Abstract
Background
Falls are a major health care problem for older people and are associated with cognitive dysfunction. Mild cognitive impairment (MCI) is an increasingly recognized clinical problem. No study has comprehensively compared normal volunteers with those with MCI for falls risk factors in both the physiological and cognitive domains.
Objective
The purpose of this cross-sectional study was to comprehensively compare falls risk factors in community-dwelling older women with and without MCI.
Design
A cross-sectional study.
Methods
158 community-dwelling women with Folstein’s Mini Mental State Examination scores of ≥ 24 were included. The Montreal Cognitive Assessment (MoCA) was used to categorise participants as either having, or not having, MCI. Each participant’s fall risk profile was assessed by the Physiological Profile Assessment (PPA). Three central executive functions were assessed: 1) set shifting by the Trail Making Test (Part B); 2) updating (i.e., working memory) by the verbal digits backward test; and 3) response inhibition by the Stroop Colour-Word Test.
Results
Both the composite PPA score and its sub-component, postural sway performance were significantly different between the two groups (P ≤ 0.03); those with MCI had higher composite PPA scores and greater postural sway. Compared with those without MCI, participants with MCI performed significantly worse on all three central executive functions tests (P ≤ 0.02).
Limitations
A screening tool was used to categorize participants as having MCI and falls risk factors were compared rather than the actual incidence of falls.
Conclusions
Falls risk screening may be prudent in older adults with MCI.
Keywords: mild cognitive impairment, physiological fall risk profile, executive function
INTRODUCTION
Falls are a major health care problem for older people and about 30% of community-dwellers over the age of 65 experience one or more falls every year 1. Older women have a higher incidence of falls compared with older men 1. The proportion of women who fall increase from about 30% in the 65 to 69 year age group to over 50% in those over the age of 85 years. The proportion of men who fall increase from 13% in the 65 to 69 year age group to approximately 30% in those aged 80 years and over 1.
Falls are not random events 2 and occur, at least in part, due to physiological impairments, such as impaired balance, muscular weakness, and slowed reaction time 3. Falls are also associated with cognitive dysfunction 4. Approximately 60% of older people with cognitive impairment fall annually; this incidence is approximately twice that of cognitively-intact peers 4, 5. Tinetti and coworkers 4 reported that compared with cognitively-intact peers, the odds of falling are 5 times greater in older adults with cognitive impairment; this compared with an odds ratio of 3.8 and 1.9 for disability in the lower extremities and impaired balance and gait, respectively. Thus, the cognitively-impaired older faller is at increased risk of major injury such as fracture and head trauma 5.
Although there has been dedicated research on falls in older adults with dementia [e.g., Alzheimer’s disease (AD)] 6, 7, very little research has focused on people with mild cognitive impairment (MCI). Consequently, the falls incidence in this population and key falls risk factors remain poorly defined. MCI is distinct from dementia and is conceptually defined as a clinical entity characterized by cognitive decline greater than that expected for an individual’s age and education level but that does not notably interfere with activities of daily living (ADLs) 8, 9. Longitudinal studies report that seniors with MCI develop Alzheimer’s disease at a rate of 10% to 30% annually 10, 11, whereas seniors without MCI develop dementia at a rate of 1% to 2% annually 10. MCI is also more prevalent than dementia 12. According to the 1994 Canadian Study of Health and Aging, 8% of Canadians aged 65 and over had dementia while 16.8% had MCI 13.
Older adults with MCI have impaired balance and gait 14, 15 as well as impaired executive functions 16; each of these impairments are associated with falls 17. Thus, falls risk screening and prevention may be a key component in the medical management of older adults with MCI. However, no single study to date has comprehensively compared older adults without MCI with those with MCI for well-recognized falls risk factors. Specifically, no previous studies have compared physiological falls risk between these two populations using a valid and reliable measure, such as the Physiological Profile Assessment (PPA)© 18 (Prince of Wales Medical Research Institute, Sydney, Australia) which is a validated tool for quantifying physiological falls risk based on a combination of physiological measures: 1) postural sway; 2) hand reaction time; 3) knee extension strength; 4) proprioception; and 5) edge contrast sensitivity. The PPA is different from clinical measures of balance and gait as it first quantifies performance within the specific physiological domains relevant to falls risk and then computes a composite falls risk score (i.e., a standardized score). A marked deficit in any one of the five physiological domains may increase falls risk (i.e., higher composite falls risk score). However, a combination of mild or moderate impairments in each of five physiological domains also may increase the risk of falling. The composite falls risk score has a 75% predictive accuracy for falls in older people 19.
Also, no previous studies have compared cognitive performance of key executive functions that are associated with falls risk between older adults with older adults without MCI and those with MCI. Previous work have identified impaired set shifting, updating (i.e., working memory), and response inhibition to be associated with increased falls risk 17, 20–22. Therefore, the purpose of this cross-sectional study was to comprehensively compare both physiological and cognitive falls risk factors in older women with and without MCI.
METHODS
Participants
The sample for this cross-sectional analysis consisted of 158 women who consented to be participants of a one-year randomized controlled trial of exercise. Women who: 1) were aged 65 to 75 years; 2) were living independently in their own home; 3) obtained a score ≥ 24 on the Mini-Mental State Examination (MMSE) 23; and 4) had a visual acuity of at least 20/40, with or without corrective lenses were recruited. Those who: 1) had a diagnosed neurodegenerative disease (e.g., AD) and/or stroke; 2) were taking psychotropic drugs; 3) did not speak and understand English; 4) had moderate to significant impairment with ADLs as determined by interview; 5) were taking cholinesterase inhibitors within the last 12 months; 6) were taking anti-depressants within the last six months; or 7) were on oestrogen replacement therapy within the last 12 months were excluded.
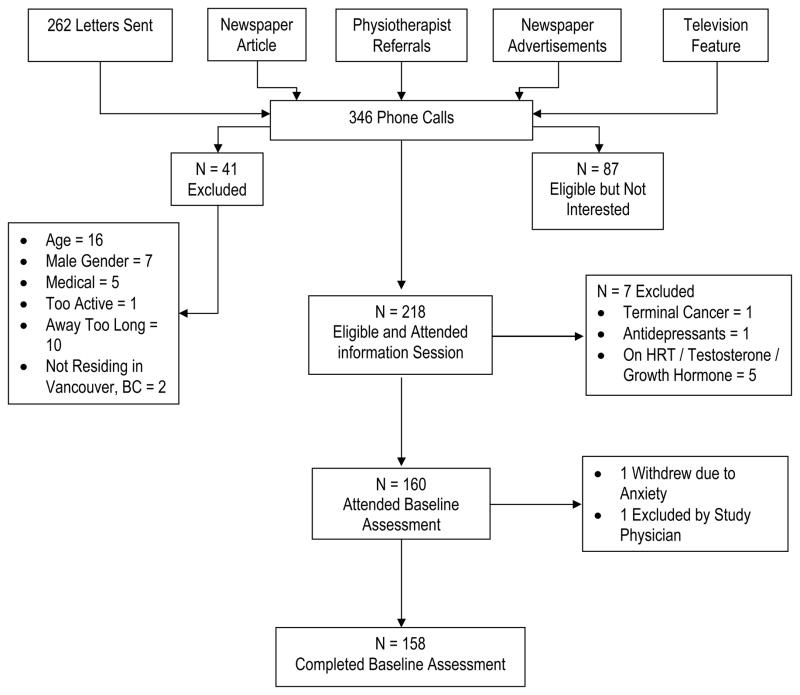
Participants were recruited through newspaper advertisements and articles, television features, flyers posted at local community centres, and advertisements through the Physiotherapy Association of British Columbia (Figure 1). All interested individuals were initially screened by phone and 41 individuals were excluded. Those who were eligible based on the telephone screen were invited to attend an information session. Two-hundred and eighteen individuals attended an information session; 7 were excluded during these sessions. One-hundred and sixty women consented and attended the baseline assessment. During the baseline assessment, one person was excluded by the study physician due to possible neurological condition and one decided to withdraw due to anxiety associated with the standard neuropsychological tests. Thus, 158 women completed the baseline assessment. The study was approved by the relevant hospital and university ethics boards and all participants provided written informed consent.
Figure 1.
Flow Chart of Participants.
Descriptive Variables
Global cognitive state was assessed using the MMSE 23. General health and socioeconomic status were ascertained by a questionnaire. Participants underwent a 15-minute physician assessment to confirm current health status and eligibility for the study. The occurrence of falls in the last 12 months was ascertained by means of an interview with the study physician.
As depression may influence performance on neuropsychological tests and has been identified in the prodromal stage and as a risk factor for developing Alzheimer’s disease 24, the 15-item Geriatric Depression Scale (GDS) 25 was used to screen for depression. A score of 11 and greater indicate severe depression 25. The Functional Comorbidity Index was calculated to estimate the degree of comorbidity associated with physical functioning 26. The Functional Comorbidity Index explains more variance in physical function scores compared with indices designed to predict mortality 26. This scale’s score is the total number of comorbidities.
Results from large prospective cohort studies indicate that regular participation in low-intensity physical activity is associated with a reduced risk of dementia 27 and with better cognitive performance among older adults 28. Thus, current level of physical activity (i.e., previous seven-day period) was determined by the Physical Activities Scale for the Elderly (PASE) self-report questionnaire 29, 30. This 10-item scale for those aged 65 years and older, measures the average number of hours per day spent participating in leisure, household, and occupational physical activities over the previous seven-day period. The time spent in each activity is multiplied by a weighted value that reflects the amount of energy expended by the respondent. These weighted values are then summed to give a composite PASE score. Higher scores indicate higher levels of physical activity. Washburn et al. 31 reported that scores may range from 0 to 400 or higher. The PASE questionnaire is valid and reliable for older adults with no serious physical limitations 29, 30. In a sample of 222 individuals, PASE scores were significantly correlated with postural balance, grip strength, leg strength, self-assessed health status, and the Sickness Impact Profile 29. The test-retest reliability coefficient of the PASE was 0.75 for self-administration and 0.68 when administered during a telephone interview 29.
General mobility was assessed by the Timed Up and Go Test (TUG) 32. Participants were instructed to rise from a standard chair with arms, walk a distance of three meters, turn, walk back to the chair and sit down again. The mean of two trials was calculated and used for statistical analysis. A TUG cut-off of at least 13.5 seconds correctly classified participants as fallers in 90% of cases 33.
Classification of Possible Mild Cognitive Impairment
There are no consensus criteria for the clinical classification of MCI 34. The Montreal Cognitive Assessment (MoCA), a brief screening tool for MCI 35 with high sensitivity and specificity, was used to categorise participants as with, or without, possible MCI. It is a 30-point test covering eight cognitive domains: 1) attention and concentration; 2) executive functions; 3) memory; 4) language; 5) visuo-constructional skills; 6) conceptual thinking; 7) calculations; and 8) orientation. Scores below 26 are considered to be indicative of possible MCI. A bonus point is given to individuals with less than 12 years of education.
Physiological Falls Risk Profile
The PPA has two versions: a comprehensive (or long) version and a screening (or short) version 18. While the comprehensive version provides information on a broader array of physiological functions than the short form, the two versions provide the same composite falls risk score. Physiological falls risk profile was assessed in this study by the short form of the PPA. The short form takes 15 minutes to administer and includes: 1) postural sway; 2) hand reaction time; 3) knee extension strength; 4) proprioception; and 5) edge contrast sensitivity. These five physiological functions were identified from discriminant function analyses as being the most important for discriminating between fallers and non-fallers in both institutional and community settings 36–38.
The PPA is a valid and reliable measure of falls risk in older people 18. Based on a participant’s performance, the PPA computes a composite falls risk score (standardized score) that has a 75% predictive accuracy for falls in older people. The composite PPA score is derived from discriminant function analysis using the data from large-scale studies 36–38. The discriminant function is made up of weighted scores of the five key components. These weightings (i.e., canonical correlation coefficients) are − 0.33 for edge contrast sensitivity, 0.20 for joint position sense, − 0.16 for isometric quadriceps strength, 0.47 for hand reaction time, and 0.51 for postural sway on foam rubber mat with eyes open. Composite PPA scores below 0 indicate a low risk of falling, scores between 0 and 1 indicate a mild risk of falling, scores between 1 and 2 indicate a moderate risk of falling and scores above 2 indicate a high risk of falling. Table 1 describes the tests from the short-form PPA assessment. The test-retest reliability (i.e., intraclass correlation coefficient) for each of the five key PPA components is 0.57 for postural sway, 0.69 for hand reaction time, 0.97 for knee extension strength, 0.50 for proprioception, and 0.81 for edge contrast sensitivity 18.
Table 1.
PPA Short-Form Assessment.
| PPA Task | Description | Measure |
|---|---|---|
| Postural Sway | Individuals stood as still as possible for 30 seconds on 15cm thick medium-density foam rubber mat with their eyes open, wearing the Lord swaymeter. Sway was recorded on a sheet of millimetre graph paper fastened to the top of an adjustable height table. | Total sway path (mm) was determined from the path traced. |
| Quadriceps Strength | A simple strain gauge assessed dominant quadriceps (isometric) strength to the nearest 0.5 kilogram. Participants were seated with the hip and the knee joint at 90 degrees of flexion. | The best of three trials (kg). |
| Hand Reaction Time | Used a light as the stimulus and depression of a switch by the finger as the response. | The average of 10 trials (ms). |
| Proprioception | Seated participants with eyes closed were asked to align the lower limbs on either side of a 60 by 60 cm by 1-cm-thick clear acrylic sheet standing on edge and inscribed with a protractor. | The difference (deg) in matching the great toes. |
| Edge Contrast Sensitivity | The Melbourne Edge Test was used. This test presents 20 circular patterns containing edges with reducing contrast. Correct identification of the orientation of the edge on the patches provides a measure of contrast sensitivity in decibel units (dB), where dB=−10log10 contrast. | Number of the last correctly identified circle (dB). |
Cognitive Performance of Executive Functions
This study focused on three central executive functions: 1) set shifting; 2) updating (i.e., working memory); and 3) response inhibition 39. While these three executive functions are moderately correlated with one another, they are clearly separable 39. These functions are often hypothesized to contribute to performance of complex “frontal” tasks. They are also highly specific and can be defined in a fairly precise manner 39. Set shifting requires one to go back and forth between multiple tasks or mental sets 39. Updating involves monitoring incoming information for relevance to the task at hand and then appropriately updating the informational content by replacing old, no longer relevant information with new incoming information. Response inhibition involves deliberately inhibiting dominant, automatic, or prepotent responses. Previous studies have demonstrated that poor set shifting and response inhibition are predictive of falls 17, 20.
Set Shifting
The Trail Making Test (Part B) was used to assess set shifting 40. This standardized test of set shifting consists of a page with encircled numbers and letters (the numbers extend from 1 to 13 and the letters from A to L). Participants were instructed to draw a line as quickly and as accurately as possible from 1 to A, A to 2, 2 to B, B to 3, and so on, until they completed the task. The amount of time (in seconds) it took to complete the task was recorded. Faster Trail Making Test (Part B) times are indicative of better set shifting.
Updating
The verbal digits backward test was used to assess updating 41. This test consists of seven pairs of random number sequences that the assessor reads aloud at the rate of one per second and the participant’s task is to repeat each sequence in an exactly reversed order. The sequence begins with three digits and increases by one at a time up to a length of nine digits. The test includes two sequences of each length and testing ceases when the participant fails to recollect any two with the same length. The score recorded, ranging from 0 to14, is the number of successful sequences. Higher scores indicate better updating.
Response Inhibition
The Stroop Colour-Word Test 42 was used to assess response inhibition. Lezak 43 has found that people who do poorly on this test have difficulty concentrating and warding off distractions. For the Stroop Colour-Word Test, participants were shown a page with Colour-Words printed in incongruent coloured inks (e.g., the word “blue” printed in red ink). Participants were asked to name the ink colour in which the words are printed (while ignoring the word itself). The time (in seconds) participants took to read 112 words and this measure was used for statistical analysis was recorded. Faster times indicate better response inhibition.
Data Analyses
Data were analyzed using SPSS Windows Version 15.0 (SPSS Inc., Chicago, IL). Descriptive data are reported for variables of interest. Comparisons of group characteristics were undertaken using a Chi Square test for differences in proportions and Students t-tests for differences in means. The level of association between the three executive functions, the composite PPA score, and the five PPA components were determined using the Pearson product moment coefficient of correlation. Alpha was set at P ≤ 0.05.
To minimize the overall probability of making a type I error, between-group differences in physiological falls risk profile and cognitive performance of executive functions were established using two separate multivariate analysis of variance analyses (MANOVA). For the physiological falls risk profile MANOVA, the composite PPA score and the five key PPA components were entered as the dependent variables and age as a covariate. For the cognitive performance of executive functions MANOVA, the Trail Making Test (Part B) completion time, the verbal digits backward test score, and the Stroop Colour-Word Test completion time were entered as the dependent variables and age and education as covariates. If the MANOVA demonstrated a significant group effect, then between-group differences on individual outcomes measures were then determined by analysis of variance (ANOVA). The overall alpha level was set at P ≤ 0.05.
RESULTS
The mean age of the entire cohort was 69.6 (SD = 3.0; Table 2). They had a mean number of two self-reported chronic conditions; arthritis and low bone mass were the two most common chronic conditions. Fifty-two (32.9%) participants reported one or more falls in the last 12 months.
Table 2.
Descriptive Statistics for Key Descriptors (N = 158).
| Variable * | MoCA Score ≥ 26 (n = 86) Mean (SD) |
MoCA Score < 26 (n = 72) Mean (SD) |
|---|---|---|
| Age (year) | 69.7 (2.8) | 69.5 (3.2) |
| Height (cm) | 161.8 (6.8) | 160.8 (9.1) |
| Mass (kg) | 72.0 (14.5) | 66.4 (5.4) ‡ |
| Body Mass Index (m/kg2) | 27.4 (4.9) | 25.8 (5.4) ‡ |
| MMSE Score (max. 30 pts) | 28.9 (1.2) | 28.3 (1.4) § |
| Montreal Cognitive Assessment Score (max. 30 pts) | 27.4 (1.5) | 22.6 (2.0) || |
| Geriatric Depression Scale (max. 15 pts) | 0.43 (1.4) | 0.72 (2.1) |
| Education: Less than Grade 9 † | 1 (1.2) | 3 (4.2) |
| Education: Grades 9 to 12 without Certificate or Diploma † | 4 (4.6) | 4 (5.6) |
| Education: High School Certificate or Diploma † | 9 (10.5) | 16 (22.2) |
| Education: Trades or Professional Certificate or Diploma † | 15 (17.4) | 14 (19.4) |
| Education: University Certificate or Diploma † | 17 (19.8) | 11 (15.3) |
| Education: University Degree † | 40 (46.5) | 24 (33.3) |
| Falls in the last 12 months † | 31 (36) | 21 (29) |
| Functional Comorbidity Index (max. 18 pts) | 2.3 (1.9) | 2.0 (1.4) |
| Physical Activity Scale for the Elderly | 122.8 (63.4) | 115.5 (52.3) |
| Timed Up and Go Test (s) | 6.7 (1.4) | 6.7 (1.4) |
MMSE = Mini-Mental State Examination.
Count (%). Count = number of “yes” cases within each group. % = percent of “yes” cases within each group.
Significantly different from MoCA > 26 at P < 0.05.
Significantly different from MoCA > 26 at P < 0.01.
Significantly different from MoCA > 26 at P < 0.001.
The mean MoCA score for the entire cohort was 25.2 (SD = 3), just below the recommended cut-off score of 26 for MCI 35. Seventy-two women scored below 26 on the MoCA. Table 2 reports descriptor variables for the two groups of women. Older women with MCI had significantly lower body mass than older women without MCI (P = 0.02); they also had lower body mass index (P = 0.05). While there were no significant between-group difference in the history of falls (P = 0.40), a greater proportion of older women without MCI had a history of falling than those with MCI (i.e., 36% versus 29%). However, the accuracy of recalling falls is limited 44. Also, it is very possible that older women with MoCA scores ≥ 26 can recall their falls better than those with MoCA scores < 26.
Correlation Coefficients
The composite PPA score was significantly associated with all three executive functions (P ≤ 0.05). The correlation coefficients between variables of interest are reported in Table 3.
Table 3.
Pearson product moment coefficient matrix between composite PPA score, PPA key components, Trail Making Test (Part B), Verbal Digit Span Backward Test, and Stroop Colour-Word Test (N = 158).
| Variable | Trail Making Test (Part B) (s) | Verbal Digit Span Backward Test (max. 14 pts) | Stroop Colour-Word Test (s) |
|---|---|---|---|
| Composite PPA Score | 0.37 ** | − 0.36 ** | 0.23 ** |
| Postural Sway(mm) | 0.15 | − 0.13 | 0.11 |
| Quadriceps Strength (kg) | − 0.14 | 0.08 | − 0.08 |
| Hand Reaction Time (s) | 0.19 * | − 0.12 | 0.28 ** |
| Proprioception (deg) | 0.08 | 0.13 | − 0.003 |
| Edge Contrast Sensitivity (dB) | − 0.08 | 0.06 | 0.04 |
P < 0.05
P < 0.01.
Physiological Falls Risk Profile
The data from the PPA are summarized in Table 4. Both groups had a mean composite PPA score between 0 and 1.0, indicating a mild risk of falling. There was an overall significant difference between the two groups on physiological falls risk profile (MANOVA, Hotelling’s Trace = 0.09, P = 0.04). Both the composite PPA score and postural sway performance (P ≤ 0.03) were significantly different between the two groups. Participants with MCI had significantly higher composite PPA scores (i.e., higher physiological risk of falling) and increased postural sway compared with women without MCI. Specifically, there was an 88% and a 21% difference, respectively, in composite PPA score and postural sway performance between participants. There were no significant differences between the two groups in any of the other four key PPA components (P ≥ 0.10).
Table 4.
Descriptive Statistics and ANOVA Results Related to the Composite PPA Score and the Five Key PPA Components. Mean Values Adjusted for Age ± Standard Error (SE).
| Variable * | MoCA Score ≥ 26 (n = 86) Mean (SE); 95% CI |
MoCA Score < 26 (n = 72) Mean (SE); 95% CI |
P value |
|---|---|---|---|
| Composite PPA Score | 0.06 (0.10); − 0.14 to 0.26 | 0.51 (0.11); 0.29 to 0.72 | < 0.01 |
| Postural Sway (mm) | 114.0 (9.1); 96.0 to 132.1 | 144.2 (9.9); 124.7 to 163.7 | 0.03 |
| Quadriceps Strength (kg) | 30.3 (0.8); 28.7 to 32.0 | 28.4 (0.9); 26.6 to 30.2 | 0.11 |
| Hand Reaction Time (ms) | 264.8 (5.9); 253.1 to 276.5 | 273.4 (6.4); 260.8 to 286.1 | 0.32 |
| Proprioception (deg) | 1.0 (1.0); 0.82 to 1.2 | 1.3 (1.1); 1.1 to 1.5 | 0.10 |
| Edge Contrast (dB) | 22.9 (0.5); 22.0 to 23.8 | 21.9 (0.5); 20.9 to 22.8 | 0.13 |
PPA = Physiological Profile Assessment.
Cognitive Performance of Executive Functions
The data from the three central executive functions tests are summarized in Table 5. There was an overall significant difference between the two groups on these tests (MANOVA, Hotelling’s Trace = 0.17, P = 0.001). Participants with MCI performed significantly worse on all three central executive functions tests (P ≤ 0.04).
Table 5.
Descriptive Statistics and ANOVA Results Related to the Executive Functions Tests. Mean Values Adjusted for Age ± Standard Error (SE).
| Variable * | MoCA Score ≥ 26 (n = 86) Mean (SE); 95% CI |
MoCA Score < 26 (n = 72) Mean (SE); 95% CI |
P value |
|---|---|---|---|
| Trail Making Test (Part B) (s) | 88.2 (3.9); 80.5 to 95.9 | 115.5 (4.3); 107.1 to 124.0 | < 0.001 |
| Verbal Digits Backward Test (max. 14 pts) | 5.1 (0.2); 4.6 to 5.6 | 3.8 (0.3); 3.3 to 4.4 | 0.001 |
| Stroop Colour-Word Test (s) | 91.5 (2.7); 86.2 to 96.9 | 101.8 (3.0); 95.9 to 107.6 | 0.01 |
High Trail Making Test (Part B) time values, low Digits Backward Test scores, and high Stroop Colour-Word Test time values indicate impaired performances.
DISCUSSION
Mild cognitive impairment is increasingly recognized as a clinical problem 9, and this study found that older women with MCI demonstrated greater number of falls risk factors than older women without MCI. Specifically, older women with MCI had significantly higher composite PPA scores, which was in part due to significantly increased postural sway. Impaired executive functions are associated with falls, and our participants with MCI also performed significantly lower in tests of three central executive functions 17, 20, 45, 46. To our knowledge, this is the first study that has comprehensively compared well-recognized falls risk factors – in both the physiological and cognitive domains – between older women with and without MCI.
The observation of increased postural sway in older women with MCI concurs with those of Franssen and coworkers 14 who found that after adjusting for age, those with MCI or mild AD had significantly reduced balance and limb coordination compared with cognitively-intact individuals. The present study extends these previous findings by demonstrating that older women with MCI have a significantly worse global physiological falls risk profile, as demonstrated by a valid and reliable tool 19, than those without MCI. It should be highlighted that impaired physiological function, such as impaired balance, for those with MCI has clinical significance beyond falls risk. For example, for people with MCI current evidence suggests that impaired physiological function is also related to increased risk of AD 15.
It is possible that those with MCI may have a greater impairment of physiological function than those without MCI because of frank structural and functional brain abnormalities. Imaging studies have demonstrated that white matter lesions, global brain atrophy, frontal lobe atrophy, and reduced cerebro-arterial blood-flow are associated with both impaired mobility and impaired balance 47–50. Also, Rosano and coworkers 51 recently demonstrated that reduced gray matter volumes in regions crucial for motor control are associated with slower gait and poorer balance, and the association appears to be independent of other diffuse brain abnormalities such as white matter lesions. Although the current study is not designed to explain why those with MCI may have increased sway, there is evidence that indices of general physiological integrity, such as the ability to balance, are “biomarkers” of brain structure and function 48.
In addition to their association with impaired physiological function, structural and functional brain abnormalities are also associated with impaired cognition, including executive functions 52. For example, both lower prefrontal gray matter volume and greater levels of white matter lesions are related to impaired set shifting 53. Also, functional changes, such as changes in activation, oxygen utilization, and glucose metabolism that disrupt the frontal-subcortical neuronal systems also compromise executive functions 54.
Impaired executive functions are associated with falls 17, 20, 45, 46 and injurious falls 20. It is tempting to speculate that structural and functional brain abnormalities may underlie at least part of the association between impaired executive functions and falls. Future brain imaging studies in older adults with MCI would help test the mechanism of this association.
This study also found a significant difference in body mass – those with MCI had significantly lower body mass. This concurs with recent evidence demonstrating that weight loss precedes the diagnosis of dementia in women by several years 55. Executive functions are essential to the older person’s ability to uptake and carry out health-promoting behaviours 54, such as medication management, dietary and lifestyle changes, self-monitoring of responses, and follow-up with health care professionals. Thus, older adults with MCI may have lower body mass at least in part due to their decreased ability to initiate and sustain health-promoting behaviours. Other reasons include pre-dementia apathy, loss of initiative, and reduced olfactory function 55. It should be highlighted that low body mass is a significant risk factor for injurious falls 56. Thus, older women with MCI may be at risk for injurious falls due to both impaired executive functioning and low body mass.
A clinical implication of our results is that falls risk screening and prevention should be a key component in the clinical management of older adults with MCI. Specifically, falls risk screening in this population should include standard neuropsychological tests of executive functioning and measures of postural sway. Furthermore, effective falls prevention strategies for those with MCI should not only target physiological function but also executive functions. Current evidence suggests that cardiovascular training benefits executive functions in older adults aged 55 years and older 57.
A limitation of our study is that our participants were categorized as with, or without, MCI based on the MoCA, a screening tool for MCI, rather than on the results of comprehensive neuropsychological testing accompanied by a clinical assessment. However, neuropsychological aspects of the classification of MCI are currently poorly defined 34. It should be highlighted that a MoCA score of 26 has a sensitivity of 90% for detecting MCI 35. Another limitation is that we compared key falls risk factors between older women with MCI and without MCI rather than the actual incidence of falls. Thus, future prospective studies using falls as the primary outcome measure is needed to confirm that older adults with MCI are indeed at greater risk for falls than those without MCI.
In summary, older women with MCI – but not dementia – have greater risk for falls than those without MCI. Our novel results suggest falls risk screening may be prudent in those with MCI. If falls prevention strategies 58, 59 prove effective among those with MCI, it would have enormous clinical importance. At present, falls cannot be prevented among those with dementia 60; identifying those at risk earlier in the process may be a valuable window of opportunity for intervention.
Acknowledgments
Vancouver Foundation, BCMSF (BCM06-0035) and the Michael Smith Foundation for Health Research Establishment Grant (CI-SCH-063(05-1)CLIN) supported this study.
Teresa Liu-Ambrose and Maureen C. Ashe are MSFHR Scholars.
Karim Khan is a Canadian Institutes of Health Research New Investigator.
Sponsor’s Role: None.
Footnotes
Author Contributions:
Teresa Liu-Ambrose, Maureen Ashe, and Karim Khan: Study concept and design, acquisition of data, analysis and interpretation of data, preparation of manuscript, and critical review of manuscript.
Peter Graf and Lynn Beattie: Study concept and design, analysis and interpretation of data, preparation of manuscript and critical review of manuscript.
References
- 1.Exton-Smith A. Functional consequences of ageing: Clinical manifestations. In: Exton-Smith AN, Grimley Evans J, editors. Care of the elderly: Meeting the challenge of dependency. London: Academic Press; 1977. [Google Scholar]
- 2.Grimely-Evans J. Fallers, non-fallers and Poisson. Age and Ageing. 1990;19:268–269. doi: 10.1093/ageing/19.4.268. [DOI] [PubMed] [Google Scholar]
- 3.Carter ND, Kannus P, Khan KM. Exercise in the prevention of falls in older people: a systematic literature review examining the rationale and the evidence. Sports Med. 2001;31:427–438. doi: 10.2165/00007256-200131060-00003. [DOI] [PubMed] [Google Scholar]
- 4.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. NEJM. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. W1 NE492. [DOI] [PubMed] [Google Scholar]
- 5.van Dijk PT, Meulenberg OG, van de Sande HJ, et al. Falls in dementia patients. Gerontologist. 1993;33:200–204. doi: 10.1093/geront/33.2.200. [DOI] [PubMed] [Google Scholar]
- 6.Buchner DM, Larson EB. Falls and fractures in patients with Alzheimer-type dementia. Jama. 1987;257:1492–1495. [PubMed] [Google Scholar]
- 7.Shaw FE. Falls in cognitive impairment and dementia. Clin Geriatr Med. 2002;18:159–173. doi: 10.1016/s0749-0690(02)00003-4. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 9.Feldman HH, Jacova C. Mild cognitive impairment. Am J Geriatr Psychiatry. 2005;13:645–655. doi: 10.1176/appi.ajgp.13.8.645. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 11.Busse A, Bischkopf J, Riedel-Heller SG, et al. Mild cognitive impairment: prevalence and incidence according to different diagnostic criteria. Results of the Leipzig Longitudinal Study of the Aged (LEILA75+) Br J Psychiatry. 2003;182:449–454. [PubMed] [Google Scholar]
- 12.The Canadian Study of Health and Aging: risk factors for Alzheimer’s disease in Canada. Neurology. 1994;44:2073–2080. doi: 10.1212/wnl.44.11.2073. [DOI] [PubMed] [Google Scholar]
- 13.Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793–1796. doi: 10.1016/S0140-6736(97)01007-6. W1 LA 242. [DOI] [PubMed] [Google Scholar]
- 14.Franssen EH, Souren LE, Torossian CL, et al. Equilibrium and limb coordination in mild cognitive impairment and mild Alzheimer’s disease. J Am Geriatr Soc. 1999;47:463–469. doi: 10.1111/j.1532-5415.1999.tb07240.x. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal NT, Wilson RS, Beck TL, et al. Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Arch Neurol. 2006;63:1763–1769. doi: 10.1001/archneur.63.12.1763. [DOI] [PubMed] [Google Scholar]
- 16.Wylie SA, Ridderinkhof KR, Eckerle MK, et al. Inefficient response inhibition in individuals with mild cognitive impairment. Neuropsychologia. 2007;45:1408–1419. doi: 10.1016/j.neuropsychologia.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Rapport LJ, Hanks RA, Millis SR, et al. Executive functioning and predictors of falls in the rehabilitation setting. Archives of Physical Medicine and Rehabilitation. 1998;79:629–633. doi: 10.1016/s0003-9993(98)90035-1. [DOI] [PubMed] [Google Scholar]
- 18.Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Phys Ther. 2003;83:237–252. [PubMed] [Google Scholar]
- 19.Lord S, Sherrington C, Menz H. Risk factors and strategies for prevention. Cambridge: Cambridge University Press; 2001. A physiological profile approach for falls prevention. Falls in older people; pp. 221–238. [Google Scholar]
- 20.Nevitt MC, Cummings SR, Hudes ES. Risk factors for injurious falls: a prospective study. J Gerontol. 1991;46:M164–170. doi: 10.1093/geronj/46.5.m164. [DOI] [PubMed] [Google Scholar]
- 21.Liu-Ambrose T, Ahamed Y, Graf P, et al. Older fallers with poor executive functioning overestimate their postural limits. Archives of Physical Medicine and Rehabilitation. 2008 doi: 10.1016/j.apmr.2007.11.052. In Press. [DOI] [PubMed] [Google Scholar]
- 22.Persad CC, Giordani B, Chen HC, et al. Neuropsychological predictors of complex obstacle avoidance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 1995;50:272–277. doi: 10.1093/geronb/50b.5.p272. [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Berger AK, Fratiglioni L, Winblad B, et al. Alzheimer’s disease and depression: preclinical comorbidity effects on cognitive functioning. Cortex. 2005;41:603–612. doi: 10.1016/s0010-9452(08)70200-4. [DOI] [PubMed] [Google Scholar]
- 25.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–711. [PubMed] [Google Scholar]
- 26.Groll DL, To T, Bombardier C, et al. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Abbott RD, White LR, Ross GW, et al. Walking and Dementia in Physically Capable Elderly Men. JAMA. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 28.Weuve J, Kang JH, Manson JE, et al. Physical Activity, Including Walking, and Cognitive Function in Older Women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 29.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 30.Washburn RA, McAuley E, Katula J, et al. The physical activity scale for the elderly (PASE): Evidence for validity. J Clin Epidemiol. 1999;52:643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 31.Washburn R, Smith K, Jette A, et al. Physical activity scale for the elderly: Administration and scoring instruction manual. Watertown, MA: New England Research Institute; 1991. [Google Scholar]
- 32.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 33.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80:896–903. [PubMed] [Google Scholar]
- 34.Portet F, Ousset PJ, Visser PJ, et al. Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer’s Disease. J Neurol Neurosurg Psychiatry. 2006;77:714–718. doi: 10.1136/jnnp.2005.085332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 36.Lord S, Clark R, Webster I. Physiological factors associated with falls in an elderly population. JAGS. 1991;39:1194–1200. doi: 10.1111/j.1532-5415.1991.tb03574.x. [DOI] [PubMed] [Google Scholar]
- 37.Lord S, Sambrook P, Gilbert C, et al. Postural stability, falls and fractures in the elderly: Results from the Dubbo Osteoporosis Epidemiology Study. The Medical Journal of Australia. 1994;160:684–691. [PubMed] [Google Scholar]
- 38.Lord S, Ward J, Williams P, et al. Physiological factors associated with falls in older community-dwelling women. JAGS. 1994;42:1110–1117. doi: 10.1111/j.1532-5415.1994.tb06218.x. [DOI] [PubMed] [Google Scholar]
- 39.Miyake A, Friedman NP, Emerson MJ, et al. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognit Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 40.Spreen O, Strauss E. A compendium of neurological tests. 2. New York: Oxford University Press, Inc; 1998. [Google Scholar]
- 41.Wechsler D. Wechsler Adult Intelligence Scale - Revised. The Psychological Corporation, Harcourt Brace Jovanovich; 1981. [Google Scholar]
- 42.Trenerry M, Crosson B, DeBoe J, et al. Stroop Neuropsychological Screening Test: Psychological Assessment Resources. 1988. [Google Scholar]
- 43.Lezak MD. Neuropsychological Assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- 44.Cummings S, Nevitt M, Kidd S. Forgetting falls. The limited accuracy of recall of falls in the elderly. JAGS. 1988;36:613–616. doi: 10.1111/j.1532-5415.1988.tb06155.x. [DOI] [PubMed] [Google Scholar]
- 45.Lord S, Fitzpatrick R. Choice stepping reaction time: A composite measure of fall risk in older people. Journal of Gerontology. 2001;10:M627–632. doi: 10.1093/gerona/56.10.m627. [DOI] [PubMed] [Google Scholar]
- 46.Lundin-Olsson L, Nyberg L, Gustafson Y. “Stops walking when talking” as a predictor of falls in elderly people. Lancet. 1997;349:617. doi: 10.1016/S0140-6736(97)24009-2. W1 LA 242. [DOI] [PubMed] [Google Scholar]
- 47.Starr JM, Leaper SA, Murray AD, et al. Brain white matter lesions detected by magnetic resosnance imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry. 2003;74:94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rabbitt PM, Scott M, Thacker N, et al. Balance marks cognitive changes in old age because it reflects global brain atrophy and cerebro-arterial blood-flow. Neuropsychologia. 2006;44:1978. doi: 10.1016/j.neuropsychologia.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 49.Onen F, Feugeas MC, Baron G, et al. Leukoaraiosis and mobility decline: a high resolution magnetic resonance imaging study in older people with mild cognitive impairment. Neurosci Lett. 2004;355:185–188. doi: 10.1016/j.neulet.2003.10.072. [DOI] [PubMed] [Google Scholar]
- 50.Whitman GT, Tang Y, Lin A, et al. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001;57:990–994. doi: 10.1212/wnl.57.6.990. [DOI] [PubMed] [Google Scholar]
- 51.Rosano C, Aizenstein HJ, Studenski S, et al. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62:1048–1055. doi: 10.1093/gerona/62.9.1048. [DOI] [PubMed] [Google Scholar]
- 52.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- 53.Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- 54.Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: is there a link? J Gerontol A Biol Sci Med Sci. 2004;59:818–826. doi: 10.1093/gerona/59.8.m818. [DOI] [PubMed] [Google Scholar]
- 55.Knopman DS, Edland SD, Cha RH, et al. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69:739–746. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 56.Tinetti ME, Doucette J, Claus E, et al. Risk factors for serious injury during falls by older persons in the community. J Am Geriatr Soc. 1995;43:1214–1221. doi: 10.1111/j.1532-5415.1995.tb07396.x. [DOI] [PubMed] [Google Scholar]
- 57.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 58.Campbell J, Robertson M, Gardner M, et al. Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. BMJ. 1997;315:1065–1069. doi: 10.1136/bmj.315.7115.1065. W1 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Close J, Ellis M, Hooper R, et al. Prevention of falls in the elderly trial (PROFET): a randomised controlled trial. Lancet. 1999;353:93–97. doi: 10.1016/S0140-6736(98)06119-4. W1 LA 242. [DOI] [PubMed] [Google Scholar]
- 60.Shaw FE, Bond J, Richardson DA, et al. Multifactorial intervention after a fall in older people with cognitive impairment and dementia presenting to the accident and emergency department: randomised controlled trial. BMJ. 2003;326:73. doi: 10.1136/bmj.326.7380.73. W1 274. [DOI] [PMC free article] [PubMed] [Google Scholar]