Abstract
Neutrophils constitute a critical part of innate immunity and are well known for their ability to phagocytose and kill invading microorganisms. The microbicidal processes employed by neutrophils are highly effective at killing most ingested bacteria and fungi. However, an alternative non-phagocytic antimicrobial mechanism of neutrophils has been proposed whereby microorganisms are eliminated by neutrophil extracellular traps (NETs). NETs are comprised of DNA, histones, and antimicrobial proteins extruded by neutrophils during NETosis, a cell death pathway reported to be distinct from apoptosis, phagocytosis-induced cell death, and necrosis. Although multiple laboratories have reported NETs using various stimuli in vitro, the molecular mechanisms involved in this process have yet to be definitively elucidated, and many questions regarding the formation and putative role or function of NETs in innate host defense remain unanswered. It is with these questions in mind that we provide some reflection and perspective on NETs and NETosis.
Keywords: neutrophil, apoptosis, necrosis, phagocytosis, inflammation
Neutrophil Turnover and Homeostasis
Neutrophils are short-lived granulocytes that mature in bone marrow for several days (Bainton et al., 1971; Weissman et al., 2001). During maturation, these cells acquire key functional attributes, including the ability to phagocytose and kill microorganisms (Bainton et al., 1971; Glasser and Fiederlein, 1987; Weissman et al., 2001; Rosenbauer and Tenen, 2007; Pillay et al., 2010). After maturation, neutrophils are released into the bloodstream and circulate and/or marginate for 10–24 h before migrating into tissues, where they may function for an additional 1–2 days before they undergo apoptosis and are cleared by macrophages or dendritic cells (Cartwright et al., 1964; Fliedner et al., 1964; Bainton et al., 1971; Savill et al., 1989; Voll et al., 1997; Fadok et al., 1998; Huynh et al., 2002; Martin et al., 2003; Rigby and DeLeo, 2012). In addition, neutrophils in the total blood granulocyte pool (circulating and marginating) can be removed by the liver, spleen, and bone marrow, although the precise mechanism for this turnover process remains incompletely determined (reviewed by Summers et al., 2010). The neutrophil lifespan is highly regulated, as it is critical to remove spent/effete neutrophils as a means to prevent accidental release of cytotoxic molecules and associated host tissue damage (Edwards et al., 2003; Duffin et al., 2010; Bratton and Henson, 2011; Milot and Filep, 2011). Neutrophil turnover in an adult human is typically on the order of 1011 cells per day (Athens et al., 1961; Dancey et al., 1976; Rankin, 2010). While the hematopoietic system is able to regulate steady-state levels of circulating neutrophils, it can also be switched to an emergency granulopoiesis response to accommodate the increased demand for neutrophils during infection (Hirai et al., 2006; Panopoulos and Watowich, 2008).
The neutrophil lifespan is regulated by a balance of pro- and anti-apoptotic factors present in the environment. Cytokines and other factors such as interleukin (IL)-1β, IL-2, IL-4, IL-15, interferon-γ, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and lipopolysaccharide (LPS) can prolong and/or enhance neutrophil function and delay apoptosis for several days (Colotta et al., 1992; Duffin et al., 2010). Although enhancing neutrophil function and survival presumably favors elimination of invading microbes, the persistence of these cytotoxic host cells increases the potential for prolonged inflammation and host tissue damage. Therefore, it is not surprising that neutrophil turnover is a highly regulated process.
Molecular control of neutrophil turnover or apoptosis is mediated by several mechanisms, including extrinsic pathways induced by extracellular signals and intrinsic pathways induced by intracellular signals. These signals include those triggered by death receptors, which bind ligands that activate caspases to promote apoptosis, mitochondrial release of cytochrome c, and processes mediated by the BCL-2 protein family (Edwards et al., 2003; Duffin et al., 2010). Spontaneous or constitutive apoptosis in neutrophils is an example of intrinsic apoptosis. Apoptosis elicited by FAS, tumor necrosis factor (TNF)-α, or TNF-related apoptosis inducing ligand (TRAIL), caused by the binding of these extracellular ligands to the cognate receptor anchored on the cell surface, is an example of extrinsic pathway apoptosis (Kennedy and DeLeo, 2009; Duffin et al., 2010). Phagocytosis may also lead to neutrophil apoptosis (Watson et al., 1996; Kobayashi et al., 2002; Zhang et al., 2003; Kennedy and DeLeo, 2009). Neutrophil phagocytosis-induced apoptosis or phagocytosis-induced cell death (PICD) promotes the resolution of infection by disposing spent or effete neutrophils containing dead or partially digested microbes in a non-inflammatory manner (Kennedy and DeLeo, 2009). This process is described below in the context of the resolution of inflammation.
Neutrophils and the Inflammatory Response
The importance of neutrophils in the immune response is underscored by human diseases caused by defects in neutrophil function, which result in increased risk of infection from bacteria and fungi (Nauseef and Clark, 2010). For example, neutropenia, which can be medically induced by cytotoxic drugs or cancer therapy, is associated with significant morbidity (Bodey et al., 1966; Dale et al., 1979; Frøland, 1984; Tobias and Schleien, 1991). In addition, the inflammatory response, which from a cellular perspective is largely comprised of neutrophils, is critical for defense against invading microorganisms. On the other hand, timely resolution of the inflammatory response is an important process that returns the host immune system to pre-infection homeostasis. Historically, neutrophils were considered to have a passive part in inflammation resolution; however, this view has changed over time, and it is now known that neutrophils actively help to resolve inflammation by blocking and scavenging chemokines and cytokines (Ariel et al., 2006), and also produce pro-resolving lipid mediators (Ariel et al., 2006; Serhan et al., 2008). Thus, given that neutrophils contain and produce a vast array of cytotoxic molecules and contribute to the regulation of inflammation, it should not be unexpected that these cells are involved in – or are the primary cause of – a variety of inflammatory disorders. For instance, in chronic obstructive pulmonary disease, the aminopeptidase activity of leukotriene A4 hydrolase (LTA4H) is inhibited, causing accumulation of proline-glycine-proline, which in turn, promotes neutrophil recruitment and chronic lung inflammation (Weathington et al., 2006). In mouse models, recruitment of neutrophils has been shown to be involved in arthritis (Chou et al., 2010) and multiple sclerosis (Carlson et al., 2008; Liu et al., 2010). More notably, recent studies have demonstrated that neutrophils and neutrophil responses (rather than bacterial pathogens per se) are the cause of severe pneumonia and tissue destruction in animal models of bacterial respiratory tract infection (Bartlett et al., 2008; Diep et al., 2010). Thus, it is clear that unchecked neutrophil activation and neutrophil lysis are phenomena that can have a significant negative impact on health of the host.
Response to Infection
Neutrophils are recruited rapidly to the site of infection in response to chemotactic stimuli released by the host and/or invading microorganism. Inasmuch as neutrophils are the most abundant leukocyte in humans, there can be a tremendous influx of neutrophils to the site of infection. At such sites, neutrophils bind and ingest microorganisms through a process known as phagocytosis (reviewed in Rigby and DeLeo, 2012). Ingested microbes are typically destroyed by the combined effects of NADPH oxidase-derived reactive oxygen species (ROS) and cytotoxic molecules delivered from cytoplasmic granules into the phagosome. Neutrophil granules contain numerous antimicrobial peptides (AMPs) and proteins, and matrix protein-degrading proteases, including alpha-defensins, cathelicidins, azurocidin, cathepsins, lactoferrin, lysozyme, proteinase-3, gelatinase, collagenase, and elastase (Faurschou and Borregaard, 2003; Nauseef and Clark, 2010; Rigby and DeLeo, 2012). Of note, these cytotoxic agents are normally targeted into the formed phagosome, thereby limiting inadvertent extracellular release and potential damage to host tissues (Nauseef and Clark, 2010).
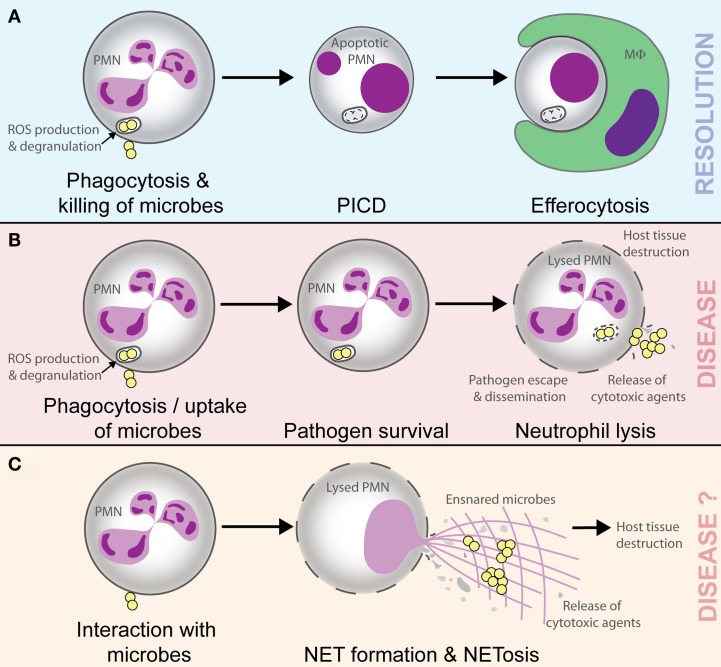
It is well documented that neutrophil PICD occurs following ingestion of numerous microorganisms in vitro (Watson et al., 1996; Colamussi et al., 1999; Engelich et al., 2001; Kobayashi et al., 2003a,b, 2012; Kennedy and DeLeo, 2009), and in vivo this phenomenon likely promotes clearance of effete neutrophils containing dead or dying microbes (Figure 1; Kobayashi et al., 2012). Importantly, this process would prevent local host tissue damage that can occur if these spent host cells are not removed and undergo lysis, and thus ultimately promotes the resolution of inflammation (Whyte et al., 1993; Savill, 1997; Kobayashi et al., 2002, 2003a, 2012; Kim et al., 2004; Iyoda et al., 2005; Ariel et al., 2006; Kobayashi and DeLeo, 2009; Rigby and DeLeo, 2012). Such a process is considered normal for neutrophils during infection and healthy for the host. On the other hand, pathogenic microorganisms circumvent killing by neutrophils, and in doing so ultimately alter the normal process of neutrophil turnover during infection, by either delaying apoptosis or causing neutrophil lysis (Kobayashi et al., 2003a, 2010; DeLeo, 2004; Voyich et al., 2005). The resulting neutrophil lysis releases tissue-damaging molecules, not only allowing pathogen survival but also exacerbating the inflammatory response (Figure 1). This process can lead to disease and can be considered unhealthy for the host. As one example, some strains of Staphylococcus aureus are known to cause lysis of human neutrophils after phagocytosis (Rogers and Tompsett, 1952; Voyich et al., 2005, 2006; Kobayashi et al., 2010). Indeed, the possibility that S. aureus survive after phagocytosis and ultimately disseminate to cause disease (which can be explained at least in part by neutrophil lysis after trafficking) has been reviewed recently (Thwaites and Gant, 2011). These authors describe neutrophils as “Trojan horses” for the dissemination or metastasis of S. aureus (Thwaites and Gant, 2011). In accordance with the observations in vitro, S. aureus is an abundant cause of pyogenic infections in humans. Therefore, the ability of S. aureus to cause neutrophil lysis is likely a component of virulence.
Figure 1.
Possible outcomes of the interaction of microbes with neutrophils. Phagocytosis and killing of microorganisms by neutrophils (polymorphonuclear leukocyte, PMN) triggers host cell apoptosis and ultimate removal by macrophages (MΦ) or dendritic cells. This process promotes resolution of the inflammatory response (A). Pathogenic microbes such as Staphylococcus aureus can cause lysis of PMN after phagocytosis, thereby facilitating escape/dissemination of the invading pathogen and release of cytotoxic molecules that cause host tissue damage and disease (B). NETs ensnare and may kill microbes, but there is accompanying lysis of neutrophils and release of cytotoxic molecules that are known to cause host tissue damage and promote inflammatory disease. In this regard, the outcome of NETosis and the formation of NETs should be similar to that in (B; i.e., disease; C).
Neutrophil Extracellular Traps and NETosis
Until fairly recently, phagocyte biologists were content with a model of neutrophil function in which these phagocytes bind, ingest, and subsequently kill microorganisms. The idea that neutrophils would extrude DNA in a cytolytic process that captures microorganisms was unheard of – until Brinkmann et al. (2004) reported the formation of structures known as neutrophil extracellular traps (NETs). These unique structures, which are discussed in detail in this issue of Frontiers in Immunology, are composed of DNA, histones, and antimicrobial proteins, and can ensnare pathogens. Since the report by Brinkmann et al. (2004), extracellular traps have been shown to be produced in vitro by a number of different cell types, including neutrophils, mast cells, eosinophils, and endothelial cells (Palić et al., 2007; von Köckritz-Blickwede et al., 2008; Yousefi et al., 2008; Chuammitri et al., 2009; Katzenback and Belosevic, 2009; Aulik et al., 2010; Gupta et al., 2010; Wardini et al., 2010; Webster et al., 2010; Lin et al., 2011; Scapinello et al., 2011). Moreover, recent studies have investigated possible mechanisms for the induction of NETs. For example, it has been reported that formation of NETs requires activation of the Raf-MEK-ERK pathway through protein kinase C (Hakkim et al., 2011) and histone citrullination (Neeli et al., 2008; Li et al., 2010; Hemmers et al., 2011; Leshner et al., 2012). These findings suggest that NETosis and formation of NETs involves specific signal transduction events. Thus, it is tempting to advocate the importance of these structures in host defense due to the apparent simplicity and elegance of the phenomenon by which they occur. However, many questions remain about the role of NETs in host defense and the molecular mechanisms underlying their formation are incompletely characterized. In addition, the evidence for formation of NETs in vivo is not very compelling, and whether the formation of NETs is of benefit to the host remains an open question. Indeed, it was suggested early on that NETs form only under extreme circumstances and can injure host tissues (Clark et al., 2007).
While use of NETs appears as an alternative mechanism for pathogen control and elimination, production of NETs and NETosis (as a cytolytic process) seems at variance with the highly regulated control of neutrophil turnover (including PICD) and homeostasis, as discussed above. That is, utilization of NETs for host defense contrasts with the considerable effort made by the host to prevent inadvertent neutrophil lysis, release of cytotoxic agents, and post-lysis sequelae, such as inflammatory disorders. Notably, NETs have been implicated in a number of pathologic processes consistent with inflammatory disorders involving lysed neutrophils and cytotoxic molecules from neutrophils. For example, NETs can cause collateral damage in the form of endothelial and tissue damage (Clark et al., 2007; Ma and Kubes, 2008; Marin-Esteban et al., 2012) and may be partially responsible for sputum viscosity and tissue damage in cystic fibrosis patients (Papayannopoulos et al., 2011). NETs have been implicated in systemic lupus erythematosus and systemic vasculitis (Hakkim et al., 2010; Amulic and Hayes, 2011; Bosch, 2011; Garcia-Romo et al., 2011; Lande et al., 2011; Villanueva et al., 2011; Knight and Kaplan, 2012; Liu et al., 2012), gout, asthma, keratinocyte damage, and lupus nephritis (Mitroulis et al., 2011; Marin-Esteban et al., 2012), and may also be involved in the hyper reaction of the immune system by triggering physiological signals and causing pre-eclampsia (Gupta et al., 2005, 2006; Brinkmann and Zychlinsky, 2007). NETs are present in transfusion-related acute lung injury (Thomas et al., 2012), atherosclerotic carotid arteries (Döring, 2012), are toxic to vasculature (Clark et al., 2007; Gupta et al., 2010; Villanueva et al., 2011; Saffarzadeh et al., 2012), and facilitate thrombosis where they could provide a scaffold for red blood cell adhesion (Fuchs et al., 2010, 2012; Van Den Berg and Reitsma, 2011; Brill et al., 2012). NETs may also contribute to cancer-associated thrombosis, since neutrophils from mice with experimentally induced cancers are more likely to form NETs than those from control mice (Demers et al., 2012). It is also of note that extracellular histones, a signature component of NETs, contribute to host death during sepsis (Xu et al., 2009). Whether the structures reported as NETs in these aforementioned inflammatory syndromes are distinct from the remains of necrotic neutrophils is unclear, but in any case the process or phenomenon is associated with a negative outcome for the host – similar to the prediction in the model described in Figure 1.
Given the association of NETs and NETosis with inflammatory disorders, and coupled with a highly regulated neutrophil turnover process, the frequency with which formation of NETs occurs should be fairly low. Indeed, even under optimal NET-inducing conditions in vitro, only one-third of activated neutrophils, and perhaps as few as 10%, make NETs (Brinkmann and Zychlinsky, 2007; Fuchs et al., 2007; Munafo et al., 2009). The kinetics of NETosis vary depending on type and concentration of stimulus, isolation procedure of neutrophils, and the sensitivity of the detection method (Fuchs et al., 2012). Despite the fact that NET formation is stimulated by pathogens, it is still not clear whether NETosis that occurs during host-pathogen interactions is a programmed mechanism, a hijacking of host pathways by pathogen-produced factors, or simply an incidental component of neutrophil lysis. For instance, S. aureus is well known to cause lysis of neutrophils in vitro and in vivo, but the pathogen has also been reported to induce NETs (Brinkmann et al., 2004; Jann et al., 2009; Yipp et al., 2012). It is also not clear whether the release of NETs always leads to cell death (and the possibility of host tissue damage) or if it is an extrusion of DNA by intact cells (Yousefi et al., 2009; Remijsen et al., 2011; Guimarães-Costa et al., 2012; Yipp et al., 2012). Although it is difficult to understand how neutrophils can remain intact and viable after release of nuclear DNA, the question of whether NET formation always causes cytolysis or can occur with intact cells is important and must be resolved by the field.
One could hypothesize that the formation of NETs represents a directed host defense mechanism. If the process is host-directed, does this suggest there is an advantage to the use of NETs for removal of microbes versus traditional phagocytosis-based uptake and subsequent killing of microbial invaders? The antimicrobial activities of NETs have been ascribed to the histones, AMPs, and other cytoplasmic components associated with extracellular DNA. However, it is important to note that NETs provide a low concentration of AMPs compared to that present in the phagosome, and NETs lack the ability to produce microbicidal ROS. Published studies to date suggest that the formation of NETs does not lead to the universal killing of all microorganisms, although NETs can reduce the burden of selected of microorganisms in vitro (Table 1). This finding is perhaps not surprising, since solubilized azurophilic granule components isolated from disrupted neutrophils have varied capacity to kill different bacterial species (Bertram et al., 1986; Joiner et al., 1989; Levy et al., 1999; Palazzolo-Ballance et al., 2008; Nordenfelt et al., 2009). Moreover, several microorganisms are known to circumvent killing by NETs using a variety of strategies, including altering bacterial surface affinity to NETs (Wartha et al., 2007; Carlin et al., 2009; Juneau et al., 2011) and secreting NET-degrading DNases (Beiter et al., 2006; Buchanan et al., 2006; Midon et al., 2011; Palmer et al., 2011). As an alternative hypothesis, the formation of NETs (especially if it requires lysis of neutrophils) could be considered an incidental event rather than something intended by the host innate immune system. An incidental process seems more consistent with our understanding of the regulation of neutrophil turnover and homeostasis.
Table 1.
Microbial susceptibility to NETs.
| Species | Susceptibility | Reference |
|---|---|---|
| VIRUSES | ||
| Feline leukemia virus | Modulates NET formation | Wardini et al. (2010) |
| Human immunodeficiency virus (HIV)-1 | Infectivity reduced | Saitoh et al. (2012) |
| Influenza A H1N1 | Modulates NET formation | Narasaraju et al. (2011) |
| BACTERIA | ||
| Actinobacillus suis | Reduction in bacterial numbers | Scapinello et al. (2011) |
| Aeromonas hydrophila | Survives | Brogden et al. (2012) |
| Bacillus anthracis | Only unencapsulated strains killed | Papayannopoulos and Zychlinsky (2009); Szarowicz and Friedlander (2011) |
| Burkholderia pseudomallei | Reduction in bacterial numbers | Riyapa et al. (2012) |
| Escherichia coli | Reduction in bacterial numbers | Grinberg et al. (2008); Marin-Esteban et al. (2012) |
| Group A streptococcus | Survives | Buchanan et al. (2006); Lauth et al. (2009) |
| Group B streptococcus | Survives | Carlin et al. (2009) |
| Haemophilus influenzae | Survives | Juneau et al. (2011) |
| Listeria monocytogenes | Reduction in bacterial numbers | Ramos-Kichik et al. (2009) |
| Mannheimia haemolytica | Reduction in bacterial numbers | Aulik et al. (2010) |
| Mycobacterium canettii | Survives | Ramos-Kichik et al. (2009) |
| Mycobacterium tuberculosis | Survives | Ramos-Kichik et al. (2009) |
| Pasteurella multocida | Reduction in bacterial numbers | Scapinello et al. (2011) |
| Porphyromonas gingivalis | Survives | Delbosc et al. (2011); Palmer et al. (2011) |
| Pseudomonas aeruginosa | Survives | von Köckritz-Blickwede et al. (2008); Douda et al. (2011); Young et al. (2011); Khatua et al. (2012) |
| Salmonella typhimurium | Reduction in bacterial numbers | Brinkmann et al. (2004) |
| Shigella flexneri | Reduction in bacterial numbers | Brinkmann et al. (2004) |
| Staphylococcus aureus | Dependent on ratio | Döring et al. (2011) |
| Staphylococcus epidermidis | Survives | Cogen et al. (2010) |
| Streptococcus pneumonia | Survives | Beiter et al. (2006); Wartha et al. (2007); Midon et al. (2011) |
| Streptococcus pyogenes | Reduction in bacterial numbers | von Köckritz-Blickwede et al. (2008) |
| Streptococcus suis | Reduction in bacterial numbers | Scapinello et al. (2011) |
| Yersinia enterocolitica | Reduction in bacterial numbers | Casutt-Meyer et al. (2010) |
| Yersinia pestis | Survives | Casutt-Meyer et al. (2010) |
| PROTOZOA | ||
| Eimeria bovis | Reduction in parasite numbers | Behrendt et al. (2010) |
| Leishmania amazonesis | Dependent on ratio | Guimarães-Costa et al. (2009) |
| Leishmania donovani | Survives | Gabriel et al. (2010) |
| Plasmodium falciparum | Trapped | Baker et al. (2008) |
| Toxoplasma gondii | Reduction in parasite numbers | Abi Abdallah et al. (2012) |
| FUNGI | ||
| Aspergillus fumigatus | Growth inhibited | McCormick et al. (2010) |
| Aspergillus nidulans | Growth inhibited | Bianchi et al. (2011) |
| Candida albicans | Growth inhibited, blastospores survive | Urban et al. (2006); Menegazzi et al. (2012) |
| Candida glabrata | Growth inhibited | Springer et al. (2010) |
| Cryptococcus gatti | Survives | Springer et al. (2010) |
Concluding Perspective
Neutrophil extracellular traps have been suggested as an alternative or additional component of the innate host defense against microorganisms. Although progress has been made, many questions related to NET formation and function remain unanswered. Do NETs commonly occur in vivo? Compelling evidence is lacking. Are NETs formed by live neutrophils or does the process (i.e., NETosis) always result in cytolysis? If it is accompanied by neutrophil lysis, how does this phenomenon fit with what we know about the control of neutrophil turnover and the host efforts to prevent inflammatory syndromes? Importantly, is the pathway that leads to the formation of NETs a host-directed mechanism or simply an incidental phenomenon in neutrophils? These and other questions can only be answered by continued investigation into the biology and function of NETs.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thea Lu, Scott D. Kobayashi, and Frank R. DeLeo are supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Mark T. Quinn is supported by a grant (GM103500) from the National Institute of General Medical Sciences, National Institutes of Health.
References
- Abi Abdallah D., Lin C., Ball C., King M., Duhamel G., Denkers E. (2012). Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect. Immun. 80, 768–777 10.1128/IAI.05730-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amulic B., Hayes G. (2011). Neutrophil extracellular traps. Curr. Biol. 21, R297–R298 10.1016/j.cub.2011.03.021 [DOI] [PubMed] [Google Scholar]
- Ariel A., Fredman G., Sun Y.-P., Kantarci A., Van Dyke T. E., Luster A. D., et al. (2006). Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat. Immunol. 7, 1209–1216 10.1038/ni1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athens J. W., Haab O. P., Raab S. O., Mauer A. M., Ashenbrucker H., Cartwright G. E., et al. (1961). Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J. Clin. Invest. 40, 989–995 10.1172/JCI104338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulik N., Hellenbrand K., Klos H., Czuprynski C. (2010). Mannheimia haemolytica and its leukotoxin cause neutrophil extracellular trap formation by bovine neutrophils. Infect. Immun. 78, 4454–4466 10.1128/IAI.00840-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Ullyot J. L., Farquhar M. G. (1971). The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J. Exp. Med. 134, 907–934 10.1084/jem.134.4.907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker V. S., Imade G. E., Molta N. B., Tawde P., Pam S. D., Obadofin M. O., et al. (2008). Cytokine-associated neutrophil extracellular traps and antinuclear antibodies in Plasmodium falciparum infected children under six years of age. Malar. J. 7, 41. 10.1186/1475-2875-7-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett A. H., Foster T. J., Hayashida A., Park P. W. (2008). α-Toxin facilitates the generation of CXC chemokine gradients and stimulates neutrophil homing in Staphylococcus aureus pneumonia. J. Infect. Dis. 198, 1529–1535 10.1086/592758 [DOI] [PubMed] [Google Scholar]
- Behrendt J. H., Ruiz A., Zahner H., Taubert A., Hermosilla C. (2010). Neutrophil extracellular trap formation as innate immune reactions against the apicomplexan parasite Eimeria bovis. Vet. Immunol. Immunopathol. 133, 1–8 10.1016/j.vetimm.2009.06.012 [DOI] [PubMed] [Google Scholar]
- Beiter K., Wartha F., Albiger B., Normark S., Zychlinsky A., Henriques-Normark B. (2006). An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr. Biol. 16, 401–407 10.1016/j.cub.2006.01.056 [DOI] [PubMed] [Google Scholar]
- Bertram T. A., Canning P. C., Roth J. A. (1986). Preferential inhibition of primary granule release from bovine neutrophils by a Brucella abortus extract. Infect. Immun. 52, 285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M., Niemiec M. J., Siler U., Urban C. F., Reichenbach J. (2011). Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J. Allergy Clin. Immunol. 127, 1243–1252e1247. 10.1016/j.jaci.2011.01.021 [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Buckley M., Sathe Y. S., Freireich E. J. (1966). Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann. Intern. Med. 64, 328–340 [DOI] [PubMed] [Google Scholar]
- Bosch X. (2011). TRAPping the cellular mechanisms of lupus. EMBO Mol. Med. 3, 578–580 10.1002/emmm.201100167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratton D., Henson P. (2011). Neutrophil clearance: when the party is over, clean-up begins. Trends Immunol. 32, 350–357 10.1016/j.it.2011.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill A., Fuchs T. A., Savchenko A. S., Thomas G. M., Martinod K., De Meyer S. F., et al. (2012). Neutrophil extracellular traps promote deep vein thrombosis in mice. J. Thromb. Haemost. 10, 136–144 10.1111/j.1538-7836.2011.04544.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D., et al. (2004). Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Zychlinsky A. (2007). Beneficial suicide: why neutrophils die to make NETs. Nat. Rev. Microbiol. 5, 577–582 10.1038/nrmicro1710 [DOI] [PubMed] [Google Scholar]
- Brogden G., Von Köckritz-Blickwede M., Adamek M., Reuner F., Jung-Schroers V., Naim H. Y., et al. (2012). β-Glucan protects neutrophil extracellular traps against degradation by Aeromonas hydrophila in carp (Cyprinus carpio). Fish Shellfish Immunol. 33, 1060–1064 10.1016/j.fsi.2012.08.009 [DOI] [PubMed] [Google Scholar]
- Buchanan J. T., Simpson A. J., Aziz R. K., Liu G. Y., Kristian S. A., Kotb M., et al. (2006). DNase expression allows the pathogen Group A Streptococcus to escape killing in neutrophil extracellular traps. Curr. Biol. 16, 396–400 10.1016/j.cub.2005.12.039 [DOI] [PubMed] [Google Scholar]
- Carlin A., Uchiyama S., Chang Y.-C., Lewis A., Nizet V., Varki A. (2009). Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood 113, 3333–3336 10.1182/blood-2008-11-187302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson T., Kroenke M., Rao P., Lane T., Segal B. (2008). The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J. Exp. Med. 205, 811–823 10.1084/jem.20072404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright G. E., Athens J. W., Wintrobe M. M. (1964). The kinetics of granulopoiesis in normal man. Blood 24, 780–803 [PubMed] [Google Scholar]
- Casutt-Meyer S., Renzi F., Schmaler M., Jann N. J., Amstutz M., Cornelis G. R. (2010). Oligomeric coiled-coil adhesin YadA is a double-edged sword. PLoS ONE 5:e15159. 10.1371/journal.pone.0015159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R. C., Kim N. D., Sadik C. D., Seung E., Lan Y., Byrne M. H., et al. (2010). Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity 33, 266–278 10.1016/j.immuni.2010.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuammitri P., Ostojić J., Andreasen C. B., Redmond S. B., Lamont S. J., Palić D. (2009). Chicken heterophil extracellular traps (HETs): novel defense mechanism of chicken heterophils. Vet. Immunol. Immunopathol. 129, 126–131 10.1016/j.vetimm.2008.12.013 [DOI] [PubMed] [Google Scholar]
- Clark S. R., Ma A. C., Tavener S. A., McDonald B., Goodarzi Z., Kelly M. M., et al. (2007). Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13, 463–469 10.1038/nm1565 [DOI] [PubMed] [Google Scholar]
- Cogen A. L., Yamasaki K., Muto J., Sanchez K. M., Crotty Alexander L., Tanios J., et al. (2010). Staphylococcus epidermidis antimicrobial (δ-toxin (phenol-soluble modulin-(γ) cooperates with host antimicrobial peptides to kill Group A Streptococcus. PLoS ONE 5:e8557. 10.1371/journal.pone.0008557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamussi M. L., White M. R., Crouch E., Hartshorn K. L. (1999). Influenza A virus accelerates neutrophil apoptosis and markedly potentiates apoptotic effects of bacteria. Blood 93, 2395–2403 [PubMed] [Google Scholar]
- Colotta F., Re F., Polentarutti N., Sozzani S., Mantovani A. (1992). Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 80, 2012–2020 [PubMed] [Google Scholar]
- Dale D. C., Guerry D., Wewerka J. R., Bull J. M., Chusid M. J. (1979). Chronic neutropenia. Medicine (Baltimore) 58, 128–144 [DOI] [PubMed] [Google Scholar]
- Dancey J. T., Deubelbeiss K. A., Harker L. A., Finch C. A. (1976). Neutrophil kinetics in man. J. Clin. Invest. 58, 705–715 10.1172/JCI108517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbosc S., Alsac J.-M., Journe C., Louedec L., Castier Y., Bonnaure-Mallet M., et al. (2011). Porphyromonas gingivalis participates in pathogenesis of human abdominal aortic aneurysm by neutrophil activation. Proof of concept in rats. PLoS ONE 6:e18679. 10.1371/journal.pone.0018679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo F. R. (2004). Modulation of phagocyte apoptosis by bacterial pathogens. Apoptosis 9, 399–413 10.1023/B:APPT.0000031448.64969.fa [DOI] [PubMed] [Google Scholar]
- Demers M., Krause D. S., Schatzberg D., Martinod K., Voorhees J. R., Fuchs T. A., et al. (2012). Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. U.S.A. 109, 13076–13081 10.1073/pnas.1200419109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep B., Chan L., Tattevin P., Kajikawa O., Martin T., Basuino L., et al. (2010). Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc. Natl. Acad. Sci. U.S.A. 107, 5587–5592 10.1073/pnas.0912403107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring P., Grumann D., Kolata J., Goosmann C., Löffler B., Völker U., et al. (2011). Neutrophil cell death induction by Staphylococcus aureus from nasal colonization and chronic furunculosis. Int. J. Med. Microbiol. 301, 40 [Google Scholar]
- Döring Y. (2012). Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 125, 1673–1683 10.1161/CIRCULATIONAHA.111.046755 [DOI] [PubMed] [Google Scholar]
- Douda D., Jackson R., Grasemann H., Palaniyar N. (2011). Innate immune collectin surfactant protein D simultaneously binds both neutrophil extracellular traps and carbohydrate ligands and promotes bacterial trapping. J. Immunol. 187, 1856–1865 10.4049/jimmunol.1004201 [DOI] [PubMed] [Google Scholar]
- Duffin R., Leitch A. E., Fox S., Haslett C., Rossi A. G. (2010). Targeting granulocyte apoptosis: mechanisms, models, and therapies. Immunol. Rev. 236, 28–40 10.1111/j.1600-065X.2010.00922.x [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Moulding D. A., Derouet M., Moots R. J. (2003). Regulation of neutrophil apoptosis. Chem. Immunol. Allergy 83, 204–224 10.1159/000071562 [DOI] [PubMed] [Google Scholar]
- Engelich G., White M., Hartshorn K. L. (2001). Neutrophil survival is markedly reduced by incubation with influenza virus and Streptococcus pneumoniae: role of respiratory burst. J. Leukoc. Biol. 69, 50–56 [PubMed] [Google Scholar]
- Fadok V. A., Bratton D. L., Konowal A., Freed P. W., Westcott J. Y., Henson P. M. (1998). Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 101, 890–898 10.1172/JCI1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faurschou M., Borregaard N. (2003). Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 5, 1317–1327 10.1016/j.micinf.2003.09.008 [DOI] [PubMed] [Google Scholar]
- Fliedner T. M., Cronkite E. P., Robertson J. S. (1964). Granulocytopoiesis. I. Senescence and random loss of neutrophilic granulocytes in human beings. Blood 24, 402–414 [PubMed] [Google Scholar]
- Frøland S. S. (1984). Bacterial infections in the compromised host. Scand. J. Infect. Dis. Suppl. 43, 7–16 [PubMed] [Google Scholar]
- Fuchs T., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., et al. (2007). Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176, 231–241 10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D., et al. (2010). Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. U.S.A. 107, 15880–15885 10.1073/pnas.1005743107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T. A., Brill A., Wagner D. D. (2012). Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler. Thromb. Vasc. Biol. 32, 1777–1783 10.1161/ATVBAHA.111.242859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel C., McMaster W. R., Girard D., Descoteaux A. (2010). Leishmania donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps. J. Immunol. 185, 4319–4327 10.4049/jimmunol.1000893 [DOI] [PubMed] [Google Scholar]
- Garcia-Romo G. S., Caielli S., Vega B., Connolly J., Allantaz F., Xu Z., et al. (2011). Netting neutrophils are major inducers of Type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20. 10.1126/scitranslmed.3001201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser L., Fiederlein R. L. (1987). Functional differentiation of normal human neutrophils. Blood 69, 937–944 [PubMed] [Google Scholar]
- Grinberg N., Elazar S., Rosenshine I., Shpigel N. (2008). Beta-hydroxybutyrate abrogates formation of bovine neutrophil extracellular traps and bactericidal activity against mammary pathogenic Escherichia coli. Infect. Immun. 76, 2802–2807 10.1128/IAI.00051-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães-Costa A., Nascimento M. T. C., Froment G. S., Soares R. P. P., Morgado F. N., Conceição-Silva F., et al. (2009). Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. U.S.A. 106, 6748–6753 10.1073/pnas.0900226106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães-Costa A. B., Nascimento M. T. C., Wardini A. B., Pinto-Da-Silva L. H., Saraiva E. M. (2012). ETosis: a microbicidal mechanism beyond cell death. J. Parasitol. Res. 2012, 929743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Hasler P., Gebhardt S., Holzgreve W., Hahn S. (2006). Occurrence of neutrophil extracellular DNA traps (NETs) in pre-eclampsia. Ann. N. Y. Acad. Sci. 1075, 118–122 10.1196/annals.1368.015 [DOI] [PubMed] [Google Scholar]
- Gupta A. K., Hasler P., Holzgreve W., Gebhardt S., Hahn S. (2005). Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum. Immunol. 66, 1146–1154 10.1016/j.humimm.2005.11.003 [DOI] [PubMed] [Google Scholar]
- Gupta A. K., Joshi M. B., Philippova M., Erne P., Hasler P., Hahn S., et al. (2010). Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 584, 3193–3197 10.1016/j.febslet.2010.06.006 [DOI] [PubMed] [Google Scholar]
- Hakkim A., Fuchs T. A., Martinez N. E., Hess S., Prinz H., Zychlinsky A., et al. (2011). Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 7, 75–77 10.1038/nnano.2011.228 [DOI] [PubMed] [Google Scholar]
- Hakkim A., Fürnrohr B., Amann K., Laube B., Abed U., Brinkmann V., et al. (2010). Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. U.S.A. 107, 9813–9818 10.1073/pnas.0909927107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmers S., Teijaro J. R., Arandjelovic S., Mowen K. A. (2011). PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS ONE 6:e22043. 10.1371/journal.pone.0022043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H., Zhang P., Dayaram T., Hetherington C., Mizuno S.-I., Imanishi J., et al. (2006). C/EBPbeta is required for “emergency” granulopoiesis. Nat. Immunol. 7, 732–739 10.1038/ni1354 [DOI] [PubMed] [Google Scholar]
- Huynh M.-L. N., Fadok V. A., Henson P. M. (2002). Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J. Clin. Invest. 109, 41–50 10.1172/JCI200211638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyoda T., Nagata K., Akashi M., Kobayashi Y. (2005). Neutrophils accelerate macrophage-mediated digestion of apoptotic cells in vivo as well as in vitro. J. Immunol. 175, 3475–3483 [DOI] [PubMed] [Google Scholar]
- Jann N., Schmaler M., Kristian S., Radek K., Gallo R., Nizet V., et al. (2009). Neutrophil antimicrobial defense against Staphylococcus aureus is mediated by phagolysosomal but not extracellular trap-associated cathelicidin. J. Leukoc. Biol. 86, 1159–1169 10.1189/jlb.0209053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Ganz T., Albert J., Rotrosen D. (1989). The opsonizing ligand on Salmonella typhimurium influences incorporation of specific, but not azurophil, granule constituents into neutrophil phagosomes. J. Cell Biol. 109, 2771–2782 10.1083/jcb.109.6.2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneau R., Pang B., Weimer K. E. D., Armbruster C., Swords W. E. (2011). Nontypeable Haemophilus influenzae initiates formation of neutrophil extracellular traps. Infect. Immun. 79, 431–438 10.1128/IAI.00660-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenback B. A., Belosevic M. (2009). Isolation and functional characterization of neutrophil-like cells, from goldfish (Carassius auratus L.) kidney. Dev. Comp. Immunol. 33, 601–611 10.1016/j.dci.2008.10.011 [DOI] [PubMed] [Google Scholar]
- Kennedy A., DeLeo F. (2009). Neutrophil apoptosis and the resolution of infection. Immunol. Res. 43, 25–61 10.1007/s12026-008-8049-6 [DOI] [PubMed] [Google Scholar]
- Khatua B., Bhattacharya K., Mandal C. (2012). Sialoglycoproteins adsorbed by Pseudomonas aeruginosa facilitate their survival by impeding neutrophil extracellular trap through siglec-9. J. Leukoc. Biol. 91, 641–655 10.1189/jlb.0511260 [DOI] [PubMed] [Google Scholar]
- Kim S., Elkon K. B., Ma X. (2004). Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity 21, 643–653 10.1016/j.immuni.2004.09.009 [DOI] [PubMed] [Google Scholar]
- Knight J. S., Kaplan M. J. (2012). Lupus neutrophils: “NET” gain in understanding lupus pathogenesis. Curr. Opin. Rheumatol. 24, 441–450 10.1097/BOR.0b013e3283546703 [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Braughton K., Palazzolo-Ballance A., Kennedy A., Sampaio E., Kristosturyan E., et al. (2010). Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. J. Innate Immun. 2, 560–575 10.1159/000317134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Braughton K., Whitney A., Voyich J., Schwan T., Musser J., et al. (2003a). Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc. Natl. Acad. Sci. U.S.A. 100, 10948–10953 10.1073/pnas.0730515100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Voyich J., Somerville G., Braughton K., Malech H., Musser J., et al. (2003b). An apoptosis-differentiation program in human polymorphonuclear leukocytes facilitates resolution of inflammation. J. Leukoc. Biol. 73, 315–322 10.1189/jlb.1002481 [DOI] [PubMed] [Google Scholar]
- Kobayashi S., DeLeo F. (2009). Role of neutrophils in innate immunity: a systems biology-level approach. Wiley Interdiscip. Rev. Syst. Biol. Med. 1, 309–333 10.1002/wsbm.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Voyich J., Buhl C., Stahl R., DeLeo F. (2002). Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: cell fate is regulated at the level of gene expression. Proc. Natl. Acad. Sci. U.S.A. 99, 6901–6906 10.1073/pnas.252610699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S. D., Rigby K. M., DeLeo F. R. (2012). “Bacteria-induced host cell death,” in Bacterial Pathogenesis: Molecular and Cellular Mechanisms, eds Locht C., Simonet M. (Norfolk: Caister Academic Press; ), 317–362 [Google Scholar]
- Lande R., Ganguly D., Facchinetti V., Frasca L., Conrad C., Gregorio J., et al. (2011). Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra19. 10.1126/scitranslmed.3001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauth X., Von Köckritz-Blickwede M., McNamara C., Myskowski S., Zinkernagel A., Beall B., et al. (2009). M1 protein allows Group A streptococcal survival in phagocyte extracellular traps through cathelicidin inhibition. J. Innate Immun. 1, 202–214 10.1159/000203645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner M., Wang S., Lewis C., Zheng H., Chen X. A., Santy L., et al. (2012). PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 3:307. 10.3389/fimmu.2012.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O., Martin S., Eichenwald E., Ganz T., Valore E., Carroll S. F., et al. (1999). Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability-increasing protein. Pediatrics 104, 1327–1333 10.1542/peds.104.6.1327 [DOI] [PubMed] [Google Scholar]
- Li P., Li M., Lindberg M., Kennett M., Xiong N., Wang Y. (2010). PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207, 1853–1862 10.1084/JEM20712OIA34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A., Rubin C., Khandpur R., Wang J., Riblett M., Yalavarthi S., et al. (2011). Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 187, 490–500 10.4049/jimmunol.1100123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Tangsombatvisit S., Rosenberg J., Mandelbaum G., Gillespie E., Gozani O., et al. (2012). Specific post-translational histone modifications of neutrophil extracellular traps as immunogens and potential targets of lupus autoantibodies. Arthritis Res. Ther. 14, R25–R25 10.1186/ar4100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Belkadi A., Darnall L., Hu T., Drescher C., Cotleur A. C., et al. (2010). CXCR2-positive neutrophils are essential for cuprizone-induced demyelination: relevance to multiple sclerosis. Nat. Neurosci. 13, 319–326 10.1038/nn.2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A. C., Kubes P. (2008). Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J. Thromb. Haemost. 6, 415–420 10.1111/j.1538-7836.2007.02865.x [DOI] [PubMed] [Google Scholar]
- Marin-Esteban V., Turbica I., Dufour G., Semiramoth N., Gleizes A., Gorges R., et al. (2012). Afa/Dr diffusely adhering Escherichia coli strain C1845 induces neutrophil extracellular traps that kill bacteria and damage human enterocyte-like cells. Infect. Immun. 80, 1891–1899 10.1128/IAI.00050-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Burdon P. C. E., Bridger G., Gutierrez-Ramos J.-C., Williams T. J., Rankin S. M. (2003). Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 19, 583–593 10.1016/S1074-7613(03)00263-2 [DOI] [PubMed] [Google Scholar]
- McCormick A., Heesemann L., Wagener J., Marcos V., Hartl D., Loeffler J., et al. (2010). NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microbes Infect. 12, 928–936 10.1016/j.micinf.2010.06.009 [DOI] [PubMed] [Google Scholar]
- Menegazzi R., Decleva E., Dri P. (2012). Killing by neutrophil extracellular traps: fact or folklore? Blood 119, 1214–1216 10.1182/blood-2011-07-364604 [DOI] [PubMed] [Google Scholar]
- Midon M., Schäfer P., Pingoud A., Ghosh M., Moon A., Cuneo M., et al. (2011). Mutational and biochemical analysis of the DNA-entry nuclease EndA from Streptococcus pneumoniae. Nucleic Acids Res. 39, 623–634 10.1093/nar/gkq802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milot E., Filep J. G. (2011). Regulation of neutrophil survival/apoptosis by Mcl-1. ScientificWorldJournal 11, 1948–1962 10.1100/2011/131539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitroulis I., Kambas K., Chrysanthopoulou A., Skendros P., Apostolidou E., Kourtzelis I., et al. (2011). Neutrophil extracellular trap formation is associated with IL-1β and autophagy-related signaling in gout. PLoS ONE 6:e29318. 10.1371/journal.pone.0029318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo D., Johnson J., Brzezinska A., Ellis B., Wood M., Catz S. (2009). DNase I inhibits a late phase of reactive oxygen species production in neutrophils. J. Innate Immun. 1, 527–542 10.1159/000235860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasaraju T., Yang E., Samy R. P., Ng H. H., Poh W. P., Liew A.-A., et al. (2011). Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 179, 199–210 10.1016/j.ajpath.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef W. M., Clark R. A. (2010). Granulocytic Phagocytes. New York: Elsevier/Churchill Livingstone [Google Scholar]
- Neeli I., Khan S., Radic M. (2008). Histone deimination as a response to inflammatory stimuli in neutrophils. J. Immunol. 180, 1895–1902 [DOI] [PubMed] [Google Scholar]
- Nordenfelt P., Bauer S., Lönnbro P., Tapper H. (2009). Phagocytosis of Streptococcus pyogenes by all-trans retinoic acid-differentiated HL-60 cells: roles of azurophilic granules and NADPH oxidase. PLoS ONE 4:e7363. 10.1371/journal.pone.0007363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzolo-Ballance A., Reniere M., Braughton K., Sturdevant D., Otto M., Kreiswirth B., et al. (2008). Neutrophil microbicides induce a pathogen survival response in community-associated methicillin-resistant Staphylococcus aureus. J. Immunol. 180, 500–509 [DOI] [PubMed] [Google Scholar]
- Palić D., Ostojić J., Andreasen C. B., Roth J. A. (2007). Fish cast NETs: neutrophil extracellular traps are released from fish neutrophils. Dev. Comp. Immunol. 31, 805–816 10.1016/j.dci.2006.11.010 [DOI] [PubMed] [Google Scholar]
- Palmer L. J., Chapple I. L., Wright H. J., Roberts A., Cooper P. R. (2011). Extracellular deoxyribonuclease production by periodontal bacteria. J. Periodontal Res. 47, 439–445 10.1111/j.1600-0765.2011.01451.x [DOI] [PubMed] [Google Scholar]
- Panopoulos A. D., Watowich S. S. (2008). Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and “emergency” hematopoiesis. Cytokine 42, 277–288 10.1016/j.cyto.2008.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V., Staab D., Zychlinsky A. (2011). Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving DNase therapy. PLoS ONE 6:e28526. 10.1371/journal.pone.0028526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V., Zychlinsky A. (2009). NETs: a new strategy for using old weapons. Trends Immunol. 30, 513–521 10.1016/j.it.2009.07.011 [DOI] [PubMed] [Google Scholar]
- Pillay J., Den Braber I., Vrisekoop N., Kwast L., De Boer R., Borghans J. A. M., et al. (2010). In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 116, 625–627 10.1182/blood-2010-01-259028 [DOI] [PubMed] [Google Scholar]
- Ramos-Kichik V., Mondragón-Flores R., Mondragón-Castelán M., Gonzalez-Pozos S., Muñiz-Hernandez S., Rojas-Espinosa O., et al. (2009). Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberculosis (Edinb.) 89, 29–37 10.1016/j.tube.2008.09.009 [DOI] [PubMed] [Google Scholar]
- Rankin S. (2010). The bone marrow: a site of neutrophil clearance. J. Leukoc. Biol. 88, 241–251 10.1189/jlb.0210112 [DOI] [PubMed] [Google Scholar]
- Remijsen Q., Kuijpers T. W., Wirawan E., Lippens S., Vandenabeele P., Vanden Berghe T. (2011). Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 18, 581–588 10.1038/cdd.2011.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby K., DeLeo F. (2012). Neutrophils in innate host defense against Staphylococcus aureus infections. Semin. Immunopathol. 34, 237–259 10.1007/s00281-011-0295-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riyapa D., Buddhisa S., Korbsrisate S., Cuccui J., Wren B. W. (2012). Neutrophil extracellular traps exhibit antibacterial activity against Burkholderia pseudomallei and are influenced by bacterial and host factors. Infect. Immun. 80, 3921–3929 10.1128/IAI.00806-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D. E., Tompsett R. (1952). The survival of staphylococci within human leukocytes. J. Exp. Med. 95, 209–230 10.1084/jem.95.6.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbauer F., Tenen D. G. (2007). Transcription factors in myeloid development: balancing differentiation with transformation. Nat. Rev. Immunol. 7, 105–117 10.1038/nri2024 [DOI] [PubMed] [Google Scholar]
- Saffarzadeh M., Juenemann C., Queisser M. A., Lochnit G., Barreto G., Galuska S. P., et al. (2012). Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS ONE 7:e32366. 10.1371/journal.pone.0032366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T., Komano J., Saitoh Y., Misawa T., Takahama M., Kozaki T., et al. (2012). Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 12, 109–116 10.1016/j.chom.2012.05.015 [DOI] [PubMed] [Google Scholar]
- Savill J. (1997). Apoptosis in resolution of inflammation. J. Leukoc. Biol. 61, 375–380 [DOI] [PubMed] [Google Scholar]
- Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. (1989). Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Invest. 83, 865–875 10.1172/JCI113970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapinello S., Brooks A. S., MacInnes J. I., Hammermueller J., Clark M. E., Caswell J. L. (2011). Bactericidal activity of porcine neutrophil secretions. Vet. Immunol. Immunopathol. 139, 113–118 10.1016/j.vetimm.2010.09.004 [DOI] [PubMed] [Google Scholar]
- Serhan C. N., Chiang N., Van Dyke T. E. (2008). Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8, 349–361 10.1038/nri2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer D. J., Ren P., Raina R., Dong Y., Behr M. J., McEwen B. F., et al. (2010). Extracellular fibrils of pathogenic yeast Cryptococcus gattii are important for ecological niche, murine virulence and human neutrophil interactions. PLoS ONE 5:e10978. 10.1371/journal.pone.0010978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers C., Rankin S. M., Condliffe A. M., Singh N., Peters A. M., Chilvers E. R. (2010). Neutrophil kinetics in health and disease. Trends Immunol. 31, 318–324 10.1016/j.it.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarowicz S. E., Friedlander A. (2011). Bacillus anthracis stimulates the formation of neutrophil extracellular traps. Mol. Biol. Cell 22, 4705. 10.1091/mbc.E11-10-0886 [DOI] [Google Scholar]
- Thomas G. M., Carbo C., Curtis B. R., Martinod K., Mazo I. B., Schatzberg D., et al. (2012). Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood 119, 6335–6345 10.1182/blood-2012-01-405183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites G. E., Gant V. (2011). Are bloodstream leukocytes Trojan Horses for the metastasis of Staphylococcus aureus? Nat. Rev. Microbiol. 9, 215–222 10.1038/nrmicro2508 [DOI] [PubMed] [Google Scholar]
- Tobias J. D., Schleien C. (1991). Granulocyte transfusions – a review for the intensive care physician. Anaesth. Intensive Care 19, 512–520 [DOI] [PubMed] [Google Scholar]
- Urban C. F., Reichard U., Brinkmann V., Zychlinsky A. (2006). Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 8, 668–676 10.1111/j.1462-5822.2006.00792.x [DOI] [PubMed] [Google Scholar]
- Van Den Berg Y. W., Reitsma P. H. (2011). Not exclusively tissue factor: neutrophil extracellular traps provide another link between chemotherapy and thrombosis. J. Thromb. Haemost. 9, 2311–2312 10.1111/j.1538-7836.2011.04502.x [DOI] [PubMed] [Google Scholar]
- Villanueva E., Yalavarthi S., Berthier C., Hodgin J., Khandpur R., Lin A., et al. (2011). Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187, 538–552 10.4049/jimmunol.1100450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll R. E., Herrmann M., Roth E. A., Stach C., Kalden J. R., Girkontaite I. (1997). Immunosuppressive effects of apoptotic cells. Nature 390, 350–351 10.1038/37022 [DOI] [PubMed] [Google Scholar]
- von Köckritz-Blickwede M., Goldmann O., Thulin P., Heinemann K., Norrby Teglund A., Rohde M., et al. (2008). Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 111, 3070–3080 10.1182/blood-2007-07-104018 [DOI] [PubMed] [Google Scholar]
- Voyich J., Braughton K., Sturdevant D., Whitney A., Sad-Salim B., Porcella S., et al. (2005). Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175, 3907–3919 [DOI] [PubMed] [Google Scholar]
- Voyich J., Otto M., Mathema B., Braughton K., Whitney A., Welty D., et al. (2006). Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J. Infect. Dis. 194, 1761–1770 10.1086/509506 [DOI] [PubMed] [Google Scholar]
- Wardini A., Guimarães-Costa A., Nascimento M. T. C., Nadaes N., Danelli M. G. M., Mazur C., et al. (2010). Characterization of neutrophil extracellular traps in cats naturally infected with feline leukemia virus. J. Gen. Virol. 91, 259–264 10.1099/vir.0.014613-0 [DOI] [PubMed] [Google Scholar]
- Wartha F., Beiter K., Albiger B., Fernebro J., Zychlinsky A., Normark S., et al. (2007). Capsule and d-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell. Microbiol. 9, 1162–1171 10.1111/j.1462-5822.2006.00857.x [DOI] [PubMed] [Google Scholar]
- Watson R. W., Redmond H. P., Wang J. H., Bouchier Hayes D. (1996). Bacterial ingestion, tumor necrosis factor-alpha, and heat induce programmed cell death in activated neutrophils. Shock 5, 47–51 10.1097/00024382-199606002-00151 [DOI] [PubMed] [Google Scholar]
- Weathington N. M., Van Houwelingen A. H., Noerager B. D., Jackson P. L., Kraneveld A. D., Galin F. S., et al. (2006). A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat. Med. 12, 317–323 10.1038/nm1361 [DOI] [PubMed] [Google Scholar]
- Webster S., Daigneault M., Bewley M., Preston J., Marriott H. (2010). Distinct cell death programs in monocytes regulate innate responses following challenge with common causes of invasive bacterial disease. J. Immunol. 185, 2968–2979 10.4049/jimmunol.1000805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman I. L., Anderson D. J., Gage F. (2001). Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu. Rev. Cell Dev. Biol. 17, 387–403 10.1146/annurev.cellbio.17.1.387 [DOI] [PubMed] [Google Scholar]
- Whyte M. K., Meagher L. C., MacDermot J., Haslett C. (1993). Impairment of function in aging neutrophils is associated with apoptosis. J. Immunol. 150, 5124–5134 [PubMed] [Google Scholar]
- Xu J., Zhang X., Pelayo R., Monestier M., Ammollo C. T., Semeraro F., et al. (2009). Extracellular histones are major mediators of death in sepsis. Nat. Med. 15, 1318–1321 10.1038/nm.2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yipp B. G., Petri B., Salina D., Jenne C. N., Scott B. N. V., Zbytnuik L. D., et al. (2012). Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 18, 1386–1393 10.1038/nm.2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. L., Malcolm K. C., Kret J. E., Caceres S. M., Poch K. R., Nichols D. P., et al. (2011). Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PLoS ONE 6:e23637. 10.1371/journal.pone.0023637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi S., Gold J. A., Andina N., Lee J. J., Kelly A. M., Kozlowski E., et al. (2008). Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 14, 949–953 10.1038/nm.1855 [DOI] [PubMed] [Google Scholar]
- Yousefi S., Mihalache C., Kozlowski E., Schmid I., Simon H. U. (2009). Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 16, 1438–1444 10.1038/cdd.2009.96 [DOI] [PubMed] [Google Scholar]
- Zhang B., Hirahashi J., Cullere X., Mayadas T. (2003). Elucidation of molecular events leading to neutrophil apoptosis following phagocytosis: cross-talk between caspase 8, reactive oxygen species, and MAPK/ERK activation. J. Biol. Chem. 278, 28443–28454 10.1074/jbc.M210727200 [DOI] [PubMed] [Google Scholar]