Abstract
Cardiovascular disease (CVD) is associated with cognitive deficits even in the absence of stroke. We examined the relationship between cardiac performance, as measured by cardiac output (CO) and ejection fraction (EF), and brain activity during a verbal working memory (VWM) task in elderly CVD patients who tend to be at increased risk for vascular cognitive impairments. Seventeen patients were recruited from a cohort participating in an ongoing prospective study examining the effects of CVD on cognitive function in the elderly. Participants were diagnosed with CVD (age 68±8) and completed a 2-back VWM task in a 1.5T fMRI paradigm. CO and EF were calculated from echocardiogram measures. Task-related activation was averaged in a priori regions of interest. The relationship between CO, EF, and 2-back-related activity was modeled using partial correlations (two-tailed p<.05) controlling for age and 2-back accuracy. All participants were globally cognitively intact as indicated by Mini-Mental Status Exam and Dementia Rating Scale scores. Mean accuracy on the 2-back was 78±9% while reaction time averaged 1,027±192 ms. Mean CO and EF values showed a large range (CO: 3.55 to 6.31; EF: 0.36 to 0.76) but average values were within the normal range. After controlling for age and 2-back accuracy, lower EF was related to decrease in left insula activity (r=0.61, p=0.03). There were trends for EF to be related to accuracy (r=0.47, p=0.09) and reaction time (r=−0.48, p=0.09). CO was also related to insula activity (r=0.60, p=0.04) and activity in the supplementary motor area activity (r=0.66, p=0.01). Cardiac performance was related to decreased efficiency in task related brain areas and tended to be related to performance on a VWM task in elderly patients with CVD. Results have implications for a line of investigation indicating that cardiac and systemic vascular indices could be used as proxy measures to examine mechanisms of cerebrovascular dysfunction in the elderly.
Keywords: Functional magnetic resonance imaging, FMRI, Functional neuroimaging, Verbal working memory, Cardiovascular disease, Heart disease, Ejection fraction, Cardiac output
Introduction
Cardiovascular disease (CVD) is a leading cause of death and morbidity in Western society and is a particular concern for the elderly. CVD is an umbrella term for various conditions including coronary artery disease, myocardial infarct, heart failure, and hypertension. It has been associated with cognitive deficits in the elderly even in the absence of clinically identified stroke (Moser et al. 1999; Paul et al. 2005; Rastas et al. 2006; Verhaegen et al. 2003). These vascular cognitive impairments appear to occur on a continuum ranging from mild cognitive deficits to the severe cognitive dysfunction characteristic of vascular dementia (Bowler et al. 1999; Rockwood 2002; Roman et al. 2002). While the underlying pathophysiological mechanisms of these cognitive impairments remain unclear and likely depends on a complex interaction of impaired cardiac and cerebral hemodynamic factors, the use of systemic cardiac and vascular indices as proxy measures for further examination of the mechanisms in cerebrovascular dysfunction has been promising (Cohen et al. 2009).
This approach has previously examined the influence of a variety of cardiac and systemic vascular risk factors on cognitive performance, structural integrity, and brain response to cognitive challenges in CVD. Previous studies have investigated these relationships by examining the prevalence of specific risk factors in an epidemiological context (Claus et al. 1996; de Leeuw et al. 2004; DeCarli et al. 1999; Vermeer et al. 2003). Using this epidemiological approach, studies have shown that chronic hypertension underlies structural white matter damage and reduced cognitive function (Birkenhager et al. 2001; de Leeuw et al. 2004; den Heijer et al. 2005; Vermeer et al. 2002). In non-epidemiologic settings, a significant relationship has been found between executive dysfunction in elderly individuals and worse cardiovascular health as measured by an index of cardiac performance (Jefferson et al. 2007a). Another index of CVD, atherosclerosis, as measured by intima-media-thickening (IMT) has been associated with diminished attention–executive–psychomotor performance in elderly CVD patients with normal global cognition (Haley et al. 2006). A variety of additional cardiac and systemic vascular factors and their impact on cognitive function and MRI brain abnormalities have also been examined in elderly individuals with CVD (Gunstad et al. 2005; Haley et al. 2006, 2007; Hoth et al. 2007; Jefferson et al. 2007a, b; Paul et al. 2005).
More recently, functional magnetic resonance imaging (fMRI) has been used to further characterize brain inefficiencies secondary to CVD. This technology may be valuable as a tool to be used in conjunction with epidemiological risk based efforts to detect inefficiencies in brain areas vulnerable to the impact of CVD. For instance, an attenuated blood oxygenation level-dependent (BOLD) response to a verbal working memory (VWM) challenge during fMRI was linked to worse peripheral vascular health as measured by IMT in elderly individuals with CVD who had intact global cognitive functioning (Haley et al. 2007). Similarly preliminary results also indicated that systemic vascular dysfunction as measured by brachial artery distensibility (BAR) was also associated with lower magnitude of task related fMRI activity in response to the same VWM task in a similar population (Mulligan et al. 2008). These findings are relevant since successful VWM task performance depends on attention–executive–processing speed systems which are known to be vulnerable to vascular-related damage in this population (Haley et al. 2006; Jefferson et al. 2007a).
While it appears that indices of peripheral vascular health such as IMT and BAR show sensitivity to cognitive challenges in a functional brain imaging environment, it remains unclear whether other measures of cardiac performance would also demonstrate similar effects. Decreases in cardiac health have previously been associated with decreased systemic and cerebral perfusion which alters the oxygen and nutrient supply to the brain (Saxena and Schoemaker 1993). Furthermore, impaired cardiac performance, as measured by ventricular ejection fraction and cardiac output has also been shown to be associated with diminished cognition among elderly CVD patients (Hoth et al. 2008; Jefferson et al. 2007b; Zuccala et al. 2001) as well as structural brain changes (Jefferson et al. 2007b).
The current study aimed to further expand this line of work by examining the relationship between cardiac performance and brain response to the aforementioned VWM challenge among elderly CVD patients without a history of stroke or other neurological disease. Since most elderly people with cardiovascular disease have a variety of underlying risk and etiological factors (Tranche et al. 2005) our sample consisted of a heterogeneous group of elderly patients with CVD who were recruited from an ongoing prospective study examining the effects of cardiovascular disease on cognitive function in an elderly cohort (Principal Investigator: RAC). This fMRI study was a secondary study that captured patients with a range of cardiac abnormalities who were willing and able to complete a VWM challenge in a fMRI environment. We used two common echocardiogram measures of cardiac performance; cardiac output (CO) and ejection fraction (EF). EF is a frequently used clinical indicator of heart health with strong prognosticator of morbidity and mortality and is stable day to day. EF may decrease when the heart muscle has been damaged, such as due to myocardial infarction, heart-muscle disease (cardiomyopathy), or heart valve problems. Since ejection fraction does not measure absolute forward flow of blood leaving the ventricle, we included a Doppler measure of systemic flow, cardiac output. CO changes in response to the body’s demands predominantly by increasing heart rate. In a recent factor analysis study, CO and EF were shown to be strongly related to heart function and loaded heavily as “cardiac factors” as compared to “systemic vascular factors” such as BAR, IMT and diastolic variability (Cohen et al. 2009). We hypothesized that reduced cardiac health, as reflected in CO and EF measures would be associated with decreased brain activation in related regions of interest previously shown to respond to a VWM challenge (dorsolateral prefrontal, posterior parietal, supplemental motor area, medial prefrontal gyrus, insula and posterior cingulated gyrus). Since autoregulatory mechanisms that augment blood flow to the brain change as a function of age-associated vasculature breakdown, we examined the effect of age by including it as a covariate in our analyses.
Methods
Participants
The local Institutional Review Board approved the study and all volunteers provided written informed consent. Seventeen right-handed participants (11 males and 6 females) between the ages of 57 and 84 were recruited from a sample of 181 in a prospective study examining the effects of cardiovascular disease on cognitive function in an elderly cohort (Principal Investigator: RAC). Participants were mainly Caucasian (88%) and enrolled in this study if they had a documented history of at least one of the following: a diagnosis of coronary artery disease (35%), angina pectoris (23%), previous myocardial infarction (47%), heart failure (23%), cardiac surgery (29%), arrhythmia (23%) or hypertension (59%). Determination of cardiovascular risk factors was based on self-report during a medical history interview, with verification of the presence of specific risk factors derived from medical records, discussion with the patients’ physicians, or both, when possible.
Patients were excluded from participation if they had a history of neurological disease (i.e., large vessel stroke, seizure disorder, Parkinson’s disease, clinically significant traumatic brain injury, multiple sclerosis, brain infection/ meningitis, or diagnosed dementia), major psychiatric illness (e.g., schizophrenia, bipolar disorder), substance abuse (i.e., diagnosed abuse or previous hospitalization for substance abuse), MRI contraindications (e.g., metallic implants, metal related injuries, body size), a score below the cutoff for dementia (total score <123) on the Dementia Rating Scale (DRS, Mattis 1988), or had identified lacunar lesions on MRI. Patients were also excluded if they were left handed, reported claustrophobia, exhibited below chance or invalid performance, could not lie still for one hour, had uncorrected visual impairment, or exhibited excessive head movement during the scan. Further details about the demographic and clinical characteristics of the sample can be found in Table 1.
Table 1.
Sample characteristics
| Variable | Minimum | Maximum | Mean | Standard deviation |
|---|---|---|---|---|
| Demographic-Clinical | ||||
| Age (years) | 57 | 84 | 68 | 8 |
| Education (years completed) | 12 | 18 | 15 | 2 |
| Mini mental status exam score | 26 | 30 | 29 | 2 |
| Dementia rating scale total score | 134 | 144 | 140 | 3 |
| Cardiac | ||||
| Ejection fraction | 0.36 | 0.76 | 0.60 | 0.12 |
| Cardiac output (L/min) | 3.55 | 6.31 | 4.4 | 0.78 |
Clinical procedures
All participants completed a detailed medical history questionnaire focused on CVD history. Participants completed the neuroimaging study approximately 11 months after the collection of cardiac data. For the vascular assessment, participants were asked to refrain from taking vasoactive medications (e.g., calcium channel blockers, acetocholene inhibitors and beta blockers), drinking caffeinated beverages, and smoking for 6 hours prior to the assessment. Furthermore, all participants fasted for 6 hours prior to the assessment. Prior to initiating vascular interrogation, patients remained supine for 15 min in a quiet room. A complete, transthoracic echocardiogram was obtained from each participant according to standards put forth by the American Society of Echocardiography and supervised by a board certified cardiologist (AP). From this echocardiogram data CO and EF were derived. CO is the amount of blood in liters per minute (L/min) that is pumped from the heart to the systemic circulation. SV is calculated from the Doppler-derived measure of blood exiting the heart (CO = (TVI × CSA) × HR, where TVI = time velocity integral, CSA = cross-sectional area of the left ventricular outflow tract, and HR = heart rate). While this method reflects a noninvasive procedure for obtaining CO, previous research has shown that data generated from such noninvasive procedures strongly correlates with invasive measures of CO (Moulinier et al. 1991). EF refers to the fraction of blood ejected by the left ventricle during the contraction (ejection phase) of the cardiac cycle. Here the SV is estimated by change in ventricular dimensions; any fraction of the SV lost in backward leaking of valves would not be accounted for.
Task paradigm
The 2-Back task was utilized as a measure of verbal working memory (Braver et al. 1997; Smith and Jonides 1997; Sweet et al. 2008). During this task, a series of individual consonants were visually presented for 500 ms each with a 2,500 ms interstimulus interval (Fig. 1). Consonants were arranged in random order from a list of all consonants except “L” due to ambiguity in the lowercase form. Participants were asked to determine if each consonant was the same as, or different from the previously presented reference consonant. Participants responded by pressing a button with either their right index finger (affirmative responses), or their right middle finger (negative responses). A 0-Back control condition was alternated with the 2-Back condition in a block design. Two imaging runs were presented, each consisted of four blocks of the 0-Back and four blocks of the 2-Back, lasting approximately 6 min. Task performance was assessed by measuring accuracy rates and mean reaction time.
Fig. 1.
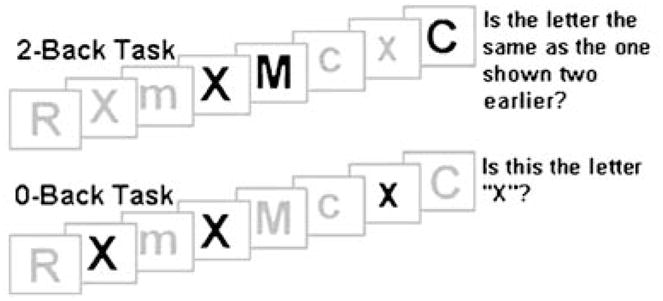
0-Back and 2-Back task stimuli. Figure caption: 0-Back control condition: This task consisted of four blocks of nine consonants of random case and order, 33% of which were targets. 2-Back experimental condition: The experimental condition consisted of four blocks, each containing 15 consonants of random case and order, 33% of which were targets
Neuroimaging procedures
Each MRI session consisted of a practice run of the 2-Back task (at least four cycles of 0-Back/2-Back), collection of structural images, and two runs of the 2-Back task. The 2-Back task was presented using E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA), back-projected onto a screen positioned at the participant’s feet, and viewed through a double-mirror attached to the head coil. Participants’ responses were collected using an MR-compatible piano-key response box.
MRI data for each participant were acquired in a single session on a 1.5T Siemens Symphony scanner equipped with a standard head coil. Functional imaging was performed using a whole brain echo-planer imaging sequence (TR=3,860 ms, TE=38 ms, FOV=192 mm2, 64×64 matrix, 48 axial slices, 3-mm slice thickness). Structural imaging sequences included a fluid-attenuated inversion recovery (TR=6,000 ms, TE=105 ms, 5-mm slice thickness, 2-mm gap) sequence for white matter hyperintensity (WMH) quantification, and a high-resolution (256×256 matrix, FOV=256 mm2, 1-mm slice thickness) magnetization prepared rapid gradient echo anatomical scans of the entire brain in the saggital plane.
FMRI data preprocessing
All EPI images were processed using Analysis of NeuroImages (AFNI) software (Cox 1996). Each time series was spatially registered to the sixth volume of the session to reduce the effects of head movement. The AFNI 3-dimensional registration program also yields information on displacement and rotation for each volume that was used later for further motion correction. Data preprocessing also included temporal smoothing, spatial filtering, and transformation to standard stereotaxic space. Task-related brain activation was determined using voxel-wise multiple regression analyses with the following parameters: a 0-Back/2-Back ideal waveform convolved with a gamma function, and covariates accounting for instruction screens, head movement, and linear trends. An a priori ROI set was created based on our published literature to examine activity in six bilateral cortical regions known to exhibit reliable activity during the 2-Back in a larger sample of 34 subjects (Sweet et al. 2008) (Table 2).
Table 2.
A priori regions of interest related to verbal working memory
| Bilateral ROIs | Center Coordinates | EF
|
CO
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Left
|
Right
|
Left
|
Right
|
|||||||
| x | y | z | r | p | r | p | r | p | r | p | |
| 1) Dorsolateral prefrontal | ±40 | 16 | 31 | 0.13 | 0.97 | −0.18 | 0.58 | 0.51 | 0.09** | 0.37 | 0.23 |
| 2) Posterior parietal | ±35 | −47 | 44 | 0.01 | 0.96 | −0.11 | 0.71 | 0.48 | 0.11 | −0.11 | 0.73 |
| 3) Supplementary motor area | ±02 | 18 | 47 | −0.22 | 0.47 | −0.10 | 0.75 | 0.51 | 0.08** | 0.66 | 0.01* |
| 4) Medial prefrontal gyrus | ±04 | 56 | 07 | 0.26 | 0.42 | 0.22 | 0.48 | −0.009 | 0.98 | −0.11 | 0.73 |
| 5) Insula | ±34 | 0 | 13 | 0.61 | 0.03* | 0.17 | 0.58 | 0.60 | 0.04* | 0.22 | 0.48 |
| 6) Posterior cingulate gyrus | ±05 | −49 | 19 | −0.04 | 0.88 | −0.19 | 0.55 | −0.03 | 0.92 | −0.03 | 0.91 |
Statistical analyses
Data were analyzed using SPSS 11.0 computer software (SPSS Inc., Chicago, IL). All variable distributions were examined using the Shapiro–Wilk test of normality recommended for small samples. Positively skewed variables were log transformed to fulfill assumptions of normality. Relationships between mean 2-Back-related activation intensity within each of the a priori ROIs and CO and EF were examined using Pearson partial correlations, controlling for the effect of age and 2-back accuracy.
Results
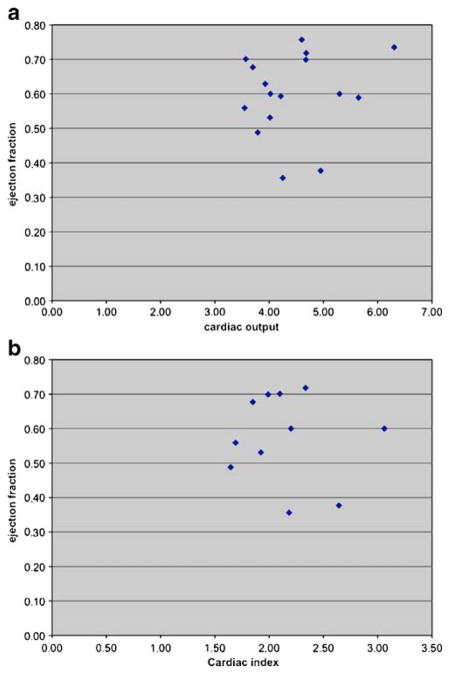
Sample characteristics are provided in Table 1. Mini-Mental Status Exam and DRS scores indicate intact global cognitive functioning according to published norms (Mattis 1988). Our cardiac measure of performance indicated that mean EF and CO were within the range of normal, with 3 patients revealing systolic ventricular dysfunction. EF dysfunction has been characterized as being mild in the 41–49% range, moderate 35–40% and <35% as severe (Lang et al. 2005; Mahadevan et al. 2008). The normal CO for an average individual at rest is usually 3–6 l/minute. As one would expect, EF and CO were not linearly correlated (r=0.17, p=0.55). As a result, they were used independently in subsequent analyses. Scatterplots for EF and CO are provided in Fig. 2a. A plot of EF against cardiac index is also provided in Fig. 2b since cardiac index relates CO to body surface area.
Fig. 2.
Scatterplot of ejection fraction vs cardiac output (a) and ejection fraction vs cardiac index (b)
All participants performed above chance on the 2-Back task. Mean accuracy was 78±9%. Mean reaction time was 1027±192 ms. Participants moved less than 1.5 mm per imaging run. Brain activity in response to the 2-Back task is shown in Fig. 3. These overlapped a priori ROIs, indicating that a priori ROIs shared common regions of activity with the observed task-related activity. Task-related effects in one ROI was not normally distributed (left insula Shapiro–Wilk=0.83, p=0.01) and was log transformed. Slower 2-Back reaction time was significantly related to worse EF (r=−0.82, p=0.001) and lower 2-Back accuracy tended to occur with lower EF (r=0.47, p=0.09). After controlling for age and 2-back accuracy, greater brain activity in left insula was also related to greater EF (r=0.61, p=0.03) and CO (r=0.60, p=0.04). There was a trend for reaction time to be inversely related to bilateral insula activation (r=−0.48, p=0.09). Supplemental motor area activation was related to greater CO, particularly on the right side (r=0.66, p=0.02). There was a trend for the dorsolateral prefrontal cortex to be related to CO (r=0.51, p=0.09). Additional correlations attempted between a priori ROIs and cardiac measures are listed in Table 2.
Fig. 3.
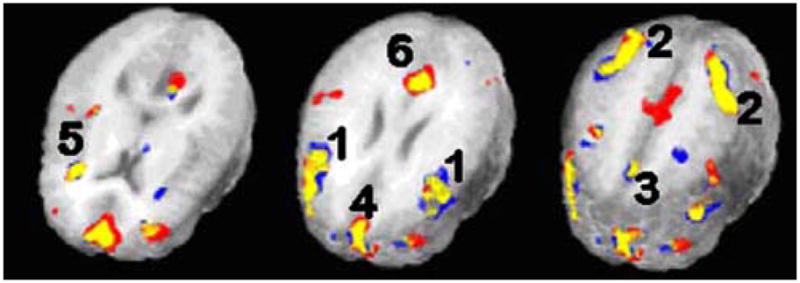
2-Back regions of interest. Note: Numbers correspond to Table 1
Discussion
The current study examined the relationship between cardiac performance as measured by cardiac output and ejection fraction, and brain response to a VWM challenge in elderly CVD patients without global cognitive impairment or a history of stroke. Since elderly individuals are at an increased risk for a wide range of vascular cognitive impairments, we aimed to extend a developing line of investigation indicating that cardiac and systemic vascular indices could be used as proxy measures to examine mechanisms in cerebrovascular dysfunction in the elderly (Cohen et al. 2009). Previous work has shown that a variety of cardiac and systemic vascular factors impact cognitive performance, structural integrity and brain response to cognitive challenges (Birkenhager et al. 2001; Claus et al. 1996; de Leeuw et al. 2004; DeCarli et al. 1999; den Heijer et al. 2005; Gunstad et al. 2005; Haley et al. 2006, 2007; Hoth et al. 2007; Jefferson et al. 2007a, b; Paul et al. 2005; Vermeer et al. 2003; Vermeer et al. 2002). Most recently, fMRI challenges have also been used to show that worse peripheral vascular health, as measured by BAR and IMT, are associated with lower magnitude of brain activity in response to VWM tasks in elderly patients with CVD (Haley et al. 2007; Mulligan et al. 2008).
Our findings further support this line of work by demonstrating that in elderly CVD patients, poorer cardiac performance, as measured by ejection fraction, is also associated with less brain activity in a task related region (i.e, insula) and tends to be associated with slower reaction times and poorer VWM accuracy. Insula activity has been frequently associated with the 2-Back task in previous literature (Braver et al. 1997; Kim et al. 2006; Sweet et al. 2008). It is also a region that has been associated with integration of autonomic functions in the cardiovascular response to mental stress. The insula is activated by cardiovascular arousal in tasks associated with physical or mental stress (Critchley et al. 2001) and during interoception induced by focusing attention to one’s heartbeats (Critchley et al. 2004). Stimulation experiments in animal models and patient populations have also suggested that the insula contains a site of cardiovascular representation that has been associated with lethal cardiac arrythmias and strokes (Oppenheimer 1992; Oppenheimer et al. 1991; Zhang and Oppenheimer 1997, 2000). The more dynamic measure of true forward flow, CO, was also associated with insula activation in addition to activation in the supplemental motor area (SMA) and a trend towards the dorsolateral prefrontal cortex (DLPFC). These are regions previously shown to be reliably associated with 2-Back activity (Braver et al. 1997; Haley et al. 2007; Sweet et al. 2008) therefore highlighting the robustness of our paradigm.
The lack of relationship between CO and performance based measures may reflect the dynamic nature of cardiac output measures. In particular, changing metabolic demands of the body can reduce cardiac output since CO reflects a relationship between stroke volume and heart rate. While stroke volume can vary by 1.5 fold, heart rate varies more (3 fold). In addition, CO is more acutely affected by the heart’s afterload. Taken together, this results in more dynamic fluctuations in CO depending on the body’s physiological demands. So, if one’s blood pressure is higher on a particular day CO will be lower unless the heart rate increases to compensate. In contrast, EF is an indicator of chronic cardiac performance and is stable on a day-to-day basis. It measures the fraction of blood ejected by the left ventricle during the contraction phase of the cardiac cycle, with estimation of stroke volume based on changes in ventricular dimensions. Any fraction of stroke volume lost in backward leaking of valves is not accounted for by this measure and EF is typically a strong prognostic indicator of morbidity and mortality. In our sample, the chronicity of cardiac health reflected by EF appears to be reflected in significant relationships noted between EF, task related brain activity and cognitive performance. Weaker performance on VWM tasks related to lower EF has also been reported recently in a similar sample of elderly individuals with CVD (Hoth et al. 2008). In the context of prior work indicating that lower cardiac performance is associated with white matter alterations in task related regions (Jefferson et al. 2007b), it appears that our findings indicate that some brain areas and corresponding functions might be differentially impacted by intermittent cardiac changes while others might be more affected by chronic changes in cardiac performance.
Furthermore, CO and EF should correlate loosely when both are abnormal and there is no valvular dysfunction. While a sample size of seventeen participants is reasonable for an fMRI study, limited power and variability in baseline values for the cardiac indices may have contributed to the lack of a relationship between CO and EF in this subset of patients. By design, the study included patients with CVD who were heterogeneous in their history and the severity of their cardiac risk factors. While this allowed for increased generalizability to elderly CVD patients in the real world who have a variety of underlying risk and etiological factors (Tranche et al. 2005), it reduced some experimental control achievable with a more homogeneous group. Also of note, while our sample was diverse in its clinical characteristics it was more limited in its sociodemographic diversity. The sample was predominantly college educated and Caucasian which is not representative of the ethnic and socieconomic diversity of patients with CVD. The number of a priori tests of our hypotheses is also considered a limitation to this study. Although we attempted to guard against Type I experiment-wise error by using a priori ROIs and by limiting tests to six bilateral regions, these were performed for CO, EF, 2-back accuracy, and 2-back reaction times. The significant p-values were modest; however, the effects EF and CO on brain activity were strong, ranging from r=.51 to .66 even after controlling for age and performance. Future large-scale and more representative studies are warranted to confirm the associations noted here and to further detect significant relationships between measures of cardiac health and task-related brain activity.
Future larger sample sizes might also be able to detect a trend that we observed between insula activity and reaction time performance, which if significant, could have implicated a mediating role for EF in the relationship between insula activity and reaction time performance. While causal interpretations cannot be drawn from the current correlational and cross-sectional data, the observed associations lend some support for the value of using fMRI to detect subtle cardiac related changes in brain function early in the disease process and while cognitive functioning is globally intact. This line of work may enable further understanding of a population vulnerable to the negative effects of reduced cardiac performance on the brain and aid in prevention efforts geared towards minimizing the effects of these systemic abnormalities on the brain. The current findings are however only an initial step in understanding these relationships and future large-scale prospective studies will be important in further determining the nature and direction of these relationships.
Acknowledgments
Funding Source: R01-AG017975 (RAC)
This work was supported by the following grants F32AG022773 (ALJ), F32HL074568 (JJG), F32NS042404 (LHS), T32AG020498 (BAJ, APH).
Contributor Information
Farzin Irani, Email: firani@upenn.edu, Department of Psychiatry and Human Behavior, Brown University, Providence, RI, USA. Department of Psychiatry, University of Pennsylvania, Philadelphia, PA, USA.
Lawrence H. Sweet, Email: lawrence_sweet@brown.edu, Department of Psychiatry and Human Behavior, Brown University, Providence, RI, USA. Butler Hospital TRG, Brown University Medical School, 345 Blackstone Bvld., Providence, RI 02906, USA
Andreana P. Haley, Department of Psychology, University of Texas, Austin, TX, USA
John J. Gunstad, Department of Psychology, Kent State University, Kent, OH, USA
Beth A. Jerskey, Department of Psychiatry and Human Behavior, Brown University, Providence, RI, USA
Richard C. Mulligan, Department of Psychiatry and Human Behavior, Brown University, Providence, RI, USA
Angela L. Jefferson, Department of Neurology, Alzheimer’s Disease Center, Boston University School of Medicine, Boston, MA, USA
Athena Poppas, Department of Psychiatry and Human Behavior, Brown University, Providence, RI, USA.
Ronald A. Cohen, Department of Psychiatry and Human Behavior, Brown University, Providence, RI, USA
References
- Birkenhager WH, Forette F, Seux ML, Wang JG, Staessen JA. Blood pressure, cognitive functions, and prevention of dementias in older patients with hypertension. Archives of Internal Medicine. 2001;161(2):152–156. doi: 10.1001/archinte.161.2.152. [DOI] [PubMed] [Google Scholar]
- Bowler JV, Steenhuis R, Hachinski V. Conceptual background to vascular cognitive impairment. Alzheimer Disease and Associated Disorders. 1999;13(Suppl 3):S30–S37. [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5(1):49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Claus JJ, Breteler MM, Hasan D, Krenning EP, Bots ML, Grobbee DE, et al. Vascular risk factors, atherosclerosis, cerebral white matter lesions and cerebral perfusion in a population-based study. European Journal of Nuclear Medicine. 1996;23(6):675–682. doi: 10.1007/BF00834530. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Poppas A, Forman DE, Hoth KF, Haley A, Gunstad J, et al. Vascular and cognitive functions associated with cardiovascular disease in the elderly. Journal of Clinical and Experimental Neuropsychology. 2009;31(1):96–110. doi: 10.1080/13803390802014594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computational Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neuroanatomical basis for first- and second-order representations of bodily states. Nature Neuroscience. 2001;4(2):207–212. doi: 10.1038/84048. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- de Leeuw FE, Richard F, de Groot JC, van Duijn CM, Hofman A, Van Gijn J, et al. Interaction between hypertension, apoE, and cerebral white matter lesions. Stroke. 2004;35(5):1057–1060. doi: 10.1161/01.STR.0000125859.71051.83. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, et al. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30(3):529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Launer LJ, Prins ND, van Dijk EJ, Vermeer SE, Hofman A, et al. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology. 2005;64(2):263–267. doi: 10.1212/01.WNL.0000149641.55751.2E. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Cohen RA, Tate DF, Paul RH, Poppas A, Hoth K, et al. Blood pressure variability and white matter hyperintensities in older adults with cardiovascular disease. Blood Pressure. 2005;14(6):353–358. doi: 10.1080/08037050500364117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley AP, Forman DE, Poppas A, Hoth KF, Gunstad J, Jefferson AL, et al. Carotid artery intima-media thickness and cognition in cardiovascular disease. International Journal of Cardiology. 2006;121(2):148–154. doi: 10.1016/j.ijcard.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley AP, Sweet LH, Gunstad J, Forman DE, Poppas A, Paul RH, et al. Verbal working memory and atherosclerosis in patients with cardiovascular disease: an fMRI study. Journal of Neuroimaging. 2007;17(3):227–233. doi: 10.1111/j.1552-6569.2007.00110.x. [DOI] [PubMed] [Google Scholar]
- Hoth KF, Tate DF, Poppas A, Forman DE, Gunstad J, Moser DJ, et al. Endothelial function and white matter hyperintensities in older adults with cardiovascular disease. Stroke. 2007;38(2):308–312. doi: 10.1161/01.STR.0000254517.04275.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth KF, Poppas A, Moser DJ, Paul RH, Cohen RA. Cardiac dysfunction and cognition in older adults with heart failure. Cognitive and Behavioral Neurology. 2008;21(2):65–72. doi: 10.1097/WNN.0b013e3181799dc8. [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Poppas A, Paul RH, Cohen RA. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiology of Aging. 2007a;28(3):477–483. doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Tate DF, Poppas A, Brickman AM, Paul RH, Gunstad J, et al. Lower cardiac output is associated with greater white matter hyperintensities in older adults with cardiovascular disease. Journal of the American Geriatrics Society. 2007b;55(7):1044–1048. doi: 10.1111/j.1532-5415.2007.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Whyte J, Wang J, Rao H, Tang KZ, Detre JA. Continuous ASL perfusion fMRI investigation of higher cognition: quantification of tonic CBF changes during sustained attention and working memory tasks. NeuroImage. 2006;31(1):376–385. doi: 10.1016/j.neuroimage.2005.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. Journal of the American Society of Echocardiography. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mahadevan G, Davis RC, Frenneaux MP, Hobbs FDR, Lip GYH, Sanderson JE, et al. Left ventricular ejection fraction: are the revised cut-off points for defining systolic dysfunction sufficiently evidence based? Heart. 2008;94:426–428. doi: 10.1136/hrt.2007.123877. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia rating scale (DRS) Odessa: Psychological Assessment Resources; 1988. [Google Scholar]
- Moser DJ, Cohen RA, Clark MM, Aloia MS, Tate BA, Stefanik S, et al. Neuropsychological functioning among cardiac rehabilitation patients. Journal of Cardiopulmonary Rehabilitation. 1999;19(2):91–97. doi: 10.1097/00008483-199903000-00002. [DOI] [PubMed] [Google Scholar]
- Moulinier L, Venet T, Schiller NB, Kurtz TW, Morris RC, Jr, Sebastian A. Measurement of aortic blood flow by Doppler echocardiography: day to day variability in normal subjects and applicability in clinical research. Journal of the American College of Cardiology. 1991;17(6):1326–1333. doi: 10.1016/s0735-1097(10)80143-3. [DOI] [PubMed] [Google Scholar]
- Mulligan RC, Haley AP, Jerskey BA, Irani F, Poppas A, Sweet L, et al. Endothelial function predicts fMRI activity in the medial prefrontal cortex [Poster] Journal of the International Neuropsychological Society. 2008;15(51):48. [Google Scholar]
- Oppenheimer S. The insular cortex and the pathophysiology of stroke-induced cardiac changes. Canadian Journal of Neurological Sciences. 1992;19(2):208–211. [PubMed] [Google Scholar]
- Oppenheimer SM, Wilson JX, Guiraudon C, Cechetto DF. Insular cortex stimulation produces lethal cardiac arrhythmias: a mechanism of sudden death? Brain Research. 1991;550(1):115–121. doi: 10.1016/0006-8993(91)90412-o. [DOI] [PubMed] [Google Scholar]
- Paul RH, Gunstad J, Poppas A, Tate DF, Foreman D, Brickman AM, et al. Neuroimaging and cardiac correlates of cognitive function among patients with cardiac disease. Cerebrovascular Diseases. 2005;20(2):129–133. doi: 10.1159/000086803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastas S, Pirttila T, Viramo P, Verkkoniemi A, Halonen P, Juva K, et al. Association between blood pressure and survival over 9 years in a general population aged 85 and older. Journal of the American Geriatrics Society. 2006;54(6):912– 918. doi: 10.1111/j.1532-5415.2006.00742.x. [DOI] [PubMed] [Google Scholar]
- Rockwood K. Vascular cognitive impairment and vascular dementia. Journal of the Neurological Sciences. 2002;203–204:23–27. doi: 10.1016/s0022-510x(02)00255-1. [DOI] [PubMed] [Google Scholar]
- Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurology. 2002;1(7):426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- Saxena PR, Schoemaker RG. Organ blood flow protection in hypertension and congestive heart failure. American Journal of Medicine. 1993;94(4A):4S–12S. [PubMed] [Google Scholar]
- Smith EE, Jonides J. Working memory: a view from neuroimaging. Cognitive Psychology. 1997;33(1):5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- Sweet LH, Paskavitz JF, Haley AP, Gunstad JJ, Mulligan RC, Nyalakanti PK, et al. Imaging phonological similarity effects on verbal working memory. Neuropsychologia. 2008;46(4):1114–1123. doi: 10.1016/j.neuropsychologia.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Tranche S, Galgo A, Mundet X, Sanchez-Zamorano MA, Karen AP. Cardiovascular risk factors in type 2 diabetic patients: multifactorial intervention in primary care. Kidney International Supplement. 2005;(93):S55–S62. doi: 10.1111/j.1523-1755.2005.09313.x. [DOI] [PubMed] [Google Scholar]
- Verhaegen P, Borchelt M, Smith J. Relation between cardiovascular and metabolic disease and cognition in very old age: cross-sectional and longitudinal findings from the berlin aging study. Health Psychology. 2003;22(6):559–569. doi: 10.1037/0278-6133.22.6.559. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2002;33(1):21–25. doi: 10.1161/hs0102.101629. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34(5):1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Oppenheimer SM. Characterization, distribution and lateralization of baroreceptor-related neurons in the rat insular cortex. Brain Research. 1997;760(1–2):243–250. doi: 10.1016/s0006-8993(97)00284-9. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Oppenheimer SM. Electrophysiological evidence for reciprocal insulo-insular connectivity of baroreceptor-related neurons. Brain Research. 2000;863(1–2):25–41. doi: 10.1016/s0006-8993(00)02068-0. [DOI] [PubMed] [Google Scholar]
- Zuccala G, Onder G, Pedone C, Cocchi A, Carosella L, Cattel C, et al. Cognitive dysfunction as a major determinant of disability in patients with heart failure: results from a multicentre survey. On behalf of the GIFA (SIGG-ONLUS) Investigators. Journal of Neurology, Neurosurgery and Psychiatry. 2001;70(1):109– 112. doi: 10.1136/jnnp.70.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]