Abstract
Objective
To assess associations between abacavir (ABC) use and systemic inflammation.
Design
Retrospective case-control study.
Methods
MACS & WIHS cohort participants who initiated ABC were matched, using propensity score methods, to ABC-unexposed persons. Levels of hsCRP(μg/mL), IL-6(pg/mL), and D-dimer (μg/mL) were measured from pre-HAART and on-HAART plasma. Random-effects models compared markers by ABC exposure and by changes from pre-HAART levels.
Results
Biomarkers were measured in N=508 matched pairs (328 women; 180 men). Pre-HAART levels did not differ by exposure group except that hsCRP levels were higher among WIHS women who subsequently used ABC (p=0.04). Regardless of ABC use, mean hsCRP increases and D-dimer reductions were seen when comparing pre- to on-HAART levels, in the overall group (28% and -27%), for MACS men (28% and -31%) and for WIHS women (29% and -24% (p<0.01 for all); IL-6 levels declined in MACS men (p=0.02). No adjusted biomarker level differences existed by ABC exposure at the on-HAART visit. HIV RNA reductions correlated with D-dimer (r = 0.14, p < 0.01) and IL-6 (r = 0.12, p < 0.01) reductions. Associations between ABC use and mean biomarker levels were modified by pre-HAART ART experience. Renal dysfunction was equally likely among non-ABC and ABC recipients.
Discussion
ABC use was not associated with plasma elevations in hsCRP, IL-6 and d-dimer. Mechanisms other than increased systemic inflammation may account for ABC’s reported association with increased cardiovascular disease. HAART -associated reductions in D-dimer and IL-6 were apparent regardless of ABC use and were correlated with HIV RNA reductions.
Keywords: HIV infection, inflammation, HAART, abacavir, cytokines
INTRODUCTION
Reports from several large observational cohorts of HIV-infected persons have linked the recent use (within six months) of certain nucleoside reverse transcriptase inhibitor antiretrovirals (NRTI’s), most notably abacavir (ABC), with increased risk for coronary events 1-8. In these groups, recent ABC use was strongly and independently associated with increased risk for the occurrence of myocardial infarctions (MI’s).
Subsequent analyses of data from the Strategies for Management of Anti-Retroviral Therapy (SMART) study suggested that ABC use was independently associated with increased plasma levels of C-reactive protein (hsCRP) and interleukin 6 (IL-6), biomarkers that are associated with systemic inflammation2-4. These observations have led to hypotheses that ABC’s unique association with increased cardiovascular-related death is mediated pathophysiologically, at least in part, through its pro-inflammatory effects. However, data from several other studies have failed to demonstrate associations between ABC use and either increases in CVD or increases in systemic levels of inflammatory biomarkers9-12.
To investigate whether use of ABC independently increases an inflammatory response, we comparatively and longitudinally evaluated plasma levels of inflammatory biomarkers among HIV-infected persons who did versus did not receive ABC-containing HAART regimens. Stored specimens collected prospectively from before and after highly active antiretroviral therapy (HAART) initiation permitted the examination of within-person changes. These within-cohort and cross-cohort comparisons were undertaken among women participating in the Women’s Interagency HIV Study (WIHS) and the Multicenter AIDS Cohort Study (MACS).
METHODS
Selection of Study Participants
The MACS and WIHS cohorts have been previously described13, 14. Briefly, both studies prospectively schedule participants for semi-annual study visits where medical and therapy histories, behavioral assessments, and biological specimens are collected as part of standardized protocols. Individuals eligible for the current study were HIV seropositive participants who initiated HAART during study follow-up. HAART initiators were restricted to persons for whom ≤1year had elapsed between the last date at which they reported no HAART use and the date at which first HAART use was reported. Persons who reported use of any non-HAART ABC regimen prior to 1st HAART use, and person-visits without specimens available, were excluded. HAART was defined in accordance with US Department of Health and Human Services (DHHS) treatment guidelines (www.aidsinfo.gov) as use of at least two nucleoside reverse transcriptase inhibitors (NRTI) in combination with at least one protease inhibitor (PI) or one non-nucleoside reverse transcriptase inhibitor (NNRTI); one NRTI in combination with at least one PI and at least one NNRTI; or an ABC or tenofovir containing regimen with at least three NRTI in the absence of both PI and NNRTI, except for the three NRTI regimens consisting of abacavir/tenofovir/lamivudine or didanosine/tenofovir/lamivudine. Antiretroviral (ART) regimens reported at each semi-annual visit for HAART initiators were categorized as either HAART including abacavir (ABC HAART), HAART not including abacavir (non-ABC HAART), or non-HAART.
Figure 1 depicts the study design. There were1,508 individuals (567 MACS, 941 WIHS) initiated HAART during study follow-up and contributed 11,703 HAART person-visits (5136 MACS, 6567 WIHS). From these, we identified 508 ‘ABC-exposed’ individuals (180 MACS, 328 WIHS) who reported ABC use.
Figure 1.
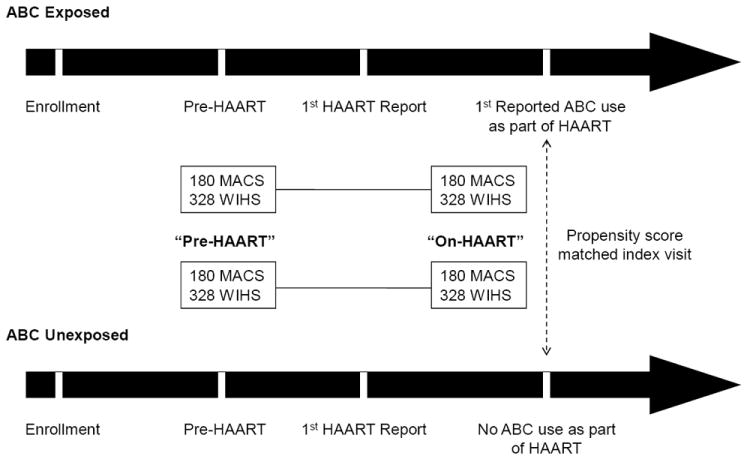
Study Design
To identify an appropriate comparison group of ABC-unexposed individuals, we utilized propensity score methodology14. Propensity score methods extend traditional matching methods to eliminate imbalances, and thus confounding, between exposed and unexposed groups in a large set of measured variables. Briefly, we took into account all HAART study visits from HAART initiation to the first report of ABC use or to the last study visit if ABC use was not reported. Since visits occurred semi-annually, ABC initiation could have taken place <1 to 6 months before the visit at which ABC use was first reported. Propensity scores for each person-visit at which HAART use was reported were obtained from logistic regression models where the outcome was ABC HAART initiation. Predictors included age, calendar year, cigarette use, body mass index (BMI), high density lipoprotein cholesterol level (HDL), race/ethnicity, hepatitis C virus (HCV) antibody status, antiretroviral (ART) experience at HAART initiation, duration of HAART use, the proportion of visits between HAART initiation and the last eligible visit where HAART was not reported, CD4+ cell count/mm3(CD4), and plasma HIV RNA levels. Propensity score matching was undertaken separately within each cohort (MACS men and WIHS women). For each ABC initiator, we identified a non-ABC HAART user by selecting the participant visit with a propensity score value closest to and no more than 3% different than the propensity score at the visit at which ABC initiation was first reported. While the visits of ABC users prior to ABC initiation were included in model construction so the same individual could contribute to both exposure groups, each matched pair consisted of two individuals, one exposed and one unexposed. The last available visit within one year prior to first report of HAART was defined as the baseline (pre-HAART) visit. In summary, our selection identified 2 visits for each ABC initiator (pre-HAART and on-HAART) matched to 2 visits of a non-ABC HAART user within the same cohort.
Plasma levels of high sensitivity hsCRP, D-dimer, and IL-6 were measured for each propensity score-matched patient pair at the pre-HAART and on-HAART visits using stored specimens collected in CPT heparin tubes for MACS men and CPT citrate tubes for WIHS women.
HsCRP, IL-6 and D-dimer were chosen as biomarkers because they showed important associations with mortality in the SMART study, and appeared to explain much of the association of ABC with CVD events2-4, 15, 16. HsCRP is a well established CVD risk factor in general populations, adding risk information to traditional risk factors such as lipids and blood pressure 15, 17. Levels were measured at the University of Vermont Laboratory for Clinical Biochemistry Research (LCBR) using a nephelometric immunoassay (Dade-Behring BN II), with an analytical coefficient of variance (CV) of <6%. Consensus cut-points for CVD risk are: <1.0 mg/L, low risk; 1.0-3.0 mg/L, intermediate risk; >3.0 mg/L, high risk 15,17. IL-6 is a proinflammatory cytokine with many important activities related to atherosclerosis and CVD 4. Levels have been used to explore CVD risk, and IL-6 is a particularly strong risk factor for mortality18, 19. It was measured by chemiluminescent ELISA (R&D Systems) with a CV of <14%. The LCBR multi-ethnic reference range is 0.5 pg/ml – 3.0 pg/ml. D-dimer, which results from the plasmin-mediated lysis of fibrin formed during ongoing coagulation, is an integrated marker of ongoing coagulation and fibrinolysis, and has also been shown to be a CVD risk factor 19,20. In addition, D-dimer is a risk factor for venous thrombosis 20. It was measured using an immunoturbidometric assay (Stago STA-R), with a CV of <15%. The LCBR multi-ethnic reference range is 0.07 ug/ml – 0.7 ug/ml.
Statistical Analysis
Summary statistics for markers measured at pre-HAART and on-HAART (ABC and non-ABC) visits were computed for all matched pairs and also separately for matched pairs within each cohort. Marker measurements showed considerable skewness, necessitating the use of log tranformations. Random-effects models were used to compare log-transformed plasma biomarker levels at the pre-HAART and on-HAART visits and to evaluate changes in these levels among ABC initiators as compared to non-ABC users21. Capitalizing on the repeated measurements for each individual, change was defined as the log-transformed ratio of the biomarker level at the on-HAART visit to the level at the pre-HAART visit – this is equivalent to the difference in log-transformed marker levels at the two visits. Models included random-effect terms for each pair, were weighted by the inverse of the propensity scores22, 23 and were adjusted for age, calendar year of the index visit, time elapsed between baseline and index visits, smoking status, BMI, HDL levels, race/ethnicity, HCV antibody status, ART experience at HAART initiation, CD4, HIV RNA level, cohort, and ABC exposure. Likelihood ratio tests (LRT) were used to evaluate whether or not each individual factor contributed significantly to the model fit or not. The LRT method was also used to evaluate specific pre-specified interactions between ABC exposure and: HIV RNA levels, ART experience at HAART initiation, HCV antibody status, and BMI. Lastly, we also examined the association between the change in biomarker levels and the change in log10 HIV RNA levels between the pre- and on-HAART visits using Spearman correlation coefficients. Analyses were conducted using all data and repeated among those with detectable (>80 copies/ml) values at the pre-HAART visit. All analyses were conducted in SAS version 9 (Cary, NC).
The MACS and WIHS protocols are reviewed annual by each participating institution’s institutional review board.
RESULTS
We measured hsCRP, D-dimer, and IL-6 levels in 508 propensity score matched participant pairs (ABC-exposed versus non-ABC exposed persons); 180 pairs contributed by MACS men who first reported HAART between 11/1995 and 3/2008 and 328 pairs contributed by WIHS women who first reported HAART between 9/1996 and 2/2008. The mean elapsed time between the pre-HAART and on HAART visits was 4.2 years. Characteristics included in the propensity score models as well as additional patient features are shown in Table 1 for MACS men and in Table 2 for WIHS women. In these tables, characteristics of HAART users initiating ABC are compared to eligible person-visits from all HAART users not reporting ABC use (middle columns), and to those persons selected using propensity-score matching methods (right columns). Prior to matching, MACS men who received ABC-containing HAART had significantly more pre-HAART ART-experience, a higher proportion of visits not on HAART, lower median CD4, higher median plasma HIV RNA levels, and a lower proportion of visits with HIV RNA ≤500 copies/ml when compared to MACS men who received non-ABC containing HAART. After matching, the two MACS exposure groups (ABC- and non-ABC HAART recipients) were comparable on all matching characteristics except the duration of HAART use (time from HAART initiation to on-HAART visit). MACS ABC users and non-users also had comparable nadir CD4, evidence of hypertension, history of diabetes mellitus, and report of cardiovascular disease.
Table 1.
Demographics and characteristics of abacavir users and all eligible and selected non-users in the Multicenter AIDS Cohort Study who initiated HAART from November 1995 through March 2008.
| Abacavir users
|
Abacavir non-users
|
||||
|---|---|---|---|---|---|
| (n=180 men) | Eligible (4,956 person-visits for 567 men) | Matched (n=180 men) | |||
| % or Median (IQR) | % or Median (IQR) | p-value* | % or Median (IQR) | p-value* | |
| Characteristics used for propensity scores | |||||
| Race/ethnicity | 0.790 | 0.392 | |||
| White | 76 | 77 | 82 | ||
| African-American | 14 | 15 | 12 | ||
| Hispanic\other | 10 | 8 | 6 | ||
| Age (years) | 47 (42-52) | 46 (41-51) | 0.499 | 47 (42-52) | 0.637 |
| Calendar date | 2001 (1999-2003) | 2001 (1998–2005) | 0.761 | 2001 (1999-2005) | 0.340 |
| Reported smoking | 25 | 23 | 0.515 | 24 | 0.903 |
| BMI (kg/m2) | 25 (23-27) | 24 (23–27) | 0.881 | 24 (23-27) | 0.700 |
| HDL (mg/dl) | 40 (33-46) | 42 (35-50) | 0.066 | 39 (32-46) | 0.747 |
| Unknown | 32 | 27 | 0.161 | 34 | 0.737 |
| Hepatitis C antibody positive | 6 | 9 | 0.181 | 7 | 0.660 |
| ART-experience pre-HAART | 79 | 65 | <0.001 | 73 | 0.138 |
| Duration of HAART (years) | 3.1 (1.5-5.4) | 3.0 (1.1–5.8) | 0.363 | 2.5 (0.8-5.1) | 0.002 |
| Proportion of visits not on HAART | 0 (0-0.17) | 0 (0-0.06) | <0.001 | 0 (0-0.17) | 0.915 |
| CD4+ T-cell count (cells/mm3) | 404 (272-567) | 477 (316-667) | 0.001 | 403 (246-572) | 0.916 |
| HIV RNA (copies/ml) | 1,852 (<50-27,526) | <50 (<50-2,121) | <0.001 | 1,219 (<50-40,202) | 0.674 |
| Percentage ≤ 500 copies/ml | 40 | 68 | <0.001 | 44 | 0.393 |
| Other Characteristics | |||||
| Nadir CD4 cell count (cells/mm3) | 213 (115-294) | 209 (108-299) | 0.850 | ||
| History of diabetes mellitus | 14 | 9 | 0.140 | ||
| Hypertension (SBP≥140 or DBP≥90) | 26 | 16 | 0.070 | ||
| History of Self-reported cardiovascular disease | 9 | 9 | 1.000 | ||
HAART: highly active antiretroviral therapy; IQR: interquartile range; SBP: systolic blood pressure; DBP: diastolic blood pressure
Reported p-values were obtained from χ2 tests of homogeneity for percentages and from Wilcoxon rank sum tests for medians. P-values represent statistical differences between abacavir users and nonusers (both before and after propensity score selection).
Table 2.
Demographics and characteristics of abacavir users and all eligible and selected non-users in the Women’s Interagency HIV Study who initiated HAART from September 1996 through 2008.
| Abacavir users
|
Abacavir non-users
|
||||
|---|---|---|---|---|---|
| (n=328 women) | Eligible (6,269 person-visits for 879 women) | Matched (n=328 women) | |||
| % or Median (IQR) | % or Median (IQR) | p-value* | % or Median (IQR) | p-value* | |
| Characteristics used for propensity scores | |||||
| Race/ethnicity | 0.062 | 0.752 | |||
| White | 13 | 19 | 15 | ||
| African-American | 56 | 53 | 56 | ||
| Hispanic\other | 30 | 28 | 28 | ||
| Age (years) | 42 (37-47) | 42 (36–48) | 0.900 | 42 (36-48) | 0.750 |
| Calendar date | 2001 (2000-2003) | 2001 (1999–2004) | 0.007 | 2002 (1999-2005) | 0.331 |
| Reported smoking | 46 | 44 | 0.334 | 47 | 0.937 |
| BMI (kg/m2) | 26 (23-31) | 27 (23–32) | 0.100 | 26 (22-31) | 0.466 |
| HDL (mg/dl) | 42 (33-50) | 48 (38-61) | <0.001 | 39 (31-52) | 0.684 |
| Unknown | 77 | 77 | 0.786 | 75 | 0.463 |
| Hepatitis C antibody positive | 33 | 39 | 0.062 | 35 | 0.659 |
| ART-experience pre-HAART | 82 | 78 | 0.127 | 79 | 0.374 |
| Duration of HAART (years) | 3.0 (1.5-4.8) | 2.7 (1.0–5.5) | 0.958 | 3.1 (0.9-6.0) | 0.527 |
| Proportion of visits not on HAART | 0.11 (0-0.32) | 0 (0-0.15) | <0.001 | 0.11 (0-0.33) | 0.855 |
| CD4+ T-cell count (cells/mm3) | 307 (171-464) | 413 (247-605) | <0.001 | 304 (186-481) | 0.549 |
| HIV RNA (copies/ml) | 5,100 (360–36,000) | 140 (<80–5,200) | <0.001 | 4,500 (190 - 37,000) | 0.416 |
| Percentage ≤ 500 copies/ml | 28 | 58 | <0.001 | 28 | 0.931 |
| Other Characteristics | |||||
| Nadir CD4 cell count (cells/mm3) | 167 (79-272) | 191 (90-279) | 0.220 | ||
| History of diabetes mellitus | 16 | 10 | 0.030 | ||
| Hypertension (SBP≥140 or DBP≥90) | 17 | 14 | 0.210 | ||
| History of self-reported cardiovascular disease | 5 | 5 | 0.860 | ||
HAART: highly active antiretroviral therapy; IQR: interquartile range; SBP: systolic blood pressure; DBP: diastolic blood pressure
Reported p-values were obtained from χ2 tests of homogeneity for percentages and from Wilcoxon rank sum tests for medians. P-values represent statistical differences between abacavir users and nonusers (both before and after propensity score selection).
Prior to ABC initiation, WIHS women who received ABC-containing HAART had lower median serum HDL cholesterol levels, a higher proportion of visits during which they were not receiving HAART, lower median CD4, higher median plasma HIV RNA levels, and a lower proportion of visits with HIV RNA ≤500 copies/ml when compared to non-ABC HAART person-visits. After matching, WIHS exposure groups were comparable on all matching characteristics, nadir CD4, evidence of hypertension, and report of cardiovascular disease. However, a higher proportion of ABC users had a history of diabetes mellitus (16% vs. 10%, p=0.03).
Comparisons of MACS men and WIHS women illustrated cohort differences other than biological sex: WIHS women were more likely to be non-white, HCV co-infected, younger, have lower CD4, greater median BMI, and a history of diabetes mellitus (p<0.01 for all comparisons). For these reasons, while the main analysis was conducted on the combined data and adjusted for cohort, we also undertook analyses stratified by cohort.
Figure 2 displays the geometric mean marker levels for the combined and individual cohorts. Pre-HAART biomarker levels in the ABC-exposed and –unexposed groups are displayed as a single mean since they were similar and nearly all were statistically indistinguishable; the only exception was observed among WIHS participants for whom hsCRP levels were slightly higher among women who subsequently used ABC (p=0.04). Substantial mean increases in hsCRP and reductions in D-dimer levels were seen when comparing pre-HAART to on-HAART time points, for both the overall group (28% and -27%) and for MACS men (28% and -31%) and WIHS women (29% and -24%) separately (p<0.01 for all). IL-6 levels, however, declined in MACS men but not in WIHS women, with the latter demonstrating low IL-6 levels at both visits (geometric mean was 1.78 pg/ml pre-HAART and 1.89 pg/ml on-HAART).
Figure 2.
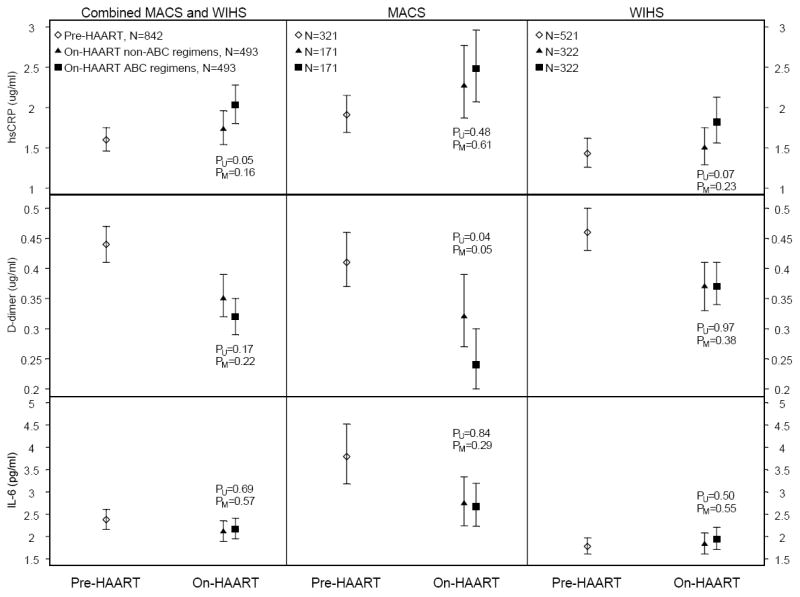
Pre and on-HAART biomarker geometric means and 95% confidence intervals overall and stratified by cohort
These trends were seen in both ABC and non-ABC HAART users. As described above, we conducted two types of analyses to compare these groups. First, we compared mean biomarker levels of persons receiving ABC HAART with levels from the corresponding matched visit of persons receiving non-ABC HAART. Figure 2 shows p-values from univariate (PU) and multivariate (PM) random-effects models for comparisons of biomarker levels at the on-HAART visit between ABC and non-ABC users. The only significant adjusted difference was for D-dimer among MACS men, for whom ABC users had lower levels than non-ABC users (PM = 0.05).
Our second analysis evaluated individual biomarker level changes between pre-HAART and on-HAART time points. Table 3 depicts changes from the pre- to on-HAART visits as percent differences - (on-HAART level – pre-HAART level)/pre-HAART level)*100, i.e. the percent increase or decrease in marker levels relative to the pre-HAART level as well as adjusted mean differences between non-ABC and ABC HAART users from random-effects models. Table 3A shows results from all 508 matched pairs and Table 3B shows results among a subgroup of 184 individuals who were ART-naïve at HAART initiation (not matched). Among all pairs, there were no significant differences in pre-HAART to on-HAART biomarker changes between exposure groups after adjustment. Results were similar for each cohort separately, except that within the MACS there was a marginally greater reduction in D-dimer levels among ABC HAART initiators compared to non-ABC HAART initiators (adjusted difference in change of -24%, p=0.06).
Table 3.
Changes in plasma biomarker levels from pre-HAART to on-HAART by abacavir exposure and cohort among (A) all 508 patient pairs and (B)184 persons who were ART-naïve at HAART initiation.
| A. All pairs (N=508) | B. Individuals ART-Naïve at HAART Initiation (N=184) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Change from Pre-HAART Visit | Change from Pre-HAART Visit | ||||||||
| Non-ABC HAART | ABC HAART | Adjusted Difference of Change | p-value1 | Non-ABC HAART | ABC HAART | Adjusted Difference of Change | p-value2 | ||
| hsCRP | |||||||||
| MACS | 19% | 33% | 4% | 0.81 | -1% | -6% | 6% | 0.88 | |
| WIHS | 21% | 16% | -2% | 0.79 | 41% | -3% | -38% | 0.14 | |
| Combined | 21% | 22% | -1% | 0.93 | 20% | -4% | -20% | 0.35 | |
| D-dimer | |||||||||
| MACS | -23% | -41% | -24% | 0.06 | -38% | -57% | -23% | 0.34 | |
| WIHS | -19% | -20% | 7% | 0.36 | -28% | -26% | -8% | 0.68 | |
| Combined | -20% | -28% | -7% | 0.29 | -33% | -40% | -7% | 0.67 | |
| IL-6 | |||||||||
| MACS | -29% | -27% | 7% | 0.72 | -32% | -63% | -15% | 0.69 | |
| WIHS | -2% | 2% | -3% | 0.72 | 8% | -17% | -38% | 0.07 | |
| Combined | -12% | -9% | -9% | 0.24 | -12% | -38% | -21% | 0.26 | |
From linear mixed models of log-transformed on-HAART to pre-HAART ratios with random effect terms for each pair adjusted for ABC HAART exposure, age, calendar year, time between baseline and index visits, smoking, BMI, HDL, CD4 cell count, HIV RNA, and cohort.
From linear mixed models of log-transformed on-HAART to pre-HAART ratios with random effect terms for each individual adjusted for ABC HAART exposure, age, calendar year, time between baseline and index visits, smoking, BMI, HDL, CD4 cell count, HIV RNA, and cohort for WIHS (124 observations for 105 individuals) and combined models (204 observations for 184 individuals). Models for MACS were adjusted for the same factors but did not include random effects terms (80 observations for 79 individuals).
We evaluated four potential modifiers of the association between ABC use and biomarker levels: HIV RNA levels, ART experience at HAART initiation, HCV antibody status, and BMI. Only ART experience status at the time of HAART initiation was a significant modifier. While this group is a minority in the MACS and WIHS, the vast majority of patients in the current treatment era are ART naïve at HAART initiation. The first and second columns of Table 3B show how observed changes from the pre-HAART visit differ for non-ABC and ABC HAART users in the ART-naïve subgroup compared to changes for the complete matched sample shown in the first and second columns of Table 3A. As in the complete matched sample, the estimated adjusted differences of change do not differ significantly between non-ABC and ABC HAART users in the ART naïve subgroup. However, in this subgroup there was a trend toward greater reductions in biomarkers at the on-HAART visit among ABC HAART recipients (Table 3B column 3 compared to Table 3A column 3). We evaluated whether ABC recipients were more likely to ever have had a history of renal dysfunction (estimated GFR<60) using the Modified Diet in Renal Disease (MDRD) equation. Among WIHS women there was no difference in history of renal dysfunction when stratifying by ABC exposure (data not shown). Among MACS men, there was a higher proportion of ABC users with a history of GFR<60 than non-ABC users (9% for ABC users vs. 4% for non-ABC users, p=0.04). However, if persons who had only one GFR estimate <60 (with all other GFR estimates >60) were re-categorized as not having renal dysfunction, differences in the proportion of MACS men with renal dysfunction in the ABC versus non-ABC groups disappeared (3% vs. 2%, respectively, p=0.46).
Prior to HAART initiation, WIHS participants had lower hsCRP and IL-6 levels than MACS participants (1.39 vs. 1.88, p<0.01; 1.88 vs. 3.75, p<0.01) but similar D-dimer levels (0.46 vs. 0.41, p=0.10). At the pre-HAART visit, there were 6 WIHS women who were pregnant, 1 (0.3%) in the non-ABC group and 5 (2%) in the ABC group, p=0.10. At the on-HAART visit there were 16 women who were pregnant, 7 (2%) in the non-ABC group and 9 (3%) in the ABC group, p=0.63. No differences in any biomarkers levels were seen at either visit when comparing pregnant to non-pregnant women.
Finally, we examined the association of change in plasma HIV RNA levels between the two visits (mean change = -1.4 log10 copies/ml) and changes in biomarker levels using Spearman correlation coefficients. Greater reductions in plasma HIV RNA were significantly correlated with reductions in D-dimer (r = 0.14, p < 0.01) and IL-6 (r = 0.12, p < 0.01) but not with changes in hsCRP (r = -0.01, p=0.70). Results were similar when restricted to persons with HIV RNA values above the limit of detection (80copies/ml) at the pre-HAART visit.
DISCUSSION
In the WIHS and the MACS, we observed changes in the levels of pro-inflammatory biomarkers hsCRP, IL-6 and D-dimer between the baseline (pre-HAART) and index (on HAART) visits, a mean elapsed time of 4.2 years. D-dimer and IL-6 levels decreased, hsCRP levels increased. These changes were comparable among persons who initiated ABC versus non-ABC containing HAART and reflected, at least in part, the effect of HAART in reducing plasma HIV viral burden.
This work should not be interpreted as either refuting or supporting hypotheses suggesting associations between recent use of abacavir (or any other ART for that matter) and cardiovascular disease in general or specific cardiovascular disease endpoints. We did not undertake evaluations of use of ABC and cardiovascular disease nor the assessment of plasma biomarker levels in relationship to specific clinical events. Such evaluations necessarily require evaluations of large numbers of patients followed over long periods of time during which sufficiently substantial numbers of clinical endpoints occur so as to permit meaningful comparisons based upon exposure. However, our work does suggest that, if recent ABC use is indeed associated with increased risk for adverse cardiovascular events, systemic inflammation is not likely the sole or primary means by which its effects are mediated. It remains entirely possible, as preliminary work from other groups has suggested, that abacavir’s effects upon vasculature may be mediated by other mechanisms such as reduced endothelial function, i.e. flow-mediated arterial dilation24.
Changes in biomarker levels observed from the pre-HAART to the on-HAART visit suggest that there may be an effect upon biomarkers exerted by HAART use itself that, at least in terms of the observed reductions in D-dimer levels (as well as the reductions in IL-6 levels seen among MACS men, a group in whom better virologic control was achieved overall compared to WIHS women), corroborates observations that HIV itself, or more specifically HIV plasma viremia, is associated with plasma elevations in inflammatory biomarkers25 Indeed, when we examined the association of HIV RNA changes with biomarker level changes between the two visits, we verified that greater HIV RNA reductions were significantly correlated with reductions in D-dimer and IL-6.
Observed increases in plasma levels of hsCRP from the pre-HAART to on-HAART visit are more difficult to explain and may reflect the influence of multiple other non-HIV-related cofactors and co-morbidities which themselves can increase biomarker levels. For example, among WIHS participants, the extent to which higher prevalences of chronic co-infection with HCV (more than one-third of participants overall), smoking (nearly one half of participants), obesity, or recreational drug use may have accounted for observed differences in plasma hsCRP levels is unclear26. Similarly, among MACS men, who are on average older than WIHS women, non-HIV cardiovascular comorbidities such as hypertension (seen in one quarter of participants) or even aging itself may have exerted an effect on hsCRP levels. Data exist from the MACS demonstrating a positive, though modest, association between plasma hsCRP levels and plasma lipids, including non-HDL cholesterol and LDL27, and age-associated hsCRP increases 28.
In analyses limited to persons whose first HAART was also the first exposure to antiretroviral therapy, a group representative of contemporary HAART initiators, there were no significant differences in pre-HAART to on-HAART biomarker level changes when comparing ABC to non-ABC HAART users, though adjusted biomarker level changes differences were more pronounced in this “ART naïve prior to HAART” group than in the overall group.
Of note, despite biomarker level changes seen from pre-HAART to on-HAART visits and the cohort-based differences observed, mean absolute plasma levels of each biomarker were within generally accepted “normal” ranges for healthy adults, both at baseline and index visits. In this regard, it becomes somewhat more difficult to assign a clinical relevance to HAART-associated changes or cohort-associated differences in observed biomarker levels, particularly since we did not (and could not because of a low number of observed events) correlate our findings with risks for cardiovascular events.
There were cohort-based differences in biomarker levels and changes in levels (in the case of IL-6) over time. WIHS women had significantly lower IL-6 and CRP levels at the baseline visit and MACS men experienced greater IL-6 decreases. It is difficult to surmise upon the extent to which these differences are consistent, predictable, and based upon gender versus reflective of differences intrinsic to these specific cohorts. MACS men and WIHS women differed in important ways other than gender. WIHS women were younger, more likely to be non-white, more likely to be chronically co-infected with hepatitis C virus, had higher BMI’s, lower CD4 and higher HIV RNA levels at both baseline and index visits than MACS men. Future work will need to further explore whether gender-based biomarker level differences reliably exist and the extent to which cohort-based differences in other non-HIV factors modify these differences.
There were other limitations to our analysis. “Official" reference ranges for the plasma biomarkers measured do not exist, and values obtained may vary depending upon several factors, including assay reagents and biological samples used. Furthermore, the statistical models that we employed do not account for measurements that outside assay ranges; however, given the small proportion of observations that were outside of “normal “ ranges, we believe it unlikely that they would have influenced our interpretations.
Obviously, we did not evaluate mechanisms other than increases in systemic inflammation (or, in the case of D-dimer, coagulation and fibrinolysis) by which abacavir use may enhance cardiovascular disease risk. Several recent reports29,30 have even suggested an independent role of HIV infection as a risk for atherosclerosis, with the magnitude of increased risk unclear but possibly similar to that which has been attributed to smoking, diabetes mellitus, or advancing age 31.
In summary, changes in plasma levels of the proinflammatory biomarkers hsCRP, D-dimer, and IL-6 were not observed in association with ABC-based HAART use. Pathophysiologic mechanisms other than increases in systemic inflammation should be explored to explain reported increased rates of myocardial infarctions observed in association with ABC use. We did note that HAART use in general was associated with decreases in D-dimer and IL-6, which may reflect reduced systemic inflammation consequent to HAART-induced HIV suppression, and increases in hsCRP, which are more difficult to explain. Our observed cohort-based differences in biomarker levels beg for further exploration of the effects of gender and of other non-HIV-related co-morbidities upon systemic inflammation in HIV-infected persons.
Acknowledgments
Funding Source
The National Institite of Allergy and Infectious Diseases (NIAID) supported the MACS and the WIHS data collection and analysis through grants with the participating institutions.
Role of Authors
Dr. Palella: study design, data interpretation, manuscript preparation
Dr. Gange: study design, data analysis, manuscript preparation
Ms. Benning: study design, data analysis, manuscript preparation
Dr. Jacobson: study design, data analysis
Dr. Kaplan: data interpretation, manuscript preparation
Dr. Landay: study design, data interpretation
Dr. Tracy: biomarker assay performance, data interpretation, manuscript preparation
Dr. Elion: study design, data interpretation, manuscript preparation
References
- 1.D;A:D Study Group. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008 Apr 26;371(9622):1417–26. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The SMART/INSIGHT and the D:A:D Study Investigators. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. AIDS. 2008;22(14):F17–24. doi: 10.1097/QAD.0b013e32830fe35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lundgren J, Reiss P, Worm S, Weber R, El-Sadr W, De Wit S, et al. Risk of Myocardial Infarction with Exposure to Specific ARV from the PI, NNRTI, and NRTI Drug Classes: The D:A:D. Study 16th Conference on Retroviruses and Opportunistic Infections (CROI 2009); February 8 - 11, 2009; Montreal, Canada. Abstract 44LB. [Google Scholar]
- 5.Lang S, Mary-Krause M, Cotte L, 2, Gilquin G, Partisani M, Simon A, et al. Impact of Specific NRTI and PI Exposure on the Risk of Myocardial Infarction: A Case-Control Study Nested within FHDH ANRS CO4. 16th Conference on Retroviruses and Opportunistic Infections (CROI 2009); February 8 - 11, 2009; Montreal, Canada. Abstract 43LB. [Google Scholar]
- 6.Cooper D, Bloch M, Humphries A, Amin J, Baker D, Emery S, et al. Simplification with Fixed-dose Tenofovir/Emtricitabine or Abacavir/Lamivudine in Adults with Suppressed HIV Replication: The STEAL Study, a Randomized, Open-label, 96-Week, Non-inferiority Trial. 16th Conference on Retroviruses and Opportunistic Infections (CROI 2009); February 8 - 11, 2009; Montreal, Canada. Abstract 576. [Google Scholar]
- 7.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay C. 5th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; July 19-22, 2009; Cape Town, South Africa. Abst. TUPEB175. [Google Scholar]
- 8.Belloso W, HOrellana LC, Losso MH, Grinsztejn B, Veloso VG, 4, Ismerio Moreira R, et al. Predictive factors of serious vascular events in HIV patients in LATINA (LATInamerican Network on AIDS). 5th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; July 19-22, 2009; Cape Town, South Africa. Abstr.TUPEB116. [Google Scholar]
- 9.Benson C, Ribaudo H, Zheng E, Koletar S, Smurzynski M, Bosch R, et al. No Association of Abacavir Use with Risk of Myocardial Infarction or Severe Cardiovascular Disease Events: Results from ACTG A5001. 16th Conference on Retroviruses and Opportunistic Infections (CROI 2009); February 8 - 11, 2009; Montreal, Canada. Abstract 721. [Google Scholar]
- 10.McComsey G, Smith K, Patel P, Bellos N, Sloan L, Lackey P, et al. Similar Reductions in Markers of Inflammation and Endothelial Activation after Initiation of Abacavir/Lamivudine or Tenofovir/Emtricitabine: The HEAT Study. 16th Conference on Retroviruses and Opportunistic Infections (CROI 2009); February 8-11, 2009; Montreal, Canada. Abst. 732. [Google Scholar]
- 11.Bedimo R, Westfall A, Drechsler H, Tebas P. Abacavir use and risk of acute myocardial infarction and cerebrovascular disease in the HAART era. 5th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; July 19-22, 2009; Cape Town, South Africa. Abst. MOAB202. [Google Scholar]
- 12.Martinez E, Larrousse M, Perez I, Lonca M, Podzamczer D, Gutierrez F, et al. No evidence for recent abacavir/lamivudine use in promoting inflammation, endothelial dysfunction, hypercoagulability, or insulin resistance in virologically suppressed HIV-infected patients: a substudy of the BICOMBO randomized clinical trial (ISRCTN6189). 5th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; July 19-22, 2009; Cape Town, South Africa. Abst. MOAB203. [Google Scholar]
- 13.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 14.Bacon MC, von Wyl V, Alden C, et al. The Women’s Interagency HIV Study: An observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 16.Schuett H, Luchtefeld M, Grothusen C, Grote K, Schieffer B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb Haemost. 2009;102(2):215–222. doi: 10.1160/TH09-05-0297. [DOI] [PubMed] [Google Scholar]
- 17.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH., Jr Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101(15):1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 19.Lowe GD, Sweetnam PM, Yarnell JW, Rumley A, Rumley C, Bainton D, et al. C-reactive protein, fibrin D-dimer, and risk of ischemic heart disease: the Caerphilly and Speedwell studies. Arterioscler Thromb Vasc Biol. 2004;24(10):1957–1962. doi: 10.1161/01.ATV.0000141842.27810.a9. [DOI] [PubMed] [Google Scholar]
- 20.Cushman M, Folsom AR, Wang L, Aleksic N, Rosamond WD, Tracy RP, et al. Fibrin fragment D-dimer and the risk of future venous thrombosis. Blood. 2003;101(4):1243–1248. doi: 10.1182/blood-2002-05-1416. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum PR, Rubin Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. The American Statistician. 1985;39:33–38. [Google Scholar]
- 22.Laird N, Ware J. Biometrics. 1982 Dec;38(4):963–974. [PubMed] [Google Scholar]
- 23.Rosenbaum PR. Model-Based Direct Adjustment. Journal of the American Statistical Association. 1987 Jun;82(398):387–394. [Google Scholar]
- 24.Hsue P, Wu Y, Schnell A, Ganz P, Hunt P, Hatano H, et al. Association of Abacavir and HIV Disease Factors with Endothelial Function in Patients on Long-term Suppressive ART. 16th Conference on Retroviruses and Opportunistic Infections (CROI 2009); February 8 - 11, 2009; Montreal, Canada. Abstract 723. [Google Scholar]
- 25.Henry K, Kitch D, Dube M, Zackin R, Parker R, Sprecher D, et al. Adult AIDS Clinical Trials Group. C-reactive protein levels over time and cardiovascular risk in HIV-infected individuals suppressed on an indinavir-based regimen: AIDS Clinical Trials Group 5056s. AIDS. 2004;18:2434–2437. [PubMed] [Google Scholar]
- 26.Reingold J, Wanke C, Kotler D, Lewis C, Tracy R, Heymsfield S, et al. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. AIDS. 2008 Jun 1;48(2):142–8. doi: 10.1097/QAI.0b013e3181685727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riddler S, Smit E, Cole S, Li R, Chmiel J, Dobs A, et al. Impact of HIV Infection and HAART on Serum Lipids in Men. JAMA. 2003 Jun;289:2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 28.Lau B, Sharrett R, Kingsley L, Post W, Palella F, Visscher B, et al. C-Reactive Protein Is a Marker for Human Immunodeficiency Virus Disease Progression. Arch Intern Med. 2006 Jan;166:64–70. doi: 10.1001/archinte.166.1.64. [DOI] [PubMed] [Google Scholar]
- 29.Kingsley L, Cuervo-Rojas J, Munoz A, Palella F, Post W, Witt M, et al. Subclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: Multicenter AIDS Cohort Study. AIDS. 2008 Aug 20;22(13):1589–1599. doi: 10.1097/QAD.0b013e328306a6c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan R, Kingsley L, Gange S, Benning L, Jacobson L, Lazar J, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008 Aug 20;22(13):1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grunfeld C, Delaney J, Wanke C, Currier J, Scherzer R, Biggs M. HIV Infection Is an Independent Risk Factor for Atherosclerosis Similar in Magnitude to Traditional Cardiovascular Disease Risk Factors. 16th Conference on Retroviruses and Opportunistic Infections (CROI 2009); February 8 - 11, 2009; Montreal, Canada. Abstract 146. [Google Scholar]


