INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer in the United States and the second most common cause of cancer death, although incidence and mortality is decreasing with increased CRC screening and polypectomy of adenomas [1-4]. Previous studies have established that patients with a first degree relative (FDR) with CRC are at a 2 – 3 fold higher risk of CRC compared to the general population (1). This has led to recommendations for earlier colonoscopy screening among individuals with a family history of CRC. Specifically, current multi society guidelines (American Cancer Society, American College of Gastroenterology, American Gastroenterological Association, and American Society for Gastrointestinal Endoscopy, American College of Physicians, and American College of Radiology) recommend that patients with a FDR (defined as a parent, sibling, or child) with colon cancer before the age of 60 should be advised to undergo screening colonoscopy starting at least at age 40, or 10 years before the index case, and repeated every 5 years (2). Patients with a FDR with CRC at or above the age of 60 should be advised to have screening colonoscopy starting at age 40, and repeated every 10 years (2).
These recommendations are primarily based upon a prospective study by Fuchs et al.(3), which indicated that FDRs of CRC patients had a risk of CRC at age 40 that was similar to the risk of CRC in average-risk patients at the age of 50. Although the data from this study demonstrated that individuals with a family history of CRC had an increased risk of developing CRC compared to age-matched individuals without a family history of CRC (RR = 1.72; 95% CI: 1.34-2.19), most of the increased risk was derived from individuals with a FDR diagnosed with CRC before the age of 45 (RR = 5.37; 95% CI: 1.98-14.6), and it is unclear how many of these individuals might have had a familial CRC syndrome, specifically HNPCC. In fact, individuals with a FDR diagnosed with CRC after the age of 60 did not have a significant increase in their risk of developing CRC (RR = 1.35; 95% CI: 0.81-2.25) . This has led to some conflicting recommendations with the 2009 American College of Gastroenterology CRC guidelines recommending that patients with a single FDR with CRC diagnosed ≥ 60 should undergo the same CRC screening as an average risk individual (4).
The conflicting recommendations for individuals with a family history of CRC are partly because of the lack of data about the prevalence of CRC and adenomas among 40-49 year old individuals with a FDR with CRC. No previous studies have stratified prevalence of adenomas or large adenomas based on age of FDR at time of CRC diagnosis. Several small studies have quantified the prevalence of adenomas in 50-75 year olds with a FDR diagnosed with CRC and reported a prevalence of 27-39% vs 13-26% in controls (5-8). After a thorough literature search, a single study based on 228 German subjects assessed prevalence of adenomas in 40-50 year olds with a family history of CRC and reported a higher prevalence (18.9%) of adenomas in subjects with a family history of CRC compared to controls (8.2%) (9).
Since the lifetime prevalence of CRC is approximately 6%, literally millions of individuals will have a positive family history of CRC in the USA and will be told to undergo colonoscopy starting at least at age 40. It seems prudent to investigate this issue in order to validate these recommendations. Therefore, in this retrospective study, we quantify the prevalence of adenomas and advanced adenomas (adenomas > 10 mm) and risk factors for adenomas in 40- to 49-year-old patients undergoing first screening colonoscopy because of a family history of CRC in a FDR at the University of Michigan from 1999 to 2009.
METHODS
Study Design
This is a retrospective study based on review of patient medical records and endoscopic databases. Data was gathered from the University of Michigan endoscopy unit and satellite endoscopy centers located at the University of Michigan East Ann Arbor (Ann Arbor, MI) and Livonia (Livonia, MI) Medical Procedure Centers. All colonoscopies were conducted by board-certified gastroenterology staff or gastroenterology fellows under direct supervision of staff gastroenterologists. A review of the subject’s electronic medical record was conducted to assess eligibility and abstract demographic and clinical information. All data were kept on University of Michigan secure laptops; patient information was stored with their medical record number as the only identifier. IRB approval was obtained before commencement of the data collection.
Study Population
Using the endoscopy unit’s database, with the assistance of the endoscopy unit’s technical staff, all colonoscopies performed between January 1st, 1999 and June 1st, 2009, in 40-49 year old individuals for a family history of CRC was compiled. A thorough review of the patient’s electronic medical record was conducted to ensure study eligibility.
Inclusion criteria included: (a) 40-49 years old; (b) indication for screening colonoscopy documented as “family history of CRC”; (c) documented history of CRC in FDR. Exclusion criteria included: (a) prior colonoscopy for any reason in the previous 10 years (b) personal history of adenomas or CRC; (c) history of familial adenomatous polyposis, hereditary non-polyposis colorectal cancer (HNPCC), or inflammatory bowel disease; (d) documented history of overt or occult GI bleeding, iron deficiency anemia, or unexplained weight loss in six months before colonoscopy.
If available, the age of the FDR at diagnosis of CRC was recorded, but absence of this information was not an exclusion criteria. If multiple family members were diagnosed with CRC, the earliest diagnosis of CRC in a first-degree relative was reported as the age of FDR at diagnosis with CRC. Colonoscopies which had poor / inadequate preps or were incomplete and were not followed up with a colonoscopy within the following 6 months were also excluded.
If a study subject had multiple colonoscopies, we looked at their first one available in our records. If the first colonoscopy was done within the age bracket of 40-49, and it was the first one done in the preceding 10 years, we included the data from that procedure. If we had documentation that a patient had had a colonoscopy at an outside facility, and we did not have records of that procedure, the patient was excluded from the study.
Colonoscopy prep and technique
For the period from 1999 till 2006, endoscopes manufactured by PENTAX were utilized for most procedures. These scopes were not High Definition (HD) imaging capable, and also did not have Narrow Band imagining (NBI) available. From 2006-2009, endoscopes manufactured by OLYMPUS were utilized for the colonoscopies, many of which were HD and NBI capable, along with digital magnification. The field of view available on the Olympus endoscopes was 170 degrees, as opposed to 140 degrees on the Pentax endoscopes. Data regarding the type of prep used was not recorded in our study, although Polyethylene glycol (PEG) based preps are used most commonly at our center. Data regarding whether split prep was utilized is not recorded for these procedures, although use of split prep did not become routine until 2010 at our institution. There has been a program in place to encourage a withdrawal time greater than 6 minutes since mid 2008, although routine recording of withdrawal time did not begin until 2009.
Data Extraction
Colonoscopy procedure data was collected from endoscopy reports in our electronic medical records. Data was collected on the patient’s bowel preparation quality (reported as excellent, good, adequate, fair, poor, and inadequate), procedure completion status (ie. visualization of the ileocecal valve and the appendiceal orifice), number and size of polyps, and the presence of a gastroenterology fellow. The name of the exact faculty member present during the colonoscopy was not recorded in our data set. However, all our faculty members perform procedures with the fellows in scheduled blocks, and the fellows perform procedures only with University of Michigan academic faculty. Recommendations for interval between screening colonoscopies was recorded for exams completed between 2006-09, and this recommendation was compared to the multi-society guideline recommendations. Pathological interpretation of polyp tissue was retrieved from the electronic medical record. Advanced adenoma (AA) was defined as an adenoma ≥ 10 mm in size. Based upon the policy of the GI pathologists (Henry Applebaum, MD, and Joel Greenson, MD) at the University of Michigan, villous histology and high grade dysplasia are not reported. Therefore, advanced adenomas are solely defined as adenomas ≥ 10 mm in this study.
Data about gender, race/ethnicity, and age at time of colonoscopy were collected for all study patients. Data was also collected on study patients body mass index (kg/m2), presence/absence of diabetes (Type I or Type II), chronic use of aspirin (> 2 tablets/week), hormone replacement therapy, or statins at the time of colonoscopy.
Statistical Analysis
The patient’s age, body mass index, number of relatives diagnosed with CRC, and the number and diameter of adenomas were reported as continuous variables. Gender, prior diagnosis of diabetes, aspirin and NSAID usage, hormone replacement therapy, statin usage, presence of a gastroenterology fellow, and presence of polyps and adenomas were dichotomized. Age of the FDR at the time of diagnosis with CRC was diagnosed as a continuous variable and as a dichotomous variable as well, with the dichotomous variable depicting if the diagnosis was at or above the age of 60 or not. Quality of preparation was dichotomized into adequate (reported as excellent, good, fair or adequate) verse inadequate (reported as poor or inadequate).
Difference in prevalence rates of adenomas or AAs’ across various groups was measured using the chi-square test. Multivariable logistic regression was used to measure the impact of family history of CRC on adenoma prevalence; this model was adjusted for age of the patient, age of the first-degree relative diagnosed with CRC, race/ethnicity, gender, BMI, aspirin and statin medicine use. The statistical analysis was conducted using STATA software, version 10.0 (STATA Corp).
RESULTS
Study Population
A total of 1302 patients in the 40 to 49 year age group underwent colonoscopy at the University of Michigan endoscopy centers between January 1st, 1999 and June 1st, 2009, because of a family history of colorectal cancer in a FDR. Six hundred sixty-two (50.8%) of these patients met various exclusion criteria (Figure 1), leaving 640 patients for the study population. The demographic and clinical characteristics of the study population are summarized in table 1. The patients were mostly Caucasian (81.4%) with similar M:F ratio (46.6% vs 53.4%) and mean age = 44.5 +/- 2.9. Age of FDR at time of diagnosis of CRC was available for most study patients (86%) with similar numbers of patients having a FDR diagnosed with CRC before age 60 (54%) or at/above age 60 (46%). Information regarding the clinical risk factors was available for 593 patients (92.7%) and demonstrated mean BMI = 28.3 +/- 6.5 with fewer than 10% of study patients diagnosed with diabetes (2.4%) or chronic ongoing use of statins (5.6%), aspirin (4.6%), or hormone replacement therapy (4.2%). Three hundred sixty-two colonoscopies were performed between 2007-2009 (56.6%) and 278 (43.4%) between 1999 and 2006.
Figure 1.
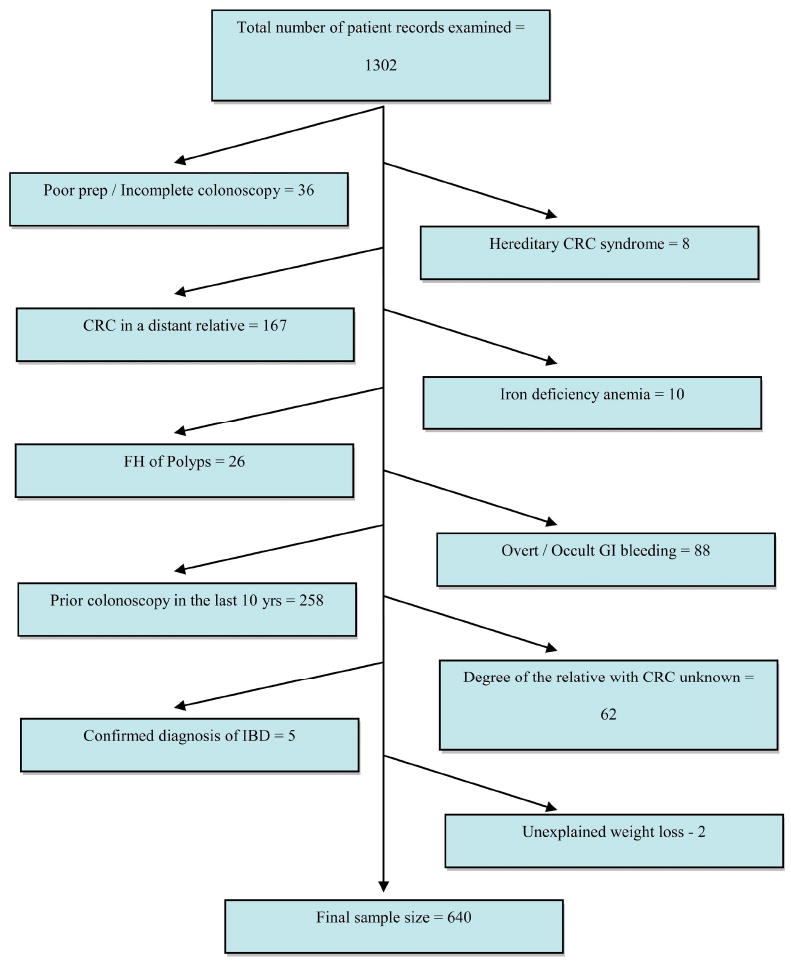
Schematic representations of various reasons why patients were excluded from the study and how we arrived at the final sample size.
Table 1.
Demographic and clinical characteristics.
| Age | All (N = 640) | ‘99-‘06 (N=278) | ‘07-‘09 (N=362) |
|---|---|---|---|
| Mean (SD) | 44.5 (2.9) | 44.4 (2.9) | 44.6 (3) |
| 40-44y – N (%) | 314 (49.1) | 145 (52.2) | 169 (46.7) |
| 45-49y – N (%) | 326 (50.9) | 133 (47.8) | 193 (53.3) |
| Sex* | All (N = 640) | ‘99-‘06 (N=278) | ‘07-‘09 (N=362) |
| Male | 298 (46.4) | 146 (52.2) | 152 (42) |
| Female | 342 (53.4) | 132 (47.5) | 210 (58) |
| BMI | All (N = 293) | ‘99-‘06 (N=10) | ‘07-’09 (N=283) |
| Mean (SD) | 28.3 (6.5) | 30.4 (4.8) | 28.3 (6.6) |
| BMI ≥ 30 – N (%) | 100 (34.2) | 4 (40) | 96 (33.9) |
| BMI < 30 – N (%) | 193 (65.8) | 6 (60) | 187 (66.1) |
| Race** | All (N = 640) | ‘99-‘06 (N= 278) | ‘07-’09 (N= 362) |
| Caucasian | 521 (81.4) | 239 (86) | 282 (77.9) |
| African American | 34 (5.3) | 12 (4.3) | 22 (6.1) |
| Hispanic | 16 (2.5) | 2 (0.7) | 14 (3.9) |
| Asian | 43 (6.7) | 13 (4.7) | 30 (8.3) |
| Native American | 0 (0) | - | - |
| Other/Unknown | 26 (4.1) | 12 (4.3) | 14 (3.9) |
| Clinical | All (N=593) | ‘99-‘06 (N= 258) | ‘07-’09 (N= 335) |
| Diabetes *** | 14 (2.4) | 2 (0.8) | 12 (3.6) |
| Aspirin use | 27 (4.6) | 14 (5.4) | 13 (3.9) |
| HRT£ | 25 (4.2) | 19 (7.4) | 6 (1.8) |
| Statin use££ | 33 (5.6) | 6 (2.3) | 27 (8.1) |
Differences statistically significant across the 2 time periods; P = 0.008
Differences statistically significant across the 2 time periods; P = 0.021
Differences statistically significant across the 2 time periods; P = 0.026
Differences statistically significant across the 2 time periods; P = 0.001
Differences statistically significant across the 2 time periods; P = 0.003
Adenoma Prevalence
The overall prevalence of adenomas (any size) was 15.4% and AA was 3.3%. The adenoma prevalence rate was significantly higher in 45 – 49 year old patients compared to the 40 - 44 year old group (21.5% vs 9.2%, p < 0.001, Table 2) and AA prevalence also trended higher in the 45-49 age group (4% vs 2.5%, p =NS). Men had a higher prevalence of adenomas versus women (18.8% vs 12.6%; P = 0.03). The prevalence rate was significantly lower in individuals with a FDR was diagnosed with CRC before the age of 60 vs individuals with a FDR diagnosed with CRC at or above age 60 years old (12.4% vs 19%, p = 0.034). Patients with FDRs diagnosed with CRC ≥ 60 were older (44.9 ± 3 yrs) compared to patients with a FDR diagnosed with CRC < 60 (44 ± 2.9; P = 0.0002). Among the patients whose FDR was diagnosed ≥ 60, 58.1% were in the 45-49 age bracket vs 43.3% among those who had younger FDRs (p = 0.001). Adenoma detection rates were higher in 2007-2009 versus 1999-2006 (18.8% vs 11.2%, p = 0.008) and trended higher for AA detection rates (3.9% vs 2.5%, p = NS). One case of CRC, an invasive rectal adenocarcinoma, was detected in the entire study population (prevalence 0.15%).
Table 2.
Prevalence of adenomas and advanced adenomas.
| N | Adenoma (any size) N (%) |
Advanced adenoma N(%) |
|
|---|---|---|---|
| Overall Prevalence | 640 | 99 (15.4) | 21 (3.3) |
| Prevalence by age of the patient | |||
| 40-44y | 314 | 29 (9.2)* | 8 (2.5) |
| 45-49y | 326 | 70 (21.5)* | 13 (4) |
| Prevalence by the age of FDR at the time of diagnosis of CRC | |||
| FDR < 60y | 298 | 37 (12.4)** | 14 (4.7) |
| FDR ≥ 60y | 253 | 48 (19)** | 7 (2.8) |
| Prevalence by the time since colonoscopy was performed | |||
| 2007 - 2009 | 362 | 68 (18.8)*** | 14 (3.9) |
| 1999 – 2006 | 278 | 31 (11.2)*** | 7 (2.5) |
Differences statistically significant across the 2 age groups; P < 0.001
Differences statistically significant across the 2 groups; P = 0.034
Differences statistically significant across the time periods; P = 0.008
Risk factors for adenomas
The multiple logistic regression analysis assessed the association between adenomas (any size) and multiple potential risk factors, including age, gender, race, BMI, age of the FDR at diagnosis, number of FDRs with CRC, presence of a GI fellow during the colonoscopy, number of years elapsed since the last colonoscopy (must be > 10 years), presence of diabetes, chronic on-going aspirin use, HRT, or statin use. [Note: please see Methods section for complete description of use of continuous or dichotomous variables for each potential risk factor.] The crude and adjusted Odds Ratios (OR) are reported in table 3. The only independent predictors for adenomas were patient age at time of colonoscopy (OR= 1.16, p = 0.02) and male gender (OR= 2.3, p = 0.02). Colonoscopies performed in the last 3 years (2007-2009) had a higher prevalence of adenoma, but this factor lost its significance when adjusted for other confounders. Age of the FDR at time of CRC diagnosis was not associated with adenoma prevalence nor was more than 1 FDR with CRC.
Table 3.
Factors impacting the prevalence of adenomas and advanced adenomas.
| Adenoma | ||
|---|---|---|
| Crude | Adjusted | |
| Age | 1.18 (1.09 -1.27) | 1.16 (1.03-1.31) |
| Male sex | 1.6 (1.04 – 2.07) | 2.1 (1.06-4.4) |
| Race (Caucasian Vs Other) | 1.46 (0.74 -2.6) | 1.34 (0.5-3.4) |
| Obesity | 1.4 (0.75-2.6) | 1.67 (0.8-3.45) |
| Diabetes | 0.9 (0.2-4.2) | 0.56 (0.08-3.9) |
| Aspirin use | 0.7 (0.2-2.3) | 0.26 (0.03-2.3) |
| HRT | 0.75 (0.2-2.5) | - |
| Statin use | 1.8 (.8-4.3) | 1.53 (0.44-5.3) |
| ≥ 2 FDR’s with CRC | 0.64 (0.2 -2.17) | 1.72 (0.33-8.8) |
| Age of the FDR at diagnosis > 60 | 1.6 (1.03-2.6) | 2.01 (0.94-4.27) |
| Presence of a GI fellow during procedure | 1.1 (0.7-1.7) | 1.05 (0.46-2.4) |
| Colonoscopy performed in 2007-2009 Vs earlier | 1.88 (1.2-2.9) | 0.43 (.09-1.93) |
Recommended interval for follow-up colonoscopy screening
In 2007-2009, 189 colonoscopies on study patients were normal without any polyps identified (Table 4). The follow-up recommendation was appropriate based on 2006 multi-society guidelines in 64% (121/189) of cases. Among study patients with inappropriate recommendations for repeat colonoscopy, most study patients were instructed to return for repeat colonoscopy sooner than 5 years (41/68; 61.2%) whereas a substantial minority of these study patients (23/68; 34.2%) did not receive any recommendation about timing of repeat colonoscopy. Among study patients with a normal colonoscopy and a documented history of FDR diagnosed with CRC at age ≥ 60, only 32.7% (25/77) were instructed that repeat screening colonoscopy should be performed in 10 years (per the guideline recommendations), whereas the majority of these study patients (55.8%; 43/77) were instructed to get repeat screening colonoscopy in 5 years.
Table 4.
Appropriateness of the recommended follow-up interval after the screening colonoscopies included in our study. The appropriateness is based on compliance with the recent multi society CRC screening guidelines(2). This analysis was only conducted for the colonoscopies performed in the last 3 years, and which were completely normal (no polyps detected).
| Recommended Interval | N (%) |
|---|---|
| Appropriate | 121 /189 (64) |
| Inappropriate | 68/189 (36) |
| If Inappropriate - | |
| Called back too soon | 41/68 (61.2) |
| Called back too late | 3/68 (4.5) |
| No follow up recommendation given | 24/68 (35.2) |
Impact of Gastroenterology Fellow on Colonoscopy Findings and Recommendations for Repeat Screening Colonoscopy
Gastroenterology (GI) fellows were present in 32.7% (209/640) of the colonoscopies performed. The adenoma and advanced adenoma detection rates were not significantly different in this group compared to the colonoscopies performed without the presence of a GI fellow. However, during 2007-2009, the percentage of cases getting a follow up recommendation in compliance with the 2006 multi society screening guidelines (2) was significantly higher in cases where a GI fellow was present (Table 5).
Table 5.
Impact of the presence of GI fellows on the appropriateness of the recommended follow up interval after the screening colonoscopies. The appropriateness is based on compliance with the recent multi society CRC screening guidelines (2). This analysis was only conducted for the colonoscopies performed in 2006-2009.
| Appropriate Follow up All Colonoscopies N (%) |
Appropriate Follow up Normal Colonoscopies N (%) |
|
|---|---|---|
| Fellow Present | 69/97 (71.1) | 36/47 (76.6) |
| Attending only | 143/250 (57.2) | 85/142 (59.9) |
| P | 0.017 | 0.038 |
DISCUSSION
This study quantifies the prevalence of adenomas (any size) and AAs among 40-49 year olds undergoing their first screening colonoscopy because of a FDR with CRC. This is an important clinical issue since current national multi society guidelines recommend screening colonoscopy at age 40 in these individual, and literally millions of US residents have or will have a family history of CRC. Yet, there is minimal data about colonoscopic findings among 40-49 year olds with a family history of CRC. This is the largest study and the first US-based study to specifically look at this 40-49 year old population with a family history of CRC. Using stringent inclusion and exclusion criteria, we identified 640 asymptomatic 40-49 year olds with a family history of CRC who underwent their first screening colonoscopy at the University of Michigan, and we found that the prevalence of adenomas (any size) =15.4% and AA = 3.3%.
Overall, this prevalence appears lower than commonly quoted prevalences of adenomas and AAs in average-risk, asymptomatic 50-59 year olds (2, 4, 10). Although an increase in adenoma detection was noted during the 2007-2009 period vs 1999-2006 period, this was not a significant difference after adjustment for confounders in our multiple logistic regression analysis. The difference in adenoma (any size) found during the 2007-2009 period could be because of multiple different factors, including the use of improved endoscopic equipment, increased awareness of endoscopic withdrawal time and the importance of adenoma detection rates. Finally, there are some important demographic differences between the 1999-2006 group and the 2007-2009 group, such as a significantly lower proportion of males, and that of Caucasians, in the later group (Table 1).
The only other study directly addressing this issue is based on 228 German subjects, and it reported a higher prevalence (18.9%) of adenomas in 40–50 year olds with a FDR with CRC compared to 40-49 year old controls (8.2%) (9). Two other studies quantified the prevalence of adenomas and AA in 40-49 year old average risk individuals in the US (10, 11). The first study, published in 2002, retrospectively looked at the prevalence of adenomas and AA in 906 asymptomatic 40-49 year olds and reported an adenoma prevalence = 11% and an AA prevalence = 3.5% (11). However, family history of CRC was not collected, so it is unclear how many of these individuals had a FH of CRC and it is possible that some of these individuals were specifically referred for colonoscopy because of FH of CRC. A second study retrospectively looked at 553 screening colonoscopies done from January 2004 through March 2006 for average risk patients in the 40 – 49 year age group in the US and reported an adenoma prevalence = 14% and an AA prevalence = 2% (10). Overall, the prevalence of adenoma (any size) and AA does not appear substantially higher among 40-49 year olds with a FH of CRC in our study versus average risk 40-49 year olds in other studies. However, if we consider the prevalence rates seen over the 2007-2009 period as the ‘current’ rates, the prevalence in this group is similar to prevalence of adenomas in 40-49 year olds with a family history of CRC in the German study and is comparable to the prevalence of adenomas in 50-59 year old average risk individuals in early screening colonoscopy studies.
In our study, the prevalence of adenomas (any size) was significantly higher in the 45-49 year old age group vs 40-44 year age group (21.5% vs 9.2%). A similar trend was seen for AA (4% vs 2.5%). No prior study has reported adenoma prevalence rates in different age groups within the 40-49 year olds with a FH of CRC in a FDR. Also, in our risk factor analysis, patient’s age and male gender both significantly increased the prevalence of adenomas after adjusting for other potential confounders. Therefore, our results suggest that the increased risk of adenomas may rise in these patients around the age of 45. Further studies with a larger sample size will be needed to prove or disprove this hypothesis.
Another important issue examined by our study is the impact of the age of the FDR at diagnosis of CRC on the prevalence of adenomas and AA in 40-49 year olds. Current multi-society guidelines recommend that patients with a FDR with CRC diagnosed before the age of 60 should get screened every 5 years, as opposed to every 10 years for those in which the FDR was diagnosed at or after the age of 60 (2). The rationale behind this recommendation is the relative risk of CRC in 40-49 year olds seems to increase when the age of FDR at diagnosis of CRC is lower (12). When evaluating the prevalence of adenomas in 40-49 year olds, we observed the opposite trend – patients with FDR diagnosed with CRC < 60 had a lower prevalence of adenomas compared to individuals whose FDRs were diagnosed with CRC ≥ 60. The most likely reason for this observation is that patients with FDRs diagnosed with CRC ≥ 60 were older (44.9 ± 3 yrs) compared to patients with a FDR diagnosed with CRC < 60 (44 ± 2.9; P = 0.0002). However, in the multiple logistic regression model, age of FDR < 60 years old did not increase the risk of adenomas (any size) after adjusting for confounding factors, including age of study patient and male gender. In fact, age of FDR ≥ 60 just missed being a risk factor that was significantly associated with adenoma prevalence. Therefore, our data do not support a higher prevalence of adenomas in 40-49 year old individuals with a FDR diagnosed with CRC < 60 years old and do not support the recommendation for more frequent colonoscopic screening (every 5 years) in this population.
Our data also suggests that endoscopists at our center do not follow guideline recommendations for repeat screening in these patients. We only evaluated this question for the 2007-09 period in our database analysis. We chose this timeframe because physician awareness and adherence to guideline recommendations may have been delayed after publication of the 2003 multi-society guidelines. In order to be conservative, our analysis (Table 4) only evaluated individuals with normal colonoscopies who had age of FDR at diagnosis of CRC documented. Therefore, recommendations for repeat screening colonoscopies should be based solely on the age of FDR at time of diagnosis of CRC. The endoscopists were compliant with guideline recommendations in only 64% of cases. Among patients who received recommendations that were not consistent with guidelines, patients were usually instructed to return sooner than recommended by guidelins. To the best of our knowledge, this is the first study that has assessed compliance with guidelines for screening FDRs of sporadic CRC cases. Our group has previously conducted a survey of knowledge and acceptance of colon polyp surveillance guidelines among gastroenterologists (13). In that survey, up to 40% of the gastroenterologists were not aware of the 2003 guidelines. Furthermore, many gastroenterologists who did know the recommended interval for repeat screening colonoscopy stated that they routinely told patients to return for repeat colonoscopy sooner than recommended by guidelines. (e.g., Recommending that patients with FDR diagnosed with CRC ≥ 60 years old to get repeat screening colonoscopy in 5 years.) (13). We did observe that presence of fellows during the procedure improves the compliance rate with guidelines. Relative lack of evidence supporting current guideline recommendations, frequent changes in the guidelines, lack of awareness of the guidelines, and fear of malpractice liability may be some of the factors that may underlie the poor compliance shown in our study. Future studies will need to go beyond simple queries about adherence with guideline recommendations and probe the underlying issues that impede adherence to these recommendations.
Our study has several limitations that need to be acknowledged. Our study is underpowered to identify factors associated with advanced adenomas. It is a retrospective study based on review of patient medical records. It relies on the recall of study patients when they reported their FH information to their physicians. It also may be impaired by health professionals incorrectly reporting that information, as evidenced by the large number of cases that were excluded after a review of their charts revealed that they were actually patients with distant relatives with CRC (Figure 1). The age of the FDR at the time of diagnosis of CRC was not available for all patients, and it is possible that it was erroneously recorded in the medical record of some patients. Nevertheless, this situation does replicate the experiences of gastroenterologists in practice.
In conclusion, we reviewed the records of 640 asymptomatic 40-49 year old individuals with FDR with CRC who had their first screening colonoscopy at the University of Michigan Hospital from 1999-2009. Among 640 study patients, the overall prevalence of adenomas (any size) = 15.4% and AA = 3.3%, which is similar to prevalences reported for average-risk 40-49 year olds in other studies. Overall, our findings do not support screening this group of individuals differently than the average risk population. However, we do not think that our data are adequate to lead to new guideline recommendations because of the inherent limitations of retrospective studies.. In our opinion, current data are inadequate to make evidence-based recommendations about appropriate colonoscopy screening among individuals with a family history of CRC. Since family history of CRC is increasingly common among our patients, it is crucial to conduct multi-center, prospective studies of 40-49 year old individuals with a family history of CRC and average-risk 40-49 year old individuals who undergo screening colonoscopy. These studies should have adequate sample size to produce precise estimates of adenoma prevalence in both groups and these studies should prospectively assess multiple risk factors which may impact development of adenomas, including tobacco use, race, and BMI.
Acknowledgments
Grant Support: Philip Schoenfeld, 1K24DK084208 NIDDK, NIH and VA HSR&D NDA 06-205.
Acronyms
- AA
Advanced Adenomas
- BMI
Body Mass Index
- CI
Confidence Interval
- CRC
Colorectal Cancer
- FDR
First Degree Relative
- HNPCC
Hereditary non-polyposis colorectal cancer
- NSAID
Non steroidal anti inflammatory drug
- OR
Odds Ratio
- RR
Relative Risk
- SD
Standard Deviation
Footnotes
Institutions Participating in the Study: University of Michigan, Ann Arbor VA Healthcare System
Meeting Presentations: American College of Gastroenterology 2009; Digestive Disease Week 2009, 2010
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer. 2006 Jan;42(2):216–27. doi: 10.1016/j.ejca.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008 May-Jun;58(3):130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Speizer FE, Willett WC. A prospective study of family history and the risk of colorectal cancer. N Engl J Med. 1994 Dec 22;331(25):1669–74. doi: 10.1056/NEJM199412223312501. [DOI] [PubMed] [Google Scholar]
- 4.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009 Mar;104(3):739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 5.Guillem JG, Forde KA, Treat MR, Neugut AI, O’Toole KM, Diamond BE. Colonoscopic screening for neoplasms in asymptomatic first-degree relatives of colon cancer patients. A controlled, prospective study. Dis Colon Rectum. 1992 Jun;35(6):523–9. doi: 10.1007/BF02050530. [DOI] [PubMed] [Google Scholar]
- 6.Gaglia P, Atkin WS, Whitelaw S, Talbot IC, Williams CB, Northover JM, et al. Variables associated with the risk of colorectal adenomas in asymptomatic patients with a family history of colorectal cancer. Gut. 1995 Mar;36(3):385–90. doi: 10.1136/gut.36.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sauar J, Hausken T, Hoff G, Bjorkheim A, Foerster A, Mowinckel P. Colonoscopic screening examination of relatives of patients with colorectal cancer. I. A comparison with an endoscopically screened normal population. Scand J Gastroenterol. 1992 Aug;27(8):661–6. doi: 10.3109/00365529209000136. [DOI] [PubMed] [Google Scholar]
- 8.Pariente A, Milan C, Lafon J, Faivre J. Colonoscopic screening in first-degree relatives of patients with ‘sporadic’ colorectal cancer: a case-control study. The Association Nationale des Gastroenterologues des Hopitaux and Registre Bourguignon des Cancers Digestifs (INSERM CRI 9505) Gastroenterology. 1998 Jul;115(1):7–12. doi: 10.1016/s0016-5085(98)70358-0. [DOI] [PubMed] [Google Scholar]
- 9.Menges M, Fischinger J, Gartner B, Georg T, Woerdehoff D, Maier M, et al. Screening colonoscopy in 40- to 50-year-old first-degree relatives of patients with colorectal cancer is efficient: a controlled multicentre study. Int J Colorectal Dis. 2006 May;21(4):301–7. doi: 10.1007/s00384-005-0032-2. [DOI] [PubMed] [Google Scholar]
- 10.Rundle AG, Lebwohl B, Vogel R, Levine S, Neugut AI. Colonoscopic screening in average-risk individuals ages 40 to 49 vs 50 to 59 years. Gastroenterology. 2008 May;134(5):1311–5. doi: 10.1053/j.gastro.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med. 2002 Jun 6;346(23):1781–5. doi: 10.1056/NEJM200206063462304. [DOI] [PubMed] [Google Scholar]
- 12.Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001 Oct;96(10):2992–3003. doi: 10.1111/j.1572-0241.2001.04677.x. [DOI] [PubMed] [Google Scholar]
- 13.Saini SD, Nayak RS, Kuhn L, Schoenfeld P. Why don’t gastroenterologists follow colon polyp surveillance guidelines?: results of a national survey. J Clin Gastroenterol. 2009 Jul;43(6):554–8. doi: 10.1097/MCG.0b013e31818242ad. [DOI] [PubMed] [Google Scholar]


