Abstract
Recent literature on thalamic aphasia and thalamic activity during neuroimaging is selectively reviewed followed by a consideration of recent anatomic and physiological findings regarding thalamic structure and functions. It is concluded that four related corticothalamic and/or thalamocortical mechanisms impact language processing: (1) selective engagement of task-relevant cortical areas in a heightened state of responsiveness in part through the nucleus reticularis (NR), (2) passing information from one cortical area to another through corticothalamo-cortical mechanisms, (3) sharpening the focus on task-relevant information through corticothalamo-cortical feedback mechanisms, and (4) selection of one language unit over another in the expression of a concept, accomplished in concert with basal ganglia loops. The relationship and interaction of these mechanisms is discussed and integrated with thalamic aphasia and neuroimaging data into a theory of thalamic functions in language.
Keywords: Thalamus, Language, Aphasia, Basal Ganglia
1. Introduction
Small infarcts limited almost entirely to the ventral anterior nucleus of the dominant thalamus and small hemorrhages in the posterior portion of the dominant thalamus almost always cause aphasia acutely and can cause lasting, though often mild language symptoms. Cases of thalamic aphasia usually involve lesions of the dominant thalamus as opposed to the nondominant thalamus. Even though the location of the lesion may vary within the thalamus, the aphasias from anterior versus posterior thalamic locations can have striking similarities. What causes these symptoms? What is the role of the dominant thalamus in language? The author has been involved in attempts to answer these questions for more than two decades (e.g., Crosson, 1984, 1999; Nadeau & Crosson, 1997). However, recent discoveries regarding thalamic mechanisms require a reconsideration of these issues.
The author and his colleagues have always felt that the answers to these questions lie not only in the symptoms of patients with thalamic aphasia, but also in an understanding of the cellular and physiological mechanisms of the thalamus. Attempting to integrate recent cellular and physiological research into a theory about the role of the dominant thalamus in language is a humbling experience. Of necessity, cellular and much of the physiological research must be done almost exclusively in animals that do not possess the complex language systems of humans. Thus, while thalamic mechanisms can be addressed by this research, the ability to directly assess the relationship of these mechanisms to language functions is rare. This fact forces us to rely almost entirely on inference as a tool for addressing how the resulting discoveries can be applied to language mechanisms. There are perils in such a reliance on inference, and many investigators would shun the task. Nontheless, the author believes that there is considerable heuristic value in trying to understand the role of thalamic mechanisms in language. First, attempts to explain the role of the thalamus in language could lead to new methods for assessing thalamic aphasias that lead to greater insight about underlying mechanisms. Second, models incorporating cellular and physiological mechanisms can lead to testable hypotheses which can be applied to studies of thalamic aphasia. Third, human neuroimaging studies are beginning to add to our understanding of the role of the thalamus in language, and a heuristic consideration of the role of thalamic mechanisms in language could suggest ways of testing hypotheses with neuroimaging studies. Finally, understanding how thalamic mechanisms might contribute to language enriches our knowledge about brain language systems. Such knowledge may be useful in developing treatments for aphasia and in understanding the implications of thalamic involvement in larger lesions. In short, the benefits of attempting to integrate recent physiological research with findings in lesion studies and functional imaging studies outweigh the shortcomings. Nonetheless, it is important to understand the limitations of the endeavor.
Because the most common symptom of thalamic aphasia is semantic paraphasia, much of this treatise focuses on lexical-semantic functions and on word-finding. However, the mechanisms discussed are relevant to other language functions, including auditory-verbal comprehension, reading, and even repetition, and some examples of other such functions will be addressed. Indeed, the author believes that integration of recent literature on thalamic mechanisms allows for an integrated model that can explain much of the variability seen in language symptoms in cases of dominant thalamic infarcts. This capacity of a newly integrated model makes it a powerful heuristic tool for future research. Indeed, because of attentional aspects of the model, which are addressed below, there are significant implications for other areas of cognition.
This consideration of thalamic mechanisms in language is organized into five main parts. The first part is the current introduction. The second part will be a brief review of the core syndrome of thalamic aphasia followed by a review of other symptoms that may co-occur with the core syndrome. Some findings from recent functional imaging studies also will be integrated. The goal of this section is not only to describe the syndrome of thalamic aphasia but also to extract information about cognitive and linguistic changes that help us to understand the role of the thalamus in language. The third, and largest part of the current paper, will consist of a consideration of the nature of thalamic mechanisms that underlie language and related processes. This section will endeavor to review previous conceptualizations as well as to introduce more recent concepts for consideration. The literature reveals four mechanisms that likely have relevance to language functions: (1) engagement of cortical areas necessary to process task-relevant information in a heightened state of responsiveness, in part through the nucleus reticularis (NR), (2) passing information from one cortical area to another, (3) sharpening the focus on relevant information through corticothalamic feedback mechanisms, and (4) selection of one language unit over another in the expression of a concept, accomplished in concert with basal ganglia loops. In the fourth section of the paper, an attempt will be made to integrate the mechanisms discussed in the previous section into a model of thalamic functions in language. In the fifth and final section, hypotheses for future study will be presented for heuristic purposes, and a few final conclusions will be drawn.
2. Thalamic Aphasia and Other Considerations Regarding the Role of the Thalamus in Language
This section is meant to provide a description of thalamic aphasia and a few other studies relevant to the role of the thalamus in language. It is not meant to be an exhaustive critique of the literature, but rather, it provides an introduction for the discussion that follows.
2.1. Thalamic Aphasia Syndrome
Key points in this subsection include the variability in aphasias from dominant thalamic infarcts, the relative ubiquity of lexical semantic deficits, and the rarity of significant deficits in repetition. Before discussing the syndrome of thalamic aphasia, however, a few comments should be made about the state of the literature that can make it challenging to describe the syndrome. The crux of the matter is that the syndrome description has relied on case descriptions that vary in several aspects. One such source of variation is the time post onset at which patients have been evaluated. Thalamic aphasias can evolve rapidly. Their presentation in the emergency room or at bedside within one to two days after stroke will be different than their presentation a week or several days after the stroke. Ideally, patients would be evaluated a few days after the stroke, after the acute effects of the stroke have resolved but before gross reorganization of function takes place. Descriptions of aphasia have been garnered anywhere from hospital admission to years after the stroke, when compensatory mechanisms may obscure the role of the thalamus in language. A second variable is the quality of language evaluation. Such descriptions have varied from only the use of classical aphasia labels to clinical evaluation to standardized language assessments. Obviously, standardized assessments give us a better characterization of the syndrome. Relying on classical nomenclature as a descriptive tool is not advisable because thalamic aphasias often do not fit this nomenclature. Few studies have used hypothesis driven approaches to determine the nature of thalamic aphasias, though some such studies do exist. A third problem is that clinical use of the classical aphasia nomenclature as well as terms such as “fluent” and “nonfluent” can vary from place to place. Keeping in mind these problems, it does seem possible to describe a canonical syndrome of thalamic aphasia, or at least cardinal symptoms, though exceptions to this symptom cluster can be found.
A good place to start is with an early definition of thalamic aphasia. In an early review of the literature on thalamic aphasia, Crosson (1984) described three cardinal features of thalamic aphasia: (1) Output is fluent with frequent paraphasias that are primarily semantic in nature. At times, paraphasias are so severe as to deteriorate into jargon. (2) Auditory-verbal comprehension is less impaired than this kind of output normally would indicate. (3) Repetition is minimally impaired.1
Some years later, Crosson (1992) addressed how well cases in the literature fit these characterizations of thalamic aphasia. Cases of dominant thalamic hemorrhage generally seemed to fit the cardinal symptoms described by Crosson (1984) fairly well. Cases of dominant thalamic hemorrhage causing aphasia frequently occurred in the posterior thalamus, around the pulvinar; though, one also can expect pressure effects with intracerebral hemorrhage. Although aphasia was frequent after dominant thalamic infarct, the cases cohered less well around the cardinal symptoms. It would not be surprising if cases of thalamic aphasia varied depending upon the nucleus that is injured because different thalamic nuclei are connected in different ways. However, in his review of the literature, Crosson (1992) noted that posterior infarcts and anterior infarcts were equally likely not to express the syndrome mentioned by Crosson (1984). On the other hand, the author has seen the thalamic aphasia syndrome described by Crosson (1984) in a case of infarction of the ventral anterior nucleus extending into surrounding nuclei (i.e., polar artery territory: Nadeau & Crosson, 1997; Raymer, Moberg, Crosson, Nadeau, & Rothi, 1997), in a case of infarction in the dorsomedial, centromedian, and parafascicular nuclei (i.e., paramedian artery territory: Nadeau & Crosson, 1997; Raymer et al., 1997), and in a case of hemorrhagic infarction in the dorsal lateral nucleus and pulvinar (Crosson et al., 1986). Hence, it is difficult to localize this syndrome, when it does occur, to any one thalamic nucleus or set of neighboring nuclei. Crosson (1992) noted that the most frequent departure from the syndrome he described in 1984 was that auditory-verbal comprehension was more severely impaired than the syndrome description suggested. Minimally impaired or unimpaired repetition was clearly evident in most cases of thalamic infarction reviewed at that time.
Over the last several years, case studies and case series of thalamic aphasia have continued to be published (e.g., Cox & Heilman, 2011; Karussis, Leker, & Abramsky, 2000; Kuljic-Obradovic, 2003; Maeshima et al., 2001; Marien, Abutalebi, Engelborghs, & De Deyn, 2005; Perren, Clarke, & Bogousslavsky, 2005; Radanovic, Azambuja, Mansur, Porto, & Scaff, 2003; Radanovic & Scaff, 2003; Weisman, Hisama, Waxman, & Blumenfeld, 2003), and an additional review has appeared as well (Carrera & Bogousslavsky, 2006). Variability in language measurement continues. In general, the results are fairly consistent with Crosson’s (1992) assessment of the thalamic aphasia literature. Specifically, cases of thalamic hemorrhage more frequently resemble the thalamic aphasia syndrome described by Crosson (1984), and cases of thalamic infarction show greater variability in symptom picture.
One facet of the infarction cases mentioned more often than previously is nonfluent output. One important clue about nonfluent output in thalamic aphasia is time post-stroke. Specifically, Karussis et al. (2000) performed neurological evaluations on eight cases of left thalamic lesion at admission (3 hemorrhagic, 5 ischemic). Of the seven cases of thalamic aphasia, all were described as having nonfluent aphasias (three transcortical motor aphasia, four “motor” aphasia). All cases of transcortical motor aphasia had lesions in the polar artery territory (centered in the ventral anterior nucleus). Cases of “motor” aphasia had lesions in the polar artery territory (n=1), the intralaminar nuclei (n=2), and anterior choroidal artery territory (n=1). Kuljic-Obradovic (2003) gave the Boston Diagnostic Examination, the Boston Naming Test, the Token Test, and a verbal fluency test to nine patients with aphasia and thalamic lesions (3 hemorrhages, 1 hemorrhagic infarct, 5 infarcts) four to eight days post-stroke. Results were compared to patients with aphasia and either striatocapsular (n=15) or periventricular white mater (n=8) lesion, who showed aphasia symptoms. Thalamic cases showed preserved fluency, repetition, and grammar compared to the non-thalamic cases, which showed nonfluent output with preserved repetition. Naming was most severely impaired in the thalamic cases, and in those patients, verbal paraphasias (substitution of one word for another) were more common than other types of paraphasias. Thus, as a group, the thalamic aphasia symptoms described were similar to the syndrome described by Crosson (1984). Unfortunately, lesion location within the thalamus was not studied. Radanovic and Scaff (2003) gave the Boston Diagnostic Aphasia Examination and Boston Naming Test to seven cases of thalamic aphasia (4 left hemorrhage, 1 right hemorrhage, 2 left infarct) three weeks to eight years after stroke. All cases would be classified as fluent according to the Boston Diagnostic Aphasia Examination. Comprehension varied from moderately impaired to unimpaired, and repetition varied from mildly impaired to unimpaired, except for one case that was more severely impaired on repetition of phrases. Naming varied from moderately impaired to unimpaired. To the degree that the cases in these three studies are representative of thalamic aphasias, patients can show nonfluent aphasia soon after stroke but within a period of a few days, patients are more commonly described as having fluent output. This is consistent with the author’s experience. However, we must not over-generalize in drawing conclusions, since Cox and Heilman (2011) described a case with decreased fluency in output eight months post left posterior thalamic hemorrhage.
One other phenomenon is useful in understanding the variability in reports of fluent vs. nonfluent output in thalamic aphasia. Often, the literature has discussed thalamic connections to the cortex as if they were homogenous within a particular nucleus. For example, we might cite the connections of the dorsal medial nucleus as primarily with the prefrontal cortex. However, Goldman-Rakic and Porrino (1985) divided the monkey prefrontal association cortex into nine cytoarchitectonically discrete segments. After injecting horseradish peroxidase or fluorescent dyes into these distinct areas in 12 macaques, they traced the connections of prefrontal association cortex with the ventral anterior nucleus, the dorsal medial nucleus, and the pulvinar. For each of the three thalamic nuclei, the overwhelming majority of cytoarchitectonic prefrontal areas received projections from discrete, largely non-overlapping subdivisions of the thalamic nuclei. Hence, whether language is nonfluent or fluent may depend on whether the portion(s) of these nuclei that project to language-related frontal cortex (e.g., Broca’s area) are injured. The degree of damage to the segment projecting to language related cortex may determine how seriously the lesion affects language processes.
In the newer cases of thalamic aphasia mentioned above, it should be noted that repetition is usually minimally impaired though exceptions to this rule of thumb exist (e.g., case 3 of Radanovic et al., 2003 or cases 4 or 8 of Karussis et al., 2000). The case of Weisman et al. (2003) was specifically characterized by poor repetition in the presence of intact naming and comprehension and the absence of paraphasias after an infarct in the left ventralateral thalamus, though the ability to repeat recovered within a week. It was not unusual in the more recent cases for there to be substantial impairment of comprehension.
A frequently mentioned aspect of thalamic aphasia is the semantic nature of deficits and paraphasias (e.g., Cox & Heilman, 2011; Crosson, 1984, 1992; Mariën et al., 2005; Nadeau & Crosson, 2007; Radanovic, 2003). Conceptually driven studies of language mechanisms in thalamic aphasia are rare, but the Raymer and colleagues’ (1997) study is a notable exception and addressed the issue of semantic errors in thalamic aphasia. They gave a battery of word processing tests to two patients with left thalamic lesions (one in the polar artery territory, one in the paramedian artery territory). The battery was designed to distinguish between deficits at the purely lexical level, deficits at the purely semantic level, and deficits at the lexical-semantic interface. According to the theory employed, lexical processing involves word forms. Further, dissociable lexicons involve both modality of input (i.e., phonological input lexicon vs. orthographic input lexicon) and mode of output (i.e., phonological output lexicon vs. orthographic output lexicon). Meaning is attached to words only when the familiar word forms are processed by the semantic system, which confers meaning on words and objects. Damage to the semantic system results in deficits across modality of input and mode of output because meaning cannot be attached to words in any form after such damage. Raymer and colleagues’ (1997) patients performed normally on tasks that could be performed solely on the basis of lexical processing (oral word reading, writing to dictation), suggesting that deficits were not purely lexical in nature. They also performed normally on matching pictures of objects to both auditory and written words, confirming both that the semantic system per se was not compromised and that the ability to access semantic information on the basis of the phonological and orthographic input lexicons was intact. However, patients made more errors in naming than neurologically normal subjects by several standard deviations regardless of input modality (picture vs. definition) or mode of output (spoken vs. written). Both patients made a significantly greater proportion of errors on low as opposed to medium or high frequency items. Most of the errors involved either semantic errors or semantic plus visual errors (84% for one patient, 63% in the other patient). Since both the lexical and semantic systems seemed to be intact, the authors concluded that the problem was at the lexical-semantic interface. Based upon the analysis of Nadeau and Crosson (1997), they hypothesized that the deficit involved a loss of the ability of a frontal-thalamic system to selectively engage those cortical components needed to perform the picture-naming task. We will discuss this system in detail below. It is worth noting that more conceptually driven studies like Raymer and colleagues’ (1997) study are more likely to shed light on thalamic mechanisms involved in language than continuation of simple clinical-pathological correlation studies.
2.2. Other Language Symptoms
Other kinds of language symptoms are occasionally mentioned in cases of dominant thalamic lesion. For example, category-specific deficits in naming occasionally have been reported in cases of dominant thalamic lesion. Both Lucchelli and De Renzi (1992) and Moreaud et al. (1995) reported cases of category-specific naming deficits for proper nouns in the dominant polar artery territory. Crosson et al. (1997) reported a case of category specific naming deficit for medical objects and conditions with a small hemorrhagic lesion of the pulvinar and posterior limb of the internal capsule. The canonical explanation for category-specific deficits in naming relates to how knowledge relevant to the distinction of the items within the category in question is stored topographically (see Crosson, Cato, Sadek, & Lu, 2000 for discussion). If this explanation is true, then these findings suggest that the thalamus relates to relatively discrete areas of cortex, as demonstrated for example by the work of Goldman-Rakic and Porrino (1985) showing that discrete regions of the frontal lobe project to different, discrete regions within the ventral anterior nucleus, dorsal medial nucleus, and pulvinar of the thalamus.
Crosson (1999) also analyzed the oral reading data from a case with a lesion in the left lateral posterior nucleus and the pulvinar. The patient was found to have neglect dyslexia; i.e., he had two or more letters correct on the left side of misread words but replaced one or more letters on the right side of the word with different letters and the substitutions were real words. The fact that the patient could retrieve words correctly when they were spelled orally for him suggests that his errors were in visual processing rather than in the internal representation. Both category-specific naming deficits and neglect dyslexia have been found in cases of cortical lesion, and their existence in cases of thalamic lesion suggests that the key to understanding the role of the thalamus in language lies in understanding thalamocortical relationships.
2.3. Functional Imaging and Related Work of Kraut, Hart, et al
The key point of this section is that recent data from functional imaging studies suggest that it might be worth considering a slight modification of Raymer and colleagues’ (1997) conceptual explanation of semantic paraphasias in thalamic aphasia. Specifically, the loss of selective engagement of the components necessary for their naming tasks may have led to an inability to bind semantic features/concepts to the corresponding lexical representation. This explanation is motivated by a series of studies by Kraut, Hart and colleagues. These investigators developed a task in which subjects were given two attributes and were asked to determine if the attributes could be combined to make an object. For example, “desert” and “humps” can be combined to make “camel”, but “bullets” and “milk” do not combine to make an object. In functional magnetic resonance imaging (fMRI) studies, they demonstrated thalamic activity in attribute pairs that combined to make an object when attributes were presented as word pairs (Kraut, Kremen, Segal et al., 2002) and when attributes were presented in a word-picture pair (Kraut, Kremen, Moo et al., 2002). Thalamic activity was limited to the left side for word-word pairs but was bilateral for word-picture pairs, and it was not present for attribute pairs that did not make an object or in semantic association and semantic categorization tasks. In a follow-up study with a larger number of subjects, Assaf et al., (2006) found thalamic activity on both trials where features combined to make objects and trials where they did not, but activity was significantly greater in the left thalamus for trials in which the features combined to make an object than on trials in which they did not. In a study that investigated temporal aspects of hemodynamic responses for their task, Kraut, Calhoun, Pitcok, Cusick, and Hart (2003) showed that there were two foci of thalamic activity for attribute pairs combining to make an object: One was around the dorsal medial nucleus, and the other was in the pulvinar. Activity in the dorsal medial nucleus appeared to precede activity in the pulvinar. The authors hypothesized that the earlier activity in the dorsal medial nucleus, along with similar activity in pre-SMA, was involved in a semantic search for a match, and that the later pulvinar activity was involved in binding the features together in the process of object recognition.
Slotnick, Moo, Kraut, Lesser, and Hart (2002) had the opportunity to record thalamic activity and cortical activity during the same task. When attributes combined to make an object, there was a significant drop in alpha (7–8 Hz) at the thalamus and in multiple cortical locations 1–2 seconds after stimulus onset, followed by an increase in gamma activity (21–34 Hz) at thalamic and cortical locations 2–3 sec post stimulus onset. Average reaction time for trials was 1.57 seconds. Cortical and thalamic responses were phase locked, suggesting that they were related to one another as opposed to being independent. Slotnik et al. hypothesized that the thalamus was involved in binding the features together for object recognition through driving cortical gamma rhythms. One inconsistency between Slotnick et al. (2002) study and the Kraut et al. (2003) study is that the thalamic target for electrodes was the dorsal medial nucleus, not the pulvinar which Kraut et al. (2003) implicated in feature binding.
Hart et al. (2007) discussed a “neural hybrid model of semantic object memory” derived from these experiments in some detail. They emphasized that the semantic system is widely distributed in the cortex, a proposition that, by now, is well accepted. For example, a tool like a hammer might be associated with a visual form (a handle with a specific kind of head), with movement memories associated with swinging a hammer oneself, with visual memories of what someone swinging a hammer looks like, and with knowledge of the use of a hammer. All of these attributes are critical to understanding a hammer, are located in areas of cortex distant from one another, and are bound together to represent a hammer when the concept is internally or externally evoked. (See Kraut et al., 2006; Wierenga et al., 2009 for examples of distributed semantic processing.) Hart et al. (2007) proposed that thalamic activity drives gamma rhythms associated with the feature binding.
At this point, a summary of the role of the thalamus in language based upon the above review is in order. Lesions of the dominant thalamus frequently lead to aphasia. Generally, thalamic aphasias resolve relatively rapidly, and time post-onset (e.g., admission vs. a few days after admission) may make some difference regarding the degree or even the nature of the symptoms. The most nearly ubiquitous feature of thalamic aphasia is intact to minimally impaired repetition. Since repetition can be based upon matching items in the phonological input and output lexicons, we can conclude lexical processing generally is unimpaired or minimally impaired in thalamic aphasia. More recent descriptions of thalamic aphasia have suggested nonfluent output that may or may not be agrammatic, but this feature may fade within a week or less, at which time output may be described as fluent. Output can deteriorate to jargon in many cases relatively early in the evolution of thalamic aphasias, though this feature does not seem to be an essential characteristic of thalamic aphasias. Comprehension can vary from minimally to significantly impaired, especially at the phrase level or for longer communications. Though the nature of paraphasias is often insufficiently analyzed, semantic paraphasias are frequently mentioned. One analysis of paraphasias in two patients suggested that they are semantic substitutions. However, the underlying cause does not seem to be gross disruption of the semantic system because other functions requiring semantic representations were intact. The suggestion has been made above that semantic paraphasias could result from an inability to bind semantic attributes representing a concept to their corresponding lexical representation. The latter hypothesis is consistent with recent neuroimaging and neurophysiological research indicating that during semantic feature binding, thalamic nuclei are active and exhibit rhythmic activity in the gamma range which is linked to similar activity in multiple cortical locations. The discussion now turns to evidence suggesting probable thalamic mechanisms underlying language functions, with an emphasis on recent physiological data.
3. Thalamic Mechanisms Potentially Underlying Language and Related Processes
In the last 15 years, a good deal of research on anatomy and physiological mechanisms of the thalamus has been accomplished. Discoveries from these studies are exciting because they are shifting the paradigm of the thalamus as a simple relay of information from the periphery to a much richer understanding of how the thalamus participates in and regulates the flow of information in the brain. Before discussing four specific mechanisms that may be relevant to language functions, it is necessary to review two more general facets of thalamic functions: the two states in which thalamic neurons reside and the two types of thalamic inputs.
It has been known for some time that thalamic relay neurons reside in one of two states (e.g., McCormick & Feeser, 1990). When the relay neuron’s resting potential is relatively polarized (~−75 mv), the neuron produces high frequency bursts of firing. The relationship of driving inputs to the output of the relay neurons in this case is decidedly nonlinear. In other words, there is a poor correspondence between driving inputs and thalamocortical relay neuron firing. This state can be considered a low-fidelity (or non-attentive) mode of information transfer from the thalamus to the cortex. When the relay neuron’s resting potential is relatively depolarized (~−65 mv), rhythmic bursting is absent and there is a linear correspondence between driving input and the neurons’s output. This can be considered a high-fidelity (or attentive) mode of information transfer (Sherman & Guillery, 2006). For descriptive purposes, these states will be referred to for the remainder of this article as the low-fidelity and the high-fidelity transfer modes. Sherman has given an example of neuronal firing in the lateral geniculate nucleus (Sherman, 1996; Sherman & Guillery, 2006). Luminance was varied in a sinusoidal fashion in the retinal receptive field of a lateral geniculate neuron. In the high-fidelity mode, the neuron’s firing pattern closely approximated the sinusoidal pattern of the input, but in the low-fidelity mode, the resulting pattern of neuronal firing did not maintain the close correspondence to the input. It was recognized that these modes of firing represented attentive (high-fidelity transfer mode) and non-attentive (low-fidelity transfer mode) states (e.g., McCormick & Feezer, 1990).
The second relevant general facet of thalamic functioning is the dual nature of axonal inputs to the thalamus. They can be classified as either drivers or modulators. Drivers impact the pattern of firing of thalamic relay cells, and in the high-fidelity transfer mode, relay cells are presumed to pass information along with little degradation or change in quality. Drivers may originate from sensory input channels (e.g., from the retina via the optic tract) in lower order relays or from the cortex in higher order relays. Drivers do not give off collaterals as they pass through the nucleus reticularis (NR: a thin sheet of GABAergic neurons surrounding the thalamus). They contact only ionotropic receptors, making for rapid transfer of information across the synapse, and they contact dendrites relatively close to the cell body, giving them a more powerful influence on cell firing than contacts terminating on distal dendrites. Terminals are large, but the number of contacts of drivers on relay cells is small. Generally, drivers are glutamatergic, and cortical drivers originate from cortical layer 5. As their name suggests, modulators modulate the firing pattern of relay cells, in part through evoking the high- or low-fidelity transfer modes. As they pass through the NR on their way to other thalamic nuclei, they give off collaterals terminating in the NR. Modulators may contact either metabotropic or ionotropic receptors, and dendritic contact is made distally as well as proximally. Modulators may be glutamatergic, cholinergic, noradrenergic or GABAergic (Sherman and Guillery, 2006). Cortical modulators emerge from cortical layer 6. One reservation about the term modulator expressed by Jones (2009) is that it may be overly simplistic to classify projections from cortical layer 6 to the thalamus simply as modulators. The reason for this reservation will become clearer in the section on sharpening the focus for relevant information through corticothalamic feedback mechanisms. The distinctions made between high-fidelity and low-fidelity transfer modes and between drivers and modulators will be useful as the four thalamic mechanisms are discussed below.
3.1. Selective Engagement of Cortical Areas in a Heightened State of Responsiveness
In 1997, Nadeau and Crosson hypothesized that there was a system that engaged cortical regions needed to perform a task, while holding those regions not involved in a task in a state of relative disengagement (Figure 1). This subsection will deal with modifications of the theory necessitated by recent data. This system proposed by Nadeau and Crosson (1997) involved frontal cortex, the inferior thalamic peduncle (conveying fibers from the frontal cortex to the thalamus), the ventral anterior portion of the nucleus reticulars (NR), and the centromedian nucleus. This theory was built both on clinical-anatomic correlation for four cases of thalamic aphasia as well as an extensive literature review, but the basics of the theory are as follows. According to the theory, the frontal lobes contact the ventral anterior NR via the inferior thalamic peduncle. The NR is a thin sheet of neurons wrapping itself around the anterior and lateral aspects of the thalamus. The NR is populated by GABAergic neurons, and the external termination of these neurons is on both relay cells and interneurons in the various thalamic nuclei. Corticothalamic axons originating from layer 6 (modulators) and thalamocortical fibers both give off collaterals to the NR as they penetrate it on their way to or from the thalamus. Topographically, the NR is organized by the thalamic nuclei to which it projects. Generally, any segment of the NR receives input from thalamocortical fibers originating in the nucleus to which it projects. Through long dendritic bundles, the collaterals from the inferior thalamic peduncle contact neurons projecting to portions of the NR related to various thalamic nuclei.2
Figure 1.
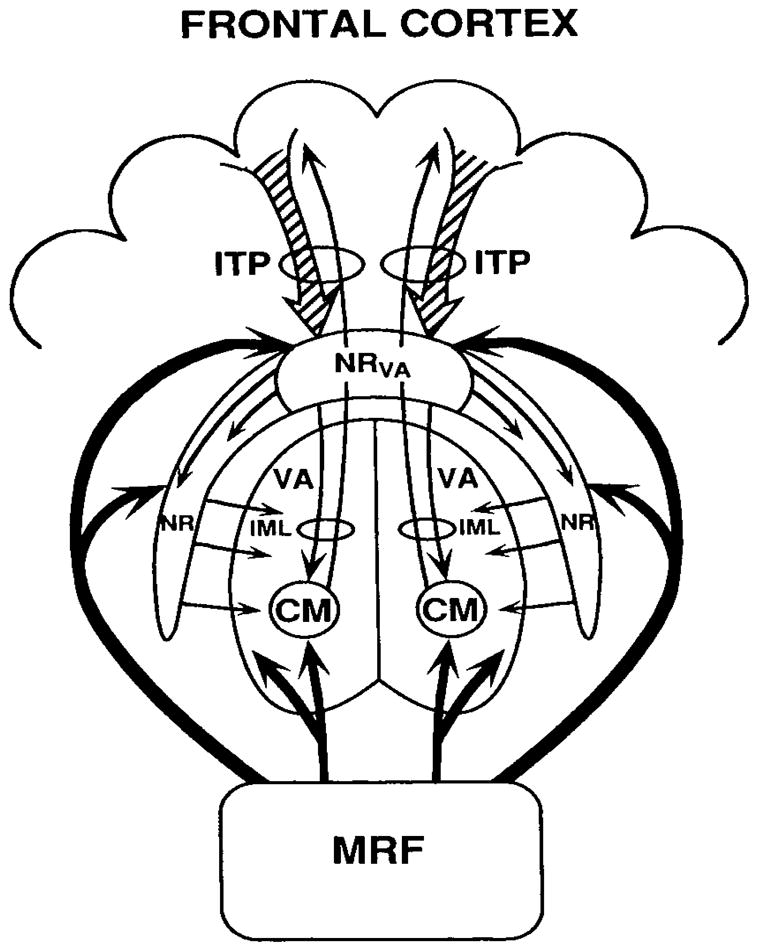
Schematic drawing of the fronto-inferior thalamic peduncle-nucleus reticularis- centromedian system that Nadeau & Crosson (1997) hypothesized to be responsible for selective engagement of cortical nets necessary for processing semantic properties of specific stimuli. CM = centromedian nucleus of the thalamus, IML = internal medullary lamina, ITP = inferior thalamic peduncle, MRF = midbrain reticular formation, NR = nucleus reticularis, NRva = ventral anterior nucleus reticularis, VA = ventral anterior nucleus of the thalamus. From S.E. Nadeau & B. Crosson. (1997). Subcortical aphasia. Brain & Language 58(3), 355–402, reprinted by permission of Elsevier.
As the theory continues, the glutamatergic frontothalamic connections can excite NR neurons, which in turn send inhibitory GABAergic fibers to the various thalamic nuclei. Through these connections, NR neurons can decrease activity in inhibitory GABAergic thalamic interneurons. In thalamocortical relay neurons, the reduction of GABAergic interneuron activity would reduce inhibitory influences, switching them to a high-fidelity transfer mode. According to the theory, in the centromedian nucleus, the reduction of inhibition of thalamocortical neurons allows them to increase firing. Unlike most other thalamic nuclei, the centromedian nucleus was thought to project diffusely to the cerebral cortex (a tenet that has since been challenged by Sadikot & Rymar, 2009), where their fibers terminate on the distal dendrites of cortical cells. These contacts could allow the increased thalamocortical activity to enhance cortical excitability in the region to which they project. At the same time that firing of specific NR neurons is increased, excited NR neurons inhibit neighboring neurons, causing them to reduce their inhibition of GABAergic thalamic interneurons which in turn increase the activity of the latter. Nadeau and Crosson (1997) suggested the term “selective engagement” for this process of temporarily engaging the topographically disparate regions of cortex necessary to perform a task, while holding areas not involved in the task in a state of relative disengagement. Considering the proposed role of the frontal cortex in this process, the authors considered selective engagement a form of attention guided by initiation of or engagement in action (i.e., intentionally guided attention). Nadeau and Crosson (1997) left it ambiguous as to whether it was the influence of the NR over higher order relay nuclei or over the centromedian nucleus that was responsible for selective engagement, though their clinical-pathological correlations favored the centromedian explanation, as just noted.
Recent evidence suggests that this widely cited theory is generally correct; however, the specifics require some modifications. First, there are two anatomic modifications that are necessary. (1) The first anatomic point regards the centromedian nucleus. In Nadeau and Crosson’s (1997) patient with a typical paramedian territory lesion, the centromedian nucleus was almost completely involved in the lesion, leading us to concentrate our attention on centromedian nucleus as the component of the intralaminar nuclei possibly involved in thalamic aphasias. However, the parafascicular nucleus also was significantly involved. In a recent review, Sadikot and Rymar (2009) reviewed available evidence and concluded that the centromedian nucleus in primates projects only to motor and premotor cortex. On the other hand, the parafascicular nucleus, which is just medial to the centromedian nucleus, projects to prefrontal cortex as well as to premotor cortex, anterior cingulate cortex, and frontal eye fields. Given that Broca’s region involves mostly cortex anterior to premotor cortex, it appears that the parafascicular nucleus may be a more appropriate candidate for inclusion in the model of selective engagement. (see Hagoort, 2006 for a discussion of the components of Broca’s region). However, the lack of evidence for projections to posterior cortices (Sadikot & Rymar, 2009) leaves doubt as to whether this nucleus has the requisite connections for selective engagement. Nadeau and Crosson (1997) noted an alternative to involvement of the centromedian (or parafascicular) nucleus in selective engagement. They noted that the pulvinar has the requisite cortical connections to participate in selective engagement, but this speculation raises questions about how the pulvinar is connected to frontal cortex. (2) Thus, the second anatomical point regards the inferior thalamic peduncle as the conduit of frontal fibers to the thalamus. We recently mapped connections from Broca’s area proper (pars triangularis and pars opercularis) to the thalamus (Ford et al., 2010). The short version is that the tracts from Broca’s area would more appropriately be classified as a part of the anterior thalamic peduncle. More specifically, the paths from pars triangularis and pars opercularis are very similar. After taking an anteromedial direction from Broca’s area, they traverse the circular sulcus and then turn tightly to take a posteromedial direction toward the thalamus. After that turn, the fibers form a sheet oriented in a superior to inferior direction. The more inferior fibers enter the anterior aspect of the thalamus from a lateral position at the ventral anterior nucleus. As this sheet of fibers traverses the anterior aspect of the thalamus from an inferior to superior position, the more superior fibers enter the ventral anterior nucleus from a superior position. We found no other bundle of fibers from Broca’s area that enters the thalamus. If we assume that in humans, the dorsal medial nucleus and the pulvinar receive fibers from Broca’s area, as is the case with other frontal cortices in the macaque (Goldman-Rakic & Porrino, 1985), then the fibers making these connections would have to traverse the ventral anterior nucleus as fibers en passage that eventually terminate in the dorsal medial nucleus or pulvinar. With the trajectory of fibers just described, it should be noted that a lesion in the polar artery territory that occupies most of the ventral anterior nucleus is likely to interrupt all fibers from Broca’s area to the thalamus.
Nadeau and Crosson’s (1997) theory of selective engagement also can be extended on the basis of neurophysiological evidence. In particular, selective engagement appears to be accompanied by gamma rhythms (20–80 Hz) in both cortical and thalamic structures. Bouyer Montaron, and Rougeul (1981) demonstrated that in an attentive state, cats showed gamma (35–45 Hz) activity in frontal (motor) cortex and parietal cortex (area 5), with intervening cortex not showing this activity.3 A thalamic nucleus was identified that also demonstrated gamma activity in high coherence with the parietal focus. When this nucleus was destroyed, gamma activity disappeared in the parietal but not in the motor focus. In response to auditory stimulation, Ribary et al. (1991) demonstrated similar 40 Hz activity in the cortex and thalamus of humans using magnetoencephalography. As noted above, Slotnik et al. (2002) demonstrated activity in the gamma frequencies that can be seen in the thalamus and cortex during a task when two presented features combine to make an object. Hart and Kraut (2007) saw the gamma activity as indicative of the feature binding process. As noted previously, the NR can be divided into sectors based upon the thalamic nuclei and cortex to which they relate. Macdonald, Fifkova, Jones, and Barth (1998) showed that stimulation (0.5 second trains of 500 Hz 0.5 ms pulses at 5–10 μA) of an NR sector in rats induced gamma frequency oscillations lasting for the duration of the stimulation specific to cortex associated with that sector. For example, stimulation of the auditory sector of the NR induced gamma oscillations (~40 Hz) in auditory but not somatosensory cortex and vice versa. Further, the area of cortex in which this gamma activity is induced can be quite specific because stimulation of the NR could evoke gamma activity in the forepaw region of the somatosensory cortex but not in other somatosensory representations.
In summary, evidence suggests that gamma activity in the cortex happens in attentive states, and this activity appears to be related to activity in associated thalamic nuclei. The fact that stimulation of an NR sector elicits gamma activity highly specific to the cortex associated with that NR sector suggests that the NR plays a role in eliciting this gamma activity. Thus, selective engagement of a neural net necessary to perform a specific task may be done through a cortico-NR-thalamo-cortical mechanism that elicits gamma rhythms in the temporarily engaged net. Pinault (2004) indicated that the mechanism by which NR evokes cortical gamma rhythms is not yet understood. Nadeau and Crosson (1997) had suggested that selective engagement might be accomplished by inhibition of inhibitory interneurons in thalamic nuclei. Subsequent anatomic data suggest that the thalamic mechanism responsible for selective engagement is neither the centromedian nucleus nor the parafascicularis nucleus. Rather, the pulvinar seems to be a more likely candidate; the functional and anatomic evidence supporting this assertion is discussed in greater detail below.
It is important to note a further aspect of selective engagement. Nadeau and Crosson (1997) suggested that selective engagement is essentially intentionally guided attention. In other words, once an intention to act is formed, the frontal lobes engage the cortical nets relevant to the intended activity. For example, if one intends to engage in a conversation, frontal cortex associated with language, via the nucleus reticularis and the pulvinar, engages cortices related to understanding and formulating language. It is proposed that gamma activity is the signature for that engagement. At the same time, areas not involved in the intended activity would be held in a state of relative disengagement so as to minimize attention to stimuli irrelevant to the intended task. It is important to note that after Nadeau and Crosson (1997) proposed selective engagement as a mechanism relevant to language, they still had difficulty explaining some aspects of thalamic aphasia. One possible resolution to this conundrum is that additional knowledge regarding thalamic mechanisms is required. In the subsections that follow, three thalamic mechanisms will be discussed that are different from, but related to selective engagement. This exposition begins with discussion of the thalamus as a mechanism for passing information from one cortical area to another, which is directly related to selective engagement.
3.2. Passing Information from One Cortical Area to Another
Sherman and Guillery (2006) divided thalamic relays into two types: first order relays and higher order relays. First order thalamic relays receive their driving afferents from ascending pathways. Examples of first order relays include fibers from the optic tract to the lateral geniculate nucleus for relaying information to primary visual cortex, fibers from the brachium of the inferior colliculus to the medial geniculate nucleus for relaying information to primary auditory cortex, and fibers from the medial lemniscus and spinal thalamic tract to the ventral posterior lateral and ventral posterior medial nuclei for relaying somatosensory information to primary somatosensory cortex. Higher order relays receive their driving afferents from layer 5 of specific cortical regions and pass information along to another area of cortex.4.
At the time that Sherman and Guillery (2006) wrote their book, the case for higher order relays was made primarily on the basis of indirect evidence, such as the morphology of putative higher-order driver synapses, their similarities to first order drivers, and their anatomic origin. The evidence was compelling, but more recent studies have put us a few steps closer to confirming the existence of corticothalamo-cortical relays. In mice, Llano and Sherman (2008) investigated connections between primary and closely associated auditory cortex (A1 and anterior auditory field (AAF), respectively), the dorsal and ventral divisions of the medial geniculate body (MGBd and MGBv, respectively), and secondary and closely associated auditory cortex (A2 and dorsoposterior region (DP), respectively).5 For tracer injections into A1 and AAF, modulators (small terminals) were found in both MGBv and MGBd, but drivers were found almost exclusively in MGBd. When MGBv was injected with a tracer, anterograde and retrograde labeling occurred in A1 and closely associated AAF. When MGBd was injected with the tracer, the densest anterograde labeling was seen in layers 1, 4, and 6 of A2 and closely associated DP, with lighter labeling of layers 1 and 6 of A1. Retrograde layer 6 labeling was seen mainly for A2 and DP, but layer 5 retrograde labeling was seen in all auditory cortices. Hence, drivers from A1 and AAF project almost exclusively to MGBd, which, in turn, projects primarily to A2 and DP. In other words, the circuitry for a higher order relay from A1/AAF to MGBd and then to A2/DP exists.
Even more telling was Theyel, Llano, and Sherman’s (2010) demonstration that a corticothalamo-cortical circuit could drive higher-order cortex in the mouse. The anatomic architecture for the experiment is represented in Figure 2. They worked with a slice of mouse brain that contained the barrel fields from primary somatosensory cortex (S1), the posteromedial nucleus of the thalamus (POm), and secondary somatosensory cortex (S2). They cut the connections between S1 and S2. They used a new optical imaging technique to observe activity. When they stimulated S1, activity in S2 and POm was evoked. POm receives layer 5 fibers from S1 and projects to S2. When they chemically inactivated POm, stimulation of S1 did not activate S2, but after washout of the chemical agent from POm, stimulation of S1 again activated S2. The stimulation of S1 that activated S2 in these experiments was in layer 5B. Layer 6 stimulation did not activate S2. Findings are consistent with a higher order relay from S1 to POm then to S2. One caveat is that Sherman and Guillery (2006) indicated that the nature of such relays can only be understood when we understand the nature of the information that traverses them, which the latter experiment did not address. But, to this point, the evidence is highly consistent with the existence of corticothalamo-cortical relays.
Figure 2.
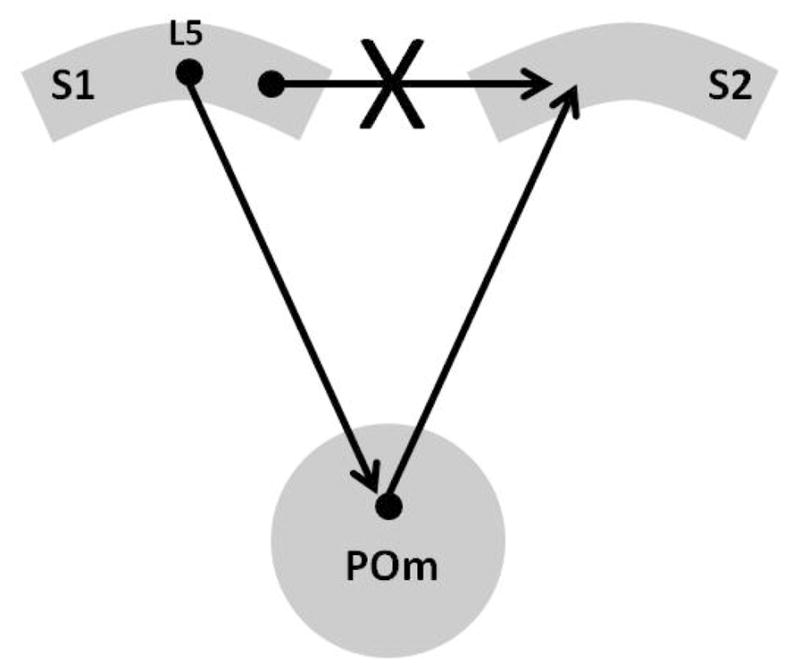
This schematic represents the experiment of Theyel et al. (2010). They cut the cortico-cortical connections between S1 and S2 in the mouse brain in vitro. Under these conditions, S2 could still be activated by stimulating S1. When the thalamic component was chemically inactivated, it was no longer possible to evoke a response in S2 by stimulating S1. After washout of the chemical inactivation, the ability to activate S2 by stimulating S1 was reestablished, demonstrating that it was the corticothalamo-cortical connection that was responsible for activating S2 during S1 stimulation. L5 = cortical layer 5, POm = posteromedial nucleus of the thalamus, S1 = primary somatosensory cortex, S2 = secondary somatosensory nucleus.
Assuming, as did Sherman and Guillery (2006), that this corticothalamo-cortical mechanism has a purpose rather than being the vestiges of some evolutionary process, a critical question arises: What purpose does passing information through the thalamus serve if there are already corticocortical connections? Sherman and Guillery (2006) entertained the notion that the corticothalamo-cortical route might transfer information whereas the cortico-cortical route might serve a modulatory function. There is no way of knowing for certain at this point in time, but the current author favors another possibility: Passing information through the thalamus serves an attentional function, not only for language but also for other cognitive processes. With respect to language, suppose that you are talking to a friend and from the other room, you hear your child yelling frantically from another room. Your conversation and your child’s yelling are competing for the same resources needed to decode the verbal content from both. You need a mechanism for temporarily giving the content of your child’s message priority so you can determine whether it requires your intervention. Fronto-NR connections, using selective engagement, can be used to put one source of information in a low-fidelity (unattended) transfer mode while raising the other to a high-fidelity (attended) transfer mode. The ability to make such a distinction between information sources could be based on the pitch or other voice quality or on the location of the information sources. The thalamus has this mechanism for prioritizing one source of information over another.
As noted above, the physiological basis of these modes of transfer lies in the resting potential of the relay cell’s membrane. The relatively polarized state is the low fidelity transfer mode while the relatively depolarized state is the high fidelity mode. It has been proposed that these states are controlled by inhibitory input from the NR to GABAergic interneurons in thalamic nuclei which decreases the inhibition of relay neuronsby these interneurons, allowing them to attain the high fidelity mode of transfer (Steriade, Domich, & Oakson, 1986). Indeed, it may be no accident that lateral geniculate neurons fire spontaneously at a rate of 30–40 spikes per sec in the high fidelity transfer mode (Sherman, 1996; Sherman & Guillery, 2006). Hence, it is suggested that selective engagement, via frontal-NR connections, places thalamic relays in the high fidelity transfer mode for which gamma rhythms are the signature. In this mode, high fidelity information can be relayed from one area of cortex to another through the thalamus. When a source of information is no longer relevant to intended activity, its corresponding thalamic relay neurons can be placed in a low fidelity transfer mode through frontal-NR mechanisms. Clearly, we are suggesting a prominent role for lateral frontal cortex in this attentional mechanism. It has not been necessary to invoke other structures thought to be involved in attention, such as medial frontal or parietal cortices (e.g., Corbetta et al., 2000) in this specific mechanism. However, they may be involved in related attention mechanisms (see section 3.4).
Given the fact that most association cortex, including frontal association cortex, is richly connected with one or more thalamic nuclei, the concept of higher order relays to transfer information between association cortices is assumed to have wide-spread applicability to a variety of systems relevant to cognition. As our primary interest in the current paper is language, we are primarily interested in left-hemisphere perisylvian language areas and related semantic networks. The connectivity of the pulvinar (e.g., Goldman-Rakic & Porrino, 1985; Jones, 2007) makes it a likely nucleus to mediate communication of information from one area of language eloquent cortex to another. Examples of putative pulvinar involvement in lexical-semantic processes is given in subsequent sections.
3.3. Sharpening the Focus through Corticothalamic Feedback Mechanisms
Over the past several years the role of feedback from cortical layer 6 cells to thalamic nuclei has been investigated. As noted above, corticothalamic input from layer 6 cells is considered to modulate thalamocortical transmission rather than drive it. Fibers originating in layer 6 give off collaterals to the NR in contrast to fibers originating in layer 5, which do not, and fibers from layer 6 terminate on both metabotropic and ionotropic receptors, in contrast to layer 5 fibers which contact only ionotropic receptors (Sherman & Guillery, 2006). It is of interest that these modulatory inputs are in much greater abundance than inputs that drive thalamic output (Sillito, Cudeiro, & Jones, 2006). Layer 6 corticothalamic input often reciprocates thalamocortical output to the same cortical focus; however, layer 6 cortical thalamic fibers also can target other related thalamic regions in a nonreciprocal fashion (e.g., Llano & Sherman, 2008). Layer 6 feedback to the thalamus can influence the firing pattern of thalamic relay neurons, for example by facilitating a change from the low- to the high-fidelity transfer mode (Sillito et al., 2006).
In their review of layer 6 corticothalamic feedback mechanisms, Sillito et al. (2006) discussed how in the visual system, area MT (a cortical region specialized for processing motion) can influence primary visual cortex through corticothalamic feedback mechanisms. MT projects to V1, including layer 6 where contact can be made with corticothalamic cells. MT cells have much larger receptive fields than V1 cells, and MT cells can be direction selective for movement, while V1 cells are not. The feedback from MT cells to V1 layer 6 projects back to an area that corresponds to the receptive field size of the MT cell rather than the smaller receptive fields of V1 cells. These characteristics of the feedback system allow MT to influence V1 cells through thalamocortical mechanisms ahead of a stimulus in a location and in the direction of movement predicted by the current direction. In this way, MT can ready “predicted” retinotopic locations to process the stimulus. The authors cited their previous work in support of their hypothesis. The basic architecture addressed in these experiments is addressed in Figure 3. They used a highly localized iontophoretic application of a GABAB receptor antagonist to MT, increasing visual response amplitude but in a dose that did not change spontaneous activity. The increased MT response amplitude led to activity increases in some V1 cells and decreases in others (Sillito et al., 2006). In a different experiment using a similar methodology (Sillito & Jones, 2002), the authors demonstrated that a small change in response magnitude of MT led to significant changes in the response magnitude of cells in the lateral geniculate nucleus (LGN), which in turn influence V1 cells. Salt et al. (in press) have shown that the metabotropic glutamate 1 (mGlu1) receptor may play some role in feedback from layer 6 to the thalamus, primarily by enhancing the amplitude of response to trains of sensory stimulation after the first stimulus in the train.
Figure 3.
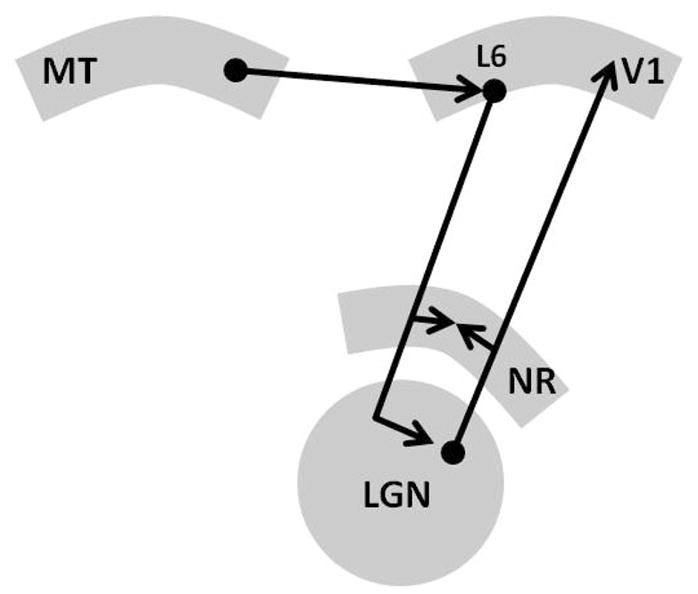
Sillito et al (2006) discussed how in the visual system, area MT can influence primary visual cortex through corticothalamic feedback mechanisms. MT projects to V1, including layer 6, where contact can be made with corticothalamic cells. The modulatory input from V1 layer 6 to the lateral geniculate nucleus in can influence the thalamocortical relay cell, allowing MT to sharpen features in M1 needed for MT to resolve movement-related information. L6 = cortical layer 6, LGN = lateral geniculate nucleus, MT = middle temporal cortex involved in visual perception of movement, NR = nucleus reticularis, V1 = primary visual cortex.
The reader is referred to the cited references for further details, but the subject under consideration here is the possibility of similar thalamic mechanisms in language. In this regard, the important consideration is that layer 6 corticothalamic feedback can enhance information about the stimulus contained in the input firing pattern and fine tune local circuitry to optimize the extraction of salient features (Sillito et al., 2006). Further, it appears that this feedback can be driven by corticocortical feedback from downstream cortical processors to upstream layer 6 neurons. Perhaps, such a mechanism could explain the neglect dyslexia in Crosson’s (1999) case of thalamic aphasia. As described above, the patient often replaced target words in oral reading with other words that had at least the same two beginning letters but differed in at least one of the final letters of the word. As in other cases of neglect dyslexia, the patient seemed to have awareness that the right side of the word existed because he replaced it with differing letter combinations than the target as opposed to just dropping the right side of the word. Hence, the deficit appeared to involve inadequate processing of features (letters or in some instances syllables) on the right side of words, which could result from disturbed corticothalamic feedback resulting in an inability to sharpen the focus on the right side of words.
Similarly, damage to such feedback mechanisms also could result in the comprehension deficits seen in some cases of thalamic aphasia. A failure to sharpen the focus on auditory-verbal information might lead to reduced processing accuracy. As envisioned, this deficit might be amplified by difficulty level. For example, in cases of complex syntax, one must relate one element of the sentence to others. A failure to sharpen the focus on the other elements might result in an inability to relate a currently processed element to other sentence elements. The variability in comprehension that is seen across cases of thalamic aphasia may be related to the importance of specific thalamic nuclei or subnuclei in comprehension. Hence, if such an important structure is damaged, comprehension will be impaired, but when it is not affected, comprehension should be less affected. Apparently, this is not the case when semantic features must be bound to a lexical item during word finding. This issue is discussed at greater length below, but briefly it may be due to the trajectory of relevant pathways through the thalamus, or to the effort required to bind semantic features to a lexical item.
3.4. Role of the Thalamus in Basal Ganglia Loops: Selection of One Word over Another
Any discussion of the role of the thalamus in language would be incomplete without briefly considering its role in basal ganglia loops. Although aphasia was reported with left basal ganglia lesions in the 1970’s, 1980’s, and 1990’s, based on the literature, Nadeau and Crosson (1997) surmised that such aphasias were the result of cortical under-perfusion. Indeed, Hillis et al. (2002) subsequently showed that aphasia did not occur acutely in left striatocapsular infarcts unless the language-eloquent cortex was underperfused as well, providing definitive proof of our prior hypothesis. This finding was relatively consistent with Copland, Chenery, and Murdoch’s (2000a) findings that patients with chronic nonthalamic subcortical lesions, including the basal ganglia, generally were not impaired on various subtests of the Western Aphasia Battery (WAB: Kertesz, 1982), a common test used to identify and classify aphasias. Yet, the findings of Copland et al. were equally definitive regarding more complex language functions as measured by the Test of Language Competence (TLC: Wigg & Secord, 1989) and the Test of Word Knowledge (ToWK: Wigg & Secord, 1992). For example, patients would attempt to resolve the meanings of semantically ambiguous sentences (one of the TLC subtests) or to give definitions for words (one of the ToWK subtests). Patients with chronic lesions of the basal ganglia and surrounding white matter were impaired on seven of the eight subtests in these two instruments.6
Further, Crosson et al. (2003) had shown that portions of the pre-SMA/Brodmann’s area 32 loop (pre-SMA/Brodmann’s area 32, dorsal caudate, ventral anterior nucleus of the thalamus) were active when generating members of a semantic category or when generating words rhyming with a given word; however, the constituents of this loop were not active when the subjects were generating nonsense syllables. It should be noted that pre-SMA and/or Brodmann’s area 32 have also shown activity for verb generation (Petersen et al., 1988) and for sentence repetition and sentence generation (Tremblay & Small, 2011), although not for word repetition (Crosson et al., 2001). These findings raise the question of what basal ganglia loops do in complex language functions and in word generation.
There are multiple basal ganglia loops starting and ending mostly (but not entirely) in discrete areas of the frontal cortex. These loops are thought to be segregated from each other at the subcortical level. Relevant frontal areas include SMA, pre-SMA, cingulate cortex, motor cortex, lateral premotor cortex, frontal eye fields, orbitofrontal cortex, and dorsolateral prefrontal cortex (Akkal, Dum, & Strick, 2007; Alexander, DeLong, & Strick, 1986; Middleton & Strick, 2000). Recently, in humans, we used diffusion weighted imaging and tractography and found that Broca’s area projects to the anterior putamen, and hence, it probably has its own basal ganglia loop. The projections of pars opercularis and pars triangularis (the two parts of Broca’s area) follow the same pathway, heading medially and slightly anteriorly around the insula and then curving in a posterior direction until they enter the putamen through its anterior aspect (Ford et al., 2010).
The direct basal ganglia loop was the first of three basal ganglia loops to be described (see Figure 5d in “Conclusions and Integration” section titled “An Integrated Model of Thalamic Language Mechanisms”). In the direct loop, frontal cortex projects excitatory (glutamatergic) fibers to the striatum (caudate nucleus, putamen, or nucleus accumbens) which in turn projects inhibitory (GABAergic) fibers to the internal segment of the globus pallidus (GPi). Subsequently, the GPi projects inhibitory (GABAergic) fibers to various thalamic nuclei, and these thalamic nuclei project back to the same cortical area from which the loop originated (through excitatory glutamatergic pathways). By tracing the inhibitory and excitatory connections of the direct loop, one can see that the net effect of exciting the direct loop is for its thalamic connections to increase excitatory input to its target area of frontal cortex.
Figure 5.
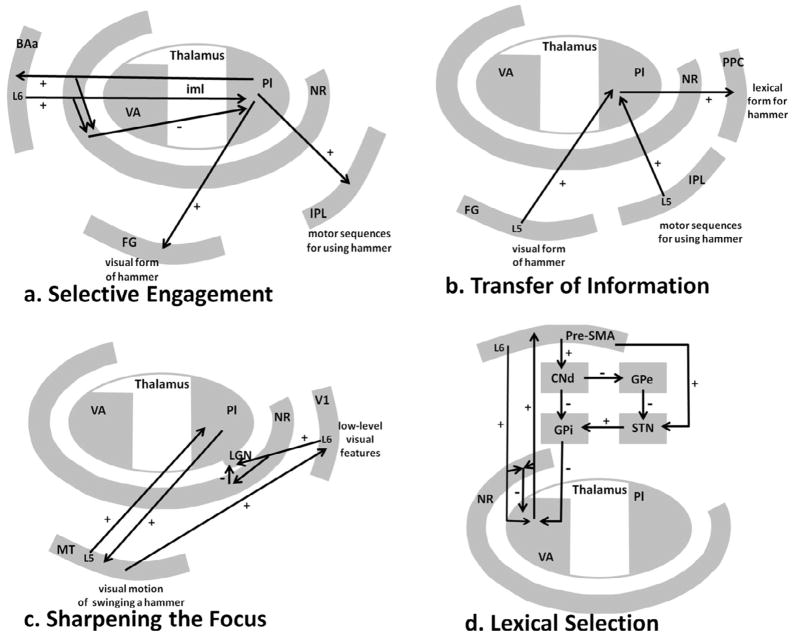
Four thalamic mechanisms proposed to affect language. (a) Selective Engagement. This schematic represents a revised selective engagement mechanism whereby anterior Broca’s area (BAa) contacts the nucleuse reticularis (NR). NR in turn places parts of the pulvinar necessary to represent features (e.g., visual form and motor sequences) of a hammer in a state of selective engagement. (b) Transfer of Information. This schematic represents passing information about the features of hammer on to a pattern associator in the inferior parietal lobe that takes the features as an input and produces the correct word as an output. (c) Sharpening the Focus. The observed motion involved in swinging a hammer can be sharpened by feedback from MR to V1 layer 6 (L6), which in turn, influences thalamocortical relays in the lateral geniculate nucleus (LGN). Not shown is that LGN relay cells, in turn, can influence V1 information necessary to identify the movement. (d) Lexical Selection. This schematic represents the hyperdirect, direct, and indirect loops for pre-SMA. Also shown is the possibility that layer 6 corticothalamo-cortical feedback might influence the output of these loops from the ventral anterior nucleus to the thalamus. BAa = anterior Broca’s area, GPi = internal globus pallidus, GPe = external globus pallidus, CNd = dorsal caudate nucleus, FG = fusiform gyrus, iml = internal medullary lamina, IPL = inferior parietal lobule, L6 = cortical layer 6, LGN = lateral geniculate nucleus, NR nucleus reticularis, Pl = pulvinar, PPC = posterior perisylvian cortex, STN = subthalamic nucleus, V1 = primary visual cortex, VA = ventral anterior nucleus.
Over the last 25 years, two additional basal ganglia loops have been described in addition to the direct loop. Indirect loops starts in the same areas of cortex as the direct loops, project glutamatergic fibers to the striatal component of the loop. The striatum, in turn, uses GABAergic fibers to project to the external globus pallidus (GPe), which has GABAergic projections to the subthalamic nucleus (STN). The subthalamic nucleus sends excitatory glutamatergic fibers to the GPi, which sends GABAergic fibers to the thalamic nucleus involved in the loop. Finally, the thalamus sends glutamatergic fibers to the area of cortex from which the indirect loop projects. The net effect of exciting the indirect loop is that its thalamic connections decrease their output to the cortical component of the loop.
The final basal ganglia loop to be discovered was the hyperdirect loop. Its frontal component sends excitatory glutamatergic fibers directly to the STN, which in turn sends glutamatergic fibers to the GPi. As in the other loops, the GPi sends GABAergic fibers to the thalamic component, which sends glutamatergic fibers to the cortex. When excitatory and inhibitory connections are traced, one can see that the net effect of exciting the hyperdirect loop is to decrease output from the loop’s thalamic segment to the cortex.
Each of the three basal ganglia loops has their own putative function (Gerfen, 1992; Mink, 1996; Nambu, 2003; Penney & Young, 1986). We described a theory (Figure 4) of how the basal ganglia participate in word generation (Crosson, Benjamin, & Levy, 2007), largely based on Nambu’s (2003) theory in the motor system, which was derived from earlier findings regarding the basal ganglia. Nambu et al. (2000) stimulated motor cortex (M1) in the macaque and recorded activity from GPi and GPe, as well as from the STN. At the GPi, there is a wave of excitation arriving roughly 7.8 ms after M1 stimulation, followed by a wave of inhibition arriving roughly 20.9 ms post M1 stimulation, and finally, followed by a late wave of excitation roughly 29.9 ms after M1 stimulation. Since the GPi inhibits thalamic activity, the result of the excitatory activity at the GPi is to reduce thalamic firing, and the result of inhibition of the GPi is to increase firing of the thalamic target. Hence, the waves of early excitation, inhibition, and late excitation at the GPi translate to waves of suppression, enhancement, and suppression of thalamocortical activity. Because the early wave of GPi excitation was preceded by an early wave of excitation at the STN, because blockade of STN activity abolished the early wave of GPi excitation, and because blockade of cortico-STN transmission with an NMDA receptor (one type of glutamate receptor) antagonist injection into the STN reduced early excitation at the GPi, the early wave of excitation was localized to the hyperdirect loop. Nambu et al. (2000) relied on previous studies to localize the wave of GPi inhibition to the direct loop. Since late GPi excitation was abolished by STN blockade, the late wave of excitation could be attributed to the STN. Both blockade of cortico-STN transmission (with an NMDA receptor antagonist) and blockade of GPe-STN transmission with bicuculline (GABA receptor antagonist)7 affected the late wave of excitation at GPi. Accordingly, both the hyperdirect and the indirect loops, respectively, were implicated in the late GPi excitation.
Figure 4.
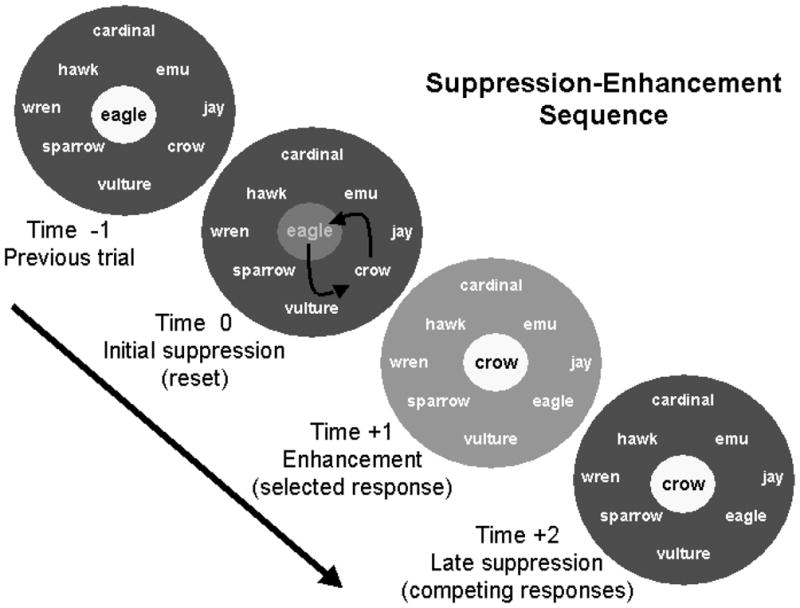
This figure is a conceptual representation of how basal ganglia loops may influence word production during a semantic fluency task. In this example, the subject is asked to produce as many birds as possible. During the previous trial (time −1), “eagle” was produced while competing birds were suppressed (white background indicates an activated concept, while gray represents suppressed concepts, with darker gray indicating greater suppression than lighter gray). When it comes time to generate a new bird (time 0), “eagle” is suppressed by the hyperdirect pathway so that “crow” can take its place. At time +1, “crow” is activated by the direct pathway, but competing responses are also somewhat activated. At time +2, competing concepts are suppressed, increasing the signal-to-noise ratio, and minimizing the probability of errors in production. From B. Crosson, M. Benjamin, & I. Levy. (2007) Role of the basal ganglia in language: Supporting Cast. In J. Hart & M. Kraut (eds.), Neural Basis of Semantic Memory, (pp. 219–233. New York: Cambridge University Press. Reprinted by permission of Cambridge University Press.
Crosson et al. (2003) found the left pre-SMA -- dorsal caudate -- (GPi) -- ventral anterior thalamus -- pre-SMA loop to be active during generation of a series of words (either members of a given category or words rhyming with a given word), but not during generation of nonsense syllables. The authors surmised that this pre-SMA loop was somehow involved in the selection of pre-existing representations (words) for production. Crosson et al. (2007) translated Nambu’s model for the motor system into a model for serial word production as follows (Figure 4): The system starts with certain words at a higher level of activation than other words either because they have already produced at least one word for the task (as in Figure 4) or because of recent experience. To allow for a switch to the production of a new, task-relevant word, a wave of suppression of thalamocortical activity resets the system to a point where activation is relatively but not quite equal between competing alternatives. As the subject searches for a new word and one begins to emerge, a wave of enhancement of thalamocortical activity enhances the activity of that choice, but to some degree also enhances the activity of closely related alternatives as well. Then, a wave of suppression reduces the activity level for all alternatives except the selected (most strongly activated) one, enhancing the probability that the selected word will be flawlessly produced. Thus, this system provides a means for enhancing the signal-to-noise ratio in serial word production, allowing words to be produced more quickly and with fewer errors in word selection. Word selection can still be performed without basal ganglia input, but it is more efficient when the basal ganglia participate. The more complex the activity is (e.g., giving definitions of both meanings of an ambiguous sentence) the more important is a favorable signal-to-noise ratio. There are some data to suggest that when word production becomes more difficult pre-SMA and/or Brodmann’s area 32 becomes more active. For example, when response competition for word production is increased, activity in this region increases (e.g., Carter et al., 2000).
4. An Integrated Model of Thalamic Language Mechanisms
This review has discussed symptom patterns in thalamic aphasia and four thalamic mechanisms that the author believes to influence language: (1) Through the NR and its connections with thalamic nuclei, frontal cortex selectively engages cortical areas necessary to perform the task at hand. (2) One area of cortex can pass information on to another area of cortex not only through corticocortical connections, but also by using the thalamus via corticothalamo-cortical pathways. (3) Layer 6 corticothalamic feedback can fine-tune local circuitry to optimize extraction of salient features from stimuli that are being processed. (4) As a part of the hyperdirect, direct, and indirect loops from pre-SMA through the basal ganglia, the ventral anterior nucleus may participate in word selection through a process that increases the signal-to-noise ratio around a selected word. It must be recognized that these proposed mechanisms represent the current state of knowledge regarding thalamic functions. By now, it should be abundantly clear that the secret to understanding the role that thalamic nuclei play in language processing lies largely in understanding cortical-thalamic relationships. Further, to this point in the review, these mechanisms for the most part have been considered separately, but it is unlikely that these four mechanisms operate independently of one another. Production (and comprehension) of 150 to 180 words per minute in spoken discourse requires that various components of language systems operate in a highly coordinated fashion. Thus, further integration will be attempted below.
Before doing so, however, one aspect of thalamic aphasia should be addressed. That is the nearly ubiquitous nature of lexical-semantic errors in thalamic aphasia. What role does the thalamus play in language that leads to the production of these kinds of errors? According to Nadeau and Crosson (1997) and Hart and Kraut (2007) the answer lies in the topographically distributed nature of semantic and lexical-semantic processing. Cortical areas processing features that make up object and action representations often are separated by large distances in the cortex. For example, most representations of “hammer” will involve visual characteristics and details best resolved in the medial fusiform gyrus of the dominant hemisphere (Wierenga et al., 2009). The concept of tools also involves our own experience of the movement required to use a tool that is likely stored in motor and premotor cortex (see Hauk et al., 2004), but the dominant parietal cortex is also likely to be involved in representation of the movement sequences, patterns, and trajectories necessary to pound a nail with a hammer (K. M. Heilman & Gonzalez Rothi, 2003). A visual representation of the movement of swinging a hammer is probably represented in or near area MT in the temporal lobe (Beauchamp & Martin, 2007; Born & Bradley, 2005). Hence, our internal representation of a hammer includes information represented in the fusiform gyrus, motor and premotor cortex, parietal cortex, middle temporal cortex, and so on. Evoking the concept of hammer involves simultaneous activation of all of these representations, and saying the word “hammer” additionally involves binding these semantic features to the lexical form. It has been proposed that the thalamus is involved in integrating the spatial and temporal contingencies necessary to bind the features and the lexical form into a lexical-semantic representation.8 How does the thalamus do this?
As noted above, Nadeau and Crosson (1997) suggested that happens through a mechanism dubbed selective engagement, whereby the frontal cortex engages those areas of cortex needed for a task through the NR and its ability to influence the state of cortical processors through thalamic nuclei. Although they favored the centromedian nucleus as the thalamic mechanism through which NR engages topologically disparate cortical processors, they left open the possibility that selective engagement could occur through the nuclei most directly connected to the cortical processors involved in the relevant processing. The author now believes that the latter explanation may be the more parsimonious explanation for the following reasons. Nadeau and Crosson (1997) identified the centromedian nucleus because it was so prominently involved in one of their four cases of thalamic aphasia and provided an explanation for why polar and paramedian artery infarcts might evoke similar symptoms, including especially lexical-semantic errors. However, since that time, the centromedian nucleus has been shown to be connected almost exclusively to motor and premotor cortex (Sadikot and Rymar, 2009), and that connectivity would be inadequate to support the engagement of semantic networks. The parafascicular nucleus was also involved in the lesion in question and has the necessary connections to frontal association cortex but lacks the connections to posterior association cortices necessary for selective engagement of semantic features (Sadikot & Rymer, 2009). Further, there are other reasons to favor activation of thalamic nuclei outside the intralaminar nuclei in the process of selective engagement.
Specifically, there is a growing consensus that the thalamus is involved in activating cortical areas involving specific tasks and that activity in the gamma range is the electroencephalographic signature for that cortical engagement (Pinault, 2004; Jones, 2009), including semantic feature binding (Hart & Kraut, 2007; Slotnick et al., 2002). As noted above, MacDonald (1998) demonstrated gamma rhythms in specific cortical areas whose corresponding sector of the NR was stimulated; hence, a relationship was established between NR activity and area-specific gamma activity in the cortex. The areas of the thalamus receiving NR input in these studies were the auditory and somatosensory thalamus, not the intralaminar nuclei. Generalizing to the language system, it seems likely that higher order relays in nuclei projecting to the language cortex, such as the pulvinar, could be involved in selective engagement, as evidenced by gamma rhythms (Slotnick et al., 2002). Finally, the point of parsimony regarding selective engagement is that putting thalamic relays connecting the topographically disparate areas into a high-fidelity transfer mode would allow them through their corticothalamo-cortical relays to transfer information between each other or perhaps to a pattern associator whose function is to integrate the input and activate the appropriate lexical item (see Rummelhart, McClelland, & Group, 1986 for a description of pattern associators). Broadly speaking, the pulvinar seems a likely location for such relays both because it has the requisite connections to frontal, posterior temporal, and inferior parietal cortices involved in language (Goldman-Rakic & Porrino, 1985; Jones, 2007) and because stimulation of the pulvinar evokes object naming failures (G. Ojemann & Fedio, 1968; G. A. Ojemann, 1977; G. A. Ojemann, Fedio, & Van Buren, 1968). Indeed, the medial pulvinar in the macaque has reciprocal connections with superior temporal cortex and posterior parietal cortex, which become language eloquent cortex in humans (Jones, 2007). The medial pulvinar also has reciprocal connections with most of the ventral visual stream of the macaque (Jones, 2007), cortex implicated in semantic processing in humans, and with most of the frontal cortex, including pre-SMA (Goldman-Rakic & Porrino, 1985).
This analysis raises one question regarding Nadeau and Crosson’s lesion data, however. The lesion of the centromedian (and parafascicular) nucleus in the frontal – NR – thalamic system was the link hypothesized to produce the same symptoms as polar artery lesions occupying primarily the ventral anterior nucleus and the ventral anterior NR. However, it will be recalled that Ford et al. (2010) demonstrated a single pathway from Broca’s area to thalamus, entering the thalamus at the ventral anterior nucleus. If Broca’s area is like other frontal cortical divisions, it projects to the dorsal medial nucleus and pulvinar as well as to the ventral anterior nucleus. Since we found no independent pathway from Broca’s area to enter the pulvinar (Ford et al., 2010), we have to assume that those connections traverse the thalamus from their point of entry at the ventral anterior nucleus, perhaps entering the internal medullary lamina as they progress posteriorly toward the pulvinar. Thus, fibers from Broca’s area to the pulvinar could be interrupted near their entry point in the ventral anterior nucleus in polar artery lesions, more posteriorly on their way to the pulvinar for paramedian artery lesions, or at the pulvinar itself for posterior thalamic lesions. Thus, any of these three lesions would compromise transfer of information and/or influence on selective engagement between Broca’s area and posterior perisylvian language areas via pulvinar relays, which could explain the similarity in symptoms between polar artery, paramedian, and pulvinar lesions causing aphasia.
Another interesting question is the parallels between the frontal – NR – thalamo – cortical system proposed by Nadeau and Crosson (1997) and modified in this article and the layer 6 thalamic feedback mechanisms discussed by Sillito et al. (2006). Are they two separate mechanisms, or is the frontal mechanism just a special case of layer 6 feedback? Based on what we know, the frontal – NR – thalamo – cortical system would have to use layer 6 fibers because layer 5 fibers do not give off collaterals to the NR (Sherman & Guillery, 2006). However, the degree to which either of these mechanisms relies on NR stimulation by layer 6 fibers vs. direct stimulation of the thalamic relay by layer 6 fibers is not known. Salt et al. (in press) showed that at least some effects of layer 6 feedback are mediated in the somatosensory thalamus and not the NR by mGlu1 receptors. However, MacDonald et al. (1998) showed that stimulation of the NR evokes gamma rhythms in cortical areas specifically related to the stimulated NR sector.
A final comparison between the function of the basal ganglia loops incorporating thalamic nuclei and layer 6 corticothalamic feedback mechanisms is in order. There is some similarity in the functions they are proposed to perform: Basal ganglia loops are thought to enhance the signal-to-noise ratio between a selected word and close semantic associates during word production (Crosson et al., 2007). Through thalamocortical fibers, layer 6 corticothalamic feedback is thought to enhance the ability of target cortex to extract the salient features of a stimulus (Sillito et al., 2006), which can also be viewed as increasing the signal-to-noise ratio for salient features. One important distinction between these mechanisms likely involves the difference between intention and attention. Intention involves the ability to select one action for execution among competing actions and to initiate that action. Attention involves the ability to select one stimulus among multiple competing stimuli and process that stimulus further (Crosson & Cohen, 2012; K. Heilman, Watson, & Valenstein, 2003). Basal ganglia loops are seen as mechanisms primarily involved in intention (Heilman et al., 2003), while layer 6 corticothalamic feedback models are based mainly on studies of sensory processing mechanisms. In other words, basal ganglia loops are involved in enhancing signal-to-noise in action-related processes while layer 6 corticothalamic feedback mechanisms may primarily be involved with improving signal-to-noise in sensory and perceptual processes.
Based on the above analyses, we can modify our previous conceptualization of thalamic activity in language (Nadeau & Crosson, 1997) as follows. Figure 5a shows this proposed modification. The literature has indicated that the anterior part of Broca’s area, pars triangularis, probably extending into pars orbitalis (BAa), participates in semantic functions (Devlin, Matthews, & Rushworth, 2003; Hagoort, 2006; Petersen, Fox, Posner, Mintun, & Raichle, 1988). We propose that through selective engagement, a possible function of BAa is to orchestrate activation of the array of cortices necessary for semantic functions during language output. It does so through its relationship with NR by switching thalamic relays in the pulvinar (Pl) connected to language and semantic cortices to the high-fidelity transfer mode, which in turn places the related cortical units into a state of increased excitability and makes them ready to process semantic features and stored lexical information. Since this is a modulatory function, and since it involves cortico-NR connections, the corticothalamic fibers from BAa must arise in layer 6. The example in the figure shows BAa activating cortices necessary to recall the construct of hammer, which include its visual form and the motor sequences necessary to use a hammer (areas for other features undoubtedly are activated as well but not shown in the figure). The signature for this process of selective engagement is gamma rhythms in the pulvinar and in the engaged cortical units.
Once the appropriate cortical units are activated they can transfer through higher-order corticothalamo-cortical relays semantic feature information regarding the construct of hammer to the dominant posterior perisylvian cortex, where the lexical form for hammer can be activated through a pattern associator whose output is the lexical item (Figure 5b)9. Fibers from BAa can be interrupted either en passage through the ventral anterior nucleus, in the internal medullary lamina as they course posteriorly, or at the pulvinar. This fact explains why lexical-semantic deficits can be seen with polar artery lesions, with paramedian artery lesions, or with posterior thalamic lesions including the pulvinar (e.g., Crosson et al., 1986; Raymer et al., 1997).
Figure 5c illustrates that layer 6 cortico-thalamic feedback can enhance input so that the salient features of an incoming stimulus are extracted. The example described by Sillito et al. (2006) will serve nicely. Suppose you are watching out the window as Johnny pounds a nail into a board, and someone in another room asks, “What is Johnny doing?” One salient feature of Johnny’s activity needed to answer this question is the nature of the motions that you see him performing as he hammers the nail into the board. MT plays a large role in identifying this motion and in order to do so, it has a relationship with the pulvinar (Jones, 2007). However, feedback from MT to layer 6 cells of V1 also activate layer 6 corticothalamic feedback to the lateral geniculate nucleus to help extract visual information necessary to identify the motion (see Sillito et al., 2006 for mechanism). Once the motion is recognized as familiar, it can be combined with other information (such as the shape of the tool in Johnny’s hand) to evoke word retrieval for the act that Johnny is performing.
Finally, as you are about ready to answer the question, you must select the word you will use to describe Johnny’s activity. The lexical items for the action (e.g., “hammering”, “pounding”, and “nailing”) are available lexical items. A variety of constraints may help determine which of these choices is in a higher state of activation. The job of the pre-SMA – basal ganglia loops illustrated in Figure 5d is to enhance the signal-to-noise ratio in the lexical selection process, decreasing the chance of errors, as described above.
5. Conclusions
A few concluding comments are in order. First, the model presented here should be taken as heuristic. Over the coming years it should and will be modified as more information about thalamic mechanisms becomes available. Second, there are questions which this model does not answer. For example, what is the role of the dominant intralaminar nuclei in language processing? Although Chatterjee et al. (1997) suggested that the intralaminar nuclei might be involved in disordered language (thought), their patient’s paramedian artery infarct may well have involved fibers coursing from the frontal lobe to the pulvinar. Another issue is whether or not connections between layer 6 corticothalamic fibers and/or thalamocortical fibers and the NR play a role in basal ganglia loops (see Figure 5d for existing circuitry). Yet one more issue is the precise mechanism whereby NR activity can generate gamma rhythms in associated cortex. A final question is why patients normally recover quite well from thalamic aphasias. The answer to this question could lie in system redundancies, either at the thalamic or the cortical level. For example, frontal patterns in thalamic projections are recapitulated in at least three thalamic nuclei (ventral anterior, dorsal medial, pulvinar: Goldman-Rakic & Porrino, 1985), and/or it may be possible that corticocortical connections can eventually be substituted for corticothalamo-cortical connections when the latter are damaged. Nonetheless, this model can account for other phenomena sometimes seen in thalamic aphasia. For example, category-specific naming deficits might result from disconnection or destruction of corticothalamo-cortical relays that pass lexical-semantic information between cortical centers critical for distinguishing category members from one another. As noted above, neglect dyslexia (e.g., Crosson, 1999) might be accounted for by a failure of layer 6 corticalthalamic feedback circuits in enhancing the right (or left) side of written words through the thalamus, and the variable comprehension problems in thalamic aphasias might be accounted for by a similar mechanism.
As noted above, one advantage to the kind of models proposed in this article is heuristic, i.e., their ability to guide future research. In other words, what testable hypotheses can be derived from the current model? A few examples in this regard are as follows:
5.1. Anatomic Hypotheses
-
1
It was suggested above that fibers from Broca’s area pierce the thalamus through the ventral anterior nucleus and then pass to the pulvinar. Ford et al. (2010) traced fibers from Broca’s area to the thalamus. It is unclear if current tractography techniques for diffusion weighted magnetic resonance (MR) imaging have the capability of or sensitivity for tracing these pathways through the thalamus on a consistent basis. However, as more sophisticated tractography algorithms become available and as the resolution and signal/noise in diffusion weighted imaging improve, it should become possible to test this hypothesis in vivo in humans. As noted above, this information is important in explaining the similarity in thalamic aphasias due to polar artery, paramedian artery, or pulvinar lesion.
-
2
It has been suggested that layer 6 corticothalamic connections may play a role in basal ganglia loops. If so, it should be possible to trace layer 6 fibers to portions of the ventral anterior nucleus to which the basal ganglia project. This information could be important in understanding how basal ganglia loops are modulated.
5.2. Physiological Hypotheses
-
3
It was suggested that there is a suppress – enhance – suppress sequence in a pre-SMA-basal ganglia loop important to increasing signal and reducing noise during lexical-semantic selection. If so, Nambu et al.’s (2000) experiment after which this hypothesis was modeled, should be able to be successfully replicated in the pre-SMA loop of macaques to determine the nature of activity in the subthalamic nucleus and internal globus pallidus after pre-SMA stimulation and what neurotransmitters and pathways are involved in this response sequence.
-
4
If the thalamus plays a major role in selective engagement and gamma rhythms are the signature for this process, then gamma rhythms should be present in the appropriate cortices for neurologically intact subjects during lexical-semantic tasks, such as Kraut et al.’s (2002, 2003) feature-binding task. Indeed, this has been seen in a patient undergoing a thalamic stimulation protocol for epilepsy (Slotnikc et al., 2002). However, what also should be seen is a lack of or interruption of gamma rhythms during this task in patients with thalamic aphasia, especially if semantic paraphasias are present to a significant degree.
5.3. Behavioral Hypotheses
-
5
It is suprising that no one has followed up on the lexical-semantic work done by Raymer et al. (1997) which systematically assessed two patients with thalamic aphasias from polar artery and paramedian artery lesions, respectively. Based on her work and the material presented in this paper, it can be hypothesized that any patient with a dominant thalamic lesion in a position to interrupt fibers between Broca’s area and the pulvinar should show similar lexical-semantic deficits. It is important to replicate and extend Raymer and colleagues’ findings in this fashion to link them to a specific thalamic topography.
-
6
if the thalamus is involved in feature binding, then patients with thalamic aphasia and semantic paraphasias should have problems on Kraut et al.’s (2002, 2003) feature-binding task when words are used. As they recover from thalamic aphasia, and their semantic paraphasias diminish, they might demonstrate a return of gamma rhythms, perhaps with some change in location from that of normal controls. Occasionally, patients with thalamic aphasia show no semantic paraphasias, even though they may have other language deficits. Perhaps, these latter patients would demonstrate normal feature binding. Such findings would lend legitimacy to Kraut et al.’s hypotheses regarding the role of the thalamus in feature binding.
These are only a few hypotheses that can be generated from the current theory. Certainly, there are others. However, one thing is obvious from these hypotheses, as well as from the discussion throughout this exposition: Discovery regarding thalamic functions relevant to language would occur at a much more rapid rate if animal model investigators, cognitive neuroscience investigators, and clinical investigators worked closely together on the issue of the role of the thalamus in language. Such collaborations would allow for generation of hypotheses in one kind of research that can be tested by a colleague doing a different kind of research. The knowledge gained from such endeavors would represent a pivotal advance in our knowledge about how the brain produces language specifically and other behaviors in general.
Highlights.
The thalamus is involved in language processes through four mechanisms.
The first selectively engages cortices needed to bind object or action features to a lexical item.
The second passes information from one cortical area to another.
The third enables cortical extraction of salient features via corticothalamo-cortical feedback.
The last enhances signal-to-noise in word production via cortico-basal ganglia-thalamic loops.
Acknowledgments
This work was supported through a Senior Research Career Scientist Award from the Department of Veterans Affairs Rehabilitation Research and Development Service (grant # B6364L) and grant # R21 DC009247 from the National Institute on Deafness and Other Communication Disorders. The author would like to thank Daniel Llano, M.D., Ph.D. for his encouragement in exploring some of the important literature for this exposition and in writing this article.
Footnotes
Cambier, Elghozi, and Graveleau (1982 as cited in Demonet, 1987) described the following features in addition to these three characteristics: reduced vocal volume, lack of spontaneity and pauses in oral expression, and frequent perseveration in word-finding. It is of interest that these additional features can be seen as characteristic frontal symptoms, but the literature does not support their frequent literature in thalamic aphasia. Hence, these symptoms will not be considered cardinal features or further discussed.
It is worth noting that Nadeau and Crosson (1997) relied on the observation of Scheibel and Scheibel (1972) who noted dendritic bundles in the dorsal lateral portion of the feline nucleus reticularis that run along its longitudinal axis. They were able to trace such bundles as far as 1,000–1,500 μm. However, Pinault (2004) noted that we still have no idea how far these bundles extend. Pinault also noted that axons from nucleus reticularis cells often project to multiple and sometimes related thalamic nuclei.
This activity could exist in a single electrode, with adjacent electrodes separated by a distance of 2 mm not showing this activity. Hence, the cortical gamma rhythms can be quite focal in their spatial distribution.
Portions of the pulvinar, the dorsal medial nucleus, and the ventral anterior nucleus may contain higher order relays. However, Sherman and Guillery (2006) pointed out that it may be difficult to classify any nucleus per se as purely first order or purely higher order. For example, portions of both the lateral and medial geniculate nuclei receive drivers from primary visual and auditory cortices, respectively, the pulvinar receives inputs from the superior colliculus, and the dorsal medial nucleus receives inputs from the mammillothalamic tract
They used morphological characteristics (large terminals for drivers, small terminals for modulators) to map inputs to the MGB.
It is worth noting that Copland et al. (2000b) and Copland (2003) also found that patients with nonthalamic subcortical lesions showed abnormalities in semantic priming at longer (≥1000 ms) interstimulus intervals but not at shorter interstimulus intervals (≤200 ms). Responses at longer intervals are thought to allow controlled (or top-down) processing, whereas responses at shorter intervals are thought to rely only on automatic processing. Controlled processing is likely to involve frontal cortices, which are more consistently involved in basal ganglia loops than other cortical regions (Middleton & Strick, 2000, 2002). Hence, one might conclude that the basal ganglia are involved in controlled processing. Such a conclusion is consistent with difficulty on more complex language tasks because their difficulty is likely to invoke more top-down processing than relatively simple language tasks.
The explanation for why a GABA receptor antagonist (bicuculline) in the STN would lead to suppression of the late excitatory wave at the GPi is somewhat complicated, but was as follows. Bicuculline removed the continuous GABAergic inhibition of STN neurons by the GPe. With the removal of this inhibition, it was no longer possible for indirect pathway activity to induce an increase in STN activity (Nambu et al., 2000).
Although the concept of feature is somewhat elusive for abstract words for which there are few sensory associations, the mechanisms discussed here are easily applied to abstract words. The key to understanding abstract representations is that they are widely distributed in a very similar fashion to concrete words. For example, studies have demonstrated that abstract words (nouns and verbs) activate a spatially distributed network of multimodal cortices in the frontal, parietal, temporal, and occipital lobes. There is a great deal of overlap between areas in these regions that process concrete words and have been shown to be involved in semantic processing. Representations of abstract words appear more limited to the left hemisphere than those for concrete words, there is greater involvement of inferior frontal cortex than for concrete words, and abstract words are associated with even more widespread cortical activity than concrete words (Binder et al., 2005; Pexman et al., 2007; Rodriguez-Ferreiro et al, 2011). The important point, however, is that it is necessary to bind information from a widely distributed collection of cortical areas to a lexical item in order to retrieve an abstract word. This process for abstract words probably works in much the same as for concrete words.
Hagoort (2006) proposed that Broca’s region was a “unification space for language,” which has some similarities to the current proposal. However, he focused on the unification of lexical-semantic information with syntactic elements and structures. Here, the current model focuses on lexical-semantic functions because they are common in thalamic aphasia and because of the work of Kraut, Hart and colleagues cited earlier. Since agrammatism is rarely a feature of thalamic aphasia, we have not focused on syntactic functions. Hagoort’s concept about Broca’s area is useful, but syntax does not seem to require as much support of thalamic mechanisms as do lexical-semantic processes.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J Neurosci. 2007;27(40):10659–10673. doi: 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Assaf M, Calhoun VD, Kuzu CH, Kraut MA, Rivkin PR, Hart J, Jr, et al. Neural correlates of the object-recall process in semantic memory. Psychiatry Res. 2006;147(2–3):115–126. doi: 10.1016/j.pscychresns.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Martin A. Grounding object concepts in perception and action: evidence from fMRI studies of tools. Cortex. 2007;43(3):461–468. doi: 10.1016/s0010-9452(08)70470-2. [DOI] [PubMed] [Google Scholar]
- Binder JR, Westbury CF, McKiernan KA, Possing ET, Medler DA. Distinct brain systems for processing concrete and abstract concepts. J Cog Neurosci. 2005;17(6):905–917. doi: 10.1162/0898929054021102. [DOI] [PubMed] [Google Scholar]
- Born RT, Bradley DC. Structure and function of visual area MT. Annu Rev Neurosci. 2005;28:157–189. doi: 10.1146/annurev.neuro.26.041002.131052. [DOI] [PubMed] [Google Scholar]
- Bouyer JJ, Montaron MF, Rougeul A. Fast fronto-parietal rhythms during combined focused attentive behaviour and immobility in cat: cortical and thalamic localizations. Electroencephalogr Clin Neurophysiol. 1981;51(3):244–252. doi: 10.1016/0013-4694(81)90138-3. [DOI] [PubMed] [Google Scholar]
- Cambier H, Elghozi D, Graveleau P. Neuropsychologie des Lesions du thalamus. Raport de Neurologie: Congres de Psychiatrie et de Neurologie de Langue Francaise.1982. [Google Scholar]
- Carrera E, Bogousslavsky J. The thalamus and behavior: effects of anatomically distinct strokes. Neurology. 2006;66(12):1817–1823. doi: 10.1212/01.wnl.0000219679.95223.4c. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger A, Noll D, Cohen JD. Parsing executive processes: Strategic vs. evaluative functions of the anterior cingulate cortex. Proc Nat Acad of Sci, USA. 2000;97(4):1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Yapundich R, Mennemeier M, Mountz JM, Inampudi C, Pan JW, et al. Thalamic thought disorder: on being “a bit addled”. Cortex. 1997;33(3):419–440. doi: 10.1016/s0010-9452(08)70228-4. [DOI] [PubMed] [Google Scholar]
- Copland DA. The basal ganglia and semantic engagement: Potential insights from semantic priming in individuals with subcortical vascular lesions, Parkinson’s disease, and cortical lesions. Journal of the International Neuropsychological Society. 2003;9:1041–1052. doi: 10.1017/S1355617703970081. [DOI] [PubMed] [Google Scholar]
- Copland DA, Chenery HJ, Murdoch BE. Persistent deficits in complex language function following dominant nonthalamic subcortical lesions. Journal of Medical Speech-Language Pathology. 2000a;8:1–14. [Google Scholar]
- Copland DA, Chenery HJ, Murdoch BE. Processing Lexical Ambiguities in Word Triplets: Evidence of Lexical-Semantic Deficits Following Dominant Nonthalamic Subcortical Lesions. Neuropsychology. 2000b;14(3):379–390. doi: 10.1037//0894-4105.14.3.379. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Cox DE, Heilman KM. Dynamic-intentional thalamic aphasia: A failure of lexical-semantic self-activation. Neurocase. 2011;17(4):313–317. doi: 10.1080/13554794.2010.504731. [DOI] [PubMed] [Google Scholar]
- Crosson B. Role of the dominant thalamus in language: a review. Psychol Bull. 1984;96(3):491–517. [PubMed] [Google Scholar]
- Crosson B. Subcortical Functions in Language and Memory. New York: Guilford; 1992. [Google Scholar]
- Crosson B. Subcortical mechanisms in language: lexical-semantic mechanisms and the thalamus. Brain Cogn. 1999;40(2):414–438. doi: 10.1006/brcg.1999.1088. [DOI] [PubMed] [Google Scholar]
- Crosson B, Benefield H, Cato MA, Sadek JR, Moore AB, Wierenga CE, et al. Left and right basal ganglia and frontal activity during language generation: contributions to lexical, semantic, and phonological processes. J Int Neuropsychol Soc. 2003;9(7):1061–1077. doi: 10.1017/S135561770397010X. [DOI] [PubMed] [Google Scholar]
- Crosson B, Benjamin M, Levy IF. Role of the basal ganglia in language and semantics: Supporting cast. In: Hart JJ, Kraut M, editors. Neural Basis of Semantic Memory. New York: Cambridge University Press; 2007. pp. 219–243. [Google Scholar]
- Crosson B, Cato MA, Sadek JR, Lu L. Organization of semantic knowledge in the human brain: Toward a resolution in the next millennium. Brain and Cognition. 2000;42:146–148. doi: 10.1006/brcg.1999.1186. [DOI] [PubMed] [Google Scholar]
- Crosson B, Cohen ML. Language and communication disorders associated with communication deficits. In: Peach RK, Shapiro LP, editors. Cognition and Acquired Language Disorders. St. Louis, MO: Elsevier Mosby; 2012. pp. 167–182. [Google Scholar]
- Crosson B, Moberg PJ, Boone JR, Rothi LJ, Raymer A. Category-specific naming deficit for medical terms after dominant thalamic/capsular hemorrhage. Brain Lang. 1997;60(3):407–442. doi: 10.1006/brln.1997.1899. [DOI] [PubMed] [Google Scholar]
- Crosson B, Parker JC, Kim AK, Warren RL, Kepes JJ, Tully R. A case of thalamic aphasia with postmortem verification. Brain Lang. 1986;29(2):301–314. doi: 10.1016/0093-934x(86)90050-7. [DOI] [PubMed] [Google Scholar]
- Crosson B, Sadek JR, Maron L, Gökçay D, Mohr CM, Auerbach EJ, Freeman AJ, Leonard CM, Briggs RW. Relative Shift in Activity from Medial to Lateral Frontal Cortex during Internally versus Externally Guided Word Generation. J Cog Neurosci. 2001;13:272–283. doi: 10.1162/089892901564225. [DOI] [PubMed] [Google Scholar]
- Demonet J-F. Unpublished These Pour le Doctorat D’Etat en Medicine. Universite Paul Sabatier-Toulouse III; 1987. Les Aphasies Sous-Corticales: Etude Linguistique, Radiologique et Hemodynamique de 31 Observations. [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MF. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci. 2003;15(1):71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Ford AA, McGregor K, Trinastic J, Seeds L, White K, Crosson B. Broca’s Area Basal Ganglia Loops. Society for Neuroscience Abstracts On Line. 2010:Poster #400.411. [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Porrino LJ. The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol. 1985;242(4):535–560. doi: 10.1002/cne.902420406. [DOI] [PubMed] [Google Scholar]
- Hagoort P. On Broca, Brain, and Binding. In: Grodzinsky Y, Amunts K, editors. Broca’s Region. New York: Oxford University Press; 2006. pp. 242–253. [Google Scholar]
- Hart J, Jr, Anand R, Zoccoli S, Maguire M, Gamino J, Tillman G, et al. Neural substrates of semantic memory. J Int Neuropsychol Soc. 2007;13(5):865–880. doi: 10.1017/S135561770707110X. [DOI] [PubMed] [Google Scholar]
- Hart J, Kraut M. Neural hybrid model of semantic object memory (version 1.1) In: Hart J, Kraut M, editors. Neural Basis of Semantic Memory. Cambridge, UK: Cambridge University Press; 2007. pp. 331–359. [Google Scholar]
- Hauk O, Johnsrude I, Pulvermuller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–307. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Heilman K, Watson R, Valenstein E. Neglect and related disorders. In: Heilman K, Valenstein E, editors. Clinical Neuropsychology. New York: Oxford University Press; 2003. pp. 296–346. [Google Scholar]
- Heilman KM, Gonzalez Rothi LJ. Apraxia. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. New York: Oxford University Press; 2003. pp. 215–235. [Google Scholar]
- Hillis AE, Wityk RJ, Barker PB, Beauchamp NJ, Gailloud P, Murphy K, et al. Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion. Brain. 2002;125(Pt 5):1094–1104. doi: 10.1093/brain/awf113. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. Cambridge, UK: Cambridge University Press; 2007. The lateral posterior and pulvinar nuclei; pp. 1009–1075. [Google Scholar]
- Jones EG. Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Ann N Y Acad Sci. 2009;1157:10–23. doi: 10.1111/j.1749-6632.2009.04534.x. [DOI] [PubMed] [Google Scholar]
- Karussis D, Leker RR, Abramsky O. Cognitive dysfunction following thalamic stroke: a study of 16 cases and review of the literature. J Neurol Sci. 2000;172(1):25–29. doi: 10.1016/s0022-510x(99)00267-1. [DOI] [PubMed] [Google Scholar]
- Kraut MA, Calhoun V, Pitcock JA, Cusick C, Hart J., Jr Neural hybrid model of semantic object memory: implications from event-related timing using fMRI. J Int Neuropsychol Soc. 2003;9(7):1031–1040. doi: 10.1017/S135561770397007X. [DOI] [PubMed] [Google Scholar]
- Kraut MA, Kremen S, Moo LR, Segal JB, Calhoun V, Hart J., Jr Object activation in semantic memory from visual multimodal feature input. J Cogn Neurosci. 2002;14(1):37–47. doi: 10.1162/089892902317205302. [DOI] [PubMed] [Google Scholar]
- Kraut MA, Kremen S, Segal JB, Calhoun V, Moo LR, Hart J., Jr Object activation from features in the semantic system. J Cogn Neurosci. 2002;14(1):24–36. doi: 10.1162/089892902317205294. [DOI] [PubMed] [Google Scholar]
- Kraut MA, Pitcock JA, Calhoun V, Li J, Freeman T, Hart J., Jr Neuroanatomic organization of sound memory in humans. J Cogn Neurosci. 2006;18(11):1877–1888. doi: 10.1162/jocn.2006.18.11.1877. [DOI] [PubMed] [Google Scholar]
- Kuljic-Obradovic DC. Subcortical aphasia: three different language disorder syndromes? Eur J Neurol. 2003;10(4):445–448. doi: 10.1046/j.1468-1331.2003.00604.x. [DOI] [PubMed] [Google Scholar]
- Llano DA, Sherman SM. Evidence for nonreciprocal organization of the mouse auditory thalamocortical-corticothalamic projection systems. J Comp Neurol. 2008;507(2):1209–1227. doi: 10.1002/cne.21602. [DOI] [PubMed] [Google Scholar]
- Lucchelli F, De Renzi E. Proper name anomia. Cortex. 1992;28(2):221–230. doi: 10.1016/s0010-9452(13)80050-0. [DOI] [PubMed] [Google Scholar]
- Macdonald KD, Fifkova E, Jones MS, Barth DS. Focal stimulation of the thalamic reticular nucleus induces focal gamma waves in cortex. J Neurophysiol. 1998;79(1):474–477. doi: 10.1152/jn.1998.79.1.474. [DOI] [PubMed] [Google Scholar]
- Maeshima S, Ozaki F, Okita R, Yamaga H, Okada H, Kakishita K, et al. Transient crossed aphasia and persistent amnesia after right thalamic haemorrhage. Brain Inj. 2001;15(10):927–933. doi: 10.1080/02699050110065646. [DOI] [PubMed] [Google Scholar]
- Marien P, Abutalebi J, Engelborghs S, De Deyn PP. Pathophysiology of language switching and mixing in an early bilingual child with subcortical aphasia. Neurocase. 2005;11(6):385–398. doi: 10.1080/13554790500212880. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Feeser HR. Functional implications of burst firing and single spike activity in lateral geniculate relay neurons. Neuroscience. 1990;39(1):103–113. doi: 10.1016/0306-4522(90)90225-s. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31(2–3):236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal-ganglia ‘projections’ to the prefrontal cortex of the primate. Cerebral Cortex. 2000;12:926–935. doi: 10.1093/cercor/12.9.926. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50(4):381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Moreaud O, Pellat J, Charnallet A, Carbonnel S, Brennen T. Deficiency in the reproduction and learning proper names after left tubero-thalamic ischemic lesion. Rev Neurol (Paris) 1995;151(2):93–99. [PubMed] [Google Scholar]
- Nadeau SE, Crosson B. Subcortical aphasia. Brain Lang. 1997;58(3):355–402. doi: 10.1006/brln.1997.1707. discussion 418–323. [DOI] [PubMed] [Google Scholar]
- Nambu A. A new dynamic model of the cortico-basal ganglia loop. Prog Brain Res. 2003;143:461–466. doi: 10.1016/S0079-6123(03)43043-4. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, et al. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol. 2000;84(1):289–300. doi: 10.1152/jn.2000.84.1.289. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Fedio P. Effect of stimulation of the human thalamus and parietal and temporal white matter on short-term memory. J Neurosurg. 1968;29(1):51–59. doi: 10.3171/jns.1968.29.1.0051. [DOI] [PubMed] [Google Scholar]
- Ojemann GA. Asymmetric function of the thalamus in man. Ann N Y Acad Sci. 1977;299:380–396. doi: 10.1111/j.1749-6632.1977.tb41923.x. [DOI] [PubMed] [Google Scholar]
- Ojemann GA, Fedio P, Van Buren JM. Anomia from pulvinar and subcortical parietal stimulation. Brain. 1968;91(1):99–116. doi: 10.1093/brain/91.1.99. [DOI] [PubMed] [Google Scholar]
- Penney JB, Jr, Young AB. Striatal inhomogeneities and basal ganglia function. Mov Disord. 1986;1(1):3–15. doi: 10.1002/mds.870010102. [DOI] [PubMed] [Google Scholar]
- Perren F, Clarke S, Bogousslavsky J. The syndrome of combined polar and paramedian thalamic infarction. Arch Neurol. 2005;62(8):1212–1216. doi: 10.1001/archneur.62.8.1212. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331(6157):585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Pexman PM, Hargreaves IS, Edwards JD, Henry LC, Goodyear BG. Neural correlates of concreteness in semantic categorization. J Cog Neurosci. 2007;19(8):1407–1419. doi: 10.1162/jocn.2007.19.8.1407. [DOI] [PubMed] [Google Scholar]
- Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res Brain Res Rev. 2004;46(1):1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Radanovic M, Azambuja M, Mansur LL, Porto CS, Scaff M. Thalamus and language: interface with attention, memory and executive functions. Arq Neuropsiquiatr. 2003;61(1):34–42. doi: 10.1590/s0004-282x2003000100006. [DOI] [PubMed] [Google Scholar]
- Radanovic M, Scaff M. Speech and language disturbances due to subcortical lesions. Brain Lang. 2003;84(3):337–352. doi: 10.1016/s0093-934x(02)00554-0. [DOI] [PubMed] [Google Scholar]
- Raymer AM, Moberg P, Crosson B, Nadeau S, Rothi LJ. Lexical-semantic deficits in two patients with dominant thalamic infarction. Neuropsychologia. 1997;35(2):211–219. doi: 10.1016/s0028-3932(96)00069-3. [DOI] [PubMed] [Google Scholar]
- Ribary U, Ioannides AA, Singh KD, Hasson R, Bolton JP, Lado F, et al. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci U S A. 1991;88(24):11037–11041. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriquez-Ferreiro J, Gennari SP, Davies R, Cuetos F. Neural correlates of concreteness in semantic categorization. J Cog Neurosci. 2011;23(1):106–118. doi: 10.1162/jocn.2010.21414. [DOI] [PubMed] [Google Scholar]
- Rummelhart DE, McClelland JL, Group tPR. Foundations. Vol. 1. Cambridge, MA: MIT Press; 1986. Parallel Distributed Processing: Explorations in the Microstructure of Cognition. [Google Scholar]
- Sadikot AF, Rymar VV. The primate centromedian-parafascicular complex: anatomical organization with a note on neuromodulation. Brain Res Bull. 2009;78(2–3):122–130. doi: 10.1016/j.brainresbull.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Salt TE, Jones HE, Andolina IM, Copeland CS, Clements JT, Knoflach F, et al. Potentiation of sensory responses in ventrobasal thalamus in vivo via selective modulation of mGlu1 receptors with a positive allosteric modulator. Neuropharmacology. doi: 10.1016/j.neuropharm.2011.11.015. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Specialized organizational patterns within the nucleus reticularis thalami of the cat. Exp Neurol. 1972;34(2):316–322. doi: 10.1016/0014-4886(72)90177-x. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Dual response modes in lateral geniculate neurons: mechanisms and functions. Vis Neurosci. 1996;13(2):205–213. doi: 10.1017/s0952523800007446. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the Thalamus. Cambridge, MA: MIT Press; 2006. [Google Scholar]
- Sillito AM, Cudeiro J, Jones HE. Always returning: feedback and sensory processing in visual cortex and thalamus. Trends Neurosci. 2006;29(6):307–316. doi: 10.1016/j.tins.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Jones HE. Corticothalamic interactions in the transfer of visual information. Philos Trans R Soc Lond B Biol Sci. 2002;357(1428):1739–1752. doi: 10.1098/rstb.2002.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Kraut MA, Lesser RP, Hart J., Jr Interactions between thalamic and cortical rhythms during semantic memory recall in human. Proc Natl Acad Sci U S A. 2002;99(9):6440–6443. doi: 10.1073/pnas.092514899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Domich L, Oakson G. Reticularis thalami neurons revisited: Activity changes during shifts in states of vigilance. Journal of Neuroscience. 1986;6:68–81. doi: 10.1523/JNEUROSCI.06-01-00068.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci. 2010;13(1):84–88. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay P, Small SL. Motor response selection in overs sentence production: A functional MRI study. Frontiers in Psychology. 2011;2 (article 253):1–14. doi: 10.3389/fpsyg.2011.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman D, Hisama FM, Waxman SG, Blumenfeld H. Going deep to cut the link: cortical disconnection syndrome caused by a thalamic lesion. Neurology. 2003;60(11):1865–1866. doi: 10.1212/01.wnl.0000066051.14414.ff. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Perlstein WM, Benjamin M, Leonard CM, Rothi LG, Conway T, et al. Neural substrates of object identification: Functional magnetic resonance imaging evidence that category and visual attribute contribute to semantic knowledge. J Int Neuropsychol Soc. 2009;15(2):169–181. doi: 10.1017/S1355617709090468. [DOI] [PubMed] [Google Scholar]
- Wigg EH, Secord W. Test of Language Competence--Expanded. New York: Psychological Corporation; 1989. [Google Scholar]
- Wigg EH, Secord W. Test of Word Knowledge. Columbus, OH: Charles E. Merrill; 1992. [Google Scholar]