Abstract
Sixty treatment-seeking individuals with methamphetamine (MA) dependence entered a randomized, placebo-controlled, double-blind clinical trial of oral dextroamphetamine (d-AMP) as a replacement therapy for MA dependence. The subjects took 60 mg sustained-release d-AMP for 8 weeks, during which time they received eight 50-min sessions of individual psychotherapy. Adverse events and urine toxicology for MA were assessed two times a week. There were no serious adverse events. Urine samples containing <1,000 ng/ml of MA were classified as negative for MA. The MA-negative scores in the d-AMP group (3.1 ± SD 4.6) were no higher than those in the placebo group (3.3 ± SD 5.3; P > 0.05). However, withdrawal and craving scores were significantly lower in the d-AMP group (P < 0.05 for both). Although subjects taking d-AMP did not reduce their use of MA, the significant reductions observed in withdrawal and craving scores in this group support the need for further exploration of d-AMP as a pharmacologic intervention for MA dependence, possibly at higher doses.
Methamphetamine (MA) dependence is endemic worldwide and produces serious public health consequences.1–4 The annual economic impact of MA in the United States in terms of lost productivity, environmental damage, law enforcement costs, and health-care expenses, is estimated to be more than $20 billion.5
Persons addicted to MA have one of two distinct patterns of use. Some pursue a chronic, periodic pattern of self-administration throughout the day;6 others follow a “binge and crash” pattern, paying little heed to the need for food and sleep until they run out of the drug or are too exhausted to continue its use. Both patterns result in substantial accumulation of MA in the brain because of the comparatively short intervals between doses relative to the long half-life of the drug. A withdrawal syndrome, often referred to as the “crash,” has been demonstrated; this consists of strong craving, electroencephalography abnormalities, depression, alterations in sleep patterns, hypersomnolence, and hyperphagia.7,8 The clinical features of chronic MA use are associated with neurotransmitter depletion. These signs and symptoms include depression, fatigue, poor concentration, loss of self-esteem, decreased libido, mild Parkinsonian features (myoclonus, tremor, bradykinesis), and insomnia.
Behavioral interventions have limited effectiveness, and patients treated for MA dependence have a high rate of relapse.9 Although several medications have been tested, none has been proven to significantly reduce MA use in MA-dependent patients seeking treatment.10–12 A promising approach that has not been thoroughly assessed is substitution therapy. Substitution therapies are well established in other addictions: methadone and buprenorphine reduce the use of heroin,13 and nicotine replacement therapy doubles smoking cessation rates.14
The exact mechanism of efficacy for substitution therapies is not yet understood, although several possibilities have been suggested. Such medications attenuate withdrawal symptoms,15–17 potentially promoting engagement and retention in behavioral and psychosocial treatment; however, research findings to date have been mixed.18,19 These substitute medications are cross-tolerant with the respective abused substances that they replace and similarly discriminated, but they typically have slower onsets of action and longer half-lives and are less reinforcing. The relatively stable pharmacokinetic profiles of the replacement medications provide more constant drug levels as compared with the erratic concentrations characteristic of illicit drugs, possibly attenuating the subjective experiences of both intoxication and withdrawal.
No substitution therapy has been established for MA dependence.20 Dextroamphetamine (d-AMP) is one of several agonist-like replacement therapies that have shown promise in preclinical studies as well as in laboratory studies in humans.21 Oral d-AMP has been prescribed for thousands of MA users in England and Wales22 and has been associated with decreases in intravenous (IV) MA use and criminal activity19 and with increased treatment engagement.23–25 Prior to the commencement of this study, there had been no placebo-controlled randomized trial of d-AMP for treatment of MA dependence.
The goal of our study was to determine the safety and efficacy of 60 mg sustained-release (SR) d-AMP in treating MA dependence. We selected the dosage of 60 mg per day on the basis of the available research to date. In a retrospective study of MA-dependent adults in treatment, an average of 43 mg of oral d-AMP was found to be as effective in reducing IV use of MA as methadone had proved to be in reducing IV heroin use.26 MA-experienced subjects who received a daily dose of 40 mg immediate-release d-AMP experienced improved treatment retention, reduced MA use, and less use of injection as a route of MA administration.23 Previous studies also found that the use of d-AMP decreased problematic behaviors associated with drug use,19 including IV administration of the drug,26 thereby suggesting that d-AMP is an effective intervention in those with more severe dependence, who tend to use the IV route.
RESULTS
Clinical characteristics
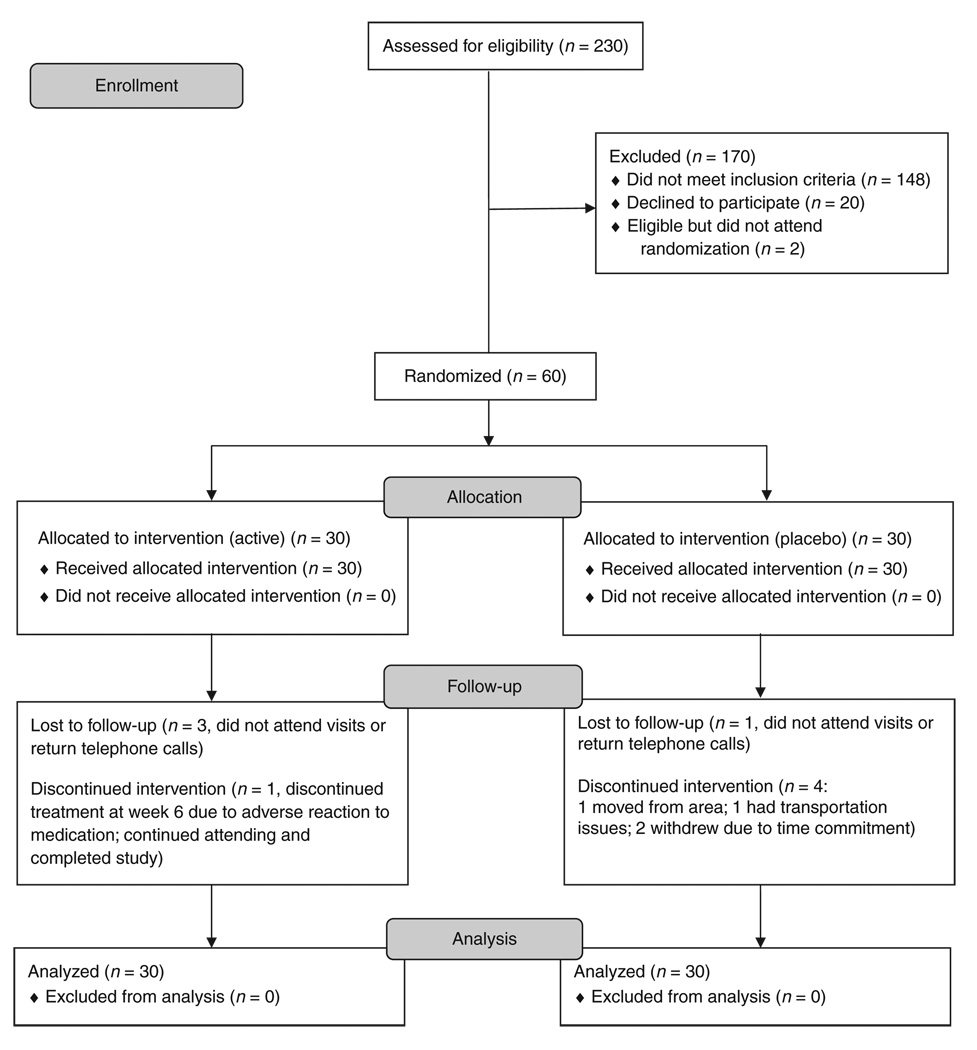
Of 230 subjects screened, 60 were randomized to either a placebo group (n = 30) or a d-AMP group (n = 30; see Figure 1 for study enrollment flow). The placebo and d-AMP groups did not differ significantly with respect to race, average age, or days of use of MA in the 30 days preceding screening. The majority of the subjects were Caucasian and male, with similar average ages (see Table 1 for participant demographics). At enrollment, subjects in the placebo group reported using MA a mean of 15.3 ± 8.5 days out of the prior 30, and d-AMP group subjects reported 18.9 ± 9.7 days of use. The quantity of MA used per day in the 30 days prior to enrollment by subjects in the placebo group was 319 ± 353 mg; those in the d-AMP group used 307 ± 283 mg.
Figure 1.
Subject flow diagram.
Table 1.
Clinical characteristics of the subjects
| Placebo | Active | P value | |
|---|---|---|---|
| n | 30 | 30 | |
| Age (years), mean (SD) | 37.5 (7.2) | 37.0 (7.2) | 0.75 |
| Male, n (%) | 19 (63%) | 15 (50%) | 0.43 |
| Caucasian, n (%) | 21 (70%) | 20 (67%) | 1 |
| Smoking as primary route of administration, n (%) | 20 (67%) | 24 (80%) | 0.38 |
| AWQ withdrawal score (0–40), mean (SD) | 14.9 (6.9) | 14.6 (8.0) | 0.72 |
| ADHD diagnosis, n (%) | 4 (13%) | 5 (17%) | 1 |
ADHD, attention deficit hyperactivity disorder; AWQ, Amphetamine Withdrawal Questionnaire.
Safety of d-AMP
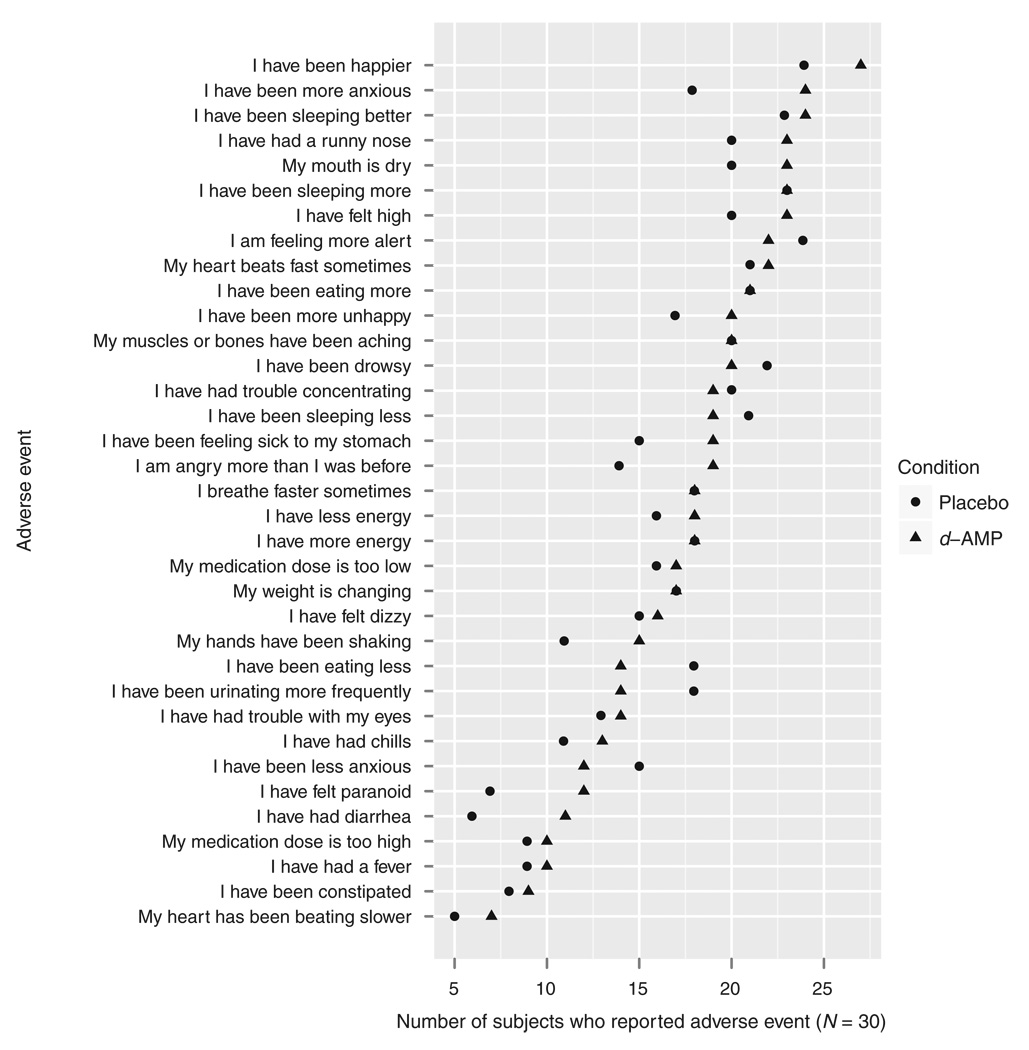
No serious adverse events occurred during the trial. No adverse events were more likely to be reported by subjects in the d-AMP group than by subjects in the placebo group (Figure 2). Dosing condition also did not significantly affect heart rate (F1,57 = 0.008, P = 0.929) or blood pressure (systolic: F1,57 = 0.709, P = 0.403; diastolic: F1,57 = 1.1488, P = 0.288). The number of subjects with clinically significant abnormalities in electrocardiography results was similar in the two groups: one in the d-AMP group and two in the placebo group.
Figure 2.
Adverse events by group. d-AMP, dextroamphetamine.
MA use
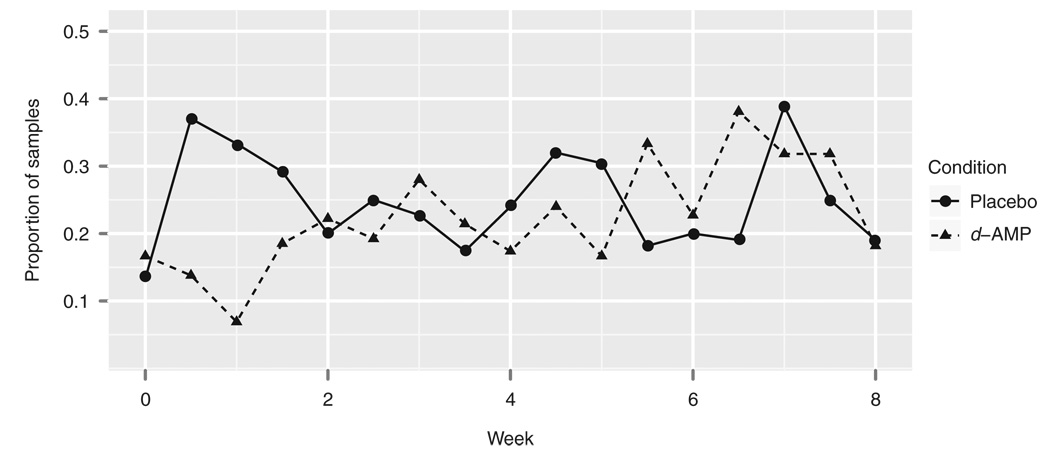
No differences were found between the placebo and d-AMP groups with respect to measures of MA use. The number of self-reported MA-abstinent days did not differ significantly by group. Of a possible 56 days of abstinence, the placebo group reported 27.5 ± 16.8 days of abstinence, whereas the d-AMP group reported 27.2 ± 17.3 days (Mann–Whitney U-test: W = 464, P = 0.842). Similarly, the number of MA-negative urine test results did not differ significantly by group (Mann–Whitney U-test: W = 441, P = 0.894). Of a possible 16 urine test results, the placebo group had 3.2 ± 5.0 MA-negative results, and the d-AMP group had 2.9 ± 4.3 MA-negative results (see Figure 3). Of 16 possible instances of no new use, the placebo group had 2.9 ± 4.9 instances, whereas the d-AMP group had 2.6 ± 4.2.
Figure 3.
Proportions of methamphetamine-negative urine samples by time and group. d-AMP, dextroamphetamine.
Withdrawal and craving
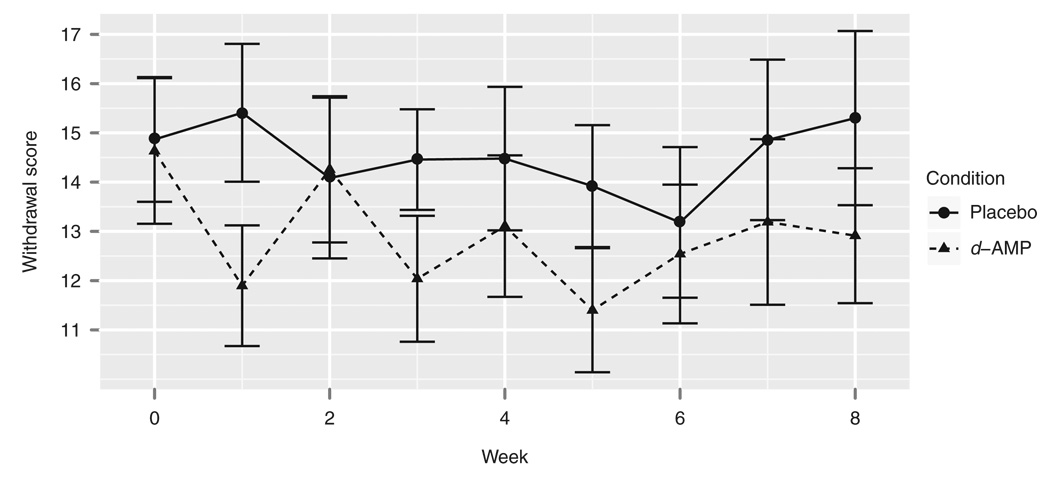
d-AMP reduced MA withdrawal symptoms as measured by the Amphetamine Withdrawal Questionnaire (AWQ; Figure 4). In a linear model in which the group and time predicted AWQ score, there was a main effect of group (F1,431 = 5.741, P = 0.018). This model indicated significantly attenuated withdrawal symptoms in the d-AMP group (t = −2.421, P = 0.016). d-AMP also reduced subjects’ craving as measured by visual analog scale (Figure 5). In a linear model in which group and time predicted MA craving, there were main effects of group (F1,408 = 5.116, P = 0.024) as well as of time (F8,408 = 3.110, P = 0.002). No significant interaction was found between group and time. This model indicated significantly lower craving scores in the d-AMP group (t = −2.302, P = 0.022).
Figure 4.
Amphetamine Withdrawal Questionnaire scores by time and group. d-AMP, dextroamphetamine.
Figure 5.
Methamphetamine craving visual analog scale scores by time and group. d-AMP, dextroamphetamine.
Medication adherence and attendance at psychosocial treatment sessions
There was no significant effect of group on medication adherence or attendance at psychosocial treatment sessions. The percentage of dispensed medication taken did not significantly differ between the groups. Placebo subjects took 73.6% ± 15.2% and d-AMP subjects took 74.2% ± 12.2% of the dispensed medication (Mann–Whitney U-test: W = 457, P = 0.923). The number of psychosocial treatment sessions attended was also not affected by group. Of a possible nine sessions, placebo group subjects attended 5.1 ± 3.0, and d-AMP group subjects attended 5.6 ± 3.0 sessions (Mann–Whitney U-test: W = 408, P = 0.535). At the first visit after day 1, all the subjects were asked which group they thought they were randomized to; 67% of the subjects in each group believed that they had been randomized to d-AMP.
DISCUSSION
The goal of our study was to determine the safety and efficacy of 60 mg SR d-AMP as substitution therapy for MA dependence. A variety of preclinical and clinical data indicate that this may be a useful approach for the treatment of MA dependence and could also serve to guide dose selection. Although we found that the overall effect of d-AMP on MA use was not significant, the results show that 30 mg d-AMP taken twice daily is safe for MA users. The main finding of the study is that d-AMP reduces withdrawal symptoms and craving for MA. Although reductions in withdrawal and craving scores do not by themselves confirm the efficacy of d-AMP as a substitution therapy, the findings in these domains do support the need for further exploration of d-AMP as a treatment for MA dependence. Both withdrawal symptoms and craving are postulated to increase relapse rates among MA users.27–30 Therefore, these findings suggest that one avenue for further consideration of d-AMP’s efficacy is among more dependent, long-term MA users, who have been shown to experience more severe withdrawal symptoms.31
We limited our dose to the maximum dose of SR d-AMP approved for use in the United States. A recent randomized placebo-controlled trial of d-AMP for MA dependence in Australia32 used a design in which subjects could be titrated to a maximum dose of 110 mg/day. Significant improvements in treatment retention and reduction in MA dependence severity scores were reported. As compared with placebo, d-AMP significantly attenuated withdrawal symptoms, and a trend was noted for decrease in MA use. Severity of MA dependence was significantly reduced in the d-AMP group.
The primary limitation of this study was the moderate size of the sample. Although it may be argued that an effect that is detected in a study with only 30 subjects per group is not clinically significant, even a small effect could be useful in guiding medication development efforts in a condition such as this, for which there is no effective pharmacotherapy. In addition, given the time and expense required to conduct addiction treatment trials, developing more sensitive outcome measures of drug use should be a priority.33
There are several indicators of tolerance to d-AMP in our study sample. In a previous study, in which low doses of immediate-release d-AMP (0.035–16 mg) were given to amphetamine-naive subjects, the drug produced stimulant-like effects, including subjective ratings of “stimulated” and “like drug,” and increased diastolic and systolic blood pressure.34,35 In our study, d-AMP administration had no significant effect on heart rate or blood pressure. Our subjects reported using >300 mg/day of MA, substantially more than the average daily amount of ≥50 mg reported in another study of MA-dependent adults.36 Although we could not measure the purity of the MA used by our subjects, the absence of any effect on heart rate and blood pressure after the d-AMP dose, considered alongside self-reports of high doses of MA used, suggests that this population may have significant tolerance to amphetamines. The absence of any significant difference between the two groups with respect to adherence to the regimen implies that the oral d-AMP dispensed to the treatment group had not been diverted elsewhere; this also suggests tolerance to d-AMP. Our data are consistent with findings to date suggesting that, if d-AMP is to be effective for the treatment of MA dependence in highly amphetamine-tolerant individuals, it would have to be administered at higher doses.37,38
METHODS
Study design
This was a placebo-controlled, double-blind, randomized clinical trial. Subjects received either 60 mg d-AMP SR or placebo daily for 8 weeks. This was given as a single dose on the first day and as two equally divided doses on subsequent days. Urn randomization was used to assign subjects to study groups so as to balance the groups with respect to baseline variables.39 Factors controlled for were current attention deficit hyperactivity disorder, AWQ (score <18 vs. ≥19), and days of MA use in the 30 days prior to randomization (<12 days vs. ≥13 days). For the latter two variables, median splits were determined on the basis of our previous work with MA. The trial was conducted at a single site, the Addiction & Pharmacology Research Laboratory in San Francisco, CA. The protocol was approved by the institutional review board of California Pacific Medical Center Research Institute and was registered at clinicaltrials.gov (identifier: NCT00630682).
Subjects
The study criteria required that subjects be between the ages of 18 and 50 years, meet Diagnostic and Statistical Manual of Mental Disorders IV–Text Revision criteria for MA dependence, be seeking treatment for MA dependence, and provide at least one MA-positive urine sample during screening. Exclusion criteria included serious medical illness, pregnancy, use of any investigational medication in the previous 30 days, a court mandate to participate in drug abuse treatment, and attendance in a drug or alcohol dependence treatment program within the 30 days prior to screening. Subjects were also excluded for the following diagnoses as determined by the Diagnostic Interview Schedule: current alcohol, opiate, or sedative-hypnotic dependence disorder requiring medical detoxification; presence of severe post-traumatic stress disorder, mania, or hypomania within the past 90 days; and lifetime history of schizophrenia, schizophreniform, or schizoaffective disorder.
Study medication
d-AMP is a US Food and Drug Administration–approved stimulant indicated for the treatment of attention deficit hyperactivity disorder and narcolepsy. It is not detected as MA in urine or other specimens and is available commercially in sustained-release form. Peak blood levels are reached 8–10 h after the dose is taken.40
We used d-AMP SR capsules manufactured by Barr Laboratories, Pomona, NY. The Drug Prod Services Laboratory at University of California, San Francisco, prepared both the active and the placebo capsules. d-AMP capsules for the study were made by repackaging 15-mg SR capsules purchased from Barr Laboratories into 30-mg capsules. The latter were identical in appearance to the placebo capsules, which were filled with lactose.
Psychosocial treatment
Subjects received 50-min, manual-based, individual motivational enhancement therapy sessions once a week for 9 weeks. This intensive therapy41 is a modification of Miller’s three-session treatment for alcohol-dependent subjects.42
MA urinalysis
Urine samples were collected two times a week and assayed for MA qualitatively on site with immunoassay devices (Redwood Biotech, Santa Rosa, CA); the cutoff value was 1,000 ng/ml. Urine samples that tested positive were then sent to Quest Diagnostics (Nichols Institute, San Juan Capistrano, CA) for quantification of MA levels using gas chromatography/mass spectrometry. The subjects were asked at each visit to report the days of MA use since the previous study visit.
Although the presence of MA in urine indicates use at some time, it may be due to carryover from the preceding sample. Using known pharmacokinetic properties of benzoylecgonine, Preston et al.43 developed a set of rules to identify urine samples that indicate new use of cocaine. On the basis of pharmacokinetic data for MA, we have generated the following rules to determine whether a urine sample indicates new use of MA. New use is indicated if the sample meets any of the following criteria:
Rule 1: In the first sample, MA is detected.
Rule 2: Following a sample with no MA, MA is detected in a subsequent sample.
Rule 3: Following a sample with MA detected, MA is detected in a subsequent sample in greater quantities than would be expected on the basis of mean half-life plus two standard deviations.44
Rule 4: Subject reports new use of MA since the previous sample.
For each of the three measures of use (urinalysis, self-report, and new-use criteria), we analyzed a number of statistics: number of abstinence events, time to first use, longest consecutive period of abstinence, and probability of use. For brevity, we report only the number of abstinence events.
Secondary outcome assessments
The subjects made two visits a week to the center for 8 weeks after randomization. At each visit, we collected samples for urinalysis and data on self-reported MA use. For the self-report data, a timeline follow-back instrument45 was used to record the number of days in which any MA use occurred and the quantity of MA used each day. Subjective assessments included a 100 mm visual analog craving scale and the AWQ.46 The Desires for Speed Questionnaire is a brief, modified version of the Desires for Alcohol Questionnaire. The AWQ is a brief, reliable, and valid 10-item questionnaire for the evaluation of acute signs and symptoms of MA withdrawal in newly abstinent MA users,47 including hypersomnia, MA craving, dysphoria, lack of energy, and increase in appetite. Items also tap three aspects of amphetamine withdrawal, namely, hyperarousal, reversed vegetative, and anxiety. 48 A checklist similar to the ones used in other trials of US Food and Drug Administration–approved medications21,49 was used to assess the prevalence of 34 potential adverse events, selected a priori on the basis of likelihood of occurrence and severity. Once every week, the subjects returned their medication bottles and were given new ones. Unused capsules were counted and then disposed of. A medication accountability form was also used at the weekly visit to document the dates on which medications were dispensed and returned. This form also documented the subject’s self-report about the medication dispensed, i.e., how much of the medication was ingested, stolen, lost, or returned.
Statistics
For overall study measures (e.g., total number of MA-negative urine samples, total number of therapy sessions attended), we used Mann–Whitney U-tests to determine whether there was any difference in the numbers of these events by group (medication and placebo). To determine whether dosing condition affected time-dependent measures, we made linear mixed-effects models in which baseline-corrected scores were predicted by group and time. For outcomes in which both group and time were significant predictors, we compared the conditions at individual time points using post hoc z-tests. For models in which only the dosing condition had a significant effect, we constructed new models predicting peak changes by group and used post hoc z-tests to compare the individual dosing conditions.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health (NIH) grant P50 DA018179 to J.M. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1.Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health. [12 January 2009];2006 < http://www.oas.samhsa.gov/methTabs.htm>. [PubMed]
- 2.Leamon MH, Flower K, Salo RE, Nordahl TE, Kranzler HR, Galloway GP. Methamphetamine and paranoia: the methamphetamine experience questionnaire. Am. J. Addict. 2010;19:155–168. doi: 10.1111/j.1521-0391.2009.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johanson CE, et al. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (Berl.) 2006;185:327–338. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- 4.Salo R, et al. Cognitive control and white matter callosal microstructure in methamphetamine-dependent subjects: a diffusion tensor imaging study. Biol. Psychiatry. 2009;65:122–128. doi: 10.1016/j.biopsych.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicosia N, et al. The Economic Cost of Methamphetamine Use in the United States. Santa Monica, CA: RAND Corp.; 2005. 2005. [Google Scholar]
- 6.Cho AK, Melega WP. Patterns of methamphetamine abuse and their consequences. J. Addict. Dis. 2002;21:21–34. doi: 10.1300/j069v21n01_03. [DOI] [PubMed] [Google Scholar]
- 7.Schildkraut JJ, Watson R, Draskoczy PR. Amphetamine withdrawal: depression and M.H.P.G. excretion. Lancet. 1971;2:485–486. doi: 10.1016/s0140-6736(71)92645-6. [DOI] [PubMed] [Google Scholar]
- 8.Gillin JC, Pulvirenti L, Withers N, Golshan S, Koob G. The effects of lisuride on mood and sleep during acute withdrawal in stimulant abusers: a preliminary report. Biol. Psychiatry. 1994;35:843–849. doi: 10.1016/0006-3223(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 9.Rawson RA, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99:708–717. doi: 10.1111/j.1360-0443.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- 10.Elkashef AM, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33:1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- 11.Heinzerling KG, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109:20–29. doi: 10.1016/j.drugalcdep.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shoptaw S, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96:222–232. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosten TR. Current pharmacotherapies for opioid dependence. Psychopharmacol. Bull. 1990;26:69–74. [PubMed] [Google Scholar]
- 14.Hurt RD, Lauger GG, Offord KP, Kottke TE, Dale LC. Nicotine-replacement therapy with use of a transdermal nicotine patch–a randomized double-blind placebo-controlled trial. Mayo Clin. Proc. 1990;65:1529–1537. doi: 10.1016/s0025-6196(12)62186-7. [DOI] [PubMed] [Google Scholar]
- 15.Guthrie SK. Pharmacologic interventions for the treatment of opioid dependence and withdrawal. DICP. 1990;24:721–734. doi: 10.1177/106002809002400716. [DOI] [PubMed] [Google Scholar]
- 16.Gowing L, Ali R, White JM. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst. Rev. 2009;3 doi: 10.1002/14651858.CD002025.pub4. CD002025. [DOI] [PubMed] [Google Scholar]
- 17.Nunn-Thompson CL, Simon PA. Pharmacotherapy for smoking cessation. Clin. Pharm. 1989;8:710–720. [PubMed] [Google Scholar]
- 18.Shearer J, et al. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009;104:224–233. doi: 10.1111/j.1360-0443.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 19.White R. Dexamphetamine substitution in the treatment of amphetamine abuse: an initial investigation. Addiction. 2000;95:229–238. doi: 10.1046/j.1360-0443.2000.9522299.x. [DOI] [PubMed] [Google Scholar]
- 20.Herin DV, Rush CR, Grabowski J. Agonist-like pharmacotherapy for stimulant dependence: preclinical, human laboratory, and clinical studies. Ann. N. Y. Acad. Sci. 2010;1187:76–100. doi: 10.1111/j.1749-6632.2009.05145.x. [DOI] [PubMed] [Google Scholar]
- 21.Grabowski J, et al. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- 22.Strang J, Sheridan J. Prescribing amphetamines to drug misusers: data from the 1995 national survey of community pharmacies in England and Wales. Addiction. 1997;92:833–838. [PubMed] [Google Scholar]
- 23.McBride AJ, Sullivan G, Blewetp AE, Morgan S. Amphetamine prescribing as a harm reduction measure: a preliminary study. Addict. Res. Theory. 1997;5:95–112. [Google Scholar]
- 24.Klee H, et al. The role of substitute therapy in the treatment of problem amphetamine use. Drug Alcohol Rev. 2001;20:417–429. [Google Scholar]
- 25.Shearer J, et al. Pilot randomized controlled study of dexamphetamine substitution for amphetamine dependence. Addiction. 2001;96:1289–1296. doi: 10.1046/j.1360-0443.2001.96912898.x. [DOI] [PubMed] [Google Scholar]
- 26.Charnaud B, Griffiths V. Levels of intravenous drug misuse among clients prescribed oral dexamphetamine or oral methadone: a comparison. Drug Alcohol Depend. 1998;52:79–84. doi: 10.1016/s0376-8716(98)00052-0. [DOI] [PubMed] [Google Scholar]
- 27.Stalcup SA, Christian D, Stalcup J, Brown M, Galloway GP. A treatment model for craving identification and management. J. Psychoactive Drugs. 2006;38:189–202. doi: 10.1080/02791072.2006.10399843. [DOI] [PubMed] [Google Scholar]
- 28.Galloway GP, Singleton EG. The Methamphetamine Treatment Project. How long does craving predict use of methamphetamine? Assessment of use one to seven weeks after the assessment of craving. Subst. Abuse. 2009;1:63–79. [PMC free article] [PubMed] [Google Scholar]
- 29.Hartz DT, Frederick-Osborne SL, Galloway GP. Craving predicts use during treatment for methamphetamine dependence: a prospective, repeated-measures, within-subject analysis. Drug Alcohol Depend. 2001;63:269–276. doi: 10.1016/s0376-8716(00)00217-9. [DOI] [PubMed] [Google Scholar]
- 30.Galloway GP, Singleton EG, Buscemi R, Baggott MJ, Dickerhoof RM, Mendelson JE. Methamphetamine Treatment Project. An examination of drug craving over time in abstinent methamphetamine users. Am. J. Addict. 2010;19:510–514. doi: 10.1111/j.1521-0391.2010.00082.x. [DOI] [PubMed] [Google Scholar]
- 31.McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100:1320–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- 32.Longo M, Wickes W, Smout M, Harrison S, Cahill S, White JM. Randomized controlled trial of dexamphetamine maintenance for the treatment of methamphetamine dependence. Addiction. 2010;105:146–154. doi: 10.1111/j.1360-0443.2009.02717.x. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Lopez JC, Galloway GP, Baggott MJ, Everhart T, Mendelson J. Estimating the intake of abused methamphetamines using experimenter-administered deuterium labeled R-methamphetamine: selection of the R-methamphetamine dose. Ther. Drug Monit. 2010;32:504–507. doi: 10.1097/FTD.0b013e3181db82f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoops WW, Lile JA, Robbins CG, Martin CA, Rush CR, Kelly TH. The reinforcing, subject-rated, performance, and cardiovascular effects of d-amphetamine: influence of sensation-seeking status. Addict. Behav. 2007;32:1177–1188. doi: 10.1016/j.addbeh.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makris AP, Rush CR, Frederich RC, Taylor AC, Kelly TH. Behavioral and subjective effects of d-amphetamine and modafinil in healthy adults. Exp. Clin. Psychopharmacol. 2007;15:123–133. doi: 10.1037/1064-1297.15.2.123. [DOI] [PubMed] [Google Scholar]
- 36.Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- 37.Sherman JP. Dexamphetamine for “speed” addiction. Med. J. Aust. 1990;153:306. doi: 10.5694/j.1326-5377.1990.tb136930.x. [DOI] [PubMed] [Google Scholar]
- 38.Carnwath T, Garvey T, Holland M. The prescription of dexamphetamine to patients with schizophrenia and amphetamine dependence. J. Psychopharmacol. (Oxford) 2002;16:373–377. doi: 10.1177/026988110201600414. [DOI] [PubMed] [Google Scholar]
- 39.Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J. Stud. Alcohol. Suppl. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- 40.Dexedrine® Spansule® Capsules, in Physicians’ Desk Reference. Montvale, NJ: Medical Economics Data Production Company; 1998. SmithKline Beecham Pharmaceuticals; pp. 2815–2816. [Google Scholar]
- 41.Galloway GP, Polcin D, Kielstein A, Brown M, Mendelson J. A nine session manual of motivational enhancement therapy for methamphetamine dependence: adherence and efficacy. J. Psychoactive Drugs Suppl. 2007;4:393–400. doi: 10.1080/02791072.2007.10399900. [DOI] [PubMed] [Google Scholar]
- 42.Miller WR, Zweben A, DiClemente CC, Rychtarik RG. vol. 2. Washington DC: US Government Printing Office; 1992. Motivational Enhancement Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals with Alcohol Abuse and Dependence, in NIAAA Project MATCH Monograph. DHHS Publication (ADM) 92-1894. [Google Scholar]
- 43.Preston KL, Silverman K, Schuster CR, Cone EJ. Assessment of cocaine use with quantitative urinalysis and estimation of new uses. Addiction. 1997;92:717–727. [PubMed] [Google Scholar]
- 44.Mendelson J, et al. Human pharmacology of the methamphetamine stereoisomers. Clin. Pharmacol. Ther. 2006;80:403–420. doi: 10.1016/j.clpt.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 45.Gerstein DR, Johnson RA, Harwood HJ, Fountain D, Sutter N, Malloy K. Evaluating Recovery Services: The California Drug and Alcohol Treatment Assessment (contract no. 92-001100) Sacramento, CA: California Department of Alcohol and Drug Programs; 1994. [Google Scholar]
- 46.Srisurapanont M, Jarusuraisin N, Jittiwutikan J. Amphetamine withdrawal: I. reliability, validity and factor structure of a measure. Aust. N.Z. J. Psychiatry. 1999;33:89–93. doi: 10.1046/j.1440-1614.1999.00517.x. [DOI] [PubMed] [Google Scholar]
- 47.McGregor C, et al. Mapping the nature, time-course and severity of methamphetamine withdrawal. Annual Meeting of the College on Problems of Drug Dependence; June 2003; Miami, FL. [Google Scholar]
- 48.Srisurapanont M, Jarusuraisin N, Jittiwutikan J. Amphetamine withdrawal: II. a placebo-controlled, randomised, double-blind study of amineptine treatment. Aust. N.Z. J. Psychiatry. 1999;33:94–98. doi: 10.1046/j.1440-1614.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 49.Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2009;101:34–41. doi: 10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]