:349–355. doi: 10.1002/jlb.65.3.349. [DOI] [PubMed] [Google Scholar]
- 167.Quinlan KL, Song IS, Naik SM, Letran EL, Olerud JE, Bunnett NW, et al. VCAM-1 expression on human dermal microvascular endothelial cells is directly and specifically up-regulated by substance P. J Immunol. 1999;162:1656–1661. [PubMed] [Google Scholar]
- 168.Shyjan AM, Bertagnolli M, Kenney CJ, Briskin MJ. Human mucosal addressin cell adhesion molecule-1 (MAdCAM-1) demonstrates structural and functional similarities to the alpha 4 beta 7-integrin binding domains of murine MAdCAM-1, but extreme divergence of mucin-like sequences. J Immunol. 1996;156:2851–2857. [PubMed] [Google Scholar]
- 169.Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, Tidswell M. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol. 1994;153:517–528. [PubMed] [Google Scholar]
- 170.Bellucci R, De Propris MS, Buccisano F, Lisci A, Leone G, Tabilio A, et al. Modulation of VLA-4 and L-selectin expression on normal CD34+ cells during mobilization with G-CSF. Bone Marrow Transplant. 1999;23:1–8. doi: 10.1038/sj.bmt.1701522. [DOI] [PubMed] [Google Scholar]
- 171.Prosper F, Stroncek D, McCarthy JB, Verfaillie CM. Mobilization and homing of peripheral blood progenitors is related to reversible downregulation of alpha4 beta1 integrin expression and function. J Clin Invest. 1998;101:2456–2467. doi: 10.1172/JCI188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mohle R, Murea S, Kirsch M, Haas R. Differential expression of L-selectin, VLA-4, and LFA-1 on CD34+ progenitor cells from bone marrow and peripheral blood during G-CSF-enhanced recovery. Exp Hematol. 1995;23:1535–1542. [PubMed] [Google Scholar]
- 173.Leavesley DI, Oliver JM, Swart BW, Berndt MC, Haylock DN, Simmons PJ. Signals from platelet/endothelial cell adhesion molecule enhance the adhesive activity of the very late antigen-4 integrin of human CD34+ hemopoietic progenitor cells. J Immunol. 1994;153:4673–4683. [PubMed] [Google Scholar]
- 174.Yamaguchi M, Ikebuchi K, Hirayama F, Sato N, Mogi Y, Ohkawara J, et al. Different adhesive characteristics and VLA-4 expression of CD34(+) progenitors in G0/G1 versus S+G2/M phases of the cell cycle. Blood. 1998;92:842–848. [PubMed] [Google Scholar]
- 175.Lichterfeld M, Martin S, Burkly L, Haas R, Kronenwett R. Mobilization of CD34+ haematopoietic stem cells is associated with a functional inactivation of the integrin very late antigen 4. Br J Haematol. 2000;110:71–81. doi: 10.1046/j.1365-2141.2000.02130.x. [DOI] [PubMed] [Google Scholar]
- 176.Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, et al. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 177.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 178.Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 179.Potocnik AJ, Brakebusch C, Fassler R. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity. 2000;12:653–663. doi: 10.1016/s1074-7613(00)80216-2. [DOI] [PubMed] [Google Scholar]
- 180.Hirsch E, Iglesias A, Potocnik AJ, Hartmann U, Fassler R. Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature. 1996;380:171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- 181.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Alpha4 integrins regulate the proliferation/differentiation balance of multilineage hematopoietic progenitors in vivo. Immunity. 1999;11:555–566. doi: 10.1016/s1074-7613(00)80131-4. [DOI] [PubMed] [Google Scholar]
- 182.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell. 1996;85:997–1008. doi: 10.1016/s0092-8674(00)81301-x. [DOI] [PubMed] [Google Scholar]
- 183.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23:9349–9360. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Priestley GV, Ulyanova T, Papayannopoulou T. Sustained alterations in biodistribution of stem/progenitor cells in Tie2Cre+ alpha4(f/f) mice are hematopoietic cell autonomous. Blood. 2007;109:109–111. doi: 10.1182/blood-2006-06-026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Priestley GV, Scott LM, Ulyanova T, Papayannopoulou T. Lack of alpha4 integrin expression in stem cells restricts competitive function and self-renewal activity. Blood. 2006;107:2959–2967. doi: 10.1182/blood-2005-07-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gribi R, Hook L, Ure J, Medvinsky A. The differentiation program of embryonic definitive hematopoietic stem cells is largely alpha4 integrin independent. Blood. 2006;108:501–509. doi: 10.1182/blood-2005-10-4209. [DOI] [PubMed] [Google Scholar]
- 187.Brakebusch C, Fillatreau S, Potocnik AJ, Bungartz G, Wilhelm P, Svensson M, et al. Beta1 integrin is not essential for hematopoiesis but is necessary for the T cell-dependent IgM antibody response. Immunity. 2002;16:465–477. doi: 10.1016/s1074-7613(02)00281-9. [DOI] [PubMed] [Google Scholar]
- 188.Bungartz G, Stiller S, Bauer M, Muller W, Schippers A, Wagner N, et al. Adult murine hematopoiesis can proceed without beta1 and beta7 integrins. Blood. 2006;108:1857–1864. doi: 10.1182/blood-2005-10-007658. [DOI] [PubMed] [Google Scholar]
- 189.Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci U S A. 1995;92:9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vermeulen M, Le Pesteur F, Gagnerault MC, Mary JY, Sainteny F, Lepault F. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92:894–900. [PubMed] [Google Scholar]
- 191.Bonig H, Watts KL, Chang KH, Kiem HP, Papayannopoulou T. Concurrent blockade of alpha4-integrin and CXCR4 in hematopoietic stem/progenitor cell mobilization. Stem Cells. 2009;27:836–837. doi: 10.1002/stem.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM, Harlan JM. Synergistic mobilization of hemopoietic progenitor cells using concurrent beta1 and beta2 integrin blockade or beta2-deficient mice. Blood. 2001;97:1282–1288. doi: 10.1182/blood.v97.5.1282. [DOI] [PubMed] [Google Scholar]
- 193.Craddock CF, Nakamoto B, Andrews RG, Priestley GV, Papayannopoulou T. Antibodies to VLA4 integrin mobilize long-term repopulating cells and augment cytokine-induced mobilization in primates and mice. Blood. 1997;90:4779–4788. [PubMed] [Google Scholar]
- 194.Papayannopoulou T, Priestley GV, Nakamoto B. Anti-VLA4/VCAM-1-induced mobilization requires cooperative signaling through the kit/mkit ligand pathway. Blood. 1998;91:2231–2239. [PubMed] [Google Scholar]
- 195.Papayannopoulou T, Nakamoto B. Peripheralization of hemopoietic progenitors in primates treated with anti-VLA4 integrin. Proc Natl Acad Sci U S A. 1993;90:9374–9378. doi: 10.1073/pnas.90.20.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Christopher MJ, Liu F, Hilton MJ, Long F, Link DC. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009 doi: 10.1182/blood-2008-10-184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bonig H, Wundes A, Chang KH, Lucas S, Papayannopoulou T. Increased numbers of circulating hematopoietic stem/progenitor cells are chronically maintained in patients treated with the CD49d blocking antibody natalizumab. Blood. 2008;111:3439–3441. doi: 10.1182/blood-2007-09-112052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jing D, Oelschlaegel U, Ordemann R, Holig K, Ehninger G, Reichmann H, et al. CD49d blockade by natalizumab in patients with multiple sclerosis affects steady-state hematopoiesis and mobilizes progenitors with a distinct phenotype and function. Bone Marrow Transplant. 2010;45:1489–1496. doi: 10.1038/bmt.2009.381. [DOI] [PubMed] [Google Scholar]
- 199.Zohren F, Toutzaris D, Klarner V, Hartung HP, Kieseier B, Haas R. The monoclonal anti-VLA-4 antibody natalizumab mobilizes CD34+ hematopoietic progenitor cells in humans. Blood. 2008;111:3893–3895. doi: 10.1182/blood-2007-10-120329. [DOI] [PubMed] [Google Scholar]
- 200.Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 201.Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354:911–923. doi: 10.1056/NEJMoa044396. [DOI] [PubMed] [Google Scholar]
- 202.Hauser SL, Weiner HL. Natalizumab: immune effects and implications for therapy. Ann Neurol. 2006;59:731–732. doi: 10.1002/ana.20877. [DOI] [PubMed] [Google Scholar]
- 203.Stuve O, Marra CM, Jerome KR, Cook L, Cravens PD, Cepok S, et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol. 2006;59:743–747. doi: 10.1002/ana.20858. [DOI] [PubMed] [Google Scholar]
- 204.Rice GP, Hartung HP, Calabresi PA. Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology. 2005;64:1336–1342. doi: 10.1212/01.WNL.0000158329.30470.D0. [DOI] [PubMed] [Google Scholar]
- 205.Foley J. Recommendations for the selection, treatment, and management of patients utilizing natalizumab therapy for multiple sclerosis. Am J Manag Care. 2010;16:S178–183. [PubMed] [Google Scholar]
- 206.Ransohoff RM. Natalizumab for multiple sclerosis. N Engl J Med. 2007;356:2622–2629. doi: 10.1056/NEJMct071462. [DOI] [PubMed] [Google Scholar]
- 207.Davenport RJ, Munday JR. Blocking alpha4-integrins -A small molecule approach to treatment of multiple sclerosis. J Neurol Sci. 2008;274:27–30. doi: 10.1016/j.jns.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 208.Davenport RJ, Munday JR. Alpha4-integrin antagonism--an effective approach for the treatment of inflammatory diseases? Drug Discov Today. 2007;12:569–576. doi: 10.1016/j.drudis.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 209.Jackson DY. Alpha 4 integrin antagonists. Curr Pharm Des. 2002;8:1229–1253. doi: 10.2174/1381612023394737. [DOI] [PubMed] [Google Scholar]
- 210.Yang GX, Hagmann WK. VLA-4 antagonists: potent inhibitors of lymphocyte migration. Med Res Rev. 2003;23:369–392. doi: 10.1002/med.10044. [DOI] [PubMed] [Google Scholar]
- 211.Sagi K, Izawa H, Chiba A, Okuzumi T, Yoshimura T, Tanaka Y, et al. Novel phenylalanine derivative. Ajinomoto Co., Inc; WO2003070709. Patent. 2003
- 212.Ghosh S, Panaccione R. Anti-adhesion molecule therapy for inflammatory bowel disease. Therapeutic Advances in Gastroenterology. 2010;3:239–258. doi: 10.1177/1756283X10373176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Takazoe M, Watanabe M, Kawaguchi T, Matsumoto T, Oshitani N, Matsui T, et al. Oral Alpha-4 Integrin Inhibitor (AJM300) in Patients with Active Crohn’s Disease — A Randomized, Double-Blind, Placebo-Controlled Trial. Gastroenterology. 2009;136:abstract [S1066]. [Google Scholar]
- 214.Muro F, Iimura S, Sugimoto Y, Yoneda Y, Chiba J, Watanabe T, et al. Discovery of trans-4-[1-[[2,5-Dichloro-4-(1-methyl-3-indolylcarboxamido)phenyl]acetyl]-(4S)-methoxy-(2S)-pyrrolidinylmethoxy]cyclohexanecarboxylic acid: an orally active, selective very late antigen-4 antagonist. J Med Chem. 2009;52:7974–7992. doi: 10.1021/jm901154c. [DOI] [PubMed] [Google Scholar]
- 215.Leone DR, Giza K, Gill A, Dolinski BM, Yang W, Perper S, et al. An assessment of the mechanistic differences between two integrin alpha 4 beta 1 inhibitors, the monoclonal antibody TA-2 and the small molecule BIO5192, in rat experimental autoimmune encephalomyelitis. J Pharmacol Exp Ther. 2003;305:1150–1162. doi: 10.1124/jpet.102.047332. [DOI] [PubMed] [Google Scholar]
- 216.Ramirez P, Rettig MP, Uy GL, Deych E, Holt MS, Ritchey JK, et al. BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. Blood. 2009;114:1340–1343. doi: 10.1182/blood-2008-10-184721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. 2011;208:421–428. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 220.Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011;208:251–260. doi: 10.1084/jem.20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lymperi S, Ersek A, Ferraro F, Dazzi F, Horwood NJ. Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood. 2011;117:1540–1549. doi: 10.1182/blood-2010-05-282855. [DOI] [PubMed] [Google Scholar]
- 222.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 224.Mendez-Ferrer S, Battista M, Frenette PS. Cooperation of beta(2)-and beta(3)-adrenergic receptors in hematopoietic progenitor cell mobilization. Ann N Y Acad Sci. 2010;1192:139–144. doi: 10.1111/j.1749-6632.2010.05390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 226.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Fruehauf S, Seeger T, Maier P, Li L, Weinhardt S, Laufs S, et al. The CXCR4 antagonist AMD3100 releases a subset of G-CSF-primed peripheral blood progenitor cells with specific gene expression characteristics. Exp Hematol. 2006;34:1052–1059. doi: 10.1016/j.exphem.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 228.Fruehauf S, Veldwijk MR, Seeger T, Schubert M, Laufs S, Topaly J, et al. A combination of granulocyte-colony-stimulating factor (G-CSF) and plerixafor mobilizes more primitive peripheral blood progenitor cells than G-CSF alone: results of a European phase II study. Cytotherapy. 2009;11:992–1001. doi: 10.3109/14653240903121245. [DOI] [PubMed] [Google Scholar]
- 229.Larochelle A, Krouse A, Metzger M, Orlic D, Donahue RE, Fricker S, et al. AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood. 2006;107:3772–3778. doi: 10.1182/blood-2005-09-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hidalgo A, Sanz-Rodriguez F, Rodriguez-Fernandez JL, Albella B, Blaya C, Wright N, et al. Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-dependent adhesion to fibronectin and VCAM-1 on bone marrow hematopoietic progenitor cells. Exp Hematol. 2001;29:345–355. doi: 10.1016/s0301-472x(00)00668-8. [DOI] [PubMed] [Google Scholar]
- 231.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 232.Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- 233.Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, et al. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105:40–48. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 234.Golan K, Vagima Y, Ludin A, Itkin T, Kalinkovich A, Cohen-Gur S, et al. The Chemotactic Lipid S1P Regulates Hematopoietic Progenitor Cell Egress and Mobilization Via Its Major Receptor S1P1 and by SDF-1 Inhibition In a p38/Akt/mTOR Dependent Manner. Blood (ASH Annual Meeting Abstracts) 2010;116:553. [Google Scholar]
- 235.Harun N, Thien M, Juarez JG, Bradstock KF, Bendall LJ. S1P1 Agonists for Use as Adjunct Mobilizing Agents. Blood (ASH Annual Meeting Abstracts) 2010;116:826. [Google Scholar]
- 236.Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, et al. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yanai N, Matsui N, Furusawa T, Okubo T, Obinata M. Sphingosine-1-phosphate and lysophosphatidic acid trigger invasion of primitive hematopoietic cells into stromal cell layers. Blood. 2000;96:139–144. [PubMed] [Google Scholar]
- 238.Kimura T, Boehmler AM, Seitz G, Kuci S, Wiesner T, Brinkmann V, et al. The sphingosine 1-phosphate receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cells. Blood. 2004;103:4478–4486. doi: 10.1182/blood-2003-03-0875. [DOI] [PubMed] [Google Scholar]
- 239.Xue X, Cai Z, Seitz G, Kanz L, Weisel KC, Mohle R. Differential effects of G protein coupled receptors on hematopoietic progenitor cell growth depend on their signaling capacities. Ann N Y Acad Sci. 2007;1106:180–189. doi: 10.1196/annals.1392.014. [DOI] [PubMed] [Google Scholar]
- 240.Allende ML, Tuymetova G, Lee BG, Bonifacino E, Wu YP, Proia RL. S1P1 receptor directs the release of immature B cells from bone marrow into blood. J Exp Med. 2010;207:1113–1124. doi: 10.1084/jem.20092210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23:43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 242.Lane SW, Scadden DT, Gilliland DG. The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood. 2009;114:1150–1157. doi: 10.1182/blood-2009-01-202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, et al. Chemosensitization of AML following mobilization by the CXCR4 antagonist AMD3100. Blood. 2008 doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tavor S, Petit I. Can inhibition of the SDF-1/CXCR4 axis eradicate acute leukemia? Semin Cancer Biol. 2010;20:178–185. doi: 10.1016/j.semcancer.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 245.Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling X, Frolova O, et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113:6215–6224. doi: 10.1182/blood-2008-05-158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Capoccia BJ, Shepherd RM, Link DC. G-CSF and AMD3100 mobilize monocytes into the blood that stimulate angiogenesis in vivo through a paracrine mechanism. Blood. 2006;108:2438–2445. doi: 10.1182/blood-2006-04-013755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Jujo K, Hamada H, Iwakura A, Thorne T, Sekiguchi H, Clarke T, et al. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci U S A. 2010;107:11008–11013. doi: 10.1073/pnas.0914248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Khan A, Greenman J, Archibald SJ. Small molecule CXCR4 chemokine receptor antagonists: developing drug candidates. Curr Med Chem. 2007;14:2257–2277. doi: 10.2174/092986707781696618. [DOI] [PubMed] [Google Scholar]
- 249.Kirton JP, Xu Q. Endothelial precursors in vascular repair. Microvasc Res. 2010;79:193–199. doi: 10.1016/j.mvr.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 250.Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28:299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4:62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 252.Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Ratajczak J, Kucia M. Very small embryonic-like stem cells: characterization, developmental origin, and biological significance. Exp Hematol. 2008;36:742–751. doi: 10.1016/j.exphem.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Shepherd RM, Capoccia BJ, Devine SM, Dipersio J, Trinkaus KM, Ingram D, et al. Angiogenic cells can be rapidly mobilized and efficiently harvested from the blood following treatment with AMD3100. Blood. 2006;108:3662–3667. doi: 10.1182/blood-2006-06-030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Dreger P, Haferlach T, Eckstein V, Jacobs S, Suttorp M, Loffler H, et al. G-CSF-mobilized peripheral blood progenitor cells for allogeneic transplantation: safety, kinetics of mobilization, and composition of the graft. Br J Haematol. 1994;87:609–613. doi: 10.1111/j.1365-2141.1994.tb08321.x. [DOI] [PubMed] [Google Scholar]
- 255.Korbling M, Huh YO, Durett A, Mirza N, Miller P, Engel H, et al. Allogeneic blood stem cell transplantation: peripheralization and yield of donor-derived primitive hematopoietic progenitor cells (CD34+ Thy-1dim) and lymphoid subsets, and possible predictors of engraftment and graft-versus-host disease. Blood. 1995;86:2842–2848. [PubMed] [Google Scholar]
- 256.Stroncek DF, Clay ME, Herr G, Smith J, Jaszcz WB, Ilstrup S, et al. The kinetics of G-CSF mobilization of CD34+ cells in healthy people. Transfus Med. 1997;7:19–24. doi: 10.1046/j.1365-3148.1997.d01-75.x. [DOI] [PubMed] [Google Scholar]
- 257.Gazitt Y, Freytes CO, Akay C, Badel K, Calandra G. Improved mobilization of peripheral blood CD34+ cells and dendritic cells by AMD3100 plus granulocyte-colony-stimulating factor in non-Hodgkin’s lymphoma patients. Stem Cells Dev. 2007;16:657–666. doi: 10.1089/scd.2006.0087. [DOI] [PubMed] [Google Scholar]
- 258.Hess DA, Bonde J, Craft TP, Wirthlin L, Hohm S, Lahey R, et al. Human progenitor cells rapidly mobilized by AMD3100 repopulate NOD/SCID mice with increased frequency in comparison to cells from the same donor mobilized by granulocyte colony stimulating factor. Biol Blood Marrow Transplant. 2007;13:398–411. doi: 10.1016/j.bbmt.2006.12.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Donahue RE, Jin P, Bonifacino AC, Metzger ME, Ren J, Wang E, et al. Plerixafor (AMD3100) and granulocyte colony-stimulating factor (G-CSF) mobilize different CD34+ cell populations based on global gene and microRNA expression signatures. Blood. 2009;114:2530–2541. doi: 10.1182/blood-2009-04-214403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Jin P, Wang E, Ren J, Childs R, Shin JW, Khuu H, et al. Differentiation of two types of mobilized peripheral blood stem cells by microRNA and cDNA expression analysis. J Transl Med. 2008;6:39. doi: 10.1186/1479-5876-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lin K, Ateeq HS, Hsiung SH, Chong LT, Zimmerman CN, Castro A, et al. Selective, tight-binding inhibitors of integrin alpha4beta1 that inhibit allergic airway responses. J Med Chem. 1999;42:920–934. doi: 10.1021/jm980673g. [DOI] [PubMed] [Google Scholar]
- 262.Norris V, Choong L, Tran D, Corden Z, Boyce M, Arshad H, et al. Effect of IVL745, a VLA-4 antagonist, on allergen-induced bronchoconstriction in patients with asthma. J Allergy Clin Immunol. 2005;116:761–767. doi: 10.1016/j.jaci.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 263.Baldwin JJ, McDonald E, Moriarity K, Sarko C, Machinaga N, Nakayama A, et al. Daiichi Pharmaceutical Co., Ltd; Japan: Pharmacopeia, Inc; Patent WO2001000206. VLA-4 Inhibitor compounds. 2000
- 264.Hijazi Y, Welker H, Dorr AE, Tang JP, Blain R, Renzetti LM, et al. Pharmacokinetics, safety, and tolerability of R411, a dual alpha4beta1-alpha4beta7 integrin antagonist after oral administration at single and multiple once-daily ascending doses in healthy volunteers. J Clin Pharmacol. 2004;44:1368–1378. doi: 10.1177/0091270004270147. [DOI] [PubMed] [Google Scholar]


