Introduction
The arsenal of signals that cells use to communicate with each other is large and diverse. Some signaling molecules (e.g., nitrous oxide) are small. At the other extreme are proteins such as Drosophila Decapentaplegic (Dpp, a bone morphogenic protein (BMP) homolog), Hedgehog (Hh), Branchless/Fibroblast growth factor (FGF), and Wingless (Wg, a Wnt homolog). In various contexts in many animals, these signaling proteins signal at both short and long distances after moving from producing to recipient cells. Although we are undoubtedly ignorant of many fascinating details about the processes that generate these signals in producing cells and respond to them in recipient cells, the general outlines of production and response are firmly established and many key components have been identified. In contrast, despite much experimental and theoretical work, the question of how these proteins move between cells is controversial. This question is important for elucidating the mechanisms of pattern formation, and its resolution will have broad general implications for cell-cell signaling in many contexts during development and in disease.
This essay focuses on the mechanism that distributes the Dpp morphogen across the Drosophila wing imaginal disc. Although many models have been proposed for the formation of the Dpp gradients in the wing disc, a full discussion of their particulars is beyond the scope of this essay. Instead, I focus on the proposal that Dpp diffuses freely in the extracellular space that adjoins the wing disc, and discuss why, despite its claims, a recent study titled “Free Extracellular Diffusion Creates the Dpp Morphogen Gradient of the Drosophila Wing Disc” from Zhou et al. (1) does not settle the issue. The free extracellular diffusion model posits that Dpp is released from Dpp-expressing cells, and that Dpp takes a random walk in extracellular space, eventually binding to receptors that are exposed on the outside of target cells. To study how the Dpp gradients form, Zhou et al. (1) applied sensitive visual methods to monitor the movement of an ectopically expressed fluorescent Dpp fusion protein, DppDendra2. Using classical diffusion theory and assumptions about the rates of receptor binding, the size and form of Dpp, and the nature of the extracellular environment, they report that calculations for diffusion rates of free protein conform to their experimental observations of Dpp movement. Of course, correlation is not proof of mechanism; moreover, there are several reasons for concluding that free extracellular diffusion cannot generate the distributions of signaling proteins that must exist in the wing disc. In the discussion that follows, I briefly describe several issues that should discount free extracellular diffusion as a possible mechanism, and also describe an alternative mechanism that I favor: cytoneme-mediated direct delivery.
Dpp Gradients in the Wing Disc
In the wing primordium portion of the wing disc, anterior compartment cells along the anteroposterior border produce Dpp that exits the cells and forms mirror-image concentration gradients that decline monotonically toward the disc flanks (Fig. 1). Work from many laboratories has established that Dpp in these gradients regulates target-cell gene expression in a concentration-dependent fashion. Fig. 2 depicts two of the several mechanisms that have been proposed to explain how these gradients form: free extracellular diffusion and cytoneme-mediated direct delivery. In contrast to free extracellular diffusion, the cytoneme-based mechanism posits that morphogens transfer at points of direct contact between producing and target cells even when there are many intervening cells. Cytonemes are specialized signaling filopodia (2), and in the wing disc, the Dpp receptor Thickveins is present in cytonemes that extend from target cells toward Dpp-expressing cells (3).
Figure 1.
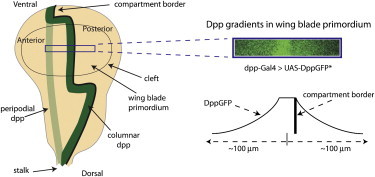
Dpp expression and Dpp gradients in the third-instar wing disc. The drawing of a wing disc (left) depicts features of the CE, with its A/P compartment border (black line), wing blade primordium, and stripe of Dpp-expressing cells (dark green) oriented dorsal side down. The relative position of the stripe of Dpp-expressing cells in the PE is depicted in light green. Dpp gradients in the region outlined by the purple box that are formed by DppGFP expressed under the control of dpp-Gal4 (upper right) are from Kicheva et al. (8) and are depicted graphically (lower right).
Figure 2.
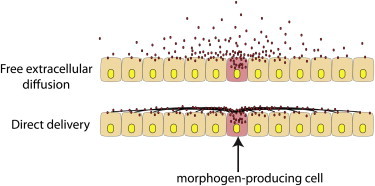
Two models of signaling protein dispersion. The drawings show morphogen protein (red) in transit from a morphogen-producing cell (pink) dispersing either by free extracellular diffusion (top) or by direct delivery (bottom) along cytonemes (black lines).
Diffusion is one Mechanism of Dispersion
There is ample evidence that morphogens such as Dpp disperse over many cell diameters from their sites of expression. Although many researchers in this field have assumed that Dpp and other morphogens diffuse across developmental fields, dispersion is not synonymous with diffusion. Diffusion is only one mechanism of dispersion, and this distinction is important. The distribution of Dpp across a field of target cells is based on hard evidence, but ascribing its movement to diffusion is not.
Morphogen Gradients Form in Tissues with Complex Anatomical Structures
The discovery that many pattern-forming genes are expressed in discrete, geographically defined groups of cells, such as the dpp stripe in wing discs, was both illuminating and seminal (see review in Tabata and Takei (18)). These expression domains, as well as the concentration gradients of the morphogens they generate, now underpin our concepts of developmental organizers. The developmental organizer at the anteroposterior (A/P) border of the wing disc is one of the most intensively studied and arguably the best understood of these organizers. It makes the Dpp that disperses across the disc (Fig. 1). In their studies, Zhou et al. (1) focused on the wing blade primordium, a region of the disc that is considered to be a planar monolayer and is bisected by a straight and relatively uniform stripe of Dpp-expressing cells. Although this apparently uncomplicated architecture has encouraged elegant models of Dpp gradient formation, the shape of the disc is in fact complex, and such models do not appropriately account for the wing disc’s anatomy. Importantly, they also do not account for the topography of its signaling landscape, i.e., the precise physical relationships between the cells that make signaling proteins and the fields of cells that receive these signals. The cytoneme mechanism satisfies both.
The third-instar wing disc is a flattened sac that is populated on one side by columnar cells (the columnar epithelium (CE) that generates the wing blade and most structures of the adult notum) and on the apposing side by squamous cells (the peripodial epithelium (PE)). The two epithelia are joined along their edges by cuboidal margin cells. The Dpp gradients that regulate the CE are generated by the columnar cells, and the best available evidence indicates that Dpp distributes along the apical surfaces that line the lumenal cavity of the wing disc (Fig. 3) (4). These gradients extend across ∼35–40 columnar cells to each flank of the disc, as much as 100–120 μm.
Figure 3.
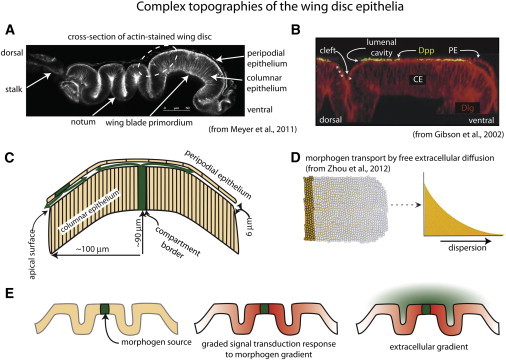
Complex topographies of the wing disc epithelia. (A) Cross section showing folds of the disc layers (from Meyer et al. (17)). The disc was stained with phalloidin and mounted on its side. (B) The fluorescent micrograph is a slightly flattened cross section of the region indicated by dashed white oval in A from a disc stained with α-Dpp antibody for external Dpp and α-Dlg (from Gibson et al. (4)). Note the presence of Dpp in the lumenal cavity, and Dpp along the surface of the CE in the lumenal cleft (stars), but the absence of Dpp in the space that forms at the lumenal cleft (arrow) between the CE and the PE. (C) The drawing depicts a cross section of the wing blade primordium, showing the spatial juxtaposition of the columnar and peripodial layers and domed shape. Green arrows represent the presumed paths of Dpp in the lumenal cavity. (D) Pictorial and graphical representations of morphogen that disperses by free extracellular diffusion (from Zhou et al. (1)). (E) Drawings depict folds of an epithelium (left), the presumed response of cells in the epithelium (red) to a continuous concentration gradient of morphogen (green, middle), and the lack of correlation of the response of the epithelium to the theoretical distribution of morphogen if it disperses by free diffusion in the extracellular space at the upper surface of the epithelium (right).
The stripe of Dpp-expressing cells that transects the CE extends beyond the ventral-most cells through the cuboidal cells at the ventral margin and to the PE, which it also transects (Figs. 1 and 3). The key point for consideration here is that the columnar and peripodial layers each have a stripe of Dpp-expressing cells, but in the late third-instar disc, the stripes in the two layers do not overlie each other—in the wing blade primordium, they are offset by ∼50–60 μm. And, despite the short distance between the layers (no more than 6 μm in the region of the wing blade primordium), the absence of a physical barrier, and the presence of the Dpp receptor on both the columnar and peripodial cells, there is no evidence of Dpp signaling across the lumenal space. The simplest model is that Dpp only targets cells in the layer where it is produced. However, because there is no evidence that Dpp produced by columnar cells is different from Dpp produced by peripodial cells, Dpp produced by either layer is likely to be competent to signal to both layers. Therefore, to generate the observed concentration gradients (Fig. 3, C and D), the Dpp produced by each layer must be constrained to signal only to the layer that produces it. It is not apparent how free extracellular diffusion in the apical lumen might be so constrained. Moreover, the tissue model that was used by Zhou et al. (1) for mathematical calculations, in which “secreted molecules diffuse along channels between cells, the ‘walls’ of which contain receptors” (5), apparently is not based on dispersion within the apical lumen. In contrast, cytonemes that track along the surfaces of the columnar and peripodial layers can transfer Dpp at points of direct contact, and if such contacts are specific to each layer, then the relative proximity of the layers is not an issue.
A second problem with free diffusion gradients is the relationship of their isocontours to the shapes of the epithelial sheets of the disc (Fig. 3 E). The small set of columnar cells that Zhou et al. (1) studied and modeled are near the Dpp source cells where the epithelium appears to be relatively flat, and the drawing of a wing disc in Fig. 1, as well as similar images from micrographs in numerous publications, give the misleading impression that the disc is a planar sheet of cells. However, whereas the PE is relatively flat, the CE has several deep folds and the wing pouch primordium region has a dome-like curvature (Fig. 3, A and C). The consequence is that the lumenal space has a complex shape; however, its shape has not been incorporated into mathematical models. Complex anatomy is not unique to the wing disc, and its relevance to morphogen dispersion mechanisms in the leg imaginal disc was previously noted by Teleman et al. (6). These authors pointed out that Dpp forms a long-range gradient that patterns the leg along the proximal-distal axis, but because the leg disc is highly folded, the disc cells that will generate the distal-most tip of the leg are very close to the cells that generate the proximal leg. Free extracellular diffusion can generate concentration gradients (Fig. 3 D), but it cannot generate concentration gradients that decline monotonically across an epithelial sheet if the sheet is folded (Fig. 3 E). In contrast to free-diffusion-generated gradients that are relevant only to idealized shapes, gradients generated by cytonemes that track along the surface of a folded epithelium can conform to the epithelium and reflect its topography.
The Wing Disc is an Unproven Ex Vivo System
The wing disc has many fortunate attributes that have been exploited to study its development, but with current technologies, it cannot be analyzed at single-cell resolution while it is inside a larva. High-resolution studies have required dissection of the larva, severing of the disc from the larval hypodermis, separation from adjoining discs and trachea, and flat mounting on a microscope slide. The disc morphology and cellular extensions of the disc cells are acutely sensitive to pressure, and even without the weight of an overlying coverslip, the face of the disc that adheres directly to a glass surface will flatten. Despite much effort, there have been no reports of de novo morphogen signaling in isolated wing discs. Therefore, although isolated discs may provide a snapshot of a disc’s state, time-dependent changes in isolated discs are not informative if we do not know whether and the extent to which signaling might be compromised by the methods that were used to prepare the discs. We also do not know which active processes in an isolated disc are faithful to normal development. Flattening the disc to remove the curvature of the domed wing blade primordium is optimal for imaging, but flattening distorts the disc and may compromise the transport of signaling proteins. Regarding the studies of Zhou et al. (1), it is unclear whether the integrity of the disc morphology was preserved, especially because in some experiments the wing discs were mounted in Ringer solution, which is optically advantageous but causes significant, irreversible, and abnormal changes to cell morphology. Furthermore, although DppDendra2 may move under their experimental conditions, there is no evidence that DppDendra2 is capable of moving from producing to target cells. Without such evidence, we cannot evaluate whether the ex vivo characterizations of active processes monitor a normal mechanism of movement that is biologically relevant.
Dpp Moves in an Uncharacterized Microenvironment
The 1970 paper by Francis Crick (7) that helped launch the field of morphogen gradient modeling predated the identification of bona fide morphogens, and assumed (incorrectly) that the relevant morphogens are low-molecular-weight organic molecules that move efficiently between and through cells. This assumption simplified calculations by treating the milieu in which the morphogen moves as inert and functionally transparent. It is more difficult to gauge the impact of the extracellular environment on a journey that free extracellular protein would make from producing to target cells. Because secreted protein morphogens might confront impermeable cell membranes and might interact with both receptor and nonreceptor components, values must be chosen for parameters such as geometric tortuosity (the effect of physical obstacles on diffusive path lengths), viscous effects (a combination of fluid viscosity and reversible interactions with immobilized components), and extracellular volume. Although these parameters can be estimated (5), direct measurements are not possible with current technologies, and no experimental data are available for comparison. In particular, we cannot have confidence in extracellular volume estimates calculated from electron micrographs that are prone to preparation artifacts.
The Uncertain Form and State of in Transit Dpp
To understand how Dpp moves, and to estimate a diffusion coefficient, it is essential to know the form of Dpp that disperses across the wing disc. Although the mathematical modeling of Zhou et al. (1) treated Dpp as a single molecule in solution, a significant fraction of Dpp in fixed discs is in large, discrete puncta (1,8), and motile Dpp in unfixed discs is also punctate (8,9). These Dpp-rich puncta have not been characterized, but it appears that Dpp is not unique in this regard: Hh and Wg are also present in motile vesicles (10,11). Without a clear understanding of either the dimensions or functions of the Dpp-containing puncta, or of the fraction of total Dpp that they represent, it is not apparent how the results from simulations of single molecules can be correlated with experimental data.
A second fundamental issue is whether Dpp that is en route to target cells is extracellular or is bound in some manner to cell membranes. There are many well-characterized extracellular proteins that are released from cells (e.g., insulin and growth hormones), as well as externalized proteins that are exposed on the external face of a plasma membrane but are not released. The question here is whether the Dpp protein that moves between the disc cells is actually extracellular. The image of the DppGFP gradient reproduced in Fig. 1 clearly shows DppGFP that has moved many cell diameters from producing cells, and the assumption has been that this protein is extracellular, that it is not directly associated with cells. Indeed, investigators have visualized the DppGFP gradient with antibody using protocols in which unfixed discs were stained to detect secreted extracellular protein (9,12). The key point, however, is that these histological methods cannot discriminate between extracellular protein that is free from any attachment to a cell and protein that is externalized but tethered to a cell membrane. The term “external staining” may be a more precise way to describe the detection of antigen when antibody is applied to unfixed tissue with such methods.
The distance between externalized protein that is tethered to the cell surface and protein that is free in the adjacent extracellular space may be small, but this difference is critical for the mechanism of movement. Dpp detected by antibody in the lumenal cavity is clearly distant from both the columnar and peripodial cell bodies shown in Fig. 3 B, but the resolution of these images is insufficient to discriminate whether the protein is free in the extracellular fluid. Furthermore, because the cytonemes that extend over the apical surfaces of the columnar cells (3,13) are not visible in these fixed preparations, these images do not resolve whether the protein is associated with cytonemes. It is interesting to note that although Dpp is in the lumenal cavity adjacent to the wing blade primordium (Fig. 3 B), Dpp does not fill the cleft at the perimeter of the wing blade primordium. Nevertheless, Dpp is present at the apical surface of the columnar cells that form the cleft and in the lumenal cavity adjacent to the cells that are dorsal to the cleft. Although such images can be misleading, the Dpp distribution in Fig. 3B is consistent with cytoneme-mediated direct delivery, but not with free extracellular diffusion.
Conclusions
This critique focuses on the experimental design and interpretations of Zhou et al. (1), but the issues it raises are relevant to most other studies of morphogen gradients, especially those that model morphogen dispersion. Modeling has made seminal contributions to concepts of morphogen signaling (7,14,15) and to identifying interesting aspects of the behavior of ligand-receptor systems (reviewed in Lander et al. (16)). However, the significance of modeled behaviors that depend upon unknown variables and unverified assumptions is uncertain. Most importantly, it seems reasonable to expect that the basic mechanisms that disperse different morphogen signaling proteins are shared and are common to the many contexts in which these proteins function. Therefore, models that cannot accommodate all of the different settings have limited biological relevance.
Acknowledgments
I thank B. Shilo, M. Noll, A. Schier, M. Gibson, M. Grabe, T. Tabata, and M. Dallman for comments on the manuscript.
This work was supported by grants from the National Institute of General Medical Sciences and the National Heart, Lung, and Blood Institute of the National Institutes of Health.
References
- 1.Zhou S., Lo W.C., Lander A.D. Free extracellular diffusion creates the Dpp morphogen gradient of the Drosophila wing disc. Curr. Biol. 2012;22:668–675. doi: 10.1016/j.cub.2012.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramírez-Weber F.A., Kornberg T.B. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. doi: 10.1016/s0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- 3.Hsiung F., Ramirez-Weber F.A., Kornberg T.B. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature. 2005;437:560–563. doi: 10.1038/nature03951. [DOI] [PubMed] [Google Scholar]
- 4.Gibson M.C., Lehman D.A., Schubiger G. Lumenal transmission of decapentaplegic in Drosophila imaginal discs. Dev. Cell. 2002;3:451–460. doi: 10.1016/s1534-5807(02)00264-2. [DOI] [PubMed] [Google Scholar]
- 5.Lander A.D., Nie Q., Wan F.Y. Do morphogen gradients arise by diffusion? Dev. Cell. 2002;2:785–796. doi: 10.1016/s1534-5807(02)00179-x. [DOI] [PubMed] [Google Scholar]
- 6.Teleman A.A., Strigini M., Cohen S.M. Shaping morphogen gradients. Cell. 2001;105:559–562. doi: 10.1016/s0092-8674(01)00377-4. [DOI] [PubMed] [Google Scholar]
- 7.Crick F. Diffusion in embryogenesis. Nature. 1970;225:420–422. doi: 10.1038/225420a0. [DOI] [PubMed] [Google Scholar]
- 8.Kicheva A., Pantazis P., González-Gaitán M. Kinetics of morphogen gradient formation. Science. 2007;315:521–525. doi: 10.1126/science.1135774. [DOI] [PubMed] [Google Scholar]
- 9.Teleman A.A., Cohen S.M. Dpp gradient formation in the Drosophila wing imaginal disc. Cell. 2000;103:971–980. doi: 10.1016/s0092-8674(00)00199-9. [DOI] [PubMed] [Google Scholar]
- 10.Callejo A., Bilioni A., Guerrero I. Dispatched mediates Hedgehog basolateral release to form the long-range morphogenetic gradient in the Drosophila wing disk epithelium. Proc. Natl. Acad. Sci. USA. 2011;108:12591–12598. doi: 10.1073/pnas.1106881108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korkut C., Ataman B., Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwank G., Dalessi S., Basler K. Formation of the long range Dpp morphogen gradient. PLoS Biol. 2011;9:e1001111. doi: 10.1371/journal.pbio.1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy S., Hsiung F., Kornberg T.B. Specificity of Drosophila cytonemes for distinct signaling pathways. Science. 2011;332:354–358. doi: 10.1126/science.1198949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meinhardt H. Models for the generation and interpretation of gradients. Cold Spring Harb. Perspect. Biol. 2009;1:a001362. doi: 10.1101/cshperspect.a001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turing A.M. The chemical basis of morphogenesis. Philos. Trans. R. Soc. Lond. 1952;237:37–72. [Google Scholar]
- 16.Lander A.D., Lo W.C., Wan F.Y. The measure of success: constraints, objectives, and tradeoffs in morphogen-mediated patterning. Cold Spring Harb. Perspect. Biol. 2009;1:a002022. doi: 10.1101/cshperspect.a002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer E.J., Ikmi A., Gibson M.C. Interkinetic nuclear migration is a broadly conserved feature of cell division in pseudostratified epithelia. Curr. Biol. 2011;21:485–491. doi: 10.1016/j.cub.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Tabata T., Takei Y. Morphogens, their identification and regulation. Development. 2004;131:703–712. doi: 10.1242/dev.01043. [DOI] [PubMed] [Google Scholar]


