Abstract
Acquired immunity to murine Chlamydia trachomatis genital tract reinfection has long been assumed to be solely dependent on cell-mediated immunity. However, in this study, we identify a previously unrecognized protective role for Ab. Immunity develops in Ab-deficient mice following the resolution of primary chlamydial genital infection. Subsequent depletion of CD4+ T cells, but not CD8+ T cells, in those immune Ab-deficient mice before secondary infectious challenge, resulted in an infection that did not resolve. Passive immunization with immune (convalescent) serum conferred a marked level of protective immunity to reinfection, which was characterized by a striking decrease in bacterial shedding, from >100,000 inclusion forming units to fewer than 10 inclusion forming units, and a shortened duration of infection. Furthermore, mAbs to the chlamydial major outer membrane protein and LPS conferred significant levels of immunity to reinfection and reduced chlamydial shedding by >100-fold. Anti-heat shock protein 60 mAb had no protective effect. In contrast to the marked protective efficacy of immune serum on reinfection, the course of primary infection was essentially unaltered by the passive transfer of immune serum. Our results convincingly demonstrate that Abs contribute importantly to immunity to chlamydial genital tract reinfection, and that Ab-mediated protection is highly dependent on CD4+ T cell-mediated adaptive changes that occur in the local genital tract tissues during primary infection. These results impact our understanding of immunity to chlamydial genital infection and may provide important insight into vaccine development.
Chlamydia trachomatis sexually transmitted infections cause considerable morbidity and socioeconomic burden worldwide. Effective control of chlamydial urogenital infection is hampered by the high frequency of asymptomatic infections and delayed diagnosis (1). Although antibiotics are effective, definitive control, or eradication, of chlamydial genital infection is likely to be achieved only through vaccination (2). Progress toward the development of an efficacious vaccine has been modest, due in part to an incomplete understanding of the adaptive immune responses required for resolving established infections and protecting against reinfection.
Genital infection of mice with Chlamydia muridarum closely mimics acute genital infection of women, and provides a reasonable model that can be used to augment our understanding of immunity to chlamydial infection (3, 4). The marked level of immunity that develops after primary infection of naive mice is highly dependent on CD4+ Th1-type cell responses (3–6). B cells and specific Ab are viewed as being inconsequential in immunity to murine chlamydial genital infection (7–9). The arguments against a protective role for Ab generally include: 1) the obligate intracellular lifestyle of chlamydiae makes them inaccessible to Ab; 2) vaccines that only elicit high-titered Ab are ineffective; and 3) cell-mediated immunity confers protection. Furthermore, Ab-deficient mice resolve primary C. muridarum chlamydial genital infection and develop marked immunity to reinfection (9, 10).
Historically, immunity to chlamydial infection has been studied by either evaluating immune responses that develop after the infection of naive mice, by passively transferring Abs or cells to naive mice, or by vaccinating naive mice and assessing resistance to infection (3). Those approaches have confirmed the dominant role of Th1 CD4+ cells in resolving chlamydial genital infection, and have directed studies away from the investigation of humoral immunity. However, by using an alternative experimental approach in which infection-resistant (immune) mice are rendered infection susceptible through T cell subpopulation depletion, we show that immunity can be conferred to genital tract reinfection, but not to primary infection, by the passive transfer of immune serum. Our data convincingly demonstrate a previously unrecognized, fundamental role for Ab in adaptive immunity to chlamydial genital tract reinfection, and provide a compelling argument for inclusion of humoral immune responses in chlamydial vaccine development.
Materials and Methods
Mice
Female wild-type C57BL/6 mice and Ab-deficient (B6.129S2-Igh-6tm1Cgn/J) mice were purchased from The Jackson Laboratory and maintained in the animal facilities at the University of Alabama (Birmingham, AL). Mice 8–15 wk old were used throughout this study. All animal procedures were in accordance with institutional policies for animal health and well-being, and were approved by the institutional animal care and use committee.
Bacterial growth and purification
C. muridarum strain Nigg (formerly C. trachomatis mouse pneumonitis biovar) was grown in HeLa 229 cells and purified by density gradient centrifugation (11).
Immune serum and mAbs to Chlamydia
Immune (convalescent) serum was prepared by infecting C57BL/6 mice vaginally with C. muridarum (described in Genital tract infection and enumeration of chlamydiae). Beginning at 28 days postinfection, and continuing at 10-day intervals until 80 days postinfection, mice were bled and serum was collected. Before passive transfer studies, the sera collected from all time points was pooled, filtered, sterilized, evaluated by ELISA, aliquotted, and stored at –80°C. Species-specific mAb to C. muridarum major outer membrane protein (MOMP; clone Mo-33b; IgG3) (12), and genus-reactive mAbs to chlamydial LPS (clone EVI-H1; IgG2a) (13) and chlamydial heat shock protein 60 (hsp60)3 (clone A57-B9; IgG1) (14) were purified from culture supernatants by immunoaffinity Sepharose 4B protein G column chromatography following the manufacturer's protocol (Zymed Laboratories).
Genital tract infection and enumeration of chlamydiae
Ab-deficient mice were injected sc with 2.5 mg of Depo-Provera (medroxyprogesterone acetate) (Pharmacia) 5 days before intravaginal inoculation of 5 × 104 inclusion forming units (IFUs) (100 ID50) of C. muridarum (10). The course of infection was monitored by enumerating the number of IFUs recovered from cervicovaginal swabs using indirect immunofluorescence (4). Fifty days after primary infection, a time when mice had resolved primary infection and acquired a marked level of resistance to reinfection (immune mice) (9), mice were begun on a treatment regimen (described in T cell subpopulation depletion) of buffer, anti-CD4 or anti-CD8 (10). Five days before secondary infectious challenge (day 51 after primary infection), mice were treated with Depo-Provera as described earlier in this section and rechallenged with 100 ID50 of C. muridarum on day 56 after primary infection, and IFUs were enumerated to monitor the course of infection.
T cell subpopulation depletion
Ab-deficient mice were depleted of CD4+ or CD8+ T cells as described in detail previously (10). Four hundred micrograms of purified anti-CD4 (clone GK1.5) or anti-CD8 (clone 2.43) mAb was injected i.p. into immune mice on days 50, 51, 52, 55, 58, 61, 64, 67, 70, 73, 76, and 79 after primary infection. Naive Ab-deficient mice were depleted similarly except injections were administered on days –6, –5, –4, –1, 2, 5, 8, 11, 14, 17, and 20 of primary infection. Following this depletion regimen, T cell subpopulations remained depleted for at least 7 days after the last anti-CD4 or anti-CD8 Ab injection.
Passive transfer of immune serum and mAbs
Immune serum or mAbs to chlamydial MOMP, LPS, or hsp60 were injected i.p. following the schedule described in the figures. Each injection of immune serum consisted of 0.5 ml, and each injection of anti-chlamydial mAbs consisted of 200 μg 4of purified Ab.
Chlamydia ELISA
Immune serum, and mAbs to chlamydial MOMP, LPS, and hsp60 were evaluated by ELISA before passive transfer as previously described (4). C. muridarum elementary bodies were used as Ag for the ELISA of immune serum, anti-MOMP, and anti-LPS, and purified recombinant chlamydial hsp60 was used as Ag to detect anti-hsp60 Ab. Serum and vaginal washes were collected at day 9 (vaginal wash) and day 10 (serum) after secondary infection, to monitor Ab levels in mice receiving passively administered Abs.
Statistical analysis
Student's t test of log-transformed data was used to analyze differences between IFU counts of control and experimental groups, and Fisher's exact test was used to compare proportions of mice that resolve infection.
Results
Passive immunization with immune serum does not prevent primary infection
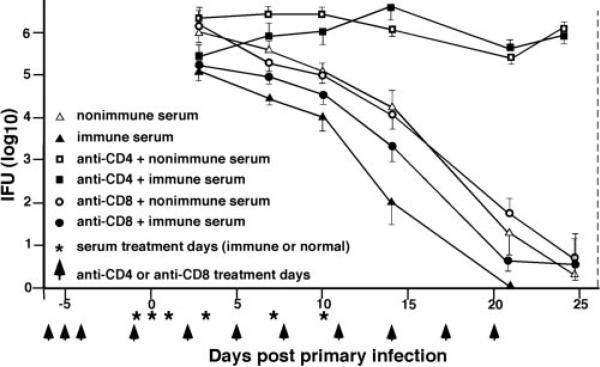
Naive, Ab-deficient mice, with or without CD4+ or CD8+ T cells, were passively immunized to evaluate the protective effect of immune serum on primary chlamydial genital infection. Immune serum had no statistically significant effect on either the course of primary infection or the level of chlamydial shedding of infected CD4-depleted Ab-deficient mice (Fig. 1). In the presence of CD4+ T cells (nondepleted or anti-CD8 depleted mice), immune serum significantly reduced ( p < 0.05) chlamydial shedding by ~10-fold (Fig. 1). Nonimmune serum had no effect. Thus, while immune serum imparted a small but measurable protective effect to primary chlamydial infection of naive mice, it failed to protect in the absence of CD4+ T cells.
FIGURE 1.
Immune serum fails to protect CD4-depleted Ab-deficient mice during a primary chlamydial genital infection. Naive Ab-deficient mice were either untreated or depleted of CD4+ or CD8+ T cells before and during the course of primary infection (as indicated by arrowheads and described in Materials and Methods). The vertical dashed line indicates minimum duration of T cell subpopulation depletion. Animals were injected i.p. with 0.5 ml of either nonimmune or immune serum on the indicated days (*). All mice were challenged with 100 ID50 of C. muridarum on day 0, and the course of infection was followed by enumerating IFUs from cervicovaginal swabs. Data are presented as the mean + SEM of IFUs recovered from five to seven mice per group. For clarity, only a single side of the SE bar is presented. Values of p < 0.05 for nondepleted mice given immune serum, compared with nondepleted mice given normal serum at all time points analyzed.
Immune serum conferred marked protective immunity to chlamydial genital tract reinfection
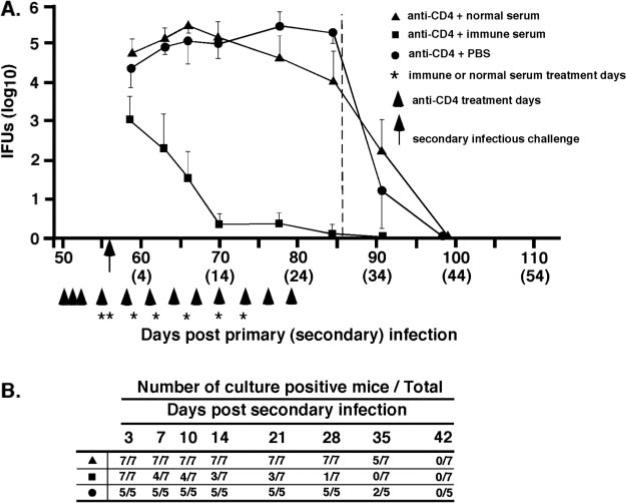
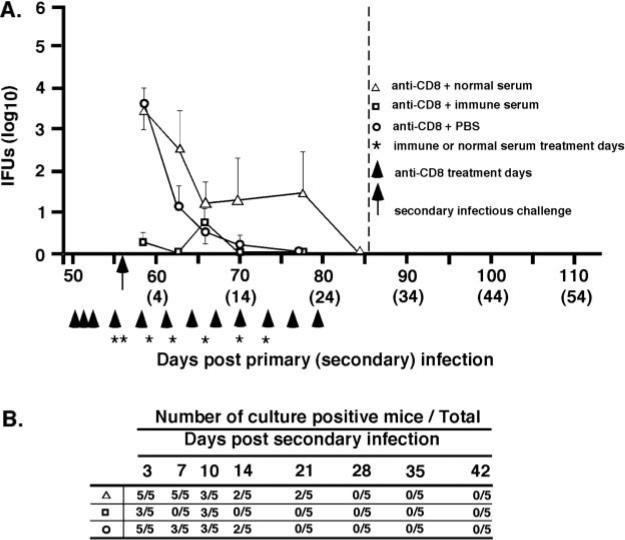
Previous studies show that following the resolution of primary genital infection, Ab-deficient mice exhibit a high level of acquired immunity to reinfection, which is characterized by much reduced shedding of infectious chlamydiae and an infection of shorter duration (9, 10). To assess the role of humoral immunity in chlamydial genital reinfection, immune Ab-deficient mice (B cell knockout mice that had previously resolved a primary infection) depleted of either CD4+ or CD8+ T cells, were passively administered either immune or nonimmune serum, and rechallenged with infectious chlamydiae. Immune serum conferred a marked level of immunity to chlamydial reinfection in CD4-depleted (Fig. 2) and CD8-depleted (Fig. 3) Ab-deficient mice, whereas nonimmune serum had no effect on the course of secondary infection. The protection observed in passively immunized Ab-deficient CD4-depleted mice was characterized by a striking decrease in bacterial shedding (IFUs decreased by 2 log10 day 3 after secondary challenge and by 5 log10 at days 14, 21, and 28), and a shortened duration of infection (Fig. 2A). Immune serum reduced the shedding of infectious chlamydiae from >100,000 IFUs to <10 IFUs. Three of seven mice treated with immune serum completely resolved infection by 7 days after reinfection and all but one mouse resolved infection during the course of immune serum therapy (Fig. 2B). Although the depletion of CD8+ T cells did not profoundly impact the course of secondary chlamydial genital infection (10), immune serum augmented protective immunity in immune Ab-deficient CD8-depleted mice (Fig. 3) and conferred nearly sterilizing immunity in those animals. A reduction in bacterial shedding of the magnitude shown here has not been previously achievable with immune serum or mAbs, and clearly demonstrates a protective role for humoral immunity to chlamydial genital reinfection. Collectively, the results presented in Figs. 1–3 support the notion that immune serum confers a marked level of protective immunity to genital tract reinfection, but not primary infection. Furthermore, the data suggest that while CD4+ T cells are not directly involved in the effector function of Ab (i.e., immune serum resolves secondary infection in the absence of CD4+ T cells), the protective response of Ab is unquestionably dependent on CD4+ T cells (i.e., immune serum has no impact the course of primary infection in CD4-depleted mice; Fig. 1).
FIGURE 2.
Immune serum protects against chlamydial genital tract reinfection in CD4-depleted Ab-deficient mice. Immune Ab-deficient mice (i.e., resolved primary infection) were treated with anti-CD4 as described in Materials and Methods and as indicated (arrowheads). The vertical dashed line indicates minimum duration of T cell subpopulation depletion. Groups of mice were injected i.p. with 0.5 ml of either normal serum (▲), immune serum (■), or PBS (●) at the indicated times (*). All mice were challenged with 100 ID50 of C. muridarum (5 × 104 IFUs) on day 56 after primary infection (arrow). A, The course of secondary infection. Data are presented as the mean number of IFUs recovered from cervicovaginal swabs collected from five or more mice per group ± SEM (for clarity, only a single side of the SE bar is presented). IFU recovery for the group of mice receiving immune sera was significantly lower (p < 0.001) than groups receiving normal serum or PBS at all enumeration time points. B, The number of culture-positive mice per total mice at each culture time point. Mice receiving immune serum completely resolved infection more quickly than mice receiving normal serum or PBS (p < 0.05 at days 14, 21, and 28 after secondary infection).
FIGURE 3.
Immune serum protects against chlamydial genital tract reinfection in CD8-depleted Ab-deficient mice. Immune Ab-deficient mice were treated with anti-CD8 as described in Materials and Methods. Refer to Fig. 2 legend for explanation of injections, challenge, and data presentation. △, Normal serum; □, immune serum; ○, PBS. A, Course of secondary infection. IFU recovery for the group of mice receiving immune sera was significantly lower at days 3 (p < 0.001) and 7 (p < 0.05) after secondary infection than groups receiving normal serum or PBS. B, Number of culture-positive mice over total mice at each culture time point. No statistically significant differences in the rate of resolution of reinfection were observed between the groups of treated mice.
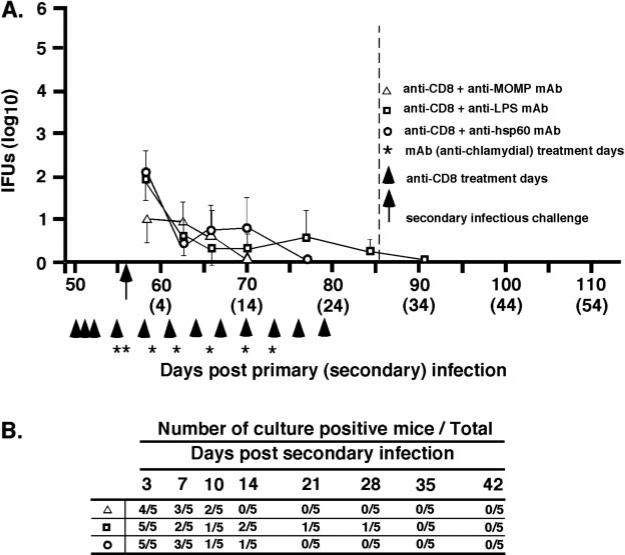
mAbs to chlamydial outer membrane Ags impart a level of immunity to reinfection
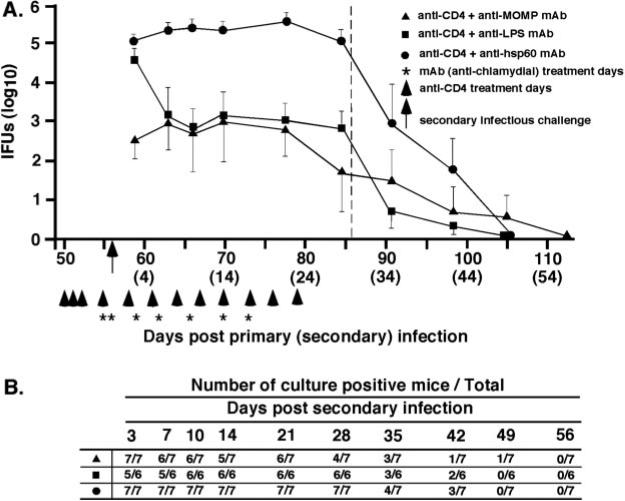
To further assess the role of Chlamydia-specific Abs in immunity to genital tract reinfection, we evaluated the protective efficacy of mAbs to MOMP (Mo-33b), LPS (EVI-H1), or hsp60 (A57-B9). Anti-MOMP and anti-LPS mAbs conferred a significant level of immunity to chlamydial reinfection in CD4-depleted Ab-deficient mice (Fig. 4). Anti-MOMP and anti-LPS mAbs reduced chlamydial shedding by 2–3 log10 IFUs compared with nontreated mice (Fig. 2A; anti-CD4+PBS) or to mice treated with anti-hsp60 mAb (Fig. 4). Anti-MOMP also reduced shedding in CD8-depleted mice (Fig. 5), although the magnitude of the reduction was modest because of the presence of protective CD4+ T cells in those mice. Although anti-MOMP and anti-LPS treatment decreased shedding of infectious chlamydiae during reinfection, the time to complete resolution of infection was not statistically different from that of mice treated with the nonprotective anti-hsp60 mAb (Figs. 4B and 5B). Thus, Abs reactive with the chlamydial cell surface (anti-MOMP and anti-LPS) were protective, whereas an Ab to a cytoplasmic Ag (anti-hsp60) was nonprotective.
FIGURE 4.
Effect of anti-chlamydial mAbs on the resolution of genital reinfection in immune, Ab-deficient CD4-depleted mice. Mice were injected i.p. with either anti-MOMP (▲), anti-LPS (■), or anti-hsp60 (●). Refer to Fig. 2 legend for explanation of injections, challenge, and data presentation. A, Course of secondary infection. Shedding of infectious chlamydiae was statistically significant (p < 0.01) for anti-MOMP (days 59, 63, 66, 70, 77, 84, and 91 postreinfection) and anti-LPS (days, 63, 66, 70, 77, 84, and 91 postreinfection) when compared with nontreated CD4-depleted mice or CD4-depleted mice given anti-hsp60 mAb. B, Number of culture-positive mice over total mice at each culture time point. No statistically significant differences in the rate of resolution of reinfection were observed between the groups of treated mice.
FIGURE 5.
Effect of mAbs to chlamydial surface Ags on the resolution of chlamydial genital tract rein-fection in immune, Ab-deficient, CD8-depleted mice. Mice were injected i.p. with anti-MOMP (△), anti-LPS (□), or anti-hsp60 (○). Refer to Fig. 2 legend for explanation of injections, challenge, and data presentation. A, Course of reinfection. B, Number of culture-positive mice over total mice at each culture time point. No statistically significant differences were observed.
Ab profile of serum and vaginal wash fluid following passive transfer of convalescent serum or mAbs
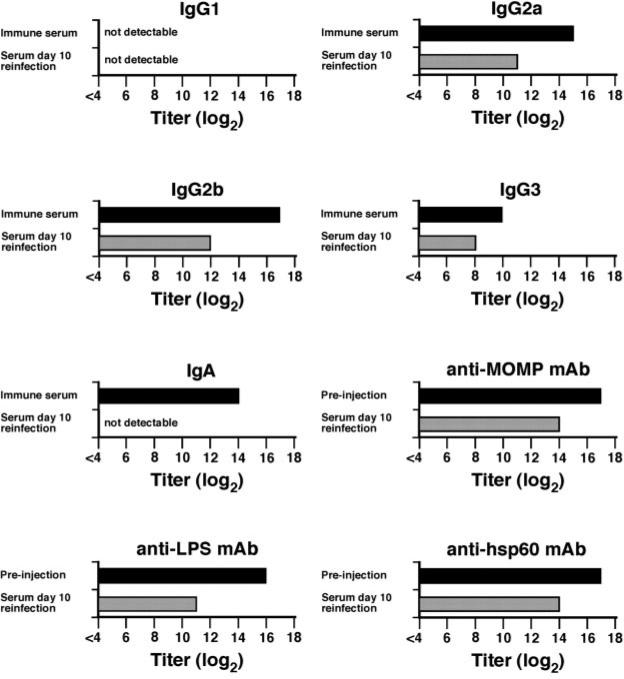
Serum and vaginal washes were collected from passively immunized mice and analyzed by ELISA. The Ab responses detected in passively immunized mice result solely from the transferred immune serum or mAbs, because these mice are genetically deficient in Ab production. Immune serum and mAbs were analyzed by ELISA before transfer, and sera collected from passively immunized mice were analyzed at 10 days after secondary infection (i.e., after five Ab injections). Before passive transfer, immune serum consisted of high-titered anti-chlamydial IgG2a, IgG2b, and IgA, moderate titer IgG3, and no detectable IgG1 (Fig. 6). After transfer, IgG2a and IgG2b titers were 4–5 log2 lower, and the IgG3 titer was 2 log2 lower. Anti-chlamydial IgA was undetectable in the serum after passive transfer, which was expected because, in rodents, the liver rapidly removes IgA and excretes it into the gut (15). Similar to the polyclonal immune serum, titers of the mAbs were reduced 3–5 log2 after passive transfer. Vaginal washes collected from mice that received immune serum were typically negative for chlamydial Ab. Only two mice had Ab responses (IgG2a and IgG2b) to chlamydiae that were above background (Fig. 7). Most mice receiving anti-MOMP had detectable Ab in their vaginal washes. Although anti-hsp60 was nonprotective, high levels of Ab were found in vaginal secretions of these mice. Thus, the Ab-mediated protection observed in our studies did not directly correlate with the presence of high-titered Abs in genital tract secretions.
FIGURE 6.
Anti-chlamydial Ab titers of immune serum and mAb preparation before and after passive transfer. Immune serum and mAb preparations were evaluated by ELISA before passive transfer (■) and at 10 days after secondary infection ( ). The anti-chlamydial titer for immune serum and preinjection (mAbs) represents the mean titers of triplicate determinations of the pool of convalescent serum or mAb preparation before passive transfer. At 10 days postreinfection, a time in which mice had received five injections of either immune serum or mAb, blood was collected and analyzed by ELISA of anti-chlamydia Ab. Those data represent the mean titers of at least seven mice per group. Error bars are omitted from the figure, but were always <0.5 log2.
). The anti-chlamydial titer for immune serum and preinjection (mAbs) represents the mean titers of triplicate determinations of the pool of convalescent serum or mAb preparation before passive transfer. At 10 days postreinfection, a time in which mice had received five injections of either immune serum or mAb, blood was collected and analyzed by ELISA of anti-chlamydia Ab. Those data represent the mean titers of at least seven mice per group. Error bars are omitted from the figure, but were always <0.5 log2.
FIGURE 7.
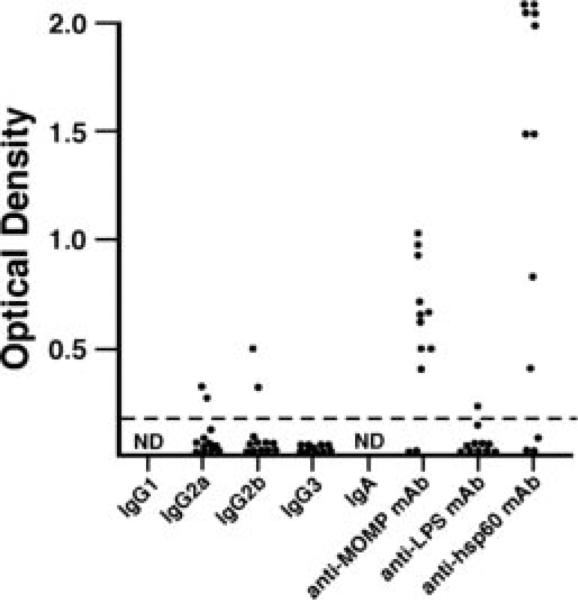
Anti-chlamydial Abs in vaginal wash fluid after passive transfer. Vaginal washes were collected at 9 days after secondary infection (i.e., a time when mice had received five injections of immune serum or mAb) and analyzed by ELISA for anti-chlamydial Ab. Data are presented as OD of vaginal washes (diluted 1/16) from individual mice.
Discussion
Immunity to intracellular microbial pathogens has long been thought to be dependent on the cellular arm of the immune system. However, several recent studies have begun to challenge that view (16–21), and show that Ab- and B cell-mediated responses contribute importantly to immunity to a number of intracellular microbial infections (reviewed in Refs. 22 and 23). The mechanisms of the protective responses vary among the pathogens, but include Abs as direct antimicrobial effector molecules, as immunomodulators of cell-mediated immunity, and as effector molecules that interact with T cell subpopulations.
Th1 CD4+ T cell responses impart a marked level of immunity to primary and secondary genital infections cause by the obligate intracellular bacterial pathogen C. trachomatis (4–6, 10, 24, 25). CD4+ T cells are much superior to CD8+ T cells in their protective effects, and adaptive immunity is neither diminished in the absence of CD8+ T cells nor is the protection conferred by CD8+ T cells of great consequence (6, 10, 26–29). The role of humoral immunity in murine chlamydial genital infection has evolved from being the focus of immune protection and vaccine development (30–32), to having a very subordinate role (7–9). Indeed, immune serum (Fig. 1) and mAbs (12) confer only a minimal level of immunity when passively administered to naive immunocompetent animals, and mice genetically incapable of producing Ab resolve primary infection and resist reinfection (9). However, recently, data derived from studies investigating adaptive immunity to murine chlamydial genital reinfection have challenged the generally accepted view that the humoral immune response is inconsequential in immunity to reinfection (10, 33). Immune wild-type mice, depleted of CD4+ T cells, remain markedly immune to re-infection, whereas immune Ab-deficient mice depleted of CD4+ T cell are unable to resolve secondary infection. Those studies demonstrated that to appreciate the protective role of B cells, immune mice rather than naive mice had to be used, and the dominant protective response of CD4+ T cells had to be removed. By doing so, the protective response mediated by B cells was revealed. The mechanism(s) responsible for immunity in the absence of CD4+ T cells was not elucidated; nevertheless, the data strongly supported the notion that B cells and/or their products were involved.
The data presented in this study demonstrate that humoral immune responses have a striking impact on immunity to chlamydial reinfection. To our knowledge, the level of immunity to reinfection conferred by immune serum has not been previously demonstrated. Although it is well documented that high-titered Ab responses are elicited after chlamydial infection and that Abs neutralize chlamydiae infectivity in cell culture (4, 34–38), neither immune serum nor purified Ab have been shown to significantly alter the course of chlamydial infection in the mouse. However, there is some precedent that establishes a protective role for Ab using other animal models of chlamydial infection (39–41).
We have demonstrated an unequivocal role for Ab in adaptive immunity to chlamydial genital reinfection. How Ab protects against chlamydial genital tract reinfection is not known, but several observations from our studies may provide important clues. First, it is important to acknowledge that CD4+ T cells are an absolute requirement for immunity. CD4+ T cell-dependent mechanisms resolve primary infection and protect against secondary infection independent of Ab, although the precise effector mechanism(s) of CD4 T cell-mediated immunity has not been defined (3). CD4+ Th cells are also needed for the affinity maturation of the Ab response, Ig class switching, and B cell memory. Thus, protective immunity does not develop in naive mice without CD4+ T cells. Secondly, in the absence of CD4+ T cells, immune serum alone has no demonstrable effect on the course of primary chlamydial genital infection (Fig. 1). Conversely, immune serum and mAbs that react to chlamydial surface Ags (MOMP, LPS) provide a marked level of protection in the absence of CD4+ T cells during reinfection (Figs. 2 and 4). Although we do not yet understand the precise mechanism(s) of Ab-mediated immunity to chlamydial re-infection, our data suggest that the protective effect of Ab is highly dependent on CD4+ T cell-mediated adaptive changes that occur in the local genital tract tissues during primary infection. In the absence of these CD4-T cell mediated changes, immune serum is not protective. Thus, although the findings regarding the protective effect of immune serum on reinfection, but not primary infection, seem contradictory, we believe the results actually support the notion that CD4+ T cells mediate changes in the local genital tract tissues during primary infection that facilitate Ab-mediated immunity.
How might Ab function in immunity to chlamydial reinfection? The direct neutralization of chlamydial elementary bodies by specific Ab to prevent attachment and subsequent intracellular infection has been shown to occur in in vitro cell culture systems (35, 36, 42). Our data argue against direct neutralization as a major effector mechanism in vivo because immune serum fails to confer even a nominal level of protective immunity against primary infection in the absence of CD4+ T cells. If immune serum protected simply by blocking elementary body attachment, some level of protection should have been observed. Similarly, Ab and complement-mediated lysis is not a plausible explanation. It is our contention that Ab is mediating its effects in cooperation with a non-T cell population that localizes to the genital tract tissue during primary infection. Perhaps mechanisms such as Fc-mediated or C3b-mediated phagocyte interactions or Ab-dependent cellular cytotoxicity responses are responsible for the marked protection conferred by immune serum during reinfection. Indeed, FcR-mediated and Ab-dependent cellular cytotoxicity mechanisms have been implicated by other investigators as playing a role in immunity to chlamydial genital tract infection (43, 44). However, whatever the mechanism, the localization and/or activation of this cell population is dependent upon CD4+ T cells. Specifically, CD4+ T cells do not contribute directly to the effector mechanism(s) of protective Ab (i.e., immune serum resolves secondary infection in the absence of CD4+ T cells; Fig. 2). Rather, we propose that CD4+ T cells act indirectly by recruiting a hereto uncharacterized cell population to genital tract tissues, and/or activating that effector cell population, which subsequently cooperates with Ab to resolve chlamydial reinfection.
In summary, this study shows that immune serum and Chlamydia-specific mAbs have a profound impact on immunity to chlamydial genital tract reinfection. Although the mechanism(s) by which Ab protects is not understood, the protective efficacy of Ab is highly dependent upon CD4+ T cell-mediated adaptive changes that occur within the genital tract tissues after primary infection. These findings are particularly germane to chlamydial vaccine development, and may provide an explanation as to why previous vaccines have not approached the protective efficacy conferred by primary infection even though high-titered Ab and cellular responses are elicited (45)—they fail to elicit responses that mimic the CD4+ T cell-dependent adaptive changes in the local genital tract tissues. The most efficacious of the experimental chlamydial vaccine consists of chlamydiae-pulsed dendritic cells, which elicit an immune response that is nearly as protective as that induced by primary infection (25). Vaccination with chlamydiae-pulsed dendritic cells results in Ab and T cell responses that reflect those observed after primary infection. Because both Ab and CD4+ T cell responses are activated after dendritic cell vaccination, it is not possible to ascertain the role of each response in protection. However, it is interesting to speculate that perhaps dendritic cells are the cell population that functions locally with specific Ab to protect against chlamydial reinfection. Our current studies provide a useful model to determine the mechanism(s) by which Ab functions in adaptive immunity to reinfection. Those studies will greatly impact our efforts in chlamydial vaccination, and perhaps will influence approaches used for vaccination against other sexually transmitted intracellular mucosal pathogens.
Footnotes
This work was supported by U.S. Public Health Service Grant AI38991 from the National Institutes of Health.
Abbreviations used in this paper: hsp60, heat shock protein 60; IFU, inclusion forming unit; MOMP, major outer membrane protein.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Black CM. Current methods of laboratory diagnosis of Chlamydia trachomatis infections. Clin. Microbiol. Rev. 1997;10:160–184. doi: 10.1128/cmr.10.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de la Maza MA, de la Maza LM. A new computer model for estimating the impact of vaccination protocols and its application to the study of Chlamydia trachomatis genital infections. Vaccine. 1995;13:119–127. doi: 10.1016/0264-410x(95)80022-6. [DOI] [PubMed] [Google Scholar]
- 3.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect. Immun. 2002;70:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect. Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-γ-dependent and -independent pathways. J. Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 6.Su H, Caldwell HD. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect. Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in γ interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect. Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansson M, Ward M, Lycke N. B-cell-deficient mice develop complete immune protection against genital tract infection with Chlamydia trachomatis. Immunology. 1997;92:422–428. doi: 10.1046/j.1365-2567.1997.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su H, Feilzer K, Caldwell HD, Morrison RP. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect. Immun. 1997;65:1993–1999. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect. Immun. 2000;68:6979–6987. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotter TW, Meng Q, Shen Z-L, Zhang Y-X, Su H, Caldwell HD. Protective efficacy of major outer membrane protein-specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect. Immun. 1995;63:4704–4714. doi: 10.1128/iai.63.12.4704-4714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldwell HD, Hitchcock PJ. Monoclonal antibody against a genus-specific antigen of Chlamydia species: location of the epitope on chlamydial lipopolysaccharide. Infect. Immun. 1984;44:306–314. doi: 10.1128/iai.44.2.306-314.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan Y, Lyng K, Zhang Y-X, Rockey DD, Morrison RP. Monoclonal antibodies define genus-specific, species-specific, and cross-reactive epitopes of the chlamydial 60-kilodalton heat shock protein (hsp60): specific immunodetection and purification of chlamydial hsp60. Infect. Immun. 1992;60:2288–2296. doi: 10.1128/iai.60.6.2288-2296.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mestecky J, Moro I, Kerr MA, Woof JM. Mucosal immunoglobulins. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal Immunology. Elsevier Academic Press; London: 2005. pp. 153–181. [Google Scholar]
- 16.Casadevall A. Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 1998;6:102–107. doi: 10.1016/s0966-842x(98)01208-6. [DOI] [PubMed] [Google Scholar]
- 17.Edelson BT, Cossart P, Unanue ER. Paradigm revisited: antibody provides resistance to Listeria infection. J. Immunol. 1999;163:4087–4090. [PubMed] [Google Scholar]
- 18.Elkins KL, Bosio CM, Rhinehart-Jones TR. Importance of B cells, but not specific antibodies, in primary and secondary protective immunity to the intracellular bacterium Francisella tularensis live vaccine strain. Infect. Immun. 1999;67:6002–6007. doi: 10.1128/iai.67.11.6002-6007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuzaki G, Vordermeier HM, Hashimoto A, Nomoto K, Ivanyi J. The role of B cells in the establishment of T cell response in mice infected with an intracellular bacteria, Listeria monocytogenes. Cell Immunol. 1999;194:178–785. doi: 10.1006/cimm.1999.1503. [DOI] [PubMed] [Google Scholar]
- 20.Teitelbaum R, Glatman-Freedman A, Chen B, Robbins JB, Unanue A, Casadevall A, Bloom BR. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc. Natl. Acad. Sci. USA. 1999;95:15688–15693. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X, Brunham RC. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J. Immunol. 1998;161:1439–1446. [PubMed] [Google Scholar]
- 22.Brady LJ. Antibody-mediated immunomodulation: a strategy to improve host responses against microbial antigens. Infect. Immun. 2005;73:671–678. doi: 10.1128/IAI.73.2.671-678.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casadevall A, Pirofski LA. New concepts in antibody-mediated immunity. Infect. Immun. 2004;72:6191–6196. doi: 10.1128/IAI.72.11.6191-6196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cotter TW, Ramsey KH, Miranpuri GS, Poulsen CE, Byrne GI. Dissemination of Chlamydia trachomatis chronic genital tract infection in γ interferon gene knockout mice. Infect. Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su H, Messer R, Whitmire W, Fischer E, Portis JC, Caldwell HD. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable Chlamydiae. J. Exp. Med. 1998;188:809–818. doi: 10.1084/jem.188.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Igietseme JU, Magee DM, Williams DM, Rank RG. Role for CD8+ T cells in antichlamydial immunity defined by Chlamydia-specific T-lymphocyte clones. Infect. Immun. 1994;62:5195–5197. doi: 10.1128/iai.62.11.5195-5197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igietseme JU, Ramsey KH, Magee DM, Williams DM, Kincy TJ, Rank RG. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Regional Immunol. 1993;5:317–324. [PubMed] [Google Scholar]
- 28.Ramsey KH, Rank RG. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect. Immun. 1991;59:925–931. doi: 10.1128/iai.59.3.925-931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starnbach MN, Bevan MJ, Lampe MF. Protective cytotoxic T-lymphocytes are induced during murine infection with Chlamydia trachomatis. J. Immunol. 1994;153:5183–5189. [PubMed] [Google Scholar]
- 30.Murdin AD, Su H, Klein MH, Caldwell HD. Poliovirus hybrids expressing neutralization epitopes from variable domains I and IV of the major outer membrane protein of Chlamydia trachomatis elicit broadly cross-reactive C. trachomatis-neutralizing antibodies. Infect. Immun. 1995;63:1116–1121. doi: 10.1128/iai.63.3.1116-1121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su H, Caldwell HD. Immunogenicity of a synthetic oligopeptide corresponding to antigenically common T-helper and B-cell neutralizing epitopes of the major outer membrane protein of Chlamydia trachomatis. Vaccine. 1993;11:1159–1166. doi: 10.1016/0264-410x(93)90080-h. [DOI] [PubMed] [Google Scholar]
- 32.Su H, Morrison RP, Watkins NG, Caldwell HD. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J. Exp. Med. 1990;172:203–212. doi: 10.1084/jem.172.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison SG, Morrison RP. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect. Immun. 2001;69:2643–2649. doi: 10.1128/IAI.69.4.2643-2649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal S, Theodor I, Peterson EM, de la Maza LM. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine. 1997;15:575–582. doi: 10.1016/s0264-410x(97)00206-5. [DOI] [PubMed] [Google Scholar]
- 35.Peeling R, Maclean IW, Brunham RC. In vitro neutralization of Chlamydia trachomatis with monoclonal antibody to an epitope on the major outer membrane protein. Infect. Immun. 1984;46:484–488. doi: 10.1128/iai.46.2.484-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson EM, Cheng X, Markoff BA, Fielder TJ, de la Maza LM. Functional and structural mapping of Chlamydia trachomatis species-specific major outer membrane protein epitopes by use of neutralizing monoclonal antibodies. Infect. Immun. 1991;59:4147–4153. doi: 10.1128/iai.59.11.4147-4153.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y-X, Stewart S, Joseph T, Taylor HR, Caldwell HD. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J. Immunol. 1987;138:575–581. [PubMed] [Google Scholar]
- 38.Zhang Y-X, Stewart SJ, Caldwell HD. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect. Immun. 1989;57:636–638. doi: 10.1128/iai.57.2.636-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rank RG, Barron AL. Humoral immune response in acquired immunity to chlamydial genital infection of female guinea pigs. Infect. Immun. 1983;39:463–465. doi: 10.1128/iai.39.1.463-465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rank RG, Batteiger BE. Protective role of serum antibody in immunity to chlamydial genital infection. Infect. Immun. 1989;57:299–301. doi: 10.1128/iai.57.1.299-301.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rank RG, White HJ, Barron AL. Humoral immunity in the resolution of genital infection in female guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infect. Immun. 1979;26:573–579. doi: 10.1128/iai.26.2.573-579.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su H, Caldwell HD. In vitro neutralization of Chlamydia trachomatis by monovalent Fab antibody specific to the major outer membrane protein. Infect. Immun. 1991;59:2843–2845. doi: 10.1128/iai.59.8.2843-2845.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore T, Ananaba GA, Bolier J, Bowers S, Belay T, Eko FO, Igietseme JU. Fc receptor regulation of protective immunity against Chlamydia trachomatis. Immunology. 2002;105:213–221. doi: 10.1046/j.0019-2805.2001.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore T, Ekworomadu CO, Eko FO, MacMillan L, Ramey K, Ananaba GA, Patrickson JW, Nagappan PR, Lyn D, Black CM, Igietseme JU. Fc receptor-mediated antibody regulation of T cell immunity against intracellular pathogens. J. Infect. Dis. 2003;188:617–624. doi: 10.1086/377134. [DOI] [PubMed] [Google Scholar]
- 45.Rank RG. Models of immunity. In: Stephens RS, editor. Chlamydia: intracellular biology, pathogenesis, and immunity. American Society for Microbiology Press; Washington, DC: 1999. pp. 239–295. [Google Scholar]