Abstract
Background
Temporary prophylactic pancreatic duct stenting effectively reduces post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP) in high-risk patients, but the optimal stent remains unclear. We compared rate of spontaneous passage, and technical difficulty of placement for 3-Fr and 5-Fr stents.
Methods
A randomized controlled trial at a single academic medical center. Patients deemed high risk for PEP randomly received 5-Fr or 3-Fr pancreatic duct stents. Primary outcome was spontaneous stent passage by 2 weeks. Secondary outcomes were ease and time for stent placement, and number of guide wires required for the entire procedure.
Results
Patients (69 female [89%]; mean age 44.9 years, standard deviation [SD] 16.8) were randomly assigned to receive 5-Fr (n = 38) and 3-Fr (n = 40) stents. Indications for stenting were similar. Seven patients in the 3-Fr group actually received a 5-Fr stent, and two in the 5-Fr group had a 3-Fr stent. Spontaneous passage or non-passage was confirmed in 64 (83%). No statistically significant difference in spontaneous passage rates was seen (5-Fr group, 68.4%; 3-Fr group 75.0%; P = 0.617). Non-passage rates were 10.5% (5-Fr group) and 10.0% (3-Fr group) (P = 1.00). The study was stopped after a futility analysis for the primary end point. Placement of 5-Fr stents was rated easier, at a mean score of 1.8 (5-Fr) vs. 3.4 (3-Fr), P < 0.001, with a trend towards being faster, 9.2 vs. 11.1 minutes (P = 0.355). Fewer guide wires were required for 5-Fr stent placement, 1.5 vs. 1.9 (P = 0.002). PEP rates did not differ (P = 0.519).
Conclusion
Placement of 5-Fr compared to 3-Fr pancreatic duct stents for PEP prophylaxis is easier, faster, and requires fewer wires. No statistically significant difference in spontaneous passage was found between the two sizes.
Introduction
Pancreatitis is the most common complication of endoscopic retrograde cholangiopancreatography (ERCP) occurring in 4% to 20% of cases [1]. Temporary prophylactic pancreatic duct stenting has been shown to decrease the rate of post-ERCP pancreatitis (PEP) in high-risk patients [2–6] and is also cost-effective [7]. Placement of a pancreatic duct stent is, however, not without complications. Failure of spontaneous passage of the stent may require a repeat endoscopic procedure for stent removal. Proximal migration and other stent-related complications may occur. Pancreatic duct and parenchymal changes mimicking chronic pancreatitis have also been reported with prophylactic pancreatic duct stenting [8].
What is the most effective stent type for PEP prophylaxis, with the fewest complications, remains controversial. A large retrospective trial suggested that a long 3-French (3-Fr) stent, compared with a short 5-French (5-Fr) stent, is associated with higher rates of spontaneous stent passage, lower rates of PEP, and lower rates of pancreatic duct changes [9]. However, a recently published randomized trial demonstrated conflicting results, suggesting that a short 5-Fr, compared with a long 3-Fr pancreatic duct stent, is associated with higher spontaneous stent passage and lower rates of PEP, although this difference was not statistically significant [10]. This study also showed that failure of attempted stent placement occurred more commonly with the 3-Fr stent, suggesting that placement may be more difficult than with the 5-Fr stent. Difficulty of placement of long 3-Fr stents may be related to the need for deeper main pancreatic duct guide wire access, and the requirement for the smaller caliber 0.018-inch wire that is harder to maneuver and visualize under fluoroscopy. A higher degree of difficulty in placing a prophylactic pancreatic duct stent is an important technical factor that may influence stent selection, since prolonged or unsuccessful pancreatic duct stent placement may increase the risk of pancreatitis [11].
The technical difficulty of placement of 5-Fr and 3-Fr pancreatic duct stents has not been compared in a randomized prospective study. Our primary study aim was to compare spontaneous passage and non-passage rates for prophylactic 5-Fr and 3-Fr pancreatic duct stents. Our secondary aim was to quantify and compare the difficulty of stent placement, and the time and the number of guide wires required for the entire procedure.
Methods
Patient selection
The study protocol was approved by the University of Michigan Institutional Review Board. Patients presenting for an ERCP between January 2007 and February 2009 were offered an opportunity to participate in this study. Patients eligible to participate in the study had to be ≥18 years of age, not pregnant, and able to provide consent. Written informed consent was obtained from all patients prior to the ERCP.
Inclusion criteria for study entry required patients to have high risk predictors of PEP, defined by: pancreatic sphincterotomy, minor papilla sphincterotomy, sphincter of Oddi dysfunction (SOD) confirmed by manometry, suspected SOD without manometry or with normal manometry findings, ampullectomy, pre-cut sphincterotomy, personal history of PEP, and difficult cannulation.
Patients were excluded from the study if they did not undergo an ERCP, did not receive a pancreatic stent during an ERCP, received a pancreatic stent for indications other than high-risk PEP prophylaxis, or received a non-study pancreatic duct stent at the discretion of the treating endoscopist.
Data collection
Data collected included patients’ age, gender, indication for the procedure, ERCP fellow involvement and level of training at the time of the procedure, sedation medication use, degree of difficulty of stent placement, number of guide wires used, results of 2-week post-procedural abdominal X-ray, requirement of follow-up endoscopy, and adverse events.
Pancreatic stents
A Zimmon 3-Fr or 5-Fr single pigtail pancreatic stent without internal flanges (Cook Endoscopy, Winston-Salem, North Carolina, USA) was placed in all patients. The preferred lengths for the 3-Fr and the 5-Fr stent were 6 cm and 5 cm, respectively; however, other lengths could be used, depending on need, at the discretion of the treating endoscopist. In cases of difficult stent placement, defined by the treating endoscopist, patients were allowed to cross over to the counterpart group.
Study design
This was a randomized, controlled clinical trial. Using a computer-generated 1:1 randomization table, patients were assigned to receive a 3-Fr or 5-Fr pancreatic stent. Allocation was concealed using an opaque envelope system until the endoscopist announced his/her intent to place a prophylactic pancreatic stent.
Specific measured outcomes
The primary end point was the spontaneous passage rate for 3-Fr and 5-Fr pancreatic stents. Secondary end points included difficulty of stent placement, number of wires used for the entire procedure, and total time required for stent placement.
Definitions
At 2 weeks (14 ± 4 days) after stent placement, spontaneous passage or non-passage was determined by the presence or absence of a stent in the pancreatic duct on abdominal X-ray.
The time required for stent placement was assessed from the point of initiation of wire insertion to successful completion of stent placement.
Difficulty of placement was assessed immediately following the procedure using a modified 5-point Likert scale [12,13] (1 = very easy, 2 = easy, 3 = average, 4 = difficult, 5 = very difficult).
Post-ERCP pancreatitis was defined by consensus criteria [14,15]: clinical evidence of pancreatitis, elevation of pancreatic enzymes to three times upper limit of normal at 24 h after the procedure, and hospital admission for 2–3 days (mild pancreatitis), 4–9 days (moderate pancreatitis) or longer than 10 days (severe pancreatitis).
Statistical analysis
We originally calculated a study sample size of 174 patients (87 in both 5-Fr and 3-Fr groups). This sample size was calculated to have 80% statistical power to detect a 19% higher spontaneous passage rate in 3-Fr relative to 5-Fr prophylactic pancreatic stents (86% to 67%, respectively) [9], using a 0.05 two-sided Fisher’s exact test.
A futility analysis, using the method of predictive power [16] was performed after 78 patients had been recruited. Because a futility analysis cannot result in the rejection of the null hypothesis, no adjustments to P values in the final analyses are required. Given the numbers of patients treated and stents retained in both study arms at the time of the futility analysis, the joint conditional probability of proportions of patients retaining stents was numerically integrated over the null hypothesis rejection region of the per-protocol final analysis, to yield its predicted power.
Data from all patients were analyzed on an intention-to-treat basis. Descriptive statistics were calculated as means with standard deviation (SD) for continuous variables and as percents for categorical variables. Statistical significance was assessed by Fisher’s exact test for binary variables and the Wilcoxon rank-sum test for continuous variables.
Results
Futility analysis
A futility analysis performed based on primary end points after enrolment of 78 patients (~40% of target sample size) revealed no statistically significant difference in spontaneous passage and non-passage rates between the 3-Fr and 5-Fr prophylactic pancreatic duct stents groups. The power for rejection of the original null hypothesis at the end of the trial was estimated to be less than 0.1%. Further enrolment of patients was therefore stopped and the study was terminated.
Patient and procedure data
A total of 234 patients consented to participation in the study (Fig. 1), of whom 78 patients (33%) at high risk for developing PEP were randomly assigned to receive 5-Fr (n = 38) or 3-Fr (n = 40) prophylactic pancreatic duct stents. The mean (SD) age was 44.9 (16.8) years and 69 (89%) of the patients were women. The demographic parameters and indications for pancreatic duct stent placement were similar for the 5-Fr and 3-Fr groups.
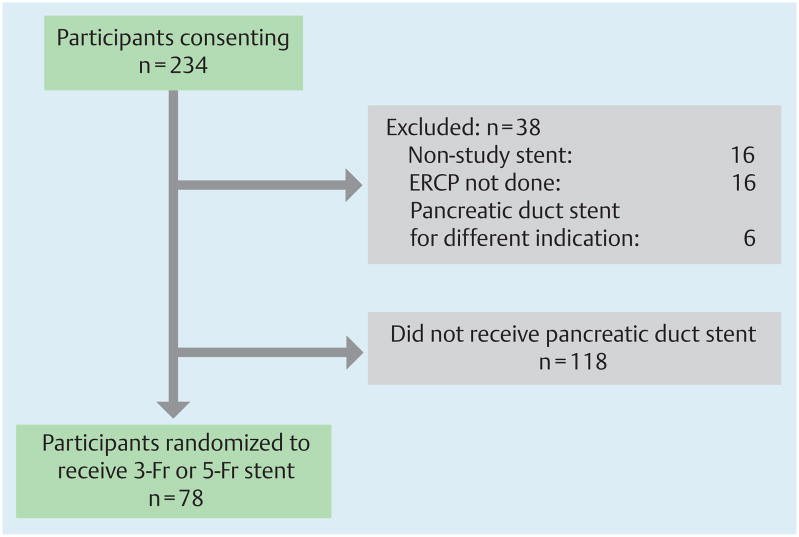
Fig. 1.
Patient flow chart represents all patients who consented to participate in the study. Numbers of patients excluded from the study, with reasons, are shown. A total of 156 were excluded. Among these four patients (2%) received a pancreatic duct stent for high-risk PEP prophylaxis but were not randomized at the request of the treating endoscopist.
A greater number of patients had undergone pancreatic sphincterotomy in the 3-Fr group and more patients had difficult biliary cannulation in the 5-Fr group (Table 1). Gastroenterology trainees (fellows) participated in 93% of the cases. Duration of fellows’ ERCP training (in months) at the time of the procedure was similar for both groups.
Table 1.
Demographic data and indications for stent placement.
| 5-Fr group n = 38 | 3-Fr group n = 40 | P value | |
|---|---|---|---|
| Female, n (%) | 35 (92 %) | 34 (85 %) | 0.482 |
| Age, years, mean (SD) | 46 (17.9) | 44 (16.4) | 0.667 |
| Primary indication for pancreatic duct stent | |||
| Pancreatic sphincterotomy | 2 | 9 | 0.030* |
| Minor papilla sphincterotomy | 7 | 5 | |
| SOD confirmed by manometry | 5 | 11 | |
| Suspected SOD with normal manometry | 4 | 3 | |
| Suspected SOD without manometry | 6 | 6 | |
| Ampullectomy | 6 | 2 | |
| Precut sphincterotomy | 1 | 2 | |
| History of post-ERCP pancreatitis | 1 | 2 | |
| Difficult cannulation | 6 | 0 | |
SD, standard deviation; SOD, sphincter of Oddi dysfunction; ERCP, endoscopic retrograde cholangiopancreatography.
Based on Fisher’s exact test of primary indication by stent type.
Stent placement was successful in 77 patients. One patient randomly assigned to the 3-Fr group had immediate stent dislodgement during the ERCP. This stent was not replaced. The majority of patients received pancreatic duct stents of the preferred length, that is 5 cm for the 5-Fr group (63%) and 6 cm for the 3-Fr group (71%).
A total of 69 patients (89%) received the type of pancreatic duct stent they had been randomly allocated to receive, while nine patients (12%) crossed over to the counterpart group at the discretion of the treating endoscopist. Seven of these patients had been originally assigned to the 3-Fr group and two to the 5-Fr group. The indication for crossing over in all patients assigned a 3-Fr stent was difficult cannulation of the pancreatic duct. In contrast, both patients assigned to a 5-Fr stent crossed over due to concern about a pancreatic duct anatomy that would not accommodate a larger diameter stent.
Stent passage
The 2-week spontaneous passage rates in the 5-Fr and the 3-Fr groups were 26/38 (68.4%) and 30/40 (75.0%) (P = 0.617). When stratified by the different stent indications, the Mantel-Haenszel chi-square test did not show significant differences between the two groups in the spontaneous passage rate (P = 0.90).
The stent non-passage rates at 2 weeks were also nearly identical, at 10.5% for the 5-Fr group and 10.0% for the 3-Fr group (P = 1.00). All patients with stent non-passage required endoscopic removal of the stent.
Seven patients had documented late spontaneous passage (21 to 31 days after stent placement). When all patients with documented spontaneous passage, either within 2 weeks or later than that, were considered (excluding patients with early stent removal), the spontaneous passage rates were 31/38 (81.6%) for the 5-Fr group and 32/40 (80.0%) for the 3-Fr group were (P = 1.000; Table 2 and Fig. 2).
Table 2.
Outcomes of stent placement during endoscopic retrograde cholangiopancreatography (ERCP) in 78 patients.
| 5-Fr group | 3-Fr group | % difference (95 %CL) | |
|---|---|---|---|
| Patients randomly allocated to stent type | 38* | 40† | |
| Spontaneous passage confirmed at 2 weeks | 26 (68.4 %) | 30 (75.0 %) | − 6.6 % (− 26.5 %, 13.4 %) |
| Non-passage confirmed at 2 weeks | 4 (10.5 %) | 4 (10.0 %) | 0.5 % (− 13.0 %, 14.0 %) |
| Late spontaneous passage | 5 (13.2 %) | 2 (5.0 %) | 8.2 % (− 4.5 %, 20.9 %) |
| Early stent removal | 3 (7.9 %) | 3 (7.5 %) | 0.4 % (− 11.4 %, 12.2 %) |
Two patients crossed over to a 3-Fr stent at the discretion of the endoscopist.
One stent dislodged during ERCP and was not replaced, and seven patients crossed over to a 5-Fr stent at the discretion of the endoscopist.
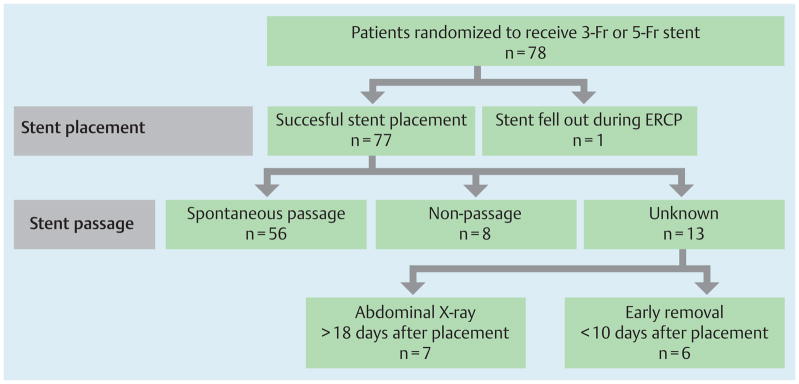
Fig. 2.
A pancreatic duct stent was successfully placed in 77 patients; 1 stent spontaneously fell out during the endoscopic retrograde cholangiopancreatography (ERCP) procedure. A total of 64 patients (82%) had spontaneous passage (n = 56) or non-passage (n = 8) documented within 2 weeks. Stent passage could not be classified in 13 patients (17%): 7 patients (5-Fr group, n = 5; 3-Fr group, n = 2) had abdominal X-rays taken > 18 days after placement, and 6 patients (5-Fr group, n = 3; 3-Fr group, n = 3) had endoscopic removal of the stent <10 days after placement. Stents were removed because of concern about stent occlusion in 5 patients (1 patient with mild post-ERCP pancreatitis). One pancreatic duct stent was exchanged during follow-up ERCP on day 2 after failed biliary cannulation on index ERCP.
There was no statistically significant difference in two-week spontaneous passage rate among patients who received 5-F and 3-F stents per-protocol (excluding patients who crossed-over) at 66.7% (24/36) and 75.8% (25/33) (P = 0.439). Likewise, there was no statistically significant difference when all patients were analyzed based on the stent they actually received at 67.4% (29/43) and 77.1% (27/35) (P = 0.450).
Use of guide wires
The number of wires needed for the entire procedure was reported in 76 patients (97%). Requirement of only one wire for the ERCP occurred more commonly with the 5-Fr compared with the 3-Fr stent, in 22 cases (59.4%) vs. 8 (20.5%), (P < 0.001), and this difference was significant even after using the Mantel-Haenszel adjustment for the stent indications. The mean (SD) number of wires required for placement of a 5-Fr stent was lower than for a 3-Fr stent, at 1.5 (0.6) vs. 1.9 (0.5) (P = 0.002).
The mean (SD) number of wires for patients who crossed over to the counterpart stent group was higher than for those who did not cross over, at 2.2 (0.6) vs. 1.6 (0.6), (P = 0.044). Among those who crossed-over there was no difference in number of wires required when they were stratified according to original assignment group.
Time required for stent placement
The time required for stent placement was reported for 68 patients (87%). Overall the mean (SD) time to place the stent was 10.2 (8.2) minutes, and was 9.2 (9.2) minutes for 5-Fr stents and 11.1 (7.7) minutes for 3-Fr stents (P = 0.355). Prolonged time requirement for stent placement occurred more frequently in the 3-Fr group and quick placement occurred more frequently in the 5-Fr group (Fig. 3).
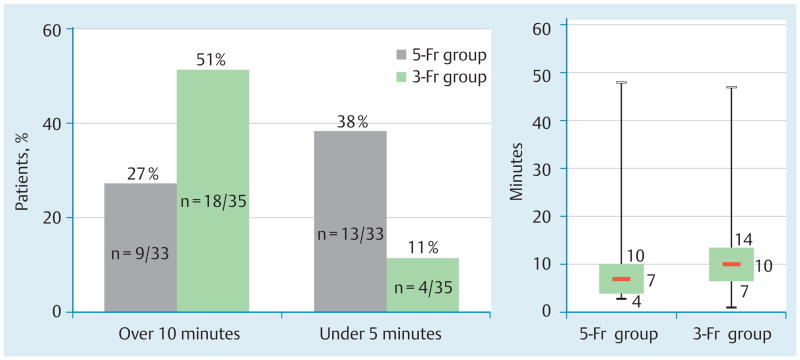
Fig. 3.
Time required for stent placement. a Requirement of greater than average amount of time (≥ 10 min) for stent placement occurred more frequently in the 3-Fr group compared with the 5-Fr group (P = 0.051). b Requirement of less than half the average amount of time for stent placement (≤ 5 min) occurred more frequently in the 5-Fr group compared with the 3-Fr group (P = 0.011). c Box plot of absolute time (minutes) required for all stent placements for 3-Fr and 5-Fr groups. Third quartile, median and first quartile data are represented numerically.
Patients who crossed over to the counterpart stent group required a longer time, an additional 3.8 min for stent placement, compared with those who did not cross over (P = 0.049). Patients who were randomly assigned a 3-Fr stent and crossed over to the 5-Fr group required 5.5 minutes longer for stent placement, compared with those randomized to receive a 5-Fr stent who crossed-over to the 3-Fr group (P = 0.030).
Difficulty of stent placement
The degree of difficulty of stent placement was reported for 73 patients (94%). Placement of a 5-Fr stent was rated as easier than placing a 3-Fr stent, with a mean (SD) Likert scale score of 1.8 (0.9) vs. 3.4 (1.2), (P < 0.001). The number of patients reported to have “difficult” or “very difficult” stent placement was lower in the 5-Fr group compared with the 3-Fr group at 3 (9%) vs. 21 (54%) (P < 0.001).
Patients who crossed over from the 3-Fr stent group to the 5-Fr group tended to have more difficult procedures compared with those who crossed over from the 5-Fr to the 3-Fr group, with a mean 4.3 (0.8) vs. 1.5 (0.7) (P = 0.053).
Other outcomes
The required dose of sedative medications was similar for the 5-Fr and 3-Fr groups, for diazepam (P = 0.380) and fentanyl (P = 0.520). Endoscopic stent removal was done in seven patients in each group (P = 1.00).
Adverse events
A total of 11 patients (14.1%) met the criteria for PEP. Only mild or moderate PEP was observed. Of the four patients in the 5-Fr group (10.5%) who developed PEP, three had moderate PEP; of the seven patients in the 3-Fr group (17.5%) who developed PEP, five had moderate PEP. There was no statistical difference in PEP rates between the 5-Fr and 3-Fr stent groups (P = 0.519).
Other adverse events noted included abdominal pain, which occurred in eight patients assigned to receive a 5-Fr stent, and in nine patients assigned to have a 3-Fr stent. One patient in each group developed post-sphincterotomy bleeding. One patient in the 5-Fr group developed internal stent migration. Post-ERCP nausea and dizziness was reported in two patients in the 3-Fr group.
Discussion
Our final cohort included 78 patients who were at high risk for developing PEP. High-risk factors included pancreatic sphincterotomy (major or minor papilla), ampullectomy, precut sphincterotomy, confirmed or suspected SOD and undergoing biliary or pancreatic sphincterotomy, history of PEP, and difficult cannulation [17–19].
Spontaneous passage of the prophylactic pancreatic duct stent was the primary end point in this study. We measured the spontaneous passage rate by calculating the proportion of patients who had spontaneous passage of the pancreatic duct stent within 2 weeks of placement without needing a secondary endoscopic procedure for stent removal. Rates of spontaneous passage were not different for the two study groups (68% of the 5-Fr group and 75% of the 3-Fr group). However, we did note that 17% of the study patients (20% of 5-Fr group and 12% of 3-Fr) could not be classified in terms of 2-week spontaneous passage rate, due to early stent removal or late documented stent passage. We acknowledge that this could affect the true SP rate in both groups. We therefore also assessed spontaneous passage indirectly by measuring the stent non-passage rate at 2 weeks. The rates were identical between the 3-Fr and 5-Fr groups, at approximately 10%. Considering the inverse of stent non-passage at 2 weeks (89.5% for 5-Fr and 90.0% for 3-Fr), our results with the 3-Fr stent were similar to those reported in a large retrospective study and recently published randomized prospective study (spontaneous passage 86% and 88%, respectively) [9,10]. Our results with the 5-Fr stent were within the range of the rates reported in the above two studies (67% and 98%, respectively). In contrast to either study, our results failed to show any meaningful differences in spontaneous passage between the two stents.
We also assessed the technical aspects of 5-Fr and 3-Fr stent placement. Procedures in the 3-Fr group in total required nearly 20% more wires, adding additional cost to the procedure. Procedures in the 3-Fr group also tended to require longer periods of time for stent insertion. We noted that quick stent placement (≤5 min) occurred more frequently with 5-Fr stents, and prolonged placement (≥10 min) with the 3-Fr stents. The endoscopist-reported degree of difficulty in placement of 3-Fr stents compared with 5-Fr stents was significantly higher. Nearly 20% of patients assigned to the 3-Fr stent group required crossover to the 5-Fr stent because of difficulty in placement.
Technical difficulty and speed of stent placement could certainly be linked to endoscopist experience and comfort with placement of a particular stent type. It is conceivable that placement of a 3-Fr stent could be associated with a steeper learning curve than a 5-Fr stent, in which case the differences in the speed and degree of difficulty of stent placement would vary among different endoscopists. In our study, among the five endoscopists who placed six or more stents, four endoscopists reported a greater mean difficulty score for placement of 3-Fr stents compared with 5-Fr stents (one endoscopist reported a similar mean difficulty score for both stents). Although trainees were involved in most of these procedures, faculty were present all of the time, did not hesitate to take over the procedure, and did not score the difficulty of stent placement on the basis of trainee inexperience. The faculty in this study are expert endoscopists with estimated total career cases of 750 to 5000 with a mean of 1700 ± 570. This suggests that the difficulty of 3-Fr stent placement is likely to be related more to the stent itself rather than to the expertise of the endoscopist who places the stent.
One reason an endoscopist may select a smaller diameter prophylactic pancreatic stent could be concern that mechanical pressure from the stent might induce irreversible pancreatic duct changes [20]. It is thought that the pressure the stent imposes on the pancreatic duct could be proportional to the diameter of the stent; this would favor the use of a smaller caliber stent. The endoscopist might also select a smaller diameter pancreatic stent in an effort to reduce the risk of PEP as has been shown in one retrospective study [9]. To date, however, there have been no prospective randomized studies in humans that assess whether irreversible pancreatic duct changes are affected by pancreatic stent size. There have also been no prospective randomized trials that have shown that smaller diameter pancreatic stents offer protection against PEP. It is important to note that our study was not designed to measure differences in ductal changes and it was not powered to detect differences in PEP rates between the 3-Fr and 5-Fr stent groups. We did note a numerically higher number of PEP events in the 3-Fr group, compared with the 5-Fr group (18% vs. 11%) that was not statistically significant. There were no events of severe PEP, and differences between minimal and moderate PEP were also not statistically significant.
PEP rates may be affected by multiple factors, including patient factors, stent factors, difficulty of cannulation, and success of cannulation. The literature suggests that an increased number of wires required for pancreatic duct stent placement, prolonged attempts at stent placement or unsuccessful pancreatic duct stent placement may be associated with higher rates of PEP [10]. Our study shows that technical aspects of stent placement that may reduce the risk of PEP favor the 5-Fr stent over the 3-Fr stent. In summary, placement of a 5-Fr stent is easier and faster than placement of a 3-Fr stent and requires fewer wires for the entire procedure. There is no difference in spontaneous stent passage between the 3-Fr and 5-Fr stents. Based on these data, we recommend placement of a 5-Fr stent in preference to 3-Fr stent for prophylaxis of PEP in high-risk patients.
Footnotes
Competing interests: None
References
- 1.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 2.Fazel A, Quadri A, Catalano MF, et al. Does a pancreatic duct stent prevent post-ERCP pancreatitis? A prospective randomized study. Gastrointest Endosc. 2003;57:291–294. doi: 10.1067/mge.2003.124. [DOI] [PubMed] [Google Scholar]
- 3.Singh P, Das A, Isenberg G, et al. Does prophylactic pancreatic stent placement reduce the risk of post-ERCP acute pancreatitis? A meta-analysis of controlled trials. Gastrointest Endosc. 2004;60:544–550. doi: 10.1016/s0016-5107(04)02013-9. [DOI] [PubMed] [Google Scholar]
- 4.Aizawa T, Ueno N. Stent placement in the pancreatic duct prevents pancreatitis after endoscopic sphincter dilation for removal of bile duct stones. Gastrointest Endosc. 2001;54:209–213. doi: 10.1067/mge.2001.115730. [DOI] [PubMed] [Google Scholar]
- 5.Smithline A, Silverman W, Rogers D, et al. Effect of prophylactic main pancreatic duct stenting on the incidence of biliary endoscopic sphincterotomy-induced pancreatitis in high-risk patients. Gastrointest Endosc. 1993;39:652–657. doi: 10.1016/s0016-5107(93)70217-5. [DOI] [PubMed] [Google Scholar]
- 6.Tarnasky PR, Palesch YY, Cunningham JT, et al. Pancreatic stenting prevents pancreatitis after biliary sphincterotomy in patients with sphincter of Oddi dysfunction. Gastroenterology. 1998;115:1518–1524. doi: 10.1016/s0016-5085(98)70031-9. [DOI] [PubMed] [Google Scholar]
- 7.Das A, Singh P, Sivak MV, Jr, Chak A. Pancreaticstent placement for prevention of post-ERCP pancreatitis: a cost-effectiveness analysis. Gastrointest Endosc. 2007;65:960–968. doi: 10.1016/j.gie.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Smith MT, Sherman S, Ikenberry SO, et al. Alterations in pancreatic ductal morphology following polyethylene pancreatic stent therapy. Gastrointest Endosc. 1996;44:268–275. doi: 10.1016/s0016-5107(96)70163-3. [DOI] [PubMed] [Google Scholar]
- 9.Rashdan A, Fogel EL, McHenry L, Jr, et al. Improved stent characteristics for prophylaxis of post-ERCP pancreatitis. Clin Gastroenterol Hepatol. 2004;2:322–329. doi: 10.1016/s1542-3565(04)00062-x. [DOI] [PubMed] [Google Scholar]
- 10.Chahal P, Tarnasky PR, Petersen BT, et al. Short 5Fr vs long 3Fr pancreatic stents in patients at risk for post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol. 2009;7:834–839. doi: 10.1016/j.cgh.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Freeman ML, Overby C, Qi D. Pancreatic stent insertion: consequences of failure and results of a modified technique to maximize success. Gastrointest Endosc. 2004;59:8–14. doi: 10.1016/s0016-5107(03)02530-6. [DOI] [PubMed] [Google Scholar]
- 12.Komorita SS. Attitude content, intensity, and the neutral point on a Likert scale. J Soc Psychol. 1963;61:327–334. doi: 10.1080/00224545.1963.9919489. [DOI] [PubMed] [Google Scholar]
- 13.Likert R. A technique for the measurement of attitudes. Arch Psychol. 1932;140:1–55. [Google Scholar]
- 14.Mallery JS, Baron TH, Dominitz JA, et al. Complications of ERCP. Gastrointest Endosc. 2003;57:633–638. doi: 10.1053/ge.2003.v57.amge030576633. [DOI] [PubMed] [Google Scholar]
- 15.Cotton PB, LG, Vennes J, Geenen JE, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 16.Spiegelhalter DJ, Freedman LS, Blackburn PR. Monitoring clinical trials: conditional or predictive power? Control Clin Trials. 1986;7:8–17. doi: 10.1016/0197-2456(86)90003-6. [DOI] [PubMed] [Google Scholar]
- 17.Masci E, Mariani A, Curioni S, Testoni PA. Risk factors for pancreatitis following endoscopic retrograde cholangiopancreatography: a meta-analysis. Endoscopy. 2003;35:830–834. doi: 10.1055/s-2003-42614. [DOI] [PubMed] [Google Scholar]
- 18.Aronson N, Flamm CR, Bohn RL, et al. Evidence-based assessment: patient, procedure, or operator factors associated with ERCP complications. Gastrointest Endosc. 2002;56 (Suppl 6):S294–S302. doi: 10.1067/mge.2002.129021. [DOI] [PubMed] [Google Scholar]
- 19.Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005;62:367–370. doi: 10.1016/j.gie.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Derfus GA, GJ, Hogan WJ. Effects of endoscopic pancreatic duct stent placement on pancreatic ductal morphology [abstract] Gastrointest Endosc. 1990;36(206A) [Google Scholar]