Abstract
We have used a transgenic cell line of Catharanthus roseus (L.) G. Don to study the relative importance of the supply of biosynthetic precursors for the synthesis of terpenoid indole alkaloids. Line S10 carries a recombinant, constitutively overexpressed version of the endogenous strictosidine synthase (Str) gene. Various concentrations and combinations of the substrate tryptamine and of loganin, the immediate precursor of secologanin, were added to suspension cultures of S10. Our results indicate that high rates of tryptamine synthesis can take place under conditions of low tryptophan decarboxylase activity, and that high rates of strictosidine synthesis are possible in the presence of a small tryptamine pool. It appears that the utilization of tryptamine for alkaloid biosynthesis enhances metabolic flux through the indole pathway. However, a deficiency in the supply of either the iridoid or the indole precursor can limit flux through the step catalyzed by strictosidine synthase. Precursor utilization for the synthesis of strictosidine depends on the availability of the cosubstrate; the relative abundance of these precursors is a cell-line-specific trait that reflects the metabolic status of the cultures.
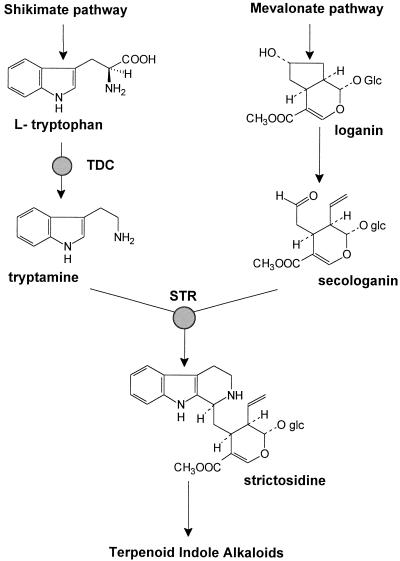
The tropical plant Catharanthus roseus (L.) G. Don (Apocynaceae) produces TIAs of high medicinal and economic value, such as ajmalicine, catharanthine, vindoline, and the bisindoles vinblastine and vincristine. Cell cultures of C. roseus have long been considered to be sources of medicinally important TIAs, but have suffered from their characteristically low productivity (Verpoorte et al., 1993). The optimization of medium composition, including the supply of biosynthetic precursors, and genetic engineering are among the various strategies that have been followed to increase alkaloid production in vitro. The precursors for the synthesis of TIAs are obtained from the shikimate and mevalonate pathways, which supply the indole tryptamine and the iridoid secologanin, respectively (Fig. 1). Tryptamine is synthesized from Trp, a step catalyzed by TDC, whereas secologanin is obtained from loganin, which is derived from the monoterpenoid geraniol. The first committed step in the biosynthesis of TIAs is the condensation of secologanin and tryptamine, catalyzed by STR, which results in the formation of strictosidine, the universal precursor of TIAs.
Figure 1.
Biosynthesis of strictosidine in C. roseus.
There are numerous reports of experiments in which precursors have been added to cell cultures of C. roseus. The reported effects of feeding various indole and terpenoid building blocks are largely inconsistent. Exogenous Trp has been reported to increase tryptamine content without affecting TIA production (Mérillon et al., 1986; Facchini and Di Cosmo, 1991); it has also been reported to cause a nearly 3-fold increase in alkaloid production in one cell line, and to reduce production in a daughter line (Zenk et al., 1977). Exogenous Trp and tryptamine negatively affected alkaloid accumulation in one study (Döller et al., 1976), whereas tryptamine had a stimulating effect in another (Krueger and Carew, 1978). Exogenous secologanin has also been shown both to have no effect on and to enhance alkaloid production (Zenk et al., 1977; Krueger and Carew, 1978; Facchini and Di Cosmo, 1991; Moreno et al., 1993). These apparently contradictory results underscore the difficulties associated with the study of a complex metabolic pathway.
We report a series of experiments designed to study the effect of feeding precursors under conditions of high-STR activity and iridoid availability. We have used cell line S10 of C. roseus, an experimental system in which flux through the crucial metabolic step of strictosidine synthesis is always unimpeded. The patterns of alkaloid accumulation and enzymatic activities of S10 are well characterized; S10 is a transgenic line that constitutively expresses the Str gene at high levels, resulting in high STR activity and the accumulation of large quantities of TIAs, including ajmalicine, catharanthine, serpentine, and tabersonine (Canel et al., 1998). We have thus been able to assess the relative importance of the two pathways that converge at the STR-catalyzed step and the influence of TDC activity on TIA production.
MATERIALS AND METHODS
Culture Media and Components
MS58 (per liter) consisted of: Murashige and Skoog salts (Murashige and Skoog, 1962), 0.1 g of myo-inositol, 0.4 mg of thiamine, 2 mg of NAA, 0.2 mg of kinetin, and 30 g of Suc; and PM consisted of: Murashige and Skoog salts devoid of phosphate and nitrate, 0.1 g of myo-inositol, 0.4 mg of thiamine, and 80 g of Suc. Suc was purchased from Duchefa (Haarlem, The Netherlands); salts, vitamins, and hormones from Merck; loganin from Extrasynthese (Genay, France); and tryptamine-HCl from Aldrich. Secologanin with a purity of 96% was obtained from crude acetone extracts of berries of Symphoricarpus sp. by elution from a silica column (Kieselgel 60, Merck) with acetone:ethyl acetate (Stevens, 1994).
Cell Lines and Culture Conditions
The transgenic cell line S10 was generated by Agrobacterium-mediated transformation of leaves of Catharanthus roseus (L.) G. Don, var Morning Mist (Blokker, The Netherlands), as described previously (Canel et al., 1998). The line carries a T-DNA constructed in binary vector pMOG22 (Mogen, Leiden, The Netherlands), which confers resistance to the antibiotic hygromycin, and contains a gus reporter gene and a recombinant version of the endogenous Str gene (accession no. X61932) under the control of the strong constitutive cauliflower mosaic virus 35S promoter. Line S10 was maintained in medium MS58 containing 50 mg/L hygromycin B for 4 months after its initiation from transgenic callus in July 1995, and has since been grown in antibiotic-free MS58. Wild-type line CRPM was established from seeds of C. roseus in 1983. Lines were maintained by periodic subculture (every 7–10 d) into 250-mL wide-mouthed Erlenmeyer flasks fitted with silicon foam stoppers (Shin Etsu, Tokyo, Japan) containing 50 mL of liquid medium in a 1:2 to 1:4 ratio. Cultures were placed on gyratory shakers (110–120 rotations per min) at 25 ± 1°C, in continuous light (2000–3600 lux). To generate sufficient biomass for inoculation of a large number of flasks, cell cultures were sequentially scaled up to 0.5- and 2-L flasks containing 100 and 500 mL of MS58, respectively. Cells in stationary phase (7–10 d old) were inoculated at the rate of 5.0 ± 0.1 g fresh weight per 50 mL of PM in 250-mL flasks. Loganin and tryptamine were added from filter-sterilized 100-mM solutions. Treated and control cultures were harvested in duplicate or triplicate; extraction and analysis were carried out after pooling the replicate samples.
Enzyme Assays
Soluble proteins were extracted from 350 mg of frozen biomass by homogenization in 350 μL of extraction buffer (0.1 m sodium phosphate, pH 7.0, 2 mm EDTA, and 4 mm DTT) in the presence of 17.5 mg of polyvinylpolypyrrolidone. Homogenization was performed in 1.5-mL microfuge tubes using hand-held plastic micropestles (Van Oostermerssen, Rijswijk, The Netherlands). A clear supernatant containing the enzymes of interest was obtained by centrifugation of the homogenate at 16,000g at 4°C for 30 min. Protein concentration was determined using the protein-staining reagent and 3550-UV Microplate Reader (Bio-Rad) and BSA as the standard. The procedures to assay the activities of STR and TDC have been described (Pennings et al., 1987, 1989).
Detection of Alkaloids and Precursors
Tryptamine was extracted from 50 mg of freeze-dried biomass using 5 mL of dichloromethane (Schripsema and Verpoorte, 1992) and detected by HPLC (Van der Heijden et al., 1987). Secologanin was extracted from 100 mg of freeze-dried biomass by incubation in 2 mL of boiling water for 2 min. Secologanin was quantified as strictosidine by HPLC (Pennings et al., 1989), after in vitro conversion in 100 μL of 0.1 mm sodium phosphate, pH 6.8, containing >15 pkat STR, 1 mm tryptamine, 1 mm EDTA, and 3 mm DTT at 30°C for 30 min (D. Hallard and R. Van der Heijden, unpublished data). For extraction of TIAs, 100 mg of freeze-dried biomass was homogenized in 15 mL of absolute ethanol. After centrifugation at 3,500g for 30 min, 10 mL of the extract was dried under reduced pressure, and the residue was dissolved in 1 mL of 1 m H3PO4. Following centrifugation of the acidic alkaloid solution at 16,000g for 5 min, 50 μL of the supernatant was analyzed by HPLC. The identity of the analytes was established by photodiode-array detection of their UV spectra (Van der Heijden et al., 1987).
RESULTS
Control cultures of S10 showed the patterns of enzymatic activity and alkaloid accumulation that are characteristic of this highly productive line. TDC activity was induced upon inoculation into PM, reaching maxima between d 3 and 5, and decreased to basal levels for the rest of the culture period. STR activity was always very high (>500 pkat/mg of soluble protein), ranging from 10 to 50 times the level found in wild-type cultures. Inoculation into PM was followed by a short lag period of about 3 d, during which the relatively high amount of TIAs already present in the inoculum increased only slightly. The rate of accumulation then increased until reaching a plateau around d 12. Tryptamine content was highest during the first few days of the production phase; it decreased rapidly as TIAs began to accumulate, and increased slightly at the end of the production period, when the rate of TIA accumulation decreased. The addition of precursors did not affect the growth of the cell cultures, as determined by measuring the amount of biomass obtained at the end of the test period. Control cultures reached 18.5 ± 3.5 g dry weight L−1 (n = 6), whereas treated cultures reached 18.6 ± 3.1 g/L (n = 19); the dry weight to fresh weight ratio was always in the range of 0.11 to 0.13. Loganin was chosen over secologanin because the latter is inefficiently utilized for strictosidine synthesis when added to the medium. Exogenous secologanin appears to be compartmentalized differently from endogenous secologanin, including that synthesized from exogenous loganin; in contrast, exogenous loganin is used in toto by C. roseus (Naudascher et al., 1989a, 1989b).
The first experiment consisted of feeding 500 μm loganin to a set of cultures either at the time of inoculation (d 0) with harvesting on d 3, or on d 11 with harvesting on d 14. Nonfed control cultures were harvested on d 3, 11, and 14. Exogenous loganin had a depleting effect on tryptamine content, which was equally dramatic on either of the two feeding dates, and was accompanied by a 2- to 4-fold increase in TIA content in the treated cells (Table I). The steady-state level of tryptamine was a small fraction, in molar equivalents, of the amount by which alkaloid content increased. From d 0 to 3, treated cells accumulated 126 μmol/L TIAs, whereas the alkaloid content of the control cultures increased only slightly; the difference in tryptamine content between the two sets of cultures at the end of that period was 6.8 μmol/L. More strikingly, in the 3-d period between d 11 and 14, during which low levels of tryptamine prevail, cells fed loganin on d 11 produced 140 μmol/L TIAs more than control cells, whereas the difference between their tryptamine content was only 5.0 μmol/L. Iridoids did not accumulate in any of the cultures.
Table I.
Effect of feeding loganin on tryptamine and TIA content of cultured C. roseus cells
| Harvest | Tryptamine
|
TIAs
|
||
|---|---|---|---|---|
| Control | Treated | Control | Treated | |
| d | μmol/L | |||
| 0 | 3.9 | — | 39.2 | — |
| 3 | 7.9 | 1.1a | 42.5 | 164.8a |
| 11 | 3.7 | — | 101.6 | — |
| 14 | 5.2 | 0.2b | 123.7 | 263.6b |
Treatment consisted of addition of 500 μm loganin. Triplicate cultures were pooled upon harvesting and analyzed as single samples.
Loganin added on d 0.
Loganin added on d 11.
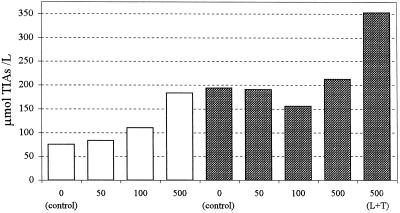
For the second experiment, we used cell cultures of S10 showing a reduced capacity to synthesize tryptamine. We have observed a significant correlation between culture browning and low tryptamine biosynthetic capacity during the course of experiments not related to precursor feeding. Nine out of 12 independently initiated cultures harvested at the onset of browning had a tryptamine content no greater than 1.5 μmol/L (mean = 1.6 ± 2.2 μmol/L), compared with a content of 6.8 ± 1.3 μmol/L measured in healthy cultures. Culture browning results from the oxidation of phenolic compounds. Browning is commonly associated with increased flux through branches of the shikimate pathway, leading to the synthesis of phenolic acids and phenylpropanoids, which compete with the indole branch for precursors and consequently impair the ability of the cells to maintain a high rate of tryptamine synthesis. We do not know the reason for the occasional spontaneous browning of the cultures, a phenomenon that is not unique to S10. Nevertheless, culture browning in S10 is valuable for indicating the occurrence of reduced tryptamine biosynthetic capacity, which provides the opportunity to carry out experiments under conditions of low tryptamine availability. The experiment was performed with 27 cultures, which remained healthily colored, out of a total of 51 that were started from the same inoculum. The remaining cultures turned brown shortly after inoculation and were discarded as browning developed. The healthy sister cultures were divided into control and treated groups. The TDC activity of the control cultures followed the normal pattern of induction upon inoculation into PM. The level of tryptamine dropped from 5.1 μmol/L on d 0 to 0.4 μmol/L on d 3. Tryptamine content remained below 1.0 μmol/L thereafter, most notably at the end of the culture period when the calculated rate of increase of TIA content was low (<10 μmol L−1 d−1). The reduced capacity to synthesize tryptamine was most apparent during the 2nd week of culture. Whereas addition of 50, 100, or 500 μm loganin on d 0 increased alkaloid content, it did not have a significant effect when done on d 11 (Fig. 2); rather, feeding loganin on d 11 resulted in the accumulation of secologanin. Alkaloid content rose considerably when tryptamine was supplied along with loganin (Fig. 2). Addition of tryptamine alone, however, did not increase TIA content.
Figure 2.
Effect of feeding loganin on TIA accumulation under conditions of low tryptamine availability. White bars represent cultures treated on d 0 and sampled on d 3; shaded bars represent cultures treated on d 11 and harvested on d 14. The concentration of exogenously added precursors is expressed in micromoles. L+T indicates that both loganin and tryptamine were added. Triplicate cultures were pooled upon harvesting and analyzed as single samples.
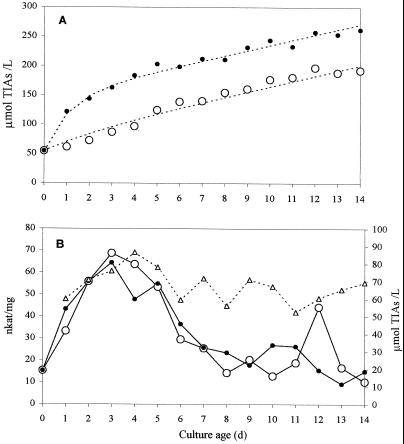
To investigate the influence of TDC activity on alkaloid production, when secologanin supply is not limiting, an experiment was performed in which one set of cultures was kept under conditions of loganin abundance for the entire production period. Two time courses of 14 d were carried out: one with loganin-fed cells and the other without feeding. Beginning on d 0, 200 μm loganin was added daily to a different set of flasks, which was harvested the following day, along with a set of control, nonfed cultures. Alkaloid accumulation was higher in fed cultures at all points during the production test (Fig. 3A). Tryptamine content followed the normal pattern, reaching a maximum of 2.4 μmol/L on d 3 in control cultures and of 1.5 μmol/L on d 2 in fed cultures. TDC activity also followed the normal pattern and was very similar in both sets of cultures, indicating that the presence of exogenous loganin did not affect the amount of active enzyme in the cells. The daily increase in alkaloid content showed a downward trend, which paralleled, but was not proportional to, the drop in TDC activity. The difference in alkaloid content between control and fed cultures was rather constant (67.4 ± 9.3 μmol/L), and was not affected by fluctuations in tryptamine content nor by changes in the level of TDC activity (Fig. 3B). Feeding 200 μm tryptamine along with loganin did not significantly increase alkaloid content relative to cultures fed only loganin, whether performed at the beginning (d 2) or near the end (d 10) of the culture period.
Figure 3.
Time course of alkaloid production (A) and TDC activity (B) under normal conditions (○) and in the presence of 200 μm exogenous loganin (•). Δ, Daily difference in alkaloid content between treated and control cultures. Duplicate or triplicate cultures were pooled upon harvesting and analyzed as single samples.
Loganin utilization was generally low; only 25 to 35% of the exogenous loganin was converted into alkaloids. Still, loganin feeding was far more effective than secologanin feeding. The capacity to utilize exogenous secologanin (500 μm) was tested in a small number of cultures, which showed an increase in TIA content that was only 10% of that achieved with loganin. Cultures of the wild-type line CRPM were also supplied with precursors. CRPM does not normally accumulate TIAs, but it did utilize exogenous loganin for alkaloid production, even more so when fed tryptamine concurrently. The alkaloid content of CRPM, however, reached levels much lower than in the transgenic line (<40 μmol/L), suggesting that other factors, possibly including the characteristically low STR activity, limit its biosynthetic capacity. The C. roseus cultures did not accumulate tryptamine and secologanin concurrently; no evidence of the existence of separate accumulation sites for these precursors was thus obtained. Alkaloids were never detected in the culture medium.
DISCUSSION
Thanks to the constitutively elevated levels of STR activity that characterize transgenic line S10, induction of the indole and iridoid pathways can be readily detected in S10 cultures by measuring tryptamine and TIA content, and the level of utilization of exogenous precursors. Induction of tryptamine synthesis takes place very rapidly after transfer to PM, whereas the iridoid pathway takes longer to become fully activated. The rapid utilization of the small tryptamine pool for TIA biosynthesis signals the flux of iridoids through to the strictosidine synthesis step. High rates of TIA accumulation can occur when TDC activity, a measure of the amount of active enzyme present in the cells, and tryptamine availability are low (Table I; Fig. 3). This indicates that the rate of TIA biosynthesis depends on the rapid turnover of tryptamine rather than on its accumulation, and that high levels of TDC are not required for this rapid turnover to occur. Under regular conditions tryptamine accumulation, as well as the rate and degree of TIA accumulation, are all independent of TDC activity; this is also the case when iridoid precursors are abundant (Fig. 3B). Our results are consistent with the lack of correlation between TDC activity and TIA biosynthesis generally found in cell cultures (Knobloch and Berlin, 1983; Mérillon et al., 1986; Eilert et al., 1987; Facchini and Di Cosmo, 1991; Islas et al., 1994). Apparently, factors other than TDC activity, such as the availability of Trp and secologanin, the activity of other tryptamine-utilizing enzymes, and tryptamine transport across the tonoplast, more strongly influence flux through the indole pathway. However, it has been reported that TDC activity in developing seedlings of Cinchona ledgeriana increases after a large pool of Trp has formed, and falls to undetectable levels once the Trp has been converted into tryptamine (Aerts et al., 1990), suggesting that, unlike in cell cultures, precisely timed changes in the level of TDC activity do play a role in alkaloid biosynthesis in the intact plant.
The utilization of tryptamine for TIA production appears to induce the cells to synthesize more tryptamine. Control cells that were not accumulating large amounts of alkaloids at the end of the production period did not show high tryptamine content, but their capacity to rapidly synthesize tryptamine became apparent upon addition of loganin. Tryptamine has been proposed to feedback inhibit TDC activity (Noé et al., 1984); its utilization may therefore have a de-inhibitory effect. We consistently detected similar levels of TDC activity in cells that were actively synthesizing alkaloids and cells that were not. The possibility that any positive influence that tryptamine utilization may have on flux through the indole pathway affects an earlier step leading to increased Trp availability merits further study.
Our results, particularly those obtained when using the tryptamine-deficient cultures (Fig. 2), show that an abundant supply of both precursors, tryptamine and secologanin, must exist for high rates of strictosidine synthesis to occur. Exogenous loganin increases alkaloid content when tryptamine is not limiting; addition of loganin causes the rapid utilization of tryptamine, at which point addition of tryptamine may have a positive effect. Loganin deficiency is limiting when tryptamine is abundant, and vice versa. This model accounts for the observations reported in the literature, except for the occasional negative effects of Trp and tryptamine on TIA accumulation, and accurately predicts the outcome of our experiments. The relative rate of biosynthesis of tryptamine and secologanin, and therefore also the limitation in the supply of either precursor, are cell-line-specific phenomena. The effect of feeding precursors depends on the metabolic status of the subject cell line, which is a function of a large number of variables that affect the steady-state concentration of a particular metabolite. Cell cultures will thus constitute more useful experimental systems when at least some of those variables can be assigned a value. The influence of the shikimate pathway on TIA biosynthesis will be more fully understood if we are able to manipulate relevant enzymatic activities in a high-STR-activity background, such as found in S10, under conditions of iridoid abundance. A number of genes encoding enzymes of the shikimate pathway are available from other plant species, constituting starting points to further study TIA biosynthesis in C. roseus.
Abbreviations:
- PM
production medium
- STR
strictosidine synthase
- TDC
Trp decarboxylase
- TIA
terpenoid indole alkaloid
Footnotes
This work was supported by the National Science Foundation under a grant awarded to C.C. in 1995.
LITERATURE CITED
- Aerts RJ, Van der Leer T, Van der Heijden R, Verpoorte R. Developmental regulation of alkaloid production in Cinchona seedlings. J Plant Physiol. 1990;13:86–91. [Google Scholar]
- Canel C, Lopes-Cardoso MI, Whitmer S, Van der Fits L, Van der Heijden R, Hoge JHC, Verpoorte R (1998) Effects of over-expression of strictosidine synthase and tryptophan decarboxylase on alkaloid production by cell cultures of Catharanthus roseus. Planta (in press) [DOI] [PubMed]
- Döller G, Alfermann AW, Reinhard E. Produktion von Indolalkaloiden in Callus-kulturen von Catharanthus roseus. Planta Med. 1976;30:14–20. doi: 10.1055/s-0028-1097686. [DOI] [PubMed] [Google Scholar]
- Eilert U, De Luca V, Kurz WGW, Constabel F. Alkaloid formation by habituated and tumorous cell suspension cultures of Catharanthus roseus. Plant Cell Rep. 1987;6:271–274. doi: 10.1007/BF00271996. [DOI] [PubMed] [Google Scholar]
- Facchini PJ, DiCosmo F. Secondary metabolite biosynthesis in cultured cells of Catharanthus roseus (L.) G. Don immobilized by adhesion to glass fibres. Appl Microbiol Biotechnol. 1991;35:382–392. doi: 10.1007/BF00172730. [DOI] [PubMed] [Google Scholar]
- Knobloch KH, Berlin J. Influence of phosphate on the formation of the indole alkaloids and phenolic compounds in cell suspension cultures of Catharanthus roseus. I. Comparison of enzyme activities and product accumulation. Plant Cell Tissue Organ Cult. 1983;2:333–340. [Google Scholar]
- Krueger RJ, Carew DP. Catharanthus roseus tissue culture: the effect of precursors on growth and alkaloid production. J Nat Prod. 1978;41:327–331. [PubMed] [Google Scholar]
- Islas I, Loyola-Vargas VM, Miranda-Ham ML. Tryptophan decarboxylase activity in transformed roots from Catharanthus roseus and its relationship to tryptamine, ajmalicine, and catharanthine accumulation during the culture cycle. In Vitro Cell Dev Biol. 1994;30P:81–83. [Google Scholar]
- Mérillon JM, Doireau P, Guillot A, Chénieux JC, Rideau M. Indole alkaloid accumulation and tryptophan decarboxylase activity in Catharanthus roseus cells cultured in three different media. Plant Cell Rep. 1986;5:23–26. doi: 10.1007/BF00269710. [DOI] [PubMed] [Google Scholar]
- Moreno PRH, Van der Heijden R, Verpoorte R. Effect of terpenoid precursor feeding and elicitation of formation of indole alkaloids in cell suspension cultures of Catharanthus roseus. Plant Cell Rep. 1993;12:702–705. doi: 10.1007/BF00233423. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol. 1962;15:473–497. [Google Scholar]
- Naudascher F, Doireau P, Guillot A, Viel C, Thiersault M. Time-course studies on the use of secologanin by Catharanthus roseus cells cultured in vitro. J Plant Physiol. 1989a;134:608–612. [Google Scholar]
- Naudascher F, Doireau P, Guillot C, Thiersault M. Time-course studies on the use of loganin by Catharanthus roseus cells cultured in vitro. J Plant Physiol. 1989b;135:366–368. [Google Scholar]
- Noé W, Mollenschot C, Berlin J. Tryptophan decarboxylase from Catharanthus roseus cell suspension cultures: purification, molecular and kinetic data on the homogeneous protein. Plant Mol Biol. 1984;3:281–288. doi: 10.1007/BF00017782. [DOI] [PubMed] [Google Scholar]
- Pennings EJM, Hegger I, Van der Heijden R, Duine JA, Verpoorte R. Assay of tryptophan decarboxylase from Catharanthus roseus cell cultures by high-performance liquid chromatography. Anal Biochem. 1987;165:33–136. doi: 10.1016/0003-2697(87)90210-7. [DOI] [PubMed] [Google Scholar]
- Pennings EJM, Van den Bosch RA, Van der Heijden R, Stevens LH, Duine JA, Verpoorte R. Assay of strictosidine synthase from plant cells by high-performance liquid chromatography. Anal Biochem. 1989;176:412–415. doi: 10.1016/0003-2697(89)90333-3. [DOI] [PubMed] [Google Scholar]
- Schripsema J, Verpoorte R. Search for factors involved in indole alkaloid production in cell suspension cultures of Tabernaemontana divaricata. Planta Med. 1992;58:245–249. doi: 10.1055/s-2006-961445. [DOI] [PubMed] [Google Scholar]
- Stevens LH (1994) Formation and conversion of strictosidine in the biosynthesis of monoterpenoid indole and quinoline alkaloids. PhD Thesis. Leiden University, The Netherlands
- Van der Heijden R, Lamping PJ, Wijnsma R, Verpoorte R. High-performance liquid chromatographic determination of indole alkaloids in a suspension cell culture of Tabernaemontana divaricata. J Chromatogr. 1987;396:287–295. [Google Scholar]
- Verpoorte R, Van der Heijden R, Schripsema J, Hoge JHC, Ten Hoopen HJG. Plant cell biotechnology for the production of alkaloids: present status and prospects. J Nat Prod. 1993;56:186–207. [Google Scholar]
- Zenk MH, El-Shagi H, Arens H, Stöckigt J, Weiler EW, Deus B (1977) Formation of the indole alkaloids serpentine and ajmalicine in cell suspension cultures of Catharanthus roseus. In W Bare, E Reinhard, MH Zenk, eds, Plant Tissue Culture and Its Bio-Technological Application. Springer-Verlag, Berlin, pp 27–44