Abstract
Antiviral prophylaxis has proved successful for prevention of cytomegalovirus (CMV) disease in solid organ transplant (SOT) patients; though emerging data suggest that antiviral agents interfere with immunity, and may inhibit immune-priming. In this context, we investigated levels and phenotype of primary CMV-specific immune responses that developed during antiviral prophylaxis in a cohort of CMV seronegative recipients (R−) of a SOT from a seropositive donor (D+). We longitudinally monitored CMV viral load, antibodies and levels of the negative immuno-modulator IL-10. PBMC were stimulated with CMV-specific peptide libraries to measure CD137 activation marker on CMV-specific T-cells and levels of PD-1 receptor, which is overexpressed on exhausted T-cells. Unexpectedly, the majority (13/18) of D+R− patients who developed a primary CMV response showed early post-transplant CMV-specific responses, though levels of PD-1 on CMV-specific T-cells remained elevated throughout prophylaxis. A strong inverse association was found between levels of plasma IL-10 and CMV-specific cellular immune responses. Our study suggests that during prophylaxis, subclinical CMV infection might have occurred in the D+R− patients, and primary CMV-specific responses were detected early post-transplant when levels of plasma IL-10 were low. Extended prophylaxis or antiviral treatment did not appear to suppress CMV-specific antibodies or T-cells, which however showed exhaustion phenotypes.
Keywords: CMV, CD137, IL-10, PD-1, SOT
Introduction
Universal antiviral prophylaxis with the orally available agent valganciclovir (VALGAN) has proved successful in limiting cytomegalovirus (CMV) disease, reducing mortality and graft rejection in high-risk CMV seronegative recipients (R−) of a solid organ transplant (SOT) from a seropositive donor (D+)[1,2]. Without antiviral prophylaxis, CMV disease occurs with incidence up to 72% in D+R− patients, during the first 3 months post-transplant, when patients are receiving intensive immunosuppressive agents for prevention of graft rejection[3]. In immunosuppressed D+R− SOT patients, CMV can replicate in the absence of adequate immunity during primary infection, predisposing these patients to a significantly increased risk of progressing to life-threatening CMV complications. With a standard 3-month course of antiviral prophylaxis, late-onset CMV disease is common after the completion of universal prophylaxis[4,5]. It typically develops between 3 to 6 months after SOT, with an incidence estimated to be approximately 17–37% among D+R− patients[6]. Late-onset CMV disease is a major clinical problem, which is associated with morbidity, substantially higher treatment costs, and is independently associated with mortality in the first year post-SOT[4,6]. A recent study has shown efficacy and safety of extended valganciclovir prophylaxis, but considerable debate remains regarding the efficacy of prolonging prophylaxis as a potential option for reducing late-onset CMV disease[6–11]
Development of antiviral resistance is a concern with longer durations of prophylaxis[12]. It has also been proposed that the development of long-term protective immunity against CMV may be compromised with the prolonged use of potent antiviral agent such as valganciclovir/ganciclovir (GCV)[13]. While there is evidence for an immunosuppressive activity of GCV[14], mainly associated with delayed CMV seroconversion and antibody maturation[15], the issue of whether antiviral prophylaxis delays, interfere and/or alters CMV-specific T-cell responses in SOT is controversial[16–20].
Breakthrough asymptomatic CMV viraemia is clinically detectable in <5% of D+R− patients during the first 3-months of antiviral prophylaxis[21–23]. In a previous study[19], we detected primary CMV-specific T-cell responses at the time of discontinuation of VALGAN prophylaxis (at 3 months post-transplant) in liver D+R− patients, suggesting that a CMV response may have started to develop early post-transplant, during antiviral prophylaxis. Interestingly, elevated inhibitory immune-signaling was also detected on the primary CMV-specific T-cells[24,25]. The state of immune-impairment was particularly marked in those liver D+R− patients who subsequently developed severe CMV-associated symptoms after discontinuation of antiviral prophylaxis[26].
The aim of the current study was to specifically define the timing of CMV-specific immune responses in high-risk D+R− patients who received antiviral prophylaxis after SOT. In this context, we sought to investigate and characterize primary CMV-specific immunity developed during VALGAN antiviral prophylaxis in a cohort of 16 kidney and 12 liver D+R− patients.
Patients and Methods
Patient population
The Institutional Review Boards (IRB) of the University of Washington Medical Center (UWMC, IRB 24704) and the City of Hope Comprehensive Cancer Center (COH, IRB 04024) approved this prospective longitudinal study. Thirty consecutive R− patients who received (in accordance with the Declaration of Istanbul) at UWMC either kidney (17) or liver (13) transplant from a D+ individual were enrolled, after informed consent was obtained (Table 1). Patients received induction therapy with daclizumab or antilymphocyte antibodies and maintenance immunosuppression with prednisone, tacrolimus or cyclosporine, and either azathioprine or mycophenolate mofetil[4]. UPN 10, who had history of hepatocellular carcinoma, was switched to sirolimus-based immunosuppression one month after transplant, since sirolimus is associated with increased survival after liver transplantation for malignancy[27]. Immunosuppression was altered due to CMV viraemia in UPN 1, and CMV disease in UPN 12, 19, 21, 26 and 30. Dose reduction of the immunosuppressive therapy was individualized as recommended[28]. Rejection was treated with steroids or/and anti-thymocyte globulin (ATG) (Table 1), as previously detailed[5]. Valganciclovir (Valcyte, Roche, Nutley, NJ) 450–900 mg/day (dose adjusted to renal function, according to manufacturer recommendation) was used as CMV prophylaxis in all patients. Antiviral resistance testing was performed as previously described when there was a clinical suspicion for resistance[29]. Specific indications for antiviral resistance testing included: failure to improve symptomatically or lack of reduction in viral load with GCV (Cytovene™ Roche, Nutley, NJ) therapy[30–32]. For UPN 05, 13 and 15 a prolonged VALGAN course (5 or 6 months) was administered, while all the other patients received a standard prophylaxis course (3 or 3.5 months). Intravenous GCV or VALGAN were used to treat CMV disease or asymptomatic viraemia (900 mg twice a day; Table 1)[4]. UPN 01 was lost to follow up at 5 months post-transplant. CMV serology at time of transplant was confirmed for all patients. UPN 17 and 18 were not included in this study because they were found to be CMV seropositive at time of transplant.
Table 1.
Population demographics, CMV treatments, viraemia, immunity, IL-10
| Patient UPN | Sex/Age | a SOT type | b Prophylaxis duration | c Viremia onset | d CMV endpoint | e IgM/ IgG | e pp65/IE-1 T-cells | g IL-10 (pg/ml) at onset |
|---|---|---|---|---|---|---|---|---|
| 01 | M/32 | K | 3.5 | 3 | none | 3 | 3 | 1 (485.5) |
| 02 | M/58 | L | 3.5 | 4.5 | none | 5 | 5 | 4.5 (12) |
| 03 | M/34 | K | 3.5 | none | none | 3.5 | 3 | none |
| 04 | F/55 | K | 3 | none | none | none | none | none |
| 05 | M/55 | K | 6 | none | none | none | none | none |
| 06⋄ | F/35 | K | 3.5 | none | none | 2 | 1.5 | 0.5 (10) |
| 07 | M/59 | L | 3 | none | none | none | none | none |
| 08 | M/61 | L | 3.5 | 3.5 | none | 3.5 | 3 | 1 (39.2) |
| 09 | M/55 | L | 3 | none | none | none | none | none |
| 10 | M/53 | L | 3.5 | 4.5 | 7: dz | 2.5 | 4.5 | 3 (19) |
| 11 | M/59 | L | 3 | none | none | none | none | none |
| 12 | M/49 | K | 3.5 | 4.5 | 4.5: dz | 2 | 4 | 3.5 (10) |
| 13 | M/41 | K | 5 | none | none | none | none | none |
| 14 | M/65 | L | 3 | none | none | none | none | none |
| 15 | M/59 | K | 6 | 1.5 | 2.5: vir | 2.5 | 1.0 | 2 (21.7) |
| 16 | M/55 | L | 3 | none | none | none | none | none |
| 19 | F/52 | K | 3 | 3.5 | 4: dz | 5 | 2.5 | 3 (25) |
| 20 | M/62 | K | 3.5 | none | none | none | none | none |
| 21⋄ | M/57 | L | 3 | 4.5 | 4.5: dz | 5 | 4.5 | 3.5 (14) |
| 22 | M/64 | K | 6 | none | none | none | 4 | none |
| 23 | M/54 | K | 3 | 4.5 | 4.5: dz | 5 | 6.5 | 3 (10) |
| 24 | M/56 | L | 3 | none | none | none | none | none |
| 25 | F/39 | L | 3 | 4.5 | none | 4.5 | 4.5 | 3 (10) |
| 26 | F/30 | K | 3.5 | 4.5 | 5: dz | 5.5 | 3 | 5 (23) |
| 27 | M/60 | L | 3 | none | none | none | 5.5 | 3 (10) |
| 28 | M/56 | K | 6 | none | none | none | 2.5 | none |
| 29 | F/59 | K | 3.5 | 6 | 6.5: dz | 6.5 | 3 | 6 (30) |
| 30 | M/71 | K | 3 | 3.5 | 3.5: dz | 3.5 | 3 | 3.5 (10) |
Anti-rejection with prednisolone and ATG at 0.5 and 1.5 months post-SOT for UPN 06, and at 1 month post-SOT for UPN 21;
Kidney (K) or liver (L) SOT type. The numbers indicate the post-SOT month:
in which antiviral prophylaxis was stopped;
of first viraemia detection;
of CMV infection requiring antiviral treatment for either disease (dz) or viremia (vir);
in which there was the first detection of CMV-specific humorale/cellularf immunity;
of first detection of IL-10, in parentheses are reported levels of IL-10 as pg/ml. Numbers in underlined italic indicate a time point during antiviral prophylaxis.
Blood specimen collection and logistics
Blood specimens were collected according to the United States Public Health Service guidelines and Helsinki doctrine, either at UWMC or at the patient’s Primary Care Provider (PCP). Specimens were shipped overnight to COH and to UWMC[19]. Blood specimens were collected bi-weekly up to 9 months post-transplant (first blood draw: 15 days after transplant). Standard hematology laboratory tests and complete blood counts with differential were performed on a Cell Dyn 3700 Abbott (Abbott Diagnostics, Abbott Park, IL). Clinical and diagnostic assays were done at UWMC, immune monitoring was performed at COH.
Viraemia
Quantitative TaqMan real time polymerase chain reaction (PCR, sensitivity 100 copies/ml) to test CMV viral load was performed as previously described in all patients for research study purposes, and the results weren’t made available to treating clinicians[33]. Throughout the study, clinicians obtained diagnostics studies for suspected CMV viraemia/disease.
Anti-CMV antibodies
Clinical serologic procedures to screen D+ and R− patients for CMV antibodies were performed by latex agglutination (CMV-scan, Becton-Dickinson, Franklin Lakes, NJ). IgM and IgG were qualitatively assessed for each recipient at multiple time points in serum, by ELISA (IgM: Trinity Biotech USA, Jamestown NY; IgG: Wampole Laboratories, Princeton, NJ, USA).
IL-10 measurements
Levels of IL-10 were quantitatively measured in the plasma of D+R− patients by “Human IL-10 ELISA Ready-SET-GO!” (assay sensitivity: 2 pg/ml; eBioscience, San Diego, USA), following manufacturer’s procedure.
Cell stimulation and surface marker staining
Peripheral blood mononuclear cells (PBMC) were stimulated with pp65 and IE-1 peptide library (JPT, Berlin, Germany), as previously described[19,26]. Cells were then stained with antibodies anti-CD8, CD4, CD137 and PD-1. For each sample ~0.5M PBMC were routinely acquired, and at least 60,000 events from CD4/CD8 T-cell gates were analyzed by FACS (FACSCanto with FACSDiva software; all from BD Biosciences, San Jose, CA)[19,24]. The number of pp65 or IE-1 peptide library specific CD4+ and CD8+ T-cells/μl was determined by multiplying the percentages of specific T-cells positive for CD137 by the relevant absolute CD4+ and CD8+ T-cell counts, based on lymphocyte absolute counts, and on PBMC surface staining.
Statistical analysis
Association analyses between IL-10, CMV-viraemia and immune-responses were performed using generalized estimating equations models (GEE; R GEE package, http://www.R-project.org) to accommodate repeated measurement in the same individuals. A GEE linear model was used to test the association of IL-10 with CMV-specific T-cell responses, to avoid reliance on the threshold of 20 pg/ml, selected a posteriori for data summary. The p values are indicated for each statistical analysis.
Results
Asymptomatic and symptomatic CMV viraemia
CMV viraemia was longitudinally measured in the whole D+R− SOT population. Fifteen D+R− patients did not have any detectable viraemia throughout the observation period. Breakthrough viraemia was detected in 3 D+R− patients during antiviral prophylaxis, though none of them showed resistance and all remained asymptomatic (Table 1). In particular, for UPN 15 (max CMV DNAemia: 6.5×104 copies/ml, at 2 months post-transplant) additional VALGAN effectively controlled CMV viraemia. In the case of UPN 01 (max CMV DNAemia: 3.5×103 copies/ml, at 3 months post-transplant) and UPN 08 (max CMV DNAemia: 2.9×104 copies/ml, at 4 months post-transplant) prospectively collected blood samples demonstrated high-grade viremia that ultimately was spontaneously controlled without any additional antiviral therapy. After prophylaxis discontinuation, asymptomatic CMV viraemia was detected in UPN 02 and 25, at 4.5 months post-transplant. CMV disease developed in 8/28 D+R− patients (28.6%,) after prophylaxis suspension, between 3.5 and 7 months after transplant, in accordance with results from a similar transplant setting[4,6,34]. In particular, UPN 10 and 12 were diagnosed with CMV colitis; UPN 12, 19, 23 and 30 with CMV syndrome; UPN 21 with CMV esophagitis; UPN 26 with CMV gastritis; UPN 29 with CMV pneumonitis and colitis.
Timing of CMV-immunity rise during prophylaxis
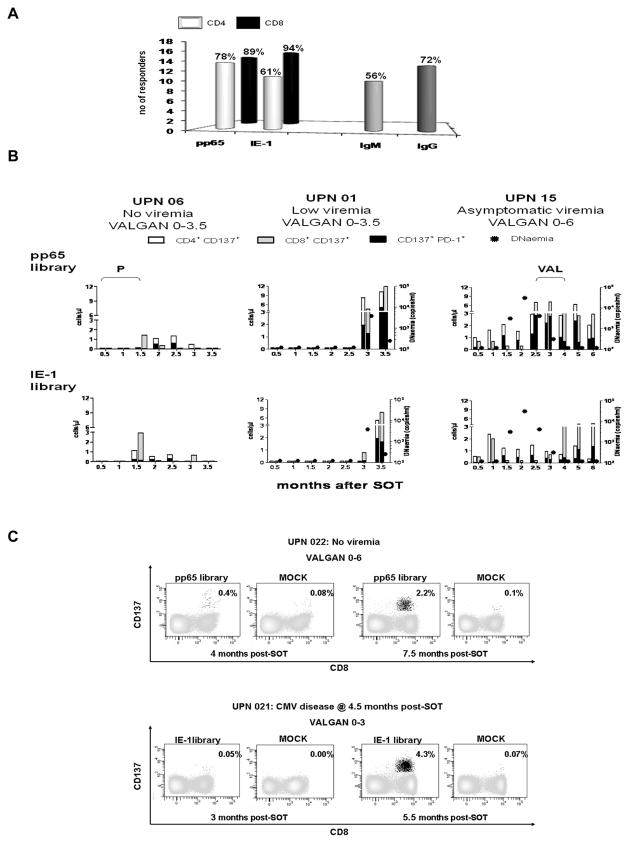
In the D+R− cohort, we measured a primary cellular and humoral CMV-specific response in a total of 18 patients (Figure 1A); while for the remaining 10 recipients there was neither CMV sero-conversion nor detection of pp65/IE-1 specific T-cells or plasma IL-10 during the observation period (Table 1). Responses to pp65 and IE-1 libraries were comparable, with dominance of CMV-specific CD8 T cells[17,19,35,36]. CMV-specific IgG were found in the large majority of the responders (72%), while only in half of them CMV-specific IgM were detected[15]. Primary CMV immunity developed during antiviral prophylaxis in thirteen of the eighteen CMV responder recipients (Table 1). Timing of humoral and cellular responses varied among patients. The earliest detection was at 1 month post-transplant for pp65 and IE-1-specific T-cells and at 2 months post-transplant for both CMV IgG and IgM antibodies. No detectable viraemia (≥100 copies/ml) was found at these early post-transplant time points. None of the early CMV responders showed leucopenia (<3000 leucocytes/mm3) preceding or at time of detection of CMV-specific immune response[38]. Five patients showed both humoral and cellular CMV-specific response during prophylaxis, while disease patients UPN 10 and 12, had CMV specific antibodies only, and UPN 19, 29 and 30 a T-cell response, with no concomitant detection of CMV antibodies. With the exception of aviremic UPN 03, 22 and 28, a wide range of plasma IL-10 levels were found at different time points post-transplant in all the recipients who developed a primary CMV response while on antiviral prophylaxis. The finding that CMV-specific immune priming can occur during antiviral prophylaxis was unexpected[17,37].
Figure 1. CMV specific responses in the population.
(A) Histograms report respectively percentages of responders to CMV pp65 and IE-1 peptide libraries (as indicated on the x axis) for both CD4 (white bars) and CD8 compartments (black bars); and the fraction of responders in which CMV-specific IgG and IgM antibodies (shown on the x axis) were detected within the total number of patients, who showed a primary CMV response during the observation period. (B) PBMC from CMV asymptomatic UPN 06 (left plots), 01 (middle plots) and 15 (right plots) were stimulated with either pp65 library (upper panel) or IE-1 library (lower panel), to measure CD137 and PD-1 surface expression on both CD4 (white bars) and CD8 (grey bars) T-cells. Each histogram bar shows the number of T-cells/μl (y axes) expressing the activation marker CD137, at the time point after SOT (month) indicated on the x axes. The black filling in each bar represents the number of T-cells/μl cells that were also expressing PD-1 (CD137+/PD-1+ T-cells). The circular filled symbols indicate viraemia levels (expressed as copies/ml of CMV DNAemia, z axes) in the recipients’ plasma at the time point after SOT (month) indicated on the x axes. For UPN 06 the z axes are not shown, since this recipient did not have any detectable CMV viraemia, and “P” indicates anti-rejection treatments at 0.5 and 1.5 month after SOT (Table 1). For UPN 15, “VAL” shows the length of enhanced VALGAN treatment administered to control viraemia (900 mg/twice a day, Table 1). The length of antiviral prophylaxis (either 3.5 or 6 months) is indicated on the top plots, for each UPN. (C) Representative flow cytometric plots showing the progressive development of a primary CMV response (as % of CD137+ CD8+ T-cells) starting either during VALGAN prophylaxis (UPN 22, pp65 specific response) or after its suspension (UPN 21, IE-1 specific response). In MOCK plots, peptide library diluent (DMSO) was added.
Post-transplant development of pp65 and IE-1 specific T-cells
The cellular CMV-specific response was evaluated by measuring the levels of the CD137 surface marker on CD8 and CD4 T-cells stimulated for 24 hours with either pp65 or IE-1 CMV antigens (Figure 1)[26,39]. Both pp65 and IE-1 specific responses were detected in CD8 and CD4 T-cells during antiviral prophylaxis, in the presence or absence of viraemia. In the case of UPN 06 (Figure 1B, left panel), steroid treatment for rejection (Table 1) was accompanied by transient lymphopenia (180 cells/mm3, at 2 months post-SOT); the patient was still able to mount detectable humoral and cellular primary CMV-specific responses at 1.5 months post-transplant, even in the absence of CMV viraemia. In UPN 01 (Figure 1B, middle panel), a predominant pp65 T-cell response was detected during VALGAN prophylaxis (at 3 months post-transplant) with simultaneous breakthrough CMV viraemia of 3.5×103 copies/ml. Interestingly, 15 days later the viraemia dropped more than 15 fold, in the presence of both increased pp65 and emerging levels of IE-1 specific T-cells. Figure 1C illustrates representative flow cytometric plots of longitudinal CMV-specific T-cell profiles in non-viremic UPN 22, who developed a pp65 specific response during VAL prophylaxis, and of CMV disease UPN 21, in whom IE-1 specific T-cell were detected after its discontinuation.
The expression of the negative immune-modulator PD-1[24,26] was up-regulated on primary pp65 and IE-1 specific T-cells that developed during VALGAN prophylaxis (Figure 1B). PD-1 expression varied among patients, was not correlated with neutropenia and could affect more than 50% of the CMV-specific T-cells. Interestingly, in the case of UPN 15 (Figure 1B, right panel), PD-1 levels strongly increased when enhanced VALGAN treatment was required to control asymptomatic viraemia. Viral replication in the presence of incompletely suppressive drug exposure has been suggested to be an important risk factor for development of antiviral resistance. Thus, re-establishment of viral suppression by increasing the drug dosage was done on a clinical basis to reduce the likelihood for emergence of resistance[40]. In this patient, the very early response mounted in the absence of viraemia, increased substantially with the rise of CMV viraemia, during enhanced VALGAN administration, and was maintained throughout the prolonged (6 months) course of antiviral prophylaxis, even after viraemia was controlled[16]. These results indicate that during antiviral administration naive CMV-specific T-cells can be detected, and are in line with previous findings showing that antivirals may not necessarily interfere with the maintenance of CMV-specific cellular immune responses during primary viraemia[16].
CMV specific antibodies during prophylaxis
Parallel to the measurements of the CMV-specific cellular response, a longitudinal qualitative study to detect the presence of CMV-specific antibodies was performed in recipient sera, using qualitative ELISA. Five kidney and two liver D+R− patients sero-converted during prophylaxis (Table 1). A complete CMV-specific IgM and IgG antibody panel was detected only in UPN 01, during antiviral prophylaxis. For UPN 08 and UPN 12, early CMV-specific IgM was followed by IgG antibody detection, after prophylaxis suspension; while for UPN 06 and 15, IgM persisted, but IgG was never detected[15]. In the case of UPN 03 and 10, only CMV-specific IgG was present in the sera of these recipients. Our findings indicate that CMV sero-conversion can occur in D+R− patients during VALGAN prophylaxis, though antivirals may have a role in delaying CMV-specific humoral response, since IgG maturation was mainly found after prophylaxis suspension[15].
IL-10 production and VALGAN administration
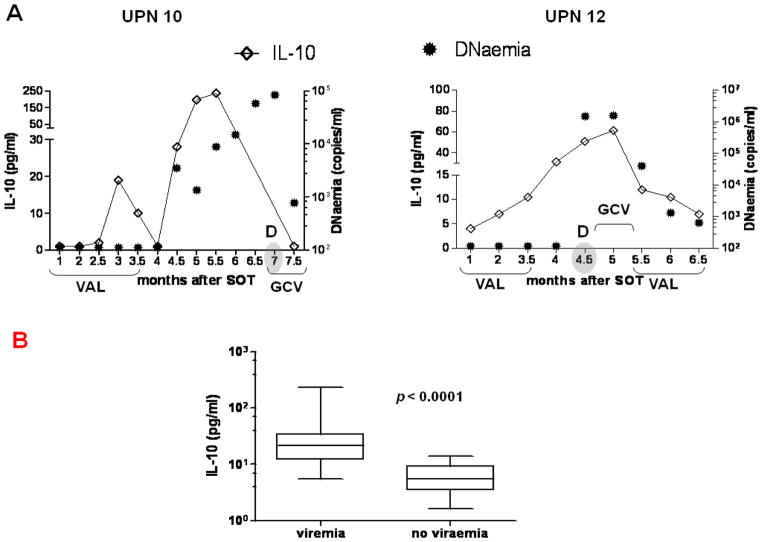
IL-10 was longitudinally monitored by quantitative ELISA in plasma[26]. High levels of plasma IL-10 were detected in all viraemic patients, who either remained asymptomatic or progressed to CMV disease (Table 1, Figure 2A)[26]. In particular, we found a highly significant difference (p<0.0001 by GEE models) between elevated levels of IL-10 measured at viraemic time points, compared to those in which viraemia was undetectable (Figure 2 B). In UPN 01 and 08, respectively 485.5 and 39.2 pg/ml of IL-10 were found immediately after SOT (Table 1 and Figure 3A, left panel). The IL-10 levels dramatically declined during prophylaxis administration, though asymptomatic viraemia eventually developed for both patients. All CMV disease patients had significant levels of IL-10 before and/or at CMV disease time points, which substantially dropped in parallel with viraemia, following GCV treatment (Figure 2 A–B)[26]. Interestingly, for both representative CMV disease UPN 10 and 12 (Figure 2A), IL-10 was detected at low levels early post-transplant, however immediately after prophylaxis suspension, IL-10 dramatically increased and CMV disease was diagnosed. Though sirolimus has been found to inhibit IL-10 signal transduction pathway[41], markedly high levels of IL-10 were detected in UPN 10, who was switched to a sirolimus-based immunosuppression (see Patient population)[27]. In general, levels of IL-10 either decreased or remained low during GCV/VALGAN treatment. When monocytopenia (<0.2 × 109/L) was detected at 2 or more consecutive blood draws, corresponding plasma measurements detected minimal levels of IL-10[42]. No correlation was found between neutropenia and IL-10 levels in patients who developed transient neutropenia (UPN 6, 8, 10, 15, 21, 23, 26, 27, 29, 30).
Figure 2. Plasma IL-10 levels.
(A) Longitudinal measurements, at the time points after SOT (months) showed on the x axes, of plasma IL-10 (line intersecting rhomboid symbols, y axes) and CMV viraemia, expressed as copies/ml of CMV DNAemia (circular filled symbols, z axes). Representative UPN 10 (left plot) and 12 (right plot), who developed CMV disease (as “D” on the plots; month of CMV disease diagnosis is shaded) are shown. The length of VALGAN (VAL) prophylaxis and GCV/VAL treatments are indicated on the x axes, for each UPN. (B) Vertical box plots show median, 25th and 75th percentiles of IL-10 measured at viremic or aviremic time points in the D+R− patients. The p values indicate the significance of the difference between the two groups, calculated using GEE models.
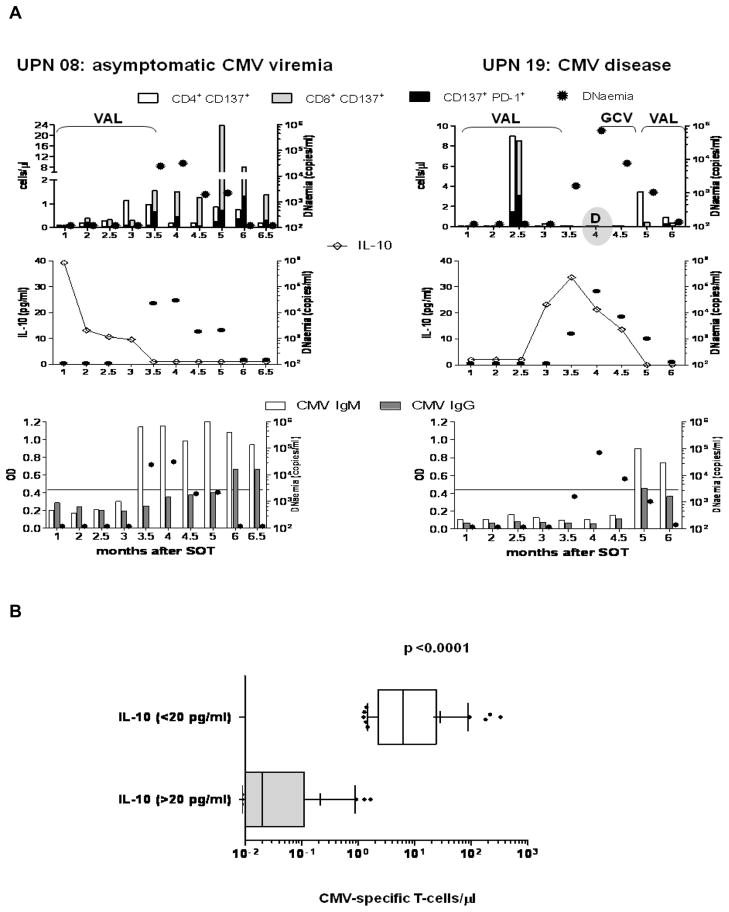
Figure 3. CMV-specific immune responses and IL-10 levels.
(A) Temporal profiles of CMV-specific T-cells (upper panel), antibodies (lower panel) and IL-10 (middle panel) for representative CMV asymptomatic UPN 08 (left plots) and CMV disease UPN 19 (right plots). CMV viraemia, expressed as copies/ml of CMV DNAemia (circular filled symbols, z axes) is shown in each plot. In the upper plots, CD137 and PD-1 surface expression on T-cells stimulated either with pp65 library (UPN 08) or IE-1 library (UPN 19) is shown, as detailed in Figure 1. In the middle plots IL-10 levels are represented as explained in Figure 2A. In the lower plots detection of CMV-specific IgM (white bars) and IgG (grey bars) is reported. The quantitative ELISA results are expressed as optical density (OD, y axes) measured at 450 nm. The line in the plots indicates the threshold of positivity for the tests. (B) The whisker plots show average (+) and 10–90 percentiles of CMV-specific T-cell levels at time points in which corresponding IL-10 measurements were either > (grey box) or < (white box) 20 pg/ml in the entire D+R− patient population. Step-function GEE models were used to calculate the p value.
CMV-specific immune responses do not develop in the presence of IL-10
D+R− patients had detectable CMV-specific IgM/IgG and pp65/IE-1 T-cells only when plasma IL-10 levels were low or undetectable. As shown in Figure 3A (left panel), pp65 T-cells were first detected at 3 months post-transplant for representative asymptomatic UPN 08, soon after IL-10 levels dropped. Fifteen days later, at the end of prophylaxis, CMV-specific IgM seroconversion was detected. While CMV viraemia began to increase, the patient was able to mount a pp65 specific T-cell response, and eventually CMV-specific IgG antibodies. At 5 months post-transplant, the viremia was ultimately controlled without GCV therapy, likely because of the robust CMV-specific primary response. Although this patient never progressed to clinical CMV symptoms, prospectively collected blood samples demonstrated high-grade viremia that ultimately was spontaneously controlled without any exogenous antiviral treatment, and this clearance of viremia was temporally related to the development of robust CMV-specific responses (Figure 3A, left panel). In contrast, we found that at time points at which IL-10 were elevated, CMV-specific T-cells either did not develop or were suppressed, as shown in the case of representative CMV disease UPN 19 (Figure 3A, right panel). For this patient early CD4 and CD8 IE-1-specific responses were detected at 2.5 months, in the absence of viremia. Fifteen days later, IL-10 abruptly increased, and the IE-1 cellular response became undetectable. Soon after prophylaxis suspension, viremia rose, and at 4 months post-transplant, CMV disease was diagnosed and GCV treatment administered. The subsequent viremia drop was accompanied by a significant decrease in IL-10, and concomitant initial restoration of IE-1-specific T-cells and detection of CMV-specific IgM/IgG antibodies.
A comprehensive statistical analysis using GEE models showed that there was a highly significant negative association between pp65/IE-1 T-cell responses and plasma IL-10 concentrations (p<0.0001). Figure 3B illustrates the striking difference detected in the whole patient population between CMV-specific T-cell responses measured at time points in which IL-10 levels were minimal (<20 pg/ml) compared to when they were higher (>20 pg/ml). As for CMV-specific IgG and IgM, the significance of the inverse association with IL-10 was marginal (p=0.02 by GEE).
Discussion
Extending anti-CMV prophylaxis to 6 months appears to provide a significant benefit in reducing the incidence of CMV disease and viremia in high-risk D+R− SOT patients, compared to 3 month prophylactic regimens[2,6]. While these results are encouraging, the impact of lengthening prophylaxis on the primary CMV immune response in D+R− SOT patients has not been established, and needs to be evaluated in a prospective clinical trial[9]. The aim of the current investigation was to define the time course of immune responses during primary CMV infection in immunosuppressed SOT recipients who received antiviral prophylaxis. Investigating whether an early post-transplant primary response against CMV has a role in protecting D+R− SOT patients from CMV complications may have important implications for the timing and regimen of antiviral prophylaxis.
We evaluated CMV-specific cellular responses by monitoring the levels of CD137 surface marker expressed on CMV-specific T-cells[26,35]. CD137 is a specific marker of recent activation, which is uniformly upregulated 24h after antigen stimulation on the surface of all T-cells, regardless of their differentiation stage or profile of cytokine secretion[39]. In the context of CMV immunity, it has been reported that the IFN-γ production of CD4 T-cells in response to the IE-1 library is frequently undetectable in healthy volunteers and SOT patients[35,43]. In contrast, both CD137+ CD4 and CD8 activated T-cells were found in the D+R− SOT patients in response to IE-1 library stimulation (Figure 1A–B and 3A, right panel). IE-1 specific CD4 T-cells may produce cytokines other than IFN-γ and/or have other functions which remain to be evaluated. Though the CD137 marker can be a convenient and informative tool in the context of clinical longitudinal studies relying on reduced amounts of patient blood specimen[26], the assay has limitations, since it does not provide insight into polyfunctional cytokine profiles and/or maturation stages of CMV specific T-cells[44–46]. Additionally, pp65 and IE-1 are largely immunodominant among CMV gene products[35], however usage of pp65 and IE-1 peptide libraries may underestimate the actual T-cell immune response against CMV, and a broader number of viral proteins and/or pool of peptides from multiple CMV antigens should be evaluated for PBMC stimulation in the context of CMV immune-monitoring[47,48].
The finding that CMV-specific immune priming occurs during antiviral prophylaxis was unexpected[17,18]. In contrast to what was previously hypothesized[18], our data suggest that the recipient does not remain naïve to CMV antigens until antiviral prophylaxis ceases. In our study, the majority (13/18) of the D+R− SOT patients, who developed a primary CMV response showed early post-transplant CMV-specific responses, even in the absence of CMV viraemia (Table 1). Our results indicate that antiviral prophylaxis may not completely suppress CMV replication. Local CMV replication at the graft site, generating undetectable levels of viremia (<100 copies/ml) may be sufficient for triggering CMV-specific immune activation[49,50].
As found in other chronic viral infections[51–53], PD-1 and IL-10 up-regulation affect both CD8 and CD4 T-cells during CMV infection, and may be followed by T-cell exhaustion and loss of antigen-specific T-cells, contributing to uncontrolled CMV replication and the development of clinical symptoms[24–26,54]. Results from the current D+R− SOT cohort show that primary pp65 and IE-1 specific T-cells expressed PD-1 during antiviral prophylaxis, both in the presence or absence of CMV viraemia (Figure 1B and 3A). Though antiviral prophylaxis is the CMV prevention strategy used at the University of Washington Transplant Program for D+R− patients, it would be of interest to assess levels of inhibitory immune-signaling in preemptively treated D+R− recipients[28].
IL-10 levels were significantly higher at viraemic time points compared to time points at which CMV viraemia was undetectable (Figure 2 and 3). Our observation is in agreement with a recent study, in which significantly higher concentrations of IL-10 were found in the plasma of viremic HIV patients[53]. In our population, plasma IL-10 levels were low at times of monocytopenia, which supports the finding that monocytes have a major role as IL-10 producers[42,53]. IL-10 production by CMV-specific T cells was not evaluated in this study due to limited patient specimens.
The mechanisms leading to IL-10 production remain unknown, and IL-10 measurements could vary because of degradation, reduced synthesis or instability[55]. Imbalanced levels of IL-10 have been described in several pathological contexts, including liver and kidney chronic disease and cancer[56–58]. Further studies will be required to assess the roles of CMV viraemia and the patient’s pre-transplant condition on the levels of IL-10. Moreover, IL-10 genotyping of SOT donors/recipients could provide valuable information since IL-10 gene polymorphism has been reported to play a role in herpes virus resistance, and a specific IL-10 genotype was found to reduce the incidence of CMV infection[59,60].
There was a strong inverse association between levels of plasma IL-10 and cellular immune responses: CMV-specific T-cells were not detectable or abruptly disappeared in the presence of levels of IL-10 >20 pg/ml (Figure 3). Levels of plasma IL-10 consistently declined in all patients either during antiviral prophylaxis or during enhanced antiviral treatment given to control CMV viraemia/disease. The strong inverse association between levels of plasma IL-10 and cellular immune responses suggest that concomitant assessment of these immune-parameters could be useful for guiding the appropriate duration of antiviral prophylaxis and may be helpful in managing high-risk D+R− SOT patients. The in vivo correlation between control of CMV viremia and development of a CMV-specific T-cell response (Figure 3A left panel) indicates that monitoring the development of a CMV-specific primary response and measurement of plasma IL-10 may have biological and clinical relevance. In this context, prolonging antiviral prophylaxis to 200 days may be appropriate in cases of elevated plasma IL-10 levels to avoid uncontrolled viraemia or late disease (Figure 2A; Figure 3A right panel). In contrast, patients who mount substantial CMV-specific T-cells and have low levels of plasma IL-10 may not require treatment if CMV viraemia is detected, since their immune system may be capable of controlling viraemia.
In conclusion, we advocate that reduction of CMV disease burden and improvement of clinical management of high-risk D+R− SOT patients will require the use of an individualized immune-guided antiviral strategy. Such a preventive strategy could be tested in a prospective interventional trial in which the duration of antiviral prophylaxis/treatment could be guided by monitoring the patient’s CMV-specific T-cell response and inhibitory immune-signaling.
Acknowledgments
These studies were partially supported by grants from the National Institute of Allergy and Infectious Diseases (NIAID; R21 AI084019-01) to CLR and APL; from the National Cancer Institute (NCI; R01-CA77544 and P01-CA30206, Project III) to DJD; from NCI (CA33572) to The City of Hope Comprehensive Cancer Center, and by The Edwin and Bea Wolfe Charitable Foundation to the Division of Translational Vaccine Research.
We acknowledge the assistance in volunteer recruiting, scheduling, and specimen shipment by UWMC General Clinical Research Center personnel including Sarah Johnson, Kristin Salmi, and Cherry Thuntarug. We thank volunteers and PCPs for kindly participating to the study. The authors wish to thank Nathan Feng for excellent technical assistance. The administrative assistance of Peter Kwon and Donna Packer is gratefully acknowledged.
Footnotes
Conflict of interest disclosure: The authors declare no competing financial interests.
Reference List
- 1.Humar A, Kumar D, Preiksaitis J, Boivin G, Siegal D, Fenton J, et al. A trial of valganciclovir prophylaxis for cytomegalovirus prevention in lung transplant recipients. Am J Transplant. 2005;5:1462. doi: 10.1111/j.1600-6143.2005.00866.x. [DOI] [PubMed] [Google Scholar]
- 2.Pescovitz MD. Valganciclovir: recent progress. Am J Transplant. 2010;10:1359. doi: 10.1111/j.1600-6143.2010.03112.x. [DOI] [PubMed] [Google Scholar]
- 3.Hodson EM, Jones CA, Webster AC, Strippoli GF, Barclay PG, Kable K, et al. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: a systematic review of randomised controlled trials. Lancet. 2005;365:2105. doi: 10.1016/S0140-6736(05)66553-1. [DOI] [PubMed] [Google Scholar]
- 4.Limaye AP, Bakthavatsalam R, Kim HW, Kuhr CS, Halldorson JB, Healey PJ, et al. Late-onset cytomegalovirus disease in liver transplant recipients despite antiviral prophylaxis. Transplantation. 2004;78:1390. doi: 10.1097/01.tp.0000145989.22373.03. [DOI] [PubMed] [Google Scholar]
- 5.Limaye AP, Bakthavatsalam R, Kim HW, Randolph SE, Halldorson JB, Healey PJ, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation. 2006;81:1645. doi: 10.1097/01.tp.0000226071.12562.1a. [DOI] [PubMed] [Google Scholar]
- 6.Humar A, Lebranchu Y, Vincenti F, Blumberg EA, Punch JD, Limaye AP, et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant. 2010;10:1228. doi: 10.1111/j.1600-6143.2010.03074.x. [DOI] [PubMed] [Google Scholar]
- 7.Palmer SM, Limaye AP, Banks M, Gallup D, Chapman J, Lawrence EC, et al. Extended valganciclovir prophylaxis to prevent cytomegalovirus after lung transplantation: a randomized, controlled trial. Ann Intern Med. 2010;152:761. doi: 10.7326/0003-4819-152-12-201006150-00003. [DOI] [PubMed] [Google Scholar]
- 8.Doyle AM, Warburton KM, Goral S, Blumberg E, Grossman RA, Bloom RD. 24-week oral ganciclovir prophylaxis in kidney recipients is associated with reduced symptomatic cytomegalovirus disease compared to a 12-week course. Transplantation. 2006;81:1106. doi: 10.1097/01.tp.0000204048.90367.97. [DOI] [PubMed] [Google Scholar]
- 9.Helantera I, Kyllonen L, Lautenschlager I, Salmela K, Koskinen P. Primary CMV infections are common in kidney transplant recipients after 6 months valganciclovir prophylaxis. Am J Transplant. 2010;10:2026. doi: 10.1111/j.1600-6143.2010.03225.x. [DOI] [PubMed] [Google Scholar]
- 10.Zamora MR. Controversies in lung transplantation: management of cytomegalovirus infections. J Heart Lung Transplant. 2002;21:841. doi: 10.1016/s1053-2498(02)00435-7. [DOI] [PubMed] [Google Scholar]
- 11.Valentine VG, Weill D, Gupta MR, Raper B, Laplace SG, Lombard GA, et al. Ganciclovir for cytomegalovirus: a call for indefinite prophylaxis in lung transplantation. J Heart Lung Transplant. 2008;27:875. doi: 10.1016/j.healun.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Zamora MR, Davis RD, Leonard C. Management of cytomegalovirus infection in lung transplant recipients: evidence-based recommendations. Transplantation. 2005;80:157. doi: 10.1097/01.tp.0000165430.65645.4f. [DOI] [PubMed] [Google Scholar]
- 13.Sun HY, Wagener MM, Singh N. Prevention of posttransplant cytomegalovirus disease and related outcomes with valganciclovir: a systematic review. Am J Transplant. 2008;8:2111. doi: 10.1111/j.1600-6143.2008.02369.x. [DOI] [PubMed] [Google Scholar]
- 14.Scholz D, Arndt R, Meyer T. Evidence for an immunosuppressive activity of ganciclovir. Transplant Proc. 1994;26:3253. [PubMed] [Google Scholar]
- 15.Steininger C, Kundi M, Kletzmayr J, Aberle SW, Popow-Kraupp T. Antibody maturation and viremia after primary cytomegalovirus infection, in immunocompetent patients and kidney-transplant patients. J Infect Dis. 2004;190:1908. doi: 10.1086/424677. [DOI] [PubMed] [Google Scholar]
- 16.Sester M, Sester U, Gartner B, Kubuschok B, Girndt M, Meyerhans A, et al. Sustained high frequencies of specific CD4 T cells restricted to a single persistent virus. J Virol. 2002;76:3748. doi: 10.1128/JVI.76.8.3748-3755.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sester M, Sester U, Gartner BC, Girndt M, Meyerhans A, Kohler H. Dominance of virus-specific CD8 T cells in human primary cytomegalovirus infection. J Am Soc Nephrol. 2002;13:2577. doi: 10.1097/01.asn.0000030141.41726.52. [DOI] [PubMed] [Google Scholar]
- 18.Singh N. Late-onset cytomegalovirus disease as a significant complication in solid organ transplant recipients receiving antiviral prophylaxis: a call to heed the mounting evidence. Clin Infect Dis. 2005;40:704. doi: 10.1086/427506. [DOI] [PubMed] [Google Scholar]
- 19.La Rosa C, Limaye AP, Krishnan A, Longmate J, Diamond DJ. Longitudinal assessment of cytomegalovirus (CMV)-specific immune responses in liver transplant recipients at high risk for late CMV disease. J Infect Dis. 2007;195:633. doi: 10.1086/511307. [DOI] [PubMed] [Google Scholar]
- 20.Snyder LD, Medinas R, Chan C, Sparks S, Davis WA, Palmer SM, et al. Polyfunctional cytomegalovirus-specific immunity in lung transplant recipients receiving valganciclovir prophylaxis. Am J Transplant. 2011;11:553. doi: 10.1111/j.1600-6143.2010.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zamora MR, Nicolls MR, Hodges TN, Marquesen J, Astor T, Grazia T, et al. Following universal prophylaxis with intravenous ganciclovir and cytomegalovirus immune globulin, valganciclovir is safe and effective for prevention of CMV infection following lung transplantation. Am J Transplant. 2004;4:1635. doi: 10.1111/j.1600-6143.2004.00571.x. [DOI] [PubMed] [Google Scholar]
- 22.Humar A, Kumar D, Preiksaitis J, Boivin G, Siegal D, Fenton J, et al. A trial of valganciclovir prophylaxis for cytomegalovirus prevention in lung transplant recipients. Am J Transplant. 2005;5:1462. doi: 10.1111/j.1600-6143.2005.00866.x. [DOI] [PubMed] [Google Scholar]
- 23.Jaksch P, Zweytick B, Kerschner H, Hoda AM, Keplinger M, Lang G, et al. Cytomegalovirus prevention in high-risk lung transplant recipients: comparison of 3- vs 12-month valganciclovir therapy. J Heart Lung Transplant. 2009;28:670. doi: 10.1016/j.healun.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 24.La Rosa C, Krishnan A, Longmate J, Martinez J, Manchanda P, Lacey SF, et al. Programmed death-1 expression in liver transplant recipients as a prognostic indicator of cytomegalovirus disease. J Infect Dis. 2008;197:25. doi: 10.1086/523652. [DOI] [PubMed] [Google Scholar]
- 25.Sester U, Presser D, Dirks J, Gartner BC, Kohler H, Sester M. PD-1 expression and IL-2 loss of cytomegalovirus- specific T cells correlates with viremia and reversible functional anergy. Am J Transplant. 2008;8:1486. doi: 10.1111/j.1600-6143.2008.02279.x. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan A, Zhou W, Lacey SF, Limaye AP, Diamond DJ, La Rosa C. Programmed death-1 receptor and interleukin-10 in liver transplant recipients at high risk for late cytomegalovirus disease. Transpl Infect Dis. 2010;12:363. doi: 10.1111/j.1399-3062.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010;51:1237. doi: 10.1002/hep.23437. [DOI] [PubMed] [Google Scholar]
- 28.Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Snydman DR, et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779. doi: 10.1097/TP.0b013e3181cee42f. [DOI] [PubMed] [Google Scholar]
- 29.Castor J, Cook L, Corey L, Jerome KR. Rapid detection directly from patient serum samples of human cytomegalovirus UL97 mutations conferring ganciclovir resistance. J Clin Microbiol. 2007;45:2681. doi: 10.1128/JCM.00526-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Limaye AP, Corey L, Koelle DM, Davis CL, Boeckh M. Emergence of ganciclovir-resistant cytomegalovirus disease among recipients of solid-organ transplants [comment] Lancet. 2000;356:645. doi: 10.1016/S0140-6736(00)02607-6. [DOI] [PubMed] [Google Scholar]
- 31.Limaye AP, Raghu G, Koelle DM, Ferrenberg J, Huang ML, Boeckh M. High incidence of ganciclovir-resistant cytomegalovirus infection among lung transplant recipients receiving preemptive therapy. J Infect Dis. 2002;185:20. doi: 10.1086/338143. [DOI] [PubMed] [Google Scholar]
- 32.Li F, Kenyon KW, Kirby KA, Fishbein DP, Boeckh M, Limaye AP. Incidence and clinical features of ganciclovir-resistant cytomegalovirus disease in heart transplant recipients. Clin Infect Dis. 2007;45:439. doi: 10.1086/519941. [DOI] [PubMed] [Google Scholar]
- 33.Boeckh M, Huang M, Ferrenberg J, Stevens-Ayers T, Stensland L, Nichols WG, et al. Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. J Clin Microbiol. 2004;42:1142. doi: 10.1128/JCM.42.3.1142-1148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.La Rosa C, Wang Z, Lacey SF, Markel SF, Sharma MC, Martinez J, et al. Characterization of Host Immunity to cytomegalovirus pp150 (UL32) Hum Immunol. 2005;66:116. doi: 10.1016/j.humimm.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lilleri D, Zelini P, Fornara C, Comolli G, Revello MG, Gerna G. Human cytomegalovirus-specific CD4+ and CD8+ T cell responses in primary infection of the immunocompetent and the immunocompromised host. Clin Immunol. 2009;131:395. doi: 10.1016/j.clim.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Singh N, Wannstedt C, Keyes L, Wagener MM, Gayowski T, Cacciarelli TV. Indirect outcomes associated with cytomegalovirus (opportunistic infections, hepatitis C virus sequelae, and mortality) in liver-transplant recipients with the use of preemptive therapy for 13 years. Transplantation. 2005;79:1428. doi: 10.1097/01.tp.0000157867.98649.f5. [DOI] [PubMed] [Google Scholar]
- 38.San Juan R, Aguado JM, Lumbreras C, Diaz-Pedroche C, Lopez-Medrano F, Lizasoain M, et al. Incidence, clinical characteristics and risk factors of late infection in solid organ transplant recipients: data from the RESITRA study group. Am J Transplant. 2007;7:964. doi: 10.1111/j.1600-6143.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- 39.Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M, et al. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110:201. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Limaye AP. Ganciclovir-resistant cytomegalovirus in organ transplant recipients. Clin Infect Dis. 2002;35:866. doi: 10.1086/342385. [DOI] [PubMed] [Google Scholar]
- 41.Nepomuceno RR, Balatoni CE, Natkunam Y, Snow AL, Krams SM, Martinez OM. Rapamycin inhibits the interleukin 10 signal transduction pathway and the growth of Epstein Barr virus B-cell lymphomas. Cancer Res. 2003;63:4472. [PubMed] [Google Scholar]
- 42.Daftarian PM, Kumar A, Kryworuchko M, Diaz-Mitoma F. IL-10 production is enhanced in human T cells by IL-12 and IL-6 and in monocytes by tumor necrosis factor-alpha. J Immunol. 1996;157:12. [PubMed] [Google Scholar]
- 43.Bunde T, Kirchner A, Hoffmeister B, Habedank D, Hetzer R, Cherepnev G, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005;201:1031. doi: 10.1084/jem.20042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim TK, St John LS, Wieder ED, Khalili J, Ma Q, Komanduri KV. Human late memory CD8+ T cells have a distinct cytokine signature characterized by CC chemokine production without IL-2 production. J Immunol. 2009;183:6167. doi: 10.4049/jimmunol.0902068. [DOI] [PubMed] [Google Scholar]
- 45.van de Berg PJ, Heutinck KM, Raabe R, Minnee RC, Young SL, van Donselaar-van der Pant KA, et al. Human cytomegalovirus induces systemic immune activation characterized by a type 1 cytokine signature. J Infect Dis. 2010;202(5):690. doi: 10.1086/655472. [DOI] [PubMed] [Google Scholar]
- 46.Makedonas G, Hutnick N, Haney D, Amick AC, Gardner J, Cosma G, et al. Perforin and IL-2 upregulation define qualitative differences among highly functional virus-specific human CD8 T cells. PLoS Pathog. 2010;6:1000798. doi: 10.1371/journal.ppat.1000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lilleri D, Zelini P, Fornara C, Comolli G, Gerna G. Inconsistent responses of cytomegalovirus-specific T cells to pp65 and IE-1 versus infected dendritic cells in organ transplant recipients. Am J Transplant. 2007;7:1997. doi: 10.1111/j.1600-6143.2007.01890.x. [DOI] [PubMed] [Google Scholar]
- 48.Walker S, Fazou C, Crough T, Holdsworth R, Kiely P, Veale M, et al. Ex vivo monitoring of human cytomegalovirus-specific CD8+ T-cell responses using QuantiFERON-CMV. Transpl Infect Dis. 2007;9:165. doi: 10.1111/j.1399-3062.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 49.Westall GP, Michaelides A, Williams TJ, Snell GI, Kotsimbos TC. Bronchiolitis obliterans syndrome and early human cytomegalovirus DNAaemia dynamics after lung transplantation. Transplantation. 2003;75:2064. doi: 10.1097/01.TP.0000069234.04901.A3. [DOI] [PubMed] [Google Scholar]
- 50.Smith JM, Corey L, Bittner R, Finn LS, Healey PJ, Davis CL, et al. Subclinical Viremia Increases Risk for Chronic Allograft Injury in Pediatric Renal Transplantation. J Am Soc Nephrol. 2010;21:1579. doi: 10.1681/ASN.2009111188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 52.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El Far M, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16:452. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sadeghi M, Daniel V, Naujokat C, Schnitzler P, Schmidt J, Mehrabi A, et al. Dysregulated cytokine responses during cytomegalovirus infection in renal transplant recipients. Transplantation. 2008;86:275. doi: 10.1097/TP.0b013e31817b063d. [DOI] [PubMed] [Google Scholar]
- 55.Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol. 2004;172:567. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]
- 56.Kakumu S, Okumura A, Ishikawa T, Yano M, Enomoto A, Nishimura H, et al. Serum levels of IL-10, IL-15 and soluble tumour necrosis factor-alpha (TNF-alpha) receptors in type C chronic liver disease. Clin Exp Immunol. 1997;109:458. doi: 10.1046/j.1365-2249.1997.4861382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chau GY, Wu CW, Lui WY, Chang TJ, Kao HL, Wu LH, et al. Serum interleukin-10 but not interleukin-6 is related to clinical outcome in patients with resectable hepatocellular carcinoma. Ann Surg. 2000;231:552. doi: 10.1097/00000658-200004000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, et al. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia--the good, the bad, and the ugly. Kidney Int. 2005;67:1216. doi: 10.1111/j.1523-1755.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 59.Hurme M, Haanpaa M, Nurmikko T, Wang XY, Virta M, Pessi T, et al. IL-10 gene polymorphism and herpesvirus infections. J Med Virol. 2003;70 (Suppl 1):S48–S50. doi: 10.1002/jmv.10320. [DOI] [PubMed] [Google Scholar]
- 60.Alakulppi NS, Kyllonen LE, Salo HM, Partanen J, Salmela KT, Laine JT. The impact of donor cytokine gene polymorphisms on the incidence of cytomegalovirus infection after kidney transplantation. Transpl Immunol. 2006;16:258. doi: 10.1016/j.trim.2006.09.007. [DOI] [PubMed] [Google Scholar]