Abstract
Chronic stress and dysfunction of the serotonergic system in the brain have been considered as two of the major risks for development of depression. In the present study, adult Fischer 344 rats were subjected to a regimen of chronic social defeat (CSD). To mimic stressful conditions some rats were not exposed to CSD but instead treated with corticosterone (CORT) in oral solution while maintained in their home cage. Protein levels of the serotonin transporter (SERT) in the dorsal raphe nucleus (DRN), hippocampus, frontal cortex and amygdala were examined by western blotting or immunofluorescence staining. The results showed that CSD up-regulated SERT protein levels in the DRN, hippocampus, frontal cortex and amygdala regions. This upregulation was abolished or prevented by adrenalectomy, or treatment with antagonists of corticosteroid receptors mifepristone and spironolactone, alone or in combination. Similarly, up-regulated SERT protein levels in these brain regions were also observed in rats treated with oral CORT ingestion, which was analogously prevented by treatment with mifepristone and spironolactone. Furthermore, both CSD- and CORT-induced upregulation of SERT protein levels in the DRN and three brain regions were attenuated by simultaneous treatment with fluoxetine, an antidepressant that specifically inhibits serotonin reuptake. The results indicate that upregulation in SERT protein levels in the DRN and forebrain limbic structures caused by CSD regimen was mainly motivated by CORT through corticosteroid receptors. The present findings demonstrate that chronic stress is closely correlated with the serotonergic system by acting on the regulation of the SERT expression in the DRN and its projection regions, which may contribute to the development of depression.
Keywords: Serotonin transporter, Corticosterone, Chronic social defeat, Rat brain, Raphe nuclei, Corticosteroid receptors
Introduction
Serotonin (5-HT) is one of the classic neurotransmitters in the central nervous system (Azmitia 2001, Gaspar et al. 2003), and plays an important role for neuronal transduction. While serotonin levels in the brain can be coordinately regulated by serotonin autoreceptors and enzymes that synthesize (tryptophan hydroxylase, TPH) or degrade (monoamine oxidase, MAO) serotonin (Watanabe et al. 2011), serotonergic transmission is also critically regulated by the recapture of released serotonin via the serotonin transporter (SERT) located in the plasma membrane. SERT is also the pharmacological target of the selective serotonin reuptake inhibitors (SSRIs), one type of antidepressant (Baudry et al. 2010). It has been documented that dysfunctional serotonergic neurotransmission is implicated in a variety of psychiatric disorders, including major depression (Mann et al. 1995, Bhagwagar et al. 2002) and anxiety (Graeff 1997). Therefore, as a key protein in the regulation of serotonergic transmission, alterations in SERT expression and function are closely related to the pathophysiology of these diseases. Given both chronic stress and a dysfunctional serotonin system have been implicated in the development of depression, examining effects of chronic stress on SERT expression in the brain will significantly advance knowledge of the molecular link between stress and the serotonin system in the brain, which may contribute to the appearance of depressive symptoms.
In the brain, serotonin neuronal cell bodies are located in the hindbrain, where serotonergic fibers project to the rest of the brain (Bethea et al. 2005). The vast majority of these clusters of cell groups are within the raphe nuclei, of which there are several main functionally and anatomically distinct subgroups including the dorsal raphe nucleus (DRN) and median raphe nucleus (MRN) (MacGillivray et al. 2010). Among them, in rats the DRN is the largest, containing approximately 50% of serotonergic neurons in the brain (Wiklund et al. 1981, Jacobs et al. 1992). The neuronal fibers from the DRN innervate the frontal and parietal cortices, amygdala, lateral septum, nucleus accumbens shell, ventral hippocampus and several hypothalamic nuclei (Lechin et al. 2006). It was reported that SERT concentrations are highest in the DRN, with lower concentrations in other raphe nuclei (Hrdina et al. 1985, Fujita et al. 1993, Hoffman et al. 1998, Rattray et al. 1999, Clark et al. 2006). In the past decades, effects of stress on SERT expression in the raphe nuclei have been studied. However, the results are controversial and stressor dependent. For example, stress sensitive cynomolgus monkeys had lower levels of SERT mRNA in the DRN (Bethea et al. 2005). Rats that experienced neonatal maternal separation showed a reduced SERT mRNA in the raphe nuclei (Lee et al. 2007). Single immobilization reduced SERT mRNA in the raphe pontis (Vollmayr et al. 2000). Single social defeat reduced the SERT densities in hippocampus, but did not change those in the midbrain where the DRN is located (Berton et al. 1999). In contrast, chronic restraint plus cold stress resulted in an increase in SERT mRNA levels in the DRN of both male and female Wistar Kyoto rats (Pare et al. 1999). Likewise, increased SERT mRNA levels in the raphe nuclei in the midbrain were observed in mice that had repeated experience of social defeats (Filipenko et al. 2002). Accordingly, these studies with inconsistent results clearly indicate the necessity of pursuing further study about effects of chronic stress on the SERT in the DRN.
During stress, multiple hormones are released including corticotropin-releasing factor (CRF), vasopressin, adrenocorticotropic hormone (ACTH), glucocorticoids [(cortisol in human and corticosterone (CORT) in rodents], oxytocin, prolactin and renin (Van de Kar et al. 1999). These hormones trigger multiple reactions to integrating homeostasis and exerting crucial life-sustaining functions. Among them, CORT is the main hormone released by the adrenal cortex in rats and serves as a master in the control of neuronal and signal transduction, as well as affects cellular and molecular events in brains by modulating the expression of many genes. It was reported that corticosteroid receptors are expressed in the DRN (Cintra et al. 1993). Also, administration of dexamethasone, a synthetic glucocorticoid hormone, resulted in an increase in SERT protein density in immortalized human B-lymphoblastoid cells, which express the human SERT (Glatz et al. 2003). Thus, identifying the exact role of CORT played during chronic stress is also essential for elucidating molecular mechanisms underlying the development of stress-related disorders.
The present study examines effects of CSD regimen on the expression of SERT in the DRN, hippocampus, frontal cortex and amygdala of rats. We also treated rats with CORT to mimic stress effect to verify whether CORT plays a main role during chronic stress. Our results reveal that CSD regimen and CORT treatment upregulated SERT protein levels in those brain regions, which may account for the reduced serotonin levels in synaptic clefts, which has been hypothesized to be causatively ascribed to the appearance of depressive symptoms.
Materials and methods
Animals
Male Fischer 344 rats, weighing about 200–250 g at the beginning of the experiment, Long-Evans retired male breeder and ovariectomized female rats were purchased from Harlan Laboratories Inc. (Indianapolis, IN, USA). All animal procedures were approved by the Animal Care and Use Committee of East Tennessee State University, and complied with the NIH Guide for the Care and Use of Laboratory Animals. Rats were maintained on a 12 hour light/dark cycle (lights on at 7:00 am) with ad libitum access to food and tap water except as specifically described below. After an acclimation period of 5 days, rats were randomly assigned to experimental groups and all experimental testing occurs in the light phase, except for half hour in the dark phase for sucrose test.
Chronic social defeat paradigm
Chronic social stress is based on the resident-intruder paradigm as described previously (Chen et al. 2012). Briefly, the experiment began (at 9:00 am of each day) by exposing an “intruder” (adult male Fischer rat) to the home cage of the “resident” (larger male Long-Evans rat) after female rats had been removed. After the “intruder” was attacked and showed the defeated behavior, the “intruder” was rescued and placed into a small protective cage within the resident’s cage. As such, the “intruder” and “resident” were separated, but still kept in the same home cage for one and half hour to remain unrestricted visual, auditory, and olfactory contact with each other without physical attack. This exposure was repeated four times in the first and fourth weeks, and two times in the second and third weeks. Some rats (as the control) were given access to the entire resident home cage when the residents have been removed (sham-exposure). Therefore, the controls were never physically attacked and defeated by the residents. One group of rats were adrenalectomized (ADX) by Harlan Laboratories Inc. before shipping to the animal facility of East Tennessee State University. The care of ADX rats was the same as reported previously (Chen et al. 2012). Briefly, ADX rats were provided with 25 µg/ml CORT in the drinking water during whole experimental period. Such small replacement dose of corticosterone has been shown to be adequate for prevention of post-adrenalectomy alterations in the central nervous system (Pace et al. 2009). Trunk blood was taken when rats were sacrificed by rapid decapitation immediately after the last session of CSD on 28th day (around 11:00 am). After rats were sacrificed, brains were removed and rapidly frozen in 2-methyl-butane on dry-ice, then stored at −80° C until dissection.
Oral treatment with CORT
In the experiment that rats were treated with CORT, after 5 day acclimation phase, while ad libitum chow remained available, drinking water was replaced with a solution containing 100 µg/ml CORT (Sigma, St. Louis, MO) at 9:00 am of each day, which was freshly prepared daily. Selection of such concentration of CORT was based on the reports in the literature, which caused an increased plasma CORT level approaching stress induced levels (Karatsoreos et al. 2010, Donner et al. 2012). Because of its hydrophobic characteristics, CORT was first dissolved in 100% ethanol, and then diluted in regular tap water to a final ethanol concentration of 2.4% ethanol. The control rats were given a 2.4% ethanol solution alone. After 3-week CORT treatment, animals were sacrificed on 22nd day by rapid decapitation and trunk blood was collected for measurement of plasma CORT (around 9:30 am). After rats were sacrificed, brains were handled by the same way as described in above paragraph.
Drug Treatment
In order to examine whether corticosteroid receptors are involved in the CSD- or CORT-induced regulation of SERT expression, relatively specific mineralocorticoid receptors (MR) antagonist spironolactone and glucocorticoid receptors (GR) antagonist mifepristone were used in these animals. Therefore, some groups of rats were treated with mifepristone (10 mg/kg, daily, s.c.) or spironolactone (15 mg/kg, daily, s.c.), either alone or in combination. To test effects of transporter blockers on expression of SERT, some groups of rats were treated with desipramine (10 mg/kg, daily, i.p.) or fluoxetine (10 mg/kg, daily, i.p.). The dose selection of these antagonists was based on our previous experiments (Chen et al. 2012). Rats in the untreated control, CSD alone, or CORT alone groups were injected with similar volumes of vehicle in the same manner. All these compounds or vehicle were administrated 10 minutes prior to the CSD regimen, or at the same time when the oral CORT solution was replaced in the morning.
Sucrose consumption test for CSD paradigm and plasma CORT determination
The methods for these two tests are the same as described before (Chen et al. 2012). Briefly, the sucrose consumption test [deprivation in the morning and two bottles of drinking solution (1% sucrose solution and tap water) provided in the one hour window between 06:30 pm and 07:30 pm] was carried out weekly on the same day of the week (Thursday) throughout the four week CSD regimen. Plasma CORT was later measured by a radioimmunoassay using a commercial kit according to the manufacturer’s instructions [ImmuChem radioimmunoassay kit, MP Biomedicals, LLC in Orangeburg, NY (formerly ICN Pharmaceuticals, Costa Mesa, CA)].
Western blotting
The frozen brain was positioned on the specimen holder in a cryostat (at −19° C), and tissue was cut in caudal to rostral direction until −8.00 mm from bregma (Paxinos and Watson, 1986). DRN tissue was then punched out (one punch for each subject) by a pre-cooled sharpened punch needle (inner diameter 1.5 mm, −20° C). As the DRN extends far caudal to −8.00 mm bregma, the region microdissected corresponds to the rostral DRN. The needle was punched 1 mm deep into the tissue. This depth corresponds to the maximal caudo-rostral extension of the DRN. The dissection of the hippocampus, frontal cortex and amygdala was performed with the similar way. The slicing coordinate is: the frontal cortex: 4.70-3.70 mm; amygdala: −2.14– −3.14 mm, and the hippocampus: −2.56 – −4.56 mm from bregma. Samples of the frontal cortex were obtained from the section after exciting the olfactory bulbs, and the hippocampus by exciting surrounding other brain tissues. The amygdala was punched from the section with a pre-cooled sharpened punch needle (inner diameter 3 mm, −20° C). Punched DRN and other brain samples were then solubilized by ultrasound (130 w, 20 kHz; 5 seconds) in sample buffer containing Tris–HCl (40 mM), sodium lauryl sulfate (SDS, 4%) and β-mercaptoethanol (5%). After centrifugation at 10,000g×5min, protein concentrations of supernatants were measured by the BCA method. Forty five µg samples per lane were loaded on 10% SDS–polyacrylamide gels for electrophoresis. Protein bands in gels were transferred to Amersham Hybond-ECL membranes by semi-dry transfer. The membranes were incubated with anti-SERT primary antibody (1:2000 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA, Cat# sc-33724, Lot# B-1711) overnight at 4°C. After washing, membranes were then further incubated with secondary antibodies (horseradish peroxidase-conjugated anti-mouse IgG, 1:10,000 dilution; Amersham Biosciences, Little Chalfont, UK). Immunoreactive bands were visualized by enhanced chemiluminescence (ECL, Amersham, Piscataway, NJ). Bands were exposed on films and scanned. Band densities were then quantified by Quantity One imaging software. Optical density values of SERT signals were compared and normalized with β-actin immunoreactivities, which were determined on the same blot. Normalized values were then averaged for all replicated gels and used to calculate the relative changes of the same gel.
Immunofluorescence staining
The process for immunofluorescence staining is the same as described before (Fan et al. 2011). Briefly, after rats were transcardially perfused with 4% paraformaldehyde, the brains were removed and stored in turn in 10% and 30% sucrose, then sectioned at 30 µm around the DRN region on a sliding microtome. For immunofluorescence staining of SERT, the sections were blocked in 5% goat normal serum in PBS/Triton for 60 min, and then incubated at 4°C overnight in PBS/Triton containing 1:200 anti-SERT primary antibodies (Calbiochem, San Diego, CA; Cat# pc-177L, Lot# D-27424). Immunoreactivity to the antigen was visualized using Alexa 488-conjugated secondary antibodies (Invitrogen, Carlsbad, CA). Immunofluorescence labeling images were taken under a Leica TCS SP2 confocal microscope system (Leica Microsystems Inc., Bannockburn, IL, USA) and semiquantitatively analyzed by using Image J software (Rasband, US National Institutes of Health, Bethesda, http://rsbweb.nih.gov/ij, 2010).
Statistics
All data are expressed as mean ± SEM. The unpaired Student’s t-test was used to analyze data of plasma CORT measurement in the experiment of CORT administration (Fig. 7A). The repeated measures ANOVA was used for data in Fig. 1B. For other experimental differences among multiple groups were compared by one-way analysis of variance (ANOVA) followed by post hoc Newman-Keuls tests for planned comparisons when there was a significant difference between groups.
Figure 7.
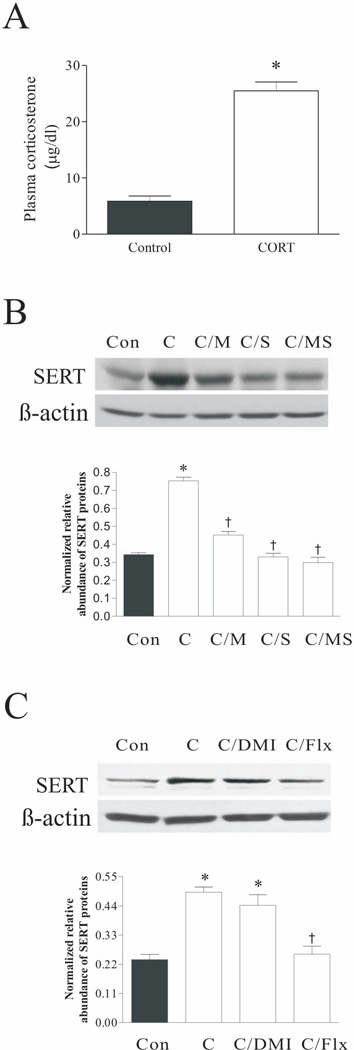
Effect of CORT treatment on plasma CORT concentrations (A), and SERT protein levels in rat DRN after CORT treatment plus corticosteroid receptor antagonists (B) or antidepressants (C). The upper panels in B and C were obtained by western blotting for SERT. The lower panels in B and C showed quantitative analysis of band densities. Values of SERT bands were normalized to those of β-actin probed on the same blot. *p<0.05, compared to the control group; † p<0.05, compared to the CORT group (n=4/group). Con: control; C: corticosterone; C/DMI: CORT plus treatment with desipramine; C/Flx: CORT plus treatment with fluoxetine; C/M: CORT plus treatment with mifepristone; C/S: CORT plus treatment with spironolactone; C/MS: CORT plus treatment with both mifepristone and spironolactone.
Figure 1.
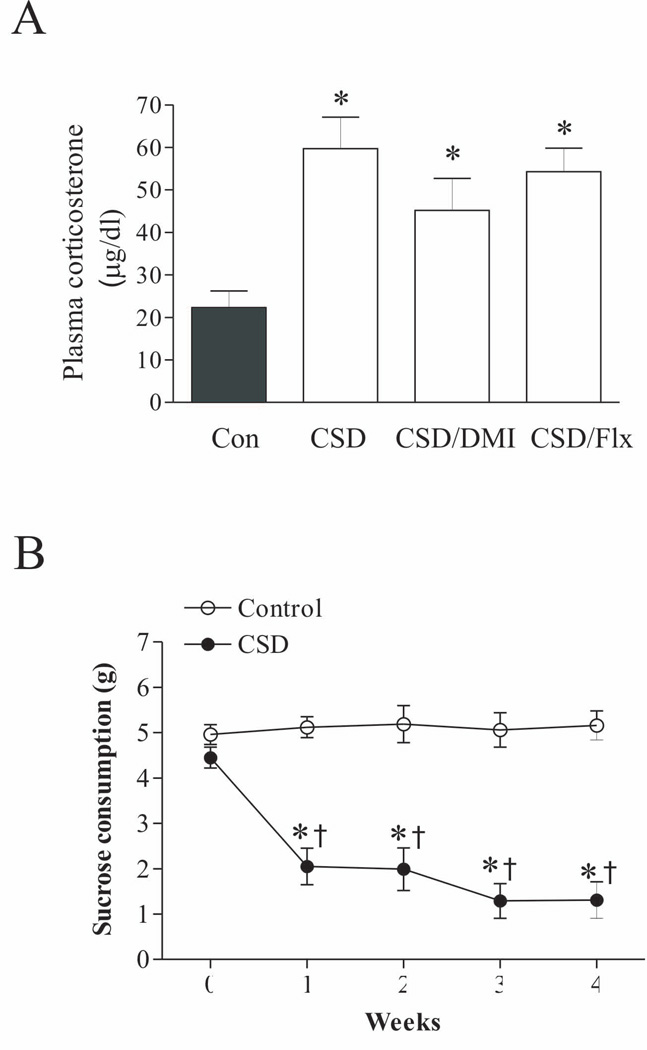
A. The graphs represent the effects of chronic social defeat (CSD) stress (n=7/group) and desipramine/fluoxetine on plasma CORT concentrations. The trunk blood was collected on the 28th day immediately after the end of last session of CSD. * p<0.01, compared to the control. Con: control; CSD/DMI: CSD plus treatment with desipramine; CSD/Flx: CSD plus treatment with fluoxetine.
B. Sucrose solution intake in the control and CSD rats (n=7/group). * p<0.05, compared to corresponding time in the control; † p<0.05, compared to the CSD group at week 0.
Results
Plasma CORT measurement and sucrose consumption test in CSD experiments
Plasma CORT levels were measured in samples collected immediately after the last CSD paradigm at the end of the four-week stress to examine the efficacy of chronic stress in inducing hormonal modification. Because effects of ADX and corticosteroid receptor antagonists on plasma CORT levels in CSD rats have been reported in our previous paper (Chen et al. 2012), we did not repeat this measurement in the current study. As shown in Figure 1A, CSD significantly affected plasma CORT levels (F3,25=13.18, p<.001). Post hoc tests revealed that plasma CORT levels were significantly elevated in the rats subjected to CSD (p<0.01), as compared to control rats. Although the plasma CORT level in the groups of treatment with desipramine or fluoxetine were lower than that in the CSD group, it was not reach the significant levels (p>0.05) and was still markedly higher than that of the control (p<0.01).
The sucrose consumption test was also used to evaluate depressive effects of CSD in rats. Similarly, we did not repeat the sucrose consumption test in CSD rats subjected to ADX and treatment with corticosteroid receptor antagonists, as similar tests have also been done as reported previously (Chen et al. 2012). Instead, in the present experiment, we only repeated the test for rats subjected to CSD regimen and in the control group. As shown in Fig. 1B, the intake of the sucrose solution was not significantly changed in non-stressed animals over the whole duration of the experiment (F4,30 = 1.89, p>0.05). However, it was significantly reduced from the first to fourth week of stress exposure in CSD rats (F4,32 =14.45, p<0.001).
Expression and localization of SERT-immunoreactive neurons in rat DRN sections
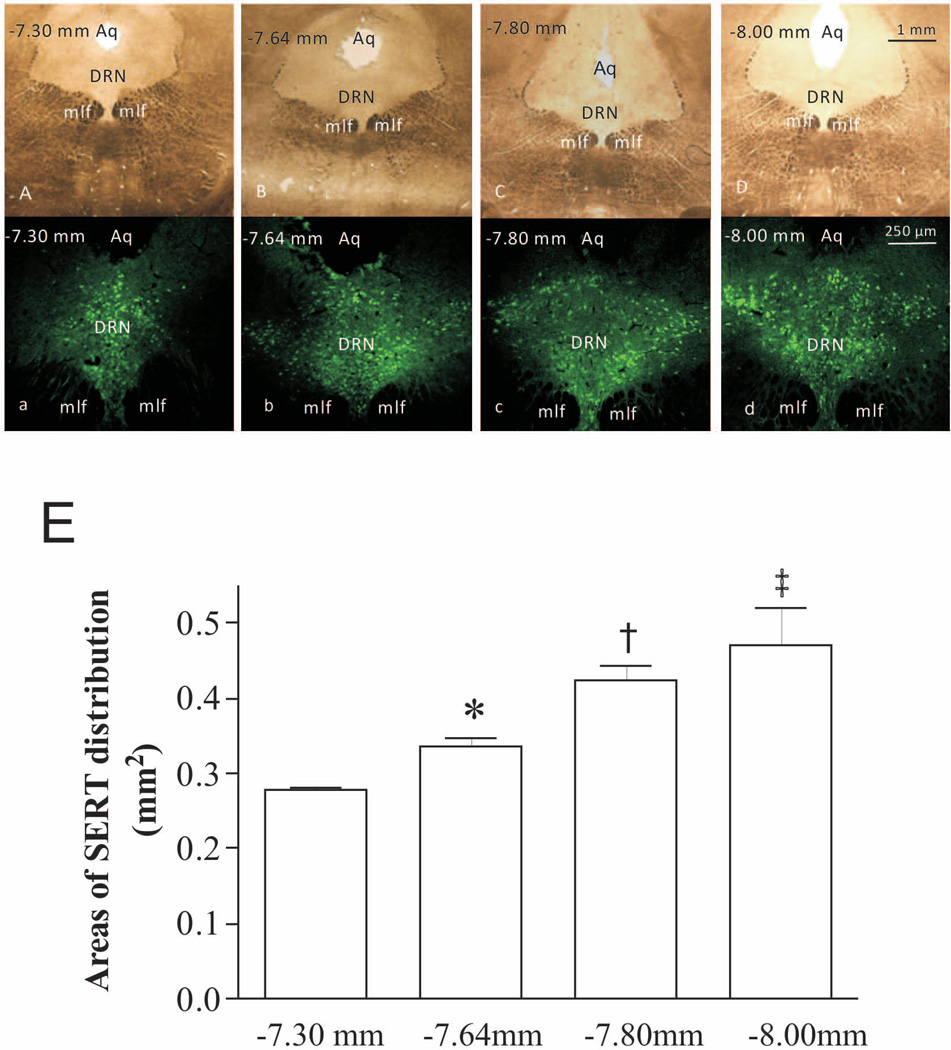
To investigate the organization and patterns of SERT-immunoreactive cells in Fischer 344 rats, coronal sections of the DRN region were subjected to immunohistochemistry with the anti-SERT antibody. As shown in Fig. 2, sections A, B, C, and D (bright field) were obtained from bregma −7.30 mm, −7.64 mm, −7.80 mm and −8.00 mm, respectively, according to the atlas figures of rat brain (Paxinos et al. 1986). Apparently, the area of SERT distribution was larger in the DRN section located at bregma −8.00 mm (Fig. 2d) than those at other locations (Figs. 2a, 2b, 2c). Areas of SERT distribution were measured, analysed and shown as the statistic graph in Fig. 2E. It revealed that there were significant differences of areas between each pair of groups (p<0.05). The regional variability in SERT expression distribution observed in this study may account for the systematic differences among animal experiments reported in the literature previously.
Figure 2.
SERT distribution in rat DRN. SERT is expressed throughout the DRN, but distributed differently in sections. (A–D): Coronal sections of the DRN were obtained from bregma −7.30 mm, −7.64 mm, −7.80 mm and −8.00 mm respectively. Microphotos were captured in bright field. (a–d): SERT immunostaining in these sections. Images were obtained by a fluorescence microscope. Aq: aqueduct; mlf: medial longitudinal fasciculus. (E): Areas of SERT distribution in immunostaining microphotos were measured by Image J software and subjected to statistical analysis. *p<0.05, compared to the bregma −7.30 mm group; † p<0.05, compared to the bregma −7.64 mm group; ‡ p<0.05, compared to the bregma −7.80 mm group (n=4/group).
Effects of CSD on SERT protein levels in the DRN region
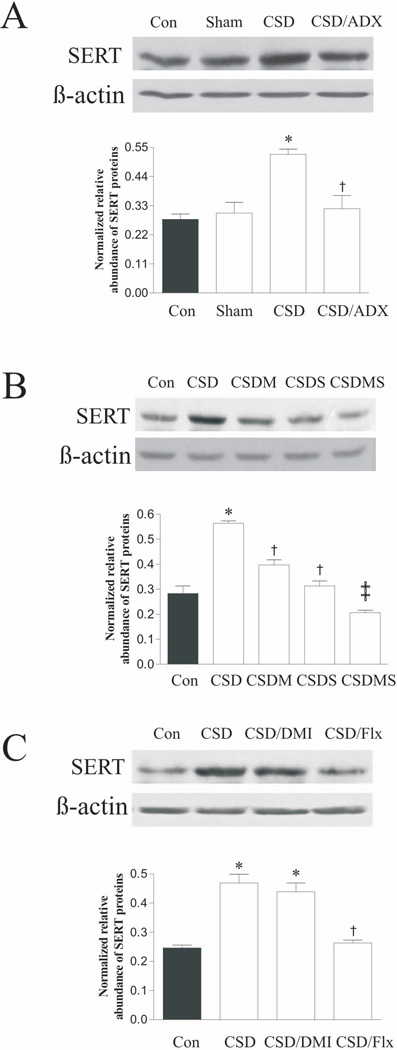
To explore the influence of CSD on the expression of SERT in rat DRN, western blotting was performed in samples punched from the DRN (about bregma −7.00 mm to −8.00 mm). As shown in Figs. 3A, 3B and 3C, quantitative analysis revealed that CSD and drug treatment significantly affected SERT protein levels in the DRN region, as revealed by statistical analysis (for Fig. 3A, F3,11=10.89, p<0.01; for Fig. 3B, F4,14=5.15, p<0.05; and for Fig. 3C, F3,11=21.67, p<0.001). We have reported that plasma CORT levels were significantly elevated in rats subjected to CSD, while relatively lower levels were detected in ADX-alone and CSD/ADX rats (Chen et al. 2012). Since CORT is the main hormone released during stress, to examine whether the altered SERT expression caused by the CSD regimen was related to plasma CORT levels, one group of rats was ADX before CSD regimen. As shown in Fig. 3A, while CSD significantly increased SERT protein levels in the DRN region, CSD/ADX rats did not show any significant alteration in SERT protein levels, as compared to the control and sham groups (both p>0.05). This suggests that the CORT released during CSD regimen was involved in CSD-induced up-regulation of SERT proteins.
Figure 3.
Effect of CSD and CSD plus ADX (A); CSD and CSD plus treatment with corticosteroid receptor antagonists (B); as well as CSD and CSD plus treatment with antidepressants (C) on SERT protein levels in rat DRN. The upper images in each figure show autoradiographs obtained by western blotting of SERT in different regions (n=4/group). The lower graph in each figure shows quantitative analysis of band densities. Values of SERT bands were normalized to those of β-actin probed on the same blot. ADX: adrenalectomy; Con: control; CSD/DMI: CSD plus treatment with desipramine; CSD/Flx: CSD plus treatment with fluoxetine; CSDM: CSD plus treatment with mifepristone; CSDS: CSD plus treatment with spironolactone; CSDMS: CSD plus treatment with both mifepristone and spironolactone. *p<0.01, compared to the control group; † p<0.05, ‡ p<0.01, compared to the CSD group.
To further examine whether the increased SERT expression caused by CSD regimen was related to corticosteroid receptors, rats were injected daily with the MR antagonist spironolactone and GR antagonist mifepristone, alone or in combination. The results showed that treatment with either mifepristone or spironolactone alone (both p<0.05), or combination of both (p<0.01) markedly attenuated the CSD-induced increase of SERT protein levels in the DRN (Fig. 3B). This demonstrates that CSD-induced up-regulation of SERT was mediated by corticosteroid receptors.
Effects of antidepressants on CSD-induced upregulation of SERT protein levels were also examined. As shown in Fig 3C, treatment with the selective SSRI fluoxetine prevented increased SERT protein levels, as compared to that of CSD rats (p<0.001). However, treatment with the norepinephrine transporter (NET) inhibitor desipramine failed to show the same effect as fluoxetine.
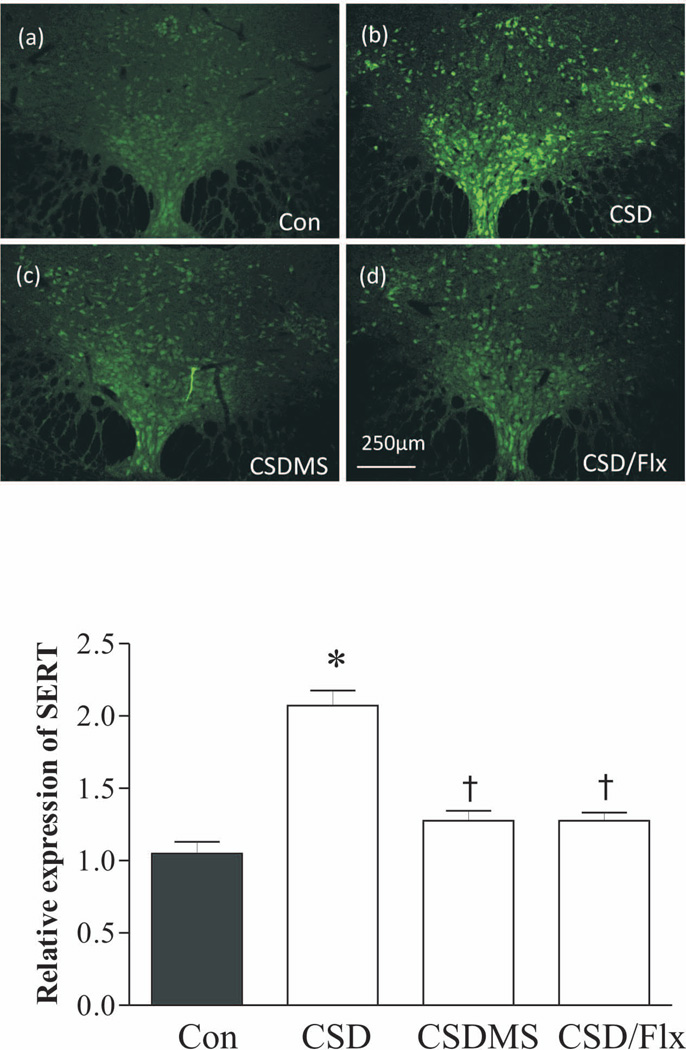
To further confirm these observations by western blotting, immunofluorescence staining was performed in the section of the DRN of rats that were subjected to CSD and CSD plus drug treatments. As shown in Fig. 4, SERT immunoreactivity was significantly elevated in the DRN sections of CSD rats, compared to the control group (p<0.05). Treatments with combined mifepristone and spironolactone, or with fluoxetine blocked CSD-induced ehnancement of SERT immunoreactivities.
Figure 4.
Effects of CSD on the immunofluorescence staining of SERT. Upper panel: SERT immunoreactivities in rat DRN were detected by a confocal microscope. Coronal DRN sections were taken at −7.80mm from bregma. Lower panel: quantitative analyses of the immunofluorescence by the Image J software. *p<0.05, compared to the control group; † p<0.05, compared to the CSD group (n=4/group). See Fig. 3 legend for abbreviations.
Effects of CSD on SERT protein levels in the hippocampus, frontal cortex and amygdala
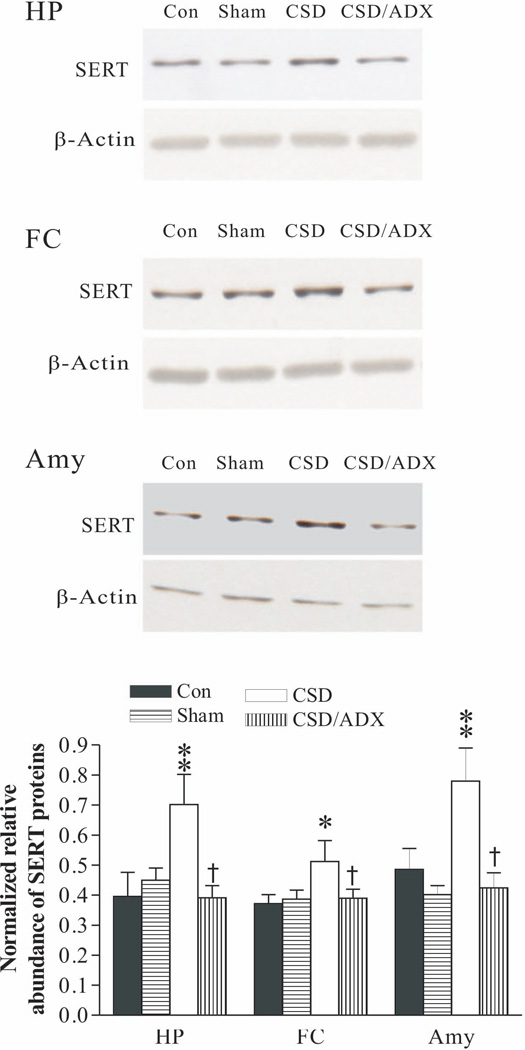
To explore whether CSD influences SERT protein levels in other brain regions, western blotting was performed in samples dissected from the hippocampus, frontal cortex and amygdala. As shown in Fig. 5, there was no significant difference in SERT protein levels between the control and ADX sham group in samples from any of these regions, indicating that the ADX surgery procedure did not significantly affect SERT protein levels. However, CSD markedly increased SERT protein levels in these regions (F3, 23= 4.16, p<0.05 for the hippocampus; F3,23=3.84, p<0.05 for the frontal cortex; F3, 22=5.52, p<0.05 for the amygdala). Similar to the effect on SERT protein levels from the DRN (Fig. 3), ADX abolished CSD-induced increase of SERT protein levels in all these regions. The Newman-Keuls test revealed that SERT protein levels in CSD/ADX group were also significantly lower than that of the CSD group (for the hippocampus and frontal, p<0.05; for the amygdala, p<0.01).
Figure 5.
Effect of CSD and CSD plus ADX on SERT protein levels in the hippocampus (HP), frontal cortex (FC) and amygdala (Amy). The upper images were obtained by western blotting for SERT. The lower panels show quantitative analysis of band densities. Values of SERT bands were normalized to those of β-actin probed on the same blot. *p<0.05, **p<0.01, compared to the control group; † p<0.05, compared to the CSD group (n=6/group). See Fig. 3 legend for abbreviations.
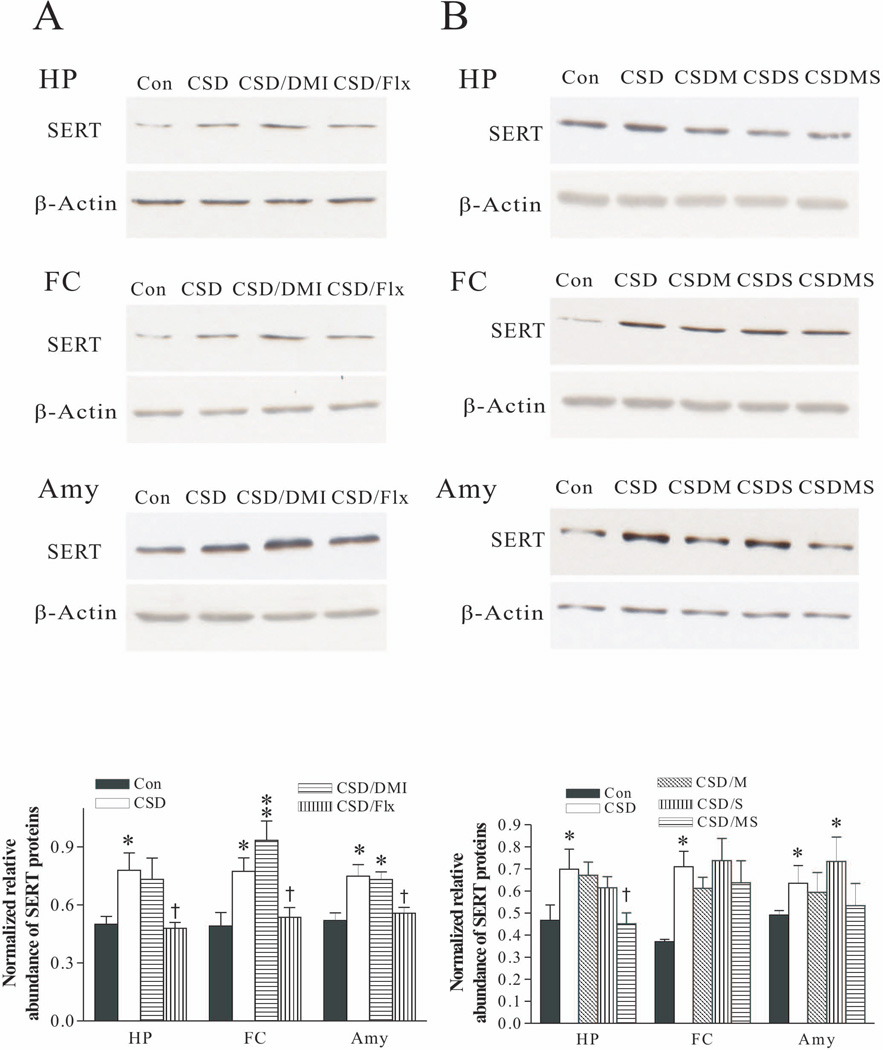
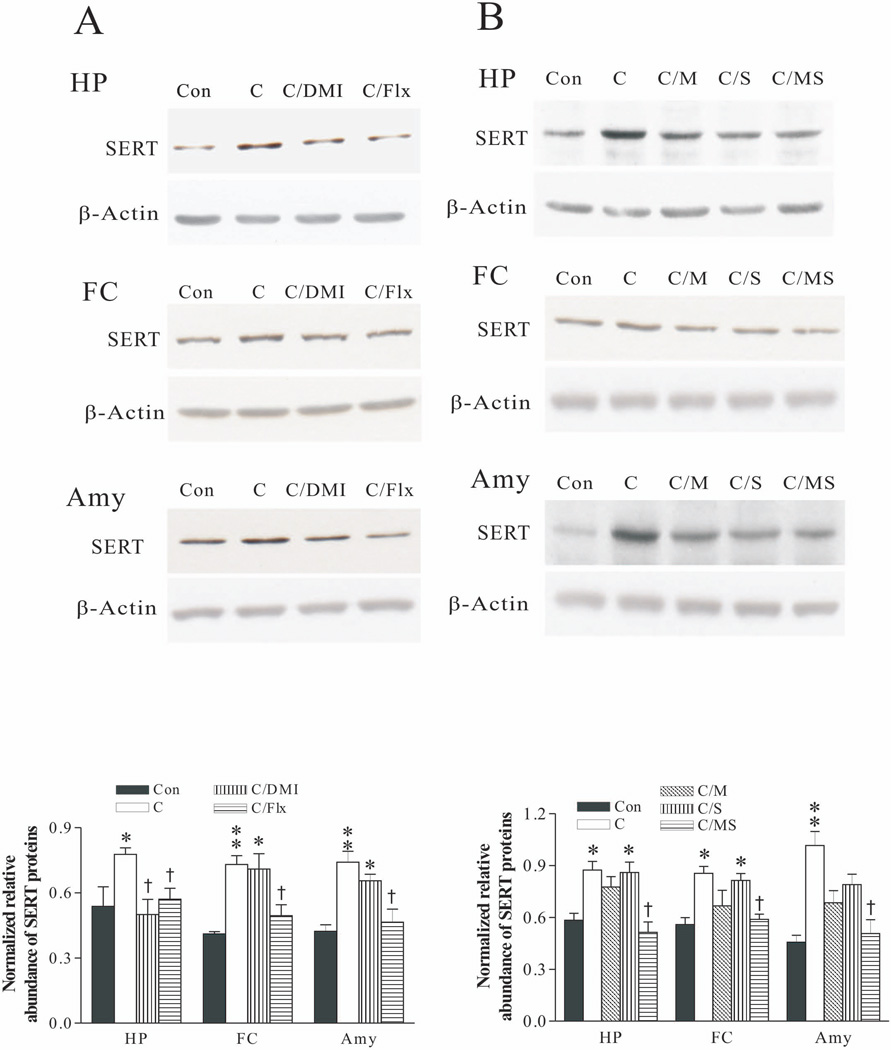
Effects of antidepressants and corticosteroid receptor antagonists on SERT protein levels in these brain regions were also examined. Analysis of the western blotting results demonstrated that while treatment with desipramine had almost no effects on CSD-induced elevation of SERT in these brain regions, treatment with fluoxetine revised CSD’s action (Fig. 6A). The effects of treatment with corticosteroid receptor antagonists on CSD-caused upregulation of SERT protein levels in these brain regions were not so consistent. Treatment with combined mifepristone and spironolactone blocked elevated SERT protein levels in the hippocampus, which was significantly lower than that of the CSD group (p<0.05). Nevertheless, the same treatment only prevented the increased SERT protein levels caused by CSD in the frontal cortex and amygdala, which did not reach a significant level, as compared to those in the CSD group. Furthermore, treatment with spironolactone alone had almost no marked effect on CSD’s action (Fig. 6B).
Figure 6.
Effect of CSD and CSD plus treatment with antidepressants (A); CSD and CSD plus treatment with corticosteroid receptor antagonists (B) on SERT protein levels in the hippocampus (HP), frontal cortex (FC) and amygdala (Amy). The upper images in A and B were obtained by western blotting for SERT. The lower panels in A and B show quantitative analysis of band densities. Values of SERT bands were normalized to those of β-actin probed on the same blot. *p<0.05, **p<0.01, compared to the control group; † p<0.05, compared to the CSD group (n=6/group). See Fig. 3 legend for abbreviations.
Effects of CORT ingestion on SERT protein levels in the DRN region
To verify whether CORT plays a key role in CSD-induced upregulation of SERT in the rat DRN, rats were given 100 µg/ml CORT in their drinking water for 3 weeks. This dose has been used in previous studies to produce a higher plasma CORT level, which is comparable to those in stressed animals (Karatsoreos et al. 2010, Bowles et al. 2012) and has been verified to cause persistent depressive-like phenotypes including sucrose cosumption tests (Gourley et al. 2009). As shown in Fig. 7A, treatment with CORT in oral drinking solution significantly increased plasma CORT levels (p<0.01). Also, although we did not measure the weight and diameter of the adrenal galnds, postmortem examination showed that there was obvious atrophy of adrenal glands in all animals chronically ingested with 100 µg/ml CORT, which is consistent with the previous observations (Karatsoreos et al. 2010, Donner et al. 2012). Similarly, western blotting analysis from the sample punched from the DRN region revealed that SERT protein levels were significantly affected by chronic ingestion of CORT and drug treatments (for Fig. 7B, F4,19=71.12, p<0.001; for Fig. 7C, F3,11=22.41, p<0.001). Post hoc tests demonstrated that administration with either mifepristone (p<0.01) or spironolactone (p<0.001) alone, or in combination of both (P<0.001) markedly attenuated the CORT-induced increase of SERT protein levels in the DRN (Fig. 5B). Treatment with fluoxetine also blocked the CORT-induced increase of SERT protein levels (Fig. 7C; p<0.001), while treatment with desipramine had no effect (p>0.05). This demonstrated that the activation of corticosteroid receptors was necessary for CORT-induced upregulation of SERT expression in the DRN.
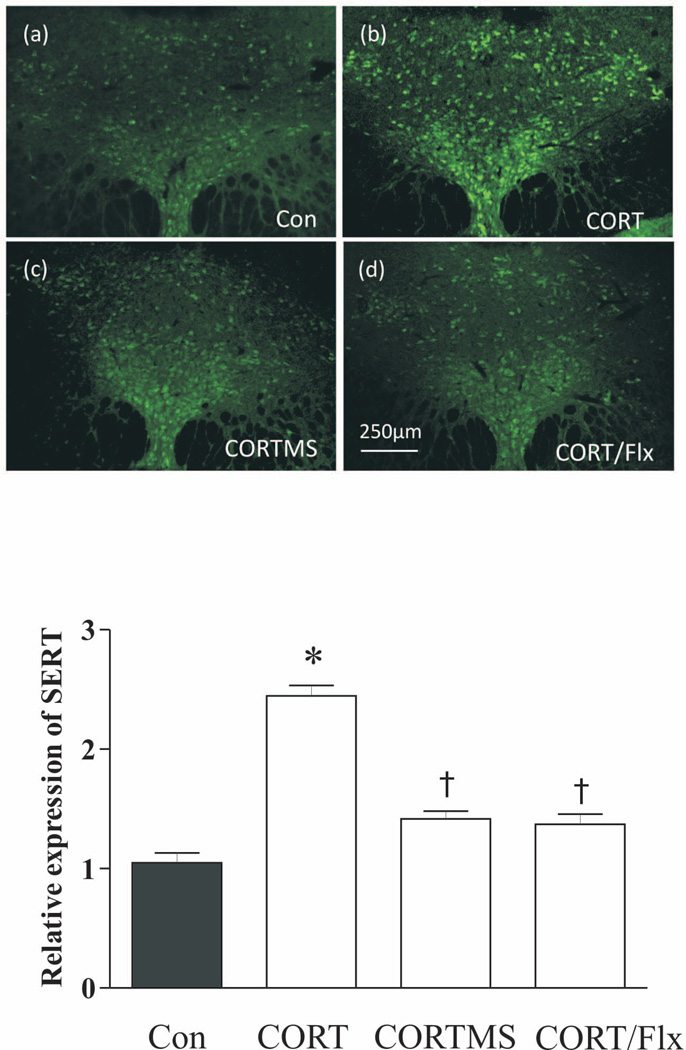
Additionally, immunofluorescence staining was performed to verify the observations described above. SERT immunoreactivity was significantly elevated in the DRN region of rats that ingested CORT in the drinking water, compared to the control groups (p<0.05). Treatments with combined mifepristone and spironolactone, or with fluoxetine abrogated CORT induced upregulation of the SERT immunoreactive signals (Fig. 8). These findings were in accordance with the results obtained by western blotting.
Figure 8.
Effect of CORT on the immunofluorescence staining of SERT. Upper panel: SERT immunoreactivities in rat DRN were detected by a confocal microscope (n=4/group). Coronal DRN sections were taken at −7.80mm from bregma. Lower panel: quantitative analyses of the immunofluorescence by the Image J software. *p<0.05, compared to the control group; † p<0.05, compared to the CORT group. Con: control; C: corticosterone; CORT/Flx: CORT plus treatment with fluoxetine; CORT/MS: CORT plus treatment with both mifepristone and spironolactone.
Effects of CORT ingestion on SERT protein levels in the hippocampus, frontal cortex and amygdala
Western blotting was performed in the samples dissected from the hippocampus, frontal cortex and amygdala of rats that were given CORT in their drinking water as mentioned above. Analysis of results showed that treatment with fluoxetine blocked CORT-induced upregulation of SERT protein levels in these brain regions, a similar effect as observed in the DRN. Interestingly, treatment with desipramine also abolished CORT’s action in the hippocampus (p<0.05), but not in the frontal cortex and amygdala (Fig. 9A). Furthermore, the CORT-induced increase of SERT protein levels in these three regions was abolished by treatment with combined mifepristone and spironolactone, but not by treatment with spironolactone alone in the hippocampus and frontal cortex (Fig. 9B).
Figure 9.
Effect of CORT and CORT plus treatment with antidepressants (A); CORT and CORT plus treatment with corticosteroid receptor antagonists (B) on SERT protein levels in the hippocampus (HP), frontal cortex (FC) and amygdala (Amy). The upper images in A and B were obtained by western blotting for SERT. The lower panels in A and B show quantitative analysis of band densities. Values of SERT bands were normalized to those of β-actin probed on the same blot. *p<0.05, **p<0.01, compared to the control group; † p<0.05, compared to the CORT group (n=6/group). See Fig. 7 legend for abbreviations.
Discussion
In the present study, protein levels of SERT in the rat DRN and three other brain regions were significantly increased by CSD regimen, as demonstrated by measurements using western blotting and immunofluorescence staining. The CSD induced up-regulation of SERT expression can be considered mainly through CORT released during CSD regimen. This notion is supported by the following observations from the present study: 1) elevated SERT protein levels were completely abolished by ADX; 2) administration of combination of the GR antagonist mifepristone and MR antagonist spironolactone prevented increase of SERT expression; 3) chronic treatment with CORT in oral drinking solution caused very similar results compared with the CSD regimen. Furthermore, treatment with SSRI fluoxetine in rats subjected to CSD regimen or CORT treatment reversed either CSD- or CORT-induced elevations of SERT expression, but not by desipramine, a specific norepinephrine transporter. This is in agreement with the fact that chronic treatment with fluoxetine reduces SERT expression in serotonergic raphe nuclei (Baudry et al. 2010). Altogether, findings of the present study provide compelling evidence for a molecular linkage between chronic stress and the serotonin system through upregulated SERT expression, which may contribute to the development of depression.
As mentioned above, expressional changes of SERT observed in animal studies varied from different laboratories using different stressors in past decades. While radioligand binding analysis showed that the density of SERT was decreased in hippocampus and unchanged in midbrain of rats (Berton et al. 1999), increased SERT mRNA levels in the raphe nuclei were observed by Northern blotting, RT-PCR, or in situ hybridization in rat stress models (Pare et al. 1999, Filipenko et al. 2002, Gardner et al. 2009). However, real-time PCR or in situ hybridization for SERT demonstrated that SERT mRNA levels were unchanged in the rat DRN region after chronic social stress (Abumaria et al. 2006) or immobilization (Vollmayr et al. 2000). One possible explanation for these discrepancies may be that dissimilar parts of the raphe nuclei were collected by different researchers. We made a series of coronal sections containing the rat DRN region according to the fourth edition of The Rat Brain in Stereotaxic Coordinates (Paxinos & Watson 1986). Four sections (Fig. 2a–d) selected from rostral to caudal (from bregma −7.30 mm to bregma −8.00 mm) showed the shape of aqueduct (Aq) shifted from pentagon to ellipse, with increasing sectional areas. The corresponding higher magnification photomicrographs displayed different patterns of SERT-immunoreactive cells. As shown in rostral DRN sections (Fig. 2a and 2b), corresponding to the left two columns of the statistical graph (Fig. 2E), SERT-immunoreactive cells only accounted for a smaller portion of the total area of sections that were usually dissected by punch needles or hypodermic needles. If the raphe nuclei were nonuniformly collected, or different sections were compared, the SERT expression may be totally different. Furthermore, there are several main raphe nuclei that are anatomically and functionally distinct. For example, compelling evidence exists that the MRN plays a different role from the DRN in physiological, neuroendocrinological and neuropharmacological functions (Lechin et al. 2006). The activation or inhibition of the DRN and MRN results in different pathophysiological responses, and even opposite behavioral responses (Hillegaart et al. 1989, Dilts et al. 1995, Andrade et al. 2004, Bielau et al. 2005, Dos Santos et al. 2005). Therefore, the experimental results may depend on the tissue dissection, extraction or preparation, as well as section selection for the DRN. Thus in the current study, we collected samples very cautiously to restrict samples to those within the DRN, especially those for western blotting.
While there are anatomical and functional distinctions among the raphe nuclei, it is known that even the DRN can also be parsed into anatomically and functionally distinct subregions. For example, the DRN in rats has been suggested to be divided as 6 subdivisions of the rostral, ventral, dorsal, caudal and interfascicular parts, as well as the lateral wings (Monti 2010). Further, the DRN contains serotonin and non-serotonin neurons with the expression of many different neurotransmitters (Jacobs & Azmitia 1992). While the present study focused on the whole DRN, it would be interesting in future studies to determine whether stress- and CORT-induced changes in SERT expression are restricted to specific subdivisions of the DRN.
The DRN is a major origin of the central serotonin system and provides widespread serotonergic innervation to the forebrain structures (Jacobs & Azmitia 1992). Such extensive efferent projection attributes to the crucial role of the DRN in the regulation of physiological and behavioral functions. In this regard, its dysfunction has been linked to a number of stress-related disorders including depression (Krishnan et al. 2010). Accordingly, DRN activity can also be modulated under the specific circumstances such as chronic stress. In the present study, CSD regimen resulted in a significant increase of SERT protein levels in the DRN and some brain regions that receive serotonergic projection such as the hippocampus, frontal cortex and amygdala, as demonstrated by western blotting and immunofluorescence analysis. This indicates that chronic stress associated with social defeat causes a long-term activation of the serotonergic system in the DRN, which in turn boosts the expression of SERT and may further lead to an increase of SERT in the projection regions. As SERT ensures the reuptake of serotonin at the synaptic cleft (Barker et al. 1995), one potential functional consequence of increased SERT would be the decrease in the serotonergic neurotransmission due to more rapid clearance of serotonin from synaptic sites. As a result, there would be a deficiency of serotonin in synaptic clefts, a proposed consequence accounted for the development of depression (Elhwuegi 2004). However, this interpretation is not completely assured. Although the present study demonstrated upregulated SERT protein levels at the serotonergic cell body location (the DRN) and some projection regions (the hippocampus, frontal cortex and amygdala), the functional consequences of such increased SERT proteins would be dependent on the location of these proteins after trafficking to axon terminals, as well as dynamically intracellular trafficking after the stimulation such as stress. That is, only an increased SERT in the plasma membrane would account for the function of reuptaking serotonin. The western blotting analysis in the present study is the result of both membrane-associated and cytosolic SERT proteins. Therefore, such increased SERT protein may not absolutely indicate enhanced reuptake of serotonin. A decrease in density of SERT-immunoreactive axons in the prefrontal cortex of suicide victims with a diagnosis of major depression by immunocytochemistry has been reported (Austin et al. 2002), which may reflect either a reduction in biosynthesis of the SERT protein, an alteration in the transport of SERT protein to the nerve terminals, or a loss of serotonin terminals containing SERTs. However, because a SERT antibody cannot distinguish SERT proteins in the cell surface from those the intracellular pools, even the immunohistochemical analysis used in some previous studies could not detect a change in SERT trafficking (Tao-Cheng et al. 1999, Miner et al. 2000). More experiments are necessary to elucidate these detailed functional consequence of CSD-induced upregulation in SERT protein levels.
Our further measurements also demonstrated that simultaneous treatment with fluoxetine abrogated CSD- or CORT-induced upregulation of SERT expression in the DRN, which provided evidence to support above notion. In line with our findings, repeated exposure to electroconvulsive shock (Shen et al. 2001), which has antidepressant effects in humans, or chronic treatment with antidepressants imipramine, fluoxetine, or tianeptine, decreased SERT mRNAs (Lesch et al. 1993, Kuroda et al. 1994, Lopez et al. 1994) and ligand binding for SERT (Baudry et al. 2010) in the rat midbrain raphe complex. Thus, CSD-induced upregulation of SERT protein levels in the DRN may be of pivotal importance for understanding the biological links among chronic stress, the serotonergic system, and depression. It is worth to mention that fluoxetine treatment had no significant effect on plasma CORT concentration (Fig. 1A), whereas attenuates CSD- and CORT-induced upregulation of SERT proteins (Figs. 3C and 7C). This conflicted result may be accounted by the different mechanisms which mediate fluoxetine’s effects. Fluoxetine is a specific inhibitor of SERT possibly through its action on the synthesis of SERT. However, fluoxetine treatment had little effect on the neuroendocrine responses to stress. There are some possible explanations. First, stress-induced corticosterone release depends on the activity and function of the hypothalamic-pituitary-adrenal (HPA) axis. Although long-term treatment of fluoxetine may result in a reduced basal tone of the HPA axis, it does not inhibit the response or sensitivity of the HPA axis to stressful stimuli (Zhang et al. 2000). Second, stress hormones are essential for regulation of various physiological processes and several neurotransmitters and receptors systems play a role in mediating stress-induced activation of neuroendocrine system. For example, noradrenergic nerves also play a role in stress-induced corticosterone secretion (Richardson Morton et al. 1990, Gaillet et al. 1991). Thus, it is not likely that stress-induced secretion of corticosterone is blocked by the alteration of a single neurotransmitter such as serotonin after SERT inhibition, or activation of a type of receptors such as serotonin receptors.
CSD has been widely accepted as a stress model of depression for its relatively high predictive validity (Buwalda et al. 2005, Huhman 2006). In the present study, while the SERT protein level in the DRN was significantly increased in CSD rats, chronic ingestion of oral CORT showed a similar increase of SERT expression. Furthermore, parallel alteration in SERT protein levels was observed after manipulation of ADX, or treatments with either mifepristone or spironolactone. This indicates that CORT released during chronic stress plays a large role for the upregulated SERT protein levels in the DRN. This is consistent with other animal studies reported previously (Pare et al. 1999, Filipenko et al. 2002, Gardner et al. 2009). So far, we do not have satisfactory explanation for the mechanism underlying CORT-induced upregulation of SERT proteins in the DRN. One possibility is concerned with the fact that glucocorticoid-regulated SERT expression is modulated by the SERT gene-promoter-linked polymorphic region (5-HTTLPR) in human, a 44-bp deletion (S)/insertion (L) of the repetitive sequence containing GC-rich, 20–~30-bp-long repeat elements (Watanabe et al. 2011). It was reported that the allele-specific differences of serotonin uptake between the L and S genotype Epstein-Barr virus (EBV)-transformed human B-lymphoblastoid cells after administration of dexamethasone were significant (Glatz et al. 2003). This suggests that 5-HTTLPR might contribute to the different changes of SERT expression observed in reaction to glucocorticoid-dependent SERT regulation in depression-related serotonergic neurotransmission. Clearly, further studies are necessary to investigate the mechanisms underlying the CSD-induced upregulation of rat SERT protein levels.
It is noteworthy that in our previous study, CSD regimen significantly increased both mRNA and protein levels of NET in the rat locus coeruleus (LC) (Chen et al. 2012). The upregulated NET expression in the LC was abolished or reduced by ADX or treatment with corticosteroid receptor antagonist mifepristone and spironolactone. A similar pattern was observed in the current study for the SERT in the DRN. An interesting fact raised by our findings is that CSD up-regulates expression of the SERT and NET in the DRN or LC with a glucocorticoid-dependent manner. Since dysfunctions of both serotonergic and noradrenergic systems are implicated in major depression, the alteration in the expression and function of SERT and NET are therefore the candidate causation of this disease. In addition, chronic stress is often a predisposing factor in the development of depression (Sapolsky 1996). Likely, the discovery of a close molecular link between these important factors may open up new avenues for future exploration for development and treatment of depression. In addition, interactions between the two monoaminergic nuclei (the LC and DRN) in the brain are of great functional importance. A topographic ordering of reciprocal connections between the DRN and LC is well documented (Peyron et al. 1996, Kim et al. 2004, Braz et al. 2009). Thus, CSD-induced activation of noradrenergic cell groups could contribute to the selective stress-induced activation of serotonergic neurons in the DRN, or vice versa. In keeping with this, it should be considered that chronic stress such as CSD likely affects two monoamine systems simultaneously.
In the present study, administration of a combination of the GR antagonist mifepristone and MR antagonist spironolactone, and in some brain regions, administration of either mifepristone or spironolactone, blocked or prevented increase of SERT expression caused by CSD or CORT treatment, indicating both receptors are involved in the upregulation of SERT expression. Because the GR is considered to be more important during stress, in which glucocorticoid concentrations rise to particularly high levels (De Kloet et al. 1998), its involvement in this regulation is expected. However, as the MR is almost completely occupied during normal physiological condition (Joels et al. 1992), we do not have a satisfactory explanation for the fact that MR also plays a role in the regulation of SERT expression observed in the present study. Nevertheless, the MR may indeed be responsive also to high levels of endogenous glucocorticoids (Anacker et al. 2011), and it may contribute to the biological effects of corticosteroid hormones in stress. It was reported that the MR also regulates the transcription of the 5-HT1A and 5-HT2A genes (Ou et al. 2001, Trajkovska et al. 2009). The precise mechanisms that underlie the regulation of SERT expression by GR and MR are still to be elusive. These receptors, like other nuclear receptors, are transcription factors. One of the main mechanisms includes direct effects on gene expression by the binding to glucocorticoid responsive elements (GRE) in the promoter region of target genes to constitute a glucocorticoid response unit to activate the transcription (Scheidereit et al. 1983, Karin 1998, Schoneveld et al. 2004). In addition, It has been reported that glucocorticoid receptors also participate in chromatin remodeling and interacts with coregulators, resulting in changes in the expression of genes that do not have GREs in the promoter (McEwan et al. 1997, Deroo et al. 2001, Lonard et al. 2007).
In conclusion, the present study demonstrates that CSD upregulates SERT protein levels in rat DRN and some brain regions which receive serotonergic projection. In this process, stress-induced release of CORT acting through corticosteroid receptors plays a key role, as ADX and treatment with corticosteroid receptor antagonists block effects of CSD regimen and treatment with CORT. The increased SERT protein levels may give rise to synaptic deficiency of serotonin, which links chronic stress with the activation of the serotonergic system and subsequently contributes to the development of depressive symptoms.
Acknowledgements
This work is supported by NIH grant MH080323.
Footnotes
The authors declare no conflict of interest regarding the work reported here.
References
- Abumaria N, Rygula R, Havemann-Reinecke U, Ruther E, Bodemer W, Roos C, Flugge G. Identification of genes regulated by chronic social stress in the rat dorsal raphe nucleus. Cell Mol Neurobiol. 2006;26:145–162. doi: 10.1007/s10571-006-9024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36:415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade TG, Macedo CE, Zangrossi H, Jr, Graeff FG. Anxiolytic-like effects of median raphe nucleus lesion in the elevated T-maze. Behav Brain Res. 2004;153:55–60. doi: 10.1016/j.bbr.2003.10.036. [DOI] [PubMed] [Google Scholar]
- Austin MC, Whitehead RE, Edgar CL, Janosky JE, Lewis DA. Localized decrease in serotonin transporter-immunoreactive axons in the prefrontal cortex of depressed subjects committing suicide. Neuroscience. 2002;114:807–815. doi: 10.1016/s0306-4522(02)00289-0. [DOI] [PubMed] [Google Scholar]
- Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull. 2001;56:413–424. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- Barker E, Blakely R. Norepinephrine and serotonin transporters. Moleculartargets of antidepressant drugs. In: Bloom F, Kupfer D, editors. Psychopharmacology. A fourth generation of progress. New York: Raven Press; 1995. pp. 321–333. [Google Scholar]
- Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- Berton O, Durand M, Aguerre S, Mormede P, Chaouloff F. Behavioral, neuroendocrine and serotonergic consequences of single social defeat and repeated fluoxetine pretreatment in the Lewis rat strain. Neuroscience. 1999;92:327–341. doi: 10.1016/s0306-4522(98)00742-8. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Streicher JM, Mirkes SJ, Sanchez RL, Reddy AP, Cameron JL. Serotonin-related gene expression in female monkeys with individual sensitivity to stress. Neuroscience. 2005;132:151–166. doi: 10.1016/j.neuroscience.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Whale R, Cowen PJ. State and trait abnormalities in serotonin function in major depression. Br J Psychiatry. 2002;180:24–28. doi: 10.1192/bjp.180.1.24. [DOI] [PubMed] [Google Scholar]
- Bielau H, Mawrin C, Krell D, et al. Differences in activation of the dorsal raphe nucleus depending on performance of suicide. Brain Res. 2005;1039:43–52. doi: 10.1016/j.brainres.2005.01.055. [DOI] [PubMed] [Google Scholar]
- Bowles NP, Hill MN, Bhagat SM, Karatsoreos IN, Hillard CJ, McEwen BS. Chronic, noninvasive glucocorticoid administration suppresses limbic endocannabinoid signaling in mice. Neuroscience. 2012;204:83–89. doi: 10.1016/j.neuroscience.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Enquist LW, Basbaum AI. Inputs to serotonergic neurons revealed by conditional viral transneuronal tracing. J Comp Neurol. 2009;514:145–160. doi: 10.1002/cne.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda B, Kole MH, Veenema AH, Huininga M, de Boer SF, Korte SM, Koolhaas JM. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev. 2005;29:83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Chen P, Fan Y, Li Y, Sun Z, Bissette G, Zhu MY. Chronic social defeat up-regulates expression of norepinephrine transporter in rat brains. Neurochem Int. 2012;60:9–20. doi: 10.1016/j.neuint.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintra A, Solfrini V, Bunnemann B, Okret S, Bortolotti F, Gustafsson JA, Fuxe K. Prenatal development of glucocorticoid receptor gene expression and immunoreactivity in the rat brain and pituitary gland: a combined in situ hybridization and immunocytochemical analysis. Neuroendocrinology. 1993;57:1133–1147. doi: 10.1159/000126480. [DOI] [PubMed] [Google Scholar]
- Clark MS, McDevitt RA, Neumaier JF. Quantitative mapping of tryptophan hydroxylase-2, 5-HT1A, 5-HT1B, and serotonin transporter expression across the anteroposterior axis of the rat dorsal and median raphe nuclei. J Comp Neurol. 2006;498:611–623. doi: 10.1002/cne.21073. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Deroo BJ, Archer TK. Glucocorticoid receptor-mediated chromatin remodeling in vivo. Oncogene. 2001;20:3039–3046. doi: 10.1038/sj.onc.1204328. [DOI] [PubMed] [Google Scholar]
- Dilts RP, Boadle-Biber MC. Differential activation of the 5-hydroxytryptamine-containing neurons of the midbrain raphe of the rat in response to randomly presented inescapable sound. Neurosci Lett. 1995;199:78–80. doi: 10.1016/0304-3940(95)12027-2. [DOI] [PubMed] [Google Scholar]
- Donner NC, Montoya CD, Lukkes JL, Lowry CA. Chronic non-invasive corticosterone administration abolishes the diurnal pattern of tph2 expression. Psychoneuroendocrinology. 2012;37:645–661. doi: 10.1016/j.psyneuen.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos L, de Andrade TG, Zangrossi H., Jr Serotonergic neurons in the median raphe nucleus regulate inhibitory avoidance but not escape behavior in the rat elevated T-maze test of anxiety. Psychopharmacology (Berl) 2005;179:733–741. doi: 10.1007/s00213-004-2120-3. [DOI] [PubMed] [Google Scholar]
- Elhwuegi AS. Central monoamines and their role in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:435–451. doi: 10.1016/j.pnpbp.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Fan Y, Huang J, Duffourc M, Kao RL, Ordway GA, Huang R, Zhu MY. Transcription factor Phox2 upregulates expression of norepinephrine transporter and dopamine beta-hydroxylase in adult rat brains. Neuroscience. 2011;192:37–53. doi: 10.1016/j.neuroscience.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipenko ML, Beilina AG, Alekseyenko OV, Dolgov VV, Kudryavtseva NN. Repeated experience of social defeats increases serotonin transporter and monoamine oxidase A mRNA levels in raphe nuclei of male mice. Neurosci Lett. 2002;321:25–28. doi: 10.1016/s0304-3940(01)02495-8. [DOI] [PubMed] [Google Scholar]
- Fujita M, Shimada S, Maeno H, Nishimura T, Tohyama M. Cellular localization of serotonin transporter mRNA in the rat brain. Neurosci Lett. 1993;162:59–62. doi: 10.1016/0304-3940(93)90559-4. [DOI] [PubMed] [Google Scholar]
- Gaillet S, Lachuer J, Malaval F, Assenmacher I, Szafarczyk A. The involvement of noradrenergic ascending pathways in the stress-induced activation of ACTH and corticosterone secretions is dependent on the nature of stressors. Exp Brain Res. 1991;87:173–180. doi: 10.1007/BF00228518. [DOI] [PubMed] [Google Scholar]
- Gardner KL, Hale MW, Lightman SL, Plotsky PM, Lowry CA. Adverse early life experience and social stress during adulthood interact to increase serotonin transporter mRNA expression. Brain Res. 2009;1305:47–63. doi: 10.1016/j.brainres.2009.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Glatz K, Mossner R, Heils A, Lesch KP. Glucocorticoid-regulated human serotonin transporter (5-HTT) expression is modulated by the 5-HTT gene-promotor-linked polymorphic region. J Neurochem. 2003;86:1072–1078. doi: 10.1046/j.1471-4159.2003.01944.x. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Taylor JR. Recapitulation and reversal of a persistent depression-like syndrome in rodents. In: Crawley Jacqueline N., et al., editors. Current protocols in neuroscience / editorial board. Unit 9 32. Chapter 9. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff FG. Serotonergic systems. The Psychiatric clinics of North America. 1997;20:723–739. doi: 10.1016/s0193-953x(05)70342-7. [DOI] [PubMed] [Google Scholar]
- Hillegaart V, Hjorth S. Median raphe, but not dorsal raphe, application of the 5-HT1A agonist 8-OH-DPAT stimulates rat motor activity. Eur J Pharmacol. 1989;160:303–307. doi: 10.1016/0014-2999(89)90505-0. [DOI] [PubMed] [Google Scholar]
- Hoffman BJ, Hansson SR, Mezey E, Palkovits M. Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Front Neuroendocrinol. 1998;19:187–231. doi: 10.1006/frne.1998.0168. [DOI] [PubMed] [Google Scholar]
- Hrdina PD, Pappas BA, Roberts DC, Bialik RJ, Ryan CL. Relationship between levels and uptake of serotonin and high affinity [3H]imipramine recognition sites in the rat brain. Can J Physiol Pharmacol. 1985;63:1239–1244. doi: 10.1139/y85-205. [DOI] [PubMed] [Google Scholar]
- Huhman KL. Social conflict models: can they inform us about human psychopathology? Horm Behav. 2006;50:640–646. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Joels M, De Kloet ER. Coordinative mineralocorticoid and glucocorticoid receptor-mediated control of responses to serotonin in rat hippocampus. Neuroendocrinology. 1992;55:344–350. doi: 10.1159/000126135. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW, McEwen BS. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology. 2010;151:2117–2127. doi: 10.1210/en.2009-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. New twists in gene regulation by glucocorticoid receptor: is DNA binding dispensable? Cell. 1998;93:487–490. doi: 10.1016/s0092-8674(00)81177-0. [DOI] [PubMed] [Google Scholar]
- Kim MA, Lee HS, Lee BY, Waterhouse BD. Reciprocal connections between subdivisions of the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Brain Res. 2004;1026:56–67. doi: 10.1016/j.brainres.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y, Watanabe Y, McEwen BS. Tianeptine decreases both serotonin transporter mRNA and binding sites in rat brain. Eur J Pharmacol. 1994;268:R3–R5. doi: 10.1016/0922-4106(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Lechin F, van der Dijs B, Hernandez-Adrian G. Dorsal raphe vs. median raphe serotonergic antagonism. Anatomical, physiological, behavioral, neuroendocrinological, neuropharmacological and clinical evidences: relevance for neuropharmacological therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:565–585. doi: 10.1016/j.pnpbp.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim HJ, Kim JG, Ryu V, Kim BT, Kang DW, Jahng JW. Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neurosci Res. 2007;58:32–39. doi: 10.1016/j.neures.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Aulakh CS, Wolozin BL, Tolliver TJ, Hill JL, Murphy DL. Regional brain expression of serotonin transporter mRNA and its regulation by reuptake inhibiting antidepressants. Brain Res Mol Brain Res. 1993;17:31–35. doi: 10.1016/0169-328x(93)90069-2. [DOI] [PubMed] [Google Scholar]
- Lonard DM, O'Malley BW. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell. 2007;27:691–700. doi: 10.1016/j.molcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Chalmers DT, Vazquez DM, Watson SJ, Akil H. Serotonin transporter mRNA in rat brain is regulated by classical antidepressants. Biol Psychiatry. 1994;35:287–290. doi: 10.1016/0006-3223(94)91262-9. [DOI] [PubMed] [Google Scholar]
- MacGillivray L, Lagrou LM, Reynolds KB, Rosebush PI, Mazurek MF. Role of serotonin transporter inhibition in the regulation of tryptophan hydroxylase in brainstem raphe nuclei: time course and regional specificity. Neuroscience. 2010;171:407–420. doi: 10.1016/j.neuroscience.2010.08.055. [DOI] [PubMed] [Google Scholar]
- Mann JJ, McBride PA, Malone KM, DeMeo M, Keilp J. Blunted serotonergic responsivity in depressed inpatients. Neuropsychopharmacology. 1995;13:53–64. doi: 10.1016/0893-133X(95)00016-7. [DOI] [PubMed] [Google Scholar]
- McEwan IJ, Wright AP, Gustafsson JA. Mechanism of gene expression by the glucocorticoid receptor: role of protein-protein interactions. Bioessays. 1997;19:153–160. doi: 10.1002/bies.950190210. [DOI] [PubMed] [Google Scholar]
- Miner LH, Schroeter S, Blakely RD, Sesack SR. Ultrastructural localization of the serotonin transporter in superficial and deep layers of the rat prelimbic prefrontal cortex and its spatial relationship to dopamine terminals. J Comp Neurol. 2000;427:220–234. doi: 10.1002/1096-9861(20001113)427:2<220::aid-cne5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Monti JM. The structure of the dorsal raphe nucleus and its relevance to the regulation of sleep and wakefulness. Sleep medicine reviews. 2010;14:307–317. doi: 10.1016/j.smrv.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Ou XM, Storring JM, Kushwaha N, Albert PR. Heterodimerization of mineralocorticoid and glucocorticoid receptors at a novel negative response element of the 5-HT1A receptor gene. J Biol Chem. 2001;276:14299–14307. doi: 10.1074/jbc.M005363200. [DOI] [PubMed] [Google Scholar]
- Pace TW, Gaylord RI, Jarvis E, Girotti M, Spencer RL. Differential glucocorticoid effects on stress-induced gene expression in the paraventricular nucleus of the hypothalamus and ACTH secretion in the rat. Stress. 2009;12:400–411. doi: 10.1080/10253890802530730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare WP, Blair GR, Kluczynski J, Tejani-Butt S. Gender differences in acute and chronic stress in Wistar Kyoto (WKY) rats. Integr Physiol Behav Sci. 1999;34:227–241. doi: 10.1007/BF02688691. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney ; Orlando: Academic Press; 1986. [Google Scholar]
- Peyron C, Luppi PH, Fort P, Rampon C, Jouvet M. Lower brainstem catecholamine afferents to the rat dorsal raphe nucleus. J Comp Neurol. 1996;364:402–413. doi: 10.1002/(SICI)1096-9861(19960115)364:3<402::AID-CNE2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Rattray M, Michael GJ, Lee J, Wotherspoon G, Bendotti C, Priestley JV. Intraregional variation in expression of serotonin transporter messenger RNA by 5-hydroxytryptamine neurons. Neuroscience. 1999;88:169–183. doi: 10.1016/s0306-4522(98)00231-0. [DOI] [PubMed] [Google Scholar]
- Richardson Morton KD, Van de Kar LD, Brownfield MS, Lorens SA, Napier TC, Urban JH. Stress-induced renin and corticosterone secretion is mediated by catecholaminergic nerve terminals in the hypothalamic paraventricular nucleus. Neuroendocrinology. 1990;51:320–327. doi: 10.1159/000125356. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Scheidereit C, Geisse S, Westphal HM, Beato M. The glucocorticoid receptor binds to defined nucleotide sequences near the promoter of mouse mammary tumour virus. Nature. 1983;304:749–752. doi: 10.1038/304749a0. [DOI] [PubMed] [Google Scholar]
- Schoneveld OJ, Gaemers IC, Lamers WH. Mechanisms of glucocorticoid signalling. Biochim Biophys Acta. 2004;1680:114–128. doi: 10.1016/j.bbaexp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Shen H, Numachi Y, Yoshida S, Toda S, Awata S, Matsuoka H, Sato M. Electroconvulsive shock regulates serotonin transporter mRNA expression in rat raphe nucleus. Psychiatry and clinical neurosciences. 2001;55:75–77. doi: 10.1046/j.1440-1819.2001.00788.x. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH, Zhou FC. Differential polarization of serotonin transporters in axons versus soma-dendrites: an immunogold electron microscopy study. Neuroscience. 1999;94:821–830. doi: 10.1016/s0306-4522(99)00373-5. [DOI] [PubMed] [Google Scholar]
- Trajkovska V, Kirkegaard L, Krey G, et al. Activation of glucocorticoid receptors increases 5-HT2A receptor levels. Exp Neurol. 2009;218:83–91. doi: 10.1016/j.expneurol.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Blair ML. Forebrain pathways mediating stress-induced hormone secretion. Front Neuroendocrinol. 1999;20:1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Keck S, Henn FA, Schloss P. Acute stress decreases serotonin transporter mRNA in the raphe pontis but not in other raphe nuclei of the rat. Neurosci Lett. 2000;290:109–112. doi: 10.1016/s0304-3940(00)01346-x. [DOI] [PubMed] [Google Scholar]
- Watanabe MA, Nunes SO, Amarante MK, Guembarovski RL, Oda JM, Lima KW, Fungaro MH. Genetic polymorphism of serotonin transporter 5-HTTLPR: involvement in smoking behaviour. J Genet. 2011;90:179–185. doi: 10.1007/s12041-011-0037-2. [DOI] [PubMed] [Google Scholar]
- Wiklund L, Leger L, Persson M. Monoamine cell distribution in the cat brain stem. A fluorescence histochemical study with quantification of indolaminergic and locus coeruleus cell groups. J Comp Neurol. 1981;203:613–647. doi: 10.1002/cne.902030405. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Raap DK, Garcia F, Serres F, Ma Q, Battaglia G, Van de Kar LD. Long-term fluoxetine produces behavioral anxiolytic effects without inhibiting neuroendocrine responses to conditioned stress in rats. Brain Res. 2000;855:58–66. doi: 10.1016/s0006-8993(99)02289-1. [DOI] [PubMed] [Google Scholar]