Abstract
Failure of axon regeneration after traumatic spinal cord injury (SCI) is attributable in part to the presence of inhibitory molecular interactions. Recent evidence demonstrates that activation of Eph signaling pathways leads to modulation of growth cone dynamics and repulsion through the activation of ephexin, a novel guanine nucleotide exchange factor (GEF). However, little is known about the expression and modulation of Eph molecular targets in the injured spinal cord. In this study, we determined the expression profile of ephexin after a moderate spinal cord contusion at thoracic level (T10) in young adult rats. Western Blot studies showed increased protein expression in injured rats at 4, and 7 days post-injury (DPI) when compared to control animals. The protein levels returned to normal at 14 DPI and remained steady until 28 DPI. However, immunoprecipitation studies of the phosphorylated ephexin demonstrated that this protein is activated by day 2 until 14 DPI. Expression of ephexin was noticeable in neurons, axons, microglia/macrophages and reactive astrocytes, and co-localized with EphA3, A4 and A7. These results demonstrate the presence of ephexin in the adult spinal cord, and its activation after SCI. Therefore, we show, for the first time, the spatio-temporal pattern of ephexin expression and activation after contusive spinal cord injury. Collectively, our data supports our previous findings on the putative non-permissive roles of Eph receptors after spinal cord injury, and the possible involvement of ephexin in the intracellular cascade of events.
Keywords: Regeneration, Eph, ephrin, trauma and guanine exchange factor
Introduction
Long-standing studies support the hypothesis that the central nervous system (CNS) has the ability to regenerate (David and Aguayo 1981). However, the normal response to injury of axons in the spinal cord is, at most, a short period of axonal sprouting followed by retraction and end-bulb formation (Bogda 1956; Fishman and Kelley 1984; Houle and Jin 2001; Busch, Horn et al. 2009). Evidence supports that the generation of a non-permissive microenvironment could be one of the main reason for the lack of axon growth observed in response to CNS injury (Bouquet and Nothias 2007). Trauma to the spinal cord triggers the expression of choindroitin sulphate proteoglycans (CSPG) such as versican (Asher, Morgenstern et al. 2002), NG2 (Jones, Yamaguchi et al. 2002; Buss, Pech et al. 2009), neurocan (Asher, Morgenstern et al. 2002; Massey, Amps et al. 2008), and brevican (Jones, Yamaguchi et al. 2002; Massey, Amps et al. 2008). Myelin associated inhibitors like NOGO, MAG and OMGp are well-known repellent molecules that also contribute to the lack of axonal regeneration observed after SCI (Grados-Munro and Fournier 2003; McGee and Strittmatter 2003; Goldshmit, Galea et al. 2004). Interestingly, inhibition of these factors results in partial regeneration and functional recovery, suggesting the presence of additional repulsive factors (Lee, Geoffroy et al.; Kim, Li et al. 2003; Simonen, Pedersen et al. 2003; Zheng, Ho et al. 2003).
Eph receptors, the largest subfamily of receptor tyrosine kinases (RTKs), and their ephrin ligands are important mediators of cell-cell communication regulating cell attachment, shape, and mobility (Lemke 1997; Klein 2001; Arvanitis and Davy 2008). They are subdivided in two groups, EphA and EphB, and their ligands are the ephrinA and B proteins, respectively (Lemke 1997; Menzel, Valencia et al. 2001). The classification is based on how the ligand is attached to the plasma membrane (1997). EphrinA ligands are glycosylphosphatidylinositol (GPI) anchored proteins, while the ephrinB ligands have a transmembrane domain. In early stages of development, Eph receptor-ephrin interactions have been found to be necessary for cell migration and formation of appropriate neural connections via repulsive activity (Wilkinson 2001; Benson, Romero et al. 2005), cell proliferation (Holmberg, Armulik et al. 2005) and apoptosis (Depaepe, Suarez-Gonzalez et al. 2005). During adulthood, the Eph/ephrin proteins continue to play roles in cell plasticity (Klein 2001; Klein 2004), including the formation and regulation of synapses (Takasu, Dalva et al. 2002; Chen, Fu et al. 2008), hippocampal plasticity associated with LTP (Henderson, Georgiou et al. 2001; Grunwald, Korte et al. 2004) and modulation of pain in the spinal cord (Battaglia, Sehayek et al. 2003).
Interestingly, these molecules are upregulated in several models of injury including excitotoxic injury in the hippocampus (Moreno-Flores and Wandosell 1999), optic nerve injury (King, Wallace et al. 2003), traumatic brain injury (Biervert, Horvath et al. 2001), multiple sclerosis (Sobel 2005) and spinal cord injury (Willson, Irizarry-Ramirez et al. 2002; Bundesen, Scheel et al. 2003; Goldshmit, Galea et al. 2004; Irizarry-Ramirez, Willson et al. 2005; Cruz-Orengo, Figueroa et al. 2006; Figueroa, Benton et al. 2006). The Eph receptors mediate the growth cone retraction by regulating the activity of different cytoplasmic GTPases through ephexin (Shamah, Lin et al. 2001).
Ephexin is one key member of the family of guanine nucleotide exchange factors (GEF). This recently described family of proteins is comprised of five members (Sahin, Greer et al. 2005) that share SH3 (Src homology 3), DH (Dbl homology) and PH (pleckstrin homology) domains. Ephexin-1 is of special interest because it catalyzes the exchange of guanine nucleotide for RhoA, Cdc42 and Rac1 equally. Studies show that basal activation of these three monomeric GTPases does not requires Eph receptors kinase activity nor ephrin ligand binding to its receptor (Shamah, Lin et al. 2001). However, once EphA receptors are activated, they induce phosphorylation of ephexin-1 through Src tyrosine kinase at tyrosine 87 (Sahin, Greer et al. 2005), which preferentially activates RhoA. Activation of RhoA stimulates actin-myosin contractility and stress fiber formation resulting in growth cone collapse (Kozma, Sarner et al. 1997; Luo 2000) and reduction in axonal regeneration (Dergham, Ellezam et al. 2002). The effect of growth cone collapse, mediated by Rho, has been observed in retinal neurons after ephrin-A5 biding (Wahl, Barth et al. 2000).
Since the Eph receptors are upregulated after CNS injury and mediate repulsive activity (Benson, Romero et al. 2005), we propose that ephexin expression and activation are altered after trauma to the spinal cord. Moreover, we aim to demonstrate that ephexin-1 is complementary to Eph and ephrin profile in the injured spinal cord and is partially responsible for the lack of axon regeneration after CNS injury.
Materials and Methods
Surgery
Adult female (200-275 grams) Sprague Dawley rats (Hilltop Lab Animals Inc, Scottdale, PA) were used. The animals were anesthetized with intramuscular injections of ketamine (Fort Dodge Animal Health, Fort Dodge, IA; 87.7mg/kg), acepromazine (Vetus Animal Health, Rockville Center, NY; 0.85 mg/kg) and xylazine (Boehringer Ingelheim, Ridgefield, CT; 4.2 mg/kg). The spinal cord was exposed through a T10 laminectomy and injured with the New York University (NYU) impactor device by dropping a 10-g rod, at a distance of 12.5 mm, onto the exposed dorsal surface of the spinal cord (Gruner 1992; Miranda, White et al. 1999; Cruz-Orengo, Figueroa et al. 2006; Figueroa, Benton et al. 2006). Control (sham) rats received a laminectomy only, exposing the cord without damaging the dura matter. Following spinal cord injury (SCI), the muscles, fascia and skin were sutured in layers and the animals returned to individual cages. Cefazolin (25 mg/kg, Bristol Myers Squibb; NY, NY) was administered intramuscularly at the time of surgery and for 7 days post-operatively to both groups. Bladders of injured animals were manually expressed three times daily until spontaneous micturition reflexes were re-established. Buprenex® (buprenorphine; Reckett & Colman Pharmaceuticals, Inc. Richmond, VA; 0.05 mg/kg, s.c.) was administered daily for three consecutive days after surgery as a post-operative analgesic (Santiago, Rosas et al. 2009). These experiments were approved by the University of Puerto Rico IACUC and followed NIH guidelines for the safe use and care of laboratory animals.
Antibody Information
Several antibodies were used in this study. Some of them were obtained from R & D System, Inc. to perform immunohistochemical studies to determine colocalization of ephexin protein with EphA3, -A4 and A7 receptors. The antibodies against EphA3 (AF640), EphA4 (AF641) and EphA7 (AF608) were anti-mouse polyclonal antibodies produced in goat, immunized with purified recombinant proteins that encoded the extracellular domain of each receptor. R & D systems, Inc. reported that IgGs specific for each Eph receptor were purified by affinity chromatography and the recommended dilution of 5 μg/ml (EphA3 & A7) and 3 μg/ml (EphA4) used to detect the presence of each receptors in ephexin expressing cells. Specificity of these antibodies was characterized by Slot blot and immunohistochemical analyses (see supplemental material 1 and 5). Each antibody recognized its specific antigen and did not cross-react with the other Eph-Fc-fusion proteins. Moreover, preabosption of the antibodies with the other Fc-fusion proteins did not affect the immunoreactivity against its specific target epitope (supplemental material 5). Although the EphA7 antibody worked well for the immunohistochemical studies, this commercial antibody did not work for Western blot studies.
Another antibody against the EphA7 receptor was made to determine the levels of this receptor in the adult spinal cord and its profile after injury by Western blot. The purified peptide CNSLESVARMTIDDV that encodes the carboxy terminus (950-963) of the EphA7 receptor (accession number: NP-599158) was analyzed by Mass-spectrophotometry and used as antigen to generate rabbit polyclonal antibodies (Biosource: project 6442). The antibody was affinity-purified and ELISA used to determine its specificity. Characterization of this antibody included amount of protein to be detected within the linear range and dilution of antibody to be used for detection of an immunoreactive band in the expected 120 kDa region. Preabsorption of the EphA7 antibody with the antigen used to generate it, did not recognize any band from the spinal cord extracts confirming its specificity.
The antibody against ephexin for Western blot and immunohistochemical studies was obtained from ECM Biosciences. This affinity-purified rabbit polyclonal antibody was raised against the carboxy-terminal region of the mouse protein, which has significant homology to the same region of the rat ephexin (ECM Biosciences, catalog number: EP2821). The dilution used for Western blot analyses was 1:1000, as recommended by the manufacturer since it recognize an 80 kDa protein band that correspond to ephexin in several cell lines and in brain tissue (ECM Biosciences). Immunohistochemical studies required a dilution of 1:500 of the ephexin antibody to determine the spatial expression of this protein in the spinal cord of adult rats. Although the commercial ephexin antibody functioned for Western blot and immunohistochemical analyzes, immunoprecipitation studies with this antibody cannot be performed (see supplemental material 2 and 3). Therefore, a custom made antibody against the phosphorylated ephexin (see supplemental material 4) to analyze the activation of this protein was used for immunoprecipitation studies. The immunogen GLL(Tyr-PO4)QEYRDKSTC phosphopeptide was used to generate rabbit polyclonal antibodies (GenScript Corporation). ELISA results performed by GenScript Corporation demonstrated that affinity purified antibodies against phospho-ephexin (versus non-phospho ephexin) were highly specific for the phosphorylated ephexin. Slot blots demonstrated the specificity of the antibody for the phosphorylated ephexin versus the non-phosphorylated peptide. Preabsorption of the antibody with the peptide used to generate it, demonstrated the specificity of the antibody for the phosphorylated ephexin (see supplemental material 4).
Additional antibodies used to monitor the levels of housekeeping genes like β-actin and GAPDH after SCI were obtained from Sigma-Aldrich. Antibodies to recognize cellular structures or phenotypes like astrocytes (anti-GFAP) (BD Biosciences), motorneurons (anti-NeuN) (Millipore), axons (anti-neurofilaments heavy chains) (Chemicon), microglia (anti-ED1) (Serotec), oligodendrocytes (anti-MAG) (Chemicon) (Cruz-Orengo, Figueroa et al. 2006; Figueroa, Benton et al. 2006; Rodriguez-Zayas, Torrado et al. 2010) were produce in mouse.
Protein extraction and Western Blot
In order to induce euthanasia, a pentobarbital (40-50 mg/kg) overdose was administered intraperitoneally and transcardial perfusion was performed using ice-cold 0.01 M phosphate buffered solution, pH 7.4 (PBS; Sigma-Aldrich, St. Louis, MO). The spinal cord segment containing the lesion epicenter (5mm) was dissected out (n=4 per time point, with their respective control groups). The dissected tissue was homogenized in ice-cold Tris lysis buffer (20 mM Tris, 150 mM NaCl, 5 mM NaF, 1 mM EDTA, 1 mM EGTA, pH 8) containing 2 μg/mL antipain, 10 μg/mL aprotinin, 5 mM benzamidine, 1mM DTT, 10 μg/mL leupeptin, 1 mM sodium orthovanadate, 1 mM PMSF, and 10 μg/mL trypsin inhibitors. After being centrifuged at 20,800g for 90 min, the supernatant fraction was analyzed. The protein concentration was determined using Bio-Rad’s DC Protein assay according to the manufacturer’s instructions (Bio-Rad Laboratories, Hercules, CA). Then, the extracted proteins (50 μg) were analyzed in a 10% polyacrylamide-SDS gel (run for 1.5 hours at 200 V, 300 mA and 10 W at room temperature) and electroblotted using a nitrocellulose membrane with transfer buffer (25 mM Tris, 192 mM glycine, 20% MeOH, pH 8.3) for 18 hours (constant voltage = 35 V). To verify transfer and reveal molecular weight markers, the nitrocellulose membrane was stained with 0.1% Ponceaus S (made in 0.1% glacial acetic acid) for 10 minutes and then rinsed with PBS 1×, three times for 10 minutes each. The nitrocellulose membrane was incubated in blocking solution (3% skim milk, 20 mM Tris-HCl, 150 mM NaCl, 0.05% Tween-20, pH 7.5) for 2 hours at room temperature. The membrane was probed overnight at 4ºC with the rabbit anti-ephexin polyclonal antiserum (1:1000; ECM Bioscience, Versailles, KY) in blocking solution. Afterwards, the membrane was washed 3 times for 10 min each in blocking solution and then HRP-conjugated donkey anti-rabbit IgG (1:10,000; Sigma, St. Louis, MO) was applied to the membrane and incubated for one hour at room temperature. The membrane was washed twice with blocking solution and twice with Tris-NaCl (10 mM Tris, 100 mM NaCl, and 0.1% Tween-20, pH 7.5) for 10 minutes each. HRP-signal was enhanced with SuperSignal West Dura extended version (Pierce, Rockford, IL) for 1 min according to manufacturer’s instruction before exposure and development. The development and densitometric analysis of the nitrocellulose membrane was performed using the Versadoc™ Imaging System and Quantity One Software (Bio-Rad Laboratories, Hercules, CA). The levels of ephexin protein were standardized to the amount of immunodetected GAPDH (1:5000; Sigma-Aldrich, St.Louis, MO, USA) at each time point studied.
EphA7 full-length receptors were evaluated from total protein homogenates that were extracted from SCI epicenters (n=3) using protease inhibitors in 1% NP-40. Aliquots of 50 μg were loaded in a 7.5% SDS-PAGE, then transferred to nitrocellulose membranes. Immunoreactivity was assessed with custom made rabbit-anti-EphA7 antibody that recognize the epitope: CNSLESVARMTIDDV (Biosource International, Hopkinton, MA, 1:50), and β-actin was analyzed with monoclonal anti-actin (Sigma, 1:1000). Both proteins were studied by anti-rabbit IgG (Sigma, 1: 10,000) and anti-mouse IgG (Sigma, 1:2000) followed by their respective secondary HRP-conjugated antibodies, as described before. Chemiluminescence was developed using SuperSignal West Dura (Pierce), and densitometric scanning was performed.
Immunoprecipitation
Animals (n=4 per time point, with their respective control groups) were sacrificed, as mentioned before, with an overdose of pentobarbital and perfused transcardially with ice-cold PBS. The lesion epicenter was dissected, followed by protein extraction and concentration determination using Bio-Rad DC protein assay. Approximately, 86.5μg of protein extract in a final volume of 500 μL was mixed with 30 μL of previously washed (3 times in lysis buffer) protein G sepharose™ beads (Amersham Biosciences,Uppsia, Sweden). The mixture was incubated for 30 min at 4ºC in a gentle rocker, and spun for 30 sec at 2,000 rpm (4ºC). The pre-cleared supernatant was transfered to a new microtube with 5μL of anti-phospho Ephexin antibody (against GLLY(PO4)QEYRDKST –NCBI References Sequence: 001104784- GenScript, Piscataway, NJ), and incubated overnight at 4ºC. Then, the supernatant-antibody mixture was transferred to a new microtube containing 40 μL of sepharose G beads (previously washed with lysis buffer and incubated with protein extract to reduce non-specific binding) and incubated at 4ºC for 2 hours in a gently-mixing shaker. The samples were centrifuged at 2,000 rpm for 20 sec (4ºC) and the supernatant was discarded. Finally, the immunocomplex was washed three times with PBS (pH 7.4) followed by two washes with TBS (1M Tris-HCl and 2M NaCl, pH 7.4). Between washes the microtubes were centrifuged at 2,000 rpm. The supernatant was removed and the pellet immunocomplex, containing the phosphorylated ephexin, was resuspended in Lammeli Sample Buffer (Bio-Rad, Hercules, CA). The samples were analyzed by SDS-PAGE and immunobloted as before with rabbit anti-ephexin polyclonal antiserum (1:1,000; ECM Bioscience, KY). Western blot studies against GAPDH were performed to monitor equal amount of cell extract at each time point.
Immunohistochemistry
Double labeling studies were performed as previously described (Cruz-Orengo et al 06b;Figueroa et al 06d). Rats (n=3) were perfused transcardially with ice-cold 0.01 M phosphate buffered saline (PBS) pH 7.4, followed by ice-cold 4% paraformaldehyde (PFA). The spinal cord was dissected and placed in 4% PFA for 2 hours at 4°C and then cryoprotected in 30% sucrose overnight. The spinal cords were mounted in tissue blocks with Tissue Freezing Medium (Triangle Biomedical Sciences, Durham, N.C.), and sectioned at 20 μm using a Leica cryostat cryocut 1800 (Nussloch, Germay).
The immunostaining was performed as Figueroa (2006). Briefly, the tissue was dried for 20 min at room temperature and the region of incubation within the slide delineated with a PAP PEN (TED PELLA.INC., Redding, CA). The sections were washed in 0.01 M PBS for 5 minutes at room temperature and post-fixed in 4% PFA for 5 minutes at 4°C. Then, the sections were washed with 0.01 M PBS three times for 10 minutes at room temperature and 0.1% Triton X-100 was added for 20 minutes at room temperature. The non-specific background was blocked in 0.01 M PBS/3% BSA serum for 3 hours at room temperature. Finally, the sections were incubated overnight at 4°C with rabbit anti-ephexin polyclonal antibody (1:500; ECM Bioscience, Versailles, KY). The following day, the slides were washed with 0.01 M PBS three times for 10 minutes at room temperature. Subsequently, secondary antibody (1:100; Rhodamine-red donkey anti-rabbit, Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) in 3% BSA was added and incubated in the dark for 2 hours at room temperature. Finally, three washes in 0.01 M PBS for 10 minutes were performed and the sections were mounted with ProLong® Antifade Kit (Molecular Probe Invitrogen, Eugene, OR, USA) and coverslipped. Cells expressing the ephexin protein were identified by double-labeling with monoclonal antibodies against NeuN (1:100; Millipore, Millipore, Massachusetts, USA), GFAP (1:100; BD Biosciences, Franklin Lakes, NJ, USA), ED-1 (1:500; Serotec, Raleigh, NC, USA), NF-H (1:1,000; Chemicon, Massachusetts, USA) and MAG (1:250; Chemicon, Massachusetts, USA), cell-specific markers for neurons, astrocytes, microglia, axons and oligodendrocytes, respectively. ALEXA-488 goat anti-mouse IgG (1:250; Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) was used to visualize these cells or structures. To abolish the possibility of bleeding signal between channels, sequential excitation and capture was performed with tissue containing multiple flourophores analyzed by laser confocal microscopy.
Additional studies were performed to visualize possible regions of EphA receptors and ephexin interaction in the injured spinal cord (n=3). Antibodies against ephexin, EphA3 (5μg/mL goat IgG; R&D Systems Minneapolis, MN, USA), EphA4 (3μg/mL goat IgG; R&D Systems Minneapolis, MN, USA) and EphA7 (5μg/mL goat IgG; R&D Systems Minneapolis, MN, USA) were used as recommended by the manufacturer. The slides were incubated with the secondary antibody Rhodamine-red donkey anti-goat IgG (1:200) to identify the receptors and with ALEXA-488 donkey anti-rabbit IgG (1:250) to visualize ephexin. The stained sections were visualized with a LSM5 Pascal Laser Scanning Microscope upright confocal system (Carl Zeiss Inc., Thornwood, NY, USA), and photomicrographs were assembled with Adobe Photoshop ® CS version 8.0 software (Adobe system Inc., San Jose, CA, USA). Analysis of ephexin fluorescence was determined with ImageJ 1.43u® software (Wayne Rashband, NIH, USA) to quantify the intensity of the signal.
Statistics
The data was expressed as the mean ± S.E.M. and statistical analyses were performed by ANOVA, followed by a post-hoc test to compare differences among samples, using InStat version 3.0 software (GraphPad Software Inc., San Diego, CA, USA). Differences were considered to be significant when p< 0.05 with 3-4 animals per time point.
Results
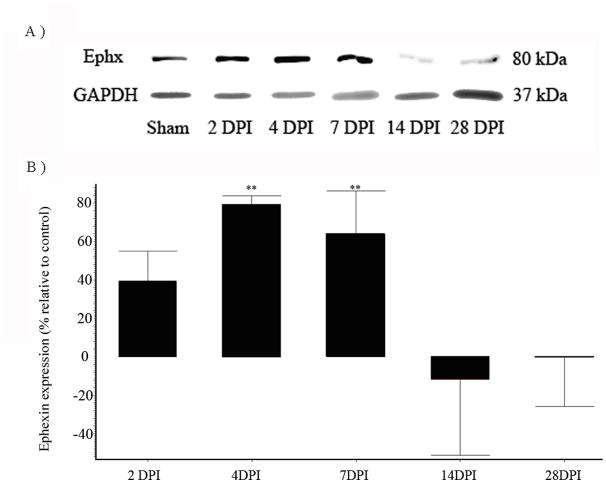
The expression profile of ephexin was determined using Western blot analysis. An immunoreactive band was observed in the expected 80kDa region (Figure 1), showing for the first time the expression of this protein in the adult spinal cord. Pre-absorption of the antibody with the peptide used to generate it eliminated the reactive band, confirming the specificity of the antibody for the ephexin protein. Fifty-micrograms of protein extract were used in our studies, finding this amount adequate after evaluating the linear range of protein detection for immunoblot assays (data not shown),
Figure 1.
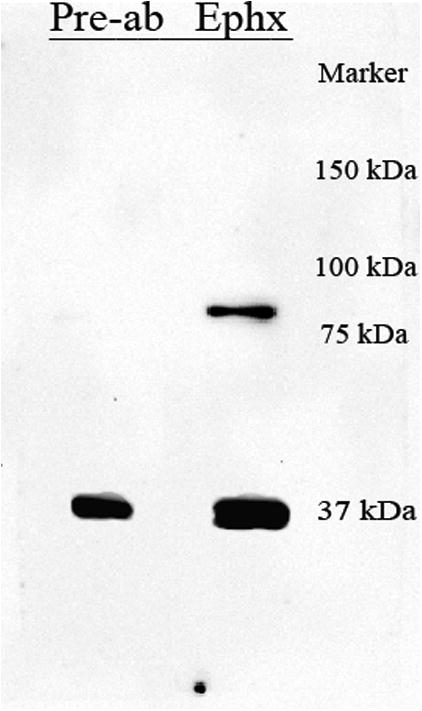
Expression of ephexin in the adult spinal cord. Proteins extracted from the spinal cord were immunobloted with a polyclonal anti-ephexin antibody, and similar fractions were incubated with the antibody pre-absorbed with the antigen used to generate it. The elimination of the 80 kDa reactive band with the pre-absorption, confirmed the specificity of the antibody and the expression of this protein in the adult spinal cord.
Western blots were performed with extracts from sham and injured animals both at different time points (2, 4, 7, 14, and 28 days post surgery), and the pattern of ephexin expression compared relative to GAPDH at each time point (Figure 2A). Densitometry analysis showed a significant increase in ephexin at 4 DPI the level increased to 79% ± 4.6 (**p<0.01), that remained elevated until 7 DPI by 64% ± 22.8 (*p<0.01) (Figure 2B). At 14 DPI, the protein expression returned to pre-injury levels and remained at basal levels until 28 DPI. ANOVA followed by Dunnett test was used to determine the significant differences among samples studies (F= 6.972 df (5,32), p< 0.0002).
Figure 2.
Spinal cord injury alters ephexin expression. Adult Sprague Dawley rats were injured with the New York University (NYU) impactor device, and proteins were extracted at different days post-injury (DPI). The extracts were analyzed by SDS-PAGE and immunoblotted for ephexin expression. SCI increases ephexin protein levels at 2, 4 and 7 DPI. Each bar represents a different time point (DPI), and all of them were compared with sham animals. Error bars show the standard error of the mean (SEM). 4 DPI (**p<0.01) and 7 DPI (**p<0.01). (n=4). ANOVA followed by Dunnett test was used to determine the significant differences among samples studies (F= 6.972 df(5,32), p= 0.0002).
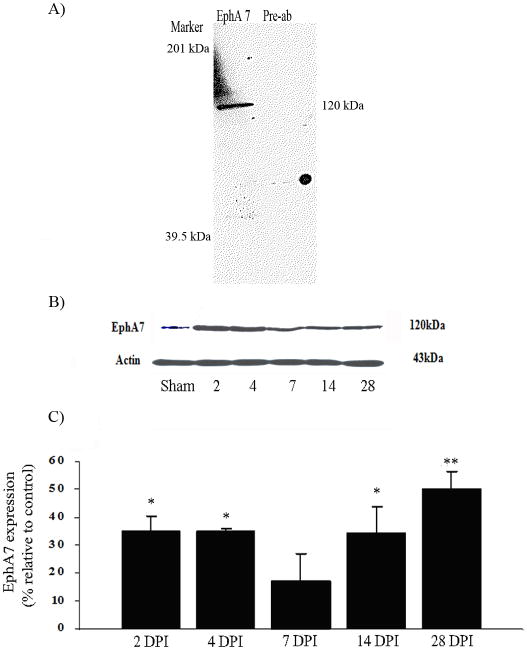
To correlate the expression pattern of ephexin with the expression profile of EphA7, the levels of tyrosine kinase receptor were studied. Antibody immunoreactivity detected a band (∼120 kDa) for EphA7 full-length expression in the spinal cord of sham animals (Figure 3A). Preabsorption of the antibody with the peptide used to generate it demonstrated the specificity for the EphA7 receptor. However, the expression was upregulated after SCI, and these differences were significant at 2 (*p<0.05), 4 (*p<0.05), 14 (*p<0.05) and 28 (**p<0.01) DPI by 36% ± 5.5, 36% ± 1, 35% ± 9.5, and 50% ± 7, respectively (Figure 3B). ANOVA followed by Dunnett test was used to determine the significant differences among samples studies (F= 6.061 df(5,12), p<0.0019). No changes were observed with β-actin expression.
Figure 3.
Spinal cord injury increased full-length EphA7 protein levels. SDS-PAGE showed a unique band at 120 kDa region for full length EphA7 compared with similar fractions that were incubated with the antibody pre-absorbed with the antigen used to generate it (A). Time course studies after SCI demonstrated that EphA7 immunoreactive protein increased relative to sham control, with no changes observed with β-actin expression (B). Densitometry analysis demonstrated a significant upregulation at 2 (*p<0.05), 4 (*p<0.05), 14 (*p<0.05) and 28 DPI (**p<0.01), n= 3 (C). ANOVA followed by Dunnett test was used to determine the significant differences among samples studies (F(5,12) = 6.061, p<0.0019).
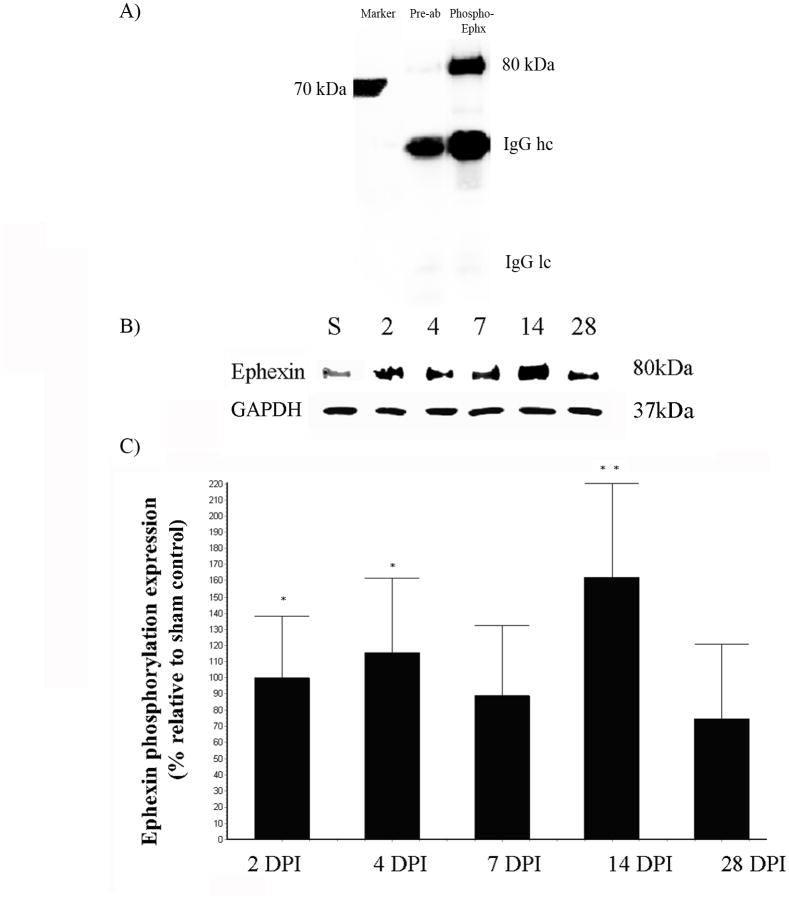
Immunoprecipitation (IPP) studies determined the phosphorylation of ephexin in the injured spinal cord, as an indicator of protein activation. The specificity of the antibody against phospho-ephexin was validated with pre-absorption of the former as described previously (data not shown). The antibody against phospho-ephexin showed a unique band at 80kDa (Figure 4A). The protein extract for IPP was analyzed at 2, 4, 7, 14 and 28 DPI to determine the pattern of ephexin activation after SCI. Extracts for each time point (n=4) were analyzed by Western blot against GAPDH to demonstrated equal amount of proteins for the immunoprecipitation assay. Densitometry analysis showed a tendency to increase in phospho-ephexin at all time points, with a significant increment at 2 (*p<0.05), 4 (*p<0.05) and 14 DPI (**p<0.01) by 99% ± 38.9, 116% ± 45.7, and 161% ± 59.0, relative to sham/control animals (Figure 4B). ANOVA followed by Dunnett test was used to determine the significant differences among samples studies (F= 4.285 df(5,27), p<0.0053).
Figure 4.
Trauma to the spinal cord induces ephexin phosphorylation. Anti-phospho Ephexin immunoprecipitated specifically the activated protein (80 kDa) from spinal cord extracts and preabsorption assay demonstrated the specificity of the antibody used (A). Immunoprecipitation showed the activation of ephexin at the lesion epicenter at 2, 4, 7, 14 and 28 DPI. Extracts for each time point were analyzed by Western blot against GAPDH to demonstrated equal amount of proteins for the immunoprecipitation assay (B). Densitometry analysis of phospho-ephexin showed a significant increase at 2 (*p<0.05), 4 (*p<0.05) and 14 DPI (**p<0.01) (C). ANOVA followed by Dunnett test was used to determine the significant differences (F= 4.285 df(5,27), p<0.0053). Error bars demonstrate the standard error of the mean (SEM), 2DPI and 4 DPI (n=5); 7 DPI and 14 DPI (n=3) and 28 DPI (n=4).
Immunohistochemical studies were used to identify the spatial profile of ephexin expression in the adult spinal cord and after injury. Single labeling immunohistochemistry and densitometric analyses were performed to compare the pattern in protein expression between sham (Figure 5A & C) and injured groups (Figure 5B & D). As observed before, the injured spinal cord at 4 DPI (Figure 5G, **p<0.05) showed more immunoreactive ephexin in experimental animals than in control groups, and the levels of ephexin at 28 DPI (Figure 5G) were similar to sham animals. ANOVA followed by Dunnett test was used to determine the significant differences (F=8.754 df(3,10), p<0.0038). Preabsorption of the antibody removed the immunoreactive signal (at 4 DPI), thus confirming the specificity of the antibody (Figure 5E & F).
Figure 5.
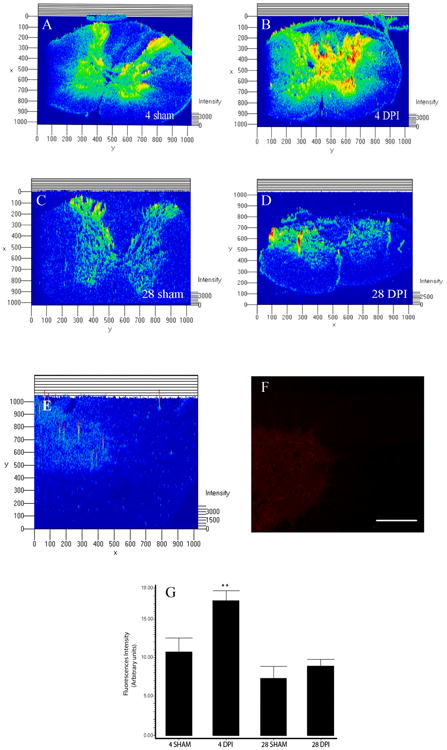
Spatial profile of ephexin expression. Densitometric analysis of photo micrographs demonstrated that contused rats at 4 DPI (B) (**p<0.01) presented more immunoreactive staining than 4 days sham (A) animals, but injured rats at 28 DPI (D) did not seem to be different than 28 days control (C) animals. ANOVA followed by Dunnett test was used to determine the significant differences (G) (F= 8.754 df(3,10), p<0.0038). Spinal cord sections at 4DPI were incubated with the antibody pre-absorbed with the antigen used to generate it (E & F) and the immunoreactive fluorescence diminished compared with sections treated with anti-ephexin antibody alone. Scale bar: 50 μm
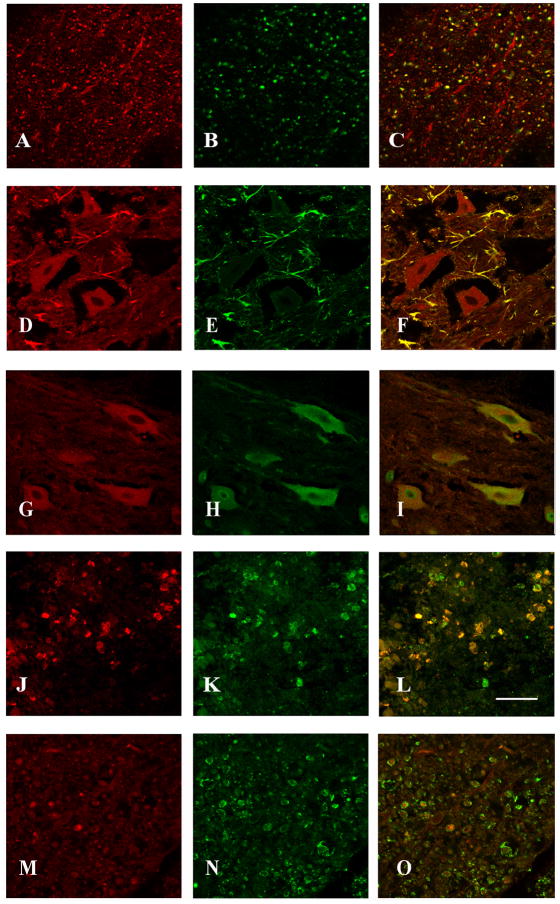
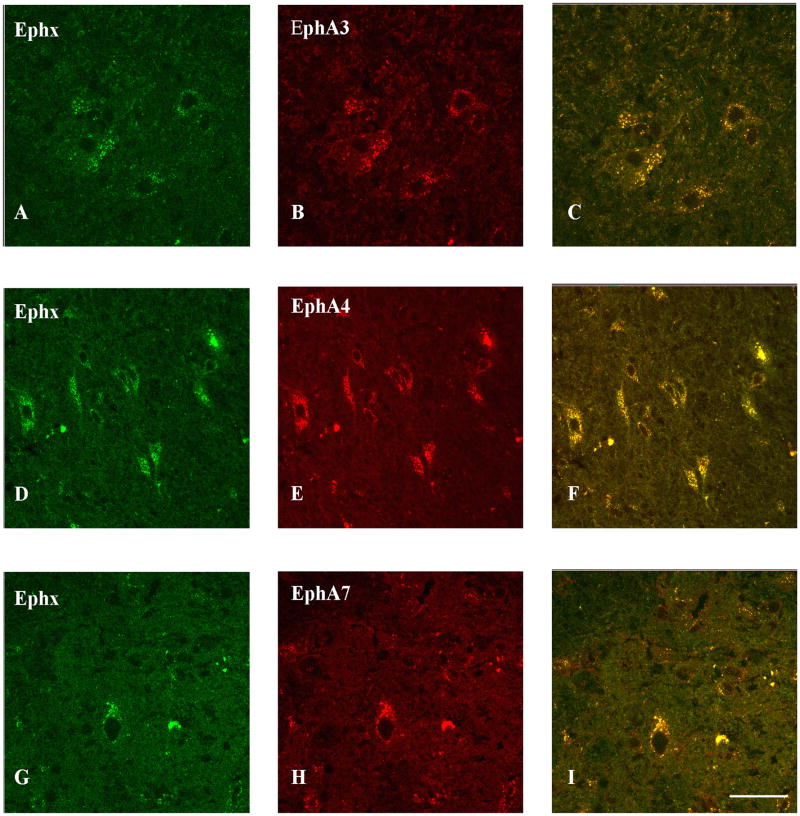
Double labeling analyses demonstrated co-localization of ephexin in NF-200 positive axons in the ventral lateral funniculus of the white matter (Figure 6A-C), GFAP-positive astrocytes (Figure 6D-F), and NeuN positive neurons (Figure 6G-I) in the ventral horn of the gray matter rostral to the lesion epicenter (also observed caudal to the lesion epicenter but the data is not shown). Ephexin is expressed in microglia/macrophages at the lesion epicenter (Figure 6J-L) but not in oligodendrocytes (Figure 6M-O). In addition, to compare the overlapping expression of ephexin with EphA receptors double label studies were made. The assays demonstrated co-localization of ephexin with EphA3 (Figure 7A-C), EphA4 (Figure 7D-F) and EphA7 (Figure 7G-I) in the same cell and in adjacent cells.
Figure 6.
Cell phenotype expressing ephexin in the injured spinal cord. Co-localization of ephexin in axons of the ventral white matter and in cells located in the gray matter such as astrocytes, neurons (close to the lesion epicenter) and macrophages (within the lesion cavity) was determined with double labeling studies and confocal microscopy (n=3). The first panel shows ephexin expression (red color; A, D, G, J), the second panel illustrates the different cellular/structural markers used (green color; NF-H (B), GFAP (E), NeuN (H), ED1 (K) and MAG (N)), and the third panel represents the merge (C, F, I, L). Ephexin (M) was not observed in myelin (N) structure of oligodendrocytes (O). Scale bar = 50μm
Figure 7.
EphA receptors interaction with ephexin after spinal cord trauma at 4 DPI. Double label analysis demonstrates the co-localization of EphA3 (B), EphA4 (E) and EphA7 (H) with ephexin (A, D and G). The yellow column (C,F and I) represents the co-localization of EphA-ephexin interaction in cells of CNS. Scale bar = 50 μm.
Discussion
Evidence demonstrates that the regulation of neurite outgrowth is an important process in order to achieve nerve regeneration after trauma to the CNS. Among the repulsive proteins that generate a non-permissive environment for axonal outgrowth after SCI are the Eph receptors and their ligands, the ephrins (Miranda, White et al. 1999; Willson, Irizarry-Ramirez et al. 2002; Bundesen, Scheel et al. 2003; Goldshmit, Galea et al. 2004; Benson, Romero et al. 2005; Irizarry-Ramirez, Willson et al. 2005; Figueroa, Benton et al. 2006). However, the intracellular mechanism activated after Eph-ephrin binding that regulates cytoskeletal elements expression and/or assembly in the regenerative axons remains unclear. Ephexin is an excellent candidate because activation of this guanine exchange factor modulates preferentially the monomeric G-protein Rho, producing growth cone collapse (Shamah, Lin et al. 2001; Sahin, Greer et al. 2005). Here, we detailed the temporal and spatial expression profile of ephexin using Western blots, immunoprecipitation and immunohistochemistry after contusive spinal cord injury.
In the present study, we have investigated the protein expression pattern of ephexin after SCI using Western Blot. The levels of ephexin increased during the sub-acute phase (Figure 2) after spinal cord trauma, suggesting that the expression of this protein mediates some of the cellular responses observed in the initial phase of axonal regeneration. Similar results were obtained from protein analysis on EphA7 receptors after SCI (Figure 3). Previous studies have shown that the expressions of several members of the Eph receptor family are significantly increased at 7 days post injury (Miranda, White et al. 1999; Willson, Irizarry-Ramirez et al. 2002; Irizarry-Ramirez, Willson et al. 2005; Cruz-Orengo, Figueroa et al. 2006; Figueroa, Benton et al. 2006). Based on the expression pattern of these receptors after SCI, our data suggest that internalization of Eph signaling could be mediated through ephexin after CNS trauma, causing growth cone collapse resulting in axonal regeneration blockade.
Our immunohistochemical studies also demonstrated that ephexin is acutely up-regulated after SCI (4 DPI) and returns to basal levels at 28 DPI (Figure 5G), confirming the Western blot analysis (Figure 2B). Disregarding the discrepancy between ephexin levels with EphA4 (Cruz-Orengo, Figueroa et al. 2007) or EphA7 receptor at 14 and 28 DPI, the phosphorylation of ephexin was up-regulated (Figure 4B), suggesting that the activation of ephexin is more important than its expression (Table 1). EphA3 receptor time course was not included in table 1 because immunohistochemistry assays were used to qualitatively establish protein levels after injury and only mRNA levels were quantified (Willson, Irizarry-Ramirez et al. 2002; Irizarry-Ramirez, Willson et al. 2005). Based on the role of ephexin during development, our results suggest that the up-regulated ephexin expression and later activation could be mediated by the Eph receptors that are expressed at the injury site modulating axon growth and/or guidance in the injury microenvironment. This conclusion is consistent with our immunohistochemical result that showed co-localization of ephexin with EphA3, A4 and A7 (Figure 7) in cells and structures of the CNS.
Table 1. Correlation of ephexin expression and activation with some members of the Eph receptors after injury.
| EphA 4 | EphA 7 | Ephexin | Ephexin-P | |
|---|---|---|---|---|
| 2 DPI | ↓ | ↑ | ↑ * | ↑ |
| 4 DPI | ↓ | ↑ | ↑ | ↑ |
| 7 DPI | = | ↑ * | ↑ | ↑ * |
| 14 DPI | ↑ * | ↑ | = | ↑ |
| 28 DPI | ↑ * | ↑ | = | ↑ * |
Tendency
= Basal level
↑ Upregulation
↓ Downregulation
Previous (Irizarry-Ramirez, Willson et al. 2005; Cruz-Orengo, Figueroa et al. 2006; Figueroa, Benton et al. 2006) and actual (Figure 6D-F) studies from our laboratory demonstrate that ephexin, EphA3, EphA4 and EphA7 full length are expressed in reactive astrocytes. These results are different to those recently reported by Puschmann (Puschmann and Turnley 2010), in which they could not detect ephexin in mouse cortical astrocytes cultures. This discrepancy could be possible due to the different types of animal species used (mouse vs. rat), time of development studied (newborn [P1-P3] vs. adult), cell type (brain cortex vs. spinal cord) or model used (in vitro vs. in vivo). Therefore, our data suggests that activation of the Eph receptors by the ephrins may promote the Eph-ephexin interaction and could affect the migration of reactive astrocytes, keeping them in place forming the glial scar (Bundesen, Scheel et al. 2003) that may reduce the size of the cyst cavity. Moreover, white matter cells in the CNS have been implicated in the neurite outgrowth repulsion due to inhibitory proteins that form a physical and chemical barrier at the lesion epicenter (Silver and Miller 2004; Niclou, Ehlert et al. 2006). Interaction of Eph receptors located in axonal structures with ephrin ligands in cells surrounding it (Benson, Romero et al. 2005) could activate ephexin and thus causing growth cone collapse.
Therefore, since the main role of ephexin is the regulation of cytoskeletal changes, it is plausible that its concomitant expression with Eph-ephrin proteins could be one of the factors responsible for the lack of regeneration observed after injury in the adult spinal cord. Furthermore, its expression pattern suggests a model for how EphA receptors may locally regulate actin dynamics after SCI. Several key downstream elements of ephexin are also activated following traumatic spinal cord injury. A key element of Eph-ephexin signaling is the small GTPase RhoA. The expression of Rho mRNA and protein are significantly enhanced in the injured adult vertebrate spinal cord one week after surgery (Sung, Miao et al. 2003). Moreover, this GTPase protein regulates the organization of the actin cytoskeleton and has been implicated in the regenerative process (Kozma, Sarner et al. 1997; Dergham, Ellezam et al. 2002). Our immunohistochemical analyses demonstrate that this inhibitory signaling molecule is present in neurons and axons after injury, probably breaking re-growth or altering essential aspects of axonal transport (Figure 6).
Our studies suggest that Eph-ephexin signaling pathways might be manipulated to improve the regenerative response to injury. The interaction between the EphA receptors and the ephrinA ligands is highly promiscuous (Himanen, Saha et al. 2007). However, there is a common intracellular signaling mechanism mediated by ephexin that initiates growth cone collapse. Therefore, future pharmacological tools to monitor the level of ephexin activation after SCI or knockdown studies will be necessary to elucidate the functional role of ephexin and its direct impact in modulating regeneration after trauma.
Supplementary Material
Acknowledgments
Grant information: MBRS-RISE Program, MBRS-SCORE (2SO66M8224), RCMI (G12RR03051), SNRP (NS39405), M-RISP (532851) and the Associate Deanship of Biomedical Sciences at the University of Puerto Rico School of Medicine.
This project was supported by MBRS-SCORE (2SO66M8224), RCMI (G12RR03051), SNRP (NS39405), M-RISP (532851) and the Associate Deanship of Biomedical Sciences at the University of Puerto Rico School of Medicine. This publication was made possible by Grant Number R25GM061838 from the National Institute of General Medical Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
We thank Luz C. Arocho, Laurivette Mosquera and Ana E. Rodriguez for their technical assistance during the surgeries. Also, we want to give a special thanks to the personnel of the Animal Resources Center (at University of Puerto Rico- Medical Sciences Campus) and the Experimental Surgery facilities. This research project is in partial fulfillment of Mr. Odrick Rosas doctoral thesis dissertation. Finally, our appreciation to Dr. Paul Shepherd for his critiques in the manuscript and to Rafael Vazquez for the statistical advice.
References
- Unified nomenclature for Eph family receptors and their ligands, the ephrins. Eph Nomenclature Committee. Cell. 1997;90(3):403–404. doi: 10.1016/s0092-8674(00)80500-0. [DOI] [PubMed] [Google Scholar]
- Arvanitis D, Davy A. Eph/ephrin signaling: networks. Genes Dev. 2008;22(4):416–429. doi: 10.1101/gad.1630408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher RA, Morgenstern DA, et al. Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J Neurosci. 2002;22(6):2225–2236. doi: 10.1523/JNEUROSCI.22-06-02225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia AA, Sehayek K, et al. EphB receptors and ephrin-B ligands regulate spinal sensory connectivity and modulate pain processing. Nat Neurosci. 2003;6(4):339–340. doi: 10.1038/nn1034. [DOI] [PubMed] [Google Scholar]
- Benson MD, Romero MI, et al. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci U S A. 2005;102(30):10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biervert C, Horvath E, et al. Semiquantitative expression analysis of ephrine-receptor tyrosine kinase mRNA's in a rat model of traumatic brain injury. Neurosci Lett. 2001;315(1-2):25–28. doi: 10.1016/s0304-3940(01)02312-6. [DOI] [PubMed] [Google Scholar]
- Bogda L. Modification of Ramon y Cajal method of silver staining of the neuroglia. Patol Pol. 1956;7(2):119–121. [PubMed] [Google Scholar]
- Bouquet C, Nothias F. Molecular mechanisms of axonal growth. Adv Exp Med Biol. 2007;621:1–16. doi: 10.1007/978-0-387-76715-4_1. [DOI] [PubMed] [Google Scholar]
- Bundesen LQ, Scheel TA, et al. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci. 2003;23(21):7789–7800. doi: 10.1523/JNEUROSCI.23-21-07789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch SA, Horn KP, et al. Overcoming macrophage-mediated axonal dieback following CNS injury. J Neurosci. 2009;29(32):9967–9976. doi: 10.1523/JNEUROSCI.1151-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss A, Pech K, et al. NG2 and phosphacan are present in the astroglial scar after human traumatic spinal cord injury. BMC Neurol. 2009;9:32. doi: 10.1186/1471-2377-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Fu AK, et al. Bidirectional signaling of ErbB and Eph receptors at synapses. Neuron Glia Biol. 2008;4(3):211–221. doi: 10.1017/S1740925X09990287. [DOI] [PubMed] [Google Scholar]
- Cruz-Orengo L, Figueroa JD, et al. Reduction of EphA4 receptor expression after spinal cord injury does not induce axonal regeneration or return of tcMMEP response. Neurosci Lett. 2007;418(1):49–54. doi: 10.1016/j.neulet.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Orengo L, Figueroa JD, et al. Blocking EphA4 upregulation after spinal cord injury results in enhanced chronic pain. Exp Neurol. 2006;202(2):421–433. doi: 10.1016/j.expneurol.2006.07.005. [DOI] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214(4523):931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Depaepe V, Suarez-Gonzalez N, et al. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435(7046):1244–1250. doi: 10.1038/nature03651. [DOI] [PubMed] [Google Scholar]
- Dergham P, Ellezam B, et al. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22(15):6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa JD, Benton RL, et al. Inhibition of EphA7 up-regulation after spinal cord injury reduces apoptosis and promotes locomotor recovery. J Neurosci Res. 2006;84(7):1438–1451. doi: 10.1002/jnr.21048. [DOI] [PubMed] [Google Scholar]
- Fishman PS, Kelley JP. The fate of severed corticospinal axons. Neurology. 1984;34(9):1161–1167. doi: 10.1212/wnl.34.9.1161. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, Galea MP, et al. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J Neurosci. 2004;24(45):10064–10073. doi: 10.1523/JNEUROSCI.2981-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grados-Munro EM, Fournier AE. Myelin-associated inhibitors of axon regeneration. J Neurosci Res. 2003;74(4):479–485. doi: 10.1002/jnr.10803. [DOI] [PubMed] [Google Scholar]
- Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9(2):123–126. doi: 10.1089/neu.1992.9.123. discussion 126-128. [DOI] [PubMed] [Google Scholar]
- Grunwald IC, Korte M, et al. Hippocampal plasticity requires postsynaptic ephrinBs. Nat Neurosci. 2004;7(1):33–40. doi: 10.1038/nn1164. [DOI] [PubMed] [Google Scholar]
- Henderson JT, Georgiou J, et al. The receptor tyrosine kinase EphB2 regulates NMDA-dependent synaptic function. Neuron. 2001;32(6):1041–1056. doi: 10.1016/s0896-6273(01)00553-0. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Saha N, et al. Cell-cell signaling via Eph receptors and ephrins. Curr Opin Cell Biol. 2007;19(5):534–542. doi: 10.1016/j.ceb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg J, Armulik A, et al. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes Dev. 2005;19(4):462–471. doi: 10.1101/gad.326905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle JD, Jin Y. Chronically injured supraspinal neurons exhibit only modest axonal dieback in response to a cervical hemisection lesion. Exp Neurol. 2001;169(1):208–217. doi: 10.1006/exnr.2001.7645. [DOI] [PubMed] [Google Scholar]
- Irizarry-Ramirez M, Willson CA, et al. Upregulation of EphA3 receptor after spinal cord injury. J Neurotrauma. 2005;22(8):929–935. doi: 10.1089/neu.2005.22.929. [DOI] [PubMed] [Google Scholar]
- Jones LL, Yamaguchi Y, et al. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22(7):2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Li S, et al. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38(2):187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- King CE, Wallace A, et al. Transient up-regulation of retinal EphA3 and EphA5, but not ephrin-A2, coincides with re-establishment of a topographic map during optic nerve regeneration in goldfish. Exp Neurol. 2003;183(2):593–599. doi: 10.1016/s0014-4886(03)00211-5. [DOI] [PubMed] [Google Scholar]
- Klein R. Excitatory Eph receptors and adhesive ephrin ligands. Curr Opin Cell Biol. 2001;13(2):196–203. doi: 10.1016/s0955-0674(00)00197-6. [DOI] [PubMed] [Google Scholar]
- Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr Opin Cell Biol. 2004;16(5):580–589. doi: 10.1016/j.ceb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Kozma R, Sarner S, et al. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17(3):1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Geoffroy CG, et al. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 66(5):663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G. A coherent nomenclature for Eph receptors and their ligands. Mol Cell Neurosci. 1997;9(5-6):331–332. doi: 10.1006/mcne.1997.0630. [DOI] [PubMed] [Google Scholar]
- Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1(3):173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- Massey JM, Amps J, et al. Increased chondroitin sulfate proteoglycan expression in denervated brainstem targets following spinal cord injury creates a barrier to axonal regeneration overcome by chondroitinase ABC and neurotrophin-3. Exp Neurol. 2008;209(2):426–445. doi: 10.1016/j.expneurol.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26(4):193–198. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- Menzel P, Valencia F, et al. Ephrin-A6, a new ligand for EphA receptors in the developing visual system. Dev Biol. 2001;230(1):74–88. doi: 10.1006/dbio.2000.0109. [DOI] [PubMed] [Google Scholar]
- Miranda JD, White LA, et al. Induction of Eph B3 after spinal cord injury. Exp Neurol. 1999;156(1):218–222. doi: 10.1006/exnr.1998.7012. [DOI] [PubMed] [Google Scholar]
- Moreno-Flores MT, Wandosell F. Up-regulation of Eph tyrosine kinase receptors after excitotoxic injury in adult hippocampus. Neuroscience. 1999;91(1):193–201. doi: 10.1016/s0306-4522(98)00568-5. [DOI] [PubMed] [Google Scholar]
- Niclou SP, Ehlert EM, et al. Chemorepellent axon guidance molecules in spinal cord injury. J Neurotrauma. 2006;23(3-4):409–421. doi: 10.1089/neu.2006.23.409. [DOI] [PubMed] [Google Scholar]
- Puschmann TB, Turnley AM. Eph receptor tyrosine kinases regulate astrocyte cytoskeletal rearrangement and focal adhesion formation. J Neurochem. 2010;113(4):881–894. doi: 10.1111/j.1471-4159.2010.06655.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Zayas AE, Torrado AI, et al. P2Y2 receptor expression is altered in rats after spinal cord injury. Int J Dev Neurosci. 2010;28(6):413–421. doi: 10.1016/j.ijdevneu.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin M, Greer PL, et al. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46(2):191–204. doi: 10.1016/j.neuron.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Santiago JM, Rosas O, et al. Molecular, anatomical, physiological, and behavioral studies of rats treated with buprenorphine after spinal cord injury. J Neurotrauma. 2009;26(10):1783–1793. doi: 10.1089/neu.2007.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamah SM, Lin MZ, et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105(2):233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Simonen M, Pedersen V, et al. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron. 2003;38(2):201–211. doi: 10.1016/s0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- Sobel RA. Ephrin A receptors and ligands in lesions and normal-appearing white matter in multiple sclerosis. Brain Pathol. 2005;15(1):35–45. doi: 10.1111/j.1750-3639.2005.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JK, Miao L, et al. A possible role of RhoA/Rho-kinase in experimental spinal cord injury in rat. Brain Res. 2003;959(1):29–38. doi: 10.1016/s0006-8993(02)03717-4. [DOI] [PubMed] [Google Scholar]
- Takasu MA, Dalva MB, et al. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science. 2002;295(5554):491–495. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- Wahl S, Barth H, et al. Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J Cell Biol. 2000;149(2):263–270. doi: 10.1083/jcb.149.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG. Multiple roles of EPH receptors and ephrins in neural development. Nat Rev Neurosci. 2001;2(3):155–164. doi: 10.1038/35058515. [DOI] [PubMed] [Google Scholar]
- Willson CA, Irizarry-Ramirez M, et al. Upregulation of EphA receptor expression in the injured adult rat spinal cord. Cell Transplant. 2002;11(3):229–239. [PubMed] [Google Scholar]
- Zheng B, Ho C, et al. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38(2):213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.