Abstract
Objective
To describe the relationship of blood pressure (BP), antihypertensive medication use, and other factors to serial measurements of retinal arteriolar diameters over time in the Beaver Dam Eye Study.
Methods
Retinal arteriolar diameter was measured by computer-assisted methods and summarized as central retinal arteriolar equivalent (CRAE) in 4573 persons aged 43–99 years at 4 examinations (each five years apart) over a 15-year interval. Associations of CRAE with risk factors measured concurrently and 5 years previously were determined using multivariate analyses.
Results
While adjusting for image quality, refraction, and lens status, age (per 10 years: β estimate −0.73, p<.001), systolic BP (SBP, per 10 mmHg: concurrent exam −2.74, p<.001; previous exam −1.75, p<0.001), smoking status (smoker vs. non-smoker: concurrent exam 4.29, p<.001; previous exam 1.63, p=0.004), body mass index (BMI, per category: concurrent exam −0.51, p=0.04; previous exam −0.22, p=0.44), and heavy drinking (current vs. past/never heavy drinker: concurrent exam −2.54, p=0.03; previous exam −2.42, p=0.02) were associated with CRAE. In the same model, there were significant interactions between concurrent and previous SBP (−0.11, p=0.003) and between concurrent and previous BMI (0.12, p=0.04). Use of calcium channel blockers at both the concurrent and past exam (vs. neither exam 1.59; P=0.01), but not other classes of antihypertensive drugs was associated with CRAE.
Conclusions
These data show retinal arteriolar diameter is independently associated with past and current SBP, calcium channel blocker use, smoking status, BMI, and heavy drinking over 5-year intervals. The relationships with CRAE were stronger for concurrent than for past measures of these variables.
Keywords: retinal vessels, retinal arteriolar narrowing, hypertension, epidemiology
Computer-assisted measurements of retinal vessel diameters from fundus photographs have increasingly been used as a tool to study cardiovascular, renal and other systemic diseases in population-based studies over the past 10 years.1–19 Retinal arteriolar diameter, measured as the width of the retinal arteriolar blood column, has been shown to be associated with past, present and future blood pressure (BP) and cardiovascular disease morbidity and mortality.1,2,9,13–21
However, most information regarding associations of BP and other factors related to retinal arteriolar diameters are from cross-sectional studies.1,2,4,10,13,14,16,22 The purpose of this report is to examine relationships of past and concurrent values of BP, antihypertensive medication use, and other factors to the central retinal arteriolar equivalent (CRAE). This report makes use of 4 examinations, each spaced 5 years apart, in the population-based Beaver Dam Eye Study.
METHODS
Population
A private census of Beaver Dam, Wisconsin was performed in 1987–1988 to identify all residents 43–84 years of age who were eligible to participate in a study of age-related eye conditions and traits.23 Four thousand nine hundred and twenty-six of 5924 persons identified (99% white) participated in the baseline examination in 1988–1990. The cohort was re-examined at 5- (n=3722), 10- (n=2962), and 15-year (n=2375) follow-up examinations. Participation rates exceeded 80% among survivors at each examination.23–26 Differences between participants and nonparticipants have been presented elsewhere.23–26 In general, participants at each examination phase were younger, had lower BP, and had fewer co-morbid conditions at baseline than nonparticipants. All data were collected with Institutional Review Board approval from the University of Wisconsin-Madison in conformity with all federal and state laws, and the study adhered to the tenets of the Declaration of Helsinki.
Procedures
Participants underwent a standardized interview and examination using the same protocols each time.27 A questionnaire including questions on the history of physician-diagnosed diabetes, cigarette smoking, hypertension, and use of medications was administered. Height, weight, and BP were measured using standardized protocols.
Refraction was performed using a modification of the Early Treatment Diabetic Retinopathy Study protocol.23 Serum total and high density lipoprotein (HDL) cholesterol levels were determined from unfrozen serum on the day they were collected.28,29 At all examinations, additional serum samples were obtained and stored at −80°C from day collected until creatinine and high sensitivity C-reactive protein (hsCRP) tests were run in 2007. The soluble vascular cell adhesion molecule-1 (VCAM-1) was measured in 2008 on baseline samples.30,31 Thus, samples from the baseline visit (1988–1990), visit 2 (1993–1995), and visit 3 (1998–2000) were stored frozen for approximately 17–19 years, 12–14 years, and 7–9 years, respectively.
Participants’ pupils were pharmacologically dilated. A slit-lamp camera (Topcon America Corporation, Paramus, NJ) was used to photograph the lens of each eye, and the photographs were graded for the presence and severity of nuclear sclerosis.32 Stereoscopic 30° color fundus photographs centered on the disc (Diabetic Retinopathy Study standard field 1) were obtained for each eye.33 Age-related macular degeneration (AMD) was graded according to the Wisconsin Age-Related Maculopathy Grading System.34,35
Retinal vessels were measured from the digitized images of field 1 slides using a semiautomated computer program designed for this project (IVAN, University of Wisconsin-Madison, Ferrier NJ).36,37 In brief, the grading protocol required vessel diameters from the six largest arterioles and six largest venules located in a zone 0.5 to 1.0 disc diameters from the disc margin.38,39 Every 6 months, the graders re-measured 50 eyes to determine inter- and intra-grader variability. Correlation coefficients were high (>0.90) for both inter- and intra-grader comparisons for both arteriolar and venular measurements (data not shown).
Definitions
For each eye graded, the measurements of the six largest arterioles were combined to calculate the CRAE.38 Image quality was evaluated as good, fair, or poor. Nuclear sclerosis was graded by comparing images to standard photographs using a 5-step scale and categorized as ≤2, 3, >3, or cataract surgery. Refraction was categorized as moderately to highly myopic (< −3 diopters), mildly myopic (−3 to −1 diopters), emmetropic (−1 to 1 diopters), mildly hyperopic (1 to 3 diopters), and hyperopic (>3 diopters). Smokers were defined as persons having smoked ≥100 cigarettes in their lifetime and had not stopped smoking by the time of examination. Non-smokers were defined as individuals who had either never smoked or who smoked in the past but had stopped smoking by the time of examination. Past and never smokers were combined into a single “non-smoker” category for all analyses. Pack-years was defined as the average number of cigarettes smoked per day divided by 20, then multiplied by the number of years smoked. Body mass index (BMI) was defined as weight in kg divided by the square of height in m. Current heavy drinkers were defined as persons who consumed ≥4 servings of alcoholic beverages daily; past heavy drinkers had consumed ≥4 servings of alcoholic beverages on a regular, daily basis in the past but not in the last year; never heavy drinkers had never consumed ≥4 servings of alcoholic beverages on a regular, daily basis. Past and never heavy drinkers were combined into a single “past/never heavy drinker” category in all analyses.
Mean arterial BP (MABP) was defined as (systolic BP [SBP] + 2 × diastolic BP [DBP]) ÷ 3.
Statistical Analysis
The purpose of our analysis was to examine previous (5 years earlier) and concurrent relationships of risk factors (Table 1) to CRAE. We hypothesized that both previous and concurrent measures of a risk factor would be independently associated with CRAE, that the association would be stronger for concurrent than for previous measures, and that the previous measure of the risk factor would modify the concurrent relationship between that variable and CRAE (i.e., there would be a significant interaction). Using SBP as an example, in concurrent analyses (Model 1 in Table 2), we modeled the relationship of SBP to CRAE at the same visit (i.e., SBP at exam 1 to CRAE at exam 1, SBP at exam 2 to CRAE at exam 2, and so on). Rather than creating four models (i.e., one for each visit), we used time-updating covariates to incorporate information from all four visits into one model. In Model 2 in Table 2, we added a term for each risk factor measured at the previous exam and an interaction term between each risk factor at the visits previous and concurrent with the CRAE measurement (i.e., SBP at visit 1 + SBP at visit 1 × SBP at visit 2, SBP at visit 2 + SBP at visit 2 × SBP at visit 3, and so on) to determine if the previous value of the risk factor modified the relationship between the concurrent risk factor and CRAE. Figures were used to show each significant relationship (Model 2 in Table 2). In Model 3, we created a single multivariate model that included terms for all risk factors where the previous value or interaction was significantly associated with CRAE.
Table 1.
Characteristics of the Beaver Dam Eye Study Cohort at Each Examination.
| Risk Factor | 1988–1990 (Visit 1) | 1993–1995 (Visit 2) | 1998–2000 (Visit 3) | 2003–2005 (Visit 4) | P valuea | ||||
|---|---|---|---|---|---|---|---|---|---|
| N=4493 | N=3223 | N=2342 | N=1817 | ||||||
|
| |||||||||
| Mean or % | SD | Mean or % | SD | Mean or % | SD | Mean or % | SD | ||
|
|
|||||||||
| Age, years | 61.3 | 10.9 | 64.4 | 10.1 | 67.5 | 9.2 | 70.5 | 8.3 | <.001 |
| Sex, male | 44.1 | 44.0 | 43.1 | 42.3 | 0.16 | ||||
| CRAE, μm | 150.0 | 14.8 | 148.6 | 14.9 | 148.3 | 14.4 | 146.6 | 14.8 | <.001 |
| CRVE, μm | 230.4 | 23.0 | 224.1 | 22.4 | 225.5 | 22.4 | 218.0 | 22.5 | <.001 |
| Systolic BP, mmHg | 131.5 | 20.2 | 128.9 | 19.2 | 130.8 | 18.8 | 130.5 | 18.2 | 0.09 |
| Diastolic BP, mmHg | 77.5 | 10.9 | 76.2 | 10.5 | 74.4 | 10.6 | 73.7 | 10.9 | <.001 |
| Mean arterial BP, mmHg | 95.5 | 12.1 | 93.8 | 11.8 | 93.2 | 11.5 | 92.7 | 11.8 | <.001 |
| Taking antihypertensive medication | 36.2 | 40.8 | 51.8 | 62.2 | <.001 | ||||
| Taking ACE inhibitor | 6.4 | 11.5 | 17.0 | 24.6 | <.001 | ||||
| Taking beta blocker | 11.5 | 11.7 | 18.4 | 28.1 | <.001 | ||||
| Taking calcium channel blocker | 4.1 | 11.7 | 14.9 | 15.9 | <.001 | ||||
| Taking glaucoma drops | 1.9 | 2.5 | 3.5 | 5.3 | <.001 | ||||
| Intraocular pressure, mmHg | 15.4 | 3.3 | 15.3 | 3.2 | 15.2 | 3.0 | 15.1 | 3.3 | <.001 |
| Body mass index, kg/m2 | 28.7 | 5.4 | 29.5 | 5.5 | 29.9 | 5.9 | 30.5 | 6.0 | <.001 |
| Refraction, diopters | 0.2 | 2.3 | 0.2 | 2.3 | 0.2 | 2.3 | 0.2 | 2.3 | 0.80 |
| Nuclear cataract present | 12.1 | 18.3 | 14.5 | 18.8 | <.001 | ||||
| History of cataract surgery | 2.9 | 6.1 | 9.6 | 14.5 | <.001 | ||||
| Current smoking | 20.3 | 15.0 | 10.4 | 9.3 | <.001 | ||||
| Pack-years smoked | 31.7 | 28.7 | 30.2 | 27.3 | 28.3 | 27.1 | 27.0 | 25.7 | <.001 |
| Current heavy drinking | 2.4 | 2.0 | 1.5 | 0.8 | <.001 | ||||
| Serum total cholesterol, mg/dL | 233.8 | 43.9 | 239.6 | 44.9 | 213.8 | 40.2 | <.001 | ||
| Serum HDL cholesterol, mg/dL | 52.2 | 17.6 | 52.6 | 16.5 | 50.8 | 16.5 | <.001 | ||
| Diabetes mellitus present | 7.1 | 8.3 | 9.5 | 12.9 | <.001 | ||||
| Serum C-reactive protein, mg/L | 4.4 | 9.7 | 4.6 | 11.2 | 0.39 | ||||
| Serum creatinine, mg/dL | 0.9 | 0.3 | 0.9 | 0.3 | 0.9 | 0.2 | 0.9 | 0.3 | 0.35 |
| Soluble VCAM-1, ng/mL | 821.6 | 294.9 | 852.2 | 278.8 | 0.006 | ||||
SD, standard deviation; CRAE, central retinal arteriolar equivalent; CRVE, central retinal venular equivalent; BP, blood pressure; ACE, angiotensin converting enzyme; HDL, high density lipoprotein; VCAM, vascular cell adhesion molecule.
Test for trend over examination phases.
Table 2.
Relationship of Risk Factors Measured Previously (5 Years Earlier) and Concurrently (Same Visit) to Central Retinal Arteriolar Equivalent in the Beaver Dam Eye Study, 1988–2005.
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| β | 95% CI | P value | β | 95% CI | P value | β | 95% CI | P value | |
|
|
|||||||||
| Associated with narrower CRAE | |||||||||
| Age, per 10 years | −1.87 | −2.15, −1.59 | <.001 | −0.73 | −1.14, −0.33 | <.001 | |||
| Sex, male | −0.18 | −0.95, 0.59 | 0.65 | ||||||
| Systolic BP, per 10 mmHg | |||||||||
| Concurrent | −1.34 | −1.46, −1.22 | <.001 | −2.69 | −3.58, −1.81 | <.001 | −2.74 | −3.71, −1.78 | <.001 |
| 5 years previously | −1.78 | −2.66, −0.90 | <.001 | −1.75 | −2.70, −0.79 | <.001 | |||
| Concurrent × 5 years previously | 0.11 | 0.04, 0.17 | 0.001 | 0.11 | 0.04, 0.18 | 0.003 | |||
| Diastolic BP, per 5 mmHg | |||||||||
| Concurrent | −1.16 | −1.26, −1.05 | <.001 | −0.76 | −1.58, 0.05 | 0.07 | |||
| 5 years previously | −0.12 | −0.90, 0.67 | 0.77 | ||||||
| Concurrent × 5 years previously | −0.02 | −0.07, 0.03 | 0.40 | ||||||
| Mean arterial BP, per 5 mmHg | |||||||||
| Concurrent | −1.14 | −1.23, −1.05 | <.001 | −1.38 | −2.19, −0.56 | 0.001 | |||
| 5 years previously | −0.67 | −1.46, 0.12 | 0.10 | ||||||
| Concurrent × 5 years previously | 0.01 | −0.03, 0.06 | 0.48 | ||||||
| BMI, per categorya | |||||||||
| Concurrent | −0.51 | −0.70, −0.32 | <.001 | −1.16 | −1.67, −0.64 | <.001 | −0.51 | −1.02, 0.00 | 0.05 |
| 5 years previously | −0.56 | −1.12, 0.01 | 0.05 | −0.22 | −0.77, 0.34 | 0.44 | |||
| Concurrent × 5 years previously | 0.20 | 0.07, 0.32 | 0.002 | 0.12 | 0.00, 0.24 | 0.04 | |||
| Heavy drinking status | |||||||||
| Concurrent | −2.25 | −3.78, −0.72 | 0.004 | −1.70 | −4.50, 1.10 | 0.23 | −2.54 | −4.79, −0.28 | 0.03 |
| 5 years previously | −2.16 | −4.40, 0.07 | 0.06 | −2.42 | −4.42, −0.42 | 0.02 | |||
| Concurrent × 5 years previously | 0.06 | −4.51, 4.63 | 0.98 | ||||||
| Associated with wider CRAE | |||||||||
| Smoking status | |||||||||
| Concurrent | 5.03 | 4.28, 5.78 | <.001 | 5.00 | 2.62, 7.37 | <.001 | 4.29 | 3.02, 5.56 | <.001 |
| 5 years previously | 1.55 | 0.38, 2.72 | 0.01 | 1.63 | 0.53, 2.74 | 0.004 | |||
| Concurrent × 5 years previously | −0.91 | −3.68, 1.86 | 0.52 | ||||||
| Not associated with CRAE | |||||||||
| Antihypertensive medication use | |||||||||
| Concurrent | 0.18 | −0.30, 0.67 | 0.46 | 0.02 | −0.69, 0.73 | 0.95 | |||
| 5 years previously | 0.69 | −0.81, 2.18 | 0.37 | ||||||
| Concurrent × 5 years previously | −0.20 | −1.86, 1.46 | 0.82 | ||||||
| Intraocular pressure, per 2 mmHg | |||||||||
| Concurrent | −0.12 | −0.27, 0.02 | 0.10 | −0.33 | −1.15, 0.49 | 0.43 | |||
| 5 years previously | −0.15 | −0.94, 0.65 | 0.72 | ||||||
| Concurrent × 5 years previously | 0.01 | −0.09, 0.11 | 0.80 | ||||||
| Total cholesterol, per categorya b | |||||||||
| Concurrent | −0.17 | −0.30, −0.04 | 0.01 | −0.09 | −0.54, 0.36 | 0.70 | |||
| 5 years previously | 0.00 | −0.38, 0.38 | >0.99 | ||||||
| Concurrent × 5 years previously | −0.01 | −0.10, 0.08 | 0.82 | ||||||
| HDL cholesterol, per categorya b | |||||||||
| Concurrent | −0.37 | −0.55, −0.19 | <.001 | −0.46 | −0.96, 0.05 | 0.08 | |||
| 5 years previously | −0.15 | −0.63, 0.33 | 0.54 | ||||||
| Concurrent × 5 years previously | 0.04 | −0.09, 0.17 | 0.52 | ||||||
| HbA1c, per 1%c | |||||||||
| Concurrent | 0.30 | 0.07, 0.52 | 0.01 | 0.07 | −1.02, 1.17 | 0.89 | |||
| 5 years previously | 0.46 | −0.82, 1.75 | 0.48 | ||||||
| Concurrent × 5 years previously | −0.01 | −0.14, 0.13 | 0.92 | ||||||
| Diabetes status | |||||||||
| Concurrent | 1.36 | 0.39, 2.32 | 0.01 | 0.65 | −0.79, 2.10 | 0.37 | |||
| 5 years previously | −0.73 | −4.29, 2.84 | 0.69 | ||||||
| Concurrent × 5 years previously | 0.98 | −3.10, 5.06 | 0.64 | ||||||
| Creatinine, per quartile | |||||||||
| Concurrent | −0.12 | −0.36, 0.11 | 0.29 | −0.05 | −0.54, 0.44 | 0.84 | |||
| 5 years previously | −0.22 | −0.74, 0.31 | 0.42 | ||||||
| Concurrent × 5 years previously | 0.00 | −0.27, 0.28 | 0.99 | ||||||
| hsCRPc, per quartile | |||||||||
| Concurrent | 0.14 | −0.19, 0.48 | 0.40 | 0.84 | −0.51, 2.20 | 0.22 | |||
| 5 years previously | 0.37 | −1.12, 1.85 | 0.63 | ||||||
| Concurrent × 5 years previously | −0.03 | −0.75, 0.69 | 0.93 | ||||||
| VCAM-1c, per quartile | |||||||||
| Concurrent | −0.07 | −0.59, 0.45 | 0.79 | 0.72 | −0.71, 2.15 | 0.33 | |||
| 5 years previously | −0.34 | −1.96, 1.28 | 0.68 | ||||||
| Concurrent × 5 years previously | −0.02 | −0.76, 0.72 | 0.96 | ||||||
BP, blood pressure; BMI, body mass index; CI, confidence interval; HbA1c, glycosylated hemoglobin A1c; HDL, high density lipoprotein; hsCRP, high sensitivity C-reactive protein; VCAM, vascular cell adhesion molecule.
Model 1: Controls for variable at current visit, age, refraction, cataract, image focus, and systolic blood pressure.
Model 2: Controls for variable at current visit, variable at past visit, interaction between visits, age, refraction, cataract, image focus, and SBP interaction.
Model 3: Controls for all variables shown plus age, refraction, cataract, and image focus
BMI: <20, 20–<23, 23–<26, 26–<29, 29–<32, 32–<35, 35–<38, 38–<41, and ≥41 kg/m2. Serum total cholesterol: <160, 160–<180, 180–<200, 200–<220, 220–<240, 240–<260, 260–<280, and ≥280 mg/dL. Serum HDL cholesterol: <30, 30–<40, 40–<50, 50–<60, 60–<70, 70–<80, 80–<90, and ≥90 mg/dL.
Measured at visits 1, 2, and 3 only.
Measured at visits 1 and 2 only.
Results are presented for the right eye only. Eyes in which one of the six largest arterioles was ungradable were excluded. Retinal photographs taken with non-Zeiss cameras, or eyes with late AMD, photocoagulation, photodynamic or any intravitreal treatment for AMD, retinal vessel occlusions, retinopathy, macular edema, other pathology, or that were aphakic or missing lens status were excluded.
All analyses were performed with SAS version 9.2 (Cary, NC). Tests for trend over multiple visits presented in Table 1 were computed using a general linear model. Models with CRAE as the outcome were analyzed using SAS Proc Mixed with unstructured correlation matrices for the error term. Each model also controlled for image focus, cataract status, and refraction at the visit where CRAE was measured because these factors may have the largest “non-disease” impact on the measurement of CRAE. Estimated CRAE and confidence intervals (CI) were calculated using the estimate statement in Proc Mixed assuming age of 65 years, good image focus, the absence of cataract or cataract surgery, and the refraction is emmetropic.
RESULTS
Of the 4926 individuals seen at baseline, 97 were excluded because they had no retinal photos of field 1 or photos were not taken using a Zeiss camera, 68 because late AMD was present or treatment for late AMD had occurred, 150 because other retinal diseases were present, 30 due to aphakia or unknown lens status, and 88 for having fewer than 6 gradable retinal arterioles, leaving 4493 individuals contributing data at visit 1. Similarly, 3223 of the 3722 participants seen at visit 2, 2342 of the 2962 participants seen at visit 3, and 1817 of the 2373 participants seen at visit 4 contributed data to the cross-sectional analyses.
Characteristics of these individuals at each interval are displayed in Table 1. Participants at later visits had narrower CRAE, lower DBP, lower serum total and HDL cholesterol, a lower frequency of current smoking and current heavy drinking, and fewer pack-years smoked. Participants at later visits had greater BMI, higher frequencies of taking antihypertensive medications, and higher frequencies of cataract, cataract surgery, and diabetes. SBP, refraction, serum creatinine, and percentages of men and women did not differ between participants at earlier and later visits.
Concurrent relationships
Model 1 in Table 2 shows the concurrent relationships between factors listed in Table 2 and CRAE at each examination. After adjustment for cataract status, refraction, and image focus, increasing age, higher SBP, DBP, MABP, and intraocular pressure, greater BMI, higher serum HDL cholesterol, serum creatinine and VCAM-1,a history of current heavy drinking, and after additional control for SBP, antihypertensive medication use were concurrently associated with narrower CRAE while current smoking and presence of diabetes were concurrently associated with wider CRAE. There was no association of sex, serum total cholesterol, or hsCRP with CRAE measured at the same visit.
Relationships of previous and concurrent BP and antihypertensive medication use to CRAE
SBP, DBP and MABP measured at the previous visit were associated with narrower CRAE independent of concurrent values of these risk variables. Adding BP measured at the previous visit to the model slightly attenuated the relationship between concurrent BP and CRAE, and the effect of the BP from the previous visit was much smaller than that of BP measured at the concurrent visit (β=−0.42 vs −1.32 for SBP, β=−0.34 vs −0.99 for DBP and β=−0.38 vs −1.07 for MABP, respectively). There was a significant interaction between the previous and concurrent SBP, but not with the previous and concurrent DBP or MABP (Model 2, Table 2). Adding this interaction term strengthened the relationship between both concurrent and previous SBP and CRAE, but concurrent SBP remained more strongly associated with concurrent CRAE than with previous SBP.
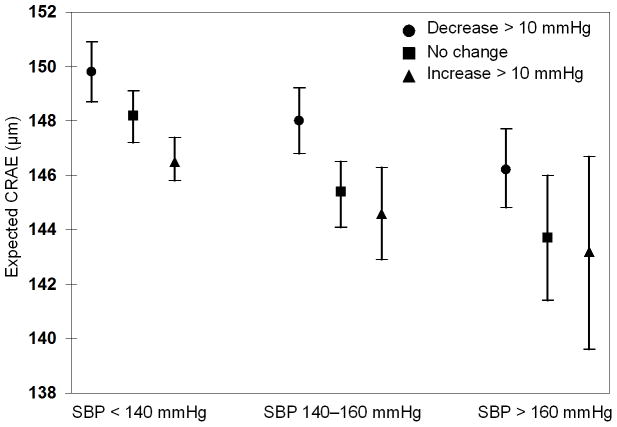
To further explore the relationship of the SBP interaction, we compared estimated values of CRAE for individuals with different previous and concurrent values of SBP as described in the Methods section. Figure 1 shows that the effect of higher and lower concurrent SBP on CRAE depends on whether an individual was normotensive or had moderate or severe hypertension at the previous visit. For example, a 10 mmHg increase in SBP is associated with a decrease in CRAE in individuals who were previously normotensive but not in individuals who were hypertensive.
Figure 1.
The relation of change over a 5-year period in systolic blood pressure (SBP) in people at three levels of SBP at the start of the period with central retinal arteriolar equivalent (CRAE) at 5-year follow-up (concurrent) examination.
Although previous use of “any” antihypertensive medication was not independently associated with CRAE measured 5 years later (Model 2 in Table 2), we hypothesized that previous and concurrent use of specific types of antihypertensive medications would be related to CRAE. Controlling for age, cataract status, refraction, image focus, and previous and concurrent SBP and their interaction, history of taking a calcium channel blocker at both visits and starting to take a calcium channel blocker were significantly associated with wider CRAE compared to individuals who did not take this medication at either visit (Table 3). There was no significant association with any other specific class of anti-hypertensive medication (e.g., beta blockers, diuretics, angiotensin-converting enzyme inhibitors). There was no relationship between change in glaucoma drop use and CRAE (data not shown).
Table 3.
Relationship of Changes in Specific Antihypertensive Medication Use to Central Retinal Arteriolar Equivalent.
| Risk Factor | β Estimatea | 95% CI | P value |
|---|---|---|---|
|
|
|||
| Diuretics | |||
| Taking both exams | −0.12 | −1.05, 0.82 | 0.81 |
| Started taking | −0.47 | −1.28, 0.34 | 0.26 |
| Stopped taking | 0.19 | −0.91, 1.28 | 0.74 |
| Not taking either exam | Reference | ||
| ACE inhibitors | |||
| Taking both exams | −0.27 | −1.42, 0.88 | 0.65 |
| Started taking | 0.86 | 0.04, 1.68 | 0.04 |
| Stopped taking | −0.49 | −1.97, 1.00 | 0.52 |
| Not taking either exam | Reference | ||
| Beta blockersb | |||
| Taking both exams | 0.17 | −0.88, 1.22 | 0.75 |
| Started taking | 0.33 | −0.50, 1.17 | 0.44 |
| Stopped taking | 0.69 | −0.68, 2.06 | 0.33 |
| Not taking either exam | Reference | ||
| Calcium channel blockers | |||
| Taking both exams | 1.59 | 0.32, 2.85 | 0.01 |
| Started taking | 0.90 | −0.04, 1.84 | 0.06 |
| Stopped taking | 1.26 | −0.21, 2.73 | 0.09 |
| Not taking either exam | Reference | ||
CI, confidence interval; ACE, angiotensin converting enzyme.
Controls for age, image focus, cataract status, refraction, current systolic blood pressure, past systolic blood pressure and current × past systolic blood pressure interaction.
Includes glaucoma drops.
Relationships of previous and concurrent other systemic factors to CRAE
There was a strong positive correlation (r>0.70, p<0.01) between past and concurrent CRAE. Over each 5-year interval, about 25% of individuals had a positive change in CRAE (widening by > 5 μ), about 35% had a negative change in CRAE (narrowing by > 5 μ) and about 40% had no change in CRAE (widening or narrowing by ≤ 5 μ). This was consistent across all pairs of examinations.
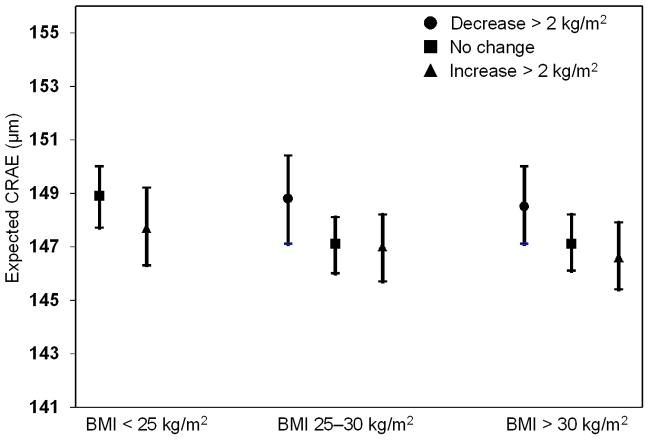
Both previous BMI and an interaction between previous and concurrent BMI were significantly associated with CRAE (Model 2 in Table 2). Adding these terms strengthened the concurrent relationship of BMI and CRAE. The association of the previous BMI was not as large as that of BMI measured at the same visit (β= −1.16 vs. −0.56). There was an interaction between previous and concurrent BMI (Figure 2). The effect of higher and lower BMI on CRAE at the same visit differed depending on the BMI status at the previous visit. For example, an increase in BMI of least 2 kg/m2 compared to no weight gain was associated with smaller CRAE in individuals who previously had a normal BMI, but an increase in BMI compared to no weight gain was not associated with smaller CRAE in individuals who were previously overweight or obese.
Figure 2.
The relation of change over a 5 year period in body mass index (BMI) in people at three levels of BMI at the start of the period with central retinal arteriolar equivalent (CRAE) at 5-year follow-up (concurrent) examination.
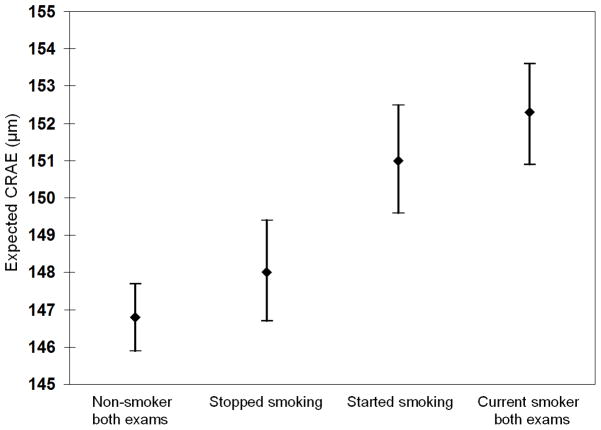
Smoking at both the concurrent and previous visits was associated with wider CRAE; however, there was no significant interaction between the two (Table 2). The effect size of a history of current smoking at the same visit where CRAE was measured was about 3.25 times larger than that of a history of past smoking (β= 5.00 vs. 1.55). Individuals who started smoking over the interval or were smokers at both visits had significantly wider CRAE than individuals who stopped smoking or were non-smokers at both visits (Figure 3).
Figure 3.
The relation of change over a 5 year period in smoking status with central retinal arteriolar equivalent (CRAE) at 5-year follow-up (concurrent) examination.
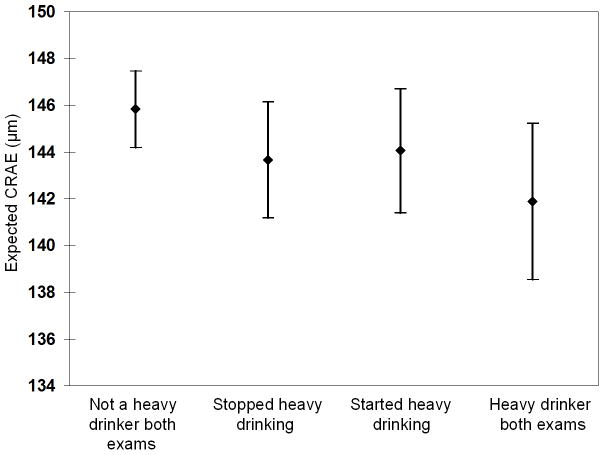
Having a history of being a heavy drinker at the previous exam was more strongly related to smaller CRAE than being a heavy drinker at the time of the concurrent examination (Model 2 in Table 2). There was no interaction between previous history of heavy drinking 5 years before CRAE was measured and at the time when the CRAE was measured. Individuals who were heavy drinkers at both exams had narrower CRAE and individuals who were not heavy drinkers at either exam had wider CRAE than individuals who either stopped or started drinking heavily (Figure 4).
Figure 4.
The relation of change over a 5 year period in heavy drinking status with central retinal arteriolar equivalent (CRAE) at 5-year follow-up (concurrent) examination.
Multivariate relationships of systemic factors to CRAE
Model 3 in Table 2 shows the multivariate relationships of previous and concurrent SBP, antihypertensive medication use, BMI, and smoking status to CRAE. Previous and concurrent SBP, heavy drinking, and BMI were associated with narrower CRAE, while previous and concurrent smoking status was associated with wider CRAE. Previous and concurrent antihypertensive medication use was no longer associated with CRAE. However, when added to the multivariate model, a history of using a calcium channel blocker at the previous and concurrent visits compared to a history of not taking a calcium channel blocker at either was significantly associated with wider CRAE (beta estimate 1.61; 95% CI 0.29 to 2.93).
The risk factors in the in the full multivariate model explained about 19% of the variance of CRAE over the 15-year period.
Multivariate relations of systemic factors when measured 10 or more years before CRAE
SBP, BMI, current smoking, and current heavy drinking were not associated with CRAE when these factors were measured 10 or 15 years before the CRAE (data not shown).
COMMENT
The Beaver Dam Eye Study offered a unique opportunity to examine the associations of the temporal relationships of BP and other factors with retinal arteriolar caliber measured using standardized protocols at multiple visits over a 15-year period. SBP, smoking status, and heavy drinking measured concurrently and 5 years previously and BMI measured concurrently were independently associated with CRAE (Table 2, Model 3). Concurrent levels of these risk factors were more strongly related to CRAE than those measured 5 or more years before.
The associations of higher SBP with narrower CRAE and interactions of SBP and hypertension with narrower CRAE in our study are consistent with previous findings in both adults and children.1,2,9,13–19,40 Our finding of a direct effect of both previous and concurrent use of calcium channel blockers but not other antihypertensive medications on CRAE was also consistent with the results of the Anglo-Scandinavian Cardiac Outcomes Trial.41 In that study, amlodipine treatment was associated with a smaller arteriolar length-diameter ratio than atenolol treatment, and the association remained significant after adjustment for age, sex, SBP, DBP, and other factors. One year of treatment with amlodipine but not atenolol in persons with hypertension led to reduction in the media-lumen ratio of the small arterioles (equivalent to a wider CRAE as measured in our study), despite the similar BP-reducing effect of both agents.42 It is thought that some calcium channel blockers have a greater effect of vasodilation (and presumably change in media-lumen proportion) of retinal blood vessels and possibly other systemic arterioles without significantly affecting systemic BP.41,43,44
In our study, starting smoking was associated with a wider retinal arteriolar diameter (increased mean CRAE) while stopping was associated with a reduction in arteriolar diameter (decreased mean CRAE), an effect consistent with previously reported associations of current smoking with wider CRAE.10,37,45,46 Smoking-induced increase in nitrous oxide production, potassium channel activation,47 and possible elastic tissue degeneration may also explain the association.48–51 The effect of smoking may also be mediated by an inflammatory effect. Ikram et al. have hypothesized that disruption of the endothelial surface layer due to inflammation secondary to smoking may result in a thinning of this layer, with an increase in the apparent intraluminal caliber of the small retinal blood vessels.10
In the Beaver Dam Eye Study, we found an inverse association of BMI with CRAE. CRAE was narrower in lean persons in whom the BMI increased, and CRAE was wider in obese persons in whom the BMI decreased over time, independent of blood pressure. This relationship has been reported for venular diameter (CRVE) but not CRAE in earlier studies.10,52 The underlying reasons for these findings are unknown and may involve complex effects on factors not measured in our study, such as changes in endothelial function associated with weight fluctuations.
In the Beaver Dam Eye Study, a history of heavy alcohol consumption was associated with narrower CRAE. This association has been inconsistently found in previous cross-sectional studies.10,36,46 A similar effect of chronic alcohol ingestion has been reported in both the cerebral and systemic circulations and has been attributed to impaired vasodilation.53–55
While this study has many unique characteristics and strengths, including long-term follow-up, high grader repeatability, and the large population-based study structure, caution is urged in interpreting the findings. One limitation was that the same individual vessels were not always measured, and when the same vessels were measured, different lengths may have been measured at different gradings; thus, the CRAE may be an overall summary of different vessels of the retina at different time points. However, the effect of this is thought to be small, as we have found statistically nonsignificant differences when we did side-by-side grading of same vessels and compared the results to the standard grading approach used in the study (Klein R, unpublished data). Second, relationships may have been attenuated by selective survival. Cardiovascular disease and retinal arteriolar narrowing have been shown to be related to mortality in the general population.10
In summary, these population-based data show, while controlling for refraction, cataract status and image quality, that older age, higher BP, heavy drinking, and greater BMI are independently associated with narrower retinal arterioles and current smoking with wider retinal arterioles. Concurrent measures of these risk factors are more strongly associated than past measures with CRAE and after 5 years the relationships are not statistically significant. These data suggest the importance in controlling for concurrent BP levels, smoking status, and BMI when examining the relationships of retinal arteriolar diameter as risk indicators of systemic disease over long periods of time. A clear message from these findings is that current exposure to these modifiable risk factors has a larger impact on the small vessel profile than previous exposure to the same factors. In other words, it suggests that a change in lifestyle (smoking, drinking) and any other modifiable factor is never too late in affecting the microvasculature. Further study is necessary to evaluate the utility of measurements of the retinal vasculature in clinical care of ocular and systemic disease.
Acknowledgments
The National Institutes of Health grant EY06594 (R Klein, BEK Klein) provided funding for entire study including collection and analyses of data; further support for data analyses was provided by Research to Prevent Blindness (R Klein and BEK Klein, Senior Scientific Investigator Awards), New York, NY.
Footnotes
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Eye Institute or the National Institutes of Health.
DISCLOSURE
None reported.
AUTHOR CONTRIBUTIONS
Conception and design (RK, TYW, BEKK), acquisition of data (RK, TYW, BEKK), analysis and interpretation of data (RK, CEM, MDK, KEL, RG, TYW, BEKK), drafting of the manuscript (RK, BEKK, TYW), critical revision of the manuscript for important intellectual content (CEM, MDK, KEL, RG, TYW), statistical expertise (CEM, MDK, KEL, RG, TYW), obtaining funding (RK, BEKK), administrative/technical/material support (RK, TYW), supervision (RK, TYW).
References
- 1.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106(12):2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 2.Sharrett AR, Hubbard LD, Cooper LS, et al. Retinal arteriolar diameters and elevated blood pressure: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999;150(3):263–270. doi: 10.1093/oxfordjournals.aje.a009997. [DOI] [PubMed] [Google Scholar]
- 3.Wong TY, Klein R, Couper DJ, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358(9288):1134–1140. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Moss SE, et al. Retinal vascular abnormalities in persons with type 1 diabetes: the Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVIII. Ophthalmology. 2003;110(11):2118–2125. doi: 10.1016/S0161-6420(03)00863-7. [DOI] [PubMed] [Google Scholar]
- 5.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002;287(9):1153–1159. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- 6.Wong TY, Knudtson MD, Klein R, Klein BE, Hubbard LD. A prospective cohort study of retinal arteriolar narrowing and mortality. Am J Epidemiol. 2004;159(9):819–825. doi: 10.1093/aje/kwh119. [DOI] [PubMed] [Google Scholar]
- 7.Ikram MK, de Jong FJ, Bos MJ, et al. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology. 2006;66(9):1339–1343. doi: 10.1212/01.wnl.0000210533.24338.ea. [DOI] [PubMed] [Google Scholar]
- 8.Wang JJ, Liew G, Klein R, et al. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J. 2007;28(16):1984–1992. doi: 10.1093/eurheartj/ehm221. [DOI] [PubMed] [Google Scholar]
- 9.Kawasaki R, Cheung N, Wang JJ, et al. Retinal vessel diameters and risk of hypertension: the Multiethnic Study of Atherosclerosis. J Hypertens. 2009;27(12):2386–2393. doi: 10.1097/HJH.0b013e3283310f7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikram MK, de Jong FJ, Vingerling JR, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004;45(7):2129–2134. doi: 10.1167/iovs.03-1390. [DOI] [PubMed] [Google Scholar]
- 11.McGeechan K, Liew G, Macaskill P, et al. Meta-analysis: retinal vessel caliber and risk for coronary heart disease. Ann Intern Med. 2009;151(6):404–413. doi: 10.7326/0003-4819-151-6-200909150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witt N, Wong TY, Hughes AD, et al. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension. 2006;47(5):975–981. doi: 10.1161/01.HYP.0000216717.72048.6c. [DOI] [PubMed] [Google Scholar]
- 13.Wong TY, Klein R, Klein BE, Meuer SM, Hubbard LD. Retinal vessel diameters and their associations with age and blood pressure. Invest Ophthalmol Vis Sci. 2003;44(11):4644–4650. doi: 10.1167/iovs.03-0079. [DOI] [PubMed] [Google Scholar]
- 14.Leung H, Wang JJ, Rochtchina E, Wong TY, Klein R, Mitchell P. Impact of current and past blood pressure on retinal arteriolar diameter in an older population. J Hypertens. 2004;22(8):1543–1549. doi: 10.1097/01.hjh.0000125455.28861.3f. [DOI] [PubMed] [Google Scholar]
- 15.Wong TY, Shankar A, Klein R, Klein BE, Hubbard LD. Prospective cohort study of retinal vessel diameters and risk of hypertension. BMJ. 2004;329(7457):79. doi: 10.1136/bmj.38124.682523.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung H, Wang JJ, Rochtchina E, et al. Relationships between age, blood pressure, and retinal vessel diameters in an older population. Invest Ophthalmol Vis Sci. 2003;44(7):2900–2904. doi: 10.1167/iovs.02-1114. [DOI] [PubMed] [Google Scholar]
- 17.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar diameter and risk for hypertension. Ann Intern Med. 2004;140(4):248–255. doi: 10.7326/0003-4819-140-4-200402170-00006. [DOI] [PubMed] [Google Scholar]
- 18.Smith W, Wang JJ, Wong TY, et al. Retinal arteriolar narrowing is associated with 5-year incident severe hypertension: the Blue Mountains Eye Study. Hypertension. 2004;44(4):442–447. doi: 10.1161/01.HYP.0000140772.40322.ec. [DOI] [PubMed] [Google Scholar]
- 19.Wang JJ, Rochtchina E, Liew G, et al. The long-term relation among retinal arteriolar narrowing, blood pressure, and incident severe hypertension. Am J Epidemiol. 2008;168(1):80–88. doi: 10.1093/aje/kwn100. [DOI] [PubMed] [Google Scholar]
- 20.Liew G, Wang JJ, Mitchell P, Wong TY. Retinal vascular imaging: a new tool in microvascular disease research. Circ Cardiovasc Imaging. 2008;1(2):156–161. doi: 10.1161/CIRCIMAGING.108.784876. [DOI] [PubMed] [Google Scholar]
- 21.Wong TY. Retinal vessel diameter as a clinical predictor of diabetic retinopathy progression: time to take out the measuring tape. Arch Ophthalmol. 2011;129(1):95–96. doi: 10.1001/archophthalmol.2010.347. [DOI] [PubMed] [Google Scholar]
- 22.Klein R, Sharrett AR, Klein BE, et al. Are retinal arteriolar abnormalities related to atherosclerosis?: The Atherosclerosis Risk in Communities Study. Arterioscler Thromb Vasc Biol. 2000;20(6):1644–1650. doi: 10.1161/01.atv.20.6.1644. [DOI] [PubMed] [Google Scholar]
- 23.Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98(8):1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 24.Klein R, Klein BE, Lee KE. Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmology. 1996;103(8):1169–1178. doi: 10.1016/s0161-6420(96)30526-5. [DOI] [PubMed] [Google Scholar]
- 25.Klein R, Klein BE, Lee KE, Cruickshanks KJ, Chappell RJ. Changes in visual acuity in a population over a 10-year period: The Beaver Dam Eye Study. Ophthalmology. 2001;108(10):1757–1766. doi: 10.1016/s0161-6420(01)00769-2. [DOI] [PubMed] [Google Scholar]
- 26.Klein R, Klein BE, Lee KE, Cruickshanks KJ, Gangnon RE. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142(4):539–549. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Klein R, Klein BE. The Beaver Dam Eye Study: Manual of Operations (Revised) 1991. pp. 182–227. NTIS Publication PB91-149823. [Google Scholar]
- 28.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20(4):470–475. [PubMed] [Google Scholar]
- 29.Lopes-Virella MF, Stone P, Ellis S, Colwell JA. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 1977;23(5):882–884. [PubMed] [Google Scholar]
- 30.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 31.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 32.Klein BE, Klein R, Linton KL, Magli YL, Neider MW. Assessment of cataracts from photographs in the Beaver Dam Eye Study. Ophthalmology. 1990;97(11):1428–1433. doi: 10.1016/s0161-6420(90)32391-6. [DOI] [PubMed] [Google Scholar]
- 33.Diabetic Retinopathy Study Research Group. Report Number 6. Design, methods, and baseline results. Report Number 7. A modification of the Airlie House classification of diabetic retinopathy. Invest Ophthalmol Vis Sci. 1981;21(1 Pt 2):1–226. [PubMed] [Google Scholar]
- 34.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98(7):1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 35.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99(6):933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 36.Wong TY, Islam FM, Klein R, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA) Invest Ophthalmol Vis Sci. 2006;47(6):2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun C, Liew G, Wang JJ, et al. Retinal vascular caliber, blood pressure, and cardiovascular risk factors in an Asian population: the Singapore Malay Eye Study. Invest Ophthalmol Vis Sci. 2008;49(5):1784–1790. doi: 10.1167/iovs.07-1450. [DOI] [PubMed] [Google Scholar]
- 38.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27(3):143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 39.Knudtson MD, Klein BE, Klein R, et al. Variation associated with measurement of retinal vessel diameters at different points in the pulse cycle. Br J Ophthalmol. 2004;88(1):57–61. doi: 10.1136/bjo.88.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell P, Cheung N, de HK, et al. Blood pressure and retinal arteriolar narrowing in children. Hypertension. 2007;49(5):1156–1162. doi: 10.1161/HYPERTENSIONAHA.106.085910. [DOI] [PubMed] [Google Scholar]
- 41.Thom S, Stettler C, Stanton A, et al. Differential effects of antihypertensive treatment on the retinal microcirculation: an anglo-scandinavian cardiac outcomes trial substudy. Hypertension. 2009;54(2):405–408. doi: 10.1161/HYPERTENSIONAHA.109.133819. [DOI] [PubMed] [Google Scholar]
- 42.Schiffrin EL, Pu Q, Park JB. Effect of amlodipine compared to atenolol on small arteries of previously untreated essential hypertensive patients. Am J Hypertens. 2002;15(2 Pt 1):105–110. doi: 10.1016/s0895-7061(01)02290-7. [DOI] [PubMed] [Google Scholar]
- 43.Noguchi M, Mori A, Sakamoto K, Nakahara T, Ishii K. Vasodilator effects of flunarizine on retinal blood vessels in anesthetized rats. Biol Pharm Bull. 2009;32(12):2068–2071. doi: 10.1248/bpb.32.2068. [DOI] [PubMed] [Google Scholar]
- 44.Christensen KL, Mulvany MJ. Vasodilatation, not hypotension, improves resistance vessel design during treatment of essential hypertension: a literature survey. J Hypertens. 2001;19(6):1001–1006. doi: 10.1097/00004872-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Kifley A, Liew G, Wang JJ, et al. Long-term effects of smoking on retinal microvascular caliber. Am J Epidemiol. 2007;166(11):1288–1297. doi: 10.1093/aje/kwm255. [DOI] [PubMed] [Google Scholar]
- 46.Liew G, Sharrett AR, Wang JJ, et al. Relative importance of systemic determinants of retinal arteriolar and venular caliber: the atherosclerosis risk in communities study. Arch Ophthalmol. 2008;126(10):1404–1410. doi: 10.1001/archopht.126.10.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iida M, Iida H, Dohi S, Takenaka M, Fujiwara H. Mechanisms underlying cerebrovascular effects of cigarette smoking in rats in vivo. Stroke. 1998;29(8):1656–1665. doi: 10.1161/01.str.29.8.1656. [DOI] [PubMed] [Google Scholar]
- 48.Frances C, Boisnic S, Hartmann DJ, et al. Changes in the elastic tissue of the non-sun-exposed skin of cigarette smokers. Br J Dermatol. 1991;125(1):43–47. doi: 10.1111/j.1365-2133.1991.tb06037.x. [DOI] [PubMed] [Google Scholar]
- 49.Rogot E, Murray JL. Smoking and causes of death among U.S. veterans: 16 years of observation. Public Health Rep. 1980;95(3):213–222. [PMC free article] [PubMed] [Google Scholar]
- 50.Sharrett AR, Sorlie PD, Chambless LE, et al. Relative importance of various risk factors for asymptomatic carotid atherosclerosis versus coronary heart disease incidence: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1999;149(9):843–852. doi: 10.1093/oxfordjournals.aje.a009900. [DOI] [PubMed] [Google Scholar]
- 51.Sharrett AR, Ding J, Criqui MH, et al. Smoking, diabetes, and blood cholesterol differ in their associations with subclinical atherosclerosis: the Multiethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2006;186(2):441–447. doi: 10.1016/j.atherosclerosis.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Liew G, Wang JJ. Retinal vascular signs in diabetes and hypertension--review. Arq Bras Endocrinol Metabol. 2007;51(2):352–362. doi: 10.1590/s0004-27302007000200027. [DOI] [PubMed] [Google Scholar]
- 53.Mayhan WG, Didion SP. Effect of chronic alcohol consumption on responses of cerebral arterioles. Alcohol Clin Exp Res. 1996;20(3):538–542. doi: 10.1111/j.1530-0277.1996.tb01089.x. [DOI] [PubMed] [Google Scholar]
- 54.Altura BM, Altura BT. Alcohol, the cerebral circulation and strokes. Alcohol. 1984;1(4):325–331. doi: 10.1016/0741-8329(84)90056-9. [DOI] [PubMed] [Google Scholar]
- 55.Potter JF, Beevers DG. The possible mechanisms of alcohol associated hypertension. Ann Clin Res. 1984;16(Suppl 43):97–102. [PubMed] [Google Scholar]