Highlights
* Neural correlates of episodic memory development was examined using fMRI. * Relational memory was examined with a pictorial paired associates task. * Brain activations during encoding and recall were examined in children ages 8–13 years. * Age-related differences in prefrontal and parietal cortex were found.
Keywords: Neurodevelopment, Episodic memory development, Relational memory, Children, Event-related fMRI
Abstract
Despite vast knowledge on the behavioral processes mediating the development of episodic memory, little is known about the neural mechanisms underlying these changes. We used event-related fMRI to examine the neural correlates of both encoding and recall processes during an episodic memory task in two different groups of school age children (8–9 and 12–13 years). The memory task was composed of an encoding phase in which children were presented with a series of unrelated pictorial pairs, and a retrieval phase during which one of these items acted as a cue to prompt recall of the paired item. Age-related differences in activations were observed for both encoding and recall. Younger children recruited additional regions in the right dorsolateral prefrontal and right temporal cortex compared to older children during successful encoding of the pairs. During successful recall, older children recruited additional regions in the left ventrolateral prefrontal and left inferior parietal cortex compared to younger children. The results suggest that the prefrontal cortex contributes to not only the formation of memories but also access to them, and this contribution changes with development. The protracted development of the prefrontal cortex has implications for our understanding of the development of episodic memory.
1. Introduction
Behavioral studies of memory in children have revealed that episodic memory (i.e., conscious memory for personally experienced events within a particular spatial–temporal context, Tulving, 1985) continues to develop into young adulthood, and is mediated by various behavioral factors (Schneider and Pressley, 1997). However, little is known about the neural systems mediating episodic memory development. The advent of non-invasive neuroimaging has enabled researchers to investigate memory processes in the healthy brain, resulting in extensive research in adult populations. Remarkably, there is a dearth of studies on the typical development of neural systems associated with episodic memory. In the present study, we addressed this gap in the literature by using fMRI to investigate age-related differences in the neural basis of encoding and recall in middle childhood.
Long-term episodic memory processes include encoding, storage (and consolidation) and retrieval. Researchers have debated which of these processes can account for age-related variability to a greater extent. That is, is poor memory performance due to encoding failure or retrieval failure and does this change across development? Bauer, 2005, Bauer, 2008 has argued that, with age, susceptibility to storage failure decreases, and “the locus of maximum age-related variability in memory traces shifts from encoding, to consolidation, to storage and then to retrieval.” There is evidence that encoding processes cannot explain all of the age-related variance because even with encoding controlled, age-related differences remain (Howe and O'Sullivan, 1997). Thus, to better understand the sources of age-related changes, it will be important to examine the neural correlates of memory during both the encoding and retrieval stages.
The medial temporal lobes (MTL) and prefrontal cortex (PFC) have consistently been identified as playing a critical role in episodic memory function through animal and human lesion studies (e.g., Gershberg and Shimamura, 1995) and neuroimaging studies in adults (Blumenfeld and Ranganath, 2007, Staresina and Davachi, 2006, but see Henson, 2005, for a list of studies with null findings in the MTL region). Current models suggest that certain PFC regions, specifically, ventrolateral (VLPFC) and dorsolateral (DLPFC) regions provide top-down influence on MTL (which binds contextual elements together) through organization and elaboration processes during encoding, and strategic search and monitoring processes during retrieval (Simons and Spiers, 2003).
Several studies have documented that both the MTL and the PFC undergo structural development through adolescence. Temporal lobe gray matter reaches its maximum thickness at around 16 years of age (Giedd, 2004). The volume of hippocampus, a medial temporal lobe structure, increases with development (Durston et al., 2001, Giedd et al., 1996), and the volume gains are specific to the posterior hippocampus (Gogtay et al., 2006). The prefrontal cortex does not reach adult levels of structure or function until late adolescence (Casey et al., 2000). Giedd (2004) found that frontal gray matter reaches maximum thickness at around age 12, and that the latest cortical gray matter loss occurs in dorsolateral prefrontal cortex and superior temporal gyrus. Even though there is extensive evidence for the structural maturation of both MTL and PFC, functional development of these regions and their role in episodic memory development need further investigation.
One paradigm widely used to examine neural correlates of successful encoding in adult populations is the subsequent memory paradigm (Sanquist et al., 1980) in which brain activation during encoding is compared for items later remembered vs. later forgotten (Brewer et al., 1998, Henson et al., 2005, Kirchhoff et al., 2000, Staresina and Davachi, 2006, Wagner et al., 1998). A limited number of studies have utilized this paradigm in children, and the evidence for relative contribution of MTL and PFC structures to encoding have been mixed. In Menon et al. (2005), the functional maturation of MTL was evident through age-related increases in connectivity of the left entorhinal cortex with the left dorsolateral PFC. There are also suggestions for the functional development of hippocampus between age 8 years and adulthood during encoding of contextual detail (Ghetti et al., 2010) and story encoding (Chiu et al., 2006). Other studies found support for the role of PFC maturation in memory encoding (Chiu et al., 2006, McAuley et al., 2007, Ofen et al., 2007). In Ofen et al., activation during successful encoding increased with age in the right DLPFC but not MTL, which was interpreted by the authors as a dissociation between these regions during development. In Maril et al. (2010), a subsequent memory effect observed in the left hippocampus declined with age in a group 7–19-year-olds. Despite strong PFC activation, activity in this the PFC was not modulated by age.
There is increasing evidence that relational processing engages the hippocampus, the parahippocampal gyrus (Davachi, 2006, Davachi and Dobbins, 2008, Davachi et al., 2003, Davachi and Wagner, 2002, Kirwan and Stark, 2004, Prince et al., 2005), and dorsolateral PFC (Blumenfeld and Ranganath, 2007, Murray and Ranganath, 2007) to a greater extent than memory for items. Even though Ofen et al. (2007) used a scene encoding task and Ghetti et al. (2010) examined encoding for item–color relations, no published study has looked at item–item relations in children in the context of fMRI. Behavioral studies suggest age-related improvements in relational memory (Lloyd et al., 2009, Lorsbach and Reimer, 2005, Sluzenski et al., 2006). In the present study, we aimed to address whether MTL involvement increases with age when the memory task requires forming item–item relations.
The extant literature on children has focused almost exclusively on neural correlates of encoding processes during development and relatively little is known about the neural correlates of retrieval in children. In adults, a recent meta-analysis of retrieval identified left parietal regions (inferior, superior, precuneus) and left frontal (inferior and middle) regions as being consistently activated (Spaniol et al., 2009). In children, there is currently only one published fMRI study of retrieval. In this study of correct and false recognition, parietal cortex was recruited in 12-year-olds and adults but not in 8-year-olds, while regions in the prefrontal cortex were recruited by adults only (Paz-Alonso et al., 2008).
In addition, neuroimaging studies with children have all made use of a recognition memory paradigm in which participants decided whether the stimulus was previously seen. Age-related improvements are more dramatic when the retrieval demand is in the form of recall as opposed to recognition (Perlmutter and Lange, 1978). Neuroimaging studies of recall in adults have revealed unique activation patterns for later recalled compared to later recognized items (Habib and Nyberg, 2007).
Our goals in the present study were to examine the neural correlates of both relational encoding and recall in a sample of 8–9-year-old and 12–13-year-old children, using a pictorial paired associates test. We focused on these two age subgroups because of continued and rapid development of recall abilities across this age range (Schneider and Pressley, 1997, pp. 238–264). Successful encoding was examined by comparing brain activity during encoding for pairs that were subsequently recalled successfully versus subsequently forgotten. To examine brain activation during cued-recall, one item of the pair was presented as a cue and the children were asked to recall the paired item. Brain activity for items successfully recalled was compared with activity for forgotten items. Based on previous research, we hypothesized that (1) during encoding, stronger activations would be found for older children in MTL regions and in frontal regions involved in cognitive control processes (e.g., VLPFC and DLPFC) and (2) during recall, stronger activations would be found for older children in parietal regions and in frontal regions involved in cognitive control processes (e.g., VLPFC and DLPFC).
2. Methods
2.1. Participants
Fifteen 8–9-year-old (7 female; M=8.73, SD=.33) and fifteen 12–13-year-old (7 female; M=12.74, SD=.36) healthy typically developing children participated in the experiment. An additional 9 children were tested but excluded from analysis due to excessive head motion artifact (over 3mm, n=6 younger group; n=1 older group) or distortion in the images (n=2). Participants were recruited from a participant pool of families who had expressed interest in research at the time of their child's birth. Participants were screened and excluded if any of the following conditions were present: a current or past history of neurological disorders or trauma, psychiatric disorders based on self and/or parental report, complicated birth history including prematurity, abnormal developmental history based on developmental milestones, history of developmental cognitive disorders including specific learning disabilities, known intellectual impairment, or uncorrected visual or auditory impairments. All participants also underwent MRI safety screening for metal implants, braces, permanent retainers, tattoos and permanent makeup. Informed consent from the participants’ parents and written assent from the participants were obtained prior to the scanning session. Participants and parents were compensated for their time and effort with $30.
2.2. Behavioral task
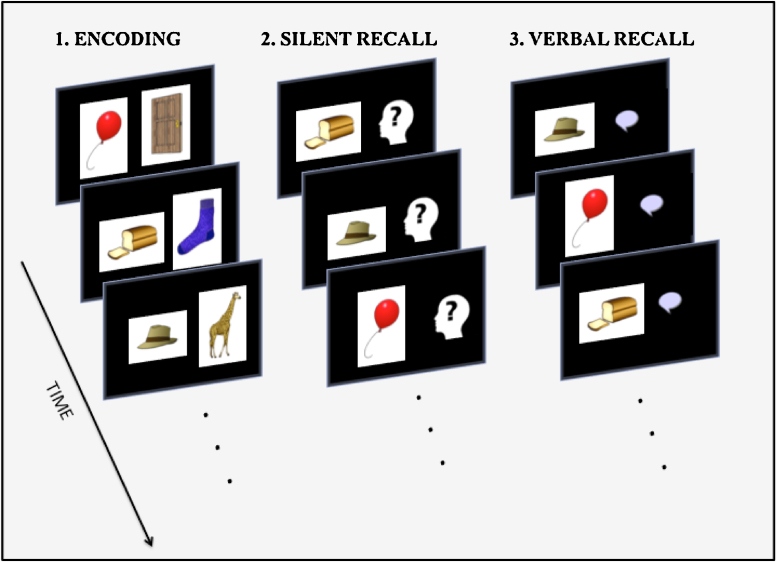
Participants performed a paired-associates picture-memory task in the scanner (adapted from Habib and Nyberg, 2007 paradigm with word pairs). Stimuli consisted of a total of 80 pairs of color drawings of everyday objects such as a banana (Rossion and Pourtois, 2004; colored versions of the Snodgrass & Vanderwart standardized image set; available at http://tarrlab.cnbc.cmu.edu/). The task included 4 runs, each of which consisted of encoding, silent recall and verbal recall phases (see Fig. 1). During the encoding phase of each run, 20 new pairs were presented consecutively. Presentation duration for each pair was 4s with an average of 2s of fixation between pairs (jittered between 1500ms and 2500ms). Children were instructed to memorize the pairs using any strategy they deemed appropriate, but were told that making a meaningful connection between the items is often very helpful. Immediately after the encoding phase, recall was assessed twice; once while brain images were being collected (silent recall) and once post-scanning (verbal recall). During the silent recall phase, the left item of each pair was presented for 4s, followed by a variable ISI (average=2s). Children were asked to mentally recall the item with which it was paired. Because obtaining a verbal response was not feasible during scanning, children were instructed to press a button on a handheld MRI compatible button response box to indicate whether they recalled the paired item. In the verbal recall phase that followed the silent recall phase of each run, the scanning was stopped and the recall task was administered again while the child was still lying in the scanner, this time with a verbal response from the child in order to assess the veracity of their memories during the scanned recall phase. Participants were instructed to verbally state the second item in the pair. Children were allowed a maximum of 6s to respond, after which the next item was presented. To ensure inter-observer agreement, two researchers recorded verbal responses from all participants. The order of stimuli was randomized for each subject during the encoding and silent recall phases of the task. Prior to scanning, participants completed a practice task with a different set of stimuli (5 pairs) with all three phases (encoding, silent recall and verbal recall) to ensure that they understood the task instructions.
Fig. 1.
Schematic representation of the memory task.
Items were later classified as “remembered” or “forgotten.” Remembered (R) items were defined as items for which the participant indicated recall with a button press during the silent recall phase and gave the correct response during the verbal recall phase. Forgotten (F) items were defined as items for which the participants did not press the button during the silent recall phase and failed to give a response during the verbal recall phase.
2.3. Imaging data acquisition
Imaging data were collected using a Siemens 3T Trio scanner and 12-channel head coil. Functional images were acquired using a gradient echo, echo-planar (EPI) imaging sequence across the whole brain in slices parallel to the AC-PC plane (T2*-weighted, 34 axial slices, TR=2000, TE=28, flip=80°, matrix=64×64, FOV=200, slice thickness=3mm, in-plane resolution=3.125mm×3.125mm×3mm). Two initial pre-stimulus data points (2TRs or 4s) were discarded. A 6-TR fixation baseline (a red cross on a black background) consisted of 2 TRs before the encoding period, 2 TRs between the encoding and recall phases, and 3TRs at the end of the recall phase. A total of 131 TRs were collected over 4min, 26s. The entire sequence (encoding, silent recall, and verbal recall) was repeated 4 times with 20 new picture pairs in each of the 4 runs. High-resolution structural images were collected using an MPRAGE pulse sequence for purposes of anatomical localization of function (T1-weigthted, 240 sagittal slices, TR=2530, TE=3.65, flip=7°, matrix=256×256, FOV=256, slice thickness=1mm, in-plane resolution=1mm×1mm×1mm, scan duration=10min, 49s).
2.4. Image processing
Imaging data were analyzed using the BrainVoyager QX (version: 2.0.8) software package (Brain Innovation, Maastricht, The Netherlands). Functional data preprocessing included slice scan time correction using cubic spline interpolation, linear trend removal, high-pass temporal filtering to remove low-frequency non-linear drifts of 2 or fewer cycles per time course, spatial smoothing with a 9mm Gaussian kernel (full-width at half-maximum), and three-dimensional motion correction. A canonical two-gamma function was used to model the hemodynamic response function (HRF). Participant's data were not included in the analysis if the head motion exceeded 3mm in any one direction. Functional data were co-registered to the anatomical volume and transformed into standard Talairach space for comparison across individuals. Statistical contrasts were performed using a general linear model (GLM). Only the trials that were classified as Remembered (R) and Forgotten (F) were included in the analysis (see Section 2.2 for description). Trials that were not categorized as R or F were not included in the model.
2.5. Image statistical analysis
Movement parameters obtained during the motion correction process were included in the GLMs as nuisance covariates. Age group (8–9 years vs. 12–13 years) was included in a second level to examine developmental differences in the subsequent memory effect and recall, and t-tests were used to compare group means. All analyses included random effects modeling with a dependent variable of percent change in signal. Whole-brain correction for multiple comparisons was accomplished through cluster-size thresholding, which was calculated using the BrainVoyager QX Cluster-level Statistical Threshold Estimator plug-in. In using this estimator, voxel-level thresholded maps were then submitted to a Monte Carlo simulation with 1000 iterations, which estimated cluster-level false-positive rates and minimum cluster size threshold needed to yield this rate (Goebel et al., 2006).
2.5.1. Overall task analysis
A first set of analyses examined task effects ignoring age group. In this analysis of the entire sample (n=30), a voxel-level threshold of p<.005 (uncorrected) was used with a cluster-level correction threshold of p<.005 for each contrast. As a result of Monte-Carlo simulations using these significance thresholds, the minimum cluster size was set at 17 voxels (498mm3) for the subsequent memory (encoding) contrast, and 29 voxels (859mm3) for the recall contrast.
2.5.2. Age-group comparison analysis
A second set of analyses examined age-group differences in activation during encoding and recall. A voxel-level threshold of p<.005 (uncorrected) was used. However, this criterion yielded in no clusters that were significantly different between the two age groups. We then conducted exploratory analyses with the more liberal voxel-level threshold of p<.05. Due to high structural and functional variability in developmental populations, it is not uncommon to use a more lenient threshold when conducting age group comparisons. Despite the more liberal initial threshold, a cluster-level correction threshold of p<.005 was still used to limit interpretation of non-meaningful differences. As a result of the Monte-Carlo simulations using these significance thresholds, the minimum cluster size was set at 49 voxels (1436mm3) for the subsequent memory (encoding) contrast, and 57 voxels (1670mm3) for the recall contrast.
2.5.3. Identifying anatomical labels for activation
Some of the task and age-group analyses resulted in large extended clusters that included multiple anatomical regions. In these cases, to identify the anatomical regions of activation, the p value was made progressively more stringent until separable peaks could be identified. The anatomical region of each peak was then identified according to Talairach coordinates. Voxels that fell within the same anatomical region were considered to belong to the same sub-cluster.
3. Results
3.1. Behavioral results
The mean percentage of trials on which the participants pressed the button during silent recall was 68.42 (SD=15.07) for the older children and 47.5 (SD=22.05) for the younger children. The mean percentage of trials on which the participants gave a verbal response during verbal recall was 66.08 (SD=15.82) for the older children and 46.92 (SD=21.06) for the younger children. In order to verify that children were pressing the button during silent recall only for the items they remembered, we compared the number of times they pressed the button to the number of items they verbally remembered. There was no statistically significant discrepancy between silent recall and verbal recall for either age group (ts(14)=.42, −1.55, for younger and older groups, respectively).
We conducted a 2 (gender: female, male)×2 (age group: 8–9, 12–13) ANOVA on the percentage of items correctly verbally recalled across all runs. There was a significant main effect of age group (F(1, 26)=8.60, p=.007, ). Recall was higher in the 12–13-year-old group (M=65.66, SD=16.10) than in the 8–9-year-old group (M=45.33, SD=21.10). There was no significant main or interaction effect with gender.
The mean percentage of trials classified as Remembered (R) was 63.25 (SD=16.34) for older children and 42.08 (SD=22.20) for younger children. The mean percentage of trials classified as Forgotten (F) was 30.17 (SD=15.18) for older children and 50.17 (SD=21.62) for younger children.
We examined reaction time differences on remembered (R) trials between the two age groups. During silent recall, older children responded significantly faster (M=1888.6ms, SD=238.76) than younger children (M=1462.13, SD=164.93), t(28)=5.69, p<.001. Importantly, both age groups were responding early in the response window, reducing the possibility that slower reaction times were leading to poor detection of remembered items.
3.2. Imaging results
3.2.1. Subsequent memory effect
Subsequent memory effects were examined by contrasting encoding activity for later remembered items to later forgotten items across the whole sample. This contrast (R>F) yielded extended activity across many regions of the brain, including parahippocampal and fusiform gyri, parietal and frontal regions (see Table 1 for list of all regions). In addition, deactivations were observed in medial frontal gyrus/anterior cingulate, precuneus and posterior cingulate. In these regions, the deactivation to remembered items was greater than deactivation to forgotten items.
Table 1.
Subsequent memory (successful encoding) activations across all participants.
| Hemisphere | BA | Talairach coordinates |
Volume (mm3) | Peak t value | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Remembered>Forgotten | |||||||
| Postcentral gyrus | Right | 4/43 | 45 | −16 | 40 | 2084 | 4.08 |
| Extended cluster | −24 | −61 | 37 | 150,048 | 7.25 | ||
| Precentral gyrus | Left | 6 | −48 | −1 | 37 | 2993 | 4.91 |
| Middle frontal gyrus | Left | 9/46 | −42 | 35 | 16 | 5618 | 4.46 |
| Inferior frontal gyrus | Left | 44 | −54 | 11 | 13 | 3895 | 4.62 |
| Inferior parietal lobule | Left | 39 | −24 | −61 | 37 | 8807 | 7.25 |
| Inferior parietal lobule | Right | 39 | 24 | −58 | 34 | 7145 | 5.59 |
| Parahippocampal gyrus | Right | 35/19 | 33 | −46 | −5 | 6050 | 4.76 |
| Fusiform gyrus | Left | 36/37 | −42 | −44 | −8 | 1843 | 5.50 |
| Fusiform/inferior occipital gyrus | Left | 18 | −33 | −76 | 1 | 2855 | 6.13 |
| Fusiform gyrus | Right | 36 | 27 | −46 | −17 | 2548 | 5.05 |
| Fusiform gyrus | Right | 19 | 24 | −76 | 1 | 3620 | 5.06 |
| Cerebellum | Left | n/a | −45 | −61 | −20 | 1992 | 5.84 |
| Cerebellum | Right | n/a | 30 | −49 | −20 | 1000 | 5.15 |
| Forgotten>Remembered | |||||||
| Medial frontal gyrus/cingulate gyrusa | Right | 8/9/32 | 9 | 41 | 13 | 15,084 | −5.94 |
| Precuneusa | Left | 31 | −6 | −64 | 25 | 2163 | −4.07 |
| Posterior cingulate gyrusa | Left | 23/31 | 0 | −25 | 34 | 917 | −3.73 |
Greater deactivation to remembered than forgotten.
3.2.2. Developmental differences in subsequent memory
Group-level whole-brain GLM analyses indicated an effect of age group in the right inferior temporal gyrus (BA 20), right fusiform gyrus (BA 20) and right middle frontal gyrus (BA 9/46) (see Table 2). Follow-up of this omnibus effect revealed that the age group differences were driven by significant memory-related effects in the 8–9year-old group only. That is, in these three regions, no significant difference between subsequently remembered versus subsequently forgotten pairs was found for the 12–13-year-old children (right inferior temporal gyrus: t(14)=1.82, p=.09; right fusiform gyrus: t(14)=1.71, p=.11; right middle frontal gyrus: t(14)=−1.17, p=.26).
Table 2.
Age group differences in brain activations during successful encoding (subsequent memory effect).
| Hemisphere | BA | Talairach coordinates |
Volume (mm3) | Peak t value | Significant SM effect | |||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Inferior temporal gyrus | Right | 20 | 63 | −13 | −14 | 2833 | −3.97 | 8–9yrs.; F>R |
| Fusiform gyrus | Right | 20 | 45 | −22 | −14 | 1467 | −4.00 | 8–9yrs.; F>R |
| Middle frontal gyrus | Right | 9/46 | 15 | 20 | 31 | 4121 | 2.69 | 8–9yrs.; R>F |
Notes: Age-group analyses were conducted with the more liberal initial voxel threshold of p<.05. Only clusters reaching a size consistent with p<.005 are included (determined by Monte Carlo simulation). SM=Subsequent Memory. 8–9 yrs.=8–9-year-old group only. F=Subsequently Forgotten, R=Subsequently Remembered.
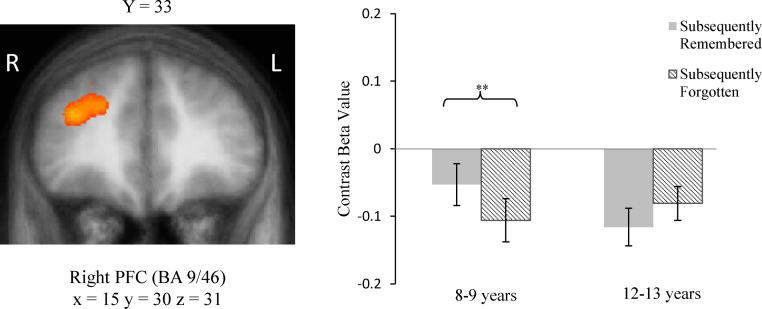
In the 8–9-year-old group, a subsequent memory effect (R>F) was observed in the dorsolateral PFC (BA 9/46: t(14)=3.64, p=.003). Further inspection of R and F means in this region revealed that the deactivation for forgotten items was greater than the deactivation for remembered items (see Fig. 2). In the remaining two regions (right inferior temporal and right fusiform cortices), a reversed subsequent memory effect was observed such that there was enhanced activity for subsequently forgotten compared to subsequently remembered pairs. Further inspection of the means revealed that this reversed subsequent memory effect resulted from significantly greater deactivation for subsequently remembered items compared to subsequently forgotten items (right inferior temporal gyrus: t(14)=−2.97, p=.01; right fusiform gyrus: t(14)=−2.40, p=.03).
Fig. 2.
Left: Age-related differences in subsequent memory activation in right PFC, p<.05, corrected. T value scale: 2.05–2.76; p-values: 0.05–0.01. Right: Average contrast beta values for each age group for the right PFC ROI from the contrast Subsequently Remembered>Subsequently Forgotten. Error bars indicate SEs. **p<.01.
In order to examine whether these age group differences were driven by differences in performance, we matched participants from the two age groups on the percentage of items correctly recalled. This matching procedure resulted in n=6 in each age group (for older children, M=62%, SD=20; for younger children, M=62%, SD=30). Using this sample, we then examined age group differences in the same 3 regions. In all regions except for the right fusiform gyrus, the age group effect remained significant even after performance was equated between groups (right inferior temporal gyrus: t(10)=−2.68, p=.02; right middle frontal gyrus: t(10)=2.88, p=.02; right fusiform gyrus: t(10)=−1.67, p=.13).
3.2.3. Successful recall
Successful recall was examined by contrasting brain activity during recall for correctly recalled pairs vs. forgotten pairs across the whole sample. Activations associated with successful recall were found in many different regions of the brain including anterior and posterior cingulate, thalamus, hippocampus, and inferior parietal region (see Table 3).
Table 3.
Activations for successful recall across all participants.
| Hemisphere | BA | Talairach coordinates |
Volume (mm3) | Peak t value | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Remembered>Forgotten | |||||||
| Anterior cingulate gyrus | Right | 32 | 18 | 35 | 4 | 3937 | 6.49 |
| Anterior cingulate gyrus | Left | 32 | −18 | 32 | 1 | 1328 | 6.18 |
| Caudate nucleus | Left | n/a | −9 | 14 | 4 | 988 | 5.84 |
| Lentiform nucleus | Left | n/a | −21 | 2 | −5 | 1859 | 5.75 |
| Thalamus | Left | n/a | −21 | −22 | 16 | 1543 | 6.28 |
| Hippocampus | Left | n/a | −27 | −10 | −7 | 1331 | 5.18 |
| Middle frontal gyrus | Left | 6 | −3 | −10 | 52 | 1816 | 5.52 |
| Inferior parietal lobule | Left | 40 | −42 | −37 | 49 | 7055 | 6.64 |
| Postcentral gyrus | Left | 1/2 | −42 | −19 | 49 | 5617 | 7.31 |
| Posterior cingulate gyrus | Left | 31/23 | −6 | −40 | 34 | 6003 | 5.83 |
| Cerebellum | Right | n/a | 18 | −49 | −23 | 7538 | 5.67 |
| Inferior temporal gyrus | Left | 37 | −51 | −49 | −2 | 1519 | 4.89 |
| Superior frontal gyrus | Left | 6 | −15 | 14 | 49 | 1927 | 4.53 |
| Precuneus | Left | 7 | −6 | −46 | 40 | 860 | 4.66 |
3.2.4. Developmental differences in successful recall
Group-level whole-brain GLM analyses indicated an effect of age group in the right middle temporal gyrus (BA 37), left inferior parietal lobule (BA 40), left middle/inferior frontal gyrus (BA 46/47), anterior cingulate (BA 32), and right cerebellum (see Table 4).
Table 4.
Age group differences in brain activations during successful recall.
| Hemisphere | BA | Talairach coordinates |
Volume (mm3) | Peak t value | Significant effect | |||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Middle temporal gyrus | Right | 37 | 45 | −58 | 19 | 2648 | −2.47 | 12–13yrs.; R>F |
| Inferior parietal lobule | Left | 40 | −57 | −22 | 34 | 2707 | −3.36 | 12–13yrs.; R>F |
| Anterior cingulate gyrusa | Left | 32 | −6 | 38 | −14 | 4327 | −3.96 | 12–13yrs.; R>F |
| Inferior/middle frontal gyrusa | Left | 46/47 | −46 | 37 | −6 | 1951 | −3.37 | 12–13yrs.; R>F |
| Cerebellum | Right | n/a | 23 | −79 | −38 | 2006 | −3.40 | 12–13yrs.; R>F |
Notes: Age-group analyses were conducted with the more liberal initial voxel threshold of p<.05. Only clusters reaching a size consistent with p<.005 are included (determined by Monte Carlo simulation). 12–13yrs.=12–13-year-old group only. F=Forgotten, R=Recalled.
Part of a larger cluster.
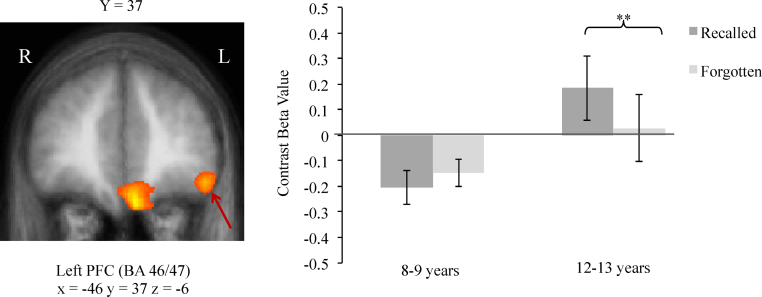
In all of these regions, only the 12–13-year-old children showed greater activity for correctly recalled versus forgotten pairs (right BA 37: t(14)=3.32, p=.005; left BA 40: t(14)=6.32, p=.00002; left BA 46/47: t(14)=3.44, p=.004; left BA 32: t(14)=3.02, p=.009; right cerebellum: t(14)=4.08, p=.001). Fig. 3 provides a depiction of this difference in the PFC region for 12–13 year olds. In contrast, in the 8–9-year-old group, none of these regions significantly differentiated between recalled and forgotten pairs (right BA 37: t(14)=0.33, p=.75; left BA 40: t(14)=1.02, p=.32; left BA 46/47: t(14)=−1.07, p=.30; left BA 32: t(14)=1.27, p=.23; right cerebellum: t(14)=.36, p=.72).
Fig. 3.
Left: Age-related differences in recall activation in left PFC (indicated by red arrow), p<.05, corrected. T value scale: 2.05–3.67; p-values: 0.05–0.001. Right: Average contrast beta values for each age group for the left PFC ROI from the contrast Recalled>Forgotten. Error bars indicate SEs. **p<.01. (For interpretation of the references to color in the figure caption, the reader is referred to the web version of the article.)
To examine whether these age group differences were driven by performance differences, we again compared our subgroups matched on recall performance (n=6 in each age group). After matching for performance, significant age group effects remained in the right middle temporal (t(10)=−2.93, p=.015), anterior cingulate (t(10)=−3.66, p=.004), the left middle/inferior frontal (t(10)=−3.67, p=.004) regions.
4. Discussion
The goal of the present study was to examine age-related differences in the neural correlates of encoding and retrieval in middle childhood. We compared two groups of children ages 8–9 years and 12–13 years on a paired associates memory task using fMRI. In this task, children were instructed to remember item-item associations in unrelated pictorial pairs. Brain activity during both successful encoding and successful recall was examined. Overall, children activated brain regions similar to what has previously been found with adults. There were also several differences between the younger and older children in our study, which we discuss in the next sections.
4.1. Encoding
Successful encoding was examined through the subsequent memory effect by comparing brain activity differences during encoding between later remembered versus later forgotten pairs. In addition to regions common to both age groups, the 8–9-year-old group activated three additional regions that were sensitive to memory (recalled vs. forgotten) compared to 12–13-year-olds. These regions included posterior sites and dorsolateral prefrontal cortex. It has been posited that during successful encoding, the DLPFC region supports organization of the to-be-remembered information (Simons and Spiers, 2003). More recent evidence suggests that DLPFC has a special role in relational encoding (Blumenfeld and Ranganath, 2007).
A DLPFC region in the left hemisphere was activated during successful encoding in both age groups. However, 8–9-year-olds recruited an additional DLPFC region in the right hemisphere that differentiated between successful versus unsuccessful encoding. Whereas the younger group showed enhanced activity in this region during successful encoding, 12–13-year-olds recruited it equally for later remembered and later forgotten items. One interpretation of these findings is that at younger ages, children need to recruit brain regions bilaterally, and with age and increased efficiency of encoding, regions recruited to accomplish the task become more focalized. A general shift from diffuse to focal brain activation with development has been suggested by several other studies (see Durston and Casey, 2006, for a review and discussion).
These findings regarding DLPFC function are not fully consistent with previous literature on the development of subsequent memory effects. In Ghetti et al. (2010), a subsequent memory effect in right DLPFC did not differ by age, even though activity in this region was less in 8-year-olds compared to older children and adults. In Ofen et al. (2007), the subsequent memory effect in bilateral PFC increased with age. However, the memory tasks used in these two studies were quite different from the one used in the present study, making direct comparisons more challenging. Our task was an intentional memory task as was the task used in Ofen et al. (but not in Ghetti et al.) but it involved making elaborative associations between two unrelated items and thus is potentially more challenging and cognitively demanding than encoding scenes (Ofen et al.) or items with their associated colors (Ghetti et al.).
It is interesting to note that, for both trial types (later remembered versus later forgotten), activity in the DLPFC region of interest fell below baseline for both age groups. The functional significance of deactivations during memory tasks is currently not well understood. For older children, there was greater deactivation for remembered compared to forgotten items in this region (although not significantly so), which is consistent with Daselaar et al. (2004), whereas for 8–9-year-olds, forgotten items resulted in greater deactivation compared to remembered items. A possible interpretation of this finding is that the younger children failed to deactivate sufficiently for remembered items, resulting in unsuccessful encoding. Further research is needed to better understand the role of deactivations in memory processes in children.
Based on previous literature, we expected to find a subsequent memory effect in the hippocampus proper. Instead we found extended activity in the surrounding parahippocampal and fusiform regions in the whole sample. Reviews of functional neuroimaging of memory have shown that MTL is not always reliably activated; indeed, many adult studies have failed to find activations in hippocampus proper (see Henson, 2005). In addition, we did not find age-related differences in the hippocampus. This result is consistent with Ofen et al. who found that activity in MTL regions did not increase with age, but is at odds with Ghetti et al. who reported increased activity in the hippocampus with age and Maril et al. (2010) who reported decreased activity in the hippocampus with age. The memory tasks used and the age range of participants differed across these studies, which may explain the mixed findings. For instance, in Maril et al., the age ranges were 7–14 years in the younger group and 15–19 years in the older group, and did not examine potential age-related differences within these groups. In Ghetti et al., four age groups (8-year-olds, 10–11-year-olds, 14-year-olds and young adults) were compared and a subsequent memory effect was found in the hippocampus and the posterior parahippocampal gyrus only in 14-year-olds and adults when the task involved the recollection of details. Thus, it is possible that we would also see subsequent memory effects in the MTL with our task if older adolescents were tested. Further research is needed to examine under what task conditions developmental changes in MTL function are most evident.
4.2. Recall
To our knowledge, this is the first study to report on the neural correlates of recall processes in typically developing children and developmental differences between middle childhood and preadolescence periods. Compared to recognition, recall processes are more effortful and tend to show a more protracted development (Perlmutter and Lange, 1978). Only one published study (Paz-Alonso et al., 2008) has examined age-related differences in brain activity associated with retrieval. These authors found greater activity in parietal regions during a recognition task for hits compared to correct rejections in 12-year-olds and adults, but not in 8-year-olds. In the same study, frontal regions (ventrolateral and dorsolateral prefrontal cortex) were recruited in adults but not in children during correct recognition. However, in our study, prefrontal regions were activated during retrieval, which might be due to higher cognitive demands associated with our recall memory task compared to the recognition task used in Paz-Alonso et al.
In our data, the regions activated in common across the two age groups are consistent with those reported in adult studies of retrieval (see Habib and Nyberg, 2007 for a similar task; see Spaniol et al., 2009, for a meta-analysis). However, there were also age-related differences. Older children showed enhanced activity during recall in ventrolateral and anterior prefrontal regions whereas younger children did not. The specific roles of the subregions of PFC in memory processes are still being actively researched, however, ventrolateral PFC activity during recollection is generally attributed to specification and elaboration of retrieval cues, which are then used for strategic search of stored representations and maintenance of retrieved information online (Badre and Wagner, 2007, Dobbins and Han, 2006, Petrides, 2002, Simons and Spiers, 2003). Thus, activations in PFC during retrieval seem to reflect cognitive control processes, which are known to develop with age (Durston and Casey, 2006). In addition, older children recruited an anterior medial PFC region. This region has been attributed to attention to one's own cognitive or affective state (Simons et al., 2005). Although speculative at this point, this difference might reflect increasing metamemory abilities with age (Schneider, 2010) and merits further investigation.
We also found evidence for enhanced recall-related activity in the parietal cortex (specifically, BA 40) for older children. Recently, researchers have begun to pay more attention to the role parietal activations play in memory-related tasks. For instance, activity in the superior parietal cortex has been attributed to top-down strategic search processes, whereas activity in the inferior parietal cortex is thought to reflect bottom-up attention processes (Cabeza, 2008, Ciaramelli et al., 2008). Thus, recruitment of the parietal region in older children in our study could reflect age-related differences in efficiency and sophistication of retrieval strategies.
4.3. Limitations and future directions
Because the task used in the present study was an intentional memory task, it is possible that the brain activation differences between the two age groups might in part reflect differences in memory strategy use. Task instructions included a suggestion to create a memorable connection between the items (elaboration) to benefit later recall. However, because we did not interview children about the types of strategies they used during this task, we do not know what percentage of children made use of the elaboration strategy. However, it is unlikely that all age-related variability is attributable to strategy use because, as reviewed in Pressley (1982), verbal elaboration strategy use does not show much developmental change after the preschool years. In addition, instructions to use a verbal elaboration strategy benefit all age groups, and there are large individual differences within any age group (Pressley, 1982).
Another limitation stems from the restrictions of MRI technology when collecting memory recall data. In contrast to recognition tests, during which participants indicate using a button-press whether or not they have seen the stimuli, tests of recall require a verbal response from the participant. However, it is challenging to collect high quality brain images while the participants are speaking, and to hear verbal responses while the scanner is collecting images. Therefore, we had to rely on self-report data from the participant, which makes it impossible to verify whether participants were actually remembering the association when they said that they were. However, strong overlap between participant report of silent retrieval and subsequent correct verbal retrieval strongly eases concerns regarding the validity of silent self-report data.
The present study employed a cross-sectional design in which we compared two samples of children from different age groups. However, a longitudinal approach is crucial in order to understand developmental changes in the contribution of brain regions to memory formation and retrieval. Thus, in future studies, the same participants should be scanned at multiple ages to examine within-individual trajectories of change.
In the present study, we chose not to include an adult age group, which could be viewed as a limitation. Our choice to study only children was motivated by two primary factors. First, equating task parameters for young children and adults significantly alters the demands of the task for children. For example, adults can typically remember longer lists of items over longer delays. The subsequent memory approach requires that sufficient numbers of both remembered and forgotten items are available for analysis. However, equating task parameters for adults and children likely would yield a very low percentage of remembered items in children and a similarly low percentage of forgotten items in adults. Task performance differences across groups have been an important and controversial issue in fMRI research (see Church et al., 2010, for a discussion of this issue). We chose to limit our age groups to those in whom who could reasonably use identical task parameters. Second, a large literature encompassing hundreds of studies has provided significant information regarding brain systems that are engaged during episodic memory in adults. Some of these studies are quite similar to the design used here with children (e.g., Habib and Nyberg, 2007) reducing the relative utility of an adult sample. Despite the potential interest in examining further developmental changes between early adolescence (12–13 years) and adulthood, the focus of our interest was on the age range during which there is strong evidence for developmental change in recall memory function. Additional age groups will undoubtedly help to further refine these initial findings.
As we learn more about the brain regions important for development of memory, it will also be important to consider connectivity between regions. Menon et al. (2005) showed that functional connectivity between MTL and PFC regions during encoding changes with age during childhood. It will be important to examine whether age-related changes in connectivity also play a role in retrieval processes. Similarly, future research will be needed to examine neural correlates of individual differences in brain activation patterns during successful remembering. Although we found strong age-related differences in the present study, within-subject variability should not be ignored (Miller et al., 2002). Future research should examine functional brain activation patterns associated with individual difference factors within a given age group.
5. Conclusion
The contribution of this study is unique in its examination of both encoding and retrieval related functional brain activity in the same sample of children and its assessment of recall as opposed to recognition processes. The results of the present study provide important insight into the contribution of functional brain maturation to the development of episodic memory in typically developing children. Our results suggest that the protracted development of PFC regions might underlie developmental differences in recall abilities. Our findings regarding increased PFC contribution to memory retrieval with age provide further insight into one possible mechanism by which control of memory increases with age and contributes to improved memory abilities. Currently, very little is known about the development of brain–behavior relations supporting episodic memory, and thus further research in this area is necessary to understand the biological mechanisms underlying this ability which is not only crucial to everyday functioning but also tends to be disrupted in many psychological disorders, such as depression, PTSD and schizophrenia.
Conflicts of interest
There are no known conflicts of interest.
Acknowledgements
Support for this research was provided by a research grant from the Center for Neurobehavioral Development at the University of Minnesota, postdoctoral training fellowship to the first author (NIH MH73129) and the Center for Magnetic Resonance Research at the University of Minnesota (NIH BTRR-P41 RR008079, P30NS057091, and the MIND Institute). The authors also thank the children and families that so generously gave of their time to make the work possible. Portions of these data were presented at the annual meeting of the Organization for Human Brain Mapping, June 2010.
References
- Badre D., Wagner A.D. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bauer P.J. Developments in declarative memory: decreasing susceptibility to storage failure over the second year of life. Psychological Science. 2005;16:41–47. doi: 10.1111/j.0956-7976.2005.00778.x. [DOI] [PubMed] [Google Scholar]
- Bauer P.J. Toward a neuro-developmental account of the development of declarative memory. Developmental Psychobiology. 2008;50:19–31. doi: 10.1002/dev.20265. [DOI] [PubMed] [Google Scholar]
- Blumenfeld R.S., Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Brewer J.B., Zhao Z., Desmond J.E., Glover G.H., Gabrieli J.D. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Role of parietal regions in episodic memory retrieval: the dual attentional processes hypothesis. Neuropsychologia. 2008;46:1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Giedd J.N., Thomas K.M. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Chiu C.-Y.P., Schmithorst V.J., Brown R.D., Holland S.K., Dunn S. Making memories: a cross-sectional investigation of episodic memory encoding in childhood using fMRI. Developmental Neuropsychology. 2006;29(2):321–340. doi: 10.1207/s15326942dn2902_3. [DOI] [PubMed] [Google Scholar]
- Church J.A., Petersen S.E., Schlaggar B.L. The “Task B Problem” and other considerations in developmental functional neuroimaging. Human Brain Mapping. 2010;31:852–862. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramelli E., Grady C.L., Moscovitch M. Top-down and bottom-up attention to memory: a hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Daselaar S.M., Prince S.E., Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. NeuroImage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context, and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L., Dobbins I.G. Declarative memory. Current Directions in Psychological Science. 2008;17:112–118. doi: 10.1111/j.1467-8721.2008.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L., Mitchell J.P., Wagner A.D. Multiple routes to memory: distinct medial temporal processes build item and source memories. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L., Wagner A.D. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. Journal of Neurophysiology. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Dobbins I.G., Han S. Cue- versus probe-dependent prefrontal cortex activity during contextual remembering. Journal of Cognitive Neuroscience. 2006;18:1439–1452. doi: 10.1162/jocn.2006.18.9.1439. [DOI] [PubMed] [Google Scholar]
- Durston S., Casey B.J. What have we learned about cognitive development from neuroimaging? Neuropsychologia. 2006;44:2149–2157. doi: 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Durston S., Hilleke E.H., Casey B.J., Giedd J.N., Buitelaar J.K., van Engeland H. Anatomical MRI of the developing brain: what have we learned? Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Gershberg F.B., Shimamura A.P. Impaired use of organization strategies in free recall following frontal lobe damage. Neuropsychologia. 1995;33:1305–1333. doi: 10.1016/0028-3932(95)00103-a. [DOI] [PubMed] [Google Scholar]
- Ghetti S., DeMaster D.M., Yonelinas A.P., Bunge S.A. Developmental differences in medial temporal lobe function during memory encoding. Journal of Neuroscience. 2010;30:9548–9556. doi: 10.1523/JNEUROSCI.3500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd J.N. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. The Journal of Comparative Neurology. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Goebel R., Esposito F., Formisano E. Analysis of functional image analysis contest (FIAC) data with BrainVoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Human Brain Mapping. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Habib R., Nyberg L. Neural correlates of availability and accessibility in memory. Cerebral Cortex. 2007;18:1720–1726. doi: 10.1093/cercor/bhm201. [DOI] [PubMed] [Google Scholar]
- Henson R. A mini-review of fMRI studies of human medial temporal lobe activity associated with recognition memory. Quarterly Journal of Experimental Psychology. 2005;58B:340–360. doi: 10.1080/02724990444000113. [DOI] [PubMed] [Google Scholar]
- Henson R.N., Hornberger M., Rugg M.D. Further dissociating the processes involved in recognition memory: an FMRI study. Journal of Cognitive Neuroscience. 2005;17:1058–1073. doi: 10.1162/0898929054475208. [DOI] [PubMed] [Google Scholar]
- Howe M.L., O'Sullivan J.T. What children's memories tell us about recalling our childhoods: a review of storage and retrieval processes in the development of long term retention. Developmental Review. 1997;17:148–204. [Google Scholar]
- Kirchhoff B.A., Wagner A.D., Maril A., Stern C.E. Prefrontal–temporal circuitry for episodic encoding and subsequent memory. Journal of Neuroscience. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan C.B., Stark C.E.L. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14:919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd M.E., Doydum A.O., Newcombe N.S. Memory binding in early childhood: evidence for a retrieval deficit. Child Development. 2009;80:1321–1328. doi: 10.1111/j.1467-8624.2009.01353.x. [DOI] [PubMed] [Google Scholar]
- Lorsbach T.C., Reimer J.F. Feature binding in children and young adults. Journal of Genetic Psychology. 2005;166(3):313–327. doi: 10.3200/GNTP.166.3.313-328. [DOI] [PubMed] [Google Scholar]
- Maril A., Davis P.E., Koo J.J., Reggev N., Zuckerman M., Ehrenfeld L., Mulkern R.V., Waber D.P., Rivkin M.J. Developmental fMRI study of episodic verbal memory encoding in children. Neurology. 2010;75:2110–2116. doi: 10.1212/WNL.0b013e318201526e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley T., Brahmbhatt S., Barch D.M. Performance on an episodic encoding task yields further insight into functional brain development. NeuroImage. 2007;34:815–826. doi: 10.1016/j.neuroimage.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Menon V., Boyett-Anderson J.M., Reiss A.L. Maturation of medial temporal lobe response and connectivity during memory encoding. Cognitive Brain Research. 2005;25:379–385. doi: 10.1016/j.cogbrainres.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Miller M.B., Van Horn J., Wolford G.L., Handy T.C., Valsangkar-Smyth M., Inati S., Grafton S., Gazzaniga M.S. Extensive individual differences in brain activations during episodic retrieval are reliable over time. Journal of Cognitive Neuroscience. 2002;14(8):1200–1214. doi: 10.1162/089892902760807203. [DOI] [PubMed] [Google Scholar]
- Murray L.J., Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. Journal of Neuroscience. 2007;27:5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N., Kao Y.-C., Sokol-Hessner P., Kim H., Whitfield-Gabrieli S., Gabrieli J. Development of the declarative memory system in the human brain. Nature Neuroscience. 2007;10(9):1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- Paz-Alonso P., Ghetti S., Donohue S.E., Goodman G., Bunge S.A. Neurodevelopmental correlates of true and false recognition. Cerebral Cortex. 2008;18:2208–2216. doi: 10.1093/cercor/bhm246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter M., Lange G. A developmental analysis of recall-recognition distinctions. In: Ornstein P.A., editor. Memory Development in Children. Erlbaum; Hillsdale, NJ: 1978. pp. 243–258. [Google Scholar]
- Petrides M. The mid-ventrolateral prefrontal cortex and active mnemonic retrieval. Neurobiology of Learning and Memory. 2002;78:528–538. doi: 10.1006/nlme.2002.4107. [DOI] [PubMed] [Google Scholar]
- Pressley M. Elaboration and memory development. Child Development. 1982;53:296–309. [Google Scholar]
- Prince S.E., Daselaar S.M., Cabeza R. Neural correlates of relational memory: successful encoding and retrieval of semantic and perceptual associations. Journal of Neuroscience. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B., Pourtois G. Revisiting Snodgrass and Vanderwart's object set: the role of surface detail in basic-level object recognition. Perception. 2004;33:217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Sanquist T.F., Rohrbaugh J.W., Syndulko K., Lindsley D.B. Electrophysiological signs of levels of processing: perceptual analysis and recognition memory. Psychophysiology. 1980;17:568–576. doi: 10.1111/j.1469-8986.1980.tb02299.x. [DOI] [PubMed] [Google Scholar]
- Schneider W. Metacognition and memory development in childhood and adolescence. In: Waters H.S., Schneider W., editors. Metacognition, Strategy Use & Instruction. Guilford Press; New York, NY: 2010. pp. 54–83. [Google Scholar]
- Schneider W., Pressley M. Lawrence Erlbaum Associates; Mahwah, New Jersey: 1997. Memory Development Between Two and Twenty. [Google Scholar]
- Simons J.S., Owen A.M., Fletcher P.C., Burgess P.W. Anterior prefrontal cortex and the recollection of contextual information. Neuropsychologia. 2005;43:1774–1783. doi: 10.1016/j.neuropsychologia.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Simons J.S., Spiers H.J. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews Neuroscience. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Sluzenski J., Newcombe N.S., Kovacs S.L. Binding, relational memory, and recall of naturalistic events: a developmental perspective. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32:89–100. doi: 10.1037/0278-7393.32.1.89. [DOI] [PubMed] [Google Scholar]
- Spaniol J., Davidson P.S.R., Kim A.S.N., Han H., Moscovitch M., Grady C.L. Event-related fMRI studies of episodic memory encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1799. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Staresina B.P., Davachi L. Differential encoding mechanisms for subsequent associate recognition and free recall. Journal of Neuroscience. 2006;26:9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26:1–12. [Google Scholar]
- Wagner A.D., Schacter D.L., Rotte M., Koutstaal W., Maril A., Dale A.M., Rosen B.R., Buckner R.L. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]