Abstract
To what extent does our visual-spatial attention change with age? In this regard, it has been previously reported that relative to young controls, seniors show delays in attention-related sensory facilitation. Given this finding, our study was designed to examine two key questions regarding age-related changes in the effect of spatial attention on sensory-evoked responses in visual cortex –– are there visual field differences in the age-related impairments in sensory processing, and do these impairments co-occur with changes in the executive control signals associated with visual spatial orienting? Therefore, our study examined both attentional control and attentional facilitation in seniors (aged 66 to 74 years) and young adults (aged 18 to 25 years) using a canonical spatial orienting task. Participants responded to attended and unattended peripheral targets while we recorded event-related potentials (ERPs) to both targets and attention-directing spatial cues. We found that not only were sensory-evoked responses delayed in seniors specifically for unattended events in the left visual field as measured via latency shifts in the lateral occipital P1 elicited by visual targets, but seniors also showed amplitude reductions in the anterior directing attentional negativity (ADAN) component elicited by cues directing attention to the left visual field. At the same time, seniors also had significantly higher error rates for targets presented in the left vs. right visual field. Taken together, our data thus converge on the conclusion that age-related changes in visual spatial attention involve both sensory-level and executive attentional control processes, and that these effects appear to be strongly associated with the left visual field.
Keywords: Aging, Visual-spatial attention, Attentional control, Event-related potentials
Introduction
Age-related changes in visual-spatial attention have been well documented (e.g., Kok, 2000; Lincourt, Folk, & Hoyer, 1997), with seniors showing marked deficits in the ability to modulate visual sensory processing in a top-down manner (Curran et al., 2001). However, the extent of these deficits remains unclear. In particular, if seniors have sensory-related impairments in visual-spatial attention, are these problems at a purely sensory level in visual cortex, or might they co-occur with impairments in the volitional orienting of attention at an executive, control level? This possibility is not unfounded, as a general degradation of executive cognitive functioning is one of the hallmarks of the human aging process (e.g., Flicker, Ferris, & Reisberg, 1993; Gazzaley & D’Esposito, 2007; Koss et al., 1991; Nettelbeck & Rabbitt, 1992) and attentional control processes in prefrontal cortex are also known to decline with age (e.g., West & Schwarb, 2006). Given these issues, we wanted to address two specific questions regarding age-related changes in visual-spatial attention.
First, if seniors show impairments in the effect of visual spatial attention on sensory processing in visual cortex, are there visual field asymmetries in these impairments? The question arises because aging has been specifically associated with a greater rate of decline in cognitive functions localized in the right cerebral hemisphere relative to the left (e.g., Cherry et al., 2005; Lux et al., 2008). With respect to visual spatial attention, the neurocognitive processes associated with spatial orienting also show strong laterality effects, such as is manifest in the strong prevalence of left visual neglect following damage to the right cerebral hemisphere (e.g., Bublak, Redel, & Finke, 2006; Reuter-Lorenz, Kinsbourne, & Moscovitch, 1990), and the ability of the right hemisphere to orient attention to both visual hemifields but the left hemisphere only to the right visual field (e.g., Mangun et al., 1994). Nevertheless, previous studies examining differences in visual-spatial attention with age have collapsed data cross visual field (e.g., Curran et al., 2001; Lorenzo-Lopez et al., 2002), thus leaving open the question of whether there may be age-related visual asymmetries in visual-spatial orienting.
Second, to what extent are the reported age-related deficits in the effect of visual attention on sensory-level processing preceded by complimentary deficits in the control of visual-spatial orienting itself? In other words, given that executive control signals are the necessary antecedents to attention-related changes in visual sensory responses (e.g., Corbetta & Shulman, 2002; Green & Macdonald, 2008; Hopfinger, Buonocore, & Mangun, 2000), are seniors showing problems relative to young adults only at a visual sensory level (e.g., Curran et al., 2001), or might these problems in visual cortex co-occur with deficits in executive control of visual-spatial attention as well?
To address these questions we had both young (under 30 years of age) and senior (over 65 years of age) participants perform a canonical spatial orienting task (Posner, 1980) while we recorded their brains electrical responses via event-related potentials (ERPs). For each trial participants maintained central fixation as a cue was presented centrally that predicted the visual field location (left or right upper quadrant) of a pending target that required a simple manual response indicating which side of fixation it was presented on. In this paradigm, we assessed the neurocognitive processes underlying the control of attentional orienting by examining the ERP responses to the attention-directing cues, with data analysis focusing on two components of interest, the early directing attentional negativity (EDAN) and the anterior directing attentional negativity (ADAN). Both of these components are assessed by comparing scalp electrode locations ipsilateral vs. contralateral to the visual field indicated by the visual cue; electrode sites contralateral to the cued hemifield are expected to yield more negative ERP amplitudes relative to the mirror ipsilateral sites (e.g., Green & McDonald, 2006; Jongen, Smulders, & Van der Heiden, 2007; Seiss et al., 2007). In terms of what the components capture functionally, the EDAN is thought to reflect the evaluation and interpretation of an attention-directing cue (e.g., Jongen, Smulders, & Van der Heiden, 2007) and is widely distributed over the scalp typically around 280–320 milliseconds (ms) post-cue (e.g., Jonge, Smulders, & Van der Heiden, 2007; Seiss et al, 2007; Talsma et al., 2005; van Velzen & Eimer, 2003). In contrast, the ADAN is believed to reflect the act of actually orienting attention itself to the cued location and is localized to frontal-central lateral sites at approximately 350–400 ms post-cue (e.g., Jongen, Smulders, & Van der Heiden, 2007; Seiss et al., 2007; Talsma et al., 2005; van Velzen & Eimer, 2003).
In turn, we assessed the facilitatory effects of attention on sensory/perceptual processing by comparing ERP responses to visual targets as a function of whether they were in an attended (or cued) versus unattended (or uncued) location. In particular, the sensory-level effects of visual spatial attention are typically measured via two main ERP components, the lateral occipital P1 and N1 components. The P1 typically peaks around 100 ms post-stimulus and is believed to reflect the magnitude of the initial sensory-evoked response in visual cortex, likely in the V3/V4 region (e.g., Heinze et al., 1994; Woldorff et al., 1997), whereas the N1 typically peaks around 170–200 msec post-stimulus and has been tied to the initial perceptual/discriminative analysis of visual events (e.g., Vogel & Luck, 2000). For both components, the amplitude scales with the amount of attention oriented to the visual field location of the ERP-eliciting stimulus (e.g., Mangun & Hillyard, 1991; Handy & Mangun, 2000; Luck et al., 1994). At issue here was whether these effects of attention on P1 and N1 amplitude would change with age, and in particular, whether there would be any visual field asymmetries in these age-related effects.
Methods
Participants
Fourteen community-dwelling seniors, aged 66–74 years (M = 69.3, SD = 2.67; all female) and fourteen undergraduates, aged 18–25 years (M = 20.86, SD = 1.96; 10 female) participated. For the senior group, 14% had not received a high school diploma, 36% had a high school diploma, 36% had a trades certificate or equivalent, and 14% had a university degree. All senior participants were cognitively intact, as indicated by Mini-Mental Status Examination (MMSE) scores above 26 (Folstein, Folstein, & McHugh, 1975) (M = 28.71, SD = 0.99). One undergraduate participant was left-handed and all participants had normal or corrected-to-normal vision. All participants provided written informed consent and the reported research was approved by the Clinical Research Ethics Board (CREB) at the University of British Columbia.
Apparatus and Stimuli
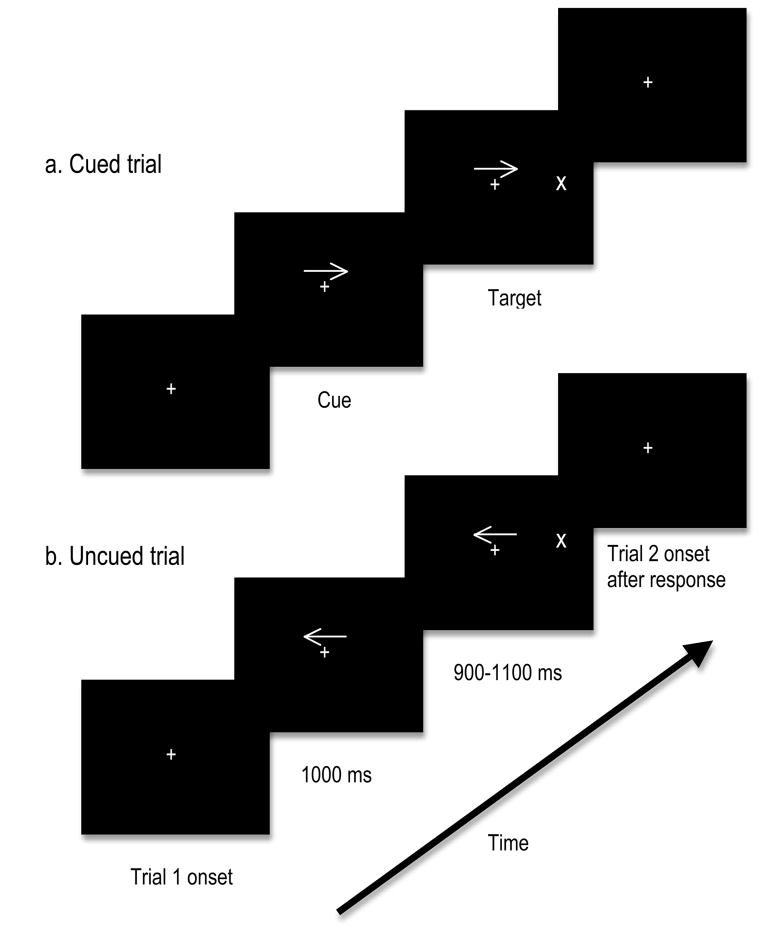
Trial sequence and timing are provided in Figure 1. Stimuli were presented on an 18 inch colour monitor placed 100 cm from the subject. Cues were 1.26° × 0.46°, presented at fixation, cueing either the left or the right target location. Targets, which were 0.92° × 0.92°, appeared either in the left visual field or the right visual field (target was 4.57° from the top of the screen, 11.31° from the bottom of the screen, and 4.86° from the left/right edge of the screen) and remained on the screen until a response was made. The cue predicted target location with 80% accuracy. After a response was made, the next trial began immediately.
Fig. 1.
Stimulus displays presented to participants. The displays shown are examples of the two different trial types: cued (top) and uncued (bottom). Targets appeared equally in the left and right visual field, with target location cued in 80% of trials by the preceding arrow.
Procedure
The task required participants to indicate via button presses whether the target appeared in the right visual field or left visual field, as quickly and accurately as possible. Participants were instructed to press one button with their left hand if the target appeared on the left, and another button with their right hand if the target appeared on the right. There were 12 blocks all together, each with 76 trials (60 cued, 12 uncued, 4 catch). Each block lasted approximately 4 minutes. Participants were instructed to keep their eyes on the central fixation point for the duration of the experiment.
Electrophysiological Recording and Analysis
During task performance, electroencephalograms (EEGs) were recorded from 32 active electrodes (Bio-Semi Active 2 system) evenly distributed over the head. All EEG activity was recorded relative to two scalp electrodes located over medial-frontal cortex (CMS/DRL), using a second order low pass filter of 0.05 HZ, with a gain of 0.5 and digitized on-line at a sampling rate of 256 samples-per-second. To ensure proper eye fixation and allow for the correction and/or removal of events associated with eye movement artifacts, vertical and horizontal electro-oculograms (EOGs) were also recorded, the vertical EOG from an electrode inferior to the right eye, and the horizontal EOG from an electrode on the right outer canthus. Off-line, computerized artifact rejection was used to eliminate trials during which detectable eye movements (> 10), blinks, muscle potentials, or amplifier blocking occurred. After artifact rejection, an average of 655 attended and 136 unattended trials were included in the analysis for each participant.
Statistical quantification of ERP data was based on mean amplitude measures relative to a −200 to 0 pre-stimulus baseline. Repeated-measures mixed-model ANOVAs were used, which had unpooled error terms in order to account for potential violations of sphericity for factors having more than 2 levels, a conservative approach that also controls for family-wise error rates (see Handy et al., 2009). Electrophysiological analysis was performed using ERPSS (UCSD; http://sdepl.ucsd.edu/erpss/doc/index.html), with electrode sites for analysis chosen based on previous research on these well-studied components (see below). In addition, because differences in latencies between young adults and seniors have been reported (e.g., Curran et al., 2001; Gilmore, 1995) amplitude analyses used latencies individually chosen based on group, according to the peak latency for seniors and young adults separately in each groups’ grand averaged waveforms.
Results
Behaviour
Reaction times (RTs) and accuracy were recorded during the experiment and results are presented in Table 1 as a function of group (senior vs. young) and trial type. Behavioural data was analyzed in a mixed-model repeated measures ANOVA using SPSS (Version 16 for MAC) with group (seniors vs. young) as a between-subjects factor, and attention (cued vs. uncued) and visual field (right vs. left) as within-subjects factors. For RTs, participants were faster to respond to attended targets compared to unattended targets. This pattern was confirmed via a main effect of attention, F(1,26) = 31.55, p < 0.001. There was also a significant attention x visual field interaction, F(1,26) = 8.48, p < 0.01. Specifically, the magnitude of the difference of RTs between attended and unattended trials was larger in the right visual field compared with the left. A main effect of group F(1,26) = 27.63, p < 0.001 indicates that seniors were significantly slower to respond compared with young controls. On average, seniors made significantly fewer mistakes than their younger counterparts. This was confirmed via a main effect of group, F(1,26) = 12.58, p = 0.002. Additionally, there was a marginal visual field x group interaction for accuracy, F(1,26) = 3.33, p = 0.08. While young adults performed slightly better in the left visual field compared to the right, seniors made disproportionally more errors in the left visual field.
Table 1.
Behavioural results for young and senior participantsa
| Young adults
|
Seniors
|
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Reaction Timesb | ||||
| Cued | ||||
| Left vfc | 337.53 | (47.27) | 449.45 | (65.04) |
| Right vf | 330.69 | (53.35) | 429.95 | (59.15) |
| Uncued | ||||
| Left vf | 389.43 | (46.93) | 469.09 | (66.79) |
| Right vf | 407.90 | (47.53) | 524.30 | (122.33) |
|
| ||||
| Accuracyd | ||||
| Cued | ||||
| Left vf | 1.14 | (1.61) | 0.64 | (0.93) |
| Right vf | 1.50 | (1.87) | 0.36 | (0.74) |
| Uncued | ||||
| Left vf | 2.36 | (2.56) | 0.50 | (0.85) |
| Right vf | 2.57 | (2.74) | 0.07 | (0.27) |
n = 14 in each group.
Mean reaction times measured in milliseconds.
vf = visual field.
Accuracy measured as mean number of errors.
Electrophysiology
Sensory-perceptual effects of attention were assessed by ERPs time-locked to visual targets. The ERP components measured for assessing early visual processing were the P1 and N1, which are the standard components used for measuring sensory gain (e.g., Mangun & Hillyard, 1991). In contrast, attentional control was assessed by examining ERPs time-locked to the attention-directing cues. The two components we focused on were the EDAN and the ADAN, both of which have been associated with the control of visual attention (Green, Teder-Salejarvi, & McDonald, 2005; Jongen, Smulders, & Van der Heiden, 2007; Seiss et al., 2007).
Sensory Facilitation
P1 Amplitude
Grand-averaged ERP waveforms for the P1 component are presented in Figure 2 and mean amplitudes are provided in Table 2. The P1 component was analyzed using electrode sites that the P1 is typically measured at (e.g., Curran et al., 2001; Jongen, Smulders, & Van der Heiden, 2007; Mangun & Hillyard, 1991). We used OL+ and OR+, which were averages of electrodes over occipital/posterior sites, with OL+ being P7, T7, and O1 and OR+ being P8, T8 and O2. The time windows used for measuring mean P1 amplitude was based on the latency of the peak in each condition within each group’s grand-averaged waveforms (i.e., seniors vs. young adults). In seniors, we examined the P1 in the right visual field at 120–160 ms post-target, and 130–170 ms in the left visual field. In young adults, both visual fields were examined at 125–165 ms post-target. P1 amplitude was examined using a mixed-model repeated-measures ANOVA with a between-subjects factor of group (senior vs. young), and within-subjects factors of visual field (left vs. right), attention (cued vs. uncued), and laterality (ipsilateral vs. contralateral sites to the visual field of the target).
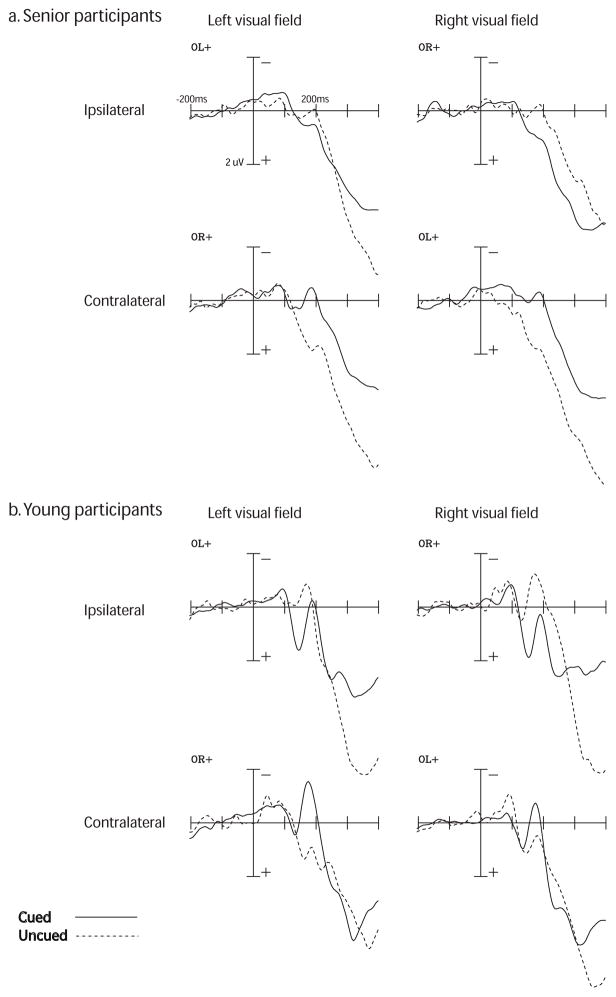
Fig. 2.
Grand-averaged ERP responses to targets for the P1 and N1 components in (a) senior and (b) young adults, as a function of visual field (left vs. right), laterality (ispilateral vs. contralateral to the visual field of the target), and cueing (cued vs. uncued). Time window is from −200 ms pre-target (baseline) to 400 ms post-target. Amplitudes are measured in uV. P1 amplitudes for seniors was measured at a time window of 90–160 ms post-target in the right visual field and 155–200 ms in the left visual field. P1 amplitude was measured for young adults at 125–165 ms post-target. The N1 component was measured at 150–200 ms post-target for both age groups. There were no significant amplitude differences between seniors and young adults for the P1 and N1 components.
Table 2.
Peak latencies and mean amplitudes for P1, N1, EDAN, and ADAN componentsa
| Component | Group | Visual field | Laterality | Cue | Peak lat. | Window | Mean amp. |
|---|---|---|---|---|---|---|---|
| P1 | Young | Left | Ipsi | Cued | 146.75 (8.90) | 125–165 | 1.28 (1.08) |
| Uncued | 131.97 (24.04) | 125–165 | -0.32 (1.32) | ||||
| Contra | Cued | 134.76 (13.85) | 125–165 | -0.15 (1.66) | |||
| Uncued | 141.73 (31.11) | 125–165 | 0.74 (1.86) | ||||
| Right | Ipsi | Cued | 151.78 (23.18) | 125–165 | 1.42 (1.77) | ||
| Uncued | 136.43 (21.31) | 125–165 | -0.15 (2.14) | ||||
| Contra | Cued | 137.83 (21.53) | 125–165 | 0.46 (1.71) | |||
| Uncued | 145.92 (20.30) | 125–165 | 0.98 (2.14) | ||||
| Seniors | Left | Ipsi | Cued | 153.17 (16.80) | 130–170 | 0.37 (0.83) | |
| Uncued | 154.01 (23.87) | 130–170 | 0.21 (1.68) | ||||
| Contra | Cued | 132.48 (16.48) | 130–170 | 0.18 (1.45) | |||
| Uncued | 162.66 (26.91) | 130–170 | 1.01 (1.54) | ||||
| Right | Ipsi | Cued | 149.82 (26.61) | 120–160 | 0.38 (1.07) | ||
| Uncued | 130.29 (24.64) | 120–160 | 0.10 (2.20) | ||||
| Contra | Cued | 144.79 (22.95) | 120–160 | -0.07 (0.96) | |||
| Uncued | 140.34 (35.55) | 120–160 | 0.95 (2.39) | ||||
| N1 | Young | Left | Ipsi | Cued | 165–205 | 0.13 (1.43) | |
| Uncued | 165–205 | -0.19 (2.06) | |||||
| Contra | Cued | 165–205 | -1.15 (2.21) | ||||
| Uncued | 165–205 | 1.15 (2.14) | |||||
| Right | Ipsi | Cued | 165–205 | 0.72 (1.95) | |||
| Uncued | 165–205 | -0.96 (3.37) | |||||
| Contra | Cued | 165–205 | -0.17 (1.81) | ||||
| Uncued | 165–205 | 0.70 (2.20) | |||||
| Seniors | Left | Ipsi | Cued | 170–210 | 0.56 (1.18) | ||
| Uncued | 170–210 | 0.05 (1.73) | |||||
| Contra | Cued | 170–210 | -0.30 (1.65) | ||||
| Uncued | 170–210 | 1.74 (1.87) | |||||
| Right | Ipsi | Cued | 160–200 | 0.96 (1.26) | |||
| Uncued | 160–200 | -0.09 (2.59) | |||||
| Contra | Cued | 160–200 | -0.17 (0.88) | ||||
| Uncued | 160–200 | 1.77 (2.79) | |||||
| EDAN | Young | Left | Ipsi | 280–340 | 0.17 (3.48) | ||
| Contra | 280–340 | -0.09 (3.71) | |||||
| Right | Ipsi | 280–340 | 0.03 (3.19) | ||||
| Contra | 280–340 | -0.14 (3.76) | |||||
| Seniors | Left | Ipsi | 280–340 | 1.03 (2.25) | |||
| Contra | 280–340 | 0.66 (2.71) | |||||
| Right | Ipsi | 280–340 | 1.46 (2.58) | ||||
| Contra | 280–340 | 0.97 (1.76) | |||||
| ADAN | Young | Left | Ipsi | 345–400 | -0.95 (3.62) | ||
| Contra | 345–400 | -1.01 (2.96) | |||||
| Right | Ipsi | 345–400 | -0.87 (2.85) | ||||
| Contra | 345–400 | -1.38 (3.80) | |||||
| Seniors | Left | Ipsi | 375–430 | 1.29 (2.71) | |||
| Contra | 375–430 | 1.16 (2.83) | |||||
| Right | Ipsi | 375–430 | 2.35 (2.49) | ||||
| Contra | 375–430 | 0.91 (2.21) |
Mean latencies and amplitudes (SD) for young adults and seniors, n = 14 per group.
Peak lat. = peak latency, measured in milliseconds. Window = time window used for amplitude analysis. Mean amp. = mean amplitude, measured in μV.
The amplitude of the P1 was consistently larger for attended versus unattended targets in ipsilateral sites (Figure 2). This was confirmed via a significant attention x laterality interaction, F(1,26) = 24.41, p < 0.001. In both visual fields, normal attention effects were seen in sites ipsilateral to the visual field of the target, where attended targets had a larger amplitude relative to unattended targets. In contrast, unattended targets had a larger amplitude than attended targets in contralateral sites. There were no significant between-groups effects, all p’s > 0.10.
P1 Latency
Latencies of the P1 are provided in Table 2. Electrode sites for the P1 latency analysis were identical to those used for the amplitude analysis. Latencies for the P1 were chosen individually on a subject-by-subject basis according to peak amplitude within an expected time window for the P1 (e.g., Mangun & Hillyard, 1991), which was 100 – 200 ms post-target. P1 latency was examined using a mixed-model repeated-measures ANOVA with a between-subjects factor of group (senior vs. young), and within-subjects factors of visual field (left vs. right), attention (cued vs. uncued), and laterality (ipsilateral vs. contralateral sites to the visual field of the target).
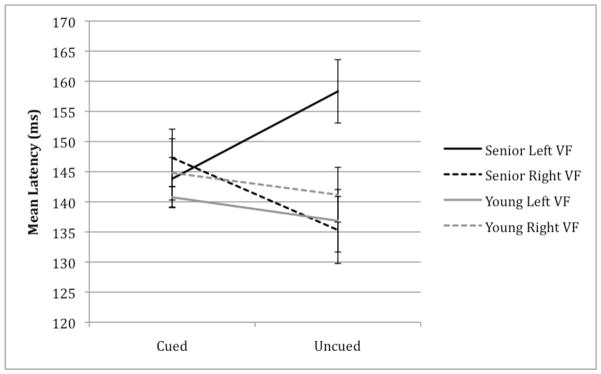
Increased latencies were exhibited by senior participants in the left visual field (Figure 2). This pattern was confirmed by a significant group x visual field x attention interaction, F(1,26) = 7.33, p= 0.01. This three-way interaction was further examined by plotting peak latencies as a function visual field and attention, separated between groups (Figure 3). While young adults had longer latencies for attended trials relative to unattended trials in both visual fields, seniors showed an interaction between visual field and attention. Specifically, unattended targets in the left visual field had delayed latencies relative to all other conditions in our senior group.
Fig. 3.
Latency of the P1 component in young and senior participants as a function of visual field (left vs. right) and cueing (cued vs. uncued). Latency measured in ms post-target. Senior participants had a significantly delayed P1 latency in the left visual field, relative to young adults.
N1 Amplitude
Grand-averaged ERP waveforms for the N1 component are presented in Figure 2 and mean amplitudes are provided in Table 2. Electrode sites for the N1 were the same as those used for the P1 analysis (i.e., OL+ and OR+). The time windows used were based on the 40 ms window following the P1. For seniors, we examined the N1 between 170–220 ms after target onset in the left visual field, and 160–200 ms in the right visual field. In young adults, the N1 was examined at 165–200 ms after target onset in both visual fields. N1 amplitude was examined using a mixed-model repeated-measures ANOVA with a between-subjects factor of group (senior vs. young), and within-subjects factors of visual field (left vs. right), attention (cued vs. uncued), and laterality (ipsilateral vs. contralateral sites to the visual field of the target).
We found an attention x laterality interaction, F(1,26) = 24.22, p < 0.001 (Figure 2). Specifically, attended targets were more negative than unattended targets measured at contralateral sites, whereas unattended targets are more negative than attended targets at ipsilateral sites. There were no between-groups differences for the N1 (all p’s > 0.10).
Attentional Control
Early directing attentional negativity (EDAN)
Grand-averaged ERP waveforms for the EDAN are presented in Figure 4 and mean amplitudes are presented in Table 2. We examined the EDAN at electrode sites F7, F8, F3, F4, T7, T8, C3, C4, P3, P4, P7, P8, O1, and O2, choosing these sites a priori based on where the EDAN is canonically measured (e.g., Jongen, Smulders, & Van der Heiden, 2007; Seiss et al., 2007; Talsma et al., 2005; van Velzen & Eimer, 2003). The latency used was 280–340 ms post-cue for seniors and young adults, guided by previous research on the EDAN (e.g., Jongen, Smulders, & Van der Heiden, 2007) and based on separate peaks identified for each group. The EDAN was examined in a mixed-model repeated-measures ANOVA with a between-subjects factor of group (senior vs. young), and within-subjects factors of visual field (right vs. left), laterality (ipsilateral vs. contralateral sites to the attended visual field), and electrode site. Interactions involving electrode site as a factor are omitted from our results because they are not directly relevant to our main questions of interest.
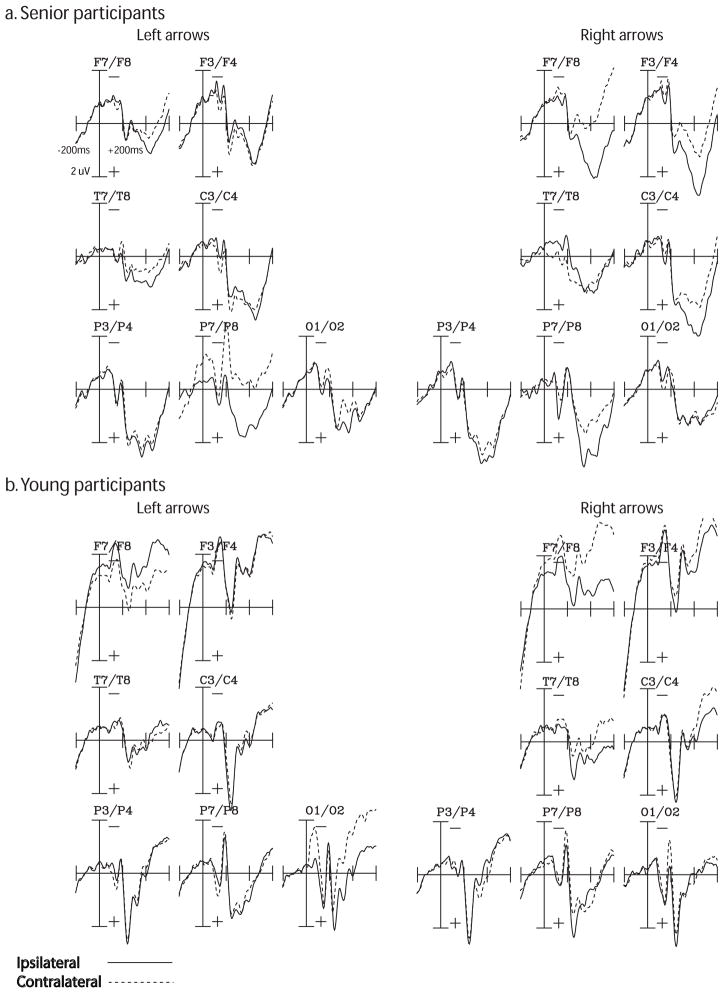
Fig. 4.
Grand-averaged ERP responses to cues for the EDAN and ADAN components in (a) senior and (b) young adults, as a function of visual field of arrows (right vs. left) and laterality (ipsilateral vs. contralateral to cued visual field). Time window is from −200 ms pre-cue (baseline) to 600 ms post-cue. Amplitudes are measured in uV. The EDAN was measured at electrode sites F7, F8, F3, F4, T7, T8, C3, C4, P3, P4, P7, P8, O1, and O2 at a latency of 280–350 ms post-cue for seniors and 280–320 ms post-cue for young adults. The ADAN was measured at electrode sites F7, F8, F3, F4, C3, and C4 at a latency of 375–430 ms post cue for seniors and 350–400 ms for young adults. The ADAN was significantly attenuated in the left visual field of seniors, as compared to young adults. There were no significant differences between groups for the EDAN component.
All participants showed the presence of an EDAN, where sites contralateral to the cued visual field were more negative in amplitude compared to ispilateral sites (Figure 4). This was confirmed via a main effect of laterality, F(1,26) = 7.45, p = 0.01. There were no significant differences between seniors and young adults, F(1,26) = 0.82, p > 0.10. To account for noisy electrodes, we re-ran the EDAN analysis excluding electrodes P7/P8 and O1/O2 and found no significant changes in the data pattern. Specifically, there was still a main effect of laterality, F(1,26) = 4.93, p = 0.04, and no between-groups differences, all p’s > 0.10.
Anterior directing attentional negativity (ADAN)
Grand-averaged ERP waveforms for the ADAN are presented in Figure 4 and mean amplitudes are presented in Table 2. Electrode sites and time windows were chosen based on previously established norms for examining the ADAN (e.g., Jongen, Smulders, & Van der Heiden, 2007; Seiss et al., 2007; Talsma et al., 2005; van Velzen & Eimer, 2003). The electrode sites we used were F7, F8, F3, F4, C3, and C4. The latency analyzed for seniors was 375–430 ms post cue and 345–400 ms for young adults, initially based on previous work on the ADAN (e.g., Jongen, Smulders, & Van der Heiden, 2007), and refined based on peaks identified for each group separately. A mixed-model repeated-measures ANOVA with a between-subjects factor of group (senior vs. young), and within-subjects factors of visual field (right vs. left), laterality (ipsilateral vs. contralateral sites to the attended visual field), and electrode site was conducted. Any interactions involving electrode site as a factor are not reported because they are tangential to the focus of our study.
Both groups of participants showed the presence of an ADAN, with larger amplitudes for contralateral sites relative to ipsilateral sites (Figure 4). This was verified by a main effect of laterality, F(1,26) = 19.80, p = 0.0001, where a greater negativity in amplitude was found for contralateral sites to the attended visual field relative to ipsilateral sites. Comparing seniors and young adults, there was a significant group x hemisphere interaction, F(1,26) = 4.36, p = 0.05. While both groups had larger amplitudes for contralateral relative to ipsilateral sites, the difference in amplitude between the two sites was reduced for seniors compared with young adults. Follow-up analysis on seniors revealed a significant visual field x laterality interaction, F(1,13) = 5.54, p < 0.04. While seniors showed normal attentional control in the right visual field, the ADAN in the left visual field was attenuated, with only a minor amplitude difference between contralateral and ipsilateral sites.
Discussion
Our study examined two key questions regarding age-related changes in the effect of spatial attention on sensory-evoked responses in visual cortex –– are there visual field differences in the age-related impairments in sensory processing that have been reported previously (e.g., Curran et al., 2001), and do these impairments co-occur with changes in the executive control signals associated with visual spatial orienting? In this regard, we found that not only were sensory-evoked responses delayed in seniors specifically for unattended events in the left visual field as measured via latency shifts in the lateral occipital P1 elicited by visual targets, but seniors also showed amplitude reductions in the ADAN component elicited by cues directing attention to the left visual field. At the same time, seniors also had significantly higher error rates for targets presented in the left vs. right visual field. Taken together, our data thus converge on the conclusion that age-related changes in visual spatial attention involve both sensory-evoked and executive attentional control processes, and that these effects appear to be strongly associated with the left visual field.
If sensory-evoked responses are specifically delayed in seniors for unattended events in the left visual field, why might this be the case? When Curran et al. (2001) reported a comparable effect in seniors based on data collapsed across visual fields, they attributed this latency shift –– which was found in the P1 component at the same ipsilateral electrode sites we report here –– to comparatively slower inter-hemispheric transfer speeds in seniors vs. young controls. While our findings are not inconsistent with this conclusion, that we found latency shifts in the P1 only for unattended events in the left visual field suggests that visual attention itself may also be a critical factor determining the speed of sensory responses in seniors. In particular, we found a delayed P1 latency when a target was presented in the left visual field but attention had been cued at the start of the trial to the right visual field. This suggests that seniors may have difficulty disengaging their visual spatial attention from the right visual field in response to the presentation of a target in the heretofore unattended left hemifield.
While our P1 data in seniors are thus consistent with selective problems in re-orienting attention to the left visual field, the ERPs time-locked to the attention-directing cues also point towards specific problems in the left visual field. To the point, both seniors and young controls showed comparable responses in the EDAN component regardless of visual field, a component which has been linked to the evaluation and interpretation of attention-directing cues (e.g., Jongen, Smulders, & Van der Heiden, 2007). This suggests that the cues themselves were being equitably evaluated regardless of the participants’ age and the visual field to which the cue was directing attention. In contrast however, seniors showed amplitude reductions in the ADAN component elicited by cues to the left, relative to their responses to right visual field cues and relative to the ADANs for both cue directions in young controls. Given that the ADAN has been tied to the actual control of orienting visual spatial attention itself (e.g., Green, Conder, & McDonald, 2008; Jongen, Smulders, & Van der Heiden, 2007; Seiss et al., 2007; but see Praamstra, Boutsen, & Humphreys, 2005), this would indicate that not only do seniors have difficulty re-orienting attention to the left visual field in response to unexpected events, but that they also show selective decrements in volitionally orienting attention to the left visual field in response to directional cues.
Importantly, these age-related changes in orienting visual spatial attention to the left visual field, which we have construed as “impairments”, are not limited to the ERP measures we report here ––we also found that seniors were significantly less accurate in their behavioral responses to left vs. right visual field targets. While we can not make definitive causal links between our ERP and performance results, the collective evidence nevertheless suggests that seniors have greater difficulty in orienting their attention to the left vs. right visual field as measured not just by ERP indices of attentional orienting processes, but also in the overt responses they make to events in this visual hemifield. Given this conclusion, it’s also thus interesting to note that our results suggest that there was a speed-accuracy trade-off between seniors and young adults. Seniors were slower to respond to targets, but were also more accurate, relative to young adults. While we expected seniors to exhibit delayed reaction times, their increased accuracy suggests that it may not be due to a general slowing in cognitive processing, but rather an increase in conservativeness in order to avoid making errors (e.g., Ratcliff & McKoon, 2008). While all participants were given the same instructions to respond both quickly and accurately, the performances differences that we observed between the two groups may be attributed to different internal priorities between seniors and young adults.
Performance issues aside, if our findings thus argue for age-related impairments in orienting visual spatial attention to the left visual field, what might be driving the constellation of effects we report here? Recent evidence has suggested that aging is specifically associated with a greater rate of decline in the right hemisphere relative to the left. For example, hemispheric asymmetries with age have been found for a variety of cognitive domains, such as memory span (e.g., Cherry et al., 2005) and global processing (e.g., Lux et al., 2008). Likewise, the neurocognitive processes associated with visual spatial attention also show strong laterality effects, such as is manifest in the strong prevalence of unilateral neglect in the left relative to the right visual field (e.g., Bublak, Redel, & Finke, 2006; Reuter-Lorenz, Kinsbourne, & Moscovitch, 1990), and the ability of the right hemisphere to orient attention to both visual hemifields but the left hemisphere only to the right visual field (e.g., Mangun et al., 1994). Pairing these two lines of evidence together, it would suggest that the visual field asymmetries in age-related changes we report may be associated with specific age-related declines in right hemisphere processing.
In closing, we also note that there are several key control issues to consider regarding our data. First, it is apparent in our results that while normal attentional modulation of the P1 ERP component is evident in sites measured ipsilateral to the visual field of the target, the effects are notably reversed in both age groups at contralateral sites. Why? In perceptually easy tasks, such as the basic Posner cueing paradigm used in this study, cueing effects are shown primarily in ipsilateral sites (e.g., Handy & Mangun, 2000; Kutas, Iragui, & Hillyard, 1994; Onofrj et al., 2001). Thus it is not surprising that we did not observe attention effects in the P1 at electrode sites contralateral to the visual field of the target. Second, the ERP waveforms of the senior participants are diminished, or “flattened out”. This attenuation of visual-evoked potentials (VEPs) and ERPs in seniors concurs with previous studies (e.g., Gilmore, 1995; Kutas et al., 1994; Nagamatsu et al., 2009). Indeed, it has been suggested that the “severely impoverished” P1 in seniors agree with both fMRI evidence that seniors have decreased activity in primary visual cortices, and behaviour declines exhibited in seniors in visual perceptual abilities (Ceponiene et al., 2008). Based on this, the morphology of ERPs in our study are consistent with those in previous studies using ERPs in seniors. Lastly, the ERP waveforms time-locked to the attention-directing cues in young adults are preceded by a large negative shift pre-baseline. This contingent negative variation (CNV) is related to the expectancy of stimulus onset (Walter et al., 1964). Importantly, comparisons of the CNV across the lifespan have revealed larger expectancy effects in young adults versus seniors (e.g., Botzel et al., 2004; Michalewski et al., 1980). Therefore, it is not surprising that we find similar effects in our study.
Conclusions
Our results suggest that impairments in visual-spatial attention extend to both aspects of covert attention: attentional control and attentional facilitation. Given the central importance of visual attention to basic processes, such as perception and action, we highlight the value of studying age-related changes in cognition and attention in order to understand the basis of the many problems associated with mobility, perception, and action.
Acknowledgments
The authors would like to thanks Dr. Olav Krigolson for his help with programming for the study and Lindsay Katarynych for her assistance recruiting and scheduling participants. Supported by grants from Michael Smith Foundation for Health Research (MSFHR) to Ms. Nagamatsu, MSFHR, Natural Sciences and Engineering Research Council of Canada (NSERC), Canadian Institutes of Health Research (CIHR) (MOB-93373) to Dr. Handy, and MSFHR, Vancouver Foundation, CIHR (MOB-93373) to Dr. Liu-Ambrose. Both Drs. Handy and Liu-Ambrose are MSHFR Scholars.
References
- Botzel K, Mayer M, Oertel WH, Paulus W. Frontal and parietal premovement slow brain potentials in Parkinson’s disease and aging. Movement Disorders. 2004;10:85–91. doi: 10.1002/mds.870100114. [DOI] [PubMed] [Google Scholar]
- Bublak P, Redel P, Finke K. Spatial and non-spatial attention deficits in neurodegenerative dieseases: Assessment based on Bundesen’s theory of visual attention (TVA) Restorative Neurology and Neuroscience. 2006;24:287–301. [PubMed] [Google Scholar]
- Ceponiene R, Westerfield M, Torki M, Townsend J. Modality-specificity of sensory aging in vision and audition: Evidence from event-related potentials. Brain Research. 2008;1215:53–68. doi: 10.1016/j.brainres.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Cherry BJ, Adamson M, Duclos A, Hellige JB. Aging and individual variation in interhemispheric collaboration and hemispheric asymmetry. Aging Neuropsychology and Cognition. 2005;12:316–339. doi: 10.1080/17444128.2005.10367004. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Curran T, Hills A, Patterson MB, Strauss ME. Effects of aging on visuospatial attention: an ERP study. Neuropsychologia. 2001;39:288–301. doi: 10.1016/s0028-3932(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Flicker C, Ferris SH, Reisberg B. A 2-year longitudinal-study of cognitive function in normal aging and alzheimers-disease. Journal of Geriatric Psychiatry and Neurology. 1993;6:84–96. doi: 10.1177/089198879300600205. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, D’Esposito M. Top-down modulation and normal aging. Annual New York Academy of Sciences. 2007;1097:67–83. doi: 10.1196/annals.1379.010. [DOI] [PubMed] [Google Scholar]
- Gilmore R. Evoked potentials in the elderly. Journal of Clinical Neurophysiology. 1995;12:132–138. doi: 10.1097/00004691-199503000-00003. [DOI] [PubMed] [Google Scholar]
- Green JJ, Conder JA, McDonald JJ. Lateralized frontal activity elicited by attention-directing visual and auditory cues. Psychophysiology. 2008;45:579–587. doi: 10.1111/j.1469-8986.2008.00657.x. [DOI] [PubMed] [Google Scholar]
- Green JJ, McDonald JJ. An event-related potential study of supramodal attentional control and crossmodal attention effects. Psychophysiology. 2006;43:161–171. doi: 10.1111/j.1469-8986.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- Green JJ, McDonald JJ. Electrical neuroimaging reveals timing of attentional control activity in human brain. PLOS Biology. 2008;6:730–738. [Google Scholar]
- Green JJ, Teder-Salejarvi WA, McDonald JJ. Control mechanisms mediating shifts of attention in auditory and visual space: A spatio-temporal ERP analysis. Experimental Brain Research. 2005;166:358–369. doi: 10.1007/s00221-005-2377-8. [DOI] [PubMed] [Google Scholar]
- Handy TC, Mangun GR. Attention and spatial selection: Electrophysiological evidence for modulation by perceptual load. Perception and Psychophysics. 2000;62:175–186. doi: 10.3758/bf03212070. [DOI] [PubMed] [Google Scholar]
- Handy TC, Nagamatsu LS, Mickelborough MJS, Liu-Ambrose TYL. Statistical strategies for translational ERP studies. In: Handy TC, editor. Brain Signal Analysis: Advances in Bioelectric and Biomagnetic Methods. Cambridge, MA: MIT Press; 2009. [Google Scholar]
- Heinze HJ, Mangun GR, Burchert W, Hinrichs H, Scholz M, Munte TF, Gos A, Scherg M, Johannes S, Hundeshagen H, Gazzaniga MS, Hillyard SA. Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature. 1994;372:543–546. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Jongen EM, Smulders FT, Van der Heiden JS. Lateralized ERP components related to spatial orienting: Discriminating the direction of attention from processing sensory aspects of the cue. Psychophysiology. 2007;44(6):968–986. doi: 10.1111/j.1469-8986.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- Kok A. Age-related changes in involuntary and voluntary attention as reflected in components of the event-related potential (ERP) Biological Psychology. 2000;54:107–143. doi: 10.1016/s0301-0511(00)00054-5. [DOI] [PubMed] [Google Scholar]
- Koss E, Haxby JV, Decarli C, Schapiro MB, Friedland RP, Rapoport SI. Patterns of performance preservation and loss in healthy aging. Developmental Neuropsychology. 1991;7:99–113. [Google Scholar]
- Kutas M, Iragui V, Hillyard SA. Effects of aging on event-related potentials (ERPs) in a visual detection task. Electrocephalography and clinical Neurophysiology. 1994;92:126–139. doi: 10.1016/0168-5597(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Lincourt AE, Folk CL, Hoyer WJ. Effects of age on voluntary and involuntary shifts of attention. Aging Neuropsychology and Cognition. 1997;4:290–303. doi: 10.1080/13825589708256654. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Lopez L, Doallo S, Vizoso C, Amenedo E, Holguin SR, Cadaveira F. Covert orienting of visuospatial attention in the early states of aging. Neuroreport. 2002;13:1459–1462. doi: 10.1097/00001756-200208070-00022. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL. Effects of spatial cueing on luminance detectability – Psychophysical and electrophysiological evidence for early selection. Journal of Experimental Psychology-Human Perception and Performance. 1994;20:887–904. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Lux S, Marshall JC, Thimm M. Differential processing of hierarchical visual stimuli in young and older healthy adults: Implications for pathology. Cortex. 2008;44:21–28. doi: 10.1016/j.cortex.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual spatial priming. Journal of Experimental Psychology - Human Perception and Performance. 1991;17(4):1057–1074. doi: 10.1037//0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Luck SJ, Plager R, Loftus W, Hillyard SA, Handy T, Clark VP, Gazzaniga MS. Monitoring the visual world – Hemispheric asymmetries and subcortical processes in attention. Journal of Cognitive Neuroscience. 1994;6:267–275. doi: 10.1162/jocn.1994.6.3.267. [DOI] [PubMed] [Google Scholar]
- Michalewski HJ, Thompson LW, Smith DBD, Patterson JV, Bowman TE, Litzelman D, Brent G. Age differences in the contingent negative variation (CNV): Reduced frontal activity in the elderly. Journal of Gerontology. 1980;35:542–549. doi: 10.1093/geronj/35.4.542. [DOI] [PubMed] [Google Scholar]
- Nagamatsu LS, Liu-Ambrose TYL, Carolan P, Handy TC. Are impairments in visual-spatial attention a critical factor for increased falls risk in seniors? An event-event potential study. Neuropsychologia. 2009;47:2749–2755. doi: 10.1016/j.neuropsychologia.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettelbeck T, Rabbitt PMA. Aging, cognitive performance, and mental speed. Intelligence. 1992;16:189–205. [Google Scholar]
- Onofrj M, Thomas A, Lacono D, D’Andreamatteo G, Paci C. Age-related changes of evoked potentials. Neurophysiol Clin. 2001;31:83–103. doi: 10.1016/s0987-7053(01)00248-9. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Boutsen L, Humphreys GW. Frontoparietal control of spatial attention and motor intention in human EEG. Journal of Neurophysiology. 2005;94:764–774. doi: 10.1152/jn.01052.2004. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, McKoon G. The diffusion decision model: Theory and data for two-choice decision tasks. Neural Computation. 2008;20:873–922. doi: 10.1162/neco.2008.12-06-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Kinsbourne M, Moscovitch M. Hemispheric control of spatial attention. Brain and Cognition. 1990;12:240–266. doi: 10.1016/0278-2626(90)90018-j. [DOI] [PubMed] [Google Scholar]
- Seiss E, Gherri E, Eardley AF, Eimer M. Do ERP components triggered during attentional orienting represent supramodal attentional control? Psychophysiology. 2007;44(6):987–990. doi: 10.1111/j.1469-8986.2007.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talsma D, Slagter HA, Nieuwenhuis S, Hage J, Kok A. The orienting of visuospatial attention: An event-related brain potential study. Cognitive Brain Research. 2005;25(1):117–129. doi: 10.1016/j.cogbrainres.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Van Velzen J, Eimer M. Early posterior ERP components do not reflect the control of attentional shifts toward expected peripheral events. Psychophysiology. 2003;40(5):827–831. doi: 10.1111/1469-8986.00083. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37:190–203. [PubMed] [Google Scholar]
- Walter WG, Winter AL, Cooper R, McCallum WC, Aldridge VJ. Contingent negative variation – Electrical sign of sensorimotor association + expectancy in human brain. Nature. 1964;203:380. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- West R, Schwarb H. The influence of aging and frontal function on the neural correlates of regulative and evaluative aspects of cognitive control. Neuropsychology. 2006;20:468–481. doi: 10.1037/0894-4105.20.4.468. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Fox PT, Matzke M, Lancaster JL, Veeraswamy S, Zamarripa F, Seabolt M, Glass T, Gao JH, Martin CC, Jerabek P. Retinotopic organization of early visual spatial attention effects as revealed by PET and ERPs. Human Brain Mapping. 1997;5:280–286. doi: 10.1002/(SICI)1097-0193(1997)5:4<280::AID-HBM13>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]