Abstract
The angiopoietin/Tie2 system has been identified as the second vascular-specific receptor tyrosine kinase system controlling vessel assembly, maturation, and quiescence. Angiopoietin-2 (Ang-2) is prominently up-regulated in the host-derived vasculature of most tumors, making it an attractive candidate for antiangiogenic intervention. Yet, the net outcome of Ang-2 functions on tumor angiogenesis is believed to be contextual depending on the local cytokine milieu. Correspondingly, Ang-2 manipulatory therapies have been shown to exert protumorigenic as well as antitumorigenic effects. To clarify the role of Ang-2 for angiogenesis and tumor growth in a definite genetic experimental setting, the present study was aimed at comparatively studying the growth of different tumors in wild-type and Ang-2–deficient mice. Lewis lung carcinomas, MT-ret melanomas, and B16F10 melanomas all grew slower in Ang-2–deficient mice. Yet, tumor growth in wild-type and Ang-2–deficient mice dissociated during early stages of tumor development, whereas tumor growth rates during later stages of primary tumor progression were similar. Analysis of the intratumoral vascular architecture revealed no major differences in microvessel density and perfusion characteristics. However, diameters of intratumoral microvessels were smaller in tumors grown in Ang-2–deficient mice, and the vasculature had an altered pattern of pericyte recruitment and maturation. Ang-2–deficient tumor vessels had higher pericyte coverage indices. Recruited pericytes were desmin and NG2 positive and predominately α-smooth muscle actin negative, indicative of a more mature pericyte phenotype. Collectively, the experiments define the role of Ang-2 during tumor angiogenesis and establish a better rationale for combination therapies involving Ang-2 manipulatory therapies.
Introduction
Cancer is a major health problem in the developed countries. One in four deaths was estimated to be caused by cancer in the United States in 2005, surpassing heart diseases as the leading cause of death for people below 85 years of age (1). Surgery, chemotherapy, and radiotherapy are the three primary therapeutic modalities in the fight against cancer. To complement established antitumor therapies, antiangiogenesis has, in the last few years, emerged as the first effective antistroma therapy (2). Vascular endothelial growth factor (VEGF), the master switch of the angiogenic cascade, has been validated as the first target for antiangiogenic intervention (3). Several strategies targeting the VEGF signaling pathway have been developed, including neutralizing antibodies to VEGF (bevacizumab) or VEGF receptors (VEGFR; DC101), soluble VEGFR/VEGFR hybrids (VEGF-Trap), and small molecular weight tyrosine kinase inhibitors of VEGFRs (BAY43-9006, SU11248, ZD6474, AZD2171, PTK787, and others; ref. 4).
Antiangiogenesis in combination with chemotherapy has rapidly become part of standard tumor therapy and is now used in the treatment of different solid tumors, including colorectal tumors, renal cell carcinomas, mammary tumors, and lung tumors. Yet, the efficacy of antiangiogenic treatment is limited, and antiangiogenic therapies are far from being exploited to their fullest extent. The search for second generation angiogenesis inhibitors concentrates on the identification of drug candidates that would combine well with anti-VEGF or anti-VEGFR therapies.
The angiopoietins have been identified as ligands for the vascular receptor tyrosine kinase Tie2 (5, 6). They control later stages of the angiogenic cascade related to vessel assembly, maturation, and quiescence (7). The angiopoietin family comprises three members: angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-2), and angiopoietin-3/4 (Ang-3/4; refs. 5, 8, 9). Ang-1 is expressed by pericytes, smooth muscle cells, and fibroblasts and acts in a paracrine manner. In contrast, Ang-2 is expressed by endothelial cells and stored in Weibel-Palade bodies from where it can be rapidly released on stimulation to act as an autocrine regulator of endothelial cell functions (5, 6, 10). Both, Ang-1 and Ang-2 are ligands of the endothelial cell tyrosine kinase receptor, Tie2/Tek (11), as well as for integrin receptors (12-14). Ang-1 and Ang-2 have been described to exert opposing functions during vessel development. Ang-1–induced Tie2 activation transduces survival signals and leads to vessel stabilization and maturation (8). In turn, Ang-2 acts as a vessel destabilizing agent that induces permeability and leads to dissociation of cell-cell contacts in cultured endothelial cells (15). Genetic experiments have solidly established Ang-2 as an antagonistic Tie2 ligand (5). Yet, the effects of Ang-2 on endothelial cell seem to be contextual because, under certain conditions, it seems to be able to act as an activator of Tie2 signaling (16).
In line with the Ang-2 contextuality model, the effects of Ang-2 on endothelial cell during angiogenesis seem to be dependent on the local cytokine milieu (17). In the presence of angiogenic activity, Ang-2 promotes angiogenesis. In contrast, in the absence of angiogenic activity, Ang-2 induces vessel regression (18, 19). As such, Ang-2 seems to be a facilitator of endothelial functions rather than exerting effector functions on its own. This concept has recently also been validated by showing that Ang-2 is required for the inflammatory program of endothelial cells. Yet, it exerts no proinflammatory effects on its own (20).
Analytic experiments in human tumors have provided evidence that Ang-2 may be associated with tumor progression. Ang-2 expression or a high Ang-2/Ang-1 ratio in the tumor stroma correlates with increased invasiveness in colon, prostate, breast, brain, and gastric carcinomas (21-25). Corresponding manipulatory experiments have shown that anti–Ang-2 therapies using peptide-Fc fusion proteins, antibodies, or an Ang-2–specific RNA aptamer inhibit tumor growth in xenograft as well as syngeneic mouse models (26, 27). These experiments suggest that Ang-2 may be an attractive target for antiangiogenic tumor therapies. Yet, the role of Ang-2 during tumor angiogenesis is mechanistically poorly understood. To study the role of Ang-2 during tumor initiation and primary tumor progression, the present study was aimed at comparatively studying the growth of syngeneic tumors in wild-type (WT) and Ang-2–deficient mice. Given that, in most tumors, Ang-2 is almost exclusively host derived and produced by the tumor-associated vasculature (7, 28), the experiments thereby facilitated the mechanistic analysis of Ang-2 function during tumor growth using a definite genetic experimental approach. The experiments surprisingly showed that Ang-2 affects the early stages of tumor formation but is dispensable for the later stages of tumor progression.
Materials and Methods
Animals
Heterozygous Ang-2/LacZ mice were kindly provided by Regeneron Pharmaceuticals. Mice were housed and maintained in the institutional animal facility. Ang-2–deficient mice bred in the C57/Bl6 background (20) and their WT littermates were used between 8 and 10 wk of age. Genotypes of WT and Ang-2–deficient mice were confirmed by PCR. All experiments were approved by the institutional and governmental Animal Care and Use Committees [RP Freiburg (35-9185.82/3/296) and RP Karlsruhe (35-9185.81/G-15/07)].
Tumor implantation
Lewis lung carcinoma (LLC) cells and B16F10 melanoma cells were obtained from American Type Culture Collection. Murine MT-ret melanoma cells were isolated and established as cell lines from MT-ret melanoma bearing mice (29). LLC cells (105), B16F10 cells (105), and MT-ret cells (105) suspended in 200 μL Matrigel (BD Matrigel Matrix, BD Biosciences)/PBS (1:1) were injected s.c. in the left and right flanks of 8- to 10-wk-old WT and Ang-2–deficient mice using a 29-gauge needle syringe. Tumor growth was quantified by caliper measurements every 2nd or 4th day. Tumor volume (in cubic centimeters) was calculated by measuring with a calliper the largest diameter (A) and its perpendicular (B) according to the formula, 0.5 × A × B2. Mice were sacrificed at day 7, day 12, or when the first tumor within an experimental group had grown to 2 cm3. Tumors were weighed and processed for morphologic analysis. For perfusion studies, 150 μg/150 μL lectin (FITC-labeled Bandeiraea simplicifolia; Sigma) was injected through the tail vein of mice 10 min before sacrifice. A perfusion index was quantified as the percentage of lectin-positive vessels per CD31-positive vessel in each microscopic field.
Immunohistochemistry
The tumor vasculature in 8-μm cryosections was detected by antimouse CD31 staining (1:500 dilution; BD Pharmingen) and a rat anti-mouse IgG conjugated with Alexa 546 (Invitrogen). Five to eight random fields in each cryosection (three sections per tumor, five to six tumors per group) were analyzed. Vessels were quantified and microvessel density (MVD) was calculated as vessels per square millimeter. Thereafter, WT values were set as 100% and relative values for Ang-2–deficient mice were calculated accordingly. For vessel diameter analysis, 50 vessels in each cryosection were analyzed and the widest diameter from each vessel was measured.
Pericytes were detected with four different antibodies: a rabbit anti-desmin polyclonal antibody (Abcam) detected by goat anti-rabbit IgG GAR Cy3 antibody (Invitrogen), a rabbit anti-mouse polyclonal NG2 antibody (Chemicon) detected by goat anti-rabbit Alexa 546 (Invitrogen), a rabbit antihuman monoclonal PDGFR-β antibody (Cell Signaling Technology) detected by goat antirabbit IgG GAR Cy3 antibody (Invitrogen), and a mouse anti–smooth muscle actin (SMA) directly labeled with Cy3 antibody (Sigma). Three to five random fields were analyzed in each section (three sections per tumor, six tumors per group).
Apoptotic cells in tissue sections were detected by MEBSTAIN-Apoptosis-Kit II (MBL International). An apoptotic index was quantified as the percentage of terminal deoxyribonucleotidyl transferase–mediated dUTP nick end labeling–positive area in each field.
Cell proliferation was detected with a monoclonal rat anti-mouse Ki-67 antibody (clone TEC-3, DAKO). A Ki-67 labeling index was quantified as the percentage of Ki-67–positive nuclei in each field.
Statistical analysis
All results were expressed as mean ± SE unless otherwise indicated. Differences between experimental groups were analyzed by unpaired Student’s t test. P < 0.05 was considered statistically significant.
Results
Host-derived Ang-2 promotes tumor growth
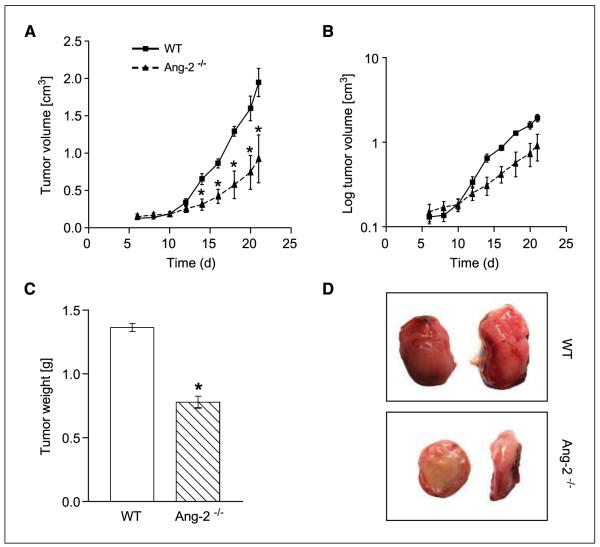
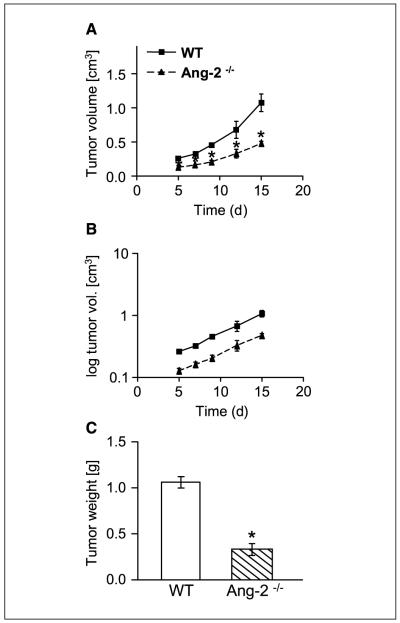
Up-regulation of host-derived Ang-2 has been shown in several types of tumors (19, 28). To address the contribution of host-derived Ang-2 toward tumor development, we comparatively studied the growth of syngeneic LLC in WT and Ang-2–deficient mice. LLC cells were s.c. implanted and tumor growth was monitored over time. In situ hybridization as well as lacZ marker staining in Ang-2 null mice confirmed the well-established expression of Ang-2 by tumor-associated endothelial cells, with most pronounced expression in the tumor periphery and in the hypoxic zone around necrotic tumor areas (data not shown). The absence of host-derived Ang-2 significantly inhibited LLC tumor growth (Fig. 1A). Yet, when plotting log-transformed tumor growth curves to assess tumor growth rates, it became evident that the tumor growth rate curves of LLC tumors grown in WT and Ang-2–deficient mice diverged between day 7 and day 12 of tumor inoculation when they had grown to 0.2 to 0.4 cm3 in size. Tumor growth rates at later time points were essentially identical as evidenced by parallel tumor growth rate curves (Fig. 1B). Growth of LLC as assessed by tumor weight at the end of the experiment was significantly reduced in Ang-2–deficient mice compared with WT mice (Fig. 1C). Yet, tumor cell proliferation and apoptosis indices during early and late stages of tumor growth were not altered in tumors grown in Ang-2–deficient mice compared with tumors grown in WT mice (data not shown), suggesting an alteration in overall tumor pattern and/or stromal organization rather than a direct or indirect effect on growth behavior of the tumor cells. This was compatible with the intriguing observation that tumors in Ang-2–deficient mice grew consistently flatter and less cuboidal than tumors grown in WT mice (Fig. 1D), indicative of an overall perturbed tumor growth pattern. Collectively, the experiments suggested that the presence of host-derived Ang-2 promoted tumor growth at an early time window of tumor development but not at later stages of tumor progression.
Figure 1.
Growth of LLC in WT and Ang-2–deficient mice. LLC tumors grew significantly slower in Ang-2–deficient mice (A). Tumor growth dissociated during early tumor growth when the tumors had grown to 0.2 to 0.4 cm3. Thereafter, tumor growth rates were almost identical as evidenced by essentially parallel curves of log-transformed tumor growth data (B). Total tumor weight was reduced in tumors grown in Ang-2–deficient mice (C). Yet, the differences in tumor weight at the end of the experiment resulted mostly from the dissociating exponential growth curves as a consequence of a dissociation of tumor growth curves during early stages of tumor growth. Tumors grown in WT mice grew cuboidal with almost spherical three-dimensional characteristics. In contrast, tumors grown in Ang-2–deficient mice had a flattened appearance (D). *, P < 0.001, compared with WT mice.
Ang-2–dependent effects on pericyte coverage and maturation
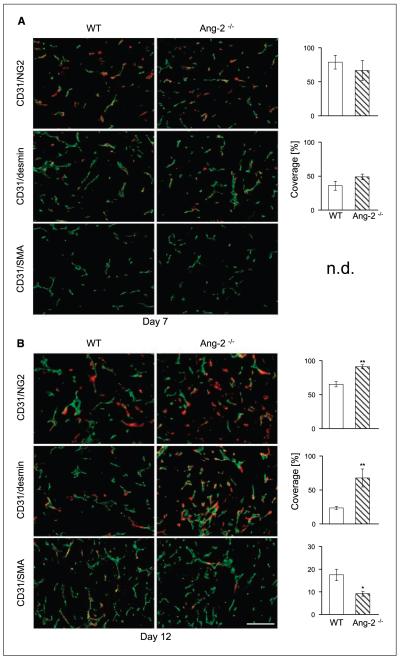
To study the consequences of Ang-2 deficiency during early tumor growth, we comparatively analyzed day 7 and day 12 LLC tumors grown in WT and Ang-2–deficient mice. MVD counts and vessel diameter were not significantly altered in day 7 and day 12 LLC tumors grown in WT and Ang-2–deficient mice (data not shown). Given the predominant role of the angiopoietin/Tie system in controlling mural cell recruitment and vessel maturation (5, 7, 8), we next focused on the analysis of mural cell recruitment. We quantitatively analyzed toward this end the presence of microvessel coverage by platelet-derived growth factor receptor-β (PDGFR-β)–, desmin-, NG2-, and α-SMA–positive mural cells. The percentage of desmin-, NG2-, and PDGFR-β–positive mural cells was similar in LLC tumors grown in WT and Ang-2–deficient mice in day 7 tumors (Fig. 2A; Supplementary Fig. S1). Coverage with PDGFR-β–positive mural cells was highest (>90%). Yet, high-resolution microscopic analysis revealed that PDGFR-β expression not only was restricted to periendothelial mural cells but also overlapped with CD31 expression (Supplementary Fig. S1), confirming previous findings that PDGFR-β is not exclusively expressed by mural cells but is also expressed by angiogenic endothelial cells (30). PDGFR-β expression was therefore included in all subsequent analyses but not strictly considered as a selective mural cell marker. Unlike PDGFR-β, expression of NG2 (60–70% positive microvessel) and desmin (30–40% positive microvessel) was restricted to mural cells and did not overlap with CD31 staining. α-SMA–positive mural cells were not detectable at day 7 of tumor growth (Fig. 1A). The distinct quantitative differences in mural cell marker positivity seemed to reflect a hierarchy of marker expression during mural cell recruitment rather than marker cell heterogeneity. This became even more evident when analyzing day 12 tumors, which exhibited a distinctly different pattern of mural cell recruitment and corresponding marker expression. As in day 7 tumors, PDGFR-β–positive microvessel staining was present in similarly high abundance in tumors grown in WT and Ang-2–deficient mice (Supplementary Fig. S1). In contrast, day 12 LLC tumors grown in Ang-2–deficient mice had significantly increased coverage of desmin- and NG2-positive pericytes (Fig. 2B). Unlike day 7 tumors, α-SMA–positive mural cells were detectable. Yet, the percentage of α-SMA–positive mural cells was higher in tumors grown in WT mice than in tumors of Ang-2–deficient mice (Fig. 2B).
Figure 2.
Effect of Ang-2 deficiency on mural cell recruitment and maturation during early stages of LLC growth. LLC tumor cells were s.c. injected in WT and Ang-2–deficient mice. Tumors were harvested 7 d (A) and 12 d (B) after tumor inoculation. Tumor sections were double-stained for the endothelial cell marker CD31 (green) and for the mural cell markers NG2, desmin, and α-SMA (red). Vessel coverage was calculated as the percentage of NG2-, desmin-, and α-SMA–positive vessels compared with the number of CD31-positive vessels. Mural cell coverage was similar in tumors grown in WT and Ang-2–deficient mice at day 7 (A). In contrast, day 12 tumors grown in Ang-2–deficient mice had higher coverage by NG2- and desmin-positive and less coverage by α-SMA–positive mural cells (B). n.d., not detected. *, P < 0.05; **, P < 0.01. Bar, 100 μm.
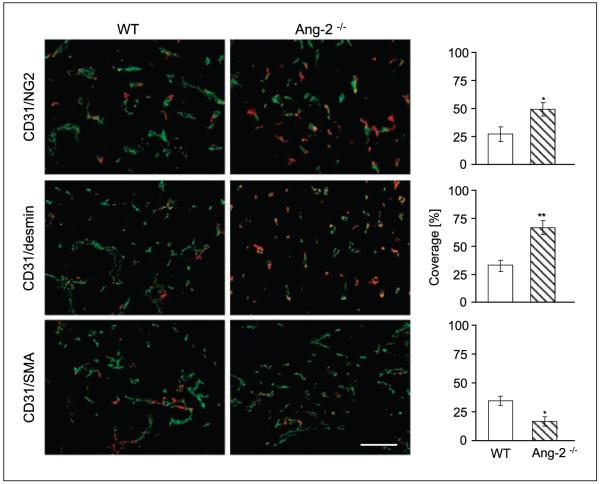
The above analytic experiments of early-stage tumors had suggested that the divergence of tumor growth between WT and Ang-2–deficient mice observed between day 7 and day 12 was associated with a distinct Ang-2–dependent mural cell recruitment and maturation phenotype. Based on these findings, we next asked if Ang-2 deficiency led to long-lasting changes of mural cell recruitment and maturation during later stages of tumor growth, which were in terms of growth kinetics not affected by the genetic ablation of Ang-2 (Fig. 1B). These experiments revealed that late-stage tumors had similar mural cell recruitment and maturation characteristics as day 12 tumors, characterized by a higher percentage of desmin- and NG2-positive microvessels and a lower percentage of α-SMA–positive mural cells in tumors grown in Ang-2–deficient mice compared with WT mice (Fig. 3).
Figure 3.
Effect of Ang-2 deficiency on mural cell recruitment and maturation during later stages of LLC growth. Tumors were grown s.c. in WT and Ang-2–deficient mice and harvested when the first tumors had grown to 2 cm3. Tumor sections were double-stained for the endothelial cell marker CD31 (green) and for the mural cell markers NG2, desmin, and α-SMA (red). Vessel coverage was calculated as the percentage of NG2-, desmin-, and α-SMA–positive vessels compared with the number of CD31-positive vessels. As in day 12 mice, late-stage LLC tumors grown in Ang-2–deficient mice had higher coverage by NG2- and desmin-positive and less coverage by α-SMA–positive mural cells. *, P < 0.05; **, P < 0.01. Bar, 100 μm.
Ang-2 deficiency affects intratumoral microvessel architecture
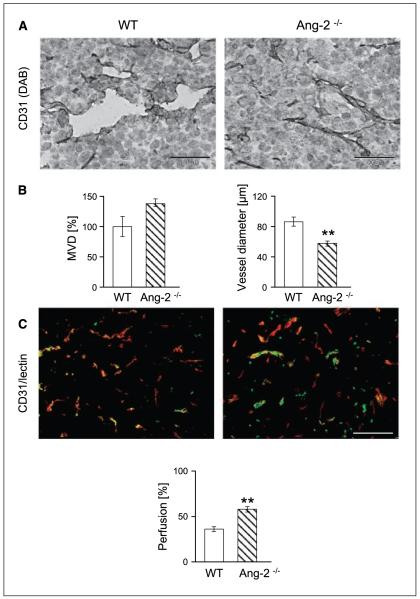
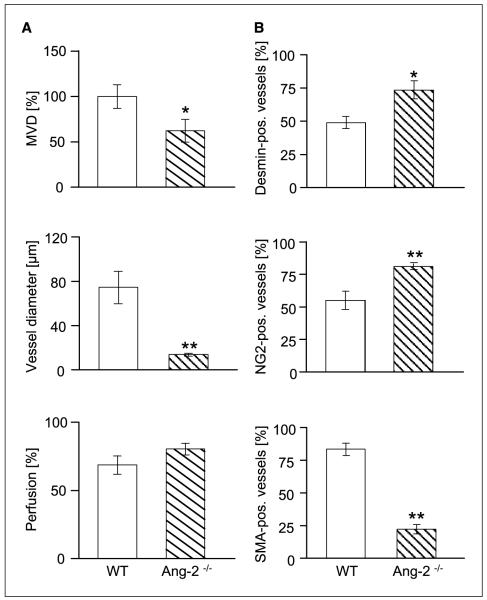
Ang-1/Tie2 signaling has previously been related to vessel diameter sensing (31-33). Correspondingly, pericyte coverage has been shown to restrict the plasticity window of a remodeling neovasculature (34). Thus, based on the observed persistent changes in mural cell recruitment and maturation in tumors grown in Ang-2–deficient mice, we examined if these led concomitantly to structural alterations of the architecture and function of the intratumoral microvascular network. Total intratumoral MVD in late-stage tumors was minimally elevated in the absence of host-derived Ang-2 (Fig. 4A and B), confirming the notion that blood vessels could grow with similar efficacy in the absence of Ang-2 as in WT mice (35). Average vessel diameters were smaller in tumors grown in Ang-2–deficient mice compared with WT mice (Fig. 4B). These changes in vessel diameter were conversely associated with significantly higher perfusion indices as assessed by FITC-lectin perfusion (Fig. 4C). Collectively, these findings suggested that the increased mural cell recruitment and maturation indices in Ang-2–deficient tumors reflected a vessel normalization phenotype with altered vessel diameters and increased perfusion.
Figure 4.
Effect of host-derived Ang-2 deficiency on MVD (A and B), diameter of intratumoral microvessels (A and B), and perfusion (C) in LLC tumors. Tumors were grown in WT and Ang-2–deficient mice and harvested when the first tumors had grown to 2 cm3. MVDs and vessel diameters were quantitated in CD31-stained tissue sections. Perfusion was assessed on the basis of FITC-lectin perfusion labeling. Ang-2 deficiency had a nonsignificant effect on intratumoral MVD. Yet, the average diameters of intratumoral microvessels were significantly reduced in tumors grown in Ang-2–deficient mice. Correspondingly, intratumoral microvessels in Ang-2–deficient mice were better perfused. **, P < 0.01. Bar, 200 μm.
Altered tumor growth and mural cell recruitment in MT-ret tumors and B16F10 melanomas grown in Ang-2–deficient mice
The comparative experiments with LLC tumors grown in WT and Ang-2–deficient mice had identified a distinct plasticity window during early tumor growth, which was affected by the presence of host-derived Ang-2 as well as persistent structural alterations of the intratumoral microvascular network indicative of a vessel normalization phenotype. To validate if these findings are related to the specific growth properties of the LLC tumor model, we used two other experimental tumor models (i.e., MT-ret and B16F10 melanomas) to study the functional consequences of host-derived Ang-2 deficiency on tumor growth.
As in LLC tumors, both MT-ret melanomas (Fig. 5A) and B16F10 melanomas (Supplementary Fig. S2A) grew slower in Ang-2–deficient mice when compared with the growth in WT mice. Yet, when plotting tumor growth data as log-transformed curves to show tumor growth rate, it became apparent that the dissociating tumor phenotype occurred during the earliest stages of tumor growth. In MT-ret melanomas, tumor growth curve had already dissociated at the earliest time points of investigation. Thereafter, tumor growth curve rates were essentially identical (Fig. 5B). In B16F10 melanomas, tumor growth in WT and Ang-2–deficient mice dissociated when tumors had grown to 0.2 to 0.4 cm3. Thereafter, tumor growth curve rates were identical or had even a tendency to converge (Supplementary Fig. S2B). The net outcome on tumor growth was most pronounced in MT-ret melanomas, which grew to much smaller total size (Fig. 5C). B16F10 melanomas had only a minor nonsignificant growth difference (Supplementary Fig. S2C). Taken together, overall tumor growth kinetics in MT-ret and B16F10 melanomas were very much compatible with the growth of LLC tumors with pronounced differences between WT and Ang-2–deficient mice during early stages of tumor growth and little to no effect during later stages of tumor progression.
Figure 5.
Growth of MT-ret melanomas in WT and Ang-2–deficient mice. Tumor growth in MT-ret melanomas dissociated during the very earliest stages of tumor growth (A). Thereafter, tumor growth rates were almost identical as evidenced by essentially parallel curves of log-transformed tumor growth data (B). Total tumor weight was strongly reduced in tumors grown in Ang-2–deficient mice (C). Yet, as in LLC tumors, the differences in tumor weight at the end of the experiment resulted mostly from the dissociating exponential growth curves as a consequence of a dissociation of tumor growth curves during early stages of tumor growth. *, P < 0.05.
Functional parameters of the intratumoral vascular phenotype of MT-ret and B16F10 melanomas were analyzed to determine MVD, vessel diameter, perfusion, and mural cell coverage (Fig. 6; Supplementary Fig. S3). Intratumoral MVD was significantly reduced in MT-ret melanomas (Fig. 6A) and nonsignificantly reduced in B16F10 tumors (Supplementary Fig. S3A). Correspondingly, average vessel diameters were strongly reduced in MT-ret tumors (Fig. 6A) and only weakly reduced in B16F10 tumors (Supplementary Fig. S3A). Lastly, the percentage of perfused vessels was not significantly altered in either of the two melanoma models (Fig. 6A; Supplementary Fig. S3A). Collectively, these varying results on angioarchitectural and functional characteristics of the tumor-associated vasculature in the three different tumor models supported the concept that Ang-2 may not have much of a direct effect on tumor vascularization, but that the net outcome of Ang-2 may primarily be dependent on the local cytokine milieu.
Figure 6.
Effect of Ang-2 deficiency on MVD, vessel diameter and perfusion (A), and mural cell recruitment and maturation (B) in MT-ret melanomas. MT-ret melanoma cells were s.c. injected in WT and Ang-2–deficient mice and harvested when the first tumors had grown to 2 cm3. MVDs and vessel diameters were quantitated in CD31-stained tissue sections. Perfusion was assessed on the basis of FITC-lectin perfusion labeling. For mural cell coverage analysis, tumor sections were double-stained for the endothelial cell marker CD31 and for the mural cell markers desmin (top), NG2 (middle), and α-SMA (bottom). Vessel coverage was calculated as the percentage of desmin-, NG2-, and α-SMA–positive vessels compared with the number of CD31-positive vessels. As in LLC tumors, microvessels in MT-ret melanomas had higher desmin and NG2 coverage and lower αSMA coverage in tumors grown in Ang-2–deficient mice.
Unlike the varying overall angioarchitectural parameters of the intratumoral vasculature, mural cell coverage characteristics showed consistent changes in all three tumor models. Approximately 45% of intratumoral microvessels were covered by desmin-positive mural cells in MT-ret and B16F10 tumors grown in WT mice (Fig. 6B; Supplementary Fig. S3B). A somewhat higher percentage of vessels were covered by NG2-positive mural cells (MT-ret, 53%; B16F10, 70%). Coverage by α-SMA–positive mural cells was similarly high in MT-ret and B16F10 tumors grown in WT mice (~75%; Fig. 6B; Supplementary Fig. S3B). Analysis of mural cell coverage in tumors grown in Ang-2–deficient mice yielded a very different pattern of mural cell recruitment and maturation: The percentage of desmin- and NG2-positive mural cells in the tumor vasculature was significantly increased in MT-ret and B16F10 tumors and the percentage of α-SMA–positive mural cells was significantly reduced in both melanoma models when tumors were grown in Ang-2–deficient mice (Fig. 6B; Supplementary Fig. S3B). The changes in mural cell recruitment and maturation in MT-ret and B16F10 melanomas consequently mirrored exactly the same changes as observed in LLC tumors.
Discussion
Ang-2–deficient mice have been reported to develop normally to term. Yet, essentially all newborn pups die within the first 14 days after birth due to lymphatic defects that lead to a lethal chylous ascites (35). The perinatal lethality of Ang-2–deficient mice has precluded tumor experiments in Ang-2–deficient mice. Consequently, experiments aimed at assessing the role of Ang-2 during tumor angiogenesis were based on the use of endogenous Ang-2–expressing WT mice in which Ang-2 was therapeutically inhibited (26), systemically overexpressed (36), or manipulated in the tumor cell compartment (12). Yet, Ang-2 is in most tumors almost exclusively produced by host endothelial cells (19, 28), acting on endothelial cells through an autocrine loop mechanism (15). Consequently, definite experiments aimed at assessing the role of host-derived Ang-2 should be based on the use of genetically manipulated mice, which either are deficient in Ang-2 production (35) or overexpress Ang-2 in the endothelial cell compartment (37).
It was recently observed that the perinatal lethality of Ang-2–deficient mice is strain dependent. Essentially all Ang-2–deficient mice in the 129/J background die postnatally within 14 days after birth (35). In contrast, the lymphatic phenotype of Ang-2–deficient mice in the C57/B16 background is much more subtle with <10% postnatal lethality (20). The present study therefore took advantage of these strain-specific differences to pursue comparative tumor experiments in adult WT and Ang-2–deficient mice.
Comparative analysis of tumor growth in WT and Ang-2–deficient mice using three different tumor models yielded the following findings: (a) Tumors grew slower in Ang-2–deficient mice compared with WT mice. (b) The growth difference between tumors grown in WT and Ang-2–deficient mice occurred during early stages of tumor development. (c) Tumor growth rates of established tumors (i.e., beyond 0.2–0.4 cm3) were similar in WT and Ang-2–deficient mice. (d) Ang-2 deficiency led to variable changes of intratumoral MVD and perfusion properties. (e) Microvessels of tumors grown in Ang-2–deficient mice were more abundantly covered by more mature pericytes.
The results of this study shed significant novel light into the complexity of Ang-2 function during tumor angiogenesis and may contribute toward a more rational therapeutic exploitation of the angiopoietin/Tie system. The intense expression of Ang-2 in tumors has long been recognized. Yet, whereas VEGF is abundantly expressed by the tumor cells in most tumors, Ang-2 is, with few exceptions, expressed by the tumor-associated endothelium and not by the tumor cells. This is in apparent contrast to a number of published reports supposedly showing Ang-2 expression by tumor cells. Most of these studies have confirmed the overall up-regulated expression of Ang-2 in tumors. Yet, few studies have done a detailed spatial analysis of Ang-2 expression, preferably by in situ hybridization on the mRNA level. Likewise, the limited ability of most antibody reagents to reliably discriminate between Ang-1 and Ang-2 put a cautionary note on some of the published Ang-2 expression data. When carefully tracing Ang-2 expression, Ang-2 is in the vast majority of human tumors expressed by the tumor associated endothelium (19, 28).5 The lack of Ang-2 expression by most tumors is conceptually of interest because it shows that there seems to be no evolutionary pressure for tumor cells to express Ang-2 during tumor progression. Correspondingly, the present study revealed that Ang-2 affects tumor growth only during early stages of tumor development but is dispensable for later stages of tumor progression. It also corresponds to the observation that Ang-2 is dispensable for embryonic blood vessel development (35).
Constitutive Ang-1/Tie2 signaling is, in the adult, required to maintain the quiescent endothelial cell phenotype (38). Ang-1 overexpression induces vessel sealing (31) and leads to wider diameter blood vessels (32, 33). Genetic experiments have established Ang-2 as functional antagonist of Ang-1/Tie2 signaling by showing that Ang-2 transgenic mice essentially phenocopy the embryonic lethal phenotype of Ang-1–deficient mice (5). Correspondingly, overexpression of Ang-2 in the endothelial cell compartment was conclusively shown to block Tie2 phosphorylation in an autocrine manner (37). Yet, recent work has also suggested that Ang-2, under certain conditions, may act as an activator of Tie2 (16). The molecular mechanisms of agonistic versus antagonistic Ang-2 functions are not understood. The findings of the present study are compatible with an antagonistic mode of action. Ang-2–mediated inhibition of Ang-1/Tie2 signaling destabilizes the quiescent endothelial cell monolayer and primes the endothelium to respond to exogenous cytokines (39). In line with this model, Ang-2–deficient tumor blood vessels are less plastic, which limits tumor growth, most pronounced during the early stages of tumor development. Likewise, Ang-2–deficient tumor blood vessels have smaller diameters corresponding to the vessel diameter sensing role of Ang-1/Tie2 signaling (32, 33).
Among the most surprising findings of the present study was the observed altered pattern of mural cell recruitment and maturation of blood vessels in tumors grown in Ang-2–deficient mice. Ang-1/Tie2 signaling has long been implicated in pericyte coverage (40-42). Yet, the mechanisms of angiopoietin-induced mural cell recruitment have not been resolved. The mechanistically best understood pathway of endothelial cell–directed mural cell recruitment is through paracrine acting PDGF-B (42, 43). Similarly, HGF (44) and HB-EGF (45) have been implicated in pericyte recruitment. The molecular crosstalk between the angiopoietin/Tie system and pericyte recruiting molecules is only poorly understood. The results of the present study suggest that the angiopoietin/Tie system may play a more direct role on mural cell recruitment than has previously been anticipated. A higher percentage of tumor microvessels of tumors grown in Ang-2–deficient mice were covered by mural cells expressing the pericyte markers desmin and NG2 compared with microvessels of tumors grown in WT mice. In contrast, a smaller fraction of these murals cells expressed α-SMA. Intense NG2 and desmin staining with reduced expression of α-SMA by tumor vessel–associated mural cells is generally interpreted to reflect a more mature pericyte phenotype. This interpretation is supported by observations suggesting that mural cells of immature tumor blood vessels may be prominently α-SMA positive, whereas capillary pericytes of mature blood vessels generally down-regulate α-SMA (46-48). Yet, NG2 expression decreased from 80% (day 12) to 50% in later stages of LLC tumor growth. This finding is in line with reduced tumor growth in the early stages. During later stages, NG2-expressing pericytes are reduced, correlating with continued sprouting and essentially identical tumor growth rates of tumors grown in WT and Ang-2–deficient mice. Collectively, the findings of this study for the first time show a molecular pathway resulting in a coordinated pattern of mural cell recruitment and maturation. The findings warrant further mechanistic analysis to focus on the molecular cross talk of angiopoietin/Tie and PDFG/PDGFR signaling during mural cell recruitment and maturation. Likewise, significant additional work needs to be done to more precisely define the hierarchy of molecular markers that govern pericyte differentiation and maturation. It is noteworthy that the repertoire of established pericyte markers PDGFRβ, NG2, desmin, and α-SMA has recently been extended by two additional markers, RGS-5 (49) and endosialin (50).
In principle, the findings of the present study support the concept that Ang-2 inhibitory therapies may have beneficial antitumorigenic effects. Yet, the efficacy of Ang-2 inhibitory therapies on established tumors during later stages of tumor progression may be limited. More importantly, angiopoietin/Tie manipulatory therapies will likely not be pursued as monotherapies but be exploited in combination with established anti-VEGF/VEGFR therapies. Anti-VEGF therapies prune the immature tumor microvasculature and normalize the tumor vascular bed to eventually facilitate better access of coadministered chemotherapeutic drugs (51). Ang-2 deficiency similarly limits the plasticity window of the growing tumor neovasculature, leading to a normalized phenotype of the remaining tumor vasculature. Thus, different therapeutic modalities may be envisioned: Inhibition of Ang-2 may act in concert with anti-VEGF/VEGFR therapies by synergistically inducing a vessel normalizing effect. Conversely, an Ang-2 gain-of-function approach may contribute to destabilizing the tumor vasculature to sensitize it to anti-VEGF/VEGFR therapies. Both approaches are presently viable options. Future work in definite mouse models with similar growth and maturation kinetics as human tumors will contribute to paving the way toward the rational development of second-generation antiangiogenic combination therapies.
Supplementary Material
Acknowledgments
Grant support: German Research Council (DFG, SFB-TR23 [Vascular Differentiation and Remodeling], project A3) and the Austrian Science Fund (FWF S9404-B11). H.G. Augustin is supported by an endowed chair from the Aventis Foundation.
Footnotes
H.G. Augustin, unpublished data.
Note:Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Kesisis G, Broxterman H, Giaccone G. Angiogenesis inhibitors. Drug selectivity and target specificity. Curr Pharm Des. 2007;13:2795–809. doi: 10.2174/138161207781757033. [DOI] [PubMed] [Google Scholar]
- 3.Ng YS, Krilleke D, Shima DT. VEGF function in vascular pathogenesis. Exp Cell Res. 2006;312:527–37. doi: 10.1016/j.yexcr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Baka S, Clamp AR, Jayson GC. A review of the latest clinical compounds to inhibit VEGF in pathological angiogenesis. Expert Opin Ther Targets. 2006;10:867–76. doi: 10.1517/14728222.10.6.867. [DOI] [PubMed] [Google Scholar]
- 5.Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 6.Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–9. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 7.Pfaff D, Fiedler U, Augustin HG. Emerging roles of the angiopoietin-Tie and the ephrin-Eph systems as regulators of cell trafficking. J Leukoc Biol. 2006;80:719–26. doi: 10.1189/jlb.1105652. [DOI] [PubMed] [Google Scholar]
- 8.Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 9.Visconti RP, Richardson CD, Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF) Proc Natl Acad Sci U S A. 2002;99:8219–24. doi: 10.1073/pnas.122109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiedler U, Scharpfenecker M, Koidl S, et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150–6. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 11.Fiedler U, Krissl T, Koidl S, et al. Angiopoietin-1 and angiopoietin-2 share the same binding domains in the Tie-2 receptor involving the first Ig-like loop and the epidermal growth factor-like repeats. J Biol Chem. 2003;278:1721–7. doi: 10.1074/jbc.M208550200. [DOI] [PubMed] [Google Scholar]
- 12.Imanishi Y, Hu B, Jarzynka MJ, et al. Angiopoietin-2 stimulates breast cancer metastasis through the α5β1 integrin-mediated pathway. Cancer Res. 2007;67:4254–63. doi: 10.1158/0008-5472.CAN-06-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cascone I, Napione L, Maniero F, Serini G, Bussolino F. Stable interaction between α5β1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J Cell Biol. 2005;170:993–1004. doi: 10.1083/jcb.200507082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlson TR, Feng Y, Maisonpierre PC, Mrksich M, Morla AO. Direct cell adhesion to the angiopoietins mediated by integrins. J Biol Chem. 2001;276:26516–25. doi: 10.1074/jbc.M100282200. [DOI] [PubMed] [Google Scholar]
- 15.Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118:771–80. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- 16.Daly C, Pasnikowski E, Burova E, et al. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci U S A. 2006;103:15491–6. doi: 10.1073/pnas.0607538103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 18.Peters S, Cree IA, Alexander R, et al. Angiopoietin modulation of vascular endothelial growth factor: effects on retinal endothelial cell permeability. Cytokine. 2007;40:144–50. doi: 10.1016/j.cyto.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Yang N, Park JW, et al. Tumor-derived vascular endothelial growth factor up-regulates angiopoietin-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer. Cancer Res. 2003;63:3403–12. [PubMed] [Google Scholar]
- 20.Fiedler U, Reiss Y, Scharpfenecker M, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-α and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235–9. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad SA, Liu W, Jung YD, et al. Differential expression of angiopoietin-1 and angiopoietin-2 in colon carcinoma. A possible mechanism for the initiation of angiogenesis. Cancer. 2001;92:1138–43. doi: 10.1002/1097-0142(20010901)92:5<1138::aid-cncr1431>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 22.Wurmbach JH, Hammerer P, Sevinc S, Huland H, Ergun S. The expression of angiopoietins and their receptor Tie-2 in human prostate carcinoma. Anticancer Res. 2000;20:5217–20. [PubMed] [Google Scholar]
- 23.Tsutsui S, Inoue H, Yasuda K, et al. Angiopoietin 2 expression in invasive ductal carcinoma of the breast: its relationship to the VEGF expression and microvessel density. Breast Cancer Res Treat. 2006;98:261–6. doi: 10.1007/s10549-005-9157-9. [DOI] [PubMed] [Google Scholar]
- 24.Zagzag D, Hooper A, Friedlander DR, et al. In situ expression of angiopoietins in astrocytomas identifies angiopoietin-2 as an early marker of tumor angiogenesis. Exp Neurol. 1999;159:391–400. doi: 10.1006/exnr.1999.7162. [DOI] [PubMed] [Google Scholar]
- 25.Etoh T, Inoue H, Tanaka S, Barnard GF, Kitano S, Mori M. Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: possible in vivo regulation via induction of proteases. Cancer Res. 2001;61:2145–53. [PubMed] [Google Scholar]
- 26.Oliner J, Min H, Leal J, et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 2004;6:507–16. doi: 10.1016/j.ccr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 27.Sarraf-Yazdi S, Mi J, Moeller BJ, et al. Inhibition of in vivo tumor angiogenesis and growth via systemic delivery of an angiopoietin 2-specific RNA aptamer. J Surg Res. 2008;146:16–23. doi: 10.1016/j.jss.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 28.Stratmann A, Risau W, Plate KH. Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. Am J Pathol. 1998;153:1459–66. doi: 10.1016/S0002-9440(10)65733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bazhin AV, Schadendorf D, Willner N, et al. Photoreceptor proteins as cancer-retina antigens. Int J Cancer. 2007;120:1268–76. doi: 10.1002/ijc.22458. [DOI] [PubMed] [Google Scholar]
- 30.Nissen LJ, Cao R, Hedlund EM, et al. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J Clin Invest. 2007;117:2766–77. doi: 10.1172/JCI32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thurston G, Suri C, Smith K, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–4. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 32.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A. 2002;99:11205–10. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kidoya H, Ueno M, Yamada Y, et al. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. 2008;27:522–34. doi: 10.1038/sj.emboj.7601982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–8. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 35.Gale NW, Thurston G, Hackett SF, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002;3:411–23. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 36.Cao Y, Sonveaux P, Liu S, et al. Systemic over-expression of angiopoietin-2 promotes tumor microvessel regression and inhibits angiogenesis and tumor growth. Cancer Res. 2007;67:3835–44. doi: 10.1158/0008-5472.CAN-06-4056. [DOI] [PubMed] [Google Scholar]
- 37.Reiss Y, Droste J, Heil M, et al. Angiopoietin-2 impairs revascularization after limb ischemia. Circ Res. 2007;101:88–96. doi: 10.1161/CIRCRESAHA.106.143594. [DOI] [PubMed] [Google Scholar]
- 38.Wong AL, Haroon ZA, Werner S, Dewhirst MW, Greenberg CS, Peters KG. Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circ Res. 1997;81:567–74. doi: 10.1161/01.res.81.4.567. [DOI] [PubMed] [Google Scholar]
- 39.Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–8. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 40.Goede V, Schmidt T, Kimmina S, Kozian D, Augustin HG. Analysis of blood vessel maturation processes during cyclic ovarian angiogenesis. Lab Invest. 1998;78:1385–94. [PubMed] [Google Scholar]
- 41.Hammes HP, Lin J, Wagner P, et al. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes. 2004;53:1104–10. doi: 10.2337/diabetes.53.4.1104. [DOI] [PubMed] [Google Scholar]
- 42.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 43.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–5. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi H, BeBusk LM, Babichev YO, Dumont DJ, Lin PC. Hepatocyte growth factor mediates angiopoietin-induced smooth muscle cell recruitment. Blood. 2006;108:1260–6. doi: 10.1182/blood-2005-09-012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iivanainen E, Nelimarkka L, Elenius V, et al. Angiopoietin-regulated recruitment of vascular smooth muscle cells by endothelial-derived heparin binding EGF-like growth factor. FASEB J. 2003;17:1609–21. doi: 10.1096/fj.02-0939com. [DOI] [PubMed] [Google Scholar]
- 46.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 48.Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type α-actin. J Cell Biol. 1991;113:147–54. doi: 10.1083/jcb.113.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamzah J, Jugold M, Kiessling F, et al. Vascular normalization in RGS5-deficient tumours promotes immune destruction. Nature. 2008;453:410–4. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 50.Christian S, Winkler R, Helfrich I, et al. Endosialin (Tem1) is a marker of tumor-associated myofibroblasts and tumor vessel-associated mural cells. Am J Pathol. 2008;172:486–94. doi: 10.2353/ajpath.2008.070623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.