Abstract
Objective
Explore the longitudinal, six-month symptom course of older adults newly started on an antidepressant or anxiolytic by non-psychiatrist physicians and enrolled in a care management program.
Method
Naturalistic cohort study of older adults (age ≥65 years) receiving pharmacotherapy and telephone care management. Participants are non-institutionalized adults participating in Pennsylvania’s Pharmaceutical Assistance Contract for the Elderly who completed telephone-based clinical assessments including demographic data, self-report on history of psychiatric treatment and adherence, and standardized symptom scales.
Results
162 participants with an average age of 77.2 years (SD 6.8) were followed and, for analysis, split into two groups by PHQ-9 score: 75 (46.3%) scoring 0–4 (minimally symptomatic group, MSG) and 87 (53.7%) ≥5 (symptomatic group, SG). Over 6 months, the SG improved with PHQ-9 scores beginning on average at 10.0 (SD 4.6) and falling to 5.4 (SD 4.2) (F(1, 86)=29.53, p <0.0001). The MSG had no significant change in depressive symptoms. Emotional health as measured by SF-12 Mental Composite Score mirrored the PHQ-9 change and lack thereof in the SG and MSG, respectively. No clinical or demographic features were associated with symptom improvement in the SG, though they were more likely to report medication adherence (66.7% v. 44.0%, χ 2(1)=8.4, p=0.0037) compared with the MSG.
Conclusions
Participation of symptomatic older adults initiated on psychotropic medication in a telephone care management program was associated with improvement in depressive symptoms and overall emotional well-being, notable findings given participants’ advanced age, state-wide distribution, and history of limited utilization of mental health care.
Keywords: care management, telepsychiatry, psychotropics, depression
Introduction
Mental health conditions are prevalent in late-life and cause significant morbidity and mortality(Bruce, 1999; Lyness et al., 1999), along with higher costs and service utilization (Unutzer et al., 1997; Luber et al., 2001). The delivery of effective mental health care to older adults is complicated by the fact that these patients are less likely to seek treatment in specialty care compared to younger counterparts with mental illness (Olfson and Pincus, 1996; Klap, Unroe, Unutzer, 2003). In addition, even if they wish to see a specialty mental health provider, workforce shortages may make finding a provider with expertise in late-life mental health difficult (Jeste et al., 1999).
As most older adults see their primary care providers at least once annually (Unutzer et al., 1999; Moak, 2011), efforts to improve mental health treatment in late-life have focused on the primary care setting. Much of this work has been on extending models of collaborative care in chronic illness (Von Korff et al., 1997) to mental health, combining the input of a mental health specialist with care management services that help primary care providers with patient education, treatment, and monitoring (Oxman, Dietrich, Schulberg, 2003). In the collaborative care approach for the treatment of depression, key components include: patient education; measurement based monitoring of outcomes, side-effects, and adherence; a psychiatrist to provide supervision of care managers; and use of an algorithm to guide treatment (Katon et al., 2010). Work has generally demonstrated that care management contributes to greater rates of guideline-adherent care with accompanying decreases in depressive symptoms as well as higher rates of medication use (Unutzer et al., 2002; Oxman, Dietrich, Schulberg, 2003; Bruce et al., 2004; Krahn et al., 2006; Bao et al., 2009).
Among the large collaborative care trials that have addressed late-life depression, the average ages for IMPACT (Unutzer et al., 2002) and PRISM-E (Krahn et al., 2006) were 71.2 and 73.9 years, respectively, while the majority (69%) of PROSPECT participants were 75 or younger (Bruce et al., 2004). There are no published studies describing the utility of care management among a majority “old old” (≥75yo) population. While the benefits of care management might be expected to persist without an age ceiling, it will be important to corroborate this as the old old population grows and demand for healthcare resources intensifies.
Although prior work on the implementation and efficacy of collaborative care models has yielded promising results, the benefits attained during clinical trials utilizing care management may not be sustained beyond the research-supported intervention. Also, while care management has been successfully implemented within large health care systems (Oslin et al., 2003; Grypma et al., 2006; Oslin et al., 2006; Reiss-Brennan et al., 2010), there are numerous obstacles to implementing care management in the community at large, from the patient and provider level up through health plans and purchasers (Pincus et al., 2001; Frank, Huskamp, Pincus, 2003; APA, 2010). Outside of these large, centrally-organized systems, there are few successful examples of care management implemented outside of a clinical trial.
In an effort to help facilitate the frequent clinical contact for initial assessment and monitoring essential to care management, investigators at the Philadelphia Veterans Affairs Medical Center (PVAMC) and the University of Pennsylvania developed the Behavioral Health Laboratory (BHL), an evidence-based clinical service administered via telephone. The BHL is designed to help identify and manage behavioral health issues in primary care using algorithm-based principles of care management to facilitate assessment, tracking, and monitoring for an array of mental health and substance abuse conditions in order to encourage patient acceptance and adherence to treatment recommendations (Oslin et al., 2003).
The Pharmaceutical Assistance Contract for the Elderly (PACE) is a program of the Pennsylvania Department of Aging that provides comprehensive prescription coverage for low-income residents 65 years or older. PACE has contracted with the BHL to provide its clinical care management service to adults enrolled in PACE and their primary care providers after the PACE enrollee fills a new prescription for psychotropic medication. Review of baseline clinical data gathered during the first 18 months of the program suggested that, while of advanced age and physically frail, nearly half of enrollees (contacted within three weeks of initiating a new psychotropic medication) did not meet criteria for any Axis I psychiatric diagnosis (Maust et al., 2011).
In order to extend our prior work and evaluate the naturalistic course of symptoms among this unique group of non-institutionalized older adults, the following analyses present 6 month longitudinal data obtained through BHL care management calls beginning with initial contact, followed by calls at weeks 2, 6, 9, and 6 months, all of which were provided as part of the contracted BHL clinical service. We hypothesized that older adults with a higher level of depressive symptoms receiving care management through the BHL would experience symptom reduction, though this would be associated with adherence, medication class, and physical function.
Methods
This cohort is drawn from low-income older adults in the state of Pennsylvania who participate in the state’s Pharmaceutical Assistance Contract for the Elderly (PACE). A sample of enrollees were initially contacted for clinical assessment by the BHL as part of a contract with PACE to provide clinical assessment and care management services. Enrollees included in this analysis were adults in a non-institutionalized setting who filled at least one new prescription (no prior pharmacy claim had been made for the index medication class in the prior nine months) for an antidepressant or anxiolytic, who agreed to receive the clinical assessment and care management services, and who also agreed to participate in the research component of this project. The research component was approved and supported by PACE, and approved by the University of Pennsylvania Institutional Review Board.
Enrollees were randomly selected for the BHL clinical assessment on a weekly basis by PACE from all claims submitted for newly-written prescriptions, stratified by medication type and county (Philadelphia area, Pittsburgh area, other). Sampling included prescriptions that may have been intended for use on an as-needed (prn) basis. Stratification by county was done to ensure that the sample was not dominated by enrollees in the state’s large urban centers. Prescriptions written by providers identified by PACE as psychiatrists were excluded from this analysis. Enrollees sampled by PACE who then agreed to participate in the BHL clinical assessment underwent an initial baseline telephone assessment administered by BHL health technicians, generally completed within three weeks of the date when PACE processed the prescription claim. This assessment was provided as part of the contracted clinical services, prior to approaching the PACE enrollee for informed consent to participate in the research component of this project.
Following the baseline assessment, enrollees were contacted by the BHL via telephone at weeks 2, 6, and 9 for follow-up monitoring, with a final contact at 6 months. In addition, all enrollees who completed the baseline interview received a follow-up call from BHL staff to complete a needs-assessment in order to connect the enrollee with available community resources. The calls at weeks 2, 6, and 9 consisted of brief (5–10 minutes), structured assessments to monitor adherence, side effects, and response to treatment (based on PHQ-9 scores). Calls continued even if an enrollee reported non-adherence with the prescribed medication. Based on symptom severity reported, BHL staff supplemented the monitoring calls with algorithm-based depression or anxiety care management to monitor and encourage patient acceptance and adherence to treatment recommendations through support, education, and motivational engagement. In addition, the care managers would utilize unstructured interventions as appropriate, such as education regarding sleep hygiene, help with problem-solving, and relaxation techniques. Following each interview, a progress report was sent to the prescribing clinician with an assessment and treatment recommendations. The 6-month call was a repeat of the baseline interview.
Domains assessed during the Baseline interview included sociodemographics along with history of depression or prior psychiatric care, and are described in detail elsewhere (Maust et al., 2011). Briefly, standardized instruments administered at baseline include: the Blessed Orientation-Memory-Concentration (BOMC) test (Katzman et al., 1983); MINI International Neuropsychiatric Interview (includes Psychosis, Mania, Generalized Anxiety Disorder (GAD), Panic Disorder, and Alcohol Abuse/Dependence modules) (Sheehan et al., 1998); Patient Health Questionnaire-9 (PHQ-9) (Kroenke, Spitzer, Williams, 2001); and Medical Outcomes Survey (SF-12), providing both a Physical Component Score (PCS) and Mental Component Score (MCS) (Ware, Kosinski, Keller, 1996).
The interview began with the BOMC; for those who scored ≥ 17 (which is indicative of moderate to severe cognitive impairment), the interview with the PACE enrollee was terminated and permission was sought to speak with a caregiver. This analysis only includes participants who completed both the baseline and 6-month interviews and does not include the caregiver interview data.
Adherence to the index medication was measured at all follow-up time points. A participant was classified as 'Yes Adherent' if at all, or nearly all, interview occasions s/he followed the prescribed dosing instructions, and the number of days taking the medication within the past seven days was consistent with prescribing instructions (which, in the case of an as-needed medication, may have meant only using it part of the time); otherwise s/he was classified as 'Not Adherent'. In some cases, a new antidepressant or anxiolytic claim sampled by PACE was for a participant that reported already receiving a psychotropic drug from the same or another class of medication. These participants remained in the analysis, though PACE claims data were obtained for each to identify other psychotropic medications prescribed in a 30-day window preceding sampling for the index medication.
For this analysis, the 87 who endorsed at least mild depressive symptoms (PHQ-9 ≥5) at baseline (Symptomatic Group, SG) were compared to the 75 least symptomatic participants (PHQ-9 <5, Minimally Symptomatic Group, MSG), as scores less than 5 generally signify absence of a depressive disorder (Kroenke, Spitzer, Williams, 2001). Baseline comparison of the SG and MSG for gender, ethnicity, financial status, marital status, past history of depression and mental health care, likely presence of GAD, adherence, and medications prescribed (antidepressant, anxiolytic, or both) was conducted using Pearson chi-square; analysis of age, depressive symptoms, PCS of the SF-12, MCS of the SF-12, and cognitive functioning was conducted using two-sample t-tests.
The comparison of 6-month to baseline scores among SG and MSG for the MCS was conducted with paired t-test. Change in PHQ-9 over time was examined with an unadjusted longitudinal mixed-effects model, using baseline as a linear term with random slope and random intercept for time, allowing incorporation of data from all participant contacts over the monitoring period. Initially an interaction model of time with group was evaluated and then models were run separately for the SG and MSG groups. A sensitivity analysis evaluating PHQ-9 in a longitudinal mixed model with all contacts over the monitoring period among all N=293 eligible potential participants, including the N=162 who completed, was also examined. The association of PHQ-9 change from baseline to 6 months with adherence to the index medication, prescribed medication class, SF-12 PCS, and selected demographic and baseline clinical characteristics was assessed with OLS regression with adjustment by baseline PHQ-9 score, separately among the SG and MSG. All tests were done using SAS 9.2.
Results
A total of 293 potential participants were eligible for the six-month interview. The six-month interview was successfully completed for 162 (55.3%) enrollees, while 57 (19.4%) refused and 37 (12.6%) could not be reached by telephone despite multiple attempts. Two (0.7%) enrollees were excluded from analysis as their BOMC scores surpassed 16 at the 6-month interview. Eight (2.7%) were unable to complete the follow-up interview due to hearing or speech impairment, nine (3.1%) were deceased, and 18 (6.1%) were not contacted due to staff error following the baseline assessment. The results that follow are for the 162 participants who completed interviews through the six-month follow-up period and consented to having their responses used for research. There was no statistically significant difference in baseline PHQ-9 scores between those who completed the follow-up (6.3, SD 5.4), those who refused (5.3, SD 5.2), or those who could not be contacted (7.1, SD 5.7) (F(2, 253) = 1.39, p=0.25).
Baseline demographic and clinical data are presented in Table 1. Of those who completed the six-month interview, slightly over half (87, 53.7%) endorsed at least mild depressive symptoms at baseline. The groups did not differ significantly by age, gender, or ethnicity, except that fewer participants in the symptomatic group reported feeling financially stable. The groups did differ significantly on most clinical features, with SG participants were more likely to report a past history of depression and meet screening criteria for a diagnosis of GAD. The SG also reported worse physical and emotional health overall, as reflected by the significantly lower PCS and MCS.
Table 1.
Baseline Sociodemographic and Clinical Characteristics of PACE Participants among the Symptomatic (PHQ-9 ≥ 5) or Minimally Symptomatic (PHQ-9 < 5) Groups
| All Baseline Interviewees N=162 |
Symptomatic Group (PHQ-9≥5) n=87 (53.7%) |
Minimally Symptomatic Group (PHQ-9<5) n=75 (46.3%) |
Test(df), p-value | |
|---|---|---|---|---|
| Age (Mean, SD) | 77.2 (6.8) | 76.8 (7.3) | 77.6 (6.2) | t(160)=0.77, p=0.45 |
| Gender | ||||
| Male | 34 (21.0%) | 21 (24.1%) | 13 (17.3%) | χ2(1)=1.12, p=0.29 |
| Female | 128 (79.0%) | 66 (75.9%) | 62 (82.7%) | |
| Ethnicity | ||||
| White | 150 (92.6%) | 82 (94.2%) | 68 (90.7%) | χ2(1)=0.76, p=0.38 |
| Non-White | 12 (7.4%) | 5 (5.8%) | 7 (9.3%) | |
|
Financial Status (have at least "enough to get along" for the duration of the study) |
141 (87.0%) | 70 (80.5%) | 71(94.7%) | χ2(1)=7.21, p=0.007 |
| Married | 43 (26.5%) | 25 (28.7%) | 18 (24.0%) | χ2(1)=0.46, p=0.50 |
|
Depressive Symptoms (PHQ-9) (Mean, SD) |
6.3 (5.4) | 10.0 (4.6) | 1.9 (1.5) | t(160)=-14.6, p<0.0001 |
| Past history of depression | 44 (27.2%) | 31 (35.6%) | 13 (17.3%) | χ2(1)=6.82, p=0.009 |
| Generalized Anxiety Disorder | 13 (8.0%) | 13 (14.9%) | 0 (0%) | χ2(1)=12.2, p=0.0005 |
| Overall General Functioning (SF-12) | ||||
| Physical Component Score (Mean, SD) | 41.6 (12.1) | 39.8 (12.2) | 43.7 (11.8) | t(160)=2.0, p=0.044 |
| Mental Component Score (Mean, SD) | 49.7 (10.5) | 44.8 (10.2) | 55.5 (7.5) | t(160)=7.5, p<0.0001 |
| In Mental Health Care in Past 2 Years | 11 (6.8%) | 7 (8.0%) | 4 (5.3%) | χ2(1)=0.47, p=0.49 |
|
Cognitive Function (BOMC) (Mean, SD) |
4.4 (3.8) | 4.6 (3.9) | 4.1 (3.8) | t(160)=-0.69, p=0.49 |
| Prescribed Medication | ||||
| Antidepressant Only | 82 (50.6%) | 45 (51.7%) | 37 (49.3%) | χ2(2)=1.18, p=0.55 |
| Anxiolytic Only | 50 (30.9%) | 24 (27.6%) | 26 (34.7%) | |
| Both | 30 (18.5%) | 18 (20.7%) | 12 (16.0%) | |
| Adherence to Index Medication | ||||
| Yes | 91 (56.2%) | 58 (66.7%) | 33 (44.0%) | χ2(1)=8.4, p=0.0037 |
| No/Not Sure | 71 (43.8%0 | 29 (33.3%) | 42 (56.0%) |
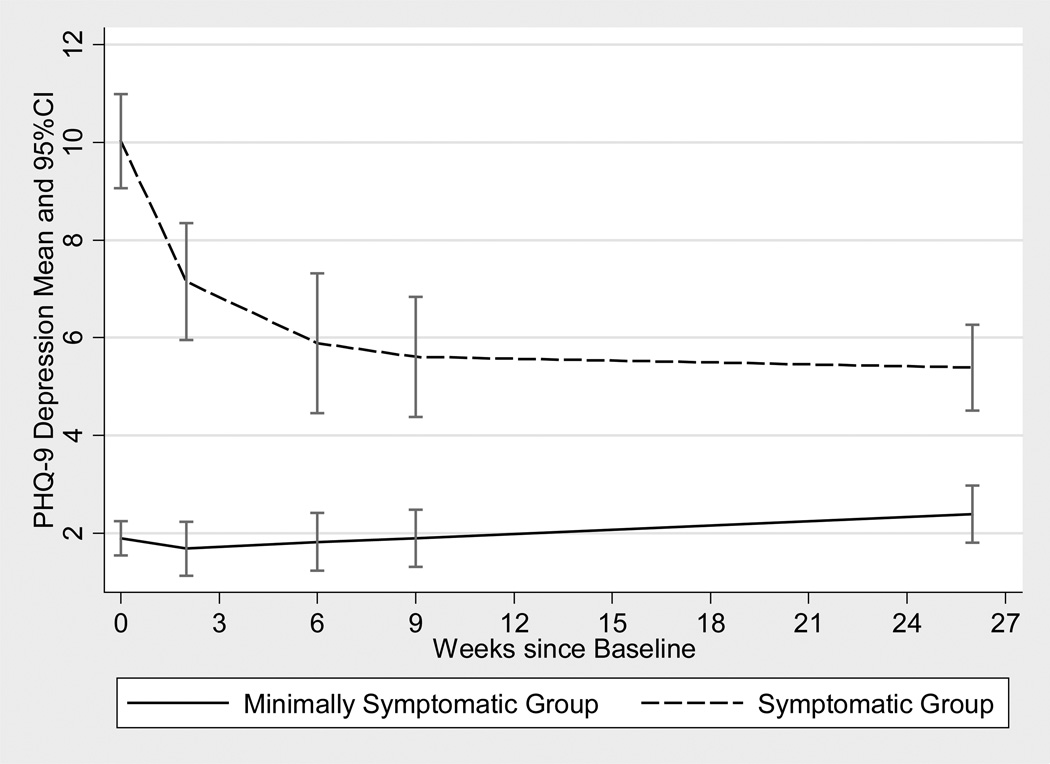
Figure 1 presents the change in PHQ-9 over time for the two symptom groups. Results from the mixed-effects model for PHQ change over time interacting with group was highly significant (F(1, 362) = 31.56, p< 0.0001). Results modeled separately for each baseline group are shown in Table 2. Among the SG, PHQ-9 demonstrated a statistically significant decrease of 0.47 per month while those in MSG showed a nonsignificant increase of 0.09 PHQ-9 score per month. Sensitivity analyses incorporating all observations for the 293 patients eligible for follow-up yielded nearly identical results, with a statistically significant decline in PHQ for those symptomatic at baseline, with no statistically significant change among the minimally symptomatic group.
Figure 1.
Longitudinal course (weeks since baseline) of PHQ-9 score means and 95% confidence intervals for Symptomatic v. Minimally Symptomatic Groups.
Table 2.
PHQ-9 Symptoms Mean (SD) at Baseline and 6 months with mixed model using all observations in the 6-month observation window.
| Baseline PHQ-9 (SD) |
6 Months PHQ-9 (SD) |
Observations | Beta est. PHQ-9 per month |
F-test (df) | p-value | |
|---|---|---|---|---|---|---|
|
All Baseline Interviewees N=162 |
6.3 (5.4) | 4.0 (3.8) | 686 | B= −0.22 | F(1,161)=17.06 | p<0.0001 |
|
Symptomatic Group (PHQ-9≥5) N=87 |
10.0 (4.6) | 5.4 (4.2) | 352 | B= −0.47 | F(1,86)=29.53 | p<0.0001 |
|
Minimally Symptomatic Group (PHQ-9<5) N=75 |
1.9 (1.5) | 2.4 (2.6) | 334 | B= 0.09 | F(1, 74)=3.36 | p=0.071 |
We tested our hypotheses that adherence, medication class, and SF-12 physical component score (continuous) were associated with improvement in SG depressive symptoms. None of these approached statistical significance. Medication class was not associated with improvement, nor was the use of both an antidepressant and an anxiolytic, though those in the SG were significantly more likely to report adherence to the new medication (58 (66.7%) v. 33 (44.0%), χ 2(1)=8.4, p=0.0037) compared to those in MSG. In addition, symptomatic participants reported equivalent improvement in depressive symptoms regardless of age, race, gender, or financial status with no significant association with any of these demographic characteristics. Similarly, cognitive functioning (continuous Blessed score) was not associated with improvement in depressive symptoms in the SG group. While presence of Generalized Anxiety Disorder (GAD) at baseline was more likely for the SG group, it was not associated with change in depressive symptoms.
Discussion
This paper presents data from 162, non-institutionalized older adults in a telephone care management program following initiation of a new antidepressant or anxiolytic. The previously reported baseline analysis of this population was striking for the low symptom burden, given the fact that a new psychotropic medication had been initiated, presumably for a perceived psychiatric disorder (Maust et al., 2011). Here, we present longitudinal data for this population, an old old and non-institutionalized group for whom outcomes in care management have not been described outside of a clinical trial or post-trial extension.
For these analyses, we split the baseline group into symptomatic (SG) vs. minimally symptomatic (MSG) with two goals in mind. First, and at the very least, we wanted to know whether the MSG would remain that way or appear worse on subsequent contacts. Worsening could suggest the baseline Behavioral Health Laboratory (BHL) telephone assessment did not fully capture the initial clinical picture that led to the provider’s prescription. In fact, depressive symptoms in the MSG remained essentially unchanged over the six months, with a corresponding lack of change on the Mental Component Score (MCS) of the SF-12, which, starting at 55.5 (SD 7.5), reflects better self-ratings of emotional well-being than the general adult population (mean of 50.0). This seems to suggest that the baseline assessment accurately captured the overall emotional health and relative lack of depressive symptoms in this group, leading to concerns about potential inappropriate psychotropic prescribing for this group. While risks associated with benzodiazepines and anticholinergic antidepressants have been described in older adults (Beers, 1997; Wang et al., 2001; Chang et al., 2008), there is a new and shifting antidepressant risk-benefit calculus, given limited evidence of benefits for antidepressants in subsyndromal (Bruce et al., 2004) or even mild to moderate depression (Fournier et al., 2010) while additional risks such as fractures (Ginzburg and Rosero, 2009; Gagne et al., 2011) and adverse impact on cognitive function (Culang et al., 2009; Sneed et al., 2010) are being described.
The second goal was to describe symptom change for the symptomatic group over the course of follow-up with telephone care management. The symptomatic participants did get better over time, with a statistically significant drop in their PHQ-9 from 10.0 (SD 4.6) to 5.4 (SD 4.2), a drop from the high-end to the low-end of mild depressive symptoms. Likewise, the MCS mirrors this improvement. It may be that the SG participants represent a distinct clinical population, especially given that they were more likely to report a history of depression, screen positive for comorbid GAD, and report lower overall physical and mental health. Interestingly, the improvement seemed to occur for all participants, with no significant predictors among the clinical or demographic variables tested. In addition, symptomatic individuals at baseline were more likely to report adherence to their index medication, perhaps suggesting that they were clearer on the need for and rationale behind the prescription. It is curious that the improvement in depressive symptoms did not vary with medication class, which could suggest that: symptoms improved regardless of medication, the short term benefits of anxiolytics apply to symptoms of mild depression, or the anxiety symptoms were most responsive as suggested by the association of GAD at baseline with improvement in the SG.
Our analysis has several important limitations. First, we do not have a definitive indication of what led the prescribers to initiate these new psychotropic medications. One assumption is that, if not prescribed for a psychiatric disorder, then perhaps the medication was intended to treat perceived emotional distress. However, the low overall symptom burden at baseline and thereafter for both groups (the baseline average PHQ-9 for the symptomatic group was just 10.0 (SD 4.6)) suggests that the perceived distress was short-lived. Another limitation is that we do not have a comparison group either without active medication or without care management. It is therefore not possible to attribute SG improvement to either the medication or some aspect of care management. In addition, while we do consider antidepressant and anxiolytic combination therapy in our models, these participants are likely prescribed multiple medications, which may vary between the groups and influence symptom change. Nevertheless, given the difficulty of treating late-life depression, it is encouraging that the symptomatic group did in fact show significant and sustained improvement over 6 months with a combination of telephone care management and newly-prescribed medication, even though starting from only a low-moderate symptomatic baseline. The effect was present despite the advanced age of participants, suggesting that telephone care management in this old old group is feasible. Lastly, a large percentage of participants who completed the baseline interview either refused the final six month interview (57, 19.4%) or were unable to be reached (37, 12.6%), despite multiple calls. However, a sensitivity analysis including all 293 of the eligible participants of the main mixed-effects analysis of change in the PHQ-9 score over time was nearly identical with the results of the N=162 completers. Further, the depressive symptoms at baseline of the N=131 non-completers did not differ significantly from those that completed follow-up through 6 months (t(291)= −0.38, p= 0.70), arguing against the possibility that we lost the more symptomatic participants.
Given the potential side-effects of CNS-active medications (Wang et al., 2001; Ensrud et al., 2002; Chang et al., 2008; Kerse et al., 2008), the benefit of initiating an anxiolytic or antidepressant in a relatively asymptomatic older adult becomes harder to justify. Going forward, we plan to suggest that patients who are minimally symptomatic at baseline and remain so upon follow-up discuss the rationale for continuing the index medication with their providers, but will continue to monitor these participants for emergence of symptoms following potential discontinuation. In this way, hopefully care management can help providers enhance the specificity of their psychotropic prescribing and minimize patient exposure to the risks of medication in the absence of a clear indication.
Conclusion
This is the first work to report that a telephone-based care management strategy could potentially be an effective tool in the management of depression in non-institutionalized older adults. Given the state-wide distribution of the participants to include those from rural areas, and the small proportion (11 (6.8%) of 162) who reported accessing mental health care in the past two years, this is an important extension of the evidence supporting the feasibility of using care management in the treatment of depression.
Key points.
Psychotropic medications are initiated in older adults with relatively low symptoms of depression.
Symptomatic older adults improve over 6 months with combination of a new medication and telephone monitoring.
This improvement is desirable and could not have been assumed given participant age and their previously limited utilization of specialty mental health care.
Acknowledgments
Funding Sources: PACE Program, Pennsylvania Department of Aging, Harrisburg, PA. DTM was supported by the NIMH-funded Clinical Research Scholars Program (R25-MH060490) of the Department of Psychiatry, University of Pennsylvania.
References
- American Psychiatric Association. A New Direction in Depression Treatment in Minnesota: DIAMOND Program, Institute for Clinical Systems Improvement, Bloomington, Minnesota. Psychiatr. Serv. 2010;61:1042–1044. doi: 10.1176/ps.2010.61.10.1042. [DOI] [PubMed] [Google Scholar]
- Bao Y, Post EP, Ten TR, Schackman BR, Bruce ML. Achieving effective antidepressant pharmacotherapy in primary care: the role of depression care management in treating late-life depression. J. Am. Geriatr. Soc. 2009;57:895–900. doi: 10.1111/j.1532-5415.2009.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers MH. Explicit Criteria for Determining Potentially Inappropriate Medication Use by the Elderly: An Update. Arch. Intern. Med. 1997;157:1531–1536. [PubMed] [Google Scholar]
- Bruce ML. The Association Between Depression and Disability. American Journal of Geriatric Psychiatry. 1999;7:8–11. [PubMed] [Google Scholar]
- Bruce ML, Ten Have TR, Reynolds CF, Katz II, Schulberg HC, Mulsant BH, Brown GK, McAvay GJ, Pearson JL, Alexopoulos GS. Reducing Suicidal Ideation and Depressive Symptoms in Depressed Older Primary Care Patients. JAMA. 2004;291:1081–1091. doi: 10.1001/jama.291.9.1081. [DOI] [PubMed] [Google Scholar]
- Chang C, Wu EC, Chang IS, Lin K. Benzodiazepine and Risk of Hip Fractures in Older People: A Nested Case-Control Study in Taiwan. American Journal of Geriatric Psychiatry. 2008;16:686–692. doi: 10.1097/JGP.0b013e31817c6a99. [DOI] [PubMed] [Google Scholar]
- Culang ME, Sneed JR, Keilp JG, Rutherford BR, Pelton GH, Devanand DP, Roose SP. Change in cognitive functioning following acute antidepressant treatment in late-life depression. Am. J. Geriatr. Psychiatry. 2009;17:881–888. doi: 10.1097/jgp.0b013e3181b4bf4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensrud KE, Blackwell TL, Mangione CM, Bowman PJ, Whooley MA, Bauer DC, Schwartz AV, Hanlon JT, Nevitt MC For The Study of Osteoporotic Fractures Research Group. Central Nervous System?Active Medications and Risk for Falls in Older Women. J. Am. Geriatr. Soc. 2002;50:1629–1637. doi: 10.1046/j.1532-5415.2002.50453.x. [DOI] [PubMed] [Google Scholar]
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, Fawcett J. Antidepressant Drug Effects and Depression Severity: A Patient-Level Meta-analysis. JAMA. 2010;303:47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RG, Huskamp HA, Pincus HA. Aligning Incentives in the Treatment of Depression in Primary Care With Evidence-Based Practice. Psychiatr. Serv. 2003;54:682–687. doi: 10.1176/appi.ps.54.5.682. [DOI] [PubMed] [Google Scholar]
- Gagne JJ, Patrick AR, Mogun H, Solomon DH. Antidepressants and Fracture Risk in Older Adults: A Comparative Safety Analysis. Clin. Pharmacol. Ther. 2011;89:880–887. doi: 10.1038/clpt.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg R, Rosero E. Risk of Fractures with Selective Serotonin-Reuptake Inhibitors or Tricyclic Antidepressants. Ann. Pharmacother. 2009;43:98–103. doi: 10.1345/aph.1L264. [DOI] [PubMed] [Google Scholar]
- Grypma L, Haverkamp R, Little S, Unützer J. Taking an evidence-based model of depression care from research to practice: Making lemonade out of depression. Gen. Hosp. Psychiatry. 2006;28:101–107. doi: 10.1016/j.genhosppsych.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Alexopoulos GS, Bartels SJ, Cummings JL, Gallo JJ, Gottlieb GL, Halpain MC, Palmer BW, Patterson TL, Reynolds CF, 3rd, Lebowitz BD. Consensus statement on the upcoming crisis in geriatric mental health: research agenda for the next 2 decades. Arch. Gen. Psychiatry. 1999;56:848–853. doi: 10.1001/archpsyc.56.9.848. [DOI] [PubMed] [Google Scholar]
- Katon W, Unützer J, Wells K, Jones L. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen. Hosp. Psychiatry. 2010;32:456–464. doi: 10.1016/j.genhosppsych.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am. J. Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- Kerse N, Flicker L, Pfaff JJ, Draper B, Lautenschlager NT, Sim M, Snowdon J, Almeida OP. Falls, Depression and Antidepressants in Later Life: A Large Primary Care Appraisal. PLoS ONE. 2008;3:e2423. doi: 10.1371/journal.pone.0002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klap R, Unroe KT, Unutzer J. Caring for mental illness in the United States: a focus on older adults. Am. J. Geriatr. Psychiatry. 2003;11:517–524. [PubMed] [Google Scholar]
- Krahn DD, Bartels SJ, Coakley E, Oslin DW, Chen H, McIntyre J, Chung H, Maxwell J, Ware J, Levkoff SE. PRISM-E: Comparison of Integrated Care and Enhanced Specialty Referral Models in Depression Outcomes. Psychiatr. Serv. 2006;57:946–953. doi: 10.1176/ps.2006.57.7.946. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luber MP, Meyers BS, Williams-Russo PG, Hollenberg JP, DiDomenico TN, Charlson ME, Alexopoulos GS. Depression and service utilization in elderly primary care patients. Am. J. Geriatr. Psychiatry. 2001;9:169–176. [PubMed] [Google Scholar]
- Lyness JM, King DA, Cox C, Yoediono Z, Caine ED. The importance of subsyndromal depression in older primary care patients: prevalence and associated functional disability. J. Am. Geriatr. Soc. 1999;47:647–652. doi: 10.1111/j.1532-5415.1999.tb01584.x. [DOI] [PubMed] [Google Scholar]
- Maust DT, Mavandadi S, Eakin A, Streim JE, Difillipo S, Snedden T, Oslin DW. Telephone-based behavioral health assessment for older adults starting a new psychiatric medication. Am. J. Geriatr. Psychiatry. 2011;19:851–858. doi: 10.1097/JGP.0b013e318202c1dc. [DOI] [PubMed] [Google Scholar]
- Moak GS. Treatment of Late-Life Mental Disorders in Primary Care: We Can Do a Better Job. J. Aging Soc. Policy. 2011;23:274–285. doi: 10.1080/08959420.2011.579503. [DOI] [PubMed] [Google Scholar]
- Olfson M, Pincus HA. Outpatient mental health care in nonhospital settings: distribution of patients across provider groups. Am. J. Psychiatry. 1996;153:1353–1356. doi: 10.1176/ajp.153.10.1353. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Ross J, Sayers S, Murphy J, Kane V, Katz IR. Screening, assessment, and management of depression in VA primary care clinics. The Behavioral Health Laboratory. J. Gen. Intern. Med. 2006;21:46–50. doi: 10.1111/j.1525-1497.2005.0267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin DW, Sayers S, Ross J, Kane VMSW, Ten Have T, Conigliaro J, Cornelius J. Disease Management for Depression and At-Risk Drinking Via Telephone in an Older Population of Veterans. Psychosom. Med. 2003;65:931–937. doi: 10.1097/01.psy.0000097335.35776.fb. [DOI] [PubMed] [Google Scholar]
- Oxman TE, Dietrich AJ, Schulberg HC. The Depression Care Manager and Mental Health Specialist as Collaborators Within Primary Care. American Journal of Geriatric Psychiatry. 2003;11:507–516. [PubMed] [Google Scholar]
- Pincus HA, Pechura CM, Elinson L, Pettit AR. Depression in primary care: linking clinical and systems strategies. Gen. Hosp. Psychiatry. 2001;23:311–318. doi: 10.1016/s0163-8343(01)00165-7. [DOI] [PubMed] [Google Scholar]
- Reiss-Brennan B, Briot PC, Savitz LA, Cannon W, Staheli R. Cost and quality impact of Intermountain's mental health integration program. J. Healthc. Manag. 2010;55:97–113. [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Sneed JR, Culang ME, Keilp JG, Rutherford BR, Devanand DP, Roose SP. Antidepressant medication and executive dysfunction: a deleterious interaction in late-life depression. Am, J. Geriatr. Psychiatry. 2010;18:128–135. doi: 10.1097/JGP.0b013e3181c796d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unutzer J, Katon W, Sullivan M, Miranda J. Treating depressed older adults in primary care: narrowing the gap between efficacy and effectiveness. Milbank Q. 1999;77:225–256. 174. doi: 10.1111/1468-0009.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unutzer J, Patrick DL, Simon G, Grembowski D, Walker E, Rutter C, Katon W. Depressive symptoms and the cost of health services in HMO patients aged 65 years and older. A 4-year prospective study. JAMA. 1997;277:1618–1623. doi: 10.1001/jama.1997.03540440052032. [DOI] [PubMed] [Google Scholar]
- Unutzer J, Katon W, Callahan CM, Williams JW, Jr, Hunkeler E, Harpole L, Hoffing M, Della Penna RD, Noel PH, Lin EH, Arean PA, Hegel MT, Tang L, Belin TR, Oishi S, Langston C IMPACT Investigators. Improving Mood-Promoting Access to Collaborative Treatment. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- Von Korff M, Gruman J, Schaefer J, Curry SJ, Wagner EH. Collaborative management of chronic illness. Ann. Intern. Med. 1997;127:1097–1102. doi: 10.7326/0003-4819-127-12-199712150-00008. [DOI] [PubMed] [Google Scholar]
- Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Hazardous Benzodiazepine Regimens in the Elderly: Effects of Half-Life, Dosage, and Duration on Risk of Hip Fracture. Am. J. Psychiatry. 2001;158:892–898. doi: 10.1176/appi.ajp.158.6.892. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Med. Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]