Abstract
Posttraumatic stress disorder (PTSD) has a well‐defined set of symptoms that can be elicited during traumatic imagery tasks. For this reason, trauma imagery tasks are often employed in functional neuroimaging studies. Here, coordinate‐based meta‐analysis (CBM) was used to pool eight studies applying traumatic imagery tasks to identify sites of task‐induced activation in 170 PTSD patients and 104 healthy controls. In this way, right anterior cingulate (ACC), right posterior cingulate (PCC), and left precuneus (Pcun) were identified as regions uniquely active in PTSD patients relative to healthy controls. To further characterize these regions, their normal interactions, and their typical functional roles, meta‐analytic connectivity modeling (MACM) with behavioral filtering was applied. MACM indicated that the PCC and Pcun regions were frequently co‐active and associated with processing of cognitive information, particularly in explicit memory tasks. Emotional processing was particularly associated with co‐activity of the ACC and PCC, as mediated by the thalamus. By narrowing the regions of interest to those commonly active across multiple studies (using CBM) and developing a priori hypotheses about directed probabilistic dependencies amongst these regions, this proposed model—when applied in the context of graphical and causal modeling—should improve model fit and thereby increase statistical power for detecting differences between subject groups and between treatments in neuroimaging studies of PTSD. Hum Brain Mapp 34:3392–3399, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: posttraumatic stress disorder, meta‐analysis, neuroimaging, trauma, imagery, connectivity
INTRODUCTION
Posttraumatic stress disorder (PTSD) is an anxiety disorder with a prototypical constellation of symptoms. It develops following a severely traumatic experience during which a person is confronted with the threat of death or serious injury to oneself or others (APA, 1994). A key feature of PTSD is frequent re‐experiencing of the trauma in the form of flashbacks, nightmares or intrusive thoughts or images. Consequently, PTSD sufferers avoid potential stimuli that may serve as triggers for re‐experiencing episodes and describe persistent arousal or anxiety (Karam et al., 2010; Rauch et al., 2006). Although the events precipitating PTSD may differ dramatically, the triad of re‐experiencing, avoidance, and anxiety is consistently observed. Moreover, PTSD is associated with an increased risk for panic disorder, social phobia, depression, and substance‐related disorders, which ultimately affect social, occupational, and other areas of daily functioning (APA, 1994).
Neuroimaging studies have explored both anatomical and functional brain alterations in PTSD patients. Anatomical differences such as smaller medial prefrontal, subgenual cingulate, and hippocampal volumes in PTSD patients have been discussed as related to PTSD pathophysiology (Bremner et al., 1995; Bremner et al., 1997; Gurvits et al., 2000; Kasai et al., 2008; Kitayama et al., 2006; Rauch et al., 2003). However, these structural findings have been inconsistent and may represent a risk factor rather than a pathophysiologic difference (Gilbertson et al., 2002).
Functional neuroimaging has been employed to study PTSD using paradigms such as fear conditioning or extinction, exposure to a description of a PTSD patient's own traumatic event, or processing of emotional facial expressions. These functional studies show both abnormal regional activation patterns and differences in functional connectivity amongst cortico‐limbic circuitry. They suggest that the neural pathology in PTSD relates to a hyperactive amygdala that is not well regulated by under‐activated ventral/medial prefrontal cortex (Etkin and Wager, 2007; Fischer et al., 2000; Rauch et al., 2006; Spoormaker et al., 2010). Moreover, PTSD patients demonstrate a unique pattern of decreased blood flow to rostral and dorsal anterior cingulate cortex, ventromedial prefrontal cortex, and thalamus when processing fearful faces (Etkin and Wager, 2007).
What remains unclear from the current neuroimaging data in PTSD is whether recall or re‐experience of trauma stimuli consistently recruits neuronal circuitry that may in turn be at the core of the experienced symptoms. The key paradigms related to this autobiographical recollection are the script imagery or related imagery tasks that elicit symptoms in response to a relevant traumatic stimulus contrasted with neutral stimuli. Identification of the brain regions consistently active during symptom provocation and their interactions would allow for a better understanding of PTSD pathophysiology and improve neurobiological assessment of pharmaceutical and behavioral treatments.
Coordinate‐based meta‐analysis (CBM) is emerging as a powerful strategy for summarizing neuroimaging literature and developing new hypotheses that can subsequently be tested in a given population, such as using effective connectivity models (Eickhoff et al., 2009a). Paradigm‐specific activation likelihood estimation (ALE) may identify brain regions consistently activated during a specific task across a number of published neuroimaging studies. CBM has recently been extended to include analyses for connectivity by estimating co‐activation likelihood and generating functional/behavioral characterizations (Eickhoff et al., 2010; Laird et al., 2009; Robinson et al., 2009). The CBM approach thus allows assessment of (1) brain regions commonly active during a particular task in a particular population, (2) task‐based functional connectivity of these regions, and (3) the cognitive functions related to activations in a region or a network of regions.
In this study, we utilized CBM methods to develop a neural model of trauma rehearsal (i.e., recall of a traumatic event and the senses associated with it) in PTSD focusing on trauma versus neutral imagery tasks, in which trauma rehearsal is elicited. These tasks were chosen because frequent re‐experiencing of a traumatic event is a defining feature of PTSD, and they elicit the traumatic experience‐specific symptoms that are indicative of the disorder. Once the regions that were uniquely associated with trauma rehearsal in PTSD were identified, hypothetical connections as indicated by co‐activation across a number of studies in normal healthy subjects recorded in BrainMap, and the mental functions related to the activation of a particular region and its connections with other regions, were analyzed.
METHOD
Study Selection
A literature search was conducted in PubMed and PsychInfo using the following terms: post‐traumatic stress disorder, PTSD, fMRI, imaging, PET, brain, and trauma. This search returned five reviews and 51 experimental studies. Studies were included in the meta‐analysis if they were published in a peer‐reviewed, English‐language journal, reported results in a stereotactic coordinate system, and employed whole‐brain acquisitions and analyses (i.e., were not limited to a priori regions of interest). The remaining studies were filtered for content to include only those that compared trauma imagery (mainly based on subject‐generated scripts) with a neutral condition. Because most studies in the literature employed emotional processing tasks (such as the emotional face discrimination task), only eight papers met the inclusion criteria, collectively reporting results from 170 PTSD patients with 106 foci of activation. Six studies investigated the neural correlates of script‐based traumatic imagery and two investigated the use of traumatic visual scenes (Table 1). Five of these studies also reported responses to a similar task in healthy controls (HC), collectively reporting results from 104 control subjects with 90 foci of activation. Control subjects' scripts represented previous stressful events, typically experiences of loss (e.g., death of a loved one) or violence (e.g., physical fight or robbery). Importantly, these events would potentially have been capable of eliciting PTSD but did not do so in the control groups. The healthy controls were included to provide information about activation patterns during this task (group analysis) but more importantly to identify regions of activity unique to PTSD patients (between group CBM contrasts).
Table 1.
The references and experimental details for the studies included in the CBMA are presented
| Study | Subjects | Imaging modality | Eyes open or closed | Stimulus modality | Response modality |
|---|---|---|---|---|---|
| Shin et al., 2009 | 8 PTSD, 8 trauma controlsa | PET | Closed | Auditory | Recall and imagine |
| Britton et al., 2005 | 16 PTSD, 15 combat controls; 14 HC | PET | NK | Auditory | Focus |
| Lanius et al., 2007 | 11 PTSD, 16 HC | fMRI | NK | Auditory | Focus, remember senses |
| Lanius et al., 2005 | 10 PTSD, 11 PTSD with flashbacks, 10 CC | fMRI | NK | Auditory | Focus, remember senses |
| Lanius et al., 2004 | 11 PTSD, 13 combat controls | fMRI | NK | Auditory | Focus, remember senses |
| Pissiota et al., 2002 | 7 M PTSD | PET | Closed | Auditory | Listen |
| Morey et al., 2008 | 39 PTSD | fMRI | Open | Visual | Button press |
| Hou et al., 2007 | 14 PTSD, 14 combat controls | fMRI | Open | Visual | Button press |
Abuse victims.
NK: Not known.
Note that the Morey et al. and Hou et al. studies differed from the others with the stimulus modality being visual and the response involving a button press. We determined that this variance in task was acceptable as the subjects were still reacting to a traumatic stimulus relative to a neutral one.
Coordinate‐Based Meta‐analysis of Trauma Versus Neutral Contrasts
Separate CBMs were conducted for each group (PTSD and HC) to identify brain regions consistently activated in the “traumatic > neutral” conditions. The eight included studies were entered into the BrainMap neuroimaging database (Fox and Lancaster, 2002; Laird et al., 2005) in a common stereotaxic coordinate system (Lancaster et al., 2007) so that their reported activations could be pooled. Convergence of activations across studies was assessed using the latest modification of a coordinate‐based ALE algorithm (Turkeltaub et al., 2002) that (a) weights the number of subjects included in the experiments assuming that larger samples allow for more reliable localization and (b) performs random‐effects inference on the ALE values (Eickhoff et al., 2009b). Permutation tests of randomly generated foci were conducted to determine the statistical significance of ALE values (uncorrected cluster‐level thresholds at p < 0.01 and cluster‐forming thresholds of p < 0.001) and identify significant regions of common activity across studies.
To determine which regions were most likely to be activated in PTSD relative to HC, a task‐based CBM was computed for the group comparison (PTSD vs. HC). The results for the experiments entered into the CBM for both groups were combined and then randomly separated into groups of the same size as the initial groups with 25,000 permutations to create null distributions against which the differences between the original groups were contrasted (Eickhoff et al., in press). Conversely, to determine whether there were regions commonly activated in the PTSD and HC groups a conjunction analysis was conducted to identify areas where both cohorts showed significantly convergent effects in the “traumatic > neutral” scripts. The conjunction analysis represents the intersection of the thresholded ALE maps for both groups (Caspers et al., 2010).
Meta‐analytic Connectivity Modeling (MACM)
Once the ROIs that are uniquely activated in PTSD patients were identified, the next phase of analysis served to hypothesize the functional connectivity amongst the regions of interest (ROIs) using meta‐analytic connectivity modeling (Robinson et al., 2009). This phase of analysis was intended to characterize whether or not these regions typically co‐activate. To do this, BrainMap was queried to find all studies in which activations were reported within the ROI (mask images of each ROI were used as “locations” in which to search) in normal, healthy subjects, regardless of the type of task eliciting that activation. Note that the intent of the MACM was to characterize the functional connectivity amongst the regions and therefore did not take into account PTSD or its symptoms. Rather, it focused only on the normal/typical activity of the ROIs. MACM infers that above‐chance co‐activation between regions in healthy control subjects performing normal mapping experiments is a measure of task‐based functional connectivity (Eickhoff et al., 2010). MACM was computed for the 6,500 experiments (at that the time of analysis) in the BrainMap database. In this approach, the ROIs in which the PTSD patients evidenced consistent activity during trauma imagery tasks were seeded so that the MACM was conducted only on the experiments in the database featuring activation in the seed ROIs (Eickhoff et al., 2010; Laird et al., 2009; Robinson et al., 2009) to derive significant co‐activations. The ensuing connectivity maps were cluster‐level FWE thresholded at P < 0.05 (cluster‐forming threshold of P < 0.001) and demonstrate the “normal” connectivity of a seed ROI to the rest of the brain.
We also explicitly tested functional connectivity between each of the ROIs directly, i.e., seed‐to‐target MACM, by computing the MACM‐ALE value in the seed (ROI 1) and the target (ROI 2) and comparing that value against a null‐distribution of random overlap between experiments showing the same characteristics as those entering the MACM analysis but located at random locations throughout grey matter. This analysis essentially provided above chance likelihood of activity in one ROI predicting activity in another ROI, thus strengthening an assumption of functional connectivity between those brain regions.
An extension of the MACM was used to limit the exploration of connectivity of the ROIs to co‐active regions across the rest of the brain. That is, the database search was limited to identify only the experiments featuring activation of two of the ROIs (i.e., both ROIs were reported to be active during an experiment); therefore, the ensuing filtered MACM maps represent the regions that were active when two seed ROIs are active (P < 0.05). For example, when the ROI 1 and ROI 2 are both active during the same experiment, the regions identified with filtered MACM are also active above chance.
Analysis of Behavioral Metadata
Metadata are stored for each study in BrainMap (cf. http://brainmap.org/scribe/ and (Fox et al., 2005; Laird et al., 2009 for detail) that summarize experimental context. The type of mental process targeted in each study is classified in one of five behavioral domains (BD): action, cognition, emotion, interoception, or perception. When appropriate, behavioral sub‐domain metadata are also included (e.g., action: inhibition). In addition, the paradigm class (PC, e.g., word generation) for the tasks employed in a study is specified. In order to characterize the functions associated with each ROI, the metadata associated with all studies that activate an ROI are analyzed for the frequency of behavioral domain “hits” relative to the domain's likelihood across the entire BrainMap database. In particular, functional roles of the ROIs are identified by significant over‐representation of BDs/PCs as assessed by a binomial test (P < 0.05), corrected for multiple comparisons using Bonferroni's method. The intent of this last phase of analysis was to add to the MACM the typical types of tasks that elicit activity in the regions that were found to be uniquely active in the CBMA in PTSD patients.
RESULTS
CBM of Traumatic Versus Neutral Stimuli
The task‐based CBM indicated consistent activity induced by trauma versus neutral stimulus processing in PTSD in several brain regions (Supporting Information Table S2), the largest of which being the mid‐cingulate cortex and the posterior cingulate and parietal cortex. The conjunction of PTSD and HC activations showed common activity in many of these regions (Fig. 1), indicating that processing of traumatic stimuli activates similar brain regions in both groups. However, the group difference contrast showed three activated regions that were unique to the PTSD group. Those regions were the right anterior cingulate cortex (ACC), the right posterior cingulate cortex (PCC), and the left precuneus (Pcun). Because these regions were identified as active only in the PTSD patients, the ACC, PCC, and Pcun will henceforth be considered ROIs (Fig. 1) and will be further characterized for their connectivity and function with MACM.
Figure 1.
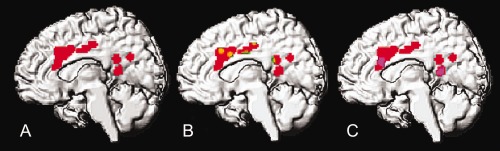
The coordinate‐based meta‐analysis (CBM) for the script imagery task in PTSD and HCs returned results identifying activity in the PTSD patients (A, red), the intersection of results for the PTSD and HC groups (B, red and green) and the brain regions active specific to the PTSD patients (C, purple), which were limited to the right anterior cingulate cortex (ACC), right posterior cingulate cortex (PCC), and the left precuneus (not pictured), i.e., PTSD regions of interest.
Meta‐analytic Connectivity Modeling
MACM maps were independently generated for the ROIs presenting whole‐brain functional connectivity for each. Again, the intent of this analysis was to characterize the “typical/normal” connectivity amongst these regions. These maps showed that the two posterior regions (PCC and Pcun, Fig. 1) share similar co‐activation patterns with virtually no overlapping activity with the ACC co‐activation pattern (Supporting Information Fig. S1) suggesting functional distinctions between them. This was confirmed with seed‐to‐target MACM, which was conducted to determine explicit co‐activation likelihoods between the three ROIs, indicating significant co‐activation between the two posterior regions (PCC, Pcun), but not with the ACC. That is, whereas experiments that activated one of the posterior regions were more likely than chance to activate the other, there was no such (conditional) relationship with the ACC.
To more narrowly define other brain regions that are co‐active with the ROIs during the same experiment, a seed‐based MACM was limited only to those experiments from BrainMap in which two of the ROIs were co‐active (i.e., co‐active pairs, PCC:Pcun = 39 experiments, PCC:ACC = 14 experiments, Pcun:ACC = 10 experiments). This analysis clarified that when the PCC:Pcun are co‐active there also was co‐activity with the left temporo‐angular region, right amygdala and middle temporal regions. When PCC:ACC were co‐active, the thalamus was also co‐active, particularly in the nuclei projecting to the prefrontal cortex (93.6% of the cluster) or the temporal lobe (Behrens et al., 2003). The 10 studies in which the ACC and Pcun were co‐activated also featured co‐activity of the right insula (cf. Supporting Information Table S2).
Analysis of Behavioral Metadata for ROIs
The behavioral metadata was analyzed to functionally characterize each ROI. The PCC and the Pcun were most likely to activate during cognitive (i.e., explicit memory, social cognition) or emotional tasks. In contrast, the ACC was active during tasks of attention, perception (somatesensation/pain), or inhibition of action (cf. Supporting Information).
The BD analysis also was applied to the ROIs as co‐active pairs to characterize the functions most likely to elicit activity of both regions during the same experiment. Pcun co‐activity, with either the PCC or the ACC, was most likely during social cognition or explicit memory tasks, whereas PCC and ACC co‐activity was most likely during emotional tasks (Fig. 2 and Supporting Information Figs. S2 and S3).
Figure 2.
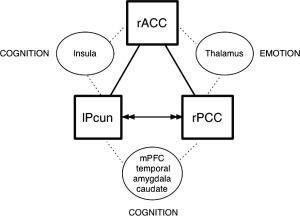
MACM indicated that (1) the three PTSD ROIs are functionally connected (lines); (2) inter‐connectivity with other regions (circles) suggests that the ROIs (squares) connectivity is mediated by those other regions (dashed lines), and (3) are behaviorally separable when co‐active within the same experiments (behavioral domains). The co‐activation of PCC:Pcun (co‐active in n = 39 experiments in BrainMap) is associated with Cognition and more specifically to social cognition, explicit memory, and interoception with particular engagement during tasks of episodic recall, theory of mind, subjective emotional picture discrimination or face monitor/discrimination. Seed to target MACM indicated reciprocal connectivity between the PCC:Pcun (i.e., activity in one region predicts activity of the other, arrows). Further, when the PCC:Pcun are co‐active, there is also co‐activity in medial prefrontal cortex (mPFC), medial and lateral temporal regions, and the caudate nucleus (Supporting Information Table S3). Co‐activation of ACC:Pcun (n = 10) is associated with cognition and particularly co‐active during explicit memory tasks during which the right insula is also co‐active. The only connection explicitly related to emotional processing was that of the PCC:ACC (n = 14) and their connection is mediated by the thalamus.
DISCUSSION
Implications for the Neuroscience of PTSD
A coordinate‐based meta‐analysis yielded an interconnected cortical network active during processing of traumatic stimuli and associated with symptoms of PTSD. As most of the experiments included in the CBM used trauma‐script imagery, the observed networks should reflect trauma‐specific activity evoked by the traumatic experience of each patient. The activation pattern consists of regions that are activated during traumatic stimulus processing in healthy control and PTSD subjects, as well as three regions that are uniquely active in PTSD.
The three ROIs were limited to medial parts of the brain (right anterior cingulate cortex, right posterior cingulate cortex, and left precuneus). The medial location of all of these regions is not surprising given previous reports that medial brain regions are inter‐connected and often functionally disconnected from lateral regions (Gilbert et al., 2010), particularly when the information being processed is self‐referential (Sajonz et al., 2010). Traumatic imagery tasks are by nature self‐referential with the patients instructed to imagine themselves in the traumatic experience.
As the CBM was driven by the traumatic imagery, it is not surprising that the ROIs were functionally associated with cognitive and emotional processing. Seed‐based MACM and behavioral domain analysis indicated that the PCC:Pcun and ACC:Pcun are most likely to be co‐active when the information being processed requires social cognition or explicit memory, whereas the ACC:PCC are co‐active when the information is emotional in nature. Our interpretation of these data is that the PCC:Pcun are functionally inter‐connected in the trauma‐processing context and related to processing of the self‐referential, autobiographical information as has been reported previously (Sajonz et al., 2010). Additionally, the posterior cingulate and precuneus are part of the “default mode network” (Laird et al., 2009), a network of regions that is activated in the absence of structured tasks, i.e., unconstrained cognition (Raichle et al., 2001) and deactivated, particularly with respect to frontal activation, during task performance (Greicius et al., 2003). Default mode activation during task performance is thought to be limited to tasks that involve autobiographical information and strongly associated with social cognition (Gusnard et al., 2001; Harrison et al., 2008; Schilbach et al., 2008; Spreng et al., 2008).
The most parsimonious explanation for the PCC:Pcun role in PTSD trauma processing is specific to the recall of the very personal traumatic event (i.e., autobiographic information that may be more relevant to PTSD patients than for control subjects). Co‐activation of default mode regions with regions engaged during task is considered to represent dynamic disequilibrium (Greicius et al., 2003) whereby regions that are usually deactivated during task performance (PCC:Pcun) have an undue influence on other regions during task performance (ACC in this case). Whether this disequilibrium is secondary to a PTSD‐specific dysfunction of the default mode network (e.g., the PTSD default mode network connectivity and baseline level of activity is different from normal) or whether the PCC:Pcun activity observed in the trauma‐task CBM is specific to the autobiographical memory requires further investigation.
In contrast to the PCC, the ACC may serve a supervisory or executive role that is engaged to manage the emotional and perceptual demands specific to the recall of a traumatic event. For example, the ACC ROI identified in the CBM is associated not only with cognition and disambiguation of emotion (Muller et al., 2011) but also with perceptual processing, particularly for monitoring or discrimination of painful stimuli. The association of PTSD and pain processing has been established in previous studies. PTSD patients have an overactivated endogenous opioid system and demonstrate stress‐induced increase in production of endogenous opioid‐mediated analgesia (Glover, 1995; Pitman et al., 1990). The ACC region identified in the present CBM has been associated with decreased μ‐opioid receptor density in PTSD patients (Liberzon et al., 2007) and negatively correlated with affective pain ratings (Zubieta et al., 2001). In fact, one genetic risk factor for PTSD, the catechol‐O‐methyltransferase (COMT) val158met polymorphism (Amstadter et al., 2009; Kolassa et al., 2010; Valente et al., 2011) is associated with diminished μ‐opioid receptor binding potential in the cingulate cortex (Zubieta et al., 2003). The convergence of findings related to pain processing and μ‐opioid associated analgesia in PTSD may relate to self‐preservation or ability to engage the biological mechanisms associated with allostasis (McEwen, 2000; Yehuda and LeDoux, 2007).
It is important to highlight that one brain region consistently implicated to be hyperactive in PTSD was not overtly identified in our analysis—the amygdala. Convergence of previous neuroimaging studies in PTSD have lead to the thought that there is an inverse co‐activation between the amygdala (hyperactivity) and the anterior cingulate cortex (hypoactivity) that is unique to PTSD relative to other anxiety disorders (4). The right amygdala was identified in our analysis, but only as it was co‐active in normal subjects with the PCC:Pcun, suggesting that although its activity is not explicitly involved in script imagery in PTSD, it is part of a network of regions that were engaged. The lack of strong amygdala activity in the present CBM was likely due to our data selection strategy. Our meta‐analysis focused on tasks using traumatic versus neutral stimuli, e.g., the trauma‐script imagery task. This task elicited the symptoms most characteristic of the disorder and is commonly used in behavioral treatments. By contrast, the most prevalent task used in previous studies was emotional face discrimination, a task designed to and known to elicit amygdala activation (Hariri et al., 2000). In fact, the amygdalae are highly associated with emotional task performance and a previous CBM‐MACM indicated that connectivity with the amygdala is characterized by a BD profile of high emotional task and low cognitive task loading (Robinson et al., 2009). The absence of the amygdala in the trauma‐script imagery CBM elucidated the importance of cognitive/memory and perception in this type of processing that may be separate from the immediate emotional reaction observed when subjects view emotional pictures.
Implications for CBM Methods in Future Analyses
The meta‐analytic techniques presented here allow for hypothesis development regarding the brain regions involved in a disorder, the co‐activations between these regions in typical or normal healthy conditions, and the task types most likely eliciting that activation. By limiting our CBM to studies in which subjects were exposed to a traumatic stimulus and further distinguishing the activity of PTSD from that of HC, the three ROIs are likely those involved with the development and maintenance of PTSD symptoms. The utility of the CBM approach is to narrow the focus of neuroimaging analysis with a data‐driven approach to identify the brain regions most likely to be involved in traumatic stimulus processing. MACM provides further information about the connectivity that can be assumed amongst the brain regions and thereby allow for hypothesis testing using causal modeling approaches.
CONCLUSIONS
The more practical intent of this work is to provide a data‐driven approach for selecting volumes of interest based on common activation as well as behavioral and paradigm profiles from meta‐analysis of neuroimaging findings. Ongoing work using the model generated here will determine its utility in differentiating PTSD from other populations using discriminant function or classifier analyses—having an increased likelihood of model fit by choosing highly relevant ROIs will increase the statistical power for detection of differences between populations. In addition, the model will be tested as an “a priori” model in causal modeling methods to evaluate treatment effects in PTSD that center on reduction of symptoms (proposed in Neumann et al., 2010, 2011). For example, the most widely accepted behavioral and pharmaceutical treatments for PTSD target reduction of the fearful response triggered by autobiographically relevant traumatic stimuli, i.e., cognitive processing therapy (e.g., Resick et al., 2009) and prolonged exposure (e.g., Rauch et al., 2009). As such, this model will be used to identify changes in brain connectivity as the causal result of treatments aimed at reducing the disabling symptoms of PTSD, a common and enduring finding in positive treatment effects measured via neuroimaging (Ma et al., 2010).
Supporting information
Supporting Information
REFERENCES
- Amstadter AB, Nugent NR, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, Gelernter J (2009): Association between COMT, PTSD, and increased smoking following hurricane exposure in an epidemiologic sample. Psychiatry 72:360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA (1994):Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC:American Psychiatric Association. [Google Scholar]
- Behrens TE, Johansen‐Berg H, Woolrich MW, Smith SM, Wheeler‐Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, et al. (2003): Non‐invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature neuroscience 6:750–757. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS (1995): Functional neuroanatomical correlates of the effects of stress on memory. J Trauma Stress 8:527–553. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS (1997): Magnetic resonance imaging‐based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biol Psychiatry 41:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB (2010): ALE meta‐analysis of action observation and imitation in the human brain. Neuroimage 50:1148–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K (2009a): A systems perspective on the effective connectivity of overt speech production. Philosophical transactions. Series A, Mathematical, physical, and engineering sciences 367:2399–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009b): Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30:2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, Behrens TE (2010): Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci 30:6409–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT (2011): Co‐activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage 57:938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD (2007): Functional neuroimaging of anxiety: a meta‐analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164:1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Andersson JL, Furmark T, Fredrikson M (2000): Fear conditioning and brain activity: A positron emission tomography study in humans. Behav Neurosci 114:671–680. [DOI] [PubMed] [Google Scholar]
- Fox PT, Laird AR, Lancaster JL (2005): Coordinate‐based voxel‐wise meta‐analysis: Dividends of spatial normalization. Report of a virtual workshop. Hum Brain Mapp 25:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL (2002): Opinion: Mapping context and content: The BrainMap model. Nat Rev Neurosci 3:319–321. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Gonen‐Yaacovi G, Benoit RG, Volle E, Burgess PW: Distinct functional connectivity associated with lateral versus medial rostral prefrontal cortex: A meta‐analysis. Neuroimage (in press). [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK (2002): Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 5:1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover H (1995): A different opinion regarding the use of opiate antagonists in PTSD: Comments on “An unusual reaction to opioid blockade with naltrexone in a case of post‐traumatic stress disorder”. J Trauma Stress 8:483–489. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvits TV, Gilbertson MW, Lasko NB, Tarhan AS, Simeon D, Macklin ML, Orr SP, Pitman RK (2000): Neurologic soft signs in chronic posttraumatic stress disorder. Arch Gen Psychiatry 57:181–186. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001): Medial prefrontal cortex and self‐referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 98:4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC (2000): Modulating emotional responses: Effects of a neocortical network on the limbic system. Neuroreport 11:43–48. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Lopez‐Sola M, Hernandez‐Ribas R, Deus J, Ortiz H, Soriano‐Mas C, Yucel M, Pantelis C, Cardoner N (2008): Consistency and functional specialization in the default mode brain network. Proc Natl Acad Sci USA 105:9781–9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam EG, Andrews G, Bromet E, Petukhova M, Ruscio AM, Salamoun M, Sampson N, Stein DJ, Alonso J, Andrade LH, et al. (2010): The role of criterion A2 in the DSM‐IV diagnosis of posttraumatic stress disorder. Biol Psychiatry 68:465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK (2008): Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat‐related posttraumatic stress disorder. Biol Psychiatry 63:550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama N, Quinn S, Bremner JD (2006): Smaller volume of anterior cingulate cortex in abuse‐related posttraumatic stress disorder. J Affect Disord 90:171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa IT, Kolassa S, Ertl V, Papassotiropoulos A, De Quervain DJ (2010): The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol‐o‐methyltransferase Val(158)Met polymorphism. Biol Psychiatry 67:304–308. [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT (2009): Investigating the functional heterogeneity of the default mode network using coordinate‐based meta‐analytic modeling. J Neurosci 29:14496–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Lancaster JL, Fox PT (2005): BrainMap: The social evolution of a human brain mapping database. Neuroinformatics 3:65–78. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas‐Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT (2007): Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Phan KL, Britton JC, Fig LM, Bueller JA, Koeppe RA, Zubieta JK (2007): Altered central micro‐opioid receptor binding after psychological trauma. Biol Psychiatry 61:1030–1038. [DOI] [PubMed] [Google Scholar]
- Ma L, Wang B, Narayana S, Hazeltine E, Chen X, Robin DA, Fox PT, Xiong J (2010): Changes in regional activity are accompanied with changes in inter‐regional connectivity during 4 weeks motor learning. Brain Res 1318:64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2000): Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology 22:108–124. [DOI] [PubMed] [Google Scholar]
- Muller VI, Habel U, Derntl B, Schneider F, Zilles K, Turetsky BI, Eickhoff SB (2011): Incongruence effects in crossmodal emotional integration. Neuroimage 54:2257–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J, Fox PT, Turner R, Lohmann G (2010): Learning partially directed functional networks from meta‐analysis imaging data. Neuroimage 49:1372–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J, Turner R, Fox PT, Lohmann G (2011): Exploring functional relations between brain regions from fMRI meta‐analysis data: Comments on Ramsey, Spirtes, and Glymour. Neuroimage 57:331–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, van der Kolk BA, Orr SP, Greenberg MS (1990): Naloxone‐reversible analgesic response to combat‐related stimuli in posttraumatic stress disorder. A pilot study. Arch Gen Psychiatry 47:541–544. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, Whalen PJ, Makris N (2003): Selectively reduced regional cortical volumes in post‐traumatic stress disorder. Neuroreport 14:913–916. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA (2006): Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research—Past, present, and future. Biol Psychiatry 60:376–382. [DOI] [PubMed] [Google Scholar]
- Rauch SA, Grunfeld TE, Yadin E, Cahill SP, Hembree E, Foa EB (2009): Changes in reported physical health symptoms and social function with prolonged exposure therapy for chronic posttraumatic stress disorder. Depress Anxiety 26:732–738. [DOI] [PubMed] [Google Scholar]
- Resick PA, Iverson KM, Artz CE (2009): Participant reactions to a pretreatment research assessment during a treatment outcome study for PTSD. J Trauma Stress 22:316–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT (2009): Metaanalytic connectivity modeling: Delineating the functional connectivity of the human amygdala. Hum Brain Mapp 31:173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajonz B, Kahnt T, Margulies DS, Park SQ, Wittmann A, Stoy M, Strohle A, Heinz A, Northoff G, Bermpohl F (2010): Delineating self‐referential processing from episodic memory retrieval: Common and dissociable networks. Neuroimage 50:1606–1617. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska‐Jagiela A, Fink GR, Vogeley K (2008): Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn 17:457–467. [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, Sturm A, Andrade KC, Schroter MS, Goya‐Maldonado R, Holsboer F, Wetter TC, Samann PG, Czisch M (2010): The neural correlates and temporal sequence of the relationship between shock exposure, disturbed sleep and impaired consolidation of fear extinction. J Psychiatr Res 44:1121–1128. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS (2008): The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: A quantitative meta‐analysis. J Cogn Neurosci 21:489–510. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. Neuroimage 16:765–780. [DOI] [PubMed] [Google Scholar]
- Valente NL, Vallada H, Cordeiro Q, Bressan RA, Andreoli SB, Mari JJ, Mello MF (2011): Catechol‐O‐methyltransferase (COMT) val158met polymorphism as a risk factor for PTSD after urban violence. J Mol Neurosci 43:516–523. [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J (2007): Response variation following trauma: A translational neuroscience approach to understanding PTSD. Neuron 56:19–32. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS (2001): Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 293:311–315. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D (2003): COMT val158met genotype affects mu‐opioid neurotransmitter responses to a pain stressor. Science 299:1240–1243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


